Abstract
Immune checkpoint inhibitors (ICIs) revolutionized the management of mismatch repair-deficient (dMMR)/microsatellite instability-high (MSI-H) gastrointestinal (GI) cancers. Based on notable results observed in the metastatic setting, several clinical trials investigated ICIs as neoadjuvant treatment (NAT) for localized dMMR/MSI-H GI cancers, achieving striking results in terms of clinical and pathological responses and creating the opportunity to spare patients from neoadjuvant chemotherapy and/or radiotherapy and even surgical resection. Nevertheless, these impressive findings are mainly derived from small proof of concept phase II studies and there are still several open questions to address. Moreover, dMMR/MSI-H represents a limited subgroup accounting for less than 10% of GI cancers. Consequently, many efforts have been produced to investigate neoadjuvant ICIs also in mismatch repair-proficient/microsatellite stable (MSS) cancers, considering the potential synergistic effect in combining immune-targeted agents with standard therapies such as chemo and/or radiotherapy. However, results for combining ICIs to the standard of care in the unselected population are still unsatisfactory, without improvements in event-free survival in esophago-gastric adenocarcinoma for the addition of pembrolizumab to chemotherapy, and sometimes limited benefit in patients with locally advanced rectal cancer. Therefore, a major challenge will be to identify among the heterogenous spectrum of this disease, those patients that could take advantage of neoadjuvant immunotherapy and deliver the most effective treatment. In this review we discuss the rationale of NAT in GI malignancies, summarize the available evidence regarding the completed trials that evaluated this treatment strategy in both MSI-H and MSS tumors. Finally, we discuss ongoing studies and future perspectives to render neoadjuvant immunotherapy another arrow in the quiver for the treatment of locally advanced GI tumors.
Keywords: Immunotherapy, Immune Checkpoint Inhibitors, Gastrointestinal Neoplasms, Radiotherapy, Programmed Cell Death 1 Receptor
Introduction
Over the last decades, immunotherapy, and specifically immune checkpoint inhibitors (ICIs), has become one of the pillars of cancer treatment, with increasing applications in different malignancies, both in palliative and in the curative setting. As for gastrointestinal (GI) cancers, including esophageal, gastric and colorectal, ICIs have been approved by Food and Drug Agency only in the metastatic setting, including pembrolizumab for first-line deficient mismatch repair (dMMR)/microsatellite high (MSI-H) colorectal cancer (CRC), nivolumab +/− ipilimumab for second-line for dMMR/MSI-H CRC, nivolumab plus chemotherapy for first-line esophago-gastric cancer (EGC), pembrolizumab for pretreated patients with programmed death-ligand 1 (PD-L1) positive EGC, nivolumab +/− chemo or ipilimumab for first-line esophageal squamous cell cancers (ESCC) irrespective of PD-L1 status, pembrolizumab for pretreated dMMR/MSI-H cancers (including EGC, biliary tract and small bowel cancers).1–6 Similar approvals have been granted by the European Medicines Agency, with some limitations for the use of nivolumab in addition to chemotherapy/ipilimumab for first-line EGC and ESCC, according to PD-L1 expression.7
Based on this promising background, ICIs have been widely investigated as part of multimodal treatments in locally advanced (LA) cancer. In this context, GI cancers represent an ideal clinical setting to test ICIs since neoadjuvant treatment (NAT) represents the current standard approach for LA EGC and rectal cancer.8–10 Several studies have been published, and others are ongoing, although no ICI approval occurred in NAT yet. In this review, we will focus exclusively on the use of ICIs in the neoadjuvant setting of EGC and CRC, by critically reviewing the current evidence and addressing future development in the field.
Principle of immunotherapy
ICIs have revolutionized the cancer treatment scenario by prolonging the survival of patients. It is focused on the development of agents able to stimulate or suppress the immune system, in a specific manner, to fight off a wide spectrum of diseases, including cancers.11 Immune cells recognize and eliminate tumor cells, inhibiting cancer development and progression. However, studies made throughout the past two decades have demonstrated that our immune system can paradoxically limit and promote tumor development and progression.12 13 This specific process is referred to “cancer immune editing” and consists of three different phases: elimination, equilibrium, and escape.14 Innate and adaptive immune systems cooperate to recognize and destroy cancer cells in the elimination phase. Second, the equilibrium phase is mediated by the adaptive immune system, preventing tumor cells development, and it is also characterized by some rare tumor cell variants that survived to the elimination phase. Finally, immunologically sculpted tumors gain the ability to survive immune surveillance and progressively grow establishing an immunosuppressive tumor microenvironment and lastly, becoming clinically apparent.15
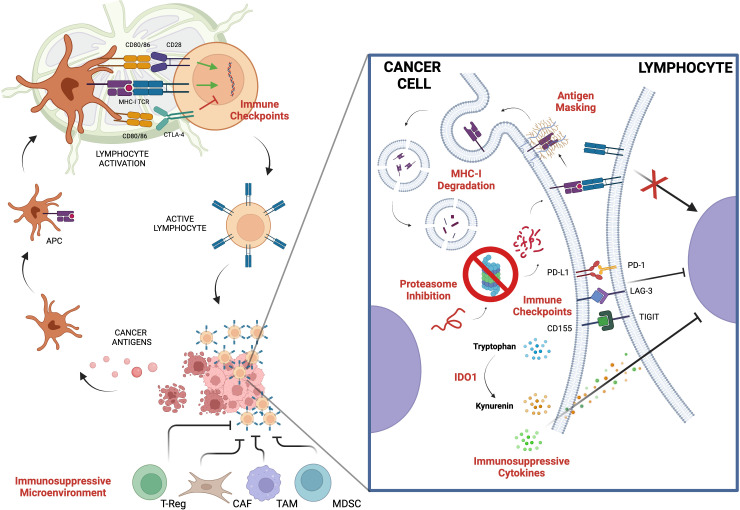
Notably, cancer cells are surrounded by many cell types including immune cells which constitute the tumor immune microenvironment whose composition, location and density are now recognized to be prognostic for patients’ survival and predictive for responses to treatment.16 Otherwise, several immune escape mechanisms have been identified: a reduced immune recognition due to the lack of specific antigens or alterations in their presentation, the promotion of an immune tolerant microenvironment formation affecting cytokine levels, and the upregulation of immune-checkpoint molecules such as programmed cell death 1 (PD-1) or PD-L1 (figure 1). In fact, immune response is finely regulated at multiple levels, which act as checkpoints and negatively downregulate T-cell activation, to preserve self-tolerance.17
Figure 1.
