Abstract
East Africa (Musa spp.), notably Musa acuminata, “Matooke” a staple and economically important food in the region. Here, 12 selected M. acuminata peels extract (MAPE) bioactive compounds were studied for hepatoprotective potentials in aluminium chloride-induced hepatoxicity in adult BALB/c mice. GC–MS analysis was used to identify active components of MAPE. In silico estimation of the pharmacokinetic, the GCMS-identified compounds' toxicity profile and molecular docking were compared with the standard (Simvastatin) drug. Hepatotoxicity was induced using aluminium-chloride treated with MAPE, followed by biochemical and histopathological examination. Twelve bioactive compounds 2,2-Dichloroacetophenone (72870), Cyclooctasiloxane 18993663), 7-Hydroxy-6,9a-dimethyl-3-methylene-decahydro-azuleno[4,5-b]furan-2,9-dione (534579), all-trans-alpha-Carotene (4369188), Cyclononasiloxane (53438479), 3-Chloro-5-(4-methoxyphenyl)-6,7a-dimethyl-5,6,7,7a-tetrahydro-4H-furo[2,3-c]pyridin-2-one (536708), Pivalic acid (6417), 10,13-Octadecadienoic acid (54284936), Ethyl Linoleate (5282184), Oleic acid (5363269), Tirucallol (101257), Obtusifoliol (65252) were identified by GC–MS. Of these, seven were successfully docked with the target proteins. The compounds possess drug likeness potentials that do not inhibits CYP450 isoforms biotransformation. All the docked compounds were chemoprotective to AMES toxicity, hERGI, hERGII and hepatotoxicity. The animal model reveals MAPE protective effect on liver marker’s function while the histological studies show regeneration of the disoriented layers of bile ducts and ameliorate the cellular/histoarchitecture of the hepatic cells induced by AlCl3. The findings indicate that MAPE improved liver functions and ameliorated the hepatic cells' cellular or histoarchitecture induced by AlCl3. Further studies are necessary to elucidate the mechanism action and toxicological evaluation of MAPE’s chronic or intermittent use to ascertain its safety in whole organism systems.
Keywords: In-silico, Pharmacokinetic, Therapeutic-potential, M. acuminata peels, Aluminium-chloride-induced-hepatotoxicity, BALB/c mice
Introduction
Liver disease, often referred to as toxic hepatitis or hepatotoxicity, is becoming a global concern with limited efficacious pharmacotherapies (Manfo et al. 2014). The prevalence of liver disorders is increasing, and the effectiveness of current medical therapies is low. Due to its primary function as the “metabolic factory” in the body, the liver is the most exposed organ to xenobiotics (Bird and Kiourtis 2022). Any substance, regardless of its intended use, has the potential to affect the liver (Manfo et al. 2014; Bird and Kiourtis 2022). However, hepatotoxicity, among other things, may result from the use of drugs, herbal supplements, and exposure to chemicals, solvents, and/or chronic use of alcohol.
Since antiquity, people have utilized herbal remedies to treat illnesses (Onohuean et al. 2018, 2021a, b, 2022a). Some plant extracts and natural substances are used as hepatoprotective active principles (Zhang et al. 2013; Xu et al. 2018; Bachar et al. 2020), while others could cause liver toxicity when taken to treat illnesses unrelated to the liver with valerian as a typical example (Fontana 2014; Bethesda 2017; Tox 2020). Again, one of the challenges with the use of folkloric medicine in the mitigation and treatment of human ailments is lack of standardization, poorly established pharmacological principles (pharmacokinetic and pharmacodynamics), and lack of identification/isolation of the effective secondary metabolites as well the toxicological properties (Onohuean and Igere 2023). At the same time, most herbal formulations lack long-term information on their effectiveness. Lack of regulation for herbal formulations, lack of evidence-based reports on their efficacy, the establishment of probable synergistic effects, numerous drug interactions, intrinsic toxicity, and ambiguity about quality, identity, and authenticity are among the difficulties facing researchers today. In silico methodologies such as computational therapeutics with pharmacological approaches that integrate biological analyses is a rapidly growing technique that may offer novel ideas in drug development. Therefore, researchers need to tackle these formidable tasks with cutting-edge research methods and clinical studies.
The peels of a green banana native to East Africa (Musa spp.), notably Musa acuminata, also known as “Matooke” in southwest Uganda, “ebitooke” in Tanzania and “igitoki” in Rwanda, is one of the main staple and economically important foods in the region (Englberger et al. 2003; Birabwa et al. 2010; Kitavi et al. 2016). Studies have shown M. acuminata to be effective in treating many illnesses, including fever, cough, bronchitis, dysentery, allergies, STDs, and some non-communicable diseases. The known pharmacological actions embrace antioxidant, antidiabetic, immunomodulatory, hypolipidemic, anticancer, and antibacterial properties, including anti-HIV activity (Whitney et al. 2015; Sreejith et al. 2016; Mathew and Negi 2017). However, the therapeutic (e.g. hepatoprotective) effect of M. acuminata remains unknown. Although the antioxidant system may be modified, the mechanisms of action involved in the chemoprotection have not been studied. Our study evaluates the in-silico pharmacokinetic parameters and therapeutic potential of MAPE following aluminium chloride-induced hepatoxicity in adult BALB/c mice.
Materials and methods
Plant collection and authentication
M. acuminata (matooke peels) were collected from Kampala International University Western-Campus, view point canteen in Bushenyi-Ishaka municipality of Bushenyi district and authenticated by a taxonomist. A specimen number (HOPE-PHA-2019/04) was deposited at the School of Pharmacy Herbarium, Kampala International University (KIU), Western Campus, Uganda.
Preparation of extract
Fresh M. acuminata peels were collected using sterile polythene bags and transported to the Department of Pharmacology and Toxicology Laboratory, School of Pharmacy, KIU. They were washed with running tap water to remove detritus, allowed to drain, and shade-dried at room temperature for 6 weeks. Dried plant materials were reduced to powder using a mortar and pestle. The powdered peels (50 g) were macerated in absolute ethanol (800 mL) for 72 h, during which the solution was intermittently shaken twice every 24 h. After that, the solution was filtered using Whatman No. 1 filter paper, and the filtrate was concentrated and evaporated to dryness in vacuo using a rotary evaporator (BuchiLab, Switzerland) regulated at 40 °C to obtain MAPE. The MAPE was stored at 4 °C in a refrigerator (Nn 2015; Sankeshwari et al. 2018).
GC–MS analysis of ethanol extract of matooke peels
The active components of the ethanol extract of M. acuminata peels were analysed using Gas Chromatography (Agilent 7890B, USA) and Mass Spectrometer (5977A MSD). MassHunter program was used for the data analysis. Column information: 30-m-long HP-5 fused-silica capillary column with a 0.250-μm-thick layer. Inlet: Operated in pulse splitless mode at 250 °C (Temp) and 48.745 kPa (Pressure); 1 L, Helium (99.999% Purity), Average Velocity of 36.262 cm/s, Auxiliary Temperature of 280 °C; Oven temperature programming: raised to 240 °C at 3 °C/min from a starting temperature of 40 °C; total runtime: 67.667 min (Konappa et al. 2020; Onohuean et al. 2022a).
In silico estimation of the pharmacokinetic and toxicity profile of the GC–MS compounds
A good pharmacokinetic feature and a lack of toxicity make potential bioactive substances that do not transgress Lipinski RO5 more likely to succeed as drug candidates. The pharmacokinetic properties and toxicity profiles of the M. acuminata peel extract were predicted using the methods earlier described by (Aja et al. 2021). Briefly, the GC–MS bioactive compounds of M. acuminata were studied using the SwissAdme web server (http://www.swissadme.ch/) to check for Lipski rule of five (RO5) violations. The pharmacokinetics principles, including absorption, distribution, etc.), were explored and the toxicity of the potential GC–MS bioactive compounds was predicted using the pkCSM web server (http://biosig.unimelb.edu.au/pkcsm/prediction) (Aja et al. 2021; Onohuean et al. 2022b).
