Abstract
Using pooled vaginal microbiota data from pregnancy cohorts (N = 683 participants) in the Environmental influences on Child Health Outcomes (ECHO) Program, we analyzed 16S rRNA gene amplicon sequences to identify clinical and demographic host factors that associate with vaginal microbiota structure in pregnancy both within and across diverse cohorts. Using PERMANOVA models, we assessed factors associated with vaginal community structure in pregnancy, examined whether host factors were conserved across populations, and tested the independent and combined effects of host factors on vaginal community state types (CSTs) using multinomial logistic regression models. Demographic and social factors explained a larger amount of variation in the vaginal microbiome in pregnancy than clinical factors. After adjustment, lower education, rather than self-identified race, remained a robust predictor of L. iners dominant (CST III) and diverse (CST IV) (OR = 8.44, 95% CI = 4.06–17.6 and OR = 4.18, 95% CI = 1.88–9.26, respectively). In random forest models, we identified specific taxonomic features of host factors, particularly urogenital pathogens associated with pregnancy complications (Aerococcus christensenii and Gardnerella spp.) among other facultative anaerobes and key markers of community instability (L. iners). Sociodemographic factors were robustly associated with vaginal microbiota structure in pregnancy and should be considered as sources of variation in human microbiome studies.
Keywords: Vaginal microbiota structure, Pregnancy, Host factors, 16S rRNA gene amplicon sequence data, Meta-analysis, ECHO cohort
Subject terms: Microbial communities, Policy and public health in microbiology, Biomarkers, Medical research, Risk factors
Introduction
Our understanding of the relationship of the human microbiome to health has expanded greatly, including its role in a range of conditions with developmental origins1–6. The vaginal microbiome, in particular, has been associated with clinical outcomes such as infections in the reproductive tract and preterm birth, although not all studies have been consistent7–10. Beyond the long-term health sequelae associated with preterm birth, the human microbiome in pregnancy shapes the acquisition of the infant microbiome, which is seeded at birth and undergoes rapid assembly in early life with implications across the life course1–4. The vertical transmission of vaginal microbiota may play a role in the acquisition of both the offspring gut microbiome11 and immune development with effects on airway allergic responses2,4,12.
Both culture and culture-independent methods have demonstrated that Lactobacillus spp. dominate the vaginal microbiome in many (but not all) women13,14. Lactobacillus spp. dominance has been consistently associated with the lowest levels of genital tract inflammation across various populations studied13,15,16. Among some women, vaginal microbiota appear to be highly dynamic communities that are relatively stable over time17, particularly during pregnancy, with increased proliferation of L. crispatus and decreased diversity18–21.
Host factors may shape the microbiome in pregnancy and could provide insight into modifiable targets for intervention. However, few studies have evaluated environmental and clinical factors shaping the structure of the human vaginal microbiome across multiple diverse cohorts. Furthermore, integrating the microbiome into population-based research across multiple studies is challenging because of the variety of methods used to assess microbial communities. Results of microbiome studies in relation to preterm birth, for example, have been variable across populations, which may be driven by differences in host characteristics.
Although host factors have been shown to confound gut microbiome studies22, relatively little is known about host factors and the vaginal microbiome in pregnancy. One exception is self-reported race14,23, which has been related to community state types (CSTs) in single-cohort studies14. Specifically, individuals who self-identify as Black are more likely to exhibit diverse microbial profiles than those who identify as White24,25. Although consistently documented, the social patterning of microbiome composition research to date has not accounted for potential host confounders or the ways in which it self-identified race may be a proxy for unmeasured host exposures. Studies of large and diverse populations are thus needed to disentangle the many social determinants of health that are closely linked to self-reported race.
Meta-analysis can be used to overcome sources of bias in single site studies. However, aggregating microbiome studies can be difficult due to the heterogeneity in methods for sample collection, DNA isolation, and DNA sequencing, as well as varying bioinformatics approaches. With the recent advent of publicly available large 16S gene amplicon libraries, understanding how host factors may affect the vaginal microbiome in pregnancy will facilitate the precision and validity of large-scale population-based studies22,26. Therefore, we sought to identify host factors associated with vaginal microbiome structure in pregnancy by leveraging existing 16S rRNA gene amplicon sequence data from a diverse set of cohorts with well-characterized clinical, demographic, and biological data.
Results
Participant and sample characteristics
We utilized vaginal 16S rRNA gene sequence data from samples collected from the National Institutes of Health-funded Environmental influences on Child Health Outcomes (ECHO) cohort, which includes caregivers and children participating in multiple existing longitudinal birth cohort studies. ECHO was designed to evaluate the impact of early life exposures on child health outcomes and includes survey, medical record, and biospecimen collections27,28, as well as a subset of cohorts with vaginal 16S rRNA gene sequence data (Table 1).
Table 1.
Overview of cohorts and collected samples.
| Cohort | Sample type and collection | Sequencing, DNA isolation |
|---|---|---|
|
MARCH (U-M sites) Recruited from University of Michigan clinics |
Self-collected vaginal Starplex star dual-headed swabs at 3 times across gestation beginning at 7 to > 28 weeks gestation Clinic sample preserved in All-Protect upon collection |
16S rRNA gene (V4 region) Qiagen PowerMicrobiome DNA/RNA EP kit |
|
MARCH (non-U-M sites) Recruited from 9 other non-UM sites across Michigan |
Self-collected vaginal Starplex star dual-headed swabs and fecal (no preservative) > 28 weeks gestation Mail collection |
16S rRNA gene (V4 region) Qiagen/Mobio power soil DNA extraction kit |
|
Atlanta Recruited from prenatal public/private clinics in Atlanta |
Vaginal (self-collected, mid-vaginal), along with oral and rectal Epicentre Catchall swabs, collected at 8–14 and 24–30 weeks gestation |
16S rRNA gene (V3–V4 region) Qiagen/Mobio power soil DNA extraction kit |
|
CREW (WISC and MAAP) WISC recruited farm and rural-nonfarm families in Wisconsin. MAAP recruited families in Detroit |
Vaginal-rectal swabs sampled from clinic at > 28 weeks gestation |
16S rRNAgene (V4 region) SequalPrep Normalization Plate Kit (Thermo Fisher Scientific) |
ATLANTA Emory Atlanta African American Maternal-Child Cohort, MAAP Microbes, Allergy, Asthma, and Pets, MARCH Michigan Archive for Research on Child Health, WISC Wisconsin Infant Study Cohort.
Overall, we analyzed vaginal 16S sequence data from 683 pregnant participants across several geographically distinct areas of the United States. Based on self-report, 63% of the participants were non-White (of whom 97% self-identified as Black); 37% were White; and 1.9% were Hispanic. The distribution differed across the ECHO participating cohorts along with receipt of public insurance and maternal education (Table 2). The mean age was 28 years (SD = 5.4), 43% had a normal body mass index (≥ 18.5 and < 25 kg/m2); 42% were nulliparous; and 12% smoked during pregnancy. Antibiotic use in pregnancy was common (38%) in all trimesters of pregnancy. Approximately 12% of women had hypertension during pregnancy; 3.7% were diagnosed with gestational diabetes; and 11% had a preterm birth (Table 2).
Table 2.
Characteristics by ECHO cohort.