Key biologic mechanisms of cancer immune escaping. APC, antigen presenting cell; CAF, cancer-associated fibroblasts; CTLA4, cytotoxic T lymphocyte antigen 4; IDO1, indoleamine 2,3-dioxygenase; LAG3, lymphocyte-activation gene 3; MDSC, myeloid-derived suppressor cells; MHC, major histocompatibility complex; PD-1, programmed death 1; PD-L1, programmed death-ligand 1; TAM, tumor-associated macrophages; TCR, T-cell receptor; TIGIT, T-cell immunoreceptor with Ig and ITIM domains; T-reg: regulatory T Cell
Cytotoxic T lymphocyte antigen 4 (CTLA4) and PD-1 are considered the two main immune checkpoint receptors in cancer. Their discovery led to the development of ICIs, which have shown clinical efficacy in many cancer types. By blocking the interaction between immune cells and tumor cells, ICIs enable T cells to recognize tumor antigens and destroy cancer cells.16 Conversely, to classic cytotoxic treatments, ICIs can induce durable responses even after treatment discontinuation. This occurrence has been observed, for instance, in patients with advanced-stage melanoma treated with the anti-CTLA4 antibody ipilimumab, where a meaningful proportion of patients reached a plateau in overall survival (OS) approximately 3 years after treatment initiation.18
Neoadjuvant ICI therapy: rationale and clinical development
NAT refers to the systemic and/or locoregional treatment of LA radically resectable cancer performed before surgery. It usually consists of chemotherapy (CT), radiotherapy (RT) or a combination of both (chemoradiotherapy, CRT). The key principles behind NAT administration include an increased probability of achieving tumor downsizing with a possible safer surgical approach, higher rates of radical resections with microscopical negative residual disease (R0), and improvement in survival given its potentiality in eradicating distant micrometastases.19 Furthermore, NAT may lead to pathologic complete response (pCR) which could be associated with improved disease-free survival (DFS), or OS as reported for many types of cancer.20 Another potential advantage of NAT is that it could induce a strong translational impact allowing testing of in vivo tumor biology response.
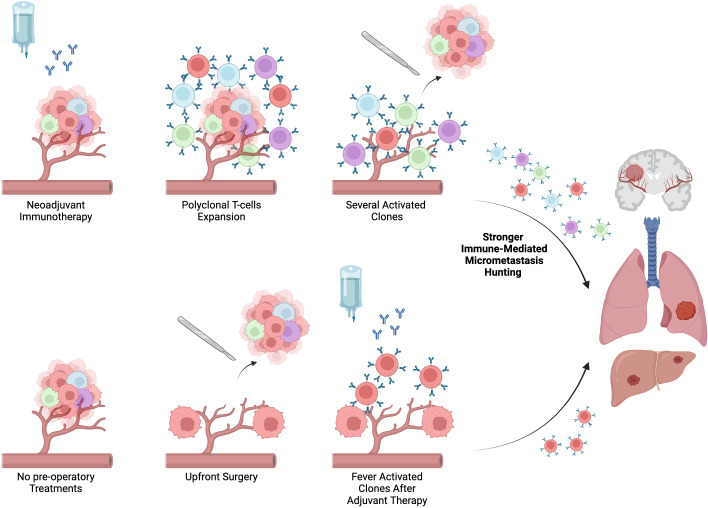
Immunotherapy has been reported to be biologically more effective as neoadjuvant rather than adjuvant treatment (figure 2). In preclinical models, CD8+T cells levels were significantly higher in both peripheral blood and organs, demonstrating stronger T-cell proliferation in the pre-surgical stage. One of the reasons potentially implicated in these results is linked to a higher release of tumor-specific antigens when primary tumor cells are exposed to ICIs. This may induce a strong vaccine effect on the immune system inducing an expansion of intratumoral tumor-specific T cells before they are released into the periphery.21 This evidence was confirmed in the clinical setting: Blank et al 22 showed the improved effect of neoadjuvant administration immunotherapy compared with adjuvant immunotherapy in melanoma.21
Figure 2.
Biologic rational for the use of immune checkpoint inhibitors in neoadjuvant setting compared with upfront surgery and adjuvant chemotherapy APC, antigen presenting cell; CAF, cancer-associated fibroblasts; CTLA-4, cytotoxic T-lymphocyte antigen 4; IDO1, indoleamine 2,3-dioxygenase; LAG-3: lymphocyte-activation gene 3; MDSC, myeloid-derived suppressor cells; MHC-I, major histocompatibility complex class I; PD-1, programmed death 1; PD-L1, programmed death-ligand 1; TAM, tumor-associated macrophages; TCR, T-cell receptor; TIGIT, T-cell immunoreceptor with Ig and ITIM domains.
Immunotherapy has been extensively studied in GI cancers, particularly after the identification of distinct subsets based on microsatellite status. Microsatellite instability (MSI) is a condition defined by hypermutability of the repetitive sequences scattered along the genome, called microsatellites. This condition is an expression of an underlying dMMR, which translates into genome-wide hypermutability.23 dMMR cancers harbor up to 100 times more mutations than the mismatch repair proficient (pMMR) ones, resulting in a highly immunogenic profile.6 In support of this hypothesis is the finding that MSI-high (MSI-H) cancers present a much higher number of infiltrating lymphocytes as well as intratumoral expression of immune checkpoints as a mechanism to inhibit immune-mediated tumor killing.24 Therefore, MSI-H cancers have been hypothesized to be more sensitive to immune system reactivation through ICIs. For those reasons, distinctions should be made between pMMR/microsatellite stable (MSS) and dMMR/MSI-H cancers in relation to both immunogenicity and chemo-refractoriness.
Neoadjuvant ICIs in radically resectable locally advanced lower-GI cancers
Rectal cancer
Treatment of locally advanced rectal cancer (LARC) requires a multidisciplinary approach with NAT. Conventional short-course radiotherapy (SCRT) (five fractions of 5 Gy over 5 days) or concomitant long-course CRT and delayed surgery lead approximately 10% of patients to obtain a pCR10 25 at surgical resection. In the case of total neoadjuvant treatment (TNT), that is a combination of systemic CT and CRT, an increased response rate (RR) was observed, with up to 30% of pCR according to prospective trials.26 27 Recently, a watch and wait strategy has been proposed for selected patients who obtained a complete clinical (radiological/endoscopic) tumor response after NAT reserving rescue surgery in case of local recurrence.28 Of course, this conservative treatment is particularly acceptable for the subset of patients with distal LARC to avoid colostomy. Therefore, novel therapeutic strategies to improve the complete response (CR) to allow a non-operative management allowing the organ preservation and reduce the risk of local/distal recurrence are warranted.
dMMR/MSI-H rectal cancer
ICIs are the standard of care for dMMR/MSI-H metastatic CRC and lead to deep and durable response.1 2 29 30 Thus, there is a strong rationale for the use of ICIs in earlier settings including LARC (table 1).
