Abstract
Based on Mader's mathematical model, the rate of capillary blood lactate concentration (νLamax) following intense exercise is thought to reflect the maximal glycolytic rate. We aimed to investigate the reliability of important variables of Mader's model (i.e. power output, lactate accumulation, predominant phosphagen contribution time frames (tP Cr)) and resulting νLamax values derived during and after a 15‐s cycling sprint. Fifty cyclists performed a 15‐s all‐out sprint test on a Cyclus2 ergometer three times. The first sprint test was considered a familiarization trial. Capillary blood was sampled before and every minute (for 8 min) after the sprint to determine νLamax. Test–retest analysis between T2 and T3 revealed excellent reliability for power output (Pmean and Ppeak; ICC = 0.99, 0.99), ∆La and νLamax with tPCr of 3.5 s (ICC = 0.91, 0.91). νLamax calculated with tPCr = tP peak (ICC = 0.87) and tP Cr = tPpeak–3.5% (ICC = 0.79) revealed good reliability. tPpeak and tPpeak–3.5% revealed only poor and moderate reliability (ICC = 0.41, 0.52). Power output and ∆La are reliable parameters in the context of this test. Depending on tPCr, reliability of νLamax varies considerably with tP Cr of 3.5 s showing excellent reliability. We recommend standardization of this type of testing especially tP Cr.
Keywords: alactic, ATP, energy, high‐intensity, metabolism, muscle
1. INTRODUCTION
Many test procedures and physiological variables exist to judge the aerobic and anaerobic metabolic components involved in high‐intensity cycling. In recent years the post‐exercise level of capillary blood lactate accumulation has received increasing interest in order to calculate the glycolytic capacity in cycling (Adam et al., 2015; Dunst et al., 2023; Quittmann, Abel, et al., 2021; Quittmann, Schwarz, et al., 2021; Yang et al., 2023), hand‐cycling (Quittmann, Abel, et al., 2021; Quittmann et al., 2018) and other endurance sports including running (Quittmann et al., 2022; Quittmann, Appelhans, et al., 2020; Quittmann, Schwarz, et al., 2021), swimming (Mavroudi et al., 2023; Teixeira et al., 2022) and rowing (Schünemann et al., 2023). In this regard the peak accumulation of capillary blood lactate within 10 min after a 15‐s all‐out sprint (often abbreviated as νLamax) has been widely discussed among athletes and within the coaching community as a feasible indicator to calculate the glycolytic capacity during sprint cycling (note: here νLamax is preferred over “ċLamax” due to its established recognition) (Heck & Schulz, 2002; Mader, 2003; Mader & Heck, 1986; Quittmann et al., 2022).
Short maximal efforts (such as 15‐s sprint cycling) rely largely on the phosphocreatine pathway as well as glycolysis to regenerate ATP rapidly (Heck & Schulz, 2002; Nevill et al., 1994; Yang et al., 2023). Therefore, the ability to produce lactate quickly is considered one important predictor of sprint performance. νLamax serves as one necessary variable for a metabolic model developed by Mader (Mader, 2003; Mader & Heck, 1986). Within Mader's model, the νLamax is thought to indirectly estimate the maximal activity of phosphofructokinase as the bottleneck of glycolysis. PFK, the principal regulator of glycolysis, is activated by the presence of ADP, AMP, and inorganic phosphate, and is inhibited by ATP, H+ ions, and citric acid (Mader, 2003).Consequently, the rate of lactate accumulation in the blood serves as an indirect indicator of glycolytic activity. This rationale underpins the standardized duration of 15 seconds for the test, which is designed to ensure maximal glycolytic activation through the accumulation of ADP and AMP, while minimizing inhibition by H+ ions.
As one consequence the elevated rate of νLamax in Mader's model is indicative of increased reliance of glycolytic energy production.
νLamax is commonly calculated as proposed by Mader (Heck & Schulz, 2002; Mader, 1984).
With Lapeakpost as the highest post‐exercise capillary blood lactate value, Lapre the capillary blood lactate value measured immediately before sprinting, t test = the sprint time (which in a 15‐s cycle sprint is set to 15 s) and tPCr as the assumed time of predominant phosphagen contribution of the test. As Mader's original model is rooted in enzymatic rates, the inclusion of a time variable in the denominator (Mader & Heck, 1986) becomes essential. This addition is imperative due to the time‐dependent nature of enzyme‐catalyzed reactions and serves to represent the duration during which glycolysis plays a role in energy production.
Based on the formula, the value of the denominator is heavily influenced by the tPCr and its determination. Unfortunately, several methods to determine tPCr exist including the time from sprint start to peak power (Ppeak) (Manunzio et al., 2016) or the time from sprint start until reaching Ppeak and subsequently declining by 3.5% (Adam et al., 2015; Quittmann, Schwarz, et al., 2021) prohibiting the comparison of results between studies. In situations where the requisite sampling rates for determining specific measures are not available, a practical duration of 3.5 seconds can be adopted, following the recommendations provided by Heck and Schulz (Heck & Schulz, 2002). Interestingly, although tPCr is a very important element in the formula its determination has not been discussed much (Dunst et al., 2023; Yang et al., 2023) and no thorough reliability investigation of the different tPCr exists so far. The process of calculating νLamax, which hinges on a foundation of physiological data, is subject to typical variability inherent in such measurements. This variability underscores the importance of ensuring high reliability in these calculations. This reliability is not just a technical concern but is fundamental for making accurate and informed interpretation where νLamax is applied. Therefore, rigorous validation and verification of these data are essential for the integrity and efficacy of decision‐making processes based on νLamax metrics.