| Overall, N = 683 | Atlanta, N = 396 | MAAP, N = 48 | MARCH, N = 123 | WISC, N = 116 | p-value | |
|---|---|---|---|---|---|---|
| Community state type | < 0.001 | |||||
| Non-L. iners Lactobacillus dominant (I, II, V) | 180 (26%) | 60 (15%) | 14 (29%) | 59 (48%) | 47 (41%) | |
| L. iners dominant (III) | 294 (43%) | 188 (48%) | 15 (31%) | 42 (34%) | 49 (42%) | |
| Diverse (IV-B, IV-C) | 207 (30%) | 146 (37%) | 19 (40%) | 22 (18%) | 20 (17%) | |
| (Missing) | < 5 | < 5 | 0 | 0 | 0 | |
| Self-reported race* | < 0.001 | |||||
| Non-White | 428 (63%) | 396 (100%) | 15 (32%) | 16 (13%) | 1 (0.9%) | |
| White | 250 (37%) | 0 (0%) | 32 (68%) | 106 (87%) | 112 (99%) | |
| Unknown or (missing) | 5 | 0 | < 5 | < 5 | < 5 | |
| Hispanic | 0.002 | |||||
| Hispanic | 13 (1.9%) | 3 (0.8%) | 3 (7.7%) | 6 (4.9%) | 1 (0.9%) | |
| Non-Hispanic | 661 (98%) | 393 (99%) | 36 (92%) | 117 (95%) | 115 (99%) | |
| (Missing) | 9 | 0 | 9 | 0 | 0 | |
| Maternal age | < 0.001 | |||||
| Mean (SD) | 27.7 (5.4) | 25.4 (4.8) | 30.4 (3.9) | 31.4 (4.8) | 30.8 (3.7) | |
| (Missing) | < 5 | < 5 | 0 | < 5 | 0 | |
| Education | < 0.001 | |||||
| BA or higher | 253 (37%) | 65 (16%) | 21 (44%) | 85 (70%) | 82 (71%) | |
| HS/GED | 171 (25%) | 153 (39%) | < 5 (8.3%) | 8 (6.6%) | 6 (5.2%) | |
| Less than HS | 64 (9.4%) | 59 (15%) | < 5 (4.2%) | < 5 (2.5%) | 0 (0%) | |
| Some college | 193 (28%) | 119 (30%) | 21 (44%) | 26 (21%) | 27 (23%) | |
| (Missing) | < 5 | 0 | 0 | 1 | 1 | |
| Public insurance | < 0.001 | |||||
| No | 229 (41%) | 88 (22%) | 42 (93%) | 99 (81%) | NA | |
| Yes | 334 (59%) | 308 (78%) | < 5 (6.7%) | 23 (19%) | NA | |
| (Missing) | 120 | 0 | < 5 | 1 | 116 | |
| Private insurance | < 0.001 | |||||
| No | 320 (57%) | 308 (78%) | 6 (13%) | 6 (4.9%) | NA | |
| Yes | 243 (43%) | 88 (22%) | 39 (87%) | 116 (95%) | NA | |
| (Missing) | 120 | 0 | < 5 | 1 | 116 | |
| Gestational diabetes | 0.6 | |||||
| No | 604 (96%) | 383 (97%) | NA | 115 (95%) | 106 (95%) | |
| Yes | 24 (3.8%) | 13 (3.3%) | NA | 6 (5.0%) | 5 (4.5%) | |
| (Missing) | 55 | 0 | 48 | < 5 | 5 | |
| Antibiotics ever in pregnancy | < 0.001 | |||||
| No | 322 (62%) | 215 (54%) | NA | 107 (87%) | NA | |
| Yes | 196 (38%) | 180 (46%) | NA | 16 (13%) | NA | |
| (Missing) | 165 | < 5 | 48 | 0 | 116 | |
| Antibiotics in first trimester | < 0.001 | |||||
| No | 428 (83%) | 310 (78%) | NA | 118 (96%) | NA | |
| Yes | 90 (17%) | 85 (22%) | NA | 5 (4.1%) | NA | |
| (Missing) | 165 | < 5 | 48 | 0 | 116 | |
| Antibiotics in second trimester | 0.001 | |||||
| No | 436 (84%) | 321 (81%) | NA | 115 (93%) | NA | |
| Yes | 82 (16%) | 74 (19%) | NA | 8 (6.5%) | NA | |
| (Missing) | 165 | < 5 | 48 | 0 | 116 | |
| Antibiotics in third trimester | 0.001 | |||||
| No | 456 (88%) | 338 (86%) | NA | 118 (96%) | NA | |
| Yes | 62 (12%) | 57 (14%) | NA | 5 (4.1%) | NA | |
| (Missing) | 165 | < 5 | 48 | 0 | 116 | |
| Infant sex | 0.7 | |||||
| Female | 347 (51%) | 204 (52%) | 27 (56%) | 57 (47%) | 59 (51%) | |
| Male | 335 (49%) | 192 (48%) | 21 (44%) | 65 (53%) | 57 (49%) | |
| (Missing) | < 5 | 0 | 0 | < 5 | 0 | |
| Smoking in pregnancy | < 0.001 | |||||
| No | 588 (88%) | 322 (82%) | 41 (85%) | 116 (97%) | 109 (98%) | |
| Yes | 84 (12%) | 71 (18%) | 7 (15%) | 4 (3.3%) | 2 (1.8%) | |
| (Missing) | 11 | < 5 | 0 | < 5 | 5 | |
| Hypertension | < 0.001 | |||||
| No | 551 (88%) | 333 (84%) | 0 (NA%) | 110 (91%) | 108 (97%) | |
| Yes | 77 (12%) | 63 (16%) | 0 (NA%) | 11 (9.1%) | 3 (2.7%) | |
| (Missing) | 55 | 0 | 48 | < 5 | 5 | |
| Early pregnancy BMI (kg/m2) category | 0.075 | |||||
| Normal (≥ 18.5 & < 25) | 272 (43%) | 161 (41%) | 10 (37%) | 63 (52%) | 38 (45%) | |
| Obese (≥ 30) | 192 (31%) | 138 (35%) | 6 (22%) | 25 (20%) | 23 (27%) | |
| Overweight (≥ 25 & < 30) | 143 (23%) | 82 (21%) | 11 (41%) | 29 (24%) | 21 (25%) | |
| Underweight (< 18.5) | 22 (3.5%) | 15 (3.8%) | 0 (0%) | 5 (4.1%) | 2 (2.4%) | |
| (Missing) | 54 | 0 | 21 | < 5 | 32 | |
| Parity category | < 0.001 | |||||
| < 1 | 291 (43%) | 187 (47%) | 19 (40%) | 52 (43%) | 33 (28%) | |
| 1 | 221 (32%) | 114 (29%) | 23 (48%) | 45 (37%) | 39 (34%) | |
| 2 | 101 (15%) | 58 (15%) | < 5 (2.1%) | 16 (13%) | 26 (22%) | |
| > 3 | 69 (10%) | 37 (9.3%) | 5 (10%) | 9 (7.4%) | 18 (16%) | |
| (Missing) | < 5 | 0 | 0 | < 5 | 0 | |
Data shown (except maternal age) are n (%).
BA or higher college degree or higher, HS/GED high school or general equivalency degree, BMI body mass index.
*Self-identified as a proxy for lived experiences.
Fisher’s exact test; Kruskal–Wallis rank sum test; Pearson’s chi-squared test.
Maternal factors varied by cohort (Table 2), most notably for race, as the Emory Atlanta African American Maternal-Child Cohort (hereafter the ‘Atlanta cohort’) is composed entirely of Black women, while fewer than 1% of the Wisconsin Infant Study Cohort (WISC) participants are Black. The subset of the Michigan Archive for Research with Mothers on Child Health (MARCH) cohort with vaginal microbiome data was 13% Black and 87% White. Samples were independently collected at each site using study-specific protocols, stored, isolated, and sequenced using a range of methods prior to bioinformatic processing. Table 1 lists details of the cohorts’ sample types and collection, DNA isolation method, and sequencing used.
16S rRNA gene amplicon data
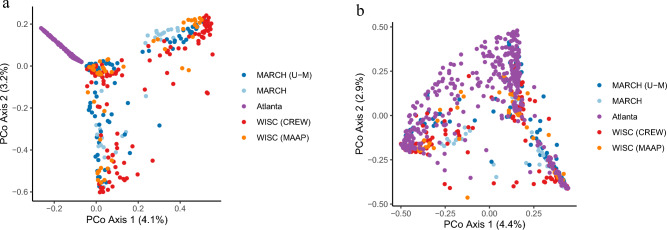
After removing one low-quality sample, the total number of reads per sample ranged from 2629 to 406,377, with a mean of 63,704 (SD = 63,970). Reads from 683 samples were denoised into amplicon sequence variants (ASVs), from which a total of 5232 phylotypes were constructed after mapping to a common phylogenetic tree constructed from full-length vaginal 16S rRNA encoding alleles. As evident in ordination plots shown in Fig. 1A, B, phylogenetic placement of the sequences (Fig. 1B) removed much of the variation that was evident across sites prior to processing in MALiAmPi (Fig. 1A), although some of the remaining site variation may also be due to inherent differences in host factors across the cohorts.
Figure 1.
(a) Principal coordinates (PCoA) analysis of Bray–Curtis distances between samples based on amplicon sequence variants (ASVs) and (b) phylotypes demonstrating that using phylogenetic placement of ASVs on a reference tree removed a large degree of variation by site.