Molecular docking of GCMS compounds and standard (Simvastatin)
Twelve of the GCMS compounds with an average %peak area ≥ 1 and the standard (Simvastatin) were fetched individually by their PubChem CID (see Table 2) into UCSF Chimera workspace and saved in pdb format. The target protein (alkaline phosphatase) with PDB ID: 7YIV was also fetched in UCSF Chimera and prepared according to the methods of Aja et al. (2021). Further, the molecular docking of the GCMS compound with the target protein was performed using AutoDock Vina Plugin Pyrx as adopted from (Onohuean et al. 2022b) with modifications. The grid box was set to Center: Dimension x (52.016:89.7177), y (1.2605:51.1835), and z (− 12.6318:81.4562). To understand the interactions between the ligands and the protein, Discovery Studio 2022 was utilized (Aja et al. 2022) (Tusubira et al. 2023).
Table 2.
GC–MS analysis and mass spectral data of ethanol extract of M. acuminata peels
| Pubchem CID | Canonical SMILES | Formula |
|---|---|---|
| 6417 | OC(=O)C(C)(C)C | C5H10O2 |
| 65252 | CC(C(=C)CC[CH]([CH]1CC[C]2([C]1(C)CCC1=C2CC[CH]2[C]1(C)CC[CH]([CH]2C)O)C)C)C | C30H50O |
| 72870 | ClC(C(=O)c1ccccc1)Cl | C8H6Cl2O |
| 101257 | CC(=CCC[CH]([CH]1CC[C]2([C]1(C)CCC1=C2CC[CH]2[C]1(C)CC[CH](C2(C)C)O)C)C)C | C30H50O |
| 534579 | CC1CCC2C(C3(C1C(O)CC3=O)C)OC(=O)C2=C | C15H20O4 |
| 536708 | COc1ccc(cc1)C1CC2=C(Cl)C(=O)OC2(CN1C)C | C16H18ClNO3 |
| 4369188 | C/C(=C\C=C\C=C(\C=C\C=C(\C=C\C1=C(C)CCCC1(C)C)/C)/C)/C=C/C=C(/C=C/C1C(=CCCC1(C)C)C)\C | C40H56 |
| 5282184 | CCCCC/C=C\C/C=C\CCCCCCCC(=O)OCC | C20H36O2 |
| 5363269 | CCCCCCCC/C=C\CCCCCCCC(=O)OCC | C20H38O2 |
| 18993663 | [Si]1O[Si]O[Si]O[Si]O[Si]O[Si]O[Si]O[Si]O1 | O8Si8 |
| 53438479 | [Si]1O[Si]O[Si]O[Si]O[Si]O[Si]O[Si]O[Si]O[Si]O1 | O9Si9 |
| 54284936 | CCCCC=CCC=CCCCCCCCCC(=O)O | C18H32O2 |
Experimental animals
The animals were acclimatized for 7 days before the start of the experiment. The animals were bred at KIU's Biomolecules, Metagenomics, Endocrine, and Tropical Diseases Research Group (BMETDREG) laboratory, Department of Pharmacology and Toxicology. The experimental animals were handled in strict adherence to the procedure for animal care as recommended by the Ethics and Research Committee, Kampala International University. The experimental protocol was approved by the KIU-Ethics and Research Committee and conducted in accordance with the guidelines given by the Uganda National Council for Higher Education (UNCHE).
Experimental design
A total of 60 male mice (150-day old; 26–34 g) were randomly allotted to six groups (n = 10), and treated as shown in Table 1. To induce hepatotoxicity, the mice were administered 10 mg/kg body weight of AlCl3 intraperitoneally for 14 days. After induction, mice were treated with standard drugs: zinc 20 mg (Agog Pharma LTD, India) and simvastatin (Denk Pharma GmbH & Co. KG, Germany) and extract (MAPE) from day 15 to day 36 (Table 1). After the test duration, the animals were perfused with sodium pentobarbital (150 mg/kg, i.p), euthanized and sacrificed. Blood samples were obtained via cardiac puncture and placed into plain tubes for biochemical analysis. The livers were eviscerated and fixed in buffered formalin for histopathological examination.
Table 1.
Experimental design
| Treatment groups | Dose (mg/kg) | Duration (days) |
|---|---|---|
| Group 1 (negative control) | AlCl3 + distilled H2O (5 mL/kg) | 15–36 |
| Group 2 (low dose) | AlCl3 + MAPE (100 mg/kg, p.o) | 15–36 |
| Group 3 (middle dose) | AlCl3 + MAPE (200 mg/kg, p.o) | 15–36 |
| Group 4 (high dose) | AlCl3 + MAPE (400 mg/kg, p.o) | 15–36 |
| Group 5 (positive control) | AlCl3 + Zn + SIMV (20 mg/kg each, p.o) | 15–36 |
| Group 6 (placebo) | Distilled water (5 mL/kg) | 01–36 |
MAPE M. acuminata peels extract, SIMV simvastatin
Biochemical analysis
Aspartate transaminase (AST) and alanine aminotransferase (ALT) activities were determined at 340 nm according to the methods described by Kumar and Gill (2018). Alkaline phosphatase (ALP) was determined at 405 nm according to the method described by Tietz et al. (1983). Also, gamma-glutamyl transferase (GGT) activity was determined using the method described by Gjerde and Mørland (1985). Total plasma protein was determined at 530 nm as earlier described by Tietz et al. (1983). Also, serum albumin was measured at 630 nm, as described by Webster et al. (1974). Serum total cholesterol and triglyceride levels of the treated mice were measured using the methods described by Tietz et al. (1983). Low-density lipoprotein (LDL) was estimated from the formula of Friedewald et al. (1972). These biochemical investigations were done using automated analysers and Fortress Diagnostic Kits® according to standard manufacturer procedures and protocols at Kampala International University Teaching Hospital, Ishaka-Bushenyi, Uganda.
Histopathological assessment of the liver
From each sodium pentobarbital (150 mg/kg, i.p) perfused, euthanized and sacrificed mice, the liver was immediately excised, freed from the adventitia, blotted with tissue paper, weighed, sectioned and fixed in 4% paraformaldehyde overnight. Afterwards, the liver tissues were post-fixed overnight at 4 °C in the same fixative and used for histological studies (Rajamohamedsait and Sigurdsson 2012). The fixed sections were dehydrated with alcohol, cleared with xylene, infiltrated and mounted with paraffin wax, sectioned, rehydrated, stained with haematoxylin and eosin (H&E) and mounted with coverslips for histopathological examination under a high-powered light microscope at a magnification of 400. To avoid bias, the doses and treatments given to the different groups of experimental animals were not disclosed to the pathologist.
Statistical analysis
Generated data were statistically analysed using GraphPad Prism version 8.0.2. While the antioxidant results were subjected to two-way ANOVA and Tukey post hoc test, others were analysed using one-way ANOVA and LSD post hoc test. All statistical significance was considered at p < 0.05.
Results
GC–MS analysis of ethanol extract of M. acuminata peels
See Table 2.