So far a limited number of studies including a low sample sizes (n = 17–23) assessed the reliability of νLamax in amateur running (n = 18); (Quittmann, Schwarz, et al., 2021), hand‐cycling (n = 18) (Quittmann, Abel, et al., 2021), rowing (n = 17) (Held et al., 2023) and cycling (n = 18–23; sport students) (Adam et al., 2015; Quittmann, Abel, et al., 2021; Quittmann, Schwarz, et al., 2021) but these included different sprint durations, amount of involved active muscle mass (i.e. upper and lower body muscle) and different ttest‐ tPCr time portion for νLamax calculation. Based on the available information, the question arises how reliable the νLamax quantification is in experienced amateur cyclists when employing different tPCr.
Therefore, the goal of the present experiment was to investigate the reliability of important variables of Mader's model derived during and after 15‐s sprint cycling performance (i.e. peak and mean power output, post‐exercise capillary blood lactate measurements and different tPCr measurements). We hypothesize that the corresponding parameters related to νLamax‐testing display robust reliability allowing for their further application in science and practice.
2. METHODS
2.1. Participants
A cohort of n = 50 (n = 30 male, n = 20 female) experienced cyclists with more than 3 years of regular cycling exercise (>2 sessions per week) were recruited for this study. All participants were experienced in road cycling with clipless pedals and cycled regularly as exercise. Prior to the study, the participants were informed of the protocol and gave their written informed consent to participate. All procedures were approved by the ethical committee of Exercise Science & Training of the Faculty of Human Sciences (EV2024/1–1004) and conducted in accordance with the Declaration of Helsinki (Harriss & Atkinson, 2009). Participants characteristics are given in Table 1.
TABLE 1.
Mean ± SD age, body stature, selected anthropometric data and peak oxygen uptake of participants.
| Variable | All (n = 50) |
|---|---|
| Age (years) | 31.2 ± 7.8 |
| Height (cm) | 177.5 ± 9.3 |
| Body mass (kg) | 71.7 ± 12.4 |
| Body fat (%) | 13.9 ± 4.8 |
| Fat‐free mass (kg) | 61.9 ± 11.5 |
| Maximum oxygen uptake (mL/kg/min) | 55.4 ± 7.6 |
2.2. Experimental design
Three visits to the laboratory were required which were at least 48 h apart and completed within a period of 2 weeks. Figure 1 illustrates the timeline and all testing procedure for each visit.
FIGURE 1.
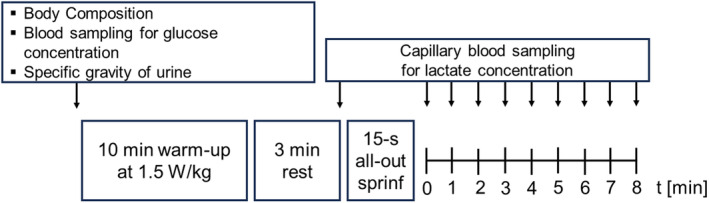
Illustration of the time line of all study procedures.
All participants were instructed to keep a nutrition diary and to repeat their usual diet for each visit within the 24 h before each visit (Jeacocke & Burke, 2010). Additionally, all were instructed to stay adequately hydrated, to eat a carbohydrate‐rich meal (i.e. a banana and a jam sandwich) no less than 3 h before each visit and to refrain from caffeine consumption. Each participant received 35 g of a carbohydrate mixture (IsoFast, DextroEnergy, Krefeld, Germany) dissolved in 500 mL of water to drink ad libitum during warm‐up and recovery periods. All participants provided a urine sample in a cup first when visiting the laboratory. Hydration status was then promptly tested via urine specific gravity analysis employing a dipstick (One step 10, DFI Co., Gyeongsangnam‐do, South Korea).
During the first visit, body composition that is, fat‐free mass (FFM) of all participants were measured employing eight‐electrode impedance analysis (InBody 720, Biospace, Des Moines, Iowa, USA).
All cycle sprints were conducted on a Cyclus2 ergometer (RBM, Leipzig, Germany) and their own personal road bike. The Cyclus2 is an electromagnetically braked ergometer and measures power with an accuracy error of 2% according to the manufacturer. All cyclists used their own shoes and pedals for all tests. For all three visits, all cyclists warmed up for 10 min cycling at 1.5 W/kg body mass and resting for 3 min (Quittmann, Schwarz, et al., 2020).
The all‐out cycle sprint was performed in a seated position utilizing the large chainring (if applicable) of the participant's bike and the 15‐tooth cog of the ergometer. Recording of the test started with cadence of >30 RPM. The ergometer software was set to isokinetic mode and 130 RPM (Adam et al., 2015; Nitzsche et al., 2018; Quittmann, Schwarz, et al., 2020).
Capillary blood samples of the left earlobe were sampled twice during the resting period, after the warm‐up and once directly after the sprint as well as every minute for 9 min after the 15‐s cycle sprint. Lactate concentration was measured amperometric‐enzymatically employing Biosen C‐Line (EKF Diagnostics, Barleben, Germany).