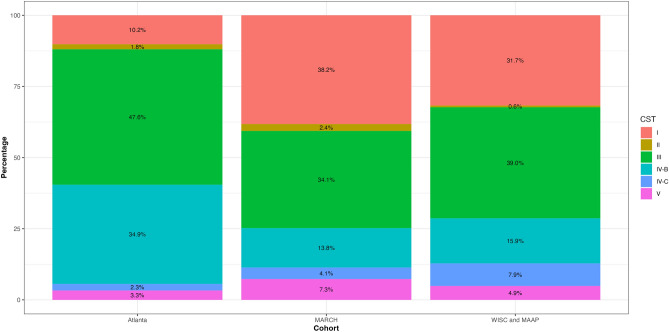
The prevalence of CSTs varied by cohort
Vaginal phylotypes were classified using the VAginaL community state typE Nearest CentroId clAssifier (VALENCIA)11. Four CSTs were Lactobacillus dominant: CST I, L. crispatus; CST II, L. gasseri; CST III, L. iners; and CST V, L. jenseni. The remainder were diverse polymicrobial communities: CST IV-B, characterized by high Gardnerella spp., low Candidatus Lachnocurva vaginae (formerly known as BVAB1), and moderate relative abundance of Fannyhessea vaginae (previously known as Atopobium vaginae), and CST IV-C, characterized by a diverse array of facultative and anaerobic bacteria and low relative abundances of Lactobacillus spp., G. vaginalis, Fannyhessea vaginae, and Ca. L. vaginae. While almost all CSTs were found in each cohort, the prevalence of each CST significantly varied by cohort (Fig. 2). For the Atlanta cohort, the most common was CST III (47.6%), followed by CST IV-B (34. 9%), whereas in the MARCH cohort, the most prevalent was CST I (38.2%), followed by CST III (34.1%). Although CST III and CST I were also the most common in WISC, the distribution was distinct from that in the MARCH cohort (Fig. 2). In contrast, non-L. iners Lactobacillus-dominant CSTs were less prevalent in the Atlanta cohort (Fig. 2).
Figure 2.
Community state types (CSTs) by ECHO cohort. CST I, Lactobacillus crispatus dominant; CST II, L. gasseri dominant; CST III, L. iners dominant; CST IV-B, diverse, characterized by high relative abundance of Gardnerella spp., low relative abundance of Candidatus Lachnocurva vaginae (formerly known as BVAB1), and moderate relative abundance of Fannyhessea vaginae (previously Atopobium vaginae); CST IV-C, diverse array of facultative and strictly anaerobic bacteria, with low relative abundance of Lactobacillus spp., G. vaginalis, Fannyhessea vaginae, and Ca. L. vaginae); CST V, L. jenseni dominant.
Host factors associated with vaginal microbiota structure across cohorts
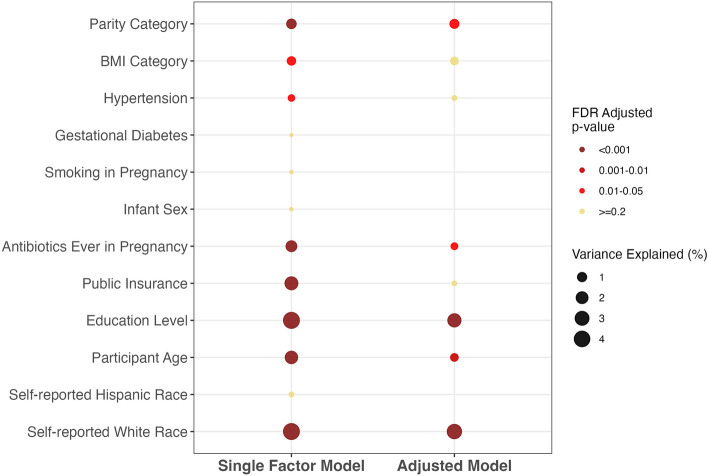
In single-factor, unadjusted permutational multivariate analysis of variance (PERMANOVA) models, both education and self-reported race accounted for the largest variance explained (4.28% and 4.26%, respectively; false discovery rate (FDR)-adjusted p-value < 0.001) in vaginal microbiota structure in pregnancy, followed by public insurance receipt (3% variance, FDR-adjusted p-value < 0.001) (Fig. 3). Antibiotics in pregnancy and age significantly contributed to 2% of the variance in vaginal microbiota structure (FDR-adjusted p-value < 0.01, each), while parity and smoking in pregnancy explained smaller amounts (Fig. 3). Although still significant after adjustment for multiple comparisons, early pregnancy BMI and hypertension each accounted for less than 1% of the variance in community structure. Self-reported Hispanic ethnicity, gestational diabetes, and sex of the infant were not associated with global community structure in pregnancy (Fig. 3).
Figure 3.
Single-adjusted (left) and multifactor-adjusted (left) permutational multivariate analysis of variance (PERMANOVA) pooled estimates of vaginal microbiota structure in pregnancy using Bray–Curtis distances across all cohorts. Only factors that were significantly associated with composition (p < 0.05) in single-factor models were retained for inclusion in the multifactor model using backward selection. FDR false discovery rate.
Host factors independently associated with vaginal microbiota structure
We next used multifactor PERMANOVA models to assess the independent effects of host factors on vaginal communities. In aggregate, host factors accounted for nearly 12% of the overall variance in vaginal microbiota structure in pregnancy. Education and self-reported race remained the most robust host factors associated with vaginal microbiota structure in pregnancy (4% of variance for each, FDR-adjusted p < 0.01) (Fig. 3). Parity, antibiotic use in pregnancy, and age remained independent predictors of vaginal microbiome structure in pregnancy but slightly less so after accounting for other host factors (FDR-adjusted p-values 0.01 to < 0.05, respectively). The effects of early pregnancy BMI, hypertension, and public insurance receipt, in contrast, became attenuated after adjustment (Fig. 3).
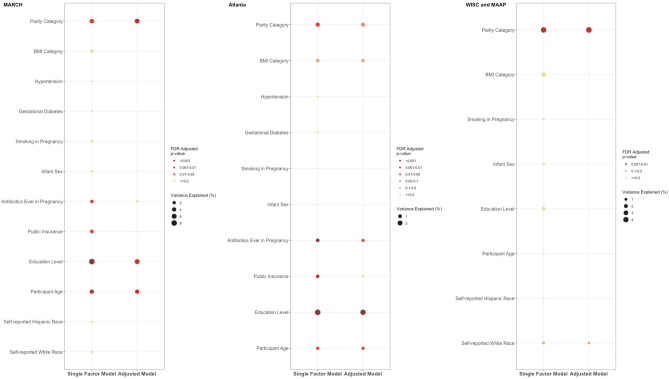
Host factors associated with vaginal microbiota structure were largely conserved across cohorts
We next generated cohort-specific PERMANOVA models to visualize results for each cohort independently (Fig. 4). The results were largely consistent, especially in the MARCH and Atlanta cohorts in which data on the host factors were well-aligned, further validating their independent effects on microbiome variation. Specifically, education and parity exhibited consistently robust associations with the vaginal microbiota community structure within each cohort (Fig. 4). Of note, self-reported race was significant in the pooled PERMANOVA model but not in the cohort-specific models, likely due to the substantial differences in its distribution between cohorts (Fig. 4 and Table 2). Some of the associations with host factors in the other cohorts were diminished in the WISC cohort, and others, such as antibiotic use in pregnancy, were not available.
Figure 4.
Associations between vaginal microbiota community structure and host factors across cohorts. Single-adjusted (left) and multifactor-adjusted (left) PERMANOVA estimates of vaginal microbiota structure in pregnancy using Bray–Curtis distances by individual cohort. Only factors that were significantly associated with composition (p < 0.05) in single-factor models were retained for inclusion in the multifactor model using backward selection.
Taxonomic differences associated with robust host factors in pregnancy
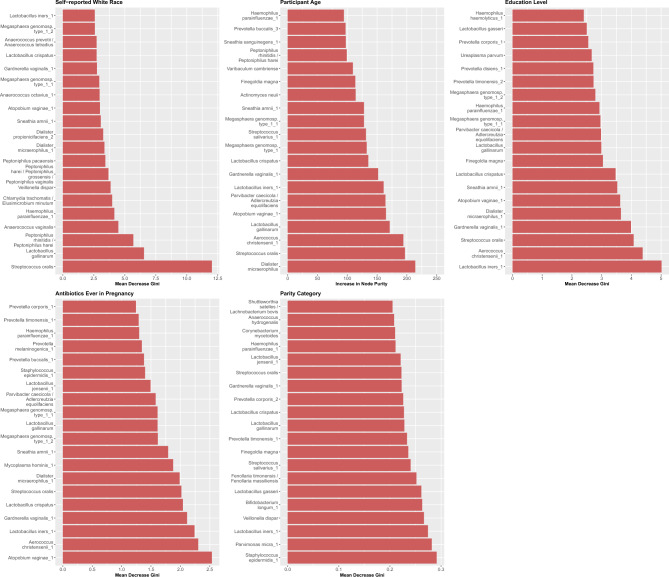
We also examined taxonomic differences associated with host factors in pregnancy that may drive variation in vaginal microbiota structure. For each host factor that remained robust in the fully adjusted models of global community variance, we used random forest models to rank the most predictive phylotypes, which were classified to the species level. Of the taxa most predictive of educational attainment, L. iners was the most discriminant, followed by A. christensenii, Streptococcus oralis, and G. vaginalis (Fig. 5). The taxa most predictive of self-identified race were S. oralis followed by Lactobacillus gallinarum (Fig. 5). Fannyhessea vaginae (previously Atopobium vaginae) was the most predictive of antibiotic use in pregnancy followed by A. christensenii, L. iners, and G. vaginalis (Fig. 5). Staphylococcus epidermidis, Parvimonas micra, and L. iners were among the most predictive of parity. Staphylococcus epidermidis, P. micra, and L. iners were among the most predictive of parity, while Dialister micraerophilus was most predictive of host age (Fig. 5). Receiver operating curves (ROCs) for the top 20 taxa from the random forest models demonstrated areas under the curve (AUCs) ranging from 0.973 for self-identified race to 0.6114 for parity (Supplementary Fig. 1).