Table 1.
Completed studies of neoadjuvant immunotherapy in colorectal cancer
| Trial number | Phase | Sample size | Treatments | Outcomes |
| NCT04165772 | Single arm phase II | 12 | Dostarlimab9 cycles > CRT > Surgery |
CR: 100% |
| NCT04304209 | Single arm phase II | 17 | Sintilimab 4 cycles > sintilimab 4 cycles > W&W/surgery Or Sintilimab 4 cycles > surgery > CAPOX +/− sintilimab |
CR: 75% PD: 6% |
| Xaio et al | Retrospective | 73 | ICIs > surgery ICIs > W&W |
ORR: 84.9% (CR: 23.3%; PR: 61.6%) pCR: 57% |
| Zhang et al | Retrospective | 32 | ICIs > surgery ICIs > W&W |
cCR: three patients MPR: 86.2% pCR:75% |
| Trojan et al | Case report | 1 | Nivolumab + ipilimumab 1 cycles | CR: 100% |
| Averectal (NCT03503630) | Single arm phase II | 44 | SCRT > FOLFOX + avelumab 6 cycles > surgery | pCR: 37.5% |
| NCT04231552 | Single arm phase II | 30 | SCRT> CAPOX + camrelizumab 2 cycles > surgery | pCR: 100% (MSI-H) pCR: 46.2% (MSS) |
| TORCH (NCT04518280) | Randomized non-comparative phase II study | 11 | SCRT > CAPOX + camrelizumab 6 cycles > surgery/W&W Or CAPOX + camrelizumab 2 cycles > SCRT > CAPOX + camrelizumab 4 cycles > surgery/W&W |
cCR: two patients pCR: 77.8% |
| AVANA (NCT03854799) |
Single arm phase II | 101 | CRT + avelumab > avelumab (up to 6 cycles) > surgery | pCR: 22% (ITT population) pCR: 8% (MSS) |
| VOLTAGE-A (NCT02948348) |
Single arm phase II | 42 | CRT + nivolumab > nivolumab (up to 5 cycles) > surgery | pCR: 60% (MSI-H) pCR: 30% (MSS) |
| PANDORA (NCT04083365) |
Single arm phase II | 26 | CRT > durvalumab 3 cycles > surgery | pCR: 50% |
| NCT04911517 | Single arm phase II | 26 | CRT + tislelizumab > surgery | pCR: 50% |
| NRG-GI002 (NCT02921256) |
Randomized phase II | 180 | FOLFOX 4 cycles > CRT +/− pembrolizumab | pCR: 31.9% (pembrolizumab arm) pCR: 29.4% (control arm) |
| NICHE (NCT03026140) |
Single arm phase II | 35 | Nivolumab + ipilimumab +/− celecoxib > surgery | pCR: 60% (MSI-H) pPR: 27% (MSS) |
| NICHE 2 (EudraCT 016-002940-17) |
Single arm phase II | 112 | Nivolumab + ipilimumab > surgery | MPR: 95% pCR: 67% |
| PICC (NCT03926338) |
Non-comparative randomized phase II study | 34 | Toripalimab ± celecoxib > surgery toripalimab (6 months perioperative treatment) |
pCR: 88% (toripalimab+celecoxib) pCR: 65% (toripalimab) |
| NICOLE (NCT04123925) |
Single arm phase II | 22 | Nivolumab 2 cycles > surgery | MPR: 15.8% (MSS) MPR: 0% (MSI-H) |
| Pei et al | Case series | 11 | ICIs > surgery | MPR: 100% PCR: 90.9% |
cCR, clinical complete response; CR, complete response; CRT, chemoradiotherapy; ICIs, immune check-point inhibitors; ITT, intention-to-treat; MPR, major pathological response; MSI-H, microsatellite instability-high; MSS, microsatellite stable tumors; ORR, overall response rate; pCR, pathological complete response; PD, progressive disease; SCRT, short-course radiotherapy; W&W, watch and wait.
The frequency of dMMR/MSI-H status is lower in rectal cancer (RC) compared with colon cancer (CC). In a large population including more than 5000 cases of RC dMMR/MSI-H was observed in 2.7% of tumor samples (147/5547).31 In this scenario, Cercek reported impressive results of NAT with the anti-PD-1 dostarlimab in a population of dMMR/MSI-H LARC.32 Overall, 12 patients with stage II/III MSI-H were enrolled and were candidate to receive 6 months of dostarlimab followed by CRT and surgery. In the case of clinical CR (cCR) after dostarlimab CRT and surgery could be avoided. Fascinatingly, at the time of the first report all 12 patients obtained a cCR after 6 months of dostarlimab and no sign of recurrence was observed at the first data cut-off. The trial is ongoing and still recruiting, and mature data with a longer follow-up are awaited. Similarly, NAT with four sintilimab was investigated in a population of Chinese patients with dMMR LARC.33 Overall, six patients were treated with radical surgery, in no cases RT or CT was administered. Remarkably, clinical, or pCR was observed in 12 out 16 patients (75%), only in one case (6%) tumor enlargement was reported. In a case series of LA MSI-H CRC, 73 patients were treated with neoadjuvant ICIs. In the subgroup with rectal cancer 17/18 (94.4%) had pCR or partial response (PR).34 All together, these findings strongly support the use of neoadjuvant ICIs in dMMR/MSI-H LARC with organ sparing in most cases, reserving surgery in case of persistent/progressive disease. The major limitation of these prospective/retrospective studies is the small number of patients included and relatively short follow-up, therefore larger prospective studies and longer follow-up are warmly waited (table 2).
Table 2.
