Abstract
Introduction
Atopic dermatitis (AD) is characterized by intense itch and other symptoms that negatively impact quality of life (QoL). This study evaluates the effect of upadacitinib (an oral selective Janus kinase inhibitor) monotherapy on patient-reported outcomes (PROs) among adults and adolescents with moderate-to-severe AD over 16 weeks.
Methods
This integrated analysis of the double-blind, placebo-controlled periods of phase 3 monotherapy clinical trials Measure Up 1 (NCT03569293) and Measure Up 2 (NCT03607422) assessed itch (Worst Pruritus Numerical Rating Scale [WP-NRS] and SCORing Atopic Dermatitis [SCORAD]), skin pain and symptom severity (AD Symptom Scale), symptom frequency (Patient-Oriented Eczema Measure), sleep (AD Impact Scale [ADerm-IS] and SCORAD), daily activities and emotional state (ADerm-IS), QoL (Dermatology Life Quality Index [DLQI] and Children’s DLQI), mental health (Hospital Anxiety and Depression Scale), and patient impressions (Patient Global Impression of Severity, Patient Global Impression of Change, and Patient Global Impression of Treatment).
Results
Data from 1683 patients (upadacitinib 15 mg, n = 557; upadacitinib 30 mg, n = 567; placebo, n = 559) were analyzed. A greater proportion of patients receiving upadacitinib versus placebo experienced improvements in itch (≥ 4-point improvement on WP-NRS) by week 1 (upadacitinib 15 mg, 11.2%; upadacitinib 30 mg, 17.7%; placebo, 0.5%; P < 0.001), with response rates sustained through week 16 (upadacitinib 15 mg, 47.1%; upadacitinib 30 mg, 59.8%; placebo, 10.4%; P < 0.001). Improvements were similar for PROs assessing skin pain/symptoms, sleep, daily activities, QoL, emotional state, mental health, and patient impressions of disease severity and treatment. Responses generally improved rapidly (within 1–2 weeks), increased through weeks 4–6, and were maintained through week 16.
Conclusions
Once-daily oral upadacitinib monotherapy improved response rates across PROs compared with placebo. Upadacitinib therapy resulted in rapid, sustained improvements in PROs measuring symptom burden and QoL in adults and adolescents with moderate-to-severe AD.
Trial Registration
ClinicalTrials.gov identifiers, NCT03569293 and NCT03607422.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-024-01157-5.
Keywords: Atopic dermatitis, Itch, Patient-reported outcome measures, Quality of life, Randomized controlled trials, Upadacitinib
Plain Language Summary
Atopic dermatitis, or eczema, is characterized by itchy, dry, inflamed skin. These symptoms often make it difficult for patients to get adequate sleep. Patients with atopic dermatitis may also experience anxiety, depression, reduced self-confidence, social isolation, disruption to daily activities like school and work, and decreased quality of life. Many atopic dermatitis symptoms, including itch and psychological impact, are difficult for doctors to assess. Thus, it is important to consider patients’ descriptions of their symptoms and quality of life, particularly when assessing treatment benefit. Upadacitinib is an orally administered drug approved to treat moderate-to-severe atopic dermatitis. We investigated how upadacitinib (15 mg or 30 mg) given once daily to adults and adolescents with moderate-to-severe atopic dermatitis in the Measure Up 1 and 2 clinical trials impacts their symptoms and quality of life over a 16-week period. We compared changes in patient-reported itch, pain, sleep, daily activities, emotional state, mental health, and overall quality of life among patients in the clinical trials who received upadacitinib with those in the same studies who received a dummy (placebo) treatment. Upadacitinib improved patient-reported symptoms and quality of life early in the clinical trials, often within the first 1–2 weeks. The extent of the improvements increased through weeks 4–6 of treatment and lasted through week 16. Patients who received upadacitinib reported greater improvements in symptoms and quality of life than did patients who received placebo. Upadacitinib treatment resulted in rapid and lasting improvements in the well-being of patients with atopic dermatitis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-024-01157-5.
Key Summary Points
| Why carry out this study? |
| Atopic dermatitis is associated with burdensome symptoms including itch, pain, sleep disruption, social and emotional difficulties, and impaired quality of life. |
| This study evaluated the effect of once-daily upadacitinib monotherapy on patient-reported outcomes among adults and adolescents with moderate-to-severe atopic dermatitis over 16 weeks. |
| What was learned from the study? |
| Within 1–2 weeks of initiating upadacitinib therapy, patients experienced rapid improvements in itch, skin pain, sleep, daily activities, emotional state, quality of life, mental health, and impression of disease severity and treatment; patient-reported outcomes continued to improve through weeks 4–6, with improvements maintained through week 16. |
| Upadacitinib results in rapid and sustained improvements in burdensome symptoms and quality of life for adults and adolescents with moderate-to-severe atopic dermatitis. |
Introduction
Atopic dermatitis (AD) is a chronic, inflammatory skin condition characterized by pruritus and skin lesions with typical eczematous morphology that affects approximately 2.7% of the global population and up to 20% of children [1, 2]. Patients diagnosed with moderate-to-severe AD experience intense symptoms that impair health-related quality of life (HRQoL) [3, 4]. Patient-reported outcomes (PROs) are important when physicians seek to understand the full impact of AD on patients’ lives and the benefit of AD treatment. PROs consider patients’ perspectives and can enrich shared decision-making discussions between physicians and patients. PROs allow the impact of treatment on symptoms such as itch and HRQoL to be readily discussed with patients, which is crucial given that reducing itch is one of the highest priority goals for patients as it has a significant negative impact on HRQoL [4–6]. PROs can be used to evaluate three of the four assessment domains (quality of life, symptoms, and eczema control) recommended by the international Harmonising Outcome Measures for Eczema (HOME) group for reporting clinical trials in AD [7]. Using PROs as criteria to create an optimized AD treatment plan is also recommended by treat-to-target guidelines and the recently defined minimal disease activity criteria [8–11].
Upadacitinib, an orally administered, selective Janus kinase inhibitor, is approved to treat patients with moderate-to-severe AD [12, 13]. The efficacy and safety of upadacitinib monotherapy and combination therapy with topical corticosteroids to treat patients with AD was previously demonstrated [14–18]. Here, we evaluate how upadacitinib monotherapy to treat patients with moderate-to-severe AD affects a comprehensive suite of PROs, including both symptoms and HRQoL measures such as social, emotional, and mental well-being. In this integrated analysis, we used data from two pivotal phase 3 clinical trials (Measure Up 1 and Measure Up 2) and assessed the effect of upadacitinib monotherapy administered once daily on patient-reported symptom burden and HRQoL in adults and adolescents with moderate-to-severe AD through 16 weeks of treatment. PROs were assessed as frequently as weekly, providing a detailed analysis of timing that may allow for more informed shared decision-making for physicians and patients with AD.