Figure 5.
Random forest panel plots of the top ranked vaginal taxonomic features predictive of host factors.
Vaginal CSTs were associated with host factors
Due to their clinical relevance, we also tested the independent and joint associations between host factors and vaginal CSTs using a series of nested multinomial logistic regression models. CSTs were collapsed into three categories: L. iners-dominant (CST III), diverse (CST IV-B and IV-C) and non–L. iners Lactobacillus-dominant (i.e. CST I, II, V) CSTs, with the latter serving as the reference category. Prior to multivariable adjustment, we assessed the associations between host factors and CSTs for model selection (Supplementary Table 1). In the unadjusted model, self-identified White race was associated with a reduced odds of L. iners dominant (OR = 0.28, 95% CI = 0.19–0.42) and diverse CSTs (OR = 0.21, 95% CI = 0.14–0.32) compared to that of non-L. iners Lactobacillus-dominant CSTs (Table 3, Model 1). However, after adjustment for maternal education, the effect of race became attenuated and was no longer significant (Table3, Model 3). After adjustment for age, antibiotic use in pregnancy, and self-identified race, less than high school education was associated with L. iners dominant and diverse CSTs (OR = 7.81, 95% CI = 2.21–27.5 and OR = 8.84, 95% CI = 2.46–31.7, respectively) (Table 3). Other host factors that remained significantly associated with L. iners CSTs compared to non-L. iners Lactobacillus-dominant CSTs included parity and antibiotic use. Antibiotic use in pregnancy was also associated with increased odds of having both L. iners-dominant and diverse CSTs compared to non-L. iners Lactobacillus-dominant CSTs (CSTs (OR = 3.24 95% CI = 1.66–6.30 and OR = 4.24. 95% CI = 2.15–8.35, respectively).
Table 3.
Host factors are independently associated with vaginal community state types in pregnancy.
| Model 1 (n = 680) | Model 2 (n = 680) | Model 3 (n = 680) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR diverse CST | 95% CI | p-value | OR L. iners CST | 95% CI | p-value | OR diverse CST | 95% CI | p-value | OR L. iners CST | 95% CI | p-value | OR diverse CST | 95% CI | p-value | OR L. iners CST | 95% CI | p-value | |
| White | ||||||||||||||||||
| No | – | – | – | – | – | – | – | – | – | – | – | – | ||||||
| Yes | 0.21 | 0.14, 0.32 | < 0.001 | 0.28 | 0.19, 0.42 | < 0.001 | 0.33 | 0.20, 0.55 | < 0.001 | 0.43 | 0.28, 0.68 | < 0.001 | 0.48 | 0.28, 0.82 | 0.007 | 0.61 | 0.38, 0.99 | 0.046 |
| Maternal age | 0.91 | 0.87, 0.96 | < 0.001 | 0.92 | 0.88, 0.96 | < 0.001 | 0.97 | 0.92, 1.02 | 0.2 | 0.97 | 0.93, 1.02 | 0.3 | ||||||
| Education level | ||||||||||||||||||
| BA or higher | – | – | – | – | ||||||||||||||
| HS/GED | 3.48 | 1.73, 6.99 | < 0.001 | 3.71 | 1.92, 7.17 | < 0.001 | ||||||||||||
| Less than HS | 8.46 | 2.60, 27.6 | < 0.001 | 6.17 | 1.91, 19.9 | 0.002 | ||||||||||||
| Some college/assoc | 2.58 | 1.43, 4.67 | 0.002 | 4.01 | 2.36, 6.82 | < 0.001 | ||||||||||||
| Parity category | ||||||||||||||||||
| 1 + | ||||||||||||||||||
| 0 | ||||||||||||||||||
| Antibiotics ever in pregnancy | ||||||||||||||||||
| No | ||||||||||||||||||
| Yes | ||||||||||||||||||
| Model 4 (n = 680) | Model 5 (n = 516) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR diverse CST | 95% CI | p-value | OR L. iners CST | 95% CI | p-value | OR diverse CST | 95% CI | p-value | OR L. iners CST | 95% CI | p-value | |||||||
| White | ||||||||||||||||||
| No | – | – | – | – | – | – | – | – | ||||||||||
| Yes | 0.47 | 0.28, 0.81 | 0.006 | 0.59 | 0.37, 0.97 | 0.036 | 0.7 | 0.34, 1.43 | 0.3 | 0.7 | 0.36, 1.35 | 0.3 | ||||||
| Maternal age | 0.96 | 0.91, 1.01 | 0.12 | 0.96 | 0.91, 1.01 | 0.093 | 0.97 | 0.90, 1.03 | 0.3 | 0.98 | 0.92, 1.05 | 0.6 | ||||||
| Education level | ||||||||||||||||||
| BA or higher | – | – | – | – | – | – | – | – | ||||||||||
| HS/GED | 3.4 | 1.69, 6.84 | < 0.001 | 3.53 | 1.82, 6.86 | < 0.001 | 5.2 | 2.22, 12.2 | < 0.001 | 6.62 | 2.94, 14.9 | < 0.001 | ||||||
| Less than HS | 7.82 | 2.39, 25.6 | < 0.001 | 5.43 | 1.67, 17.6 | 0.005 | 8.84 | 2.46, 31.7 | < 0.001 | 7.81 | 2.21, 27.5 | 0.001 | ||||||
| Some college/assoc | 2.52 | 1.39, 4.57 | 0.002 | 3.86 | 2.26, 6.58 | < 0.001 | 4.18 | 1.88, 9.26 | < 0.001 | 8.44 | 4.06, 17.6 | < 0.001 | ||||||
| Parity category | ||||||||||||||||||
| 1 + | – | – | – | – | – | – | – | – | ||||||||||
| 0 | 0.67 | 0.43, 1.06 | 0.09 | 0.55 | 0.36, 0.84 | 0.006 | 0.68 | 0.38, 1.19 | 0.2 | 0.55 | 0.32, 0.95 | 0.032 | ||||||
| Antibiotics ever in pregnancy | ||||||||||||||||||
| No | – | – | – | – | ||||||||||||||
| Yes | 4.24 | 2.15, 8.35 | < 0.001 | 3.24 | 1.66, 6.30 | < 0.001 | ||||||||||||
OR odds ratio, CI confidence interval, BA or higher college degree or higher, HS/GED high school or general equivalency degree.
Multinomial logistic regression models. Reference is non-L. iners Lactobacillus dominant (I, II, V) community state type.
Model 1 is adjusted for self-identified White race.
Model 2 is adjusted for self-identified White race, host age.
Model 3 is adjusted for self-identified White race, host age, educational attainment.
Model 4 is adjusted for self-identified White race, host age, educational attainment, parity.
Model 5 is adjusted for self-identified White race, host age, educational attainment, parity, and antibiotics use in pregnancy.
Discussion
Our results identified new associations between microbiota structure and host factors overall as well as with specific taxonomic signatures. We also verified some associations, such as host self-identified race and age, that had been previously described and further tested their independent effects after adjustment for other factors. The most robust factors associated with vaginal microbiota structure in pregnancy were education, parity, and self-identified race, followed by prenatal antibiotic use. Together, education, age, self-identified race, antibiotic use, and parity explained a moderate amount of variance in prenatal vaginal community structure. Our results corroborate single-site studies linking vaginal community patterns and maternal education and age and parity23,29–32. They are also consistent with prior smaller studies on host socio-demographics and the structure of other microbial niches as well as those among non-pregnant populations.33
While self-identified race remained significantly associated with global microbiome structure, which was consistent with prior research,14,25, it was no longer significantly associated with vaginal community states in fully adjusted models. Race may reflect a range of unmeasured exposures, including maternal stress exposures related to discrimination and structural racism. In a recent paper, combined effects of individual and neighborhood-level measures of socioeconomic status were associated with vaginal microbiome composition30. Similar to our results, social host factors (i.e., education and self-identified race) were more closely related to the pregnancy microbiome than clinical factors (i.e., gestational diabetes, hypertension, early-pregnancy BMI, and antibiotics during pregnancy). These results suggest that exposures that occur over a longer period of time, compared to the relatively short period of gestation, have greater effects on the microbiota in pregnancy. Alternatively, social factors may be more influential because they reflect the physiological effects of multiple interacting host factors and unmeasured environmental factors, including urbanicity34, pollution, racial segregation, diet, and chronic stress35,36. Host factors associated with vaginal microbiota structure were also largely conserved across the MARCH and Atlanta cohorts, which are demographically and racially diverse.
