Abstract
Introduction
The treatment options for moderate to severe psoriasis (msPsO) in China have been greatly increased with the approvals of biologics. However, the unmet needs and treatment preferences of systemic treatments for msPsO in China remain unclarified.
Methods
Fifty dermatologists and 300 patients with msPsO (41% with severe psoriasis) were surveyed for effectiveness, safety, treatment convenience, and treatment preferences (using a choice-based conjoint questionnaire). Descriptive statistics and conjoint simulation analyses were employed to summarize survey information and assess treatment preferences.
Results
Both patients and dermatologists reported shorter treatment duration for oral drugs (2.7–6.2 months) than that for biologics (9.5–17.0 months). The most frequently reported treatment discontinuation reasons by the surveyed patients and dermatologists were unsatisfactory effectiveness (average 84.5%) for oral drugs and loss of efficacy over time (average 68.5%) for biologics. Commonly reported treatment inconveniences included regular lab tests for traditional oral drugs (average 71.5%) and administration assistance for biologics (average 58.0%). Injection site reactions (average 51.5%) and needle fear (average 35.5%) were frequently reported for biologics among the surveyed patients and dermatologists. Once-daily oral treatment was preferred over biweekly subcutaneous injection treatment when the two had comparable attributes (average in patients 87.1% vs. 12.9%; average in dermatologists 93.4% vs. 6.6%).
Conclusions
Unmet needs of systemic treatments remain for msPsO in China. Once-daily oral treatment is preferred over biweekly subcutaneous injections to treat msPsO when other treatment attributes are comparable.
Keywords: Moderate-to-severe psoriasis, Medical needs, Treatment preference, Choice-based conjoint analysis, Treatment administration route
Key Summary Points
| Why carry out this study? |
| The treatment options for moderate to severe psoriasis (msPsO) in China have been greatly expanded since the introduction of biologics. However, the limitations and challenges associated with these new treatments have not been well clarified from the perspectives of dermatologists and patients. |
| Surveying dermatologists and patients with msPsO for the unmet medical needs and treatment preferences could help with developing long-term disease management strategies for msPsO. |
| What was learned from the study? |
| Current systemic treatments for msPsO still face challenges related to limited treatment duration, treatment toxicity, and treatment inconvenience. Both dermatologists and patients favored once-daily oral treatments over biweekly injections when their attributes for treatment effects and side effects were similar. |
| Effective and convenient treatments are needed to address the unmet medical needs for msPsO. |
Introduction
About 0.47% of the population in China, which is roughly 6.3 million people, are estimated to have psoriasis [1]. Plaque psoriasis accounts for 80–90% psoriasis cases in China [2]. Psoriasis has a profound impact on patients’ quality of life [3], mental and emotional well-being [4], and personal relationships [5]. Additionally, patients with psoriasis are associated with comorbidities that could further worsen their health status [6]. When patients progress to moderate-to-severe psoriasis (msPsO), it may require systemic treatments to manage the condition effectively [7].
Historically, Chinese patients with psoriasis were mainly treated with methotrexate, cyclosporine, and acitretin before the first biologic was approved for psoriasis in 2006. Biologic therapies have revolutionized the treatment of psoriasis and have become an important part of the therapeutic landscape in China. Various biologic therapies, including tumor necrosis factor (TNF) inhibitors, interleukin (IL)-17 inhibitors, and IL-23 inhibitors, have been approved and used in the management of psoriasis. In recent years, additional small molecule therapies have emerged as a treatment option for psoriasis in China. Moreover, novel small molecule oral medications that target intracellular signaling pathways involved in inflammation and immune response will offer patients an alternative treatment option [8]. Although there have been important advancements in psoriasis treatment, the current treatments for msPsO may still have limitations that need to be identified and addressed. To address these issues and improve disease management, the main purpose of this study was to gain a better understanding of the unmet medical needs and treatment preferences of patients with msPsO in China.
Methods
This study was designed as a multicenter cross-sectional study to survey patients with msPsO and dermatologists for unmet medical needs and treatment preference of systemic treatments in the five tier III hospitals across China from north to south. This study was reviewed and approved by the ethics committee boards of Xiangya Hospital of Central South University (approval #202212843), The Second Affiliated Hospital of Harbin Medical University (approval #KY2023-010), Dalian Dermatological Diseases Hospital (approval #KY2003-001), Dushu Lake Hospital of Suzhou University (approval #230058), and the 8th Affiliated Hospital of Zhongshan University (approval #2023-015-01). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All included patients and dermatologists signed a formal consent form to participate in this study and allow publication of the results.