In silico estimation of pharmacokinetics and toxicity profile of M. acuminata GC–MS compounds
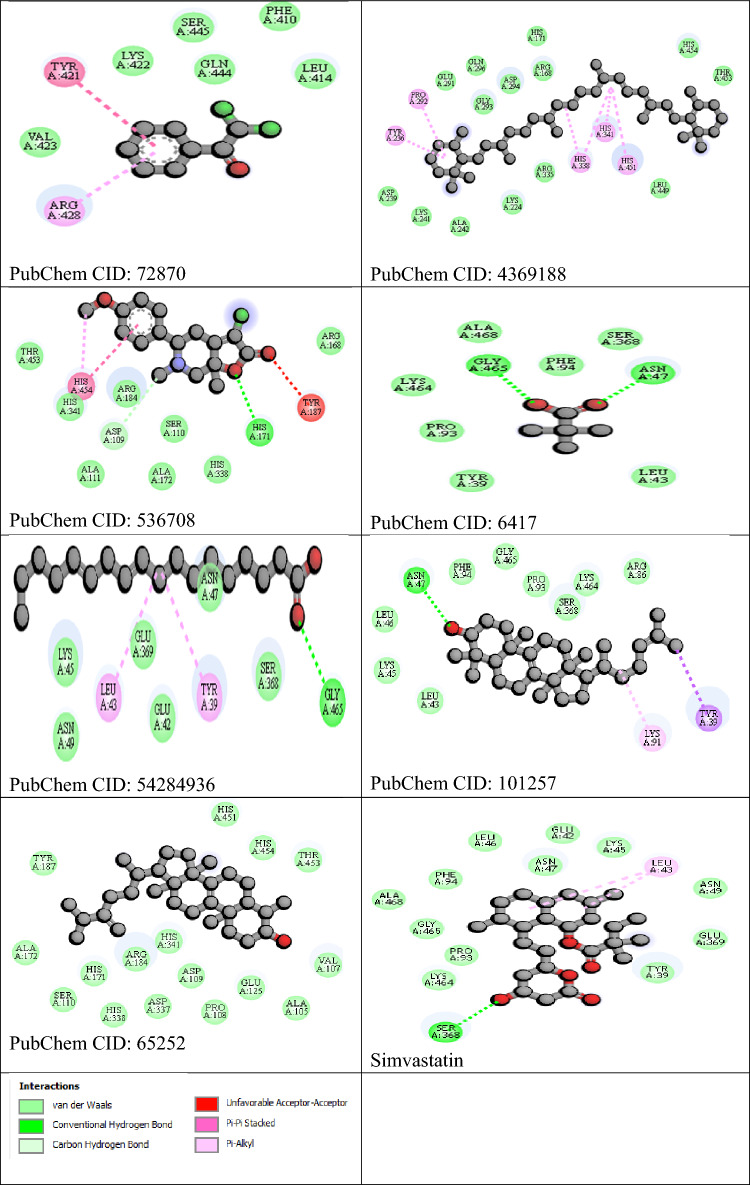
12 GCMS bioactive compounds and their canonical SMILES, formula and molecular weight (MW) are presented in Table 2. Were selected for in silico analysis, based on their molecular weights and retention time. The compound name and PubChem CID include 2,2-Dichloroacetophenone (72870), Cyclooctasiloxane (18993663), 7-Hydroxy-6,9a-dimethyl-3-methylene-decahydro-azuleno[4,5-b]furan-2,9-dione (534579), all-trans-alpha-Carotene (4369188), Cyclononasiloxane (53438479), 3-Chloro-5-(4-methoxyphenyl)-6,7a-dimethyl-5,6,7,7a-tetrahydro-4H-furo[2,3-c]pyridin-2-one (536708), Pivalic acid (6417), 10,13-Octadecadienoic acid (54284936), Ethyl Linoleate (5282184), Oleic acid (5363269), Tirucallol (101257), Obtusifoliol (65252) detailed in Table 3. Of the 12 GCMS compounds, 5 have sulfur derivatives, making them incompatible with the AutoDock Vina; hence, only 7 were successfully docked with the target proteins. The seven compounds identified three binding pockets (Fig. 1). Two of the compounds (Pivalic acid and Tirucallol) bind in the same pocket with the standard whereas five (2,2-Dichloroacetophenone, all-trans-alpha-Carotene, 3-Chloro-5-(4-methoxyphenyl)-6,7a-dimethyl-5,6,7,7a-tetrahydro-4H-furo[2,3-c]pyridin-2-one, 10,13-Octadecadienoic acid and Obtusifoliol) bind at different pockets with relatively high binding affinities (Table 3). Interaction visualized in Discovery Studio shows that the within compounds that bind in the same pockets interacted with similar amino acids using different kinds of bonds (Fig. 2).
Table 3.
Docking scores (binding affinities) of the lead molecules
| Compound name | %peak area | PubChem CID | Binding affinity (Kcal/mol) |
|---|---|---|---|
| Simvastatin (standard) | – | 54454 | − 7.4C |
| 2,2-Dichloroacetophenone | 2.35 | 72870 | − 5.1A |
| Cyclooctasiloxane | 1.14 | 18993663 | ∞ |
| 7-Hydroxy-6,9a-dimethyl-3-methylene-decahydro-azuleno[4,5-b]furan-2,9-dione | 1.64 | 534579 | ∞ |
| All-trans-alpha-Carotene | 1.01 | 4369188 | − 8.0B |
| Cyclononasiloxane | 1.05 | 53438479 | ∞ |
| 3-Chloro-5-(4-methoxyphenyl)-6,7a-dimethyl-5,6,7,7a-tetrahydro-4H-furo[2,3-c]pyridin-2-one | 1.23 | 536,708 | − 6.4B |
| Pivalic acid | 1.00 | 6417 | − 3.8C |
| 10,13-Octadecadienoic acid | 1.08 | 54284936 | − 4.2B |
| Ethyl linoleate | 1.97 | 5282184 | ∞ |
| Oleic acid | 1.99 | 5363269 | ∞ |
| Tirucallol | 1.93 | 101257 | − 7.9C |
| Obtusifoliol | 2.06 | 65252 | − 7.0B |
APocket A; BPocket B; CPocket C; ∞ Not compatible with AutoDock
Fig. 1.
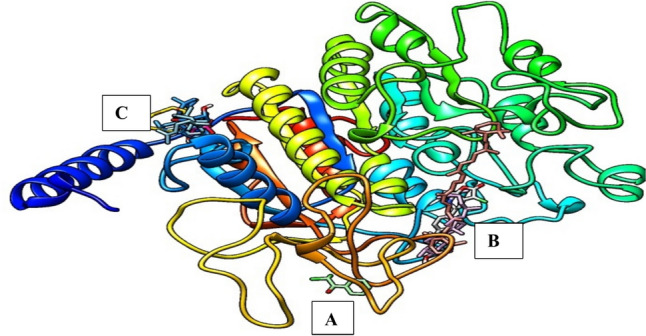
Display of the binding pose of the ligands with the pockets of the target protein
Fig. 2.
Binding interactions between the ligands and the target proteins
Utilizing the Swissadme server, the drug-likeness was investigated by taking Lipinski RO5's physicochemical characteristics into account. According to Nisha et al. (2016), a compound’s lipophilicity can be determined by its iLogP value, which is the logarithm of the compound's n-octanol–water partition coefficient. Lower molecular weights also increase the absorption rate. A higher iLog P value indicates poor absorption and penetration, which also suggests reduced lipophilicity. Determining the selectivity of ligand binding is aided by hydrogen bonding. The TPSA (Topological Polar Surface Area) indicates the compound's polar atoms' surface. P-glycoprotein, in charge of drug efflux from a cell, prefers substrates with a higher TPSA, and substances with a higher TPSA are linked to decreased membrane permeability (Blake 2000). Therefore, 2,2-Dichloroacetophenone (72870), 3-Chloro-5-(4-methoxyphenyl)-6,7a-dimethyl-5,6,7,7a-tetrahydro-4H-furo[2,3-c]pyridin-2-one (536708), 10,13-Octadecadienoic acid (54284936), Obtusifoliol (65252), Pivalic acid (6417), Tirucallol (101257) compounds possesses the best drug likeness potential among the bioactive components of Musa acuminata Peels (Table 4). The Pharmacokinetics properties of the Musa acuminata Peels showed that GI absorption was low for Obtusifoliol, Tirucallol, and Oleic acid, while other compounds were high. The BBB permeant was found in all the tested compounds except Obtusifoliol, Tirucallol, and Oleic acid, whereas no Pgp substrate was found in the compounds identified in Musa acuminata Peels.