Taken together, our results suggest that it is important to account for host factors in vaginal microbiota studies, as they may drive specific facets of community structure. The taxa most predictive of level of education, L. iners, is clinically relevant as a marker of instability of vaginal microbiome structure as well as bacterial vaginosis and pregnancy complications37–40. The other most discriminant taxa inversely associated with lower education were pathogens previously associated with pregnancy complications: Aerococcus christensenii, S. oralis, and G. vaginalis41–44. Taxa most discriminant of host factors across all cohorts were consistent with polymicrobial vaginal communities and bacterial vaginosis in contrast with the hallmarks of highly stable L. crispatus-dominant communities15,45,46, which confer pathogen resistance through the production of lactic acid and hydrogen peroxide by lowering vaginal pH and inflammation and which are critical for pregnancy maintenance. Our results also indicate that when phylogenetic distance is used to cluster and taxonomically classify 16S rRNA gene data, the effect of host factors on the vaginal community structure are remarkably consistent across populations. Furthermore, the distribution of CSTs varied by cohort, and such variation could be explained by the differential distribution of host factors, which should be considered in the design and analysis of future studies.
Strengths of the study include the large size and inclusion of well-characterized ECHO cohort metadata. We attempted to removed site-specific biases by using a common bioinformatic pipeline that included phylogenetic mapping and well-curated full-length 16S rRNA gene vaginal references. While we acknowledge that some technical sources of variation may have remained using this approach to harmonizing different cohorts’ 16S rRNA gene sequence data, the magnitude of variation across sites appeared to be less than it was prior to phylogenetic scaffolding. Limitations include some differences in sample collection protocols i.e., WISC and Microbes, Allergy, Asthma, and Pets (MAAP) samples, collected at group B streptococcus screenings in the third trimester, were drawn from recto-vaginal samples, which may have explained some of the differences between the host factors that remained consistent between the MARCH and Atlanta cohorts but were more attenuated in the WISC and MAAP cohort. There were also site differences in sequence length and primer kits, which may have resulted in some artifacts in the taxa we identified with host factors. In addition, limited data availability for some factors that likely affect vaginal microbiome structure precluded comparisons, such as prenatal antibiotic use in the WISC, as well as measures of diet, cohabitation/marital status, and douching practices23. Since data were sequenced prior to this study, we were not able to harmonize and account for the effect of gestational week at sample collection. Therefore, we aligned the sequence data to the first collection in pregnancy, although this varied from the first to the third trimester, which also may have explained some of the different results observed in the WISC and MAAP sites.
We disentangled host factors that may drive differences in microbial signatures across populations when their underlying distributions are differential. Our results suggest that host factors are plausible explanations for some of the inconsistent results in previous smaller studies, most of which, to date have failed to account for host factors. As such, our results have important implications for the design and analysis of future population-based studies of the vaginal microbiome in pregnancy and underscore the need to fully account for the complex relationships between host factors and the microbiome.
Methods
Study population
The ECHO cohort is a consortium of birth cohorts from across the United States designed to evaluate the impact of in utero and early-life exposures on child health outcomes and includes detailed survey, medical record, and bio-specimen collections. The design and purpose of the ECHO cohort have been previously described27,28. All ECHO cohorts with available vaginal samples that had previously undergone 16S rRNA gene amplicon sequencing were included in the present study (Table 1), which meta-analyzed 16S rRNA gene amplicon data where available (Supplementary Materials, Fig. 2) from ECHO across a diverse set of sites and populations. The Atlanta cohort participants were recruited between 8 and 14 weeks gestations from prenatal clinics affiliated with two hospitals in Atlanta, GA. The MAAP cohort recruited pregnant women during their second and third trimesters from two hospital systems in metro Detroit, MI, to understand how exposures in early life modify risk for asthma47. The Children’s Respiratory and Environmental Workgroup (CREW) includes both the WISC, drawn from rural medical centers in north-central Wisconsin48, and the MAAP cohort, drawn from urban metro-Detroit sites. The MARCH) a population-based cohort that recruited from initial prenatal appointments; for this study, we used a subset of the entire MARCH cohort that included both University of Michigan sites (MARCH U-M) and remote collection of samples from nine other sites across the state of Michigan. For this study, only those participants who provided informed consent for providing at least one vaginal swab sample during their pregnancy were included, and we analyzed the first sample collected in pregnancy.
Clinical and demographic measures
At all sites, demographic data and health-related practices were collected from prenatal surveys, and health conditions, infection, delivery information, and antibiotic use were abstracted from the medical record. In MARCH, detailed information, including the infant’s sex, infant’s birth weight, complications of pregnancy, and pre-pregnancy BMI and gestational age, was derived from the birth certificate. Host factor metadata were harmonized to include self-reported White versus non-White race, any antibiotics in pregnancy, and public insurance as a proxy for socioeconomic status since household income was collected differently across sites.
Vaginal sample collection
At the MARCH U-M sites, vaginal dual-headed dry swabs (Starplex™ Scientific S09D, Fisher Scientific) were self-collected in clinics. Immediately upon collection, AllProtect (Qiagen) preservative was added to the swabs, and they were stored for 24–48 h at −20 °C before being transported on ice for long-term storage at -80 °C. In the other MARCH sites, vaginal dual-headed dry swabs were self-collected at home, mailed to the laboratory, and archived at −80 °C. Similarly, in the Atlanta cohort, vaginal swabs were self-collected using the Sterile Catch-All Sample Swab (Epicentre), placed immediately in MoBio bead tubes, and transported on ice for archival storage at −80 °C. For both the MARCH and Atlanta cohorts, the first vaginal swab collected in pregnancy was analyzed. At the WISC and MAAP sites, vaginal/rectal swabs (Epicentre Catch-All) were collected by a provider within 6 weeks of delivery at the time of group B streptococcus screening. Swabs were stored in RNAlater (Thermo Fisher Scientific) at 4 °C for at least 24 h and then transferred to −80 °C. Prior to DNA extraction, swabs were thawed on ice and transferred to Lysing Matrix E (LME) tubes. RNAlater was transferred into a sterile tube and centrifuged at 16,000×g for 5 min at 4 °C. Pellets were re-suspended using cetyltrimethylammonium bromide buffer and transferred to the LME tube containing the swab47.
DNA extraction, library preparation, and sequencing
MARCH
DNA was extracted from the MARCH U-M samples using the PowerMag kit (Qiagen; MoBio Laboratories) optimized for the epMotion 5075 TMX (Eppendorf). DNA samples were quantified using the Quant-iT PicoGreen dsDNA Assay kit (Thermo Fisher Scientific). The V4 region of the 16S rRNA gene was amplified using the dual-index sequencing strategy outlined in the MiSeq SOP49 at the MARCH U-M Microbiome Core50. Amplicons were sequenced using 250 bp Illumina MiSeq (MiSeq Reagent 222 kit V2) for 500 cycles according to the manufacturer’s instructions with modifications for the primer set. Libraries and sequencing reagents with custom read 1/read 2 and index primers added were prepared according to Illumina’s protocol for 2 nM libraries. The final load concentration was 4 pM, spiked with 15% PhiX.
DNA was extracted from the other MARCH samples also using the DNEasy Powersoil DNA Isolation kit (Qiagen) per the Human Microbiome Project’s protocol51 and MiSeq SOP49. Polymerase chain reaction (PCR) amplification was performed on the V4 region of the 16S rRNA gene following the mothur wet lab documentation52, using primer sets SB501-SB508 and SA701-SA712 ordered from IDT. Successfully amplified triplicate PCR reactions were pooled and purified using Agencourt AMPure XP (Beckman Coulter), and the concentration of 16S rRNA gene amplicons was quantified using the Quant-IT dsDNA assay kit (Invitrogen). The purified 16S rRNA gene pool was submitted to the Michigan State University Research Technology Support Facility Genomics Core for paired-end 250 bp sequencing on the Illumina MiSeq platform using V2 chemistry.
Atlanta
Samples underwent amplification of the V3-V4 region of the 16S rRNA gene following a two-step PCR protocol53. Amplicons were sequenced on the Illumina HiSeq 2500 modified to generate 300 bp paired-end reads. Additional details have been published elsewhere8.