Survey Participants
The study included 50 dermatologists and 300 patients with msPsO visiting five regional dermatology treatment centers during March to May 2023. These five centers are affiliated with tertiary care hospitals in large cities in the northeast, east, central south, and south of China. To control geographic bias, the sample was divided evenly among the hospitals. Dermatologists had to be attending physicians or above with psoriasis experience. Patients had to be aged 18–75, diagnosed with msPsO [assessed by dermatologists according to the defined psoriasis affected body surface area (3% or above) for msPsO in the Chinese clinical guideline for psoriasis] [7], have a history of systemic treatment, and provide written consent. Exclusions applied to those in other skin disease studies or those unable to complete the survey. All study participants have signed the study consent form to enroll in the study and allow publication of the results.
Survey Package Development
This study developed survey packages for patients and dermatologists. For patients, the package included demographics, clinical data, quality of life (scored on a 0–100 scale), previous treatments, unmet needs, and treatment preferences. The questionnaire covered treatment onset time, persistence, discontinuation reasons, side effects impact, concern levels, and convenience challenges. It also addressed biologics-specific needs. A choice-based conjoint (CBC) questionnaire assessed preferences on the basis of various medication attributes. The dermatologists’ package followed the same structure, gathering data on unmet needs and preferences. Additionally, it collected specialty, treatment experience, and prescription patterns. Separate surveys for moderate and severe psoriasis allowed us to explore variations in unmet needs and preferences on the basis of disease severity.
Data Analysis
The enrolled patients with msPsO were stratified by disease severity (moderate vs. severe) for analysis of the collected survey information. Similarly, the collected survey information from the dermatologists was also analyzed by disease severity since they were surveyed accordingly. Descriptive statistical methods were employed to summarize the collected survey information for the characteristics of the enrolled patients with msPsO and dermatologists, patient’s past systemic treatments, dermatologist’s past prescriptions for systemic treatments, and responses to survey questions regarding unmet medical needs. Student t tests and chi-square tests were used only to compare patient characteristics between patients with moderate psoriasis and those with severe psoriasis. The reported time to reduce skin lesion by half, treatment persistence time, and treatment safety concern scale of systemic treatments were summarized by the average values for the surveyed patients and dermatologists, respectively. Proportions of dermatologists and patients reporting treatment discontinuation reasons, negative impact of treatment side effects, and treatment inconvenience of systemic treatments were summarized for the surveyed dermatologists and patients, respectively. This study did not conduct any comparison analysis for the surveyed information between patients and dermatologists.
The CBC questionnaire responses were analyzed using descriptive statistics, HB analysis, and preference share simulations. Descriptive statistics summarized choices based on administration routes (oral vs. subcutaneous injection). HB analysis estimated preference utilities from questionnaire data. Preference share simulations estimated preference shares of the two drugs (once-daily oral vs. biweekly subcutaneous injection) with similar attributes (20% reduction of skin lesions after 1 month of treatment, 70% patients with PASI (Psoriasis Area and Severity Index) 75 response after 3 months of treatment, 70% patients with PASI 75 response after 1 year of treatment, 10% patients experiencing reduced treatment effects during 1 year of treatment, and 3% patients requiring hospital admission for serious treatment side effects during 1 year of treatment). Scenario analysis explored the impact of different PASI 75 response rates for once-daily oral treatment on preference shares while keeping injection treatment attributes constant.
The statistical software R (version 4.2.1) was employed to conduct the described descriptive statistical data analyses, while Lighthouse Studio (version 9.14.2) was used to conduct CBC analysis.
Results
Characteristics of Surveyed Dermatologists
The enrolled dermatologists had an average age of 42.5 years (standard deviation [SD] 8.6 years), and male patients accounted for 30.0% of the participants. The professional ranks among the dermatologists were evenly distributed, with attending physicians comprising 38.0%, deputy chief physicians comprising 34.0%, and chief physicians comprising 28.0%. On average, the dermatologists had 15.4 years of experience in managing patients with psoriasis and treated approximately 29.4 patients with msPsO per week.