Methods
Study Design and Patients
An in-depth description of the Measure Up 1 (NCT03569293) and Measure Up 2 (NCT03607422) study designs, patient populations, and methods were previously reported [14]. Briefly, Measure Up 1 and Measure Up 2 are replicate, randomized, double-blind, placebo-controlled, phase 3 clinical trials evaluating once-daily, orally administered upadacitinib in patients with moderate-to-severe AD. Both studies included a 16-week double-blind period and an ongoing blinded extension period of up to 260 weeks, and were conducted in clinical centers across Europe, North America, South America, Oceania, and the Asia–Pacific region. Eligible patients were aged 12–75 years and had moderate-to-severe AD, which was defined as an Eczema Area and Severity Index (EASI) score ≥ 16, validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD) score ≥ 3, a weekly average of daily Worst Pruritus Numerical Rating Scale (WP-NRS) scores ≥ 4, and ≥ 10% body surface area involvement. All patients had to be candidates for systemic therapy.
As previously described [14], patients were stratified by baseline vIGA-AD (scores 3 and 4), geographic region (USA/Puerto Rico/Canada, Japan [Measure Up 1 only], China [Measure Up 1 only], or other), and age group (adult and adolescent). Patients were then randomized 1:1:1 to receive once-daily, orally administered upadacitinib 15 mg, upadacitinib 30 mg, or placebo. Patients, study site investigators, and study site staff were blinded to treatments throughout the double-blind period. Independent ethics committees or institutional review boards at each study site approved the study protocols, informed consent forms, and recruitment materials before patient enrollment (Supplementary Material Table 1). The studies were conducted in accordance with the International Conference for Harmonisation guidelines, applicable regulations, and the Declaration of Helsinki. All patients provided written informed consent.
Assessments
PRO assessments were used to evaluate pruritus (WP-NRS and SCORing Atopic Dermatitis [SCORAD] Itch), skin pain and other symptoms (Atopic Dermatitis Symptom Scale [ADerm-SS] Skin Pain, ADerm-SS 7-item total symptom score [TSS-7], and Patient-Oriented Eczema Measure [POEM]), sleep (Atopic Dermatitis Impact Scale [ADerm-IS] Sleep, SCORAD Sleep, and POEM Sleep), quality of life (ADerm-IS Daily Activities, ADerm-IS Emotional State, and Dermatology Life Quality Index [DLQI] for patients aged ≥ 16 years, Children’s Dermatology Life Quality Index [CDLQI] for patients aged 12–15 years), mental health (Hospital Anxiety and Depression Scale [HADS]), and patient impressions (Patient Global Impression of Severity, Patient Global Impression of Change, and Patient Global Impression of Treatment) through the 16-week double-blind period (Supplementary Material Table 2). The necessary permissions were obtained for using the reported PRO assessments. ADerm-SS and ADerm-IS were developed according to US Food and Drug Administration guidance [19]. PROs were reported as the proportion of patients achieving a clinically meaningful improvement (defined as the minimal clinically important difference), the proportion of patients achieving a minimal disease burden score threshold (scores representing no/minimal symptoms or no/minimal impact of AD on HRQoL), and the mean change from baseline and percent change from baseline. As an exploratory analysis, the percent overlap in patients who achieved minimal disease burden score thresholds for WP-NRS, DLQI, and POEM at week 16 was reported.
Statistical Analysis
This integrated analysis included pooled data from the 16-week, double-blind periods of the Measure Up 1 and Measure Up 2 studies. Sample size calculations were previously described [14]. PRO endpoints were prespecified within each clinical trial, excluding achievement of minimal disease burden threshold for ADerm-IS, ADerm-SS, and POEM total, which were assessed post hoc (Supplementary Material Table 2). Clinically meaningful improvement was assessed among patients whose baseline score was greater than or equal to the minimal clinically important difference score threshold; achievement of minimal disease burden was assessed among patients whose baseline score was greater than the minimal disease burden score threshold. Categorical endpoints were analyzed using the Cochran–Mantel–Haenszel test with non-responder imputation incorporating multiple imputation for missing data due to COVID-19 as the primary approach for handling missing data. Continuous endpoints were analyzed using the mixed-effect model repeat measurement method. Mean change from baseline and percent change from baseline were estimated using the least squares mean. Determination of the percent overlap in patients who achieved minimal disease burden score thresholds for WP-NRS, DLQI, and POEM was based on patients who reported achievement of any one of the three endpoints, included only patients with non-missing data for all three outcomes at week 16, and was agnostic to treatment group.
Results
Patients
A total of 1683 patients enrolled in the Measure Up 1 and Measure Up 2 studies were included in this analysis (n = 557, upadacitinib 15 mg; n = 567, upadacitinib 30 mg; n = 559, placebo). Baseline demographics and disease characteristics were generally balanced between upadacitinib and placebo groups (Table 1). Patient dispositions within the Measure Up 1 and Measure Up 2 studies were previously described [14].
Table 1.
Baseline demographics and disease characteristics for patients enrolled in the Measure Up 1 and Measure Up 2 studies
| Parameter | UPA 15 mg (n = 557) |
UPA 30 mg (n = 567) |
PBO (n = 559) |