WISC and MAAP
The V4 region of the 16S rRNA gene was amplified in triplicate reactions per sample using 515F/806R primers and PCR conditions previously described12,54. Pooled amplicon reactions with 5 ng were purified using the SequalPrep Normalization Plate Kit according to the manufacturer’s specifications, quantified using the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific), and pooled at equimolar concentrations. The amplicon library was constructed using the Agencourt AMPure XP system (Beckman-Coulter), quantified using the KAPA Library Quantification Kit (APA Biosystems), and diluted to 2 nM. Equimolar PhiX was added at 40% final volume to the amplicon library followed by sequencing on the Illumina NextSeq 500 platform employing a 2 × 150 bp sequencing run. Additional details have been published elsewhere for WISC38 and MAAP32.
Bioinformatics
De-duplication was performed using the dual bar-code approach. We processed raw sequences from all sites together using the DADA2 Workflow for Big Data v.1.5.2 in order to cluster them into ASVs (https://benjjneb.github.io/dada2/bigdata.html)55 . For the Atlanta and MARCH cohorts, forward and reverse reads were trimmed using lengths of 255 and 225 bp, respectively, and filtered using a minimum quality score of 2. For the WISC and MAAP cohort, reads were maintained if they exhibited a maximum expected error of 2 and a read length of at least 150 bp. We then processed the ASVs using MaLiAmPi56,57, a computational tool designed to robustly combine 16S amplicon data for meta-analysis using phylogenetic placement. Sequences are mapped to a common tree, which we constructed from full-length 16S rRNA allele data from NCBI (cached as a repository on Zenodo58). We employed a minimum overlap at six for read-joining and removed Chimeras following the dada2 protocol.
Given that samples had previously undergone isolation and sequencing at each site (Table 1), we harmonized 16S rRNA gene sequences using MaLiAmPi56,57. We used the “refpkg.nf” module in MaLiAmPi to recruit alleles from this repository and assemble them de novo via RAxMLv859. Finally, the amplicon sequence variants from DADA255 were placed onto this phylogenetic tree via pplacer60, and metrics including alpha-diversity, pairwise phylogenetic distance, and taxonomic composition were derived via the pplacer utility guppy, per the pplacer_place_classify.nf module of MaLiAmPi.
After filtering of non-bacterial taxa, the relative abundance of a total of 5,232 phylotypes was estimated61,62. With mothur (version 1.48.0)63,64, we calculated Bray–Curtis distances and principal coordinates (PCoA) plotted with RStudio (version 2023.06.0 + 421), R (version 4.3.1), and the tidyverse65 (version 2.0.0) library, which includes ggplot2 (version 3.4.2). One sample with low counts was excluded. We also classified vaginal samples into CSTs using VALENCIA11, based on similarity to reference centroids.
Statistical analysis
Demographic and clinical characteristics across cohorts are summarized in Table 2. To test for significant differences in the distribution of participant characteristics, we used chi-square or t-tests as appropriate. To identify host factors associated with vaginal microbiota structure in pregnancy, we generated single-factor PERMANOVA models based on Bray–Curtis distances overall and separately for each cohort using adonis2 implementation in R’s vegan package and dispersion using betsdispr based on 100,000 permutations.
We also used multifactor PERMANOVA models to examine the independent effects of host factors using a backward variable selection approach. We adjusted p-values for multiple comparisons using a Benjamini and Hochberg FDR criterion of p < 0.0566. To test the independent effects of host factors on vaginal CSTs, we constructed a series of nested multinomial logistic regression models from the most robust predictors of vaginal microbiome variation identified from the PERMANOVA results. In these models, we collapsed the six VALENCIA classifications into three categories: non-L. iners Lactobacillus dominant (CSTs I, II, and V [reference]), L. iners dominant (CST III), and diverse (CST IV). Covariates were selected using a criterion of p < 0.2 in the PERMANOVA models including self-identified race, education level, maternal age, receipt of antibiotics in pregnancy, and parity category. Using a stepwise forward selection approach beginning with self-identified race, we compared parameter estimates as host factors were added to subsequent models to test whether the effect of self-identified race became attenuated after adjustment for each host factor or remained independently associated with vaginal CSTs. Models were run for the pooled cohort data as well as for each cohort individually using the R multinom() function from the nnet package.
We next examined how the factors retained in the multiple adjusted models associated with the relative abundance of specific taxa within communities. Using a machine learning approach utilizing a random forest classifier for each robust host factor in the PERMANOVA models, we ranked specific taxa that contributed the largest amount of homogeneity in the nodes and leaves of the forest trees by estimating the mean decrease in Gini index and increase in node purity coefficients for categorical and continuous predictors, respectively. Gini coefficients are estimated each time the tree is split on each feature, with higher values ranking greater discrimination. Random forest classifiers were run using a test and train validation set approach, splitting the data to set aside 20% for testing (80/20 split). We generated supervised random forest model plots and validated them using ROCs with AUCs using “randomForest” based on Breiman’s random forest algorithm for classification and regression67 and “pROC” package in R. Multidimensional scaling plots of the proximity matrix were also used to rank taxa between samples. Models were tuned focusing on the optimal number of variables randomly sampled as candidates at each split (“mtry”) and the optimal number of trees (“ntree”) in R. Default values for number of variables randomly sampled as candidates at each split were the square root of the number of variables in the model from 5,228 taxa. We set the hyperparameter nTree to 500, and tuned with “caret” until the out-of-bag error stopped decreasing.
Missing data in the outcome variables were imputed by calculating the variable median for the numeric variable and the mode for the categorical variables by cohort except when it was not missing at random (i.e., for an entire cohort). All statistical analyses were conducted using R version 4.2.368. The University of Michigan IRB approved the research, which was deemed exempt and performed in accordance the Declaration of Helsinki. Informed consent was obtained from all participants.
Supplementary Information
Acknowledgements
The authors wish to thank our ECHO colleagues; the medical, nursing, and program staff; and the children and families participating in the ECHO cohorts. Please see full list of ECHO Cohort Consortium collaborators in the Supplemental Material. We would also like to acknowledge the University of Michigan Microbial Systems Laboratory for constructing and sequencing the 16S rRNA gene amplicon libraries for the MARCH U-M samples. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of the Director, National Institutes of Health, under Award Numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core), UH3OD023251 (Alshawabkeh), UH3OD023320 (Aschner), UH3OD023253 (Camargo), UH3OD023248 (Dabelea), UH3OD023313 (Deoni), UH3OD023328 (Duarte), UH3OD023318 (Dunlop), UH3OD023279 (Elliott), UH3OD023289 (Ferrara), UH3OD023282 (Gern), UH3OD023287 (Breton), UH3OD023244 (Hipwell), UH3OD023275 (Karagas), UH3OD023271 (Karr), UH3OD023347 (Lester), UH3OD023389 (Leve), UH3OD023288 (McEvoy), UH3OD023349 (O’Connor), UH3OD023285 (Kerver), UH3OD023290 (Herbstman), UH3OD023272 (Schantz), UH3OD023249 (Stanford), UH3OD023305 (Trasande), UH3OD023337 (Wright).
Author contributions
K.S.M. designed and conceived of the study. C.A.B. and B.P. analyzed the data. J.G. developed and provided consultation on the bioinformatics processing of the data. A.L.D. and N.P served as senior authors and mentors to K.S.M. for the study. All authors reviewed the findings and assisted with the interpretations of the data and the writing of the manuscript.
Data availability
The unprocessed 16S rRNA gene amplicon data are publicly available for the Atlanta cohort (https://www.ncbi.nlm.nih.gov/sra, PRJNA725416), MARCH (https://www.ncbi.nlm.nih.gov/sra, PRJNA1041860) and MAAP/WISC (European Nucleotide Archive: PRJEB46659). Select de-identified data from the ECHO Program are available through the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Data and Specimen Hub (DASH). Code for the study can be accessed publicly69. Information on study data not available on DASH can be obtained by contacting the ECHO Data Analysis Center at ECHO-DAC@rti.org with inquiries.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Kimberly McKee, Email: kimckee@umich.edu.