Characteristics of Surveyed Patients with msPsO
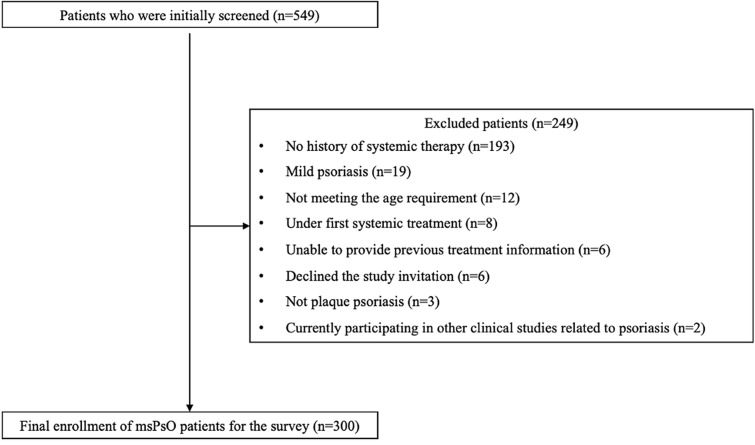
Out of 549 patients with psoriasis screened at the five study hospitals, 300 were selected on the basis of study criteria (Fig. 1). Among these, 176 had moderate psoriasis and 124 had severe psoriasis (Table 1). Patients with severe psoriasis, compared to those with moderate psoriasis, were older and had a higher proportion of skin lesion sites on various body areas. They also reported lower quality of life on the VAS (visual analogue scale) scale.
Fig. 1.
Flowchart for the enrollment of patients with msPsO in the survey study. msPsO moderate-to-severe psoriasis
Table 1.
Patient characteristics of the enrolled patients with msPsO stratified by disease severity
| Moderate psoriasis (n = 176) | Severe psoriasis (n = 124) | p value | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean ± SD | 41.7 ± 13.4 | 44.5 ± 12.1 | 0.035 |
| Male proportion, % | 60.2 | 66.9 | 0.236 |
| Skin lesion site, % | |||
| Lower limb | 76.7 | 97.6 | < 0.001 |
| Trunk | 61.9 | 87.9 | < 0.001 |
| Scalp | 60.8 | 80.6 | < 0.001 |
| Upper limb | 54.0 | 88.7 | < 0.001 |
| Facial and neck | 15.9 | 48.4 | < 0.001 |
| Nail | 17.6 | 41.1 | < 0.001 |
| Palms or soles of the feet | 15.9 | 35.5 | < 0.001 |
| Sacrococcygeal region | 11.4 | 25.0 | 0.002 |
| External genitalia | 4.5 | 8.1 | 0.206 |
| Quality of life | |||
| VAS scale, mean ± SD | 62.6 ± 24.4 | 49.6 ± 23.0 | < 0.001 |
msPsO moderate-to-severe psoriasis, SD standard deviation, VAS visual analogue scale
Survey on Pattern of Previous Systemic Treatments for msPsO
Of the enrolled 300 patients, 202 patients (67.3%) received oral treatment before (197 receiving traditional oral treatments and 5 receiving apremilast) and 133 patients (44.3%) were previously treated with six biologics. The reported past systemic treatments in patients with msPsO included traditional oral drugs (methotrexate, cyclosporine, and acitretin), apremilast, and biologics targeting TNF (adalimumab, infliximab, etanercept), IL-17 (secukinumab and ixekizumab), and IL-12/23 (ustekinumab). Acitretin was the most commonly used oral treatment (moderate psoriasis 40.9%; severe psoriasis 52.4%), and adalimumab was the most frequently received biologic treatment (moderate psoriasis 17.6%; severe psoriasis 16.1%). Similarly, dermatologists frequently prescribed acitretin (moderate psoriasis 19.6%; severe psoriasis 10.5%), while secukinumab was their most frequently chosen biologic for prescription (20.1%; severe psoriasis 27.8%), regardless of disease severity.
Survey on Treatment Effectiveness of Previous Systemic Treatments for msPsO
Patients with msPsO reported longer time to achieve a 50% skin lesion reduction with oral treatments (5.8–7.2 weeks vs. 3.4–6.5 weeks) and shorter persistence (2.7–6.2 months vs. 9.5–17.0 months) compared to biologics. Discontinuation reasons for oral treatments included unsatisfactory effects (77.2%), side effects (33.0%), and safety concerns (29.9%). Biologics were discontinued primarily because of loss of treatment effects (60.9%).
Dermatologists echoed patient responses, reporting longer onset times and shorter persistence for oral treatments (Table 2). The dermatologists reported that oral treatments took longer to achieve a 50% reduction in skin lesions (5.6–6.5 weeks vs. 2.6–6.1 weeks) and had a shorter treatment persistence (4.1–5.7 months vs. 9.7–15.7 months) compared to biologics. However, notably high proportions of dermatologists reported treatment discontinuation reasons for both oral treatments and biologics (Table 3). For instance, 92% of the surveyed dermatologists cited unsatisfactory treatment effects and side effects as reasons for discontinuing oral treatments. More than half of them noted reasons like loss of treatment effects over time (76.0%), pregnancy preparation (70.0%), financial difficulties (66.0%), deteriorating comorbidities (62.0%), patients considering the disease as cured (62.0%), paradoxical skin reactions (60.0%), breastfeeding (58.0%), injection site reactions (56.0%), and unsatisfactory treatment effects (54.0%).