2.2.1. Analysis of tP Cr
Sprint power data were processed with Winlaktat (6, mesics, Münster, Germany) to determine values for tPpeak and tPpeak–3.5%. tPpeak was calculated as the time from start of the test until peak power output was reached (Manunzio et al., 2016). tPpeak–3.5% was calculated as the time from the start of the test until peak power was reached and subsequently decreased by 3.5% or more (Quittmann, Schwarz, et al., 2021). Existing measures were employed to preclude the introduction of an additional time frame, with tPpeak–3.5% being utilized to reflect the accuracy error of an early version of the SRM ergometer (Yang et al., 2023). Additionally, a fixed tPCr of 3.5 s was used for calculations. This time frame represents the mean of Heck and Schulz's assumptions of tPCr = 3 s for a test duration of 10s and tPCr = 4 s for a test duration of 20s and is a time frame commonly used in absence of adequate sampling rates for determination of tPpeak and tPpeak–3.5%.
2.3. Statistical analyses
Raw data was processed using Microsoft Excel. Statistical analyses (mean, standard deviations, and 95% confidence intervals) were computed with GraphPad Prism (10, Boston, MA, USA). Data normality for all measured variables (Ppeak, Pmean, ∆La, FFM, body mass) was assessed using the Shapiro–Wilk test and visual inspection, without requiring further transformation.
Since T1 represented a familiarization trial we excluded this data for further reliability testing.
Intraclass correlations were calculated using JASP 0.18.1 (ICC, Model: Alpha, two‐way random; Type: absolute agreement, single measures) for all relevant measures of the cycle sprint test (Ppeak, Pmean, ∆La, tPpeak and tPpeak–3.5%) and subsequent calculations of νLamax (with tPCr = 3.5 s, tPpeak and tPpeak–3.5%). (Koo & Li, 2016; Shrout & Fleiss, 1979). Interpretation was based on the 95% confidence interval of the ICC estimate. Values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.9, and greater than 0.90 are indicative of poor, moderate, good, and excellent reliability, respectively (Koo & Li, 2016).Standard error of measurement (SEM) was calculated from the square root of the mean square error term in a repeated measures ANOVA according to the method of Stratford and Goldsmith and Eliasziw et al (Atkinson & Nevill, 1998; Eliasziw et al., 1994; Stratford & Goldsmith, 1997) for measures of the cycle sprint test (Ppeak, Pmean, ∆La, tPpeak and tPpeak–3.5%) and calculations of νLamax (with tPCr = 3.5 s, tPpeak and tPpeak–3.5%). Coefficient of Variation was calculated as the ratio of SEM to the grand mean of T2 and T3 (Weir & Vincent, 2020).
Bland–Altman plots and limits of agreement were calculated using the difference between methods compared to the average for the varying calculation methods of νLamax (with tPCr = 3.5 s, tPpeak and tPpeak–3.5%) for T2 and T3 (Martin Bland & Altman, 1986). 95% Limits of agreement and bias was calculated according to the method of Bland–Altmann for Ppeak, Pmean, ∆La, tPpeak, tPpeak–3.5% and all νLamax calculations in T2 and T3 (Martin Bland & Altman, 1986).
3. RESULTS
All mean ± SD data for all variables assessed during the 15‐s cycle sprints are summarized in Table 2.
TABLE 2.
All variables (Mean ± SD) obtained during and after the relevant (T2, T3) 15‐s cycle sprints used for analysis.
| Variable | T2 (second visit) | T3 (third visit) |
|---|---|---|
| νLamax (3.5 s) [mmol/l/s] | 0.55 ± 0.14 | 0.54 ± 0.13 |
| νLamax (tPpeak) [mmol/l/s] | 0.50 ± 0.11 | 0.49 ± 0.13 |
| νLamax (tPpeak–3.5%) [mmol/l/s] | 0.56 ± 0.13 | 0.53 ± 0.14 |
| Ppeak (W) | 970 ± 272 | 974 ± 283 |
| Pmean (W) | 758 ± 195 | 752 ± 194 |
| Total Work (J) | 11,373 ± 2927 | 11,284 ± 2912 |
| Pmean/FFM [W/kg] | 12.16 ± 1.47 | 12.06 ± 1.49 |
| LaPre [mmol/L] | 0.90 ± 0.26 | 0.80 ± 0.22 |
| Lamaxpost [mmol/L] | 7.18 ± 1.63 | 7.03 ± 1.81 |
| ∆La [mmol/l] | 6.28 ± 1.57 | 6.23 ± 1.79 |
| tPpeak [s] | 2.45 ± 0.87 | 2.29 ± 0.81 |
| tPpeak–3.5% [s] | 3.77 ± 1.35 | 3.40 ± 1.30 |
| Urine specific gravity (g/mL) | 1.020 ± 0.33 | 1.016 ± 0.013 |
| Blood glucose [mmol/L] | 5.5 ± 0.7 | 5.4 ± 0.5 |
Abbreviations: νLamax (3.5 s) = νLamax calculated with tPCr = 3.5 s; νLamax (tPpeak) = νLamax calculated with tPCr = tPpeak; νLamax (tPpeak–3.5%) = νLamax calculated with tPCr = tPpeak–3.5%; Ppeak = peak power during the sprint test; Pmean = average power during the sprint test; Pmean/FFM = average power during the sprint test normalized to fat‐free mass; Lapre = blood lactate concentration before the sprint test; Lamaxpost = maximal blood lactate concentration in 8 minutes after the sprint test; ∆La = difference between Lapre and Lamaxpost; tPpeak = time until Ppeak was reached; tPpeak–3.5% = time until Ppeak–3.5% was reached.