Table 4.
Drug likeness of the potential of Musa acuminata Peels from swissadme server
| Pubchem CID | #Heavy atoms | Aromatic heavy atoms | Fraction Csp3 | Rotatable bonds | H-bond acceptors | H-bond donors | MR | TPSA | iLOGP | Lipinski #violations | MW |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6417 | 7 | 0 | 0.8 | 1 | 2 | 1 | 27.66 | 37.3 | 1.28 | 0 | 102.13 |
| 65252 | 31 | 0 | 0.87 | 5 | 1 | 1 | 137.3 | 20.23 | 5 | 1 | 426.72 |
| 72870 | 11 | 6 | 0.12 | 2 | 1 | 0 | 46.23 | 17.07 | 1.68 | 0 | 189.04 |
| 101257 | 31 | 0 | 0.87 | 4 | 1 | 1 | 137.04 | 20.23 | 5.14 | 1 | 426.72 |
| 534579 | 19 | 0 | 0.73 | 0 | 4 | 1 | 69.79 | 63.6 | 1.85 | 0 | 264.32 |
| 536708 | 21 | 6 | 0.44 | 2 | 4 | 0 | 84.58 | 38.77 | 2.98 | 0 | 307.77 |
| 4369188 | 40 | 0 | 0.45 | 10 | 0 | 0 | 184.43 | 0 | 1.85 | 0 | 536.87 |
| 5282184 | 22 | 0 | 0.75 | 16 | 2 | 0 | 98.59 | 26.3 | 4.09 | 1 | 308.5 |
| 5363269 | 22 | 0 | 0.85 | 17 | 2 | 0 | 99.06 | 26.3 | 4.29 | 1 | 310.51 |
| 18993663 | 16 | 0 | 0.12 | 0 | 1 | 0 | 54.71 | 73.84 | 1.68 | 0 | 352.68 |
| 53438479 | 18 | 0 | 0.45 | 0 | 0 | 0 | 61.55 | 83.07 | 1.85 | 0 | 396.76 |
TPSA topological polar surface area, Log P octanol–water partition coefficient
Only 3-Chloro-5-(4-methoxyphenyl)-6,7a-dimethyl-5,6,7,7a-tetrahydro-4H-furo[2,3-c]pyridin-2-one inhibits isoforms (CYP2C19, CYP2D6, and CYP3A4), while Ethyl Linoleate and 10,13-Octadecadienoic acid are inhibitors of the isoforms CYP2D6 ability to biotransformation (Table 5).
Table 5.
Pharmacokinetics properties of the Musa acuminata Peels from swissadme server
| Pubchem CID | GI absorption | BBB permeant | Pgp substrate | CYP1A2 inhibitor | CYP2C19 inhibitor | CYP2C9 inhibitor | CYP2D6 inhibitor | CYP3A4 inhibitor |
|---|---|---|---|---|---|---|---|---|
| 6417 | High | Yes | No | No | No | No | No | No |
| 65252 | Low | No | No | No | No | No | No | No |
| 72870 | High | Yes | No | Yes | No | No | No | No |
| 101257 | Low | No | No | No | No | No | No | No |
| 534579 | High | Yes | No | No | No | No | No | No |
| 536708 | High | Yes | No | No | Yes | No | Yes | Yes |
| 4369188 | High | Yes | No | No | No | No | No | No |
| 5282184 | High | Yes | No | Yes | No | Yes | No | No |
| 5363269 | Low | No | No | Yes | No | No | No | No |
| 18993663 | High | Yes | No | Yes | No | No | No | No |
| 53438479 | High | Yes | No | No | No | No | No | No |
| 54284936 | High | Yes | No | Yes | No | Yes | No | No |
GI gastrointestinal, BBB blood–brain barrier; Pgp P-glycoprotein, CYP cytochrome P
The compounds 7-Hydroxy-6,9a-dimethyl-3-methylene-decahydro-azuleno[4,5-b]furan-2,9-dione and Pivalic acid of MAPE are inhibitors of HERG I and AMES toxicity. All the tested compounds are skin sensitive whereas all the identified compounds are chemoprotective to AMES toxicity, HERG I, HERG II and hepatotoxicity (Table 6).
Table 6.
Predicted toxicity profile of M. acuminata GC–MS compounds in pKCSM webserver
| PubChem CID | AMES toxicity | HERG I Inhibitor | HERG II Inhibitor | Hepatotoxicity | Skin Sensitization |
|---|---|---|---|---|---|
| 6417 | Yes | No | No | No | Yes |
| 65252 | No | No | No | No | Yes |
| 72870 | No | No | No | No | Yes |
| 101257 | No | No | No | No | Yes |
| 534579 | No | Yes | No | No | Yes |
| 536708 | No | No | No | No | Yes |
| 4369188 | No | No | No | No | Yes |
| 5282184 | No | No | No | No | Yes |
| 5363269 | No | No | No | No | Yes |
| 18993663 | No | No | No | No | Yes |
| PubChem CID | MTD (Human)Log (mg/kg/day) | LD50 (mol/kg) | LOAEL Log (mg/kg_bw/day) | T.pT Log(ug/L) | MT Log (mM) |
|---|---|---|---|---|---|
| 6417 | 1.109 | 2.026 | 2.047 | − 1.026 | 1.920 |
| 65252 | 0.261 | 1.501 | 1.394 | 2.026 | − 0.595 |
| 72870 | 0.540 | 2.153 | 2.011 | − 0.198 | 2.034 |
| 101257 | 0.170 | 1.481 | 1.323 | 2.004 | − 1.089 |
| 534579 | − 0.073 | 1.574 | 1.194 | 0.989 | − 2.245 |
| 536708 | − 0.708 | 1.440 | 3.181 | 0.840 | − 1.083 |
| 4369188 | − 0.810 | 1.417 | 3.259 | 0.676 | − 1.438 |
| 5282184 | − 0.791 | 1.406 | 3.330 | 0.650 | − 1.565 |
| 5363269 | − 0.810 | 1.417 | 3.259 | 0.676 | − 1.438 |
| 18993663 | 0.013 | 1.478 | 1.172 | 1.963 | − 1.070 |
AMES Mutagenicity, hERG human ether-a-go-go gene, MTD maximum tolerated dose, LD50 oral rat acute toxicity, LOAEL oral rat chronic toxicity, T.pT T. pyriformis toxicity, MT Minnow toxicity
Effect of MAPE on animal body and liver weights
The mice's body weights were determined weekly in each treatment process, as revealed in Fig. 1 below and plotted as weight (g) against weeks. Compared to controls, significant increases in body weight of the treated animals were observed in weeks 4 and 5 of the study duration Fig. 3A. Also, the results showed a significant (p < 0.05) increase in the liver weights of mice administered aluminium chloride (−C) compared to placebo (P). Extract-treated groups (T1–T3) showed a significant and dose-dependent reduction in liver weight compared to the untreated group (−C). Similarly, experimental animals administered standard drugs (simvastatin/zinc, +C) showed a significant reduction in liver weight compared to the untreated group (−C). However, no significant difference was observed between the extract-treated and standard groups Fig. 3B.
Fig. 3.
A Effect of MAPE on body weight. B Total liver weight of animals in the different groups
Effect of MAPE on liver function markers
Lipid profile
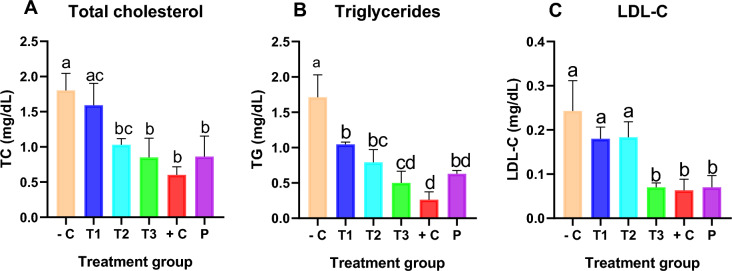
The lipid profile evaluation results are shown in Fig. 4A–C.