Principal ongoing studies of neoadjuvant immunotherapy in localized colorectal cancer
| Trial number | Phase | Sample size | Treatment | Outcome (primary endpoint) |
| PEMREC (NCT04109755) |
Single arm phase II study | 25 | SCRT + pembrolizumab (4 cycles) > surgery | pCR |
| TARZAN (NCT04017455) |
Single arm phase II study | 38 | SCRT > atezolizumab + bevacizumab > surgery | cCR |
| NCT04443543 | Non-comparative randomized phase II study | 222 | CRT (FOLFIRINOX OR XELIRI) > surgery/W&W (MSS) Or CRT (FOLFIRINOX OR XELIRI) > tislelizumab > surgery/W&W |
cCR |
| NCT05731726 | Single arm phase II study | 50 | CAPEOX + serpililumab > surgery | pCR |
| NCT03921684 | Single arm phase II study | 29 | CRT > FOLFOX + nivolumab > surgery | pCR TRAEs |
| N-PRC (NCT05576480) |
Single arm phase II study | 55 | SCRT + penpulimab > penpulimab + CAPOC (4 cycles) > surgery | pCR |
| NCT05752136 | Randomized phase II study | 102 | SCRT > CAPOX > surgery Or SCRT > CAPOX + envafolimab > surgery |
pCR |
| (OPTICAL-2) NCT05571644 |
Randomized phase II study | 82 | FOLFOXIRI + cadonilimab > surgery Or FOLFOX > surgery |
pCR |
| NCT04621370 | Non-comparative randomized phase II study | 48 | Durvalumab > SCRT > FOLFOX + durvalumab > surgery Or Durvalumab + CRT > FOLFOX + durvalumab > surgery |
pCR/cCR |
| BASKET (NCT04643041) |
Single arm phase II study | 47 | Anti PD-1 (6 cycles) > W&W | 1-year DFS rate |
| NCT04663763 | Single arm phase II study | 40 | SCRT > CAPOX + sintilimab > surgery | pCR |
| NCT05215379 | Phase II randomized study | 180 | CRT Or CRT+xintilimab > xintilimab (up to 4 cycles) |
cCR |
| NCT05507112 | Single arm phase II study | 100 | Tislelizumab + CRT | pCR |
| NCT04357587 | Single arm phase II study | 10 | Pembrolizumab + CRT >surgery |
TRG Safety |
| TORCH (NCT04518280) | Randomized non-comparative phase II study | 130 | SCRT > CAPOX + camrelizumab 6 cycles > surgery/W&W Or CAPOX + camrelizumab 2 cycles > SCRT > CAPOX + camrelizumab 4 cycles > surgery/W&W |
CR |
| PICC (NCT03926338) |
Non-comparative randomized phase II study | 69 | Toripalimab ± celecoxib > surgery toripalimab (6 months perioperative treatment) |
pCR |
| NAIO (NCT05239546) |
Single arm phase II study | 25 | Dostarlimab 12 cycles | MCR |
| NCT04625803 | Single arm phase II study | 64 | FOLFOX + camrelizumab 6 cycles > apatinib 2 months > surgery | TRG |
| NCT05662527 | Single arm phase II study | 85 | Pembrolizumab 1 cycle > surgery | pCR |
| NCT04231526 | Non-comparative randomized phase II study | 46 | Pembrolizumab 2 cycles > surgery Or Surgery |
Feasibility of neoadjuvant treatment |
| NCT05202314 | Single arm phase II study | 20 | Camrelizumab+ FOLFOX (3 cycles)/CAPOX (2 cycles) > surgery |
pCR |
. cCR, clinical complete response; CRT, chemoradiotherapy; DFS, disease-free survival; MCR, major clinical response; MSI-H, microsatellite instability; MSS, microsatellite stable tumors; NR, not reported; pCR, pathological complete response; PD-1, programmed cell death 1 ; SCRT, short course radiotherapy; TRAE, treatment-related adverse events; TRG, tumor regression grade; TRG, tumor regression grade; W&W, watch and wait.
pMMR/MSS rectal cancer
To date, ICIs demonstrated limited efficacy in advanced MSS CRC, therefore novel and more effective combinatory strategies are under investigation.35 It has been shown that RT could elicit an immune modulatory effect. The “abscopal effect” is a rare phenomenon described for the first time in 1953.36 It consists of tumor regression in a site distant from the field of irradiation. This may be due to the reactivation of the host immune response against cancer cells. Indeed, RT can increase the expression of major histocompatibility complex class I on cell membranes, and, thus, can improve antigen presentation by dendritic cells with a strong immune activation and subsequent immunogenic cell death.37 Therefore, there is a strong rationale for combining RT and ICIs. AVERECTAL is a phase II single-arm study investigating the role of SCRT, followed by six cycles of mFOLFOX-6 plus the anti PD-L1 avelumab and total mesorectal excision as TNT for LARC.38 Final results showed that 37.5% of patients achieved a pCR, while another 30% had a near-CR. Similar results were reported by two phase II study investigating SCRT followed by CAPOX plus the anti PD-1 camrelizumab or toripalimab and delayed surgery.31 39
A different treatment strategy is represented by a combination of CRT with immunotherapy. In the phase II AVANA trial, addressing the combination of avelumab plus CRT in patients with LARC, 23% of patients had a pCR and 61.5% a major pathological response (MPR).40 In the PANDORA study, 55 patients with LARC received CRT followed by three cycles of durvalumab and surgery with a CR of 32.7%.41 Conversely, negative results came up from the NRG-GI002 trial, in which 185 patients with LARC were treated with four cycles of FOLFOX followed by CRT with/without pembrolizumab before surgery42
Colon cancer
Over the last two decades, conventional treatment of localized CC has been represented by radical surgery followed by 6 months of adjuvant oxaliplatin-based CT for patients with stage III.43 In this clinical scenario, the use of neoadjuvant chemotherapy (NAC) in resectable localized CC has been investigated in the FOxTROT study.44 Patients with T3/T4 (N0-2) CC were randomly assigned to receive 6 weeks of NAC (plus panitumumab in the Rat Sarcoma Virus (RAS) wild type (wt) subgroup) followed by surgery and adjuvant CT or the standard approach. Despite initial negative results, in the final analysis the study demonstrated an improvement in 2-year recurrence rate, with significant tumor downstaging and histologic regression after NAC.
dMMR/MSI-H colon cancer
According to the FOxTROT study, low activity was observed in the MSI-H subgroup of patients, with only 7% of RR after NAC. The NICHE trial was the first exploratory study that investigated ICIs as NAT in early-stage CC.45 Patients with MSI-H and MSS CC received one administration of ipilimumab and two of nivolumab before surgery. Remarkably, at histological examination a pathological response was observed in the 100% of the 20 dMMR cancers, with 60% pCR. By contrast in the MSS population, only 4 out of 15 patients (27%) exhibited pathological responses, with three near complete and one pathologic PR. Subsequently, at the 2022 ESMO annual meeting, the results of the NICHE II study were presented.46 A total of 112 patients with dMMR localized CC were treated with a short immunotherapy induction consisting, as in the NICHE 1, of one administration of ipilimumab and two of nivolumab followed by surgery within 6 weeks. Pathological response was observed in 106/107 (99%) patients, with 67% of pCR, 95% of MPR and no disease recurrence after a median follow-up of 13.1 months.