Table 2.
Reported time to clean skin lesion by half and treatment persistence time from the surveyed patients with msPsO and dermatologists
| Patients with msPsO | Dermatologists | |||
|---|---|---|---|---|
| N | Mean ± SD/% | N | Mean ± SD/% | |
| Time to clean skin lesion by half (weeks) | ||||
| Oral treatment | ||||
| Acitretin | 85 | 5.8 ± 3.5 | 47 | 6.5 ± 3.2 |
| Methotrexate | 48 | 6.9 ± 5.4 | 46 | 6.1 ± 2.6 |
| Cyclosporine | 5 | 7.2 ± 3.3 | 44 | 5.6 ± 2.5 |
| Apremilast | 2 | 6.0 ± 2.8 | 26 | 5.8 ± 2.9 |
| Biologic treatment | ||||
| Infliximab | 7 | 5.9 ± 6.4 | 35 | 4.5 ± 1.6 |
| Adalimumab | 39 | 6.5 ± 6.4 | 49 | 5.2 ± 2.8 |
| Etanercept | 15 | 6.5 ± 4.1 | 42 | 6.1 ± 2.9 |
| Secukinumab | 36 | 4.0 ± 2.3 | 50 | 2.9 ± 1.5 |
| Ixekizumab | 19 | 3.4 ± 2.5 | 50 | 2.6 ± 1.5 |
| Ustekinumab | 9 | 3.9 ± 1.8 | 47 | 5.1 ± 3.0 |
| Guselkumab | 0 | – | 43 | 4.1 ± 2.2 |
| Treatment persistence time (months) | ||||
| Oral treatment | ||||
| Acitretin | 126 | 6.2 ± 5.8 | 47 | 4.7 ± 1.9 |
| Methotrexate | 73 | 4.9 ± 4.6 | 46 | 4.6 ± 1.9 |
| Cyclosporine | 15 | 5.5 ± 7.6 | 44 | 4.1 ± 2.0 |
| Apremilast | 5 | 2.7 ± 2.3 | 22 | 5.7 ± 2.6 |
| Biologics | ||||
| Infliximab | 8 | 17.0 ± 9.5 | 36 | 11.7 ± 5.8 |
| Adalimumab | 51 | 12.1 ± 10.0 | 48 | 11.7 ± 7.8 |
| Etanercept | 18 | 14.4 ± 10.0 | 41 | 9.7 ± 5.6 |
| Secukinumab | 43 | 10.5 ± 6.8 | 50 | 15.7 ± 9.1 |
| Ixekizumab | 25 | 9.7 ± 8.3 | 47 | 15.0 ± 9.0 |
| Ustekinumab | 15 | 9.5 ± 8.4 | 46 | 13.7 ± 6.8 |
| Guselkumab | 0 | – | 39 | 12.2 ± 6.6 |
msPsO moderate-to-severe psoriasis, SD standard deviation
Table 3.
Proportions of the surveyed patients with msPsO and dermatologists reporting treatment discontinuation reasons for traditional oral treatments and biologic treatments
| Patients with msPsO | Dermatologists | |||
|---|---|---|---|---|
| N | % | N | % | |
| Treatment discontinuation reasons for traditional oral treatments | ||||
| Unsatisfactory treatment effects | 197 | 77.2 | 50 | 92.0 |
| Treatment side effects | 197 | 33.0 | 50 | 92.0 |
| Treatment safety concern | 197 | 29.9 | 50 | 78.0 |
| Patients considering the disease as cured | 197 | 5.6 | 50 | 44.0 |
| Pregnancy preparation | 197 | 4.6 | 50 | 86.0 |
| Drug interactions | 197 | 0.0 | 50 | 68.0 |
| Breastfeeding | 197 | 0.0 | 50 | 70.0 |
| Treatment discontinuation reasons for biologic treatments | ||||
| Loss of treatment effects over time | 133 | 60.9 | 50 | 76.0 |
| Primary treatment failure | 133 | 24.1 | 50 | 54.0 |
| Paradoxical skin reactions | 133 | 9.0 | 50 | 60.0 |
| Financial difficulties | 133 | 6.8 | 50 | 66.0 |
| Treatment side effects | 133 | 5.3 | 50 | 44.0 |
| Limited treatment access | 133 | 4.5 | 50 | 24.0 |
| Pregnancy preparation | 133 | 3.8 | 50 | 70.0 |
| Treatment inconvenience | 133 | 3.8 | 50 | 36.0 |
| Needle fear | 133 | 2.3 | 50 | 14.0 |
| Patients considering the disease as cured | 133 | 1.5 | 50 | 62.0 |
| Injection site reactions | 133 | 0.8 | 50 | 56.0 |
| Drug interactions | 133 | 0.8 | 50 | 26.0 |
| Deteriorating comorbidities | 133 | 0.8 | 50 | 62.0 |
| Breastfeeding | 133 | 0.8 | 50 | 58.0 |
msPsO moderate-to-severe psoriasis, SD standard deviation
Survey on Treatment Safety of Previous Systemic Treatments for msPsO
The adverse effects of oral treatments and biologics in patients with msPsO were similar. The most frequently reported negative impacts for both included reduced quality of life (37.5% vs. 14.3%) and increased costs (15.8% vs. 28.6%). However, patients expressed greater safety concerns about oral treatments compared to biologics, reflected in their VAS scales (4.9–6.0 vs. 1.1–4.3).