Fig. 4.
A–C Effect of MAPE on liver function markers in the different groups. Each bar represents the mean ± SEM of each group of animal experiments. ap < 0.05 −C versus P; bp < 0.05 +C versus −C; cp < 0.05 T1, T2, T3 versus −C; dp < 0.05 T1, T2, T3 versus +C
A. Total cholesterol (TC): From the result (A), a dose-dependent significant (p < 0.05) decrease in mean TC was observed in the treatment groups compared with the untreated group. A comparison of the untreated and placebo groups showed a significant difference in mean TC.
B. Triglycerides (TG): There was a significant difference (p < 0.05) in the mean TG of group −C when compared with group P. Also, a dose-dependent decrease in TG was observed in the treatment groups compared with the untreated group −C. However, the standard drug group (+C) has more potent triglyceride-lowering effects compared to the extract-treated groups.
C. Low-density lipoproteins (LDL): There was a significant difference (p < 0.05) in the mean LDL of the group −C when compared with group P. A significant reduction in LDL was observed at 400 mg/kg body weight of extract compared with the negative control group (−C). There was no significant difference (p > 0.05) between the mean LDL of the groups treated with 100 and 200 mg/kg bwt of the extract compared with the untreated group.
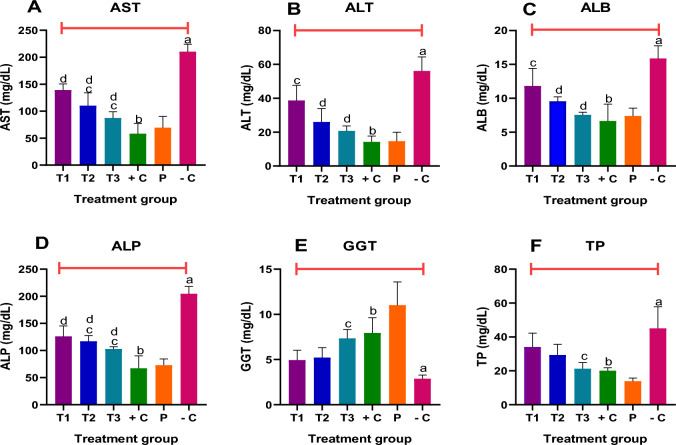
Liver enzymes parameters
The results are presented in Fig. 5A–F.
Fig. 5.
A–F Effect of MAPE on liver enzymes parameter in the different groups. Each bar represents the mean ± SEM of each group animal experiments. ap < 0.05 −C versus P; bp < 0.05 +C versus −C; cp < 0.05 T1, T2, T3 versus −C; dp < 0.05 T1, T2, T3 versus +C
A. Aspartate transaminase (AST): Compared with the placebo group (P), significant (p < 0.05) elevation in the activity of AST was observed in the negative control group (−C). Also, significant dose-dependent reductions in the activities of AST were recorded for the extract-treated (T1, T2 and T3) groups compared with the negative control (−C) group. However, these observations were less than those of the standard drug.
B. Alanine transaminase (ALT): In comparison with P, significant elevations in the activities of ALT were observed in group −C. Also, dose-dependent decreases in the activities of ALT were recorded for experimental animals in the extract-treated groups compared to −C. However, these observations were less than those of the standard drug.
C. Serum albumin (ALB): Compared to the negative control group, MAPE caused a significant dose-dependent decrease in the level of ALB at all the dose levels tested. Though with reduced potency, this observed effect was similar to that of the standard drug.
D. Alanine phosphatase (ALP): The activities of ALP in group −C were significantly (p < 0.05) higher when compared to group P. The extracts showed a dose-dependent decrease compared with the untreated group −C. The ALP-lowering effects of MAPE were similar to that of the standard drug.
E. Gamma-glutamyl transferase (GGT): The highest GGT activity was observed in group P and the lowest in group −C. Only T3 significantly decreased GGT activity when compared to −C. Meanwhile, the T1, T2, and T3 treatment groups showed an insignificant reduction of GGT compared to group +C. Therefore, only extract administrated at 400 mg/kg is effective in GGT activities by significantly reducing the effect of AlCl3 chloride-induced hepatoxicity in adult BALB/c mice.
F. Total protein (TP): Significant increases in the level of total protein were observed in the negative control relative to the placebo group. At the doses tested, MAPE caused significant dose-dependent reductions in the serum levels of TP relative to the negative control group. The observed effect was similar to that of the standard drug.
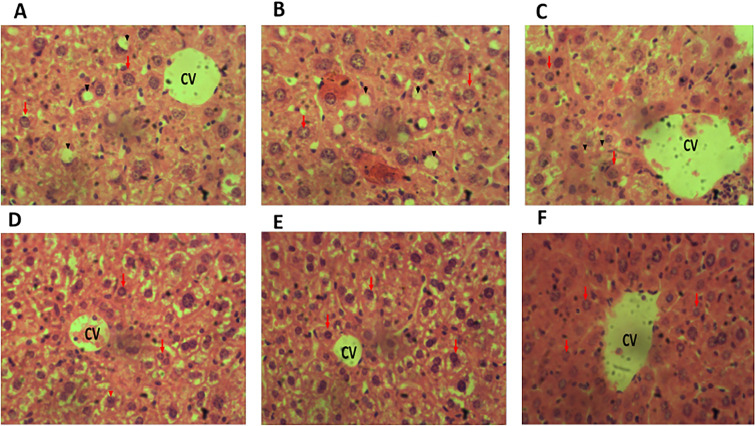
Histological studies
Histological examination of the liver of adult BALB/c mice in the positive control (standard drug; Fig. 6E), normal control (placebo; Fig. 6F), and 400 mg/kg bwt MAPE (T3; Fig. 6D) presented preserved/normal cellular architecture of the ductal cells, the normal orientation of layers of blood vessels, well-spaced hepatocytes and presence of Kupffer cells within the sinusoids. However, those of the negative control group, T1 and T2, presented a distorted histoarchitecture with the presence of fatty droplets, ruptured, degenerated and disoriented layers of bile ducts, abnormally spaced and vacuolated hepatocytes, disoriented sinusoids, tissue degeneration and hyperplasia (Fig. 6A–C respectively).
Fig. 6.