In the PICC trial, 34 patients with MSI-H LA CRC were randomly assigned to receive the anti PD-1 toripalimab (with or without celecoxib) followed by surgery and adjuvant therapy.47 Outstandingly, 15 of 17 patients (88%) in the toripalimab plus celecoxib group and 11 of 17 patients (65%) in the toripalimab monotherapy group showed pCR.
pMMR/MSS colon cancer
Limited evidence is available for ICIs as NAT in MSS CC. In the NICOLE trial, 19 patients with MSS CC (plus 3 MSI-H) received two cycles of nivolumab followed by surgery.48 MPRs were observed in three pMMR tumors, including one CR. Translational analysis showed a significant increase in lymphocytes infiltration on surgical samples compared with a baseline tumor biopsy. Recently, the promising activity of the combination of NAT with the next generation of ICIs botensilimab plus balstilimab has been reported in patients with MSS CC.49
Neoadjuvant ICIs in radically resectable locally advanced upper-GI cancers
Esophageal and gastric cancer
Less than 40% of patients with EGC are diagnosed at an early stage and could be potentially cured by surgery. However, the cure rate by means of surgery alone remains limited and perioperative treatments have been implemented. Currently, the standard of care in western countries relies on the use of FLOT regimen (fluorouracil + leucovorin + oxaliplatin + docetaxel) which improved OS compared with ECF/ECX regimens (epirubicin, cisplatin and 5-fluorouracil/capecitabine).8 Another option is represented by CRT. The CROSS trial reported a clear benefit in OS for patients treated with preoperative CRT (concomitant CRT with carboplatin and paclitaxel) compared with surgery alone, with median OS 49.4 versus 24 months and 5 years OS 47% versus 34%, respectively.9 According to preclinical data, NAT with ICIs seems to increase the number of tumor-specific lymphocytes compared with adjuvant therapy, due to the presence of the primary tumor. Moreover, the concurrent administration of CT and/or RT seems to carry an immunomodulatory effect, inducing upregulation of PD-L1 in the tumor microenvironment.19 50 However, it is important to notice that, since EGC did not show a strong immunogenic signature, ICIs have been tested in combination associated with a perioperative standard of care and this poses questions regarding the best treatment companion for ICIs and the most proper timing of introduction (table 3).
Table 3.
Completed and ongoing trial of neoadjuvant immunotherapy in esophageal and gastric cancer
| Histology | Trial number | Ph | Treatment | Sample size | Outcome (pCR rate) |
Status |
| ESCC | NCT05357846 | 3 | Tislelizumab/aclitaxel/cisplatin/radiation | 422 | NR | Ongoing |
| ESCC | NCT05043688 | 2 | Camrelizumab/albumin paclitaxel/carboplatin/radiation | 204 | NR | Not yet recruiting |
| ESCC | NCT04974047 | 2 | Tislelizumab/paclitaxel/cisplatin/radiation | 70 | NR | Ongoing |
| ESCC | NCT04973306 | 2/3 | Tislelizumab/paclitaxel/carboplatin/radiation | 176 | NR | Ongoing |
| ESCC | NCT04776590 | 2 | Tislelizumab/albumin paclitaxel/caboplatin/radiation | 30 | 46.7% | Ongoing |
| ESCC | NCT04644250 | 2 | Toripalimab/paclitaxel liposome/carboplatin/radiation | 32 | NR | Ongoing |
| ESCC | NCT04568200 | 2 | Durvalumab/paclitaxel/carboplatin/radiation | 60 | NR | Ongoing |
| ESCC | NCT04435197 | 2 | Pembrolizumab/carboplatin/paclitaxel/radiation | 143 | NR | Ongoing |
| EGJAC | NCT03544736 | 1/2 | Nivolumab/paclitaxel/carboplatin/radiation | 30 | NR | Ongoing |
| EGJAC | NCT03064490 | 2 | Pembrolizumab/paclitaxel/carboplatin/radiation | 38 | 35.7% | Completed |
| ESCC | NCT04006041 | 2 | Toripalimab/paclitaxel/cisplatin/radiation | 44 | 50% | Completed |
| ESCC | NCT05244798 | 3 | Sintilimab/albumin paclitaxel/carboplatin±radiation | 420 | NR | Not yet recruiting |
| ESCC | NCT05355168 | 1/2 | Camrelizumab/nimotuzumab/carboplatin/paclitaxel/radiation | 57 | NR | Ongoing |
| GAC/ EGJAC |
NCT03776487 | 1/2 | Ipilimumab/nivolumab/5-fluorouracil/oxaliplatin/radiation | 36 | NR | Ongoing |
| EAC/ EGJAC |
NCT03087864 | 2 | Atezolizumab/carboplatin/paclitaxel/radiation | 40 | 30.3% | Completed |
| EAC/ EGJAC | NCT02962063 | 1/2 | Durvalumab/tremelimumab/carboplatin/paclitaxel/radiation (induction FOLFOX 2 cycles) | 78 | 22.2% | Ongoing |
| Durvalumab/tremelimumab/5-fluorouracil or capecitabine/oxaliplatin/radiation (induction FOLFOX 2 cycles) | NR | |||||
| ESCC | NCT05476380 | 2 | Camrelizumab/paclitaxel/cisplatin | 39 | 17.6% | Completed |
| ESCC | NCT05302011 | 2 | Pembrolizumab/docetaxel/carboplatin or cisplatin | 30 | NR | Ongoing |
| ESCC | NCT05281003 | 2 | Pembrolizumab/paclitaxel/cisplatin | 128 | NR | Ongoing |
| ESCC | NCT05213312 | 2/3 | Nivolumab/paclitaxel or 5-fluorouracil/cisplatin | 90 | NR | Ongoing |
| ESCC | NCT05189730 | 2 | Tislelizumab/paclitaxel/cisplatin | 80 | NR | Ongoing |
| ESCC | NCT05182944 | 2 | Camrelizumab/albumin paclitaxel/cisplatin | 130 | 35.8% | Ongoing |
| ESCC | NCT05174325 | 2 | Sintilimab/albumin paclitaxel/cisplatin | 30 | NR | Ongoing |
| ESCC | NCT05050760 | NA | Camrelizumab/oxaliplatin/docetaxel/tegafur | 55 | NR | Ongoing |
| ESCC | NCT04848753 | 3 | Toripalimab/paclitaxel/cisplatin | 663 | NR | Ongoing |