ECHO Cohort Consortium:
P. Brian Smith, L. Kristin Newby, Linda Adair, Lisa P. Jacobson, Diane Catellier, Monica McGrath, Christian Douglas, Priya Duggal, Emily Knapp, Amii Kress, Courtney K. Blackwell, Maxwell A. Mansolf, Jin-Shei Lai, Emily Ho, David Cella, Richard Gershon, Michelle L. Macy, Suman R. Das, Jane E. Freedman, Simon A. Mallal, John A. McLean, Ravi V. Shah, Meghan H. Shilts, Akram N. Alshawabkeh, Jose F. Cordero, John Meeker, Leonardo Trasande, Carlos A. Camargo, Kohei Hasegawa, Zhaozhong Zhu, Ashley F. Sullivan, Dana Dabelea, Wei Perng, Traci A. Bekelman, Greta Wilkening, Sheryl Magzamen, Brianna F. Moore, Anne P. Starling, Deborah J. Rinehart, Daphne Koinis Mitchell, Viren D’Sa, Sean C. L. Deoni, Hans-Georg Mueller, Cristiane S. Duarte, Catherine Monk, Glorisa Canino, Jonathan Posner, Tenneill Murray, Claudia Lugo-Candelas, Anne L. Dunlop, Patricia A. Brennan, Christine Hockett, Amy Elliott, Assiamira Ferrara, Lisa A. Croen, Monique M. Hedderson, John Ainsworth, Leonard B. Bacharier, Casper G. Bendixsen, James E. Gern, Diane R. Gold, Tina V. Hartert, Daniel J. Jackson, Christine C. Johnson, Christine L. M. Joseph, Meyer Kattan, Gurjit K. Khurana Hershey, Robert F. Lemanske, Jr., Susan V. Lynch, Rachel L. Miller, George T. O’Connor, Carole Ober, Dennis Ownby, Katherine Rivera-Spoljaric, Patrick H. Ryan, Christine M. Seroogy, Anne Marie Singh, Robert A. Wood, Edward M. Zoratti, Rima Habre, Shohreh Farzan, Frank D. Gilliland, Irva Hertz-Picciotto, Deborah H. Bennett, Julie B. Schweitzer, Rebecca J. Schmidt, Janine M. LaSalle, Alison E. Hipwell, Kate E. Keenan, Catherine J. Karr, Nicole R. Bush, Kaja Z. LeWinn, Sheela Sathyanarayana, Qi Zhao, Frances Tylavsky, Kecia N. Carroll, Christine T. Loftus, Leslie D. Leve, Jody M. Ganiban, Jenae M. Neiderhiser, Scott T. Weiss, Augusto A. Litonjua, Cindy T. McEvoy, Eliot R. Spindel, Robert S. Tepper, Craig J. Newschaffer, Kristen Lyall, Heather E. Volk, Rebecca Landa, Sally Ozonoff, Joseph Piven, Heather Hazlett, Juhi Pandey, Robert Schultz, Steven Dager, Kelly Botteron, Daniel Messinger, Wendy Stone, Jennifer Ames, Thomas G. O’Connor, Richard K. Miller, Emily Oken, Michele R. Hacker, Tamarra James-Todd, T. Michael O’Shea, Rebecca C. Fry, Jean A. Frazier, Rachana Singh, Caitlin Rollins, Angela Montgomery, Ruben Vaidya, Robert M. Joseph, Lisa K. Washburn, Semsa Gogcu, Kelly Bear, Julie V. Rollins, Stephen R. Hooper, Genevieve Taylor, Wesley Jackson, Amanda Thompson, Julie Daniels, Michelle Hernandez, Kun Lu, Michael Msall, Madeleine Lenski, Rawad Obeid, Steven L. Pastyrnak, Elizabeth Jensen, Christina Sakai, Hudson Santos, Jean M. Kerver, Nigel Paneth, Charles J. Barone, Michael R. Elliott, Douglas M. Ruden, Chris Fussman, Julie B. Herbstman, Amy Margolis, Susan L. Schantz, Sarah Dee Geiger, Andrea Aguiar, Karen Tabb, Rita Strakovsky, Tracey Woodruff, Rachel Morello-Frosch, Amy Padula, Joseph B. Stanford, Christina A. Porucznik, Angelo P. Giardino, Rosalind J. Wright, Robert O. Wright, Brent Collett, Nicole Baumann-Blackmore, Ronald Gangnon, Daniel J. Jackson, Chris G. McKennan, Jo Wilson, Matt Altman, Judy L. Aschner, Annemarie Stroustrup, Stephanie L. Merhar, Paul E. Moore, Gloria S. Pryhuber, Mark Hudak, Ann Marie Reynolds Lyndaker, Andrea L. Lampland, Burton Rochelson, Sophia Jan, Matthew J. Blitz, Michelle W. Katzow, Zenobia Brown, Codruta Chiuzan, Timothy Rafael, Dawnette Lewis, Natalie Meirowitz, Brenda Poindexter, Tebeb Gebretsadik, Sarah Osmundson, Jennifer K. Straughen, Amy Eapen, Andrea Cassidy-Bushrow, Ganesa Wegienka, Alex Sitarik, Kim Woodcroft, Audrey Urquhart, Albert Levin, Tisa Johnson-Hooper, Brent Davidson, Tengfei Ma, Emily S. Barrett, Martin J. Blaser, Maria Gloria Dominguez-Bello, Daniel B. Horton, Manuel Jimenez, Todd Rosen, Kristy Palomares, Lyndsay A. Avalos, Yeyi Zhu, Kelly J. Hunt, Roger B. Newman, Michael S. Bloom, Mallory H. Alkis, James R. Roberts, Sunni L. Mumford, Heather H. Burris, Sara B. DeMauro, Lynn M. Yee, Aaron Hamvas, Antonia F. Olidipo, Andrew S. Haddad, Lisa R. Eiland, Nicole T. Spillane, Kirin N. Suri, Stephanie A. Fisher, Jeffrey A. Goldstein, Leena B. Mithal, Raye-Ann O. DeRegnier, Nathalie L. Maitre, Ruby H. N. Nguyen, Meghan M. JaKa, Abbey C. Sidebottom, Michael J. Paidas, JoNell E. Potter, Natale Ruby, Lunthita Duthely, Arumugam Jayakumar, Karen Young, Isabel Maldonado, Meghan Miller, Jonathan L. Slaughter, Sarah A. Keim, Courtney D. Lynch, Kartik K. Venkatesh, Kristina W. Whitworth, Elaine Symanski, Thomas F. Northrup, Hector Mendez-Figueroa, Ricardo A. Mosquera, Margaret R. Karagas, Juliette C. Madan, Debra M. MacKenzie, Johnnye L. Lewis, Brandon J. Rennie, Bennett L. Leventhal, Young Shin Kim, Somer Bishop, Sara S. Nozadi, Li Luo, Barry M. Lester, Carmen J. Marsit, Todd Everson, Cynthia M. Loncar, Elisabeth C. McGowan, Stephen J. Sheinkopf, Brian S. Carter, Jennifer Check, Jennifer B. Helderman, Charles R. Neal, and Lynne M. Smith
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-62537-7.