A–F Image of MAPE effect on liver sections in the different groups. A–F represent Group −C, T1, T2, T3, +C, and P, respectively. CV central vein, hepatocytes (red arrows), fatty droplet (arrowhead) (H&E; ×400)
Discussion
In Uganda, M. acuminata has traditionally been exploited to treat several illnesses, especially as it contains a variety of bioactive substances. The study was undertaken due to the uncertainty of the pharmacokinetic profile and pharmacological actions of the peel extract of M. acuminata. From our findings, MAPE showed promising hepatoprotective active in-silico and good pharmacokinetic profile, as well as in the experimental studies. We hypothesize that the existence of the listed compounds detected by GC–MS may be responsible for the pharmacological effects. The GC–MS elucidates the bioactive component associated with therapeutics relevant (Onohuean et al. 2022a, b). Our results show the present of 2,2-Dichloroacetophenone, Cyclooctasiloxane, 7-Hydroxy-6,9a-dimethyl-3-methylene-decahydro-azuleno[4,5-b]furan-2,9-dione, all-trans-alpha-Carotene, Cyclononasiloxane, 3-Chloro-5-(4-methoxyphenyl)-6,7a-dimethyl-5,6,7,7a-tetrahydro-4H-furo[2,3-c]pyridin-2-one, Pivalic acid, 10,13-Octadecadienoic acid, Ethyl Linoleate, Oleic acid, Tirucallol, and Obtusifoliol which are effective in hepatoprotective pharmacologic indications. However, enlisting new compounds as therapeutic candidates necessitates thoroughly comprehending their drug-likeness. The goal of the in-silico portion of this study was to forecast how similar each molecule may be to a certain medicine to help with drug discovery and the development of M. acuminata nutraceuticals. Surprisingly, four compounds (Cyclooctasiloxane, 7-Hydroxy-6,9a-dimethyl-3-methylene-decahydro-azuleno[4,5-b]furan-2,9-dione, Cyclononasiloxane, Ethyl Linoleate and Oleic acid) were not compatible with the molecular docking software (AutoDock vina plugin Pyrx) and was excluded from the studies. A similar finding has been reported by Aja et al. (2021), implying that the silicon-containing compound was not compatible with docking in (AutoDock vina plugin Pyrx) software. Thus, compounds are considered potential inhibitors only if they bind at the exact location as conventional inhibitors in their docking pose and have significant binding affinities. An indicator of a ligand's modulating impact is its capacity to directly find and bind to the receptor's active site. To render a protein inactive, inhibitors attach themselves to its active areas. Our finding reveals two compounds (Pivalic acid and Tirucallol) that perfectly bind in the same pocket as the standard drug simvastatin. Pivalic acid compound is present in pharmaceutical preparations that mostly functions in the esterified prodrugs such as atorvastatin calcium and beta-lactam antibiotics (Sonje et al. 2010), whereas tirucallol has been reported to have a topical anti-inflammatory effect by suppressing ear edema in the mouse model and inhibit nitrite production in lipopolysaccharide-stimulated macrophages (Fernandez-Arche et al. 2010). However, the impact of these two compounds on hepatotoxicity remains unknown. The test compounds and Simvastatin inhibitors bind to alkaline phosphatase at the same location, implying they are potential inhibitors of the target protein similar to the standard inhibitor. All other potent inhibitors, such as 2,2-Dichloroacetophenone, have been previously reported to inhibit pyruvate dehydrogenase kinase (PDK) and are more effective than dichloroacetate (DCA) in anti-cancer therapy (Yang et al. 2017). Trans-alpha-carotene is a known antioxidant and possesses anti-carcinogenic properties, and enhances immunity (Merhan 2017), 3-Chloro-5-(4-methoxyphenyl)-6,7a-dimethyl-5,6,7,7a-tetrahydro-4H-furo[2,3-c]pyridin-2-one, 10,13-Octadecadienoic acid has analgesic and anti-inflammatory effect (Muzahid et al. 2023) and Obtusifoliol have previously been reported to have antibacterial, anti-inflammatory, and anticancer properties (Aghaei et al. 2016).
Similar linkages were implicated; however, the typical and prospective inhibitors engage with distinct amino acids in the binding locations. Interacting bonds include hydrogen bonding, van der Waals, several types of pi-bonds, unfavourable donors, and salt bridges. Stronger interactions occur during binding that involves hydrogen bonds. All-trans-alpha-carotene and Tirucallol indicate more potential inhibitors interacting with hydrogen bonds and van der Waals. These interactions of ligands and receptors are the primary driver of cell-to-cell communication and dysregulation and modifications in diseases, which inspire targeted therapeutics development. Also, the drug-likeness potential among the bioactive components of MAPE was positive in the test of seven compounds. However, evaluating the possible inhibitors, a drug-like characteristic was more advantageous with a lower TPSA and compounds with improved blood–brain barrier (BBB) permeability are expected to have a lower TPSA value (Chico et al. 2009; Onohuean et al. 2022b). Most of the active compounds of MAPE are bioavailable in the neurological pathways, opening up the possibility of treating neurodegeneration. Given that none of the Lipinski RO5 are broken by the seven compounds, these indicate that they have a higher chance of being therapeutically successful than other inhibitor compounds derived from MAPE.
An additional predictor of the possibility of treatment success for pharmacological compounds such as these putative inhibitors is their pharmacokinetic characteristics. Obtusifoliol, Tirucallol, and Oleic acid were low for GI absorption, while other compounds were high. Also, all other compounds were BBB permeating except for Obtusifoliol, Tirucallol, and Oleic acid. When taken orally, a compound with high GI absorption may be more readily absorbed from the digestive system. The results shown most active compounds of MAPE is more absorbed from the intestinal tract due to high GI absorption when administered orally. However, Obtusifoliol, Tirucallol, and Oleic acid with low GI absorption could benefit through different methods, instead of oral delivery. Surprisingly, all the tested active compounds MADPE emerged as substrates for predicting efflux by p-glycoprotein indicating they are comparatively safe. The machinery involved in drug metabolism is CYP450. The 2,2-Dichloroacetophenone, Ethyl Linoleate, Oleic acid, Cyclooctasiloxane, and 10,13-Octadecadienoic acid where inhibitors of CYP450 isoforms (CYP1A2). Only 3-Chloro-5-(4-methoxyphenyl)-6,7a-dimethyl-5,6,7,7a-tetrahydro-4H-furo[2,3-c]pyridin-2-one inhibits isoforms (CYP2C19, CYP2D6, and CYP3A4), while Ethyl Linoleate and 10,13-Octadecadienoic acid are inhibitors of the isoforms CYP2D6 ability to biotransformation. All other compound were non-inhibitors of the isoforms CYP450. Thus, the biotransformation of pharmaceuticals that are metabolized by the CYP450 enzyme may not be interfered with by the M. acuminata GC–MS compounds that are not inhibitors of CYP450 isoforms (CYP1A2, CYP2C19, CYP2C9, CYP2D6, and CYP3A4). This implies that most of M. acuminata compounds, neither substrate nor inhibitors to the metabolizing enzyme subunits, and may not enhance or antagonized the activities of other drugs.
Active molecules' level of toxicity dictates whether or not they are appropriate to be chosen as a lead compound in drug discovery. Our finds indicate only 7-Hydroxy-6,9a-dimethyl-3-methylene-decahydro-azuleno[4,5-b]furan-2,9-dione and Pivalic acid compounds of MAPE is an inhibitors of HERG I and AMES toxicity respectively, while all the tested compounds are skin sensitive. It indicates that MADPE bioactive compounds are allergy-induced contact dermatitis can result from toxic skin sensitization. Interestingly, all the identified compounds are chemoprotective to AMES toxicity, HERG I, HERG II and hepatotoxicity. AMES testing establishes a substance's carcinogenic or mutagenic properties. Therefore, all MAPE tested compounds potential inhibitors are not known to cause cancer. One anti-drug candidate is the hERG potassium channel, which is linked to cardiac repolarization and arrhythmia (Nisha et al. 2016). The hERG inhibitory medications' negative side effects are compelling their removal from the market. However, MAPE identified compounds are not inhibitory to hERG I and hERG II except the 7-Hydroxy-6,9a-dimethyl-3-methylene-decahydro-azuleno[4,5-b]furan-2,9-dione observed to inhibit hERG I. All MAPE tested compounds have shown MTD below 0.477 and a comparable LD50 doses. The MTD > 0.477 is regarded as high, while MTD < 0.477 as low. The MTD have been utilize in determining the Phase I clinical trial starting doses for pharmaceuticals which provides an estimate of the hazardous dosage threshold of substances in humans. The assessment of long-term toxicity is crucial in identifying the maximum dosage at which no negative consequences are noted, i.e. the possible inhibitors' oral rat chronic toxicity (LOAEL). Nonetheless, the minimum amount of time needed for therapy and the bioactive concentration of a compound determine its LOAEL. T. pyriformis and Minnow toxicity characterize the Lethal concentration values (LC50) and toxic endpoints (IGC50) of compounds. Consequently, an IGC50 more than or equal to 0.5 log μg/L is considered harmful, whereas an LC50 value of less than 0.5 mM (logLC50 < − 0.3) indicates severe acute toxicity. The LOAEL, T. pyriformis and Minnow toxicity where show to be effective for MAPE compounds.