| EGJAC | NCT04813523 | 2 | Pembrolizumab/5-fluorouracil/cisplatin | 30 | NR | Ongoing |
| ESCC | NCT04807673 | 3 | Pembrolizumab/paclitaxel/cisplatin | 342 | NR | Ongoing |
| ESCC | NCT04804696 | 2 | Toripalimab/paclitaxel/cisplatin | 53 | NR | Ongoing |
| ESCC | NCT04506138 | 2 | Camrelizumab/albumin paclitaxel/carboplatin | 46 | 21.6% | Completed |
| ESCC | NCT04460066 | 1b/2 | Socazolimab or placebo/albumin paclitaxel/cisplatin | 70 | 41.4 vs 27.6% | Completed |
| ESCC | NCT04389177 | 2 | Pembrolizumab/carboplatin/paclitaxel | 50 | 41.4% | Ongoing |
| ESCC | NCT04280822 | 3 | Toripalimab or placebo/paclitaxel/cisplatin | 400 | 15.7 vs 3.2% | Ongoing |
| GAC/ EGJAC | NCT04221555 | 2 | Durvalumab/docetaxel/oxaliplatin/S-1 | 68 | 29% | Ongoing |
| ESCC | NCT03946969 | 1/2 | Sintilimab/liposomal paclitaxel/cisplatin/S-1 | 30 | 20% | Completed |
| ESCC | NCT03917966 | 2 | Camrelizumab/docetaxel/nedaplatin | 40 | 39.6% | Completed |
| EGJAC | NCT04757363 | 2 | Nivolumab/regorafenib/oxaliplatin/5-fluorouracil | 35 | N.A. | Completed |
| ESCC | NCT04666090 | 2 | Carrelizumab/albumin paclitaxel/nedaplatin/apatinib | 42 | NR | Ongoing |
| ESCC | ChiCTR2100045659 | 2 | Sintilimab/albumin paclitaxel/cisplatin | 30 | 17% | Completed |
| ESCC | NCT03985670 | 2 | Toripalimab/paclitaxel/cisplatin | 30 | 20.8% | Completed |
| ESCC | ChiCTR1900026240 | 2 | Camrelizumab/albumin paclitaxel/carboplatin | 60 | 39.2% | Completed |
| ESCC | NCT04225364 | 2 | Camrelizumab/albumin paclitaxel/cisplatin | 56 | 31.4% | Completed |
| ESCC | ChiCTR2000037488 | 2 | Tislelizumab/albumin paclitaxel/carboplatin | 45 | 50% | Completed |
| EAC/GAC/ EGJAC | NCT03399071 | 2 | Avelumab/5-fluorouracil/oxaliplatin/docetaxel | 44 | 15% (prematurely closed) | Completed |
| EAC/ EGJAC | NCT03604991 | 2/3 | Nivolumab or nivolumab/CROSS or CROSS or nivolumab/ipilimumab | 278 | NR | Ongoing |
| EAC/ EGJAC |
NCT03784326 | 1 | Atezolizumab/5-fluorouracil/oxaliplatin | 40 | 11% | Ongoing |
| GAC/EGJAC | NCT02918162 | 2 | Pembrolizumab/capecitabine or 5-fluorouracil/oxaliplatin/(optional epirubicin) | 40 | 20.6% | Completed |
.EAC, esophageal adenocarcinoma; EGJAC, esophago-gastric junction adenocarcinoma; ESCC, esophageal squamous cell carcinoma; GAC, gastric adenocarcinoma; NA, not available; NR, not reported; pCR, pathologic complete response; ph, phase.
dMMR/MSI-H EGC
The remarkable sensitivity to ICIs in MSI-H cancers encouraged clinicians to assess their efficacy also in earlier settings of EGC. A subgroup analysis from the DANTE trial, showed that MSI-H cancers were more likely to achieve a pCR, with 46% compared with 24% of MSS cancers.51 Moreover, among MSI-H cancers those treated with FLOT + atezolizumab achieved complete or subtotal regression in 80% of cases, compared with 59% of those treated with FLOT alone. It has been reported that MSI status could negatively impact responsiveness to CT, particularly to fluoropyrimidines, as shown in the previous meta-analysis.52 Thus, investigating whether perioperative CT, and the related toxicity, can be avoided if replaced with ICIs has gained high clinical interest. Two phase II trials investigated this topic. The former, the GERCOR NEONIPIGA trial, enrolled patients with radically resectable EGC who underwent preoperative immunotherapy with nivolumab plus ipilimumab and adjuvant nivolumab.53 The primary endpoint was the pCR rate, with a threshold of 20% considered acceptable. Results showed a pCR rate of 58.6%, therefore meeting the primary endpoint. Of note three patients did not undergo surgery, one due to inclusion deviation as it was metastatic at diagnosis and two due to patient refusal. All three cases achieved CR as per radiological, endoscopic, and histologic assessment. The latter one, the INFINITY trial, is an ongoing phase II study of durvalumab + tremelimumab in radically resectable MSI-H EGC.54 The therapy is administered either as NAT (cohort 1) or as definitive treatment (cohort 2). Preliminary results from cohort 1 have recently been published and reported a pCR rate of 60% and an MPR rate of 80%. All patients who achieved pCR had negative circulating tumor DNA before surgery and none of the patients who received surgery experienced disease relapse at the time of this analysis. These results spurred the investigators to move forward to assess the non-operative approach in cohort 2, which is currently recruiting patients.
Eventually, the IMHOTEP trial is an ongoing phase II trial of MSI-H solid cancers, which aims to assess whether a limited number of cycles (one or two cycles) of pembrolizumab administered preoperatively may be sufficient to reach a higher pCR rate, compared with historical control.55 After the first preliminary analysis, a pCR rate of 38.9% in the overall population (colorectal, gastroesophageal, endometrial, and other cancers) has been reported, with a 25% rate in the gastroesophageal cohort. These data were lower than those previously reported, but it should be noticed that a high percentage of patients did not undergo surgery due to their own choice after reaching a cCR, potentially impacting the rate of pCR.