Dermatologists responded similarly in the treatment safety survey. However, they more frequently cited negative impacts of treatment side effects for both oral treatments (quality of life reduction 78.0%; daily life disruption 68.0%; time costs increase 74.0%) and biologics (expenditure increase 84.0%; time costs increase 44.0%; quality of life reduction 34.0%) compared to patients. Dermatologists also had higher VAS scales for safety concerns with traditional oral treatments compared to biologics (average VAS scale 6.5 vs. 3.8). Nearly all dermatologists reported safety concerns for both traditional oral treatments (100%) and biologics (98.3%). The proportion of patients reporting safety concerns for traditional oral treatments was higher than that for biologics (80.3% vs. 62.6%).
Survey on Treatment Convenience and Treatment Access Challenges of Previous Systemic Treatments for msPsO
The surveyed patients had a lower proportion reporting treatment convenience challenges for traditional oral treatments than the surveyed dermatologists (regular lab tests 49.2% vs. 94.0%; treatment side effects needing medical attention 21.8% vs. 82.0%; treatment dosage and duration restrictions 18.3% vs. 96.0%). Biologics presented different convenience challenges, mainly related to injection administration. For example, 30.8% of the surveyed patients with biologic treatment history reported needle fear, and 68.4% patients required healthcare provider assistance for injections. Injection site reactions, like pain (31.5%) and swelling (23.3%), were commonly reported in these patients. Among the identified treatment access challenges with biologics, limited access (46.6%) was the most cited by patients, while limited affordability (86.0%) was the most frequently reported by the surveyed dermatologists.
Treatment Preference of Systemic Treatments by Treatment Administration Routes
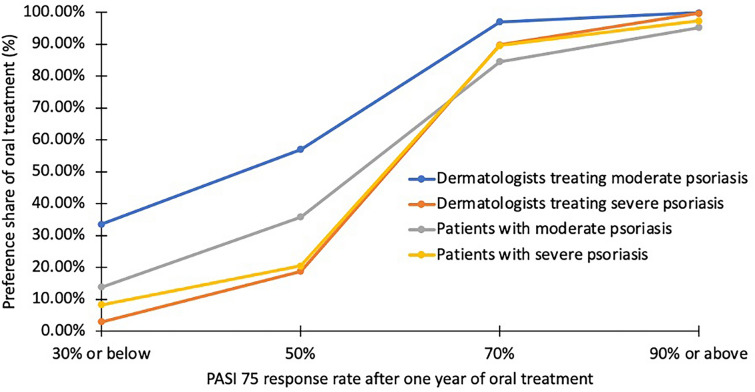
The CBC analysis showed that both patients with msPsO and dermatologists preferred oral hypothetical drugs over subcutaneous injections for moderate psoriasis (patients 34.5% vs. 30.4%, p = 0.020; dermatologists 36.3% vs. 27.7%, p = 0.009) and severe psoriasis (patients 34.3% vs. 30.6%, p = 0.078; dermatologists 32.1% vs. 28.1%, p = 0.217). Further preference share simulation analysis indicated that once-daily oral treatment was more preferred than biweekly subcutaneous injection treatment among patients with msPsO (moderate psoriasis 84.5% vs. 15.5%; severe psoriasis 89.6% vs. 10.4%) and dermatologists (moderate psoriasis 97.0% vs. 3.0%; severe psoriasis 89.8% vs. 10.2%) when treatment attributes were similar for effectiveness and safety. The scenario analysis suggested that preference for once-daily oral treatment increased with a higher PASI response rate after 1 year, irrespective of psoriasis severity, among surveyed dermatologists and patients (Fig. 2).
Fig. 2.