Overall, the in-silico approach has been used in this work in addition to the in-vivo investigation to indicate the drug-like qualities of the M. acuminata. In the aluminium chloride-induced hepatotoxicity animal (adult BALB/c mice) model, there were significant elevations in the serum levels of lipids (total cholesterol and triglycerides), non-specific liver transaminases (AST, ALT and GGT), total protein and alkaline phosphatase in the negative control. These elevations are indications of hepatotoxicities following exposure to AlCl3. When an injury to the liver occurs, these abundant transaminases in the hepatic cytosol leak into the bloodstream, which explains their marked elevations in the serum. Alkaline phosphatase is a membrane-bound enzyme that facilitates the hydrolysis of phosphate esters hydrolyses phosphate esters. The enzyme is also found in the kidneys, intestines, bone, reticuloendothelial tissues and the placenta. Though not entirely localised in the liver cells' sinusoids, the enzyme is believed to be involved in hepatic transport (Udom et al. 2020; Vroon and Israili 1990). Hepatic injury is associated with sustained elevations in ALP serum activities and may be due to either the liver's primary or secondary pathological conditions or both. For example, serum ALP and gamma-glutamyl transferase elevations are seen in cholestatic lesions secondary to intrahepatic or post-hepatic disease (Vroon and Israili 1990). Therefore, the marked elevations of these parameters, as shown by our findings, are indicative of AlCl3-induced liver toxicity.
Physiologically, cholesterol is a constituent of animal cell membranes and a precursor of various essential substances, including bile acids, and steroid hormones of adrenal gland and gonadal origins. In contrast, triglycerides are fatty acid esters of glycerol and are the main lipid component of dietary fat and animal fat depots. They are transported alongside cholesterol in the plasma. The concentrations of cholesterol and triglycerides in the plasma implicate an increase in lipoproteins levels. For example, an isolated elevation of total plasma cholesterol usually indicates elevated LDL, while an isolated elevation of plasma triglycerides indicates elevated chylomicrons and VLDLs. These lipoproteins are tagged as “bad cholesterol” and are implicated in several cardiovascular disorders. Therefore, the persistent and abnormal elevations of total plasma cholesterol and triglycerides are of huge clinical correlates, especially as they are pointers to an underlying pathology of the cardiovascular system as well as metabolic disorder. According to Vroon and Israili (1990), hypercholesterolemia and hyperlipidaemia are considerably high-risk factors for atherosclerotic plague formation and severe coronary heart disease. In this study, the finding from the biochemical analysis corroborates that of the histopathology of the liver. The administration of the MAPE to the experimental BALB/c mice at the different test levels demonstrated dose-dependent reductions in the various biomarkers of liver functions and lipid profile induced by aluminium chloride. Similarly, the histopathological assessment of the liver revealed the potent hepatoprotective activity of MAPE at 400 mg/kg. These observations recorded in the present-day study suggest the probable antidotal and ameliorative effects of the plant extract against AlCl3-induced liver toxicity.
Conclusion
The findings of this study highlight the inherent potential of MAPE to improve liver functions and ameliorate the cellular or histoarchitecture of the hepatic cells following exposure to AlCl3. Though this validates the rationale for indigenous Ugandans' ethnomedicinal use of the plant material, a toxicological evaluation of the chronic or intermittent use of the plant material is necessary to ascertain its safety profile in whole organism systems.
Author contributions
This work was carried out in collaboration between all authors. Hope Onohuean: Conceptualization, Visualization, Methodology, Investigation, Formal analysis, Writing—original draft, review and editing. Eseohe Fanny Onohuean, Eseohe Sharon IGBINOBA, Saidi ODOMAb, Ibe USMAN, Josiah Eseoghene IFIE, Abdullateef Isiaka ALAGBONSI, Afodun Adam MOYOSORE, Godswill J. UDOM, Peter Chinedu AGU: Formal analysis, Writing—original draft, Writing—review and editing, Validation. Sharon Igbinob, Hayder M. Al‑KURAISHY, Gaber El‑Saber BATIHA, Patrick Maduabuchi Aja, Akinniyi A. OSUNTOK: Supervision, Writing—review and editing, Validation;
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no competing interest.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aghaei M, Yazdiniapour Z, Ghanadian M, Zolfaghari B, Lanzotti V, Mirsafaee V. Obtusifoliol related steroids from Euphorbia sogdiana with cell growth inhibitory activity and apoptotic effects on breast cancer cells (MCF-7 and MDA-MB231) Steroids. 2016 doi: 10.1016/j.steroids.2016.07.008. [DOI] [PubMed] [Google Scholar]
- Aja PM, Agu PC, Ezeh EM, Awoke JN, Ogwoni HA, Deusdedit T, et al. Prospect into therapeutic potentials of Moringa oleifera phytocompounds against cancer upsurge: de novo synthesis of test compounds, molecular docking, and ADMET studies. Bull Natl Res Cent. 2021 doi: 10.1186/s42269-021-00554-6. [DOI] [Google Scholar]
- Aja PM, Awoke JN, Agu PC, Adegboyega AE, Ezeh EM, Igwenyi IO, et al. Hesperidin abrogates bisphenol A endocrine disruption through binding with fibroblast growth factor 21 (FGF-21), α-amylase and α-glucosidase: an in silico molecular study. J Genet Eng Biotechnol. 2022 doi: 10.1186/s43141-022-00370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachar SC, Bachar R, Jannat K, Jahan R, Rahmatullah M. Hepatoprotective natural products. Annu Rep Med Chem. 2020 doi: 10.1016/bs.armc.2020.06.003. [DOI] [Google Scholar]
- Bethesda (2017) LiverTox: clinical and research information on drug-induced liver injury [Internet] [PubMed]
- Blake JF. Chemoinformatics-predicting the physicochemical properties of “drug-like” molecules. Curr Opin Biotechnol. 2000;11(1):104–107. doi: 10.1016/S0958-1669(99)00062-2. [DOI] [PubMed] [Google Scholar]
- Birabwa R, Asten PJAV, Alou IN, Taulya G. Got matooke (Musa spp.) for christmas? Acta Hortic. 2010 doi: 10.17660/actahortic.2010.879.9. [DOI] [Google Scholar]
- Bird TG, Kiourtis C. Liver diseases fibrosis and cirrhosis. Cell Senescence Dis. 2022 doi: 10.1016/B978-0-12-822514-1.00004-3. [DOI] [Google Scholar]
- Chico LK, Van Eldik LJ, Watterson DM. Targeting protein kinases in central nervous system disorders. Nat Rev Drug Discov. 2009 doi: 10.1038/nrd2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englberger L, Darnton-Hill I, Coyne T, Fitzgerald MH, Marks GC. Carotenoid-rich bananas: a potential food source for alleviating vitamin A deficiency. Food Nutr Bull. 2003;24:303–318. doi: 10.1177/156482650302400401. [DOI] [PubMed] [Google Scholar]
- Fernandez-Arche A, Saenz MT, Arroyo M, de la Puerta R, Garcia MD. Topical anti-inflammatory effect of tirucallol, a triterpene isolated from Euphorbia lactea latex. Phytomedicine. 2010 doi: 10.1016/j.phymed.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Fontana RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology. 2014 doi: 10.1053/j.gastro.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- Gjerde H, Mørland J. Determination of gamma glutamyltransferase in completely haemolysed blood samples. Scand J Clin Lab Invest. 1985;45(7):661–664. doi: 10.3109/00365518509155275. [DOI] [PubMed] [Google Scholar]