pMMR/MSS EGC
Several trials tested the combination of ICIs with the standard of care in the setting of biomarker unselected EGC. The addition of atezolizumab to FLOT in the DANTE trial was safe in terms of surgical morbidity and mortality and demonstrated an improvement in main pathological outcomes at an interim analysis. The clinical PR rate was higher in the atezolizumab group, both in the overall population (tumor regression grade (TRG) 1a 24% vs 15%) and in cancers with high PD-L1 (combined positive score (CPS) ≥10) (TRG1a 38% vs 14%). Accordingly, macroscopical downsizing favored atezolizumab addition compared with CT alone (pT0 23% vs 15%).56 Data regarding primary endpoint progression-free survival (PFS) are not yet available and will define whether pathological outcomes translate into improved clinical outcomes. Currently, two randomized phase III clinical trials (KEYNOTE-585 and MATTERHORN) are investigating the addition of anti-PD-1/PD-L1, pembrolizumab and durvalumab, respectively, to perioperative CT in gastric or junctional adenocarcinoma, respectively.57 58 Preliminary results of a prespecified analysis from the KEYNOTE-585 trial have recently been released. pCR rate, one of the primary endpoints was significantly increased with the addition of pembrolizumab, whereas event-free survival (EFS) did not show a statistically significant improvement and, therefore, median OS was not tested. Similarly, an interim analysis has recently shown the addition of durvalumab to preoperative FLOT regimen to improve pCR rate, a secondary endpoint. Data regarding the primary endpoint EFS, and the other secondary endpoint OS are still awaited. Recently, an Asian study reported preliminary outcomes of NAC plus camrelizumab versus NAC alone in ESCC.59 Co-primary endpoints were pCR rate and 5 years OS rate. Data for pathological outcomes showed an increase in pCR and MPR rates by the addition of camrelizumab to CT (27.8% vs 10% and 43.3% vs 26.7%, respectively), but survival outcomes and relationship to PD-L1 expression are not yet available. Likewise, the ongoing HCHTOG1909 clinical trial is investigating the role of toripalimab added to neoadjuvant CT versus CT alone in ESCC.60 The interim analysis reported around five times the pCR rate with the addition of toripalimab to CT (15.7% vs 3.2%). Considering the promising results reported in phase II and III trials, questions about the best modality to associate ICIs are rising. A Chinese trial has addressed this topic by randomizing patients to receive either two or four cycles of camrelizumab in association with NAC in ESCC. Results showed superiority in terms of RR and pCR rate for the four cycles group without a significantly higher rate of toxicities.61 Moving to CRT, few studies are currently assessing the addition of ICIs in EGC. The KEYNOTE-975 is an ongoing phase III trial that randomized patients with LA EGC to receive pembrolizumab plus definitive CRT versus definitive CRT alone.62 The phase II/III trial ECOG-ACRIN-2174 is ongoing and is investigating pCR rate and DFS of EGC treated with preoperative CRT according to CROSS regimen with or without concomitant nivolumab.63 After surgery, patients will be further randomized to adjuvant nivolumab with or without ipilimumab. So far, only toxicity data are available and the addition of nivolumab to CRT did not significantly increase the rate of side effects, without any new safety concerns.
Conclusions
Over the last years, ICIs have been introduced into the clinical practice in metastatic EGC and CRC and, recently, new insights were reported in early-stage disease. NAT is a standard treatment for radically resectable LA EGC and RC, and it provides an optimal setting for ICIs administration, due to wide neo-antigens, low tumor clonalities and a naïve immune system.64 However, besides the solid biological rationale, ICIs use is far from being set as the standard of care for NAT in unselected populations.
Due to considerable differences in biology and responses to treatments, in this review, we differentiated MSS from MSI-H cancers.65 Results obtained in the MSI-H-specific subgroup have been changing the treatment scenario of GI cancers, sparing CT, RT and even surgery, with a significant benefit in survival32 53 66 The current data are changing the clinical approach to manage patients with LA radically resectable EGC and RC, even though they are still immature to support a drastic change of clinical practice.
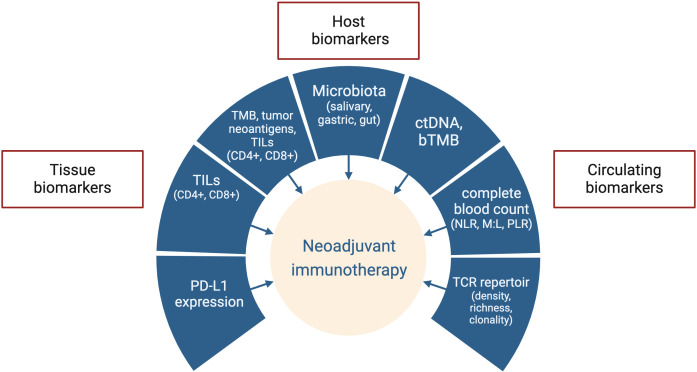
However, it is probably only a matter of time. If larger randomized clinical trials with a longer follow-up should confirm some initial impressive results in the MSI-H population, in LA EGC and RC we will assist in a de-escalation revolution. Nevertheless, it should be noted that even a proportion of MSI-H cancers did not benefit from ICIs treatment, and it needs to be rapidly investigated into details. Several biomarkers, both circulating and tissual, have been tested for their prognostic or predictive value in EGC, but results are not yet conclusive67 (figure 3). Moreover, some molecular features seem to negatively impact outcomes of MSI-H EGC as well as responses to PD-1 blockade and require further evaluations.68 69 Therefore, the implementation of molecular profiling could help to assess the real impact of concomitant mutations in MSI-H GI cancers.
Figure 3.
Biomarkers under investigation for neoadjuvant immune checkpoint inhibitors in gastrointestinal cancers. bTMB, blood-based tumor mutation burden; ctDNA, circulating tumor DNA; M:L, myeloid to lymphoid ratio; MSI, microsatellite instability; MMR, mismatch repair; NLR, neutrophil to lymphocyte ratio; PD-L1, programmed cell death-ligand 1; PLR, platelet to lymphocyte ratio; TCR, T-cell receptor; TILs, tumor infiltrating lymphocytes; TMB, tumor mutation burden.
Unfortunately, most GI cancers do not display a highly immunogenic profile, conversely to melanoma or lung cancer.70 Hence, the success of ICIs use as NAT in biomarker unselected patients remains far from being achieved.
In LARC, even though signals of synergistic clinical activity were observed, combining ICIs with multimodal NAT in MSS tumors produced fewer strickling results than those in the MSI-H subset. However, there are different crucial points to consider. First, it is still unknown what is the best NAT (SCRT followed by chemo-immunotherapy/immunotherapy vs CRT plus immunotherapy). Second, the identification of predictive biomarkers of response is required for improving patients’ selection and treatment efficacy. Third, randomized studies are needed to demonstrate the potential room for ICIs as a part of NAT in MSS LARC. Similar questions are present in LA EGC, another field where NAT is the current standard of care. The addition of ICIs to CT did not translate into a meaningful clinical benefit, as reported in the pivotal phase III KEYNOTE-585 trial. Several methodological and statistical considerations could be done to interpret those results, but at the bottom there is the failure of the “one size fits all” strategy. A reliable and precise stratification of patients according to clinical, pathologic, and molecular biomarkers is urgently required, to give the best personalized therapeutic option to any single patient. Additionally, further synergistic combinations could be worthwhile in this setting. Other signaling processes such as tumor angiogenesis play crucial a role in cancer progression and may be tackled by approved drugs,71 even though their role in NAT is not yet clinical practice. According to recent reports, the addition of the antiangiogenic agent apatinib to CT and ICIs improved pCR rate in a single-arm phase II trial of LAEGC.72 In conclusion, the addition of ICIs to NAT in GI cancers represents a thorny field for research since despite promising results, its widespread use was not associated with a global significant clinical benefit. Further strategies should be designed, rethinking the approach and trying to overcome the intrinsic low immunogenic profile of GI cancers.