3.1. Reliability calculation T2, T3
The correlation matrix of all variables T2 vs. T3 are illustrated in Figure 2. Reliability measures are summarized in Table 3.
FIGURE 2.
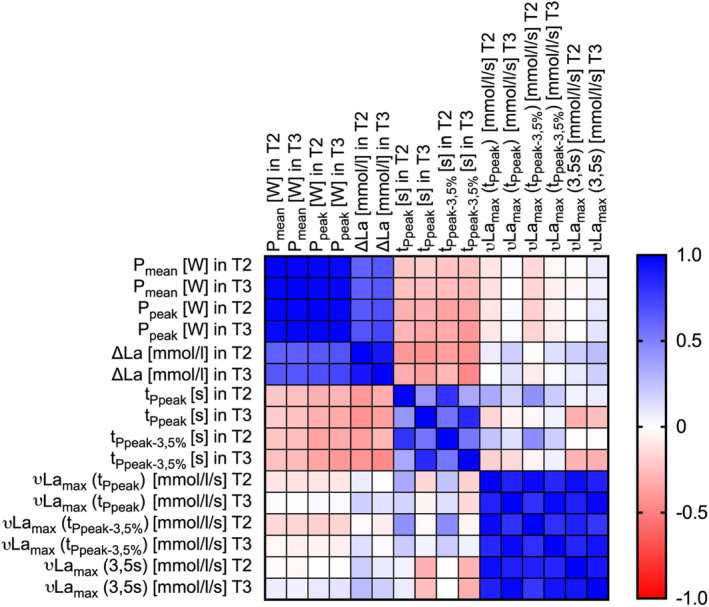
Correlation matrix of all relevant variables during the 15‐s sprint, Pmean = mean power output, Ppeak = peak power output, ∆La = difference between Lapre and Lamaxpost, tPpeak = time until Ppeak was reached, tPpeak–3.5% = time until Ppeak–3.5% was reached, νLamax (tPpeak) = νLamax calculated with tPCr = tPpeak, νLamax (tPpeak–3.5%) = νLamax calculated with tPCr = tPpeak–3.5%, νLamax (3.5 s) = νLamax calculated with tPCr = 3.5 s.
TABLE 3.
Intraclass correlation coefficient (ICC) (95% confidence interval) and standard error of measurement (SEM) calculated for T2 and T3.
| ICC | SEM | CoV | Bias | 95% LoA | |
|---|---|---|---|---|---|
| νLamax (3.5 s) mmol/l/s | 0.911 | 0.02 | 3.1% | 0.00 | −0.13 to 0.12 |
| (0.849–0.949) | |||||
| νLamax (tPpeak) [mmol/l/s] | 0.866 | 0.06 | 12.1% | −0.01 | −0.15 to 0.13 |
| (0.777–0.922) | |||||
| νLamax (tPpeak–3.5%) [mmol/l/s] | 0.794 | 0.15 | 26.4% | −0.03 | −0.23 to 0.17 |
| (0.660–0.879) | |||||
| ∆La [mmol/l] | 0.911 (0.849–0.949) | 0.22 | 3.6% | −0.05 | −1.44 to 1.35 |
| Ppeak [W] | 0.986 | 23 | 2.4% | 4.6 | −87.4 to 96.5 |
| (0.975–0.992) | |||||
| Pmean [W] | 0.986 | 30 | 3.6% | −5.9 | −69.9 to 58.0 |
| (0.975–0.992) | |||||
| tPpeak [s] | 0.414 | 0.71 | 30.0% | −0.14 | −1.91 to 1.62 |
| (0.158–0.618) | |||||
| tPpeak–3.5% [s] | 0.520 | 1.83 | 51.0% | −0.37 | −2.87 to 2.14 |
| (0.289–0.695) |
Abbreviations: νLamax (3.5 s) = νLamax calculated with tPCr = 3.5 s; νLamax (tPpeak) = νLamax calculated with tPCr = tPpeak; νLamax (tPpeak–3.5%) = νLamax calculated with tPCr = tPpeak–3.5%; ∆La = difference between Lapre and Lamaxpost; Ppeak = peak power during the sprint test; Pmean = average power during the sprint test; tPpeak = time until Ppeak was reached; tPpeak–3.5% = time until Ppeak–3.5% was reached.
Figure 3a–h illustrates the data of each variable of interest (Ppeak, Pmean, ∆La, tPpeak, tPpeak–3.5%, νLamax (3.5 s), νLamax (tPpeak), νLamax (tPpeak–3.5%)) and cyclist between T2 and T3.
FIGURE 3.