Preference shares of once-daily oral treatment based on the varied PASI 75 response rates after 1 year of oral treatment when compared to biweekly subcutaneous injection treatment in the surveyed dermatologists and patients. PASI Psoriasis Area and Severity Index
Discussion
This study surveyed the current treatment pattern for msPsO in both dermatologists and patients in five regional dermatology treatment centers across China and demonstrated that unmet treatment needs exist in current systemic therapies for msPsO in China. According to the surveyed treatment pattern for msPsO, traditional oral treatments are still the main systemic treatments for msPsO likely owing to their much cheaper acquisition costs than biologics. Of these traditional oral treatments, acitretin was the mostly used medication likely because acitretin is much safer than other oral treatments. The treatment pattern of biologics in patients with msPsO was slightly different from the prescription pattern in dermatologists likely as a result of the differences in approval time of biologics. For example, as adalimumab was approved 2 years earlier than secukinumab, this might result in more patients receiving adalimumab instead of secukinumab. Both patients and dermatologists reported slow treatment onset, short durations, side effects, and inconvenience. Biologics show faster responses but their treatment persistence time was limited (less than 2 years). Once-daily oral treatment is preferred over biweekly subcutaneous injection treatment when attributes of the two treatments for treatment effectiveness and safety are similar, highlighting that an oral treatment with competitive effectiveness and safety could address these unmet needs.
The survey results on systemic treatment effects from both patients with msPsO and dermatologists closely matched published clinical evidence. Traditional oral treatments generally required over 1.5 months to achieve a 50% reduction in skin lesions. This delayed efficacy might affect treatment compliance [9], crucial for long-term msPsO management [10]. Additionally, these oral treatments are not suitable for long-term use because of limited effectiveness and side effects, including gastrointestinal issues, liver toxicity, infection risk, and potential drug interactions [11]. Both patients and dermatologists reported treatment persistence of less than 6 months for traditional oral treatments, with side effects being the primary cause of discontinuation and inconvenience. The latest Chinese psoriasis clinical guideline mainly recommends older oral treatments for acute flare-ups or as a temporary solution [2].
Our patients with msPsO received prior treatment with all approved biologics for psoriasis, except guselkumab, which was not affordable for most Chinese patients with msPsO as a result of high costs and lack of reimbursement support. Dermatologists, on the other hand, had experience prescribing all approved biologics, including guselkumab. Thus, this study addressed the unmet medical needs of all approved biologics for msPsO, including the need for rapid treatment response. IL-17A inhibitors (secukinumab and ixekizumab) demonstrated faster treatment responses in our survey and a previous study [12]. However, the average treatment persistence associated with biologics from our survey was less than 1.5 years, limiting long-term management of msPsO. Real-world data indicated that after 1 year of treatment, drug survival rates were 66% for etanercept, 69% for adalimumab, 61% for infliximab, and 82% for ustekinumab [13]. Biologic adherence might be even lower in the Chinese real-world setting because of cost constraints. One Chinese real-world study [14] reported that 1-year drug survival rates were 67.1% for ixekizumab, 63.0% for secukinumab, 72.2% for guselkumab, and 37.1% for adalimumab, with discontinuation mainly attributed to efficacy, cost, and adverse events, aligning with our findings. Given the unlikely duration of each biologic’s treatment persistence to support long-term management of msPsO, treatment switching between biologics is common [15]. Future treatments with more durable effects may help address this unmet need.
Subcutaneous injection is another challenge; 30.8% of our patients had needle fear, and 68.4% required healthcare provider assistance for injections. Additionally, injection site reactions such as pain (31.5%) and swelling (23.3%) were common among patients treated with biologics. These negative effects related to subcutaneous injections could impact treatment compliance, with needle fear serving as a psychological barrier [16]. Fear of injections and needle-related anxiety were associated with lower treatment compliance in patients with psoriasis [17], and issues like redness, swelling, and itching at the injection site were common reasons for discontinuation and non-adherence [18].
Seeking healthcare professional assistance for biologic injections could hinder treatment compliance, discouraging long-term use. Therefore, this study conducted a CBC questionnaire among patients with msPsO and dermatologists to assess treatment preferences based on administration method. Once-daily oral treatment was strongly preferred over biweekly subcutaneous injections when attributes were comparable. Our findings are well aligned with the previous studies assessing the impact of treatment administration routes on treatment choices in patients with psoriasis or psoriasis-related diseases. One international cross-sectional qualitative study found that oral administration was the first-choice preference in 85% of patients with psoriatic arthritis living in the USA because of speed and ease of administration [19]. Another study using a similar approach (discrete choice experiment) as ours reported that treatment administration route was the strongest factor driving treatment choices for moderate psoriasis in both patients and physicians [20]. In addition, our study found an increasing preference for once-daily oral treatment with a higher PASI 75 response rate after 1 year. This finding suggests that small molecule oral treatments with comparable effects to biologics could represent a treatment advancement. Novel oral treatments are unlikely to lead to anti-drug antibodies, a major cause of biologic effectiveness loss over time [21]. They also avoid injection-related issues such as local reactions causing needle fear and reduced adherence, as well as the need for temperature-controlled transportation and storage like biologics. Novel oral treatments could potentially address the challenges associated with biologics [22] and be more appealing to both patients and dermatologists owing to their convenience.