Footnotes
Contributors: LGe: conceptualization, writing—original draft, writing—review and editing. DC: conceptualization, writing—original draft, writing—review and editing. RAO: writing—original draft, writing—review and editing. MB: writing—original draft, writing—review and editing. LGu: writing—original draft, writing—review and editing. LB: writing—review and editing. LA: writing—review and editing. FS: writing—review and editing. MGZ: writing—review and editing. CAC: writing—review and editing. NF: conceptualization, supervision, writing—review and editing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: DC received travel support from Sanofi, BMS and Merck KgA. NF declares Personal Financial Interests for ADACAP, Ipsen (Invited speaker), for ADACAP, Merck, MSD, Novartis, Pfizer, Boehringer (Advisory Board); Institutional Financial Interests: local PI of trials for 4SC, Astellas, Beigene, Fibrogen, Incyte, Ipsen, Nucana; research grants from ADACAP, Ipsen, MSD, Merck. Other Authors declare no conflict of interests.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. André T, Shiu K-K, Kim TW, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med 2020;383:2207–18. 10.1056/NEJMoa2017699 [DOI] [PubMed] [Google Scholar]
- 2. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (Checkmate 142): an open-label, Multicentre, phase 2. Lancet Oncol 2017;18:1182–91. 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janjigian YY, Shitara K, Moehler M, et al. First-line Nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, Gastro-Oesophageal junction, and Oesophageal adenocarcinoma (Checkmate 649): a randomised, open-label, phase 3 trial. Lancet 2021;398:27–40. 10.1016/S0140-6736(21)00797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of Pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial [published correction appears in JAMA Oncol. 2019 Apr 1;5(4):579]. JAMA Oncol 2018;4:e180013. 10.1001/jamaoncol.2018.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doki Y, Ajani JA, Kato K, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med 2022;386:449–62. 10.1056/NEJMoa2111380 [DOI] [PubMed] [Google Scholar]
- 6. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020;38:1–10. 10.1200/JCO.19.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Opdivo. n.d. Available: https://www.ema.europa.eu/en/medicines/human/EPAR/opdivo
- 8. Al-Batran S-E, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus Leucovorin, Oxaliplatin, and Docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (Flot4): a randomised, phase 2/3 trial. Lancet 2019;393:1948–57. 10.1016/S0140-6736(18)32557-1 [DOI] [PubMed] [Google Scholar]
- 9. van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074–84. 10.1056/NEJMoa1112088 [DOI] [PubMed] [Google Scholar]
- 10. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40. 10.1056/NEJMoa040694 [DOI] [PubMed] [Google Scholar]
- 11. Dahiya DS, Kichloo A, Singh J, et al. Current immunotherapy in gastrointestinal malignancies a review. J Investig Med 2021;69:689–96. 10.1136/jim-2020-001654 [DOI] [PubMed] [Google Scholar]
- 12. Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704. 10.1146/annurev.immunol.26.021607.090331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O’Donnell JS, Teng MWL, Smyth MJ. Cancer Immunoediting and resistance to T cell-based Immunotherapy. Nat Rev Clin Oncol 2019;16:151–67. 10.1038/s41571-018-0142-8 [DOI] [PubMed] [Google Scholar]
- 14. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 2011;331:1565–70. 10.1126/science.1203486 [DOI] [PubMed] [Google Scholar]
- 15. Mittal D, Gubin MM, Schreiber RD, et al. New insights into cancer Immunoediting and its three component phases--elimination, equilibrium and escape. Curr Opin Immunol 2014;27:16–25. 10.1016/j.coi.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fridman WH, Pagès F, Sautès-Fridman C, et al. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012;12:298–306. 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 17. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020;20:651–68. 10.1038/s41577-020-0306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of Ipilimumab in unresectable or metastatic Melanoma. J Clin Oncol 2015;33:1889–94. 10.1200/JCO.2014.56.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petricevic B, Kabiljo J, Zirnbauer R, et al. Neoadjuvant Immunotherapy in gastrointestinal cancers - the new standard of care. Semin Cancer Biol 2022;86:834–50. 10.1016/j.semcancer.2022.05.015 [DOI] [PubMed] [Google Scholar]
- 20. Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for Rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835–44. 10.1016/S1470-2045(10)70172-8 [DOI] [PubMed] [Google Scholar]
- 21. Liu J, Blake SJ, Yong MCR, et al. Improved efficacy of neoadjuvant compared to adjuvant Immunotherapy to eradicate metastatic disease. Cancer Discov 2016;6:1382–99. 10.1158/2159-8290.CD-16-0577 [DOI] [PubMed] [Google Scholar]
- 22. Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III Melanoma. Nat Med 2018;24:1655–61. 10.1038/s41591-018-0198-0 [DOI] [PubMed] [Google Scholar]
- 23. Luchini C, Bibeau F, Ligtenberg MJL, et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol 2019;30:1232–43. 10.1093/annonc/mdz116 [DOI] [PubMed] [Google Scholar]
- 24. Mlecnik B, Bindea G, Angell HK, et al. Integrative analyses of colorectal cancer show Immunoscore is a stronger Predictor of patient survival than Microsatellite instability. Immunity 2016;44:698–711. 10.1016/j.immuni.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 25. Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 2017;18:336–46. 10.1016/S1470-2045(17)30086-4 [DOI] [PubMed] [Google Scholar]
- 26. Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced Rectal cancer (RAPIDO): a randomised, open-label. Lancet Oncol 2021;22:29–42. 10.1016/S1470-2045(20)30555-6 [DOI] [PubMed] [Google Scholar]
- 27. Conroy T, Bosset J-F, Etienne P-L, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a Multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:702–15. 10.1016/S1470-2045(21)00079-6 [DOI] [PubMed] [Google Scholar]
- 28. Fernandez LM, São Julião GP, Figueiredo NL, et al. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International watch & wait database: a retrospective, international, multicentre registry study. Lancet Oncol 2021;22:43–50. 10.1016/S1470-2045(20)30557-X [DOI] [PubMed] [Google Scholar]
- 29. Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol 2020;38:11–9. 10.1200/JCO.19.02107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. André T, Lonardi S, Wong KYM, et al. Nivolumab plus low-dose Ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from checkmate 142. Ann Oncol 2022;33:1052–60. 10.1016/j.annonc.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 31. Lin Z, Cai M, Zhang P, et al. Phase II, single-arm