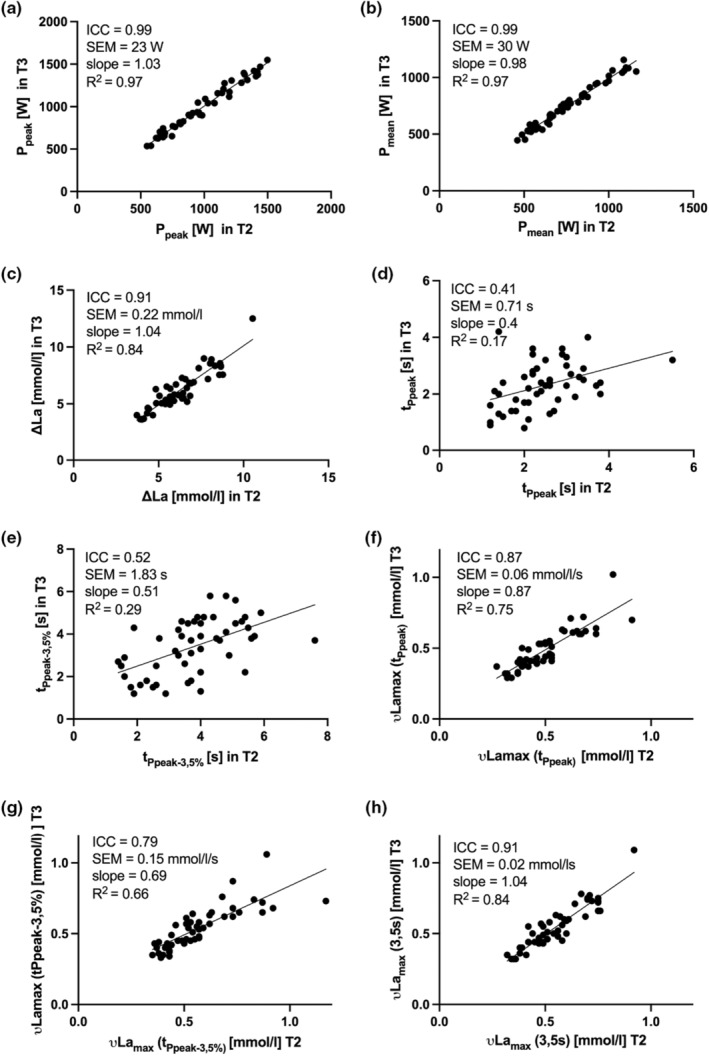
Correlation for T2 and T3 in (a) Ppeak, (b) Ppeak, (c) ∆La, (d) tPpeak, (E) tPpeak–3.5%, (f) νLa max (tPpeak), (g) νLa max (tPpeak–3.5%), (h) νLa max (3.5 s).
As main result Pmean (ICC = 0.99, CoV = 3.9%) and PPeak (ICC = 0.99, CoV = 2.4%) revealed excellent retest reliability while ∆La showed good to excellent reliability (ICC = 0.91, CoV = 3.6%). Across trials 2 and 3, νLamax revealed excellent, good and good reliability respectively for the different tPCr calculation methods: tPCr = 3.5 s (ICC = 0.91, CoV = 3.1%), tPCr = tPpeak (ICC = 0.87, CoV = 12.1%), tPCr = tpeak–3.5% (ICC = 0.79, CoV = 26.4%).
Figure 4a–f illustrates the Bland–Altman plots of νLamax values corresponding to the different calculation methods for tPCr νLamax (3.5 s), νLamax (tPpeak), νLamax (tPpeak–3.5%) in T2 and T3 respectively.
FIGURE 4.
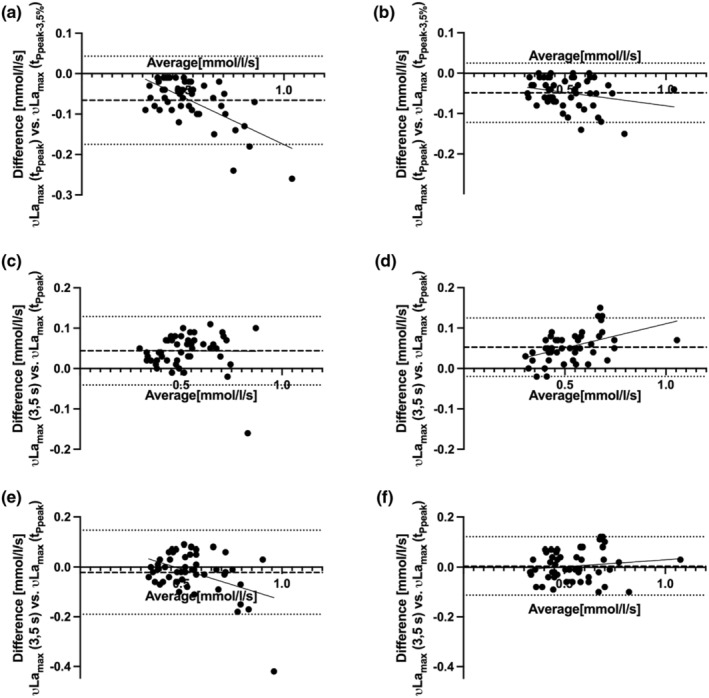
Bland–Altman analysis for νLa max (tPpeak) and νLa max (tPpeak–3.5%) in T2 (a) and T3 (b), νLa max (3,5 s) and νLa max (tPpeak) in T2 (c) and T3 (d), νLa max (3,5 s) and νLa max (tPpeak–3.5%) in T2 (e) and T3 (f).
3.2. TPCr variables
Measurements for tPpeak and tPpeak–3.5% revealed poor to moderate test–retest reliability (ICC = 0.39; =0.52, CoV = 30.0%; =51.0%, Figure 3d,e). Additionally, a weak relationship appeared between increased Ppeak and decreased tPpeak as well as tPpeak–3.5% values (Figure 5a,b). Exemplary data is displayed in Figure 5c.
FIGURE 5.