This cross-sectional survey study on past treatment experiences in patients and dermatologists has several limitations to consider. First, the nature of surveyed treatment effects and safety differs from controlled clinical studies, so while they align with clinical evidence, they are not clinical evidence themselves. Second, recall bias is possible because of patients’ long disease duration, potentially affecting response accuracy. Third, limited experience with apremilast may result in insufficient information to fully reflect its unmet medical needs. Lastly, dermatologists seemed to emphasize different aspects of unmet needs compared to patients, particularly related to safety, discontinuation reasons, convenience, and access challenges. Future studies could explore the reasons for these discrepancies and their impact on disease management.
Conclusion
Current systemic therapies for msPsO in China leave unmet treatment needs despite the availability of biologics. More effective, convenient, and safe treatment options may be needed to meet the needs of Chinese patients with msPsO. When once-daily oral treatments have attributes similar to biweekly subcutaneous injection treatments, both patients with msPsO and dermatologists in China prefer oral treatments over injections. Novel small molecular treatments with comparable efficacy to biologics may better address the medical needs of patients with msPsO in China.
Acknowledgements
We thank the participants of the study.
Authors Contribution
All authors contributed to the study conception and design. Yehong Kuang, Yuzhen Li, Chengzhi Lv, Min Li, Zhenying Zhang, Wendong Chen, Lina Ba, Xingzhi Wang, Steven Feldman, and Yichen Zhong formulated the research idea and developed the study protocol. Yehong Kuang, Yuzhen Li, Chengzhi Lv, Min Li, Zhenying Zhang, and Yi Chen conducted the study subject enrollment and the survey with the enrolled participants. Yi Chen and Wendong Chen performed the data analysis. All authors have critically reviewed the manuscript and approved its submission.
Funding
This study was funded by Bristol Myers Squibb (Princeton, NJ, USA), which had a role in the design of the study; analysis and interpretation of the data; review and approval of the manuscript; and decision to submit the manuscript for publication. Bristol Myers Squibb also funded the journal’s rapid service fee.
Data Availability
The data that support the findings of this study are not openly available due to reasons of sensitivity. The data are available from the corresponding author upon reasonable request. The data are located in controlled access data storage at Xiangya Hospital.
Declarations
Conflict of Interest
Yi Chen and Wendong Chen are the employees of Changsha Normin Medical Technology Ltd, which received funding from Bristol Myers Squibb to conduct this study. Xingzhi Wang, Lina Ba, and Yichen Zhong are the employees of Bristol Myers Squibb. Yehong Kuang, Yuzhen Li, Chengzhi Lv, Min Li, Zhenying Zhang, and Steven Feldman have nothing to disclose.
Ethical Approval
This study was reviewed and approved by the ethics committee boards of Xiangya Hospital of Central South University (approval #202212843), The Second Affiliated Hospital of Harbin Medical University (approval #KY2023-010), Dalian Dermatological Diseases Hospital (approval #KY2003-001), Dushu Lake Hospital of Suzhou University (approval #230058), and the 8th Affiliated Hospital of Zhongshan University (approval #2023-015-01). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. All included patients and dermatologists signed a formal consent form to participate in this study and allow publication of the results.
Footnotes
Prior Presentation: This study was presented as a poster in the 2024 AAD Annual Meeting that took place March 8–12 in San Diego, California, USA.
References
- 1.Damiani G, Bragazzi NL, Karimkhani Aksut C, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med (Lausanne) 2021;8:743180. doi: 10.3389/fmed.2021.743180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Committee on Psoriasis, Chinese Society of Dermatology. Guideline for the diagnosis and treatment of psoriasis in China (2022 edition). Chin J Dermatol. 2019;52:223–230.
- 3.Chen X, Zheng L, Zhang H, et al. Disease burden and quality of life in patients with psoriasis: an internet-based questionnaire survey. Chin J Dermatol. 2019;52:791–5.