References
- 1.Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. 2016;352:539–544. doi: 10.1126/science.aad9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert JA, et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535:94–103. doi: 10.1038/nature18850. [DOI] [PubMed] [Google Scholar]
- 3.Kostic AD, et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe. 2015;17:260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 5.Ridaura VK, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341:1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan A, Hunt E, MacSharry J, Murphy DM. The Microbiome and the pathophysiology of asthma. Respir. Res. 2016;17:163. doi: 10.1186/s12931-016-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J. Clin. Invest. 2011;121:4610–4617. doi: 10.1172/JCI57172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlop AL, et al. Vaginal microbiome composition in early pregnancy and risk of spontaneous preterm and early term birth among African American women. Front. Cell Infect. Microbiol. 2021;11:641005. doi: 10.3389/fcimb.2021.641005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eastment MC, McClelland RS. Vaginal microbiota and susceptibility to HIV. AIDS. 2018;32:687–698. doi: 10.1097/QAD.0000000000001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fettweis JM, et al. The vaginal microbiome and preterm birth. Nat. Med. 2019;25:1012–1021. doi: 10.1038/s41591-019-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferretti P, et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe. 2018;24:133–145.e135. doi: 10.1016/j.chom.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCauley, K. E. et al. Heritable vaginal bacteria influence immune tolerance and relate to early-life markers of allergic sensitization in infancy. Cell Rep Med.3, 112 (2022). [DOI] [PMC free article] [PubMed]
- 13.Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clin. Microbiol. Rev. 2016;29:223–238. doi: 10.1128/CMR.00075-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravel J, et al. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA. 2011;108:4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anahtar MN, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42:965–976. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buve A, Jespers V, Crucitti T, Fichorova RN. The vaginal microbiota and susceptibility to HIV. AIDS. 2014;28:2333–2344. doi: 10.1097/qad.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 17.Verstraelen, H. et al. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol.9, 116 10.1186/1471-2180-9-116 (2009). [DOI] [PMC free article] [PubMed]
- 18.Aagaard K, et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012;7:e36466. doi: 10.1371/journal.pone.0036466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiGiulio DB, et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA. 2015;112:11060–11065. doi: 10.1073/pnas.1502875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyman RW, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod. Sci. 2014;21:32–40. doi: 10.1177/1933719113488838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero R, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2:4. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vujkovic-Cvijin I, et al. Host variables confound gut microbiota studies of human disease. Nature. 2020;587:448–454. doi: 10.1038/s41586-020-2881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun, S. et al. Race, the vaginal microbiome, and spontaneous preterm birth. mSystems.7, e00017–00022 10.1128/msystems.00017-22 (2022). [DOI] [PMC free article] [PubMed]
- 24.Fettweis JM, et al. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology (Reading). 2014;160:2272–2282. doi: 10.1099/mic.0.081034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.France MT, et al. VALENCIA: A nearest centroid classification method for vaginal microbial communities based on composition. Microbiome. 2020;8:166. doi: 10.1186/s40168-020-00934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duvallet C, Gibbons SM, Gurry T, Irizarry RA, Alm EJ. Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 2017;8:1784. doi: 10.1038/s41467-017-01973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley JP, et al. Opportunities for evaluating chemical exposures and child health in the United States: The Environmental influences on Child Health Outcomes (ECHO) Program. J. Expo Sci. Environ. Epidemiol. 2020;30:397–419. doi: 10.1038/s41370-020-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gillman MW, Blaisdell CJ. Environmental influences on Child Health Outcomes, a research program of the National Institutes of Health. Curr. Opin. Pediatr. 2018;30:260–262. doi: 10.1097/mop.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wright ML, et al. Factors associated with vaginal lactobacillus predominance among African American women early in pregnancy. J. Womens Health (Larchmt). 2022;31:682–689. doi: 10.1089/jwh.2021.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dixon M, Dunlop AL, Corwin EJ, Kramer MR. Joint effects of individual socioeconomic status and residential neighborhood context on vaginal microbiome composition. Front. Public Health. 2023;11:1029741. doi: 10.3389/fpubh.2023.1029741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Virtanen S, et al. Vaginal microbiota composition correlates between pap smear microscopy and next generation sequencing and associates to socioeconomic status. Sci. Rep. 2019;9:7750. doi: 10.1038/s41598-019-44157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kervinen K, et al. Parity and gestational age are associated with vaginal microbiota composition in term and late term pregnancies. EBioMedicine. 2022;81:104107. doi: 10.1016/j.ebiom.2022.104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357–360. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vargas-Robles D, et al. Changes in the vaginal microbiota across a gradient of urbanization. Sci. Rep. 2020;10:12487. doi: 10.1038/s41598-020-69111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turpin R, et al. Perceived stress and molecular bacterial vaginosis in the National Institutes of Health Longitudinal Study of Vaginal Flora. Am. J. Epidemiol. 2021;190:2374–2383. doi: 10.1093/aje/kwab147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borgogna JC, et al. Vaginal microbiota of American Indian women and associations with measures of psychosocial stress. PLoS One. 2021;16:e0260813. doi: 10.1371/journal.pone.0260813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bloom SM, et al. Cysteine dependence of Lactobacillus iners is a potential therapeutic target for vaginal microbiota modulation. Nat. Microbiol. 2022;7:434–450. doi: 10.1038/s41564-022-01070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brooks JP, et al. Changes in vaginal community state types reflect major shifts in the microbiome. Microb. Ecol. Health Dis. 2017;28:1303265. doi: 10.1080/16512235.2017.1303265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munoz A, et al. Modeling the temporal dynamics of cervicovaginal microbiota identifies targets that may promote reproductive health. Microbiome. 2021;9:163. doi: 10.1186/s40168-021-01096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng N, Guo R, Wang J, Zhou W, Ling Z. Contribution of Lactobacillus iners to vaginal health and diseases: A systematic review. Front. Cell Infect. Microbiol. 2021;11:792787. doi: 10.3389/fcimb.2021.792787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Callahan BJ, et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc. Natl. Acad. Sci. USA. 2017;114:9966–9971. doi: 10.1073/pnas.1705899114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carlstein, C., Marie Søes, L. & Jørgen Christensen, J. Aerococcus christensenii as part of severe polymicrobial chorioamnionitis in a pregnant woman. Open Microbiol J.10, 27–31 10.2174/1874285801610010027 (2016). [DOI] [PMC free article] [PubMed]
- 43.Souza JGS, et al. Role of glucosyltransferase R in biofilm interactions between Streptococcus oralis and Candida albicans. ISME J. 2020;14:1207–1222. doi: 10.1038/s41396-020-0608-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wydall S, Durrant F, Scott J, Cheesman K. Streptococcus oralis endocarditis leading to central nervous system infection in pregnancy. Anaesth. Rep. 2021;9:e12133. doi: 10.1002/anr3.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anton L, et al. Common cervicovaginal microbial supernatants alter cervical epithelial function: Mechanisms by which Lactobacillus crispatus contributes to cervical health. Front. Microbiol. 2018;9:2181. doi: 10.3389/fmicb.2018.02181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lepargneur JP. Lactobacillus crispatus as biomarker of the healthy vaginal tract. Ann. Biol. Clin. (Paris) 2016;74:421–427. doi: 10.1684/abc.2016.1169. [DOI] [PubMed] [Google Scholar]
- 47.Panzer AR, et al. The impact of prenatal dog keeping on infant gut microbiota development. Clin. Exp. Allergy. 2023;53:833–845. doi: 10.1111/cea.14303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seroogy CM, et al. Respiratory health, allergies, and the farm environment: design, methods and enrollment in the observational Wisconsin Infant Study Cohort (WISC): A research proposal. BMC Res. Notes. 2019;12:423. doi: 10.1186/s13104-019-4448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013;79:5112–5120. doi: 10.1128/aem.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The Michigan Microbiome Project. https://microbe.med.umich.edu/about/research#:~:text=The%20mission%20of%20the%20Michigan%20Microbiome%20Project%20is,by%20stimulating%20their%20microbiomes%20to%20make%20more%20butyrate (2023)
- 51.Methé, B. A. et al. A framework for human microbiome research. Nature486, 112 (2012). [DOI] [PMC free article] [PubMed]
- 52.Sugino KY, Paneth N, Comstock SS. Michigan cohorts to determine associations of maternal pre-pregnancy body mass index with pregnancy and infant gastrointestinal microbial communities: Late pregnancy and early infancy. PLoS One. 2019;14:e0213733. doi: 10.1371/journal.pone.0213733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elovitz MA, et al. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat. Commun. 2019;10:1305. doi: 10.1038/s41467-019-09285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujimura KE, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016;22:1187–1191. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Callahan BJ, et al. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maximum Likelihood Amplicon Pipeline (MaLiAmPi): An Amplicon (PCR/16S) Microbiome Pipeline. https://github.com/jgolob/maliampi (2023)
- 57.Minot, S. S. et al. Robust harmonization of microbiome studies by phylogenetic scaffolding with MaLiAmPi. BioRxiv:2022.2007.2026.501561 10.1101/2022.07.26.501561 (2022).
- 58.Golob J. 2022. ARF / YA16Sdb collection of curated 16S rRNA alleles (2020–04-20) Zenodo. [DOI]
- 59.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsen FA, Kodner RB, Armbrust EV. pplacer: Linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinformatics. 2010;11:538. doi: 10.1186/1471-2105-11-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Version 1.48.0. https://github.com/mothur/mothur/releases/tag/v1.48.0 (2022)
- 62.Schloss PD, et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/aem.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957;27:326–349. doi: 10.2307/1942268. [DOI] [Google Scholar]
- 64.Dixon P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003;14:927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x. [DOI] [Google Scholar]
- 65.Wickham H, et al. Welcome to the Tidyverse. J. Open Source Softw. 2019;4:1686. doi: 10.21105/joss.01686. [DOI] [Google Scholar]
- 66.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 67.Breiman L. Random forests. Mach. Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 68.The R Project for Statistical Computing. https://www.R-project.org (2023)
- 69.Vaginal Microbiome Structure in Pregnancy Results from the ECHO Cohorts. https://github.com/kimckee-umich/Vaginal-Microbiome-Structure-in-Pregnancy-Results-from-the-ECHO-Cohorts (2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Golob J. 2022. ARF / YA16Sdb collection of curated 16S rRNA alleles (2020–04-20) Zenodo. [DOI]
Supplementary Materials
Data Availability Statement
The unprocessed 16S rRNA gene amplicon data are publicly available for the Atlanta cohort (https://www.ncbi.nlm.nih.gov/sra, PRJNA725416), MARCH (https://www.ncbi.nlm.nih.gov/sra, PRJNA1041860) and MAAP/WISC (European Nucleotide Archive: PRJEB46659). Select de-identified data from the ECHO Program are available through the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Data and Specimen Hub (DASH). Code for the study can be accessed publicly69. Information on study data not available on DASH can be obtained by contacting the ECHO Data Analysis Center at ECHO-DAC@rti.org with inquiries.