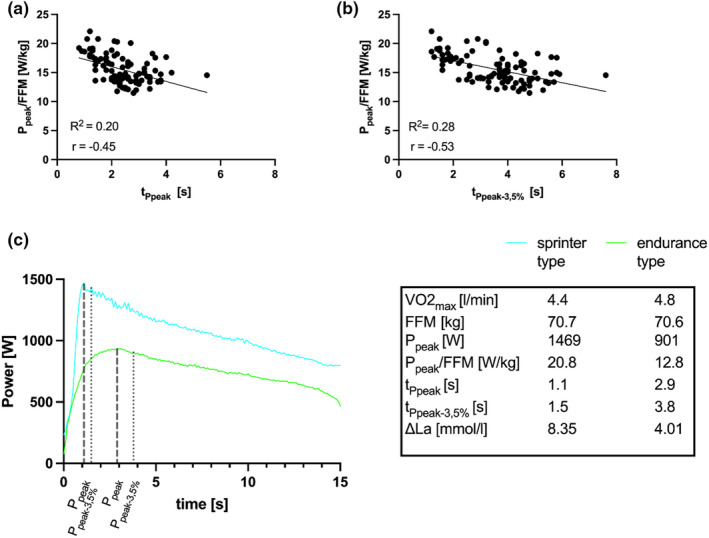
Relation between Ppeak/FFM and tPpeak (a) and tPpeak–3.5% (b), (c) example of sprint power data over 15 s, Ppeak, tPpeak, Ppeak–3.5% and tPpeak–3.5% for two male cyclists of similar stature and body mass for sprinter type versus endurance type (see legend for individual data).
4. DISCUSSION
The main findings of the present investigation are:
Test‐restest reliability for peak and mean power output was excellent
∆La and subsequently, νLamax with tPCr of 3.5 s showed excellent reliability
TPCr measures (tPpeak and tPpeak–3.5%) display poor to moderate reliability resulting in decreased reliability of the νLamax calculation employing these measures. Overall reliability of the equations was moderate to good.
4.1. Reliability assessment
The present investigation revealed excellent test–retest reliability of Ppeak and Pmean and excellent reliability for νLamax with tPCr of 3.5 s between T2 and T3.
The high test–retest reliability for maximal blood lactate accumulation in sprint cycling has been also demonstrated in earlier studies assessing reliability of νLamax with tPpeak–3.5% (Adam et al., 2015; Quittmann, Schwarz, et al., 2021). Based on our and the recent data, we may conclude that νLamax with a fixed time frame for predominant phosphagen contribution constitutes a variable with high test‐restest reliability in connection with 15‐s sprint cycling.
Given that directly measuring intramuscular lactate production in sprint cycling is presently impractical, recent and older studies have explored various metabolic pathways through simulations employing Mader's model. (Hauser et al., 2014; Ji et al., 2021; Mader & Heck, 1986). However, these simulations are highly dependent on νLamax and its calculation, i.e., the denominator and the tPCr component. The present findings showed that depending on which tPCr was employed the νLamax calculation differed extensively. Based on the findings of our study, we recommend a standardized application of tPCr, specifically in our case tPCr of 3.5 s because this variable revealed the highest degree of reliability. We assume that the integration of different time frames of tPCr may at least partly explain the discrepancy between data obtained from the simulations compared to actual performance testing (Hauser et al., 2014; Ji et al., 2021).
As mentioned before, the determination of “predominant phosphagen contribution time” is an important factor in the calculation of the maximal rate of lactate production in Mader's model. As an example, in a 15 s sprint test, a change of 1 s in the time span of predominant phosphagen contribution could influence the resulting νLamax up to 26% (Dunst et al., 2023). This represents a hypothetical time parameter that significantly impacts the denominator in the νLamax calculation. However, the application of an “predominant phosphagen contribution time span” seems disputable: depending on the transport and diffusion of lactate from the muscle into and within the blood stream an time without lactate accumulation may occur when employing and interpreting the kinetics of capillary blood lactate measurements in connection with sprint cycling. However, when considering the glycolytic activity within the working muscle cell it seems questionable whether an time span with no blood lactate accumulation exists (Brooks, 2018; Chung et al., 1998; Dunst et al., 2023).
Based on the present findings, the most reliable data was observed in connection with fixed time span, i.e. tPCr of 3.5 s. However, as elucidated before, the question remains whether this time should be measured at all. We are well aware that other quantification methods of an tPCr exists including the determination by the power curve when Ppeak is reached (Quittmann, Abel, et al., 2021), by the power curve with Ppeak–3.5% (Quittmann, Schwarz, et al., 2021), by individual determination of the force/velocity profile (Dunst et al., 2023) and by relative contribution of glycolysis while measuring phosphagen and oxidative contribution with breath‐by‐breath measurement (Yang et al., 2023). Nevertheless, to the best of our knowledge, none of these methods underwent rigorous reliability assessment in connection with sprint cycling. Noteworthy, the tPCr measures including Ppeak as a marker are influenced by the initial inertia resistance of the ergometer: in case the initial resistance is set too low for the cyclist, Ppeak is reached rapidly vice versa when the initial resistance is too high, power outputs will increase even during increasing muscular fatigue (Dunst et al., 2023). Our results show that the determination of the tPCr by the power curve (tPeak) is not a reliable measure for determination of νLamax. However, this finding may partly be caused by the employed ergometer and its settings.
Adding to that, Figure 5c shows lower tPCr in the cyclists with higher Ppeak/FFM. This is in line with the findings by Dunst et al. (Dunst et al., 2023). The decreased tPCr (in the cyclists with lower Ppeak/FFM) increases the denominator (ttest‐ tPCr) of Mader's equation, resulting in lower νLamax values compared to the cyclist with greater Ppeak/FFM and shorter tPCr. Differences between athletes in power output and ∆La may therefore appear reduced when expressed as νLamax. Therefore, the use of a fixed tPCr for the determination of νLamax seems to reveal physiological differences more consistently.
4.2. Strength and limitations
We consider our sample size (n = 50) a strength of this study. Comparable studies in the field provided a sample size of 18–23 (Hauser et al., 2014; Quittmann, Schwarz, et al., 2021; Yang et al., 2023). Further all participants considered themselves as true cyclists or triathletes, not as runners or physical education students being tested as unexperienced cyclist once or twice on a bike.
The measure of νLamax is used in endurance cycling and triathlon and therefore dependent on the accompanying body angles and acclimatization to the position and contact points on the bike. Therefore, the use of the participants own bike is necessitated which was the case in the present study in contrast to other studies (Adam et al., 2015).
Determination of tPCr is controversial and crucial for calculation of νLamax. The time frame of tPpeak–3.5% is rooted in a reduction of power output by 1%, factoring in the accuracy error of the SRM of 2.5%. In our setup, considering the error accuracy of 2% of the Cyclus2 ergometer, tPpeak–3.5% could have been used. However, considering the ongoing discussion in this matter (Dunst et al., 2023), we avoided creating an additional time frame for tPCr. Determination of tPCr by the force/velocity profile as used by Dunst et al. (Dunst et al., 2023) necessitates additional testing and the method was not available at the time of data collection. Moreover, the approach proposed by Yang et al. (Yang et al., 2023) has not undergone reliability testing and could potentially gain from the integration of a force/velocity profile analysis.
While we ensured control over hydration status through specific gravity of urine testing, body composition was assessed using bioelectrical impedance analysis due to the unavailability of more advanced methods for body composition.
4.3. Conclusion
In conclusion, the reliability of νLamax varies considerably when employing different time frames for predominant phosphagen contribution with tPCr of 3.5 s showing the highest and good level of reliability. Changes in physiology might be best represented using a fixed tPCr or absolute values in ∆La.
FUNDING INFORMATION
Authors state no funding involved.
CONFLICT OF INTEREST STATEMENT
Authors state no conflict of interest.
ACKNOWLEDGMENT
Open Access funding enabled and organized by Projekt DEAL.
Meixner, B. , Nusser, V. , Koehler, K. , Sablain, M. , Boone, J. , & Sperlich, B. (2024). Reliability of power output, maximal rate of capillary blood lactate accumulation, and phosphagen contribution time following 15‐s sprint cycling in amateur cyclists. Physiological Reports, 12, e16086. 10.14814/phy2.16086
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Adam, J. , Ohmichen, M. , Ohmichen, E. , Rother, J. , Muller, U. M. , Hauser, T. , & Schulz, H. (2015). Reliability of the calculated maximal lactate steady state in amateur cyclists. Biology of Sport, 32, 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson, G. , & Nevill, A. M. (1998). Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Medicine, 26, 217–238. [DOI] [PubMed] [Google Scholar]
- Brooks, G. A. (2018). The science and translation of lactate shuttle theory. Cell Metabolism, 27, 757–785. [DOI] [PubMed] [Google Scholar]
- Chung, Y. , Sharman, R. , Carlsen, R. , Unger, S. W. , Larson, D. , & Jue, T. (1998). Metabolic fluctuation during a muscle contraction cycle. American Journal of Physiology‐Cell Physiology, 274, C846–C852. [DOI] [PubMed] [Google Scholar]
- Dunst, A. K. , Hesse, C. , Feldmann, A. , & Holmberg, H. C. (2023). A novel approach to determining the Alactic time span in connection with assessment of the maximal rate of lactate accumulation in elite track cyclists. International Journal of Sports Physiology and Performance, 18, 157–163. [DOI] [PubMed] [Google Scholar]
- Eliasziw, M. , Young, S. L. , Woodbury, M. G. , & Fryday‐Field, K. (1994). Statistical methodology for the concurrent assessment of interrater and Intrarater reliability: Using goniometric measurements as an example. Physical Therapy, 74, 777–788. [DOI] [PubMed] [Google Scholar]
- Harriss, D. J. , & Atkinson, G. (2009). International journal of sports medicine ‐ ethical standards in sport and exercise science research. International Journal of Sports Medicine, 30, 701–702. [DOI] [PubMed] [Google Scholar]
- Hauser, T. , Adam, J. , & Schulz, H. (2014). Comparison of calculated and experimental power in maximal lactate‐steady state during cycling. Theoretical Biology & Medical Modelling, 11, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck, H. , & Schulz, H. (2002). Methoden der anaeroben Leistungsdiagnostik. Deutsche Zeitschrift Für Sportmedizin, 53, 11. [Google Scholar]
- Held, S. , Rappelt, L. , Brockherde, J. , & Donath, L. (2023). Reliability of the maximal lactate accumulation rate in rowers. International Journal of Sports Medicine, 45, 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeacocke, N. A. , & Burke, L. M. (2010). Methods to standardize dietary intake before performance testing. International Journal of Sport Nutrition and Exercise Metabolism, 20, 87–103. [DOI] [PubMed] [Google Scholar]
- Ji, S. , Sommer, A. , Bloch, W. , & Wahl, P. (2021). Comparison and performance validation of calculated and established anaerobic lactate thresholds in running. Medicina (Kaunas, Lithuania), 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo, T. K. , & Li, M. Y. (2016). A guideline of selecting and reporting intraclass correlation coefficients for reliability research. Journal of Chiropractic Medicine, 15, 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mader, A. (1984). Eine Theorie zur Berechnung der Dynamik und des steady state von Phosphorylierungszustand und Stoffwechselaktivität der Muskelzelle als Folge des Energiebedarfs.
- Mader, A. (2003). Glycolysis and oxidative phosphorylation as a function of cytosolic phosphorylation state and power output of the muscle cell. European Journal of Applied Physiology, 88, 317–338. [DOI] [PubMed] [Google Scholar]
- Mader, A. , & Heck, H. (1986). A theory of the metabolic origin of "anaerobic threshold". International Journal of Sports Medicine, 7, 20. [PubMed] [Google Scholar]
- Manunzio, C. , Mester, J. , Kaiser, W. , & Wahl, P. (2016). Training intensity distribution and changes in performance and physiology of a 2nd place finisher team of the race across America over a 6 month preparation period. Frontiers in Physiology, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin Bland, J. , & Altman, D. (1986). Statistical methods for assessing agreement between two methods of clinical measurement. The Lancet, 327, 307–310. [PubMed] [Google Scholar]
- Mavroudi, M. , Kabasakalis, A. , Petridou, A. , & Mougios, V. (2023). Blood lactate and maximal lactate accumulation rate at three Sprint swimming distances in highly trained and elite swimmers. Sports, 11, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevill, M. , Bogdanis, G. , Boobis, L. , Lakomy, H. K. A. , & Williams, C. (1994). Biochemistry of exercise IX. In Maughan R. J. & Shirreffs S. M. (Eds.), Muscle metabolism and performance during sprinting (pp. 243–260). Human Kinetics. [Google Scholar]
- Nitzsche, N. , Baumgrtel, L. , & Schulz, H. (2018). Comparison of maximum lactate formation rates in ergometer Sprint and maximum strength loads. Deutsche Zeitschrift f¸r Sportmedizin, 69, 13–18. [Google Scholar]
- Quittmann, O. J. , Abel, T. , Vafa, R. , Mester, J. , Schwarz, Y. M. , & Strüder, H. K. (2021). Maximal lactate accumulation rate and post‐exercise lactate kinetics in handcycling and cycling. European Journal of Sport Science, 21, 539–551. [DOI] [PubMed] [Google Scholar]
- Quittmann, O. J. , Abel, T. , Zeller, S. , Foitschik, T. , & Strüder, H. K. (2018). Lactate kinetics in handcycling under various exercise modalities and their relationship to performance measures in able‐bodied participants. European Journal of Applied Physiology, 118, 1493–1505. [DOI] [PubMed] [Google Scholar]
- Quittmann, O. J. , Appelhans, D. , Abel, T. , & Strüder, H. K. (2020). Evaluation of a sport‐specific field test to determine maximal lactate accumulation rate and sprint performance parameters in running. Journal of Science and Medicine in Sport, 23, 27–34. [DOI] [PubMed] [Google Scholar]
- Quittmann, O. J. , Foitschik, T. , Vafa, R. , Freitag, F. J. , Sparmann, N. , Nolte, S. , & Abel, T. (2022). Is maximal lactate accumulation rate promising for improving 5000‐m prediction in running? International Journal of Sports Medicine, 44, 268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quittmann, O. J. , Schwarz, Y. M. , Mester, J. , Foitschik, T. , Abel, T. , & Strüder, H. K. (2020). Maximal lactate accumulation rate in all‐out exercise differs between cycling and running. International Journal of Sports Medicine, 42, 314–322. [DOI] [PubMed] [Google Scholar]
- Quittmann, O. J. , Schwarz, Y. M. , Mester, J. , Foitschik, T. , Abel, T. , & Strüder, H. K. (2021). Maximal lactate accumulation rate in all‐out exercise differs between cycling and running. International Journal of Sports Medicine, 42, 314–322. [DOI] [PubMed] [Google Scholar]
- Schünemann, F. , Park, S.‐Y. , Wawer, C. , Theis, C. , Yang, W.‐H. , & Gehlert, S. (2023). Diagnostics of ν La.max and glycolytic energy contribution indicate individual characteristics of anaerobic glycolytic energy metabolism contributing to rowing performance. Metabolites, 13, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout, P. E. , & Fleiss, J. L. (1979). Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin, 86, 420–428. [DOI] [PubMed] [Google Scholar]
- Stratford, P. W. , & Goldsmith, C. H. (1997). Use of the standard error as a reliability index of interest: An applied example using elbow flexor strength data. Physical Therapy, 77, 745–750. [DOI] [PubMed] [Google Scholar]
- Teixeira, C. L. , Mezzaroba, P. V. , Peserico, C. S. , & Machado, F. A. (2022). Effect of photobiomodulation on maximal lactate production rate on swimmers: A randomized, crossover, double‐blind and placebo‐controlled study. Motriz: Revista de Educação Física, 28. [Google Scholar]
- Weir, J. P. , & Vincent, W. J. (2020). Statistics in kinesiology. Human Kinetics Publishers. [Google Scholar]
- Yang, W.‐H. , Park, S.‐Y. , Kim, T. , Jeon, H.‐J. , Heine, O. , & Gehlert, S. (2023). A modified formula using energy system contributions to calculate pure maximal rate of lactate accumulation during a maximal sprint cycling test. Frontiers in Physiology, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