Total (N = 1683) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Female | 245 (44.0) | 250 (44.1) | 261 (46.7) | 756 (44.9) |
| Male | 312 (56.0) | 317 (55.9) | 298 (53.3) | 927 (55.1) |
| Age, median (range), years | 29.0 (12–74) | 29.0 (12–75) | 30.0 (12–75) | 29.0 (12–75) |
| Age, years, n (%) | ||||
| < 18 | 75 (13.5) | 77 (13.6) | 76 (13.6) | 228 (13.5) |
| 18–64 | 454 (81.5) | 456 (80.4) | 461 (82.5) | 1371 (81.5) |
| ≥ 65 | 28 (5.0) | 34 (6.0) | 22 (3.9) | 84 (5.0) |
| Race, n (%) | ||||
| White | 366 (65.7) | 389 (68.6) | 377 (67.4) | 1132 (67.3) |
| Black or African American | 43 (7.7) | 26 (4.6) | 37 (6.6) | 106 (6.3) |
| Asian | 128 (23.0) | 133 (23.5) | 125 (22.4) | 386 (22.9) |
| American Indian/Alaska Native | 5 (0.9) | 2 (0.4) | 8 (1.4) | 15 (0.9) |
| Native Hawaiian or Other Pacific Islander | 3 (0.5) | 1 (0.2) | 2 (0.4) | 6 (0.4) |
| Multiple | 12 (2.2) | 16 (2.8) | 10 (1.8) | 38 (2.3) |
| Disease duration, mean (SD), years | 19.7 (14.7) | 20.6 (14.3) | 21.2 (14.4) | 20.5 (14.5) |
| BSA involvement, mean % (SD) | 46.8 (22.3) | 47.0 (22.6) | 46.6 (22.2) | 46.8 (22.3) |
| vIGA-AD score, n (%) | ||||
| 3 (moderate) | 280 (50.3) | 280 (49.4) | 281 (50.3) | 841 (50.0) |
| 4 (severe) | 277 (49.7) | 287 (50.6) | 278 (49.7) | 842 (50.0) |
| Previous systemic therapy,a n (%) | 275 (49.4) | 274 (48.3) | 300 (53.7) | 849 (50.4) |
| EASI score, mean (SD) | 29.6 (12.3) | 29.3 (11.7) | 29.0 (12.4) | 29.3 (12.1) |
| Average weekly WP-NRS, mean (SD) | 7.2 (1.6) | 7.3 (1.5) | 7.3 (1.6) | 7.3 (1.6) |
| HADS-A Total, mean (SD) | 7.3 (4.1) | 7.5 (4.3) | 7.3 (4.3) | 7.4 (4.2) |
| HADS-D Total, mean (SD) | 5.2 (4.0) | 5.5 (4.2) | 5.4 (4.1) | 5.4 (4.1) |
| POEM, mean (SD) | 21.2 (4.9) | 21.6 (5.0) | 21.7 (5.3) | 21.5 (5.1) |
| DLQI, mean (SD) |
16.6 (7.0) (n = 512) |
16.5 (6.9) (n = 517) |
17.1 (7.0) (n = 509) |
16.7 (7.0) (n = 1538) |
| CDLQI, mean (SD) |
14.0 (6.0) (n = 38) |
14.3 (5.5) (n = 33) |
13.5 (6.5) (n = 39) |
13.9 (6.0) (n = 110) |
| Overall SCORAD, mean (SD) | 67.4 (12.6) | 67.0 (12.7) | 67.0 (12.5) | 67.1 (12.6) |
| ADerm-SS Skin Pain, mean (SD) | 6.3 (2.2) | 6.4 (2.2) | 6.5 (2.3) | 6.4 (2.2) |
| ADerm-SS TSS-7, mean (SD) | 46.3 (13.6) | 46.3 (13.6) | 46.7 (14.0) | 46.4 (13.7) |
| ADerm-IS Sleep, mean (SD) | 18.2 (7.4) | 18.5 (7.6) | 19.1 (7.5) | 18.6 (7.5) |
| ADerm-IS Daily Activities, mean (SD) | 23.1 (10.5) | 22.8 (10.6) | 23.4 (10.6) | 23.1 (10.6) |
| ADerm-IS Emotional State, mean (SD) | 20.4 (7.9) | 20.1 (8.3) | 20.3 (8.1) | 20.3 (8.1) |
| PGIS, mean (SD) | 4.4 (1.1) | 4.4 (1.1) | 4.4 (1.1) | 4.4 (1.1) |
| Medical history, n (%) | ||||
| Acne | 56 (10.1) | 67 (11.8) | 47 (8.4) | 170 (10.1) |
| Asthma | 220 (39.5) | 221 (39.0) | 230 (41.1) | 671 (39.9) |
| Chronic sinusitis | 2 (0.4) | 2 (0.4) | 0 | 4 (0.2) |
| Allergic conjunctivitis | 28 (5.0) | 31 (5.5) | 21 (3.8) | 80 (4.8) |
| Eosinophilic oesophagitis | 1 (0.2) | 1 (0.2) | 5 (0.9) | 7 (0.4) |
| Food allergy | 165 (29.6) | 183 (32.3) | 160 (28.6) | 508 (30.2) |
| Nasal polyps | 6 (1.1) | 11 (1.9) | 16 (2.9) | 33 (2.0) |
| Allergic rhinitis | 180 (32.3) | 197 (34.7) | 195 (34.9) | 572 (34.0) |
ADerm-IS Atopic Dermatitis Impact Scale, ADerm-SS Atopic Dermatitis Symptom Scale, BSA body surface area, CDLQI Children’s Dermatology Life Quality Index, DLQI Dermatology Life Quality Index, EASI Eczema Area and Severity Index, HADS Hospital Anxiety and Depression Scale, HADS-A HADS Anxiety, HADS-D HADS Depression, PBO placebo, PGIS Patient Global Impression of Severity, POEM Patient-Oriented Eczema Measure, SCORAD SCORing Atopic Dermatitis, SD standard deviation, TSS-7 7-item total symptom score, UPA upadacitinib, vIGA-AD validated Investigator Global Assessment for Atopic Dermatitis, WP-NRS, Worst Pruritus Numerical Rating Scale
aSystemic therapy included both biologic and non-biologic systemic therapies
PRO Measures
Overall, both upadacitinib doses were associated with greater improvement in itch, skin pain and other skin symptoms, sleep, and HRQoL compared with placebo. Treatment with upadacitinib led to a rapid improvement in all PROs, with the upadacitinib 30 mg dose demonstrating numerically higher response rates compared with the upadacitinib 15 mg dose.
Pruritus
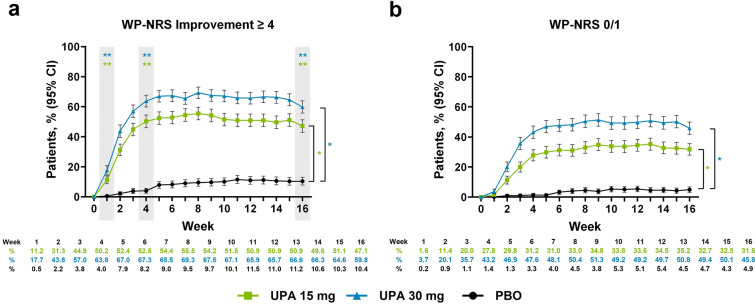
A greater proportion of patients receiving upadacitinib achieved rapid clinically meaningful improvement in itch intensity as early as week 1 of treatment compared with patients receiving placebo (upadacitinib 15 mg, 11.2%; upadacitinib 30 mg, 17.7%; placebo, 0.5%; P < 0.001; Fig. 1a). Response rates for patients treated with upadacitinib increased through week 6 and were maintained through week 16 (upadacitinib 15 mg, 47.1%; upadacitinib 30 mg, 59.8%; placebo, 10.4%; P < 0.001). Rapid response was similarly observed for the proportion of patients reporting minimal to no itch, with greater response rates for the upadacitinib 15 mg group and upadacitinib 30 mg group compared with the placebo group as early as week 1 (upadacitinib 15 mg, 1.6%; upadacitinib 30 mg, 3.7%; placebo, 0.2%; nominal P < 0.05; Fig. 1b). The greater response rate for minimal to no itch increased through week 6 and was sustained through week 16 (upadacitinib 15 mg, 31.8%; upadacitinib 30 mg, 45.8%; placebo, 4.9%; nominal P < 0.001). Similar improvements in itch intensity were observed for the percent change from baseline in WP-NRS and SCORAD itch visual analog scale, which were reduced (improved) compared with improvements in itch intensity with placebo at the earliest weekly time point assessed (week 1 for WP-NRS, nominal P < 0.001; week 2 for SCORAD itch, nominal P < 0.001; Supplementary Material Fig. 1a, b); results were similar when evaluating the mean change from baseline for WP-NRS (Supplementary Material Table 3).
Fig. 1.
Patients enrolled in the Measure Up 1 and Measure Up 2 studies who achieved a WP-NRS improvement ≥ 4a and b WP-NRS 0/1b during the double-blind period (NRI-C). Comparisons at time points in shaded boxes were multiplicity controlled. *Nominal P < 0.05 versus placebo for all time points. **Multiplicity adjusted P < 0.001 versus placebo. aAssessed in patients with WP-NRS ≥ 4 at baseline. bAssessed in patients with WP-NRS > 1 at baseline. CI confidence interval, NRI-C non-responder imputation incorporating multiple imputation for missing data due to COVID-19, PBO placebo, UPA upadacitinib, WP-NRS Worst Pruritus Numerical Rating Scale
Skin Pain and Other Symptoms
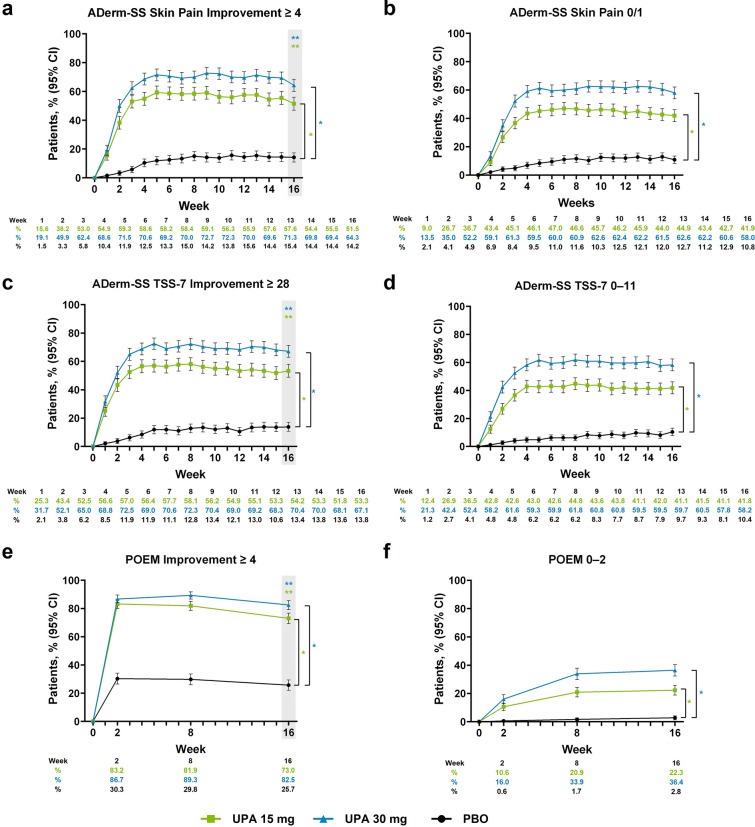
Treatment with upadacitinib led to rapid improvements in patient-reported skin pain and other skin symptoms compared with placebo. A greater proportion of patients in the upadacitinib 15 mg group and upadacitinib 30 mg group achieved clinically meaningful improvement in skin pain as early as week 1 (upadacitinib 15 mg, 15.6%; upadacitinib 30 mg, 19.1%; placebo, 1.5%; nominal P < 0.001), with response rates increasing through week 4 and sustained through week 16 (upadacitinib 15 mg, 51.5%; upadacitinib 30 mg, 64.3%; placebo, 14.2%; P < 0.001; Fig. 2a). Similar patterns of response were observed for the proportion of patients reporting minimal to no skin pain (Fig. 2b) and for the percent change from baseline and mean change from baseline (Supplementary Material Fig. 2a and Table 3).
Fig. 2.
Patients enrolled in the Measure Up 1 and Measure Up 2 studies who achieved a ADerm-SS Skin Pain Improvement ≥ 4,a b ADerm-SS Skin Pain 0/1,b c ADerm-SS TSS-7 Improvement ≥ 28,c d ADerm-SS TSS-7 0–11,d e POEM Improvement ≥ 4,e and f POEM 0–2f during the double-blind period (NRI-C). Comparisons at time points in shaded boxes were multiplicity controlled. *Nominal P < 0.001 versus placebo for all time points. **Multiplicity adjusted P < 0.001 versus placebo. aAssessed in patients with ADerm-SS Skin Pain ≥ 4 at baseline. bAssessed in patients with ADerm-SS Skin Pain ≥ 2 at baseline. cAssessed in patients with ADerm-SS TSS-7 ≥ 28 at baseline. dAssessed in patients with ADerm-SS TSS-7 ≥ 12 at baseline. eAssessed in patients with POEM ≥ 4 at baseline. fAssessed in patients with POEM scores ≥ 3 at baseline. ADerm-SS Atopic Dermatitis Symptom Scale, CI confidence interval, NRI-C non-responder imputation incorporating multiple imputation for missing data due to COVID-19, PBO placebo, POEM Patient-Oriented Eczema Measure, TSS-7 7-item Total Symptom Score, UPA upadacitinib
Patients treated with upadacitinib 15 mg or upadacitinib 30 mg also demonstrated improvement in other skin-related symptoms. A greater proportion of patients treated with upadacitinib achieved clinically meaningful improvement in skin symptoms by week 1 versus patients in the placebo group (upadacitinib 15 mg, 25.3%; upadacitinib 30 mg, 31.7%; placebo, 2.1%; nominal P < 0.001); response rates increased through week 4 and were maintained through week 16 (upadacitinib 15 mg, 53.3%; upadacitinib 30 mg, 67.1%; placebo, 13.8%; P < 0.001; Fig. 2c). Similar response patterns were observed for the proportion of patients reporting minimal to no symptoms (Fig. 2d), as well as patients achieving clinically meaningful improvement on POEM and clear or almost clear on POEM (Fig. 2e, f). For ADerm-SS TSS-7 and POEM, the percent change and mean change from baseline reflected greater improvement through week 16 for patients receiving upadacitinib 15 mg or upadacitinib 30 mg compared with placebo (nominal P < 0.001; Supplementary Material Fig. 2b, c and Table 3).
Sleep
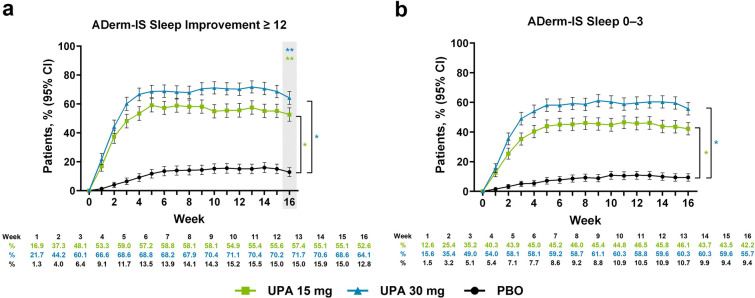
Upadacitinib treatment resulted in a greater proportion of patients achieving clinically meaningful improvement in AD-related sleep disturbance compared with patients receiving placebo at week 1 (upadacitinib 15 mg, 16.9%; upadacitinib 30 mg, 21.7%; placebo, 1.3%; nominal P < 0.001); response rates for patients treated with upadacitinib rapidly increased through week 5 and were sustained through week 16 (upadacitinib 15 mg, 52.6%; upadacitinib 30 mg, 64.1%; placebo, 12.8%; P < 0.001; Fig. 3a). Similar temporal patterns of improved response with upadacitinib versus placebo were observed for patients reporting minimal to no AD-related sleep disturbance (nominal P < 0.001; Fig. 3b). Both upadacitinib groups demonstrated a greater percent change and mean change from baseline on ADerm-IS Sleep compared with placebo at week 1; rapid improvement in response rates with upadacitinib continued through week 6 and were maintained through week 16 (nominal P < 0.001; Supplementary Material Fig. 3a and Table 3). Similar temporal patterns were observed for percent change from baseline in SCORAD visual analog scale sleep and for the proportion of patients reporting no sleep disturbance on POEM Sleep (Supplementary Material Fig. 3b, c).
Fig. 3.
Patients enrolled in the Measure Up 1 and Measure Up 2 studies who achieved a ADerm-IS Sleep Improvement ≥ 12a and b ADerm-IS Sleep 0–3b during the double-blind period (NRI-C). Comparison at time point in shaded box was multiplicity controlled. *Nominal P < 0.001 versus placebo for all time points. **Multiplicity adjusted P < 0.001 versus placebo. aAssessed in patients with ADerm-IS Sleep Domain ≥ 12 at baseline. bAssessed in patients with ADerm-IS Sleep Domain ≥ 4 at baseline. ADerm-IS Atopic Dermatitis Impact Scale, CI confidence interval, NRI-C non-responder imputation incorporating multiple imputation for missing data due to COVID-19, PBO placebo, UPA upadacitinib
Quality of Life and Mental Health
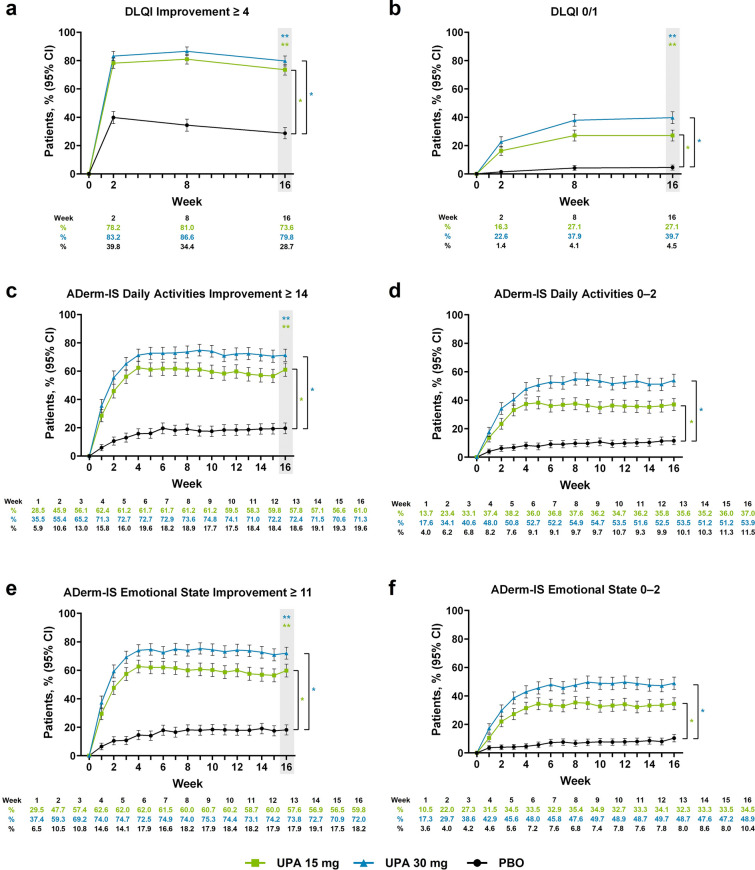
Treatment with upadacitinib led to rapid clinically meaningful improvement on DLQI compared with placebo by week 2 (upadacitinib 15 mg, 78.2%; upadacitinib 30 mg, 83.2%; placebo, 39.8%; nominal P < 0.001); improved responses with upadacitinib were sustained through week 16 (upadacitinib 15 mg, 73.6%; upadacitinib 30 mg, 79.8%; placebo, 28.7%; P < 0.001; Fig. 4a). More patients treated with upadacitinib reported that their AD had minimal or no effect on their quality of life by week 2 versus patients receiving placebo (upadacitinib 15 mg, 16.3%; upadacitinib 30 mg, 22.6%; placebo, 1.4%; nominal P < 0.001); improved responses with upadacitinib were sustained through week 16 (upadacitinib 15 mg, 27.1%; upadacitinib 30 mg, 39.7%; placebo, 4.5%; P < 0.001; Fig. 4b). Greater improvement in HRQoL with upadacitinib versus placebo was also demonstrated by the percent change and mean change from baseline on DLQI through week 16 (Supplementary Material Fig. 4a and Table 3). For patients aged 12–15 years at baseline, similar trends were observed for achievement of minimal or no disease burden on CDLQI and the percent change and mean change from baseline with upadacitinib compared with placebo (Supplementary Fig. 4b, c and Table 3).
Fig. 4.
Patients enrolled in the Measure Up 1 and Measure Up 2 studies who achieved a DLQI improvement scores ≥ 4,a b DLQI scores 0/1,b c ADerm-IS Daily Activities Improvement scores ≥ 14,c d ADerm-IS Daily Activities scores 0–2,d e ADerm-IS Emotional State Improvement scores ≥ 11,e and f ADerm-IS Emotional State scores 0–2f during the double-blind period (NRI-C). Comparisons at time points in shaded boxes were multiplicity controlled. *Nominal P < 0.001 versus placebo for all time points. **Multiplicity adjusted P < 0.001 versus placebo. aAssessed in patients with DLQI scores ≥ 4 at baseline. bAssessed in patients with DLQI scores > 1 at baseline. cAssessed in patients with ADerm-IS Daily Activities Domain scores ≥ 14 at baseline. dAssessed in patients with ADerm-IS Daily Activities Domain scores ≥ 3 at baseline. eAssessed in patients with ADerm-IS Emotional State Domain scores ≥ 11 at baseline. fAssessed in patients with ADerm-IS Emotional State Domain scores ≥ 3 at baseline. ADerm-IS Atopic Dermatitis Impact Scale, CI confidence interval, DLQI Dermatology Life Quality Index, NRI-C non-responder imputation incorporating multiple imputation for missing data due to COVID-19, PBO placebo, UPA upadacitinib
Patients treated with upadacitinib experienced rapid improvement in daily activities and emotional state. By week 1, more patients achieved clinically meaningful improvement and reported absent or minimal disease burden in daily activities and emotional state with upadacitinib compared with placebo (nominal P < 0.001; Fig. 4c–f); response rates with upadacitinib improved through week 4 and were sustained through week 16. Comparable temporal patterns were observed for the percent change from baseline and mean change from baseline on ADerm-IS Emotional State and ADerm-IS Daily Activities with upadacitinib versus placebo (Supplementary Material Fig. 4d, e and Table 3).
Improvements in symptoms of anxiety and depression were observed for patients treated with upadacitinib by week 12 and were maintained through week 16. At week 16, 45.7% and 52.7% of patients receiving upadacitinib 15 mg or upadacitinib 30 mg, respectively, achieved clinically meaningful improvement in anxiety and depression compared with 12.8% of patients receiving placebo (P < 0.001; Supplementary Material Fig. 5).
Impressions of Disease Severity and Treatment Satisfaction
A greater proportion of patients reported an improved impression of disease severity, treatment efficacy, and treatment satisfaction through week 16 with upadacitinib compared with patients receiving placebo. Greater proportions of patients receiving upadacitinib reported absent or minimal symptoms on the Patient Global Impression of Severity instrument, much or very much improved symptoms on the Patient Global Impression of Change instrument, and very or extremely satisfied with treatment on the Patient Global Impression of Treatment instrument versus patients receiving placebo (Supplementary Material Fig. 6).
Achievement of Minimal Disease Burden Thresholds Across Multiple PROs
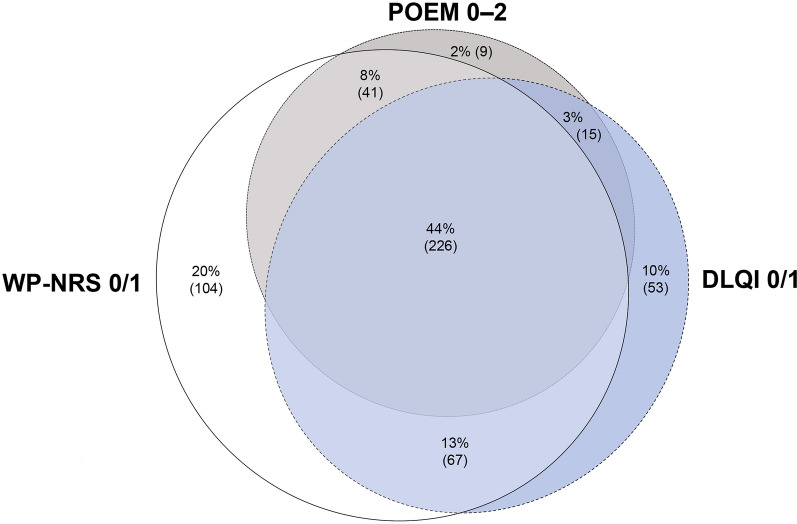
In an exploratory, illustrative analysis of patients who achieved the minimal disease burden threshold for either itch, skin pain and other skin symptoms, or quality of life at week 16, 44% of patients achieved the minimal severity threshold for all three PROs (Fig. 5). Of the included patients, 68% achieved the minimal disease burden threshold for at least two of the three PROs measuring itch, skin pain and other skin symptoms, and quality of life.
Fig. 5.
Overlap in achievement of WP-NRS 0/1, DLQI 0/1, and POEM 0–2 at week 16 for patients enrolled in the Measure Up 1 and Measure Up 2 studies. Data are reported as % (n). Percentages are calculated among patients who achieved at least one of the following endpoints at week 16: WP-NRS 0/1, DLQI 0/1, or POEM 0–2. Data from all treatment groups were combined. The analysis was based on observed cases, including only patients with non-missing data for all three outcome measures at week 16. DLQI Dermatology Life Quality Index, POEM Patient-Oriented Eczema Measure, WP-NRS Worst Pruritus Numerical Rating Scale
Discussion
Results from this integrated analysis of PROs from the Measure Up 1 and Measure Up 2 studies evaluating once-daily administration of upadacitinib 15 mg or 30 mg in adults and adolescents with moderate-to-severe AD demonstrated rapid and clinically meaningful improvements in itch, skin pain, other skin-related symptoms, sleep, HRQoL, and mental health with upadacitinib. Compared with patients receiving placebo, patients in the upadacitinib groups demonstrated greater improvements comprehensively across all PRO measures. Improvements were generally observed as early as week 1 or week 2 after upadacitinib initiation. This rapid response across PROs aligns with findings from a previous analysis of data from Measure Up 1 and Measure Up 2 independently, in which clinically meaningful improvements in itch intensity (≥ 4-point improvement in daily WP-NRS) were seen as early as day 1 or day 2 following upadacitinib initiation [14]. In general, a fast increase in response rates occurred across PROs, which plateaued around weeks 4–6, and was maintained through week 16 (the end of the double-blind period). Response rates were generally numerically greater for upadacitinib 30 mg compared with upadacitinib 15 mg across PROs.
Given the significant daily impact of AD on HRQoL, understanding patients’ individual symptoms and treatment goals through use of PROs is crucial [3, 4, 6, 20]. While there has been increased emphasis on evaluating PROs in addition to clinician-reported outcomes when considering AD treatments [7–11], as of yet, there is no clear consensus on how to use PROs to guide treatment decisions [21]. Importantly, our study evaluates the achievement of no or minimal impact of AD on patients’ lives using highly stringent minimal disease burden PRO threshold scores. The stringent minimal disease burden threshold scores applied in this analysis align with the recently developed minimal disease activity criteria, which combines the treat-to-target framework with shared decision-making [11]. The minimal disease activity criteria were developed by 87 dermatologists across 44 countries with expertise in the treatment of AD, and include optimal treatment goals that reflect minimal disease activity such as WP-NRS (≤ 1), POEM (≤ 2), and DLQI/CDLQI (≤ 1) [11]. The stringent PRO treatment goals, such as those presented in this analysis and the minimal disease activity criteria, could lead to improved HRQoL and increased patient satisfaction; in this analysis, a greater proportion of patients treated with upadacitinib versus placebo achieved the stringent minimal disease burden threshold scores.
The results presented here demonstrate rapid control of itch in patients with moderate-to-severe AD who received upadacitinib, which is consistent with findings in prior reports that upadacitinib may improve itch intensity within 1–2 days [14]. Itch is a key outcome to assess, as it is the AD symptom experienced daily by most patients and may drive the negative impacts observed on patients’ HRQoL including sleep and mental health [4]. Relief from itch is consistently ranked as a top treatment goal for patients with AD, demonstrating the heavy burden of this symptom [6, 20]. Additionally, itch can negatively impact patients’ mental health [4]; mental health concerns are thought to be a key symptom negatively impacting patients’ daily life [22]. Therefore, rapid control of itch is important and may lead to earlier improvements in patients’ well-being and quality of life.
In addition to rapid reductions in itch intensity, treatment with upadacitinib rapidly improved sleep symptoms for patients with moderate-to-severe AD, with significant improvements noted by week 1. This study comprehensively evaluated the effect of upadacitinib on sleep using multiple PRO instruments (ADerm-IS Sleep, SCORAD Sleep, and POEM Sleep), and through these instruments, similar rapid improvements in sleep were observed. Impaired sleep is a common and problematic symptom for patients with AD and is associated with worsened quality of life and mental health [23, 24], rendering the rapid effect of upadacitinib on sleep improvement particularly impactful.
Beyond itch and sleep, the multidimensional burden of AD necessitates a comprehensive assessment of diverse PROs, hence our multifaceted evaluation of the symptom and HRQoL burden experienced by patients with AD. The rapid parallel responses between sensory symptoms (itch and pain) and complex domains such as sleep and mental/emotional health shed light on the pervasive and interlinked burden experienced by patients with AD and underscore a need for early symptom control. Our analysis of patients who achieved minimal disease burden thresholds for itch, skin pain, and quality of life demonstrated an incomplete overlap of responses across domains; fewer than half of patients achieved all three PRO endpoints, and less than three-quarters achieved any two PRO endpoints. These findings underscore the importance of independently assessing multiple PROs to guide treatment decisions and evaluate efficacy. In alignment with this observation, the minimal disease activity criteria recommends patients select multiple PRO domains to inform treatment decisions [11].
The limitations of this study arise from the interdependence of PROs such as itch, sleep, and emotional well-being, thus making it challenging to determine the magnitude of improvement due to a single variable. Particularly, itch is speculated to be a mediator of much of the negative impact of AD on HRQoL [4]. Furthermore, the results presented here assess the effect of upadacitinib over a relatively short period (through the end of the 16-week double-blind period of the Measure Up 1 and Measure Up 2 studies). Additionally, some of the analyses presented were conducted post hoc (i.e., achievement of minimal disease burden thresholds on the ADerm-IS, ADerm-SS, and POEM instruments), and the studies were not designed to make statistically significant conclusions about these endpoints across different time points; however, results from the post hoc analyses are in directional agreement with the prespecified analyses assessing HRQoL. Finally, on the basis of eligibility criteria, patients enrolled in the Measure Up 1 and Measure Up 2 studies may not be fully representative of the population of patients with moderate-to-severe AD seen in real-world clinical practice; for example, this analysis includes predominantly white and Asian patients.
Conclusion
The results presented here support the efficacy of once-daily administration of upadacitinib in rapidly improving disease-specific, patient-reported symptoms and HRQoL for patients with moderate-to-severe AD. Improvements were observed as early as week 1 and were sustained through week 16 of treatment. These results provide important information on how upadacitinib affects AD-related symptoms and HRQoL, which can be applied during shared decision-making discussions between physicians and patients to support more informed treatment choices. Incorporating PROs and shared decision-making into regular practice for treatment of AD, as recommended by the treat-to-target guidelines, HOME clinical practice recommendations, and minimal disease activity criteria [9–11, 21], may lead to more optimal outcomes for patients with AD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The AbbVie and authors thank all the trial investigators and the patients who participated in this clinical trial.
Medical Writing/Editorial Assistance
Medical writing support was provided by Jennifer A Jiménez, PhD, Morgan A Gingerich, PhD, and Akua Adu-Boahene, MD, MPH, of JB Ashtin, and funded by AbbVie.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article. All authors had access to relevant data and participated in data interpretation, critically reviewed this manuscript, provided final approval for publication and take responsibility for the publication as a whole. No honoraria or payments were made for authorship.
Author Contributions
Eric L. Simpson, Jonathan I. Silverberg, Brian M. Calimlim, Wan-Ju Lee, Henrique Teixeira, Barry Ladizinski, and Xiaofei Hu participated in study concept/design. Eric L. Simpson, Vimal H. Prajapati, Andrew E. Pink, Henrique Teixeira, Barry Ladizinski, and Xiaofei Hu participated in data acquisition. Xiaofei Hu, Yang Yang, Yingi Liu, and Meng Liu participated in statistical analysis. Eric L. Simpson, Vimal H. Prajapati, Yael A. Leshem, Raj Chovatiya, Marjolein S. de Bruin-Weller, Sonja Ständer, Andrew E. Pink, Brian M. Calimlim, Wan-Ju Lee, Henrique Teixeira, Barry Ladizinski, Xiaofei Hu, Yang Yang, Yingyi Liu, Meng Liu, Ayman Grada, Andrew M. Platt, and Jonathan I. Silverberg contributed to data interpretation, critically reviewed this manuscript, and provided final approval for publication.
Funding
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. AbbVie funded the Rapid Service Fee.
Data Availability
The datasets generated during and/or analyzed during the current study are available on reasonable request via the following link: https://vivli.org/ourmember/abbvie/, then select “Home”. AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal, and Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the United States and Europe and after acceptance of this manuscript for publication. These data will be accessible for 12 months, with possible extensions considered.
Declarations
Conflicts of Interest
Eric L. Simpson has received personal fees from AbbVie, Amgen, Arena Pharmaceuticals, ASLAN, Benevolent AI-Bio Tech Limited, BiomX Ltd, Bluefin Biomedicine, Boehringer Ingelheim, Boston Consulting Group, Collective Acumen, Coronado, Dermira, Evidera, Excerpta Medica, Galderma, GSK, Forte Biosciences, Incyte Dermatologics, Janssen, Kyowa Kirin Pharmaceutical Development, LEO Pharma, Lilly, Medscape, Merck, Novartis, Ortho Galderma, Pfizer, Physicians World, Pierre Fabre Dermo Cosmetique, Regeneron, Roivant Sciences, Sanofi-Genzyme, SPARC India, Trevi therapeutics, WebMD, and Valeant. He has received grants from AbbVie, Amgen, Arcutis, ASLAN, Castle Biosciences, Celgene, CorEvitas, Dermavant, Dermira, Galderma, Incyte, Kymab, Kyowa Hakko Kirin, LEO Pharma, Lilly, Merck, Novartis, Pfizer, Regeneron, Sanofi, and TARGET-DERM outside the submitted work. Vimal H. Prajapati has served as an advisor, consultant, and/or speaker for AbbVie, Actelion, Amgen, Apogee Therapeutics, Aralez, Arcutis, Aspen, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Canadian Psoriasis Network, Celgene, Cipher, Eczema Society of Canada, Galderma, GSK, Homeocan, Janssen, LEO Pharma, Lilly, L’Oréal, Medexus, Novartis, Pediapharm, Pfizer, Sanofi Genzyme, Sun Pharma, Tribute, UCB, and Valeant. He has served as an investigator for AbbVie, Amgen, AnaptysBio, Arcutis, Arena, Asana, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Concert, Dermavant, Dermira, Galderma, Incyte, Janssen, LEO Pharma, Lilly, Nimbus Lakshmi, Novartis, Pfizer, Regeneron, Reistone, Sanofi Genzyme, Takeda, UCB, and Valeant. He has received grants from AbbVie, Bausch Health, Janssen, LEO Pharma, Novartis, and Sanofi Genzyme. Yael A. Leshem has received honoraria or fees as a consultant from AbbVie, Genentech, Janssen, Pfizer, and Sanofi. She has served as an advisory board member for AbbVie, Dexcel Pharma, Pfizer, Regeneron, and Sanofi; has received an independent research grant from AbbVie; and has, without personal compensation, provided investigator services for AbbVie, Lilly, and Pfizer. Raj Chovatiya has served as an advisor, consultant, speaker, and/or investigator for AbbVie, Arcutis, Argenx, Apogee, ASLAN, Beiersdorf, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Dermavant, Galderma, Genentech, Incyte, LEO Pharma, Lilly, L’Oréal, Nektar, Novan, Inc., Opsidio, Pfizer, Regeneron, Sanofi, and UCB. Marjolein S. de Bruin-Weller is a consultant, advisory board member, and/or speaker for AbbVie, Almirall, Amgen, Aslan, Galderma, Janssen, LEO Pharma, Lilly, Pfizer, Regeneron, and Sanofi-Genzyme. Sonja Ständer is an investigator for Celldex, Clexio, Galderma, GSK, Incyte, Kiniksa Pharmaceuticals, Menlo Therapeutics, Novartis, Sanofi Genzyme, and Trevi Therapeutics. She is a member of scientific advisory boards, consultant, and/or speakers’ bureaus for AbbVie, Almirall, Beiersdorf, Benevolent, Bionorica, Cara, Clexio Biosciences, Escient Pharmaceuticals, Galderma, Grünenthal, Kiniksa, Klinge Pharma, LEO Pharma, Lilly, Menlo Therapeutics, Pfizer, P.G. Unna Academy, Sanofi Genzyme, Symbio Research, Trevi Therapeutics, Vifor Pharma, and WebMD. Andrew E. Pink has been an advisor/speaker/investigator or has received educational support from AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Galderma, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sanofi, and UCB. Brian M. Calimlim, Wan-Ju Lee, Henrique Teixeira, Xiaofei Hu, Yang Yang, Yingyi Liu, Meng Liu, Ayman Grada, and Andrew M. Platt are full-time employees of AbbVie, and may hold AbbVie stock and/or stock options. Barry Ladizinski is a former employee of AbbVie and may hold AbbVie stock and/or stock options. He is a current employee of ONE Pharmaceuticals. Jonathan I. Silverberg receives consulting fees from AbbVie, Anacor Pharmaceuticals, GlaxoSmithKline, Lilly, Pfizer, Procter & Gamble, MedImmune, and Regeneron. He serves as an investigator in trials sponsored by Celgene, GlaxoSmithKline, Lilly, Regeneron, and Roche.
Ethics Approval
The independent ethics committee or institutional review board at each study site approved the study protocol, informed consent forms, and recruitment materials before patient enrollment (Supplementary Material Table 1). The studies were conducted in accordance with the International Conference for Harmonisation guidelines, applicable regulations, and the Declaration of Helsinki. All patients provided written informed consent to participate in this study before screening.
References
- 1.Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126(4):417–28.e2. doi: 10.1016/j.anai.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Laughter MR, Maymone MBC, Mashayekhi S, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990–2017. Br J Dermatol. 2021;184(2):304–309. doi: 10.1111/bjd.19580. [DOI] [PubMed] [Google Scholar]
- 3.Ronnstad ATM, Halling-Overgaard AS, Hamann CR, Skov L, Egeberg A, Thyssen JP. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79(3):448–56.e30. doi: 10.1016/j.jaad.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Drucker AM. Atopic dermatitis: burden of illness, quality of life, and associated complications. Allergy Asthma Proc. 2017;38(1):3–8. doi: 10.2500/aap.2017.38.4005. [DOI] [PubMed] [Google Scholar]
- 5.Copley-Merriman C, Zelt S, Clark M, Gnanasakthy A. Impact of measuring patient-reported outcomes in dermatology drug development. Patient. 2017;10(2):203–213. doi: 10.1007/s40271-016-0196-6. [DOI] [PubMed] [Google Scholar]
- 6.Augustin M, Langenbruch A, Blome C, et al. Characterizing treatment-related patient needs in atopic eczema: insights for personalized goal orientation. J Eur Acad Dermatol Venereol. 2020;34(1):142–152. doi: 10.1111/jdv.15919. [DOI] [PubMed] [Google Scholar]
- 7.Williams HC, Schmitt J, Thomas KS, et al. The HOME core outcome set for clinical trials of atopic dermatitis. J Allergy Clin Immunol. 2022;149(6):1899–1911. doi: 10.1016/j.jaci.2022.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Yeung J, Gooderham MJ, Hong HC, et al. Treat-to-target in the management of moderate-to-severe atopic dermatitis in adults: a Canadian perspective. J Am Acad Dermatol. 2023;89(2):372–5. [DOI] [PubMed]
- 9.de Bruin-Weller M, Biedermann T, Bissonnette R, et al. Treat-to-target in atopic dermatitis: an international consensus on a set of core decision points for systemic therapies. Acta Derm Venereol. 2021;101(2):adv00402. doi: 10.2340/00015555-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Bruin-Weller M, Deleuran M, Biedermann T, et al. The treat-to-target project in atopic dermatitis: one year on. Acta Derm Venereol. 2023;103:adv5382. doi: 10.2340/actadv.v103.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverberg JI, Gooderham M, Katoh N, et al. Optimizing the management of atopic dermatitis with a new minimal disease activity concept and criteria and consensus-based recommendations for systemic therapy. Br J Dermatol. 2023;188:ii16–ii17. doi: 10.1093/bjd/ljac140.022. [DOI] [Google Scholar]
- 12.Parmentier JM, Voss J, Graff C, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494) BMC Rheumatol. 2018;2:23. doi: 10.1186/s41927-018-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rinvoq® Tablets [package insert]. AbbVie GK, November 2023. https://www.rxabbvie.com/pdf/rinvoq_pi.pdf. Accessed 19 Jan 2024.
- 14.Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. doi: 10.1016/S0140-6736(21)00588-2. [DOI] [PubMed] [Google Scholar]
- 15.Simpson EL, Papp KA, Blauvelt A, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022;158(4):404–413. doi: 10.1001/jamadermatol.2022.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silverberg JI, de Bruin-Weller M, Bieber T, et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: week 52 AD Up study results. J Allergy Clin Immunol. 2022;149(3):977–87.e14. doi: 10.1016/j.jaci.2021.07.036. [DOI] [PubMed] [Google Scholar]
- 17.Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181. doi: 10.1016/S0140-6736(21)00589-4. [DOI] [PubMed] [Google Scholar]
- 18.Guttman-Yassky E, Thaçi D, Pangan AL, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–884. doi: 10.1016/j.jaci.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 19.Foley C, Tundia N, Simpson E, Teixeira HD, Litcher-Kelly L, Bodhani A. Development and content validity of new patient-reported outcome questionnaires to assess the signs and symptoms and impact of atopic dermatitis: the Atopic Dermatitis Symptom Scale (ADerm-SS) and the Atopic Dermatitis Impact Scale (ADerm-IS) Curr Med Res Opin. 2019;35(7):1139–1148. doi: 10.1080/03007995.2018.1560222. [DOI] [PubMed] [Google Scholar]
- 20.von Kobyletzki LB, Thomas KS, Schmitt J, et al. What factors are important to patients when assessing treatment response: an international cross-sectional survey. Acta Derm Venereol. 2017;97(1):86–90. doi: 10.2340/00015555-2480. [DOI] [PubMed] [Google Scholar]
- 21.Leshem YA, Chalmers JR, Apfelbacher C, et al. Measuring atopic eczema symptoms in clinical practice: the first consensus statement from the Harmonising Outcome Measures for Eczema in clinical practice initiative. J Am Acad Dermatol. 2020;82(5):1181–1186. doi: 10.1016/j.jaad.2019.12.055. [DOI] [PubMed] [Google Scholar]
- 22.Wollenberg A, Gooderham M, Katoh N, et al. Understanding the impact of atopic dermatitis on patients: a large international, ethnically diverse survey-based qualitative study. Br J Dermatol. 2023;188. 10.1093/bjd/ljac140.023.
- 23.Bawany F, Northcott CA, Beck LA, Pigeon WR. Sleep disturbances and atopic dermatitis: relationships, methods for assessment, and therapies. J Allergy Clin Immunol Pract. 2021;9(4):1488–1500. doi: 10.1016/j.jaip.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silverberg JI, Chiesa-Fuxench Z, Margolis D, et al. Epidemiology and burden of sleep disturbances in atopic dermatitis in US adults. Dermatitis. 2022;33(6s):S104–S113. doi: 10.1097/DER.0000000000000731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available on reasonable request via the following link: https://vivli.org/ourmember/abbvie/, then select “Home”. AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal, and Statistical Analysis Plan (SAP), and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time after approval in the United States and Europe and after acceptance of this manuscript for publication. These data will be accessible for 12 months, with possible extensions considered.