- 4.Hepat A, Chakole S, Rannaware A. Psychological well-being of adult psoriasis patients: a narrative review. Cureus. 2023;15(4):e37702. doi: 10.7759/cureus.37702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Yang Z, Tang K, Sun Q, Jin H. Stigmatization in patients with psoriasis: a mini review. Front Immunol. 2021;12:715839. doi: 10.3389/fimmu.2021.715839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai TF, Wang TS, Hung ST, et al. Epidemiology and comorbidities of psoriasis patients in a national database in Taiwan. J Dermatol Sci. 2011;63(1):40–46. doi: 10.1016/j.jdermsci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Committee of Psoriasis, Dermatology Branch, Chinese Medical Association. Guidelines for the diagnosis and treatment of psoriasis in China: 2019 concise edition#. Int J Dermatol Venereol. 2020;3(01):14–26.
- 8.Balogh EA, Bashyam AM, Ghamrawi RI, Feldman SR. Emerging systemic drugs in the treatment of plaque psoriasis. Expert Opin Emerg Drugs. 2020;25(2):89–100. doi: 10.1080/14728214.2020.1745773. [DOI] [PubMed] [Google Scholar]
- 9.Augustin M, Holland B, Dartsch D, Langenbruch A, Radtke MA. Adherence in the treatment of psoriasis: a systematic review. Dermatology. 2011;222(4):363–374. doi: 10.1159/000329026. [DOI] [PubMed] [Google Scholar]
- 10.Tada Y, Jo SJ, Huang YH, et al. Uncovering the unmet needs among psoriasis patients in the Asia-Pacific region. J Dermatol. 2021;48(11):1665–1674. doi: 10.1111/1346-8138.16072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balak DM, Gerdes S, Parodi A, Salgado-Boquete L. Long-term safety of oral systemic therapies for psoriasis: a comprehensive review of the literature. Dermatol Ther (Heidelb) 2020;10:589–613. doi: 10.1007/s13555-020-00409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson MC, Heron CE, Ghamrawi RI, Balogh EA, Feldman SR. Speed of psoriasis treatment response for biologic agents: a review of phase III clinical trials. J Psoriasis Psoriatic Arthritis. 2021;6(2):99–105. doi: 10.1177/2475530321999087. [DOI] [Google Scholar]
- 13.Lin PT, Wang SH, Chi CC. Drug survival of biologics in treating psoriasis: a meta-analysis of real-world evidence. Sci Rep. 2018;8(1):16068. doi: 10.1038/s41598-018-34293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Lu JJ, Zhong XY, et al. Drug survival outcomes associated with the real-world use of ixekizumab, secukinumab, guselkumab, and adalimumab for the treatment of plaque psoriasis in China: a 52-week single-center retrospective study. Clin Cosmet Investig Dermatol. 2022;15:2245–2252. doi: 10.2147/CCID.S387759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curmin R, Guillo S, De Rycke Y, et al. Switches between biologics in patients with moderate-to-severe psoriasis: results from the French cohort PSOBIOTEQ. J Eur Acad Dermatol Venereol. 2022;36(11):2101–12. [DOI] [PMC free article] [PubMed]
- 16.Duncanson E, Le Leu RK, Shanahan L, et al. The prevalence and evidence-based management of needle fear in adults with chronic disease: a scoping review. PLoS One. 2021;16(6):e0253048. doi: 10.1371/journal.pone.0253048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871–881. doi: 10.1016/j.jaad.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Thomaidou E, Ramot Y. Injection site reactions with the use of biological agents. Dermatol Ther. 2019;32(2):e12817. doi: 10.1111/dth.12817. [DOI] [PubMed] [Google Scholar]
- 19.Aletaha D, Husni ME, Merola JF, et al. Treatment mode preferences in psoriatic arthritis: a qualitative multi-country study. Patient Prefer Adherence. 2020;14:949–61. [DOI] [PMC free article] [PubMed]
- 20.Alcusky M, Lee S, Lau G, et al. Dermatologist and patient preferences in choosing treatments for moderate to severe psoriasis. Dermatol Ther (Heidelb) 2017;7:463–483. doi: 10.1007/s13555-017-0205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel V, Efimov A, Baker D, Kang AS. Immunogenicity of biologics used in the treatment of moderate to severe psoriasis. Hum Antibodies. 2021;29(3):171–178. doi: 10.3233/HAB-210447. [DOI] [PubMed] [Google Scholar]
- 22.Martin G. Novel therapies in plaque psoriasis: a review of tyrosine kinase 2 inhibitors. Dermatol Ther (Heidelb) 2023;13(2):417–435. doi: 10.1007/s13555-022-00878-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity. The data are available from the corresponding author upon reasonable request. The data are located in controlled access data storage at Xiangya Hospital.