- Kitavi M, Downing T, Lorenzen J, Karamura D, Onyango M, Nyine M, et al. The triploid East African Highland Banana (EAHB) genepool is genetically uniform arising from a single ancestral clone that underwent population expansion by vegetative propagation. Theor Appl Genet. 2016;129:547–561. doi: 10.1007/s00122-015-2647-1. [DOI] [PubMed] [Google Scholar]
- Konappa N, Udayashankar AC, Krishnamurthy S, Pradeep CK, Chowdappa S, Jogaiah S. GC–MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Sci Rep. 2020 doi: 10.1038/s41598-020-73442-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Gill KD. Basic concepts in clinical biochemistry: a practical guide. Singapore: Springer; 2018. To determine creatinine clearance. [Google Scholar]
- Manfo FPT, Nantia EA, Kuete V (2014) Hepatotoxicity and hepatoprotective effects of African medicinal plants. In: Toxicological survey of African medicinal plants. 10.1016/B978-0-12-800018-2.00011-X
- Mathew NS, Negi PS. Traditional uses, phytochemistry and pharmacology of wild banana (Musa acuminata Colla): a review. J Ethnopharmacol. 2017 doi: 10.1016/j.jep.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Merhan O. The biochemistry and antioxidant properties of carotenoids. Carotenoids. 2017 doi: 10.5772/67592. [DOI] [Google Scholar]
- Muzahid AA, Sharmin S, Hossain MS, Ahamed KU, Ahmed N, Yeasmin MS, et al. Analysis of bioactive compounds present in different crude extracts of Benincasa hispida and Cucurbita moschata seeds by gas chromatography-mass spectrometry. Heliyon. 2023 doi: 10.1016/j.heliyon.2022.e12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisha CM, Kumar A, Nair P, Gupta N, Silakari C, Tripathi T, Kumar A. Molecular docking and in silico ADMET study reveals acylguanidine 7a as a potential inhibitor of β-secretase. Adv Bioinform. 2016;2016:9258578. doi: 10.1155/2016/9258578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nn A. Medicinal and aromatic plants a review on the extraction methods use in medicinal plants. Princ Strength Limit. 2015;4:3–8. doi: 10.4172/2167-0412.1000196. [DOI] [Google Scholar]
- Onohuean H, Igere BE. Ethnopharmacology and phytochemistry of medicinal plants against enterocyte bacteria-linked infections and diarrhoea in some African countries: a systematic review. RPS Pharm Pharmacol Rep. 2023 doi: 10.1093/rpsppr/rqad041. [DOI] [Google Scholar]
- Onohuean H, Ibrahim OA, Saheed LM, Ismaila IO. Chloroform seed extract of Buchholzia coriacea (Capparaceae) ameliorates complete Freund’s adjuvant-induced chronic inflammation in rat 1 2 2 3 2 L’extrait de graines de chloroforme de Buchholzia coriacea (Capparaceae) améliore complètement l’inflammation. West Afr J Pharm. 2018;29:95–104. [Google Scholar]
- Onohuean H, Adisa RA, Alagbonsi AI. Anti-apoptotic effect of Buchholzia coriacea Engl. stem back extracts on AsPC-1 and mechanisms of action. BMC Complement Med Ther. 2021 doi: 10.1186/s12906-021-03433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onohuean H, Alagbonsi AI, Usman IM, Iceland Kasozi K, Alexiou A, Badr RH, et al. Annona muricata Linn and Khaya grandifoliola C.DC. reduce oxidative stress in vitro and ameliorate Plasmodium berghei-induced parasitemia and cytokines in BALB/c mice. J Evid Based Integr Med. 2021 doi: 10.1177/2515690X211036669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onohuean H, Bright IE, Alagbonsi AI. GC-MS biocomponents characterization and antibacterial potency of ethanolic crude extracts of Camellia sinensis. SAGE Open Med. 2022 doi: 10.1177/20503121221116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onohuean H, Onohuean FE, Igbinoba SI, Ezeonwumelu JOC, Agu PC, Ifie JE, et al. Elucidation of chemical profiles and molecular targets of Mondia whitei leave fractions bioactive as novel therapeutics: an in vitro and in silico assay. J Genet Eng Biotechnol. 2022 doi: 10.1186/s43141-022-00440-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamohamedsait HB, Sigurdsson EM. Histological staining of amyloid and pre-amyloid peptides and proteins in mouse tissue. Methods Mol Biol. 2012 doi: 10.1007/978-1-61779-551-0_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankeshwari R, Ankola A, Bhat K, Hullatti K. Soxhlet versus cold maceration: which method gives better antimicrobial activity to licorice extract against Streptococcus mutans? J Sci Soc. 2018 doi: 10.4103/jss.jss_27_18. [DOI] [Google Scholar]
- Sonje VM, Kumar L, Meena CL, Kohli G, Puri V, Jain,R et al (2010) Atorvastatin calcium. In: Profiles of drug substances, excipients and related methodology. 10.1016/S1871-5125(10)35001-1 [DOI] [PubMed]
- Sreejith PE, Linu NK, Sasikumar P, Radhakrishnan KV, Sabu M. Phytochemical studies of an endemic and critically endangered hill banana, Musa acuminata Colla (AA) “Karivazhai” fruit by GC–MS. J Chem Pharm Res. 2016;2016:164–168. [Google Scholar]
- Tietz NW, Burtis CA, Duncan P, Ervin K, Petitclerc CJ, Rinker AD, Shuey D, Zygowicz ER. A reference method for measurement of alkaline phosphatase activity in human serum. Clin Chem. 1983;29(5):751–761. doi: 10.1093/clinchem/29.5.751. [DOI] [PubMed] [Google Scholar]
- Tox L (2020) LiverTox: clinical and research information on drug-induced liver injury—tocilizumab. Natl. Inst. Diabetes Dig. Kidney Dis. [PubMed]
- Tusubira D, Munezero J, Agu PC, Ajayi CO, Oloro J, Namale N, et al. In-vivo and in-silico studies revealed the molecular mechanisms of Colocasia esculenta phenolics as novel chemotherapy against benign prostatic hyperplasia via inhibition of 5α-reductase and α1-adrenoceptor. Silico Pharmacol. 2023 doi: 10.1007/s40203-023-00141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udom GJ, Okokon JE, Udobang JA, Obot DN, Asuquo IK. Nephrotoxicity assessment of Dr Iguedo Goko Cleanser® in exposed Wistar rats. Asian J Res Med Pharm Sci. 2020;9(1):30–40. doi: 10.9734/ajrimps/2020/v9i130144. [DOI] [Google Scholar]
- Vroon D, Israili Z (1990) Clinical methods: the history, physical, and laboratory examinations, 3rd edn [PubMed]
- Webster D, Bignell AH, Attwood EC. An assessment of the suitability of bromocresol green for the determination of serum albumin. Clin Chim Acta. 1974;53(1):101–108. doi: 10.1016/0009-8981(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Whitney CW, Bahati J, Gebauer J (2015) Value of plants in Ugandan homegardens; results of homegardens inventories and participatory ethnobotany investigations. In: Botany 2015; Science and plants for people
- Xu GB, Xiao YH, Zhang QY, Zhou M, Liao SG. Hepatoprotective natural triterpenoids. Eur J Med Chem. 2018 doi: 10.1016/j.ejmech.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Yang Z, Hu X, Zhang S, Zhang W, Tam KY. Pharmacological synergism of 2,2-dichloroacetophenone and EGFR-TKi to overcome TKi-induced resistance in NSCLC cells. Eur J Pharmacol. 2017 doi: 10.1016/j.ejphar.2017.08.037. [DOI] [PubMed] [Google Scholar]
- Zhang F, Han B, Li P, Lin Z, Yin D, Li Y, et al. Design, synthesis and hepatoprotective activity of analogs of the natural product goodyeroside A. Molecules. 2013 doi: 10.3390/molecules18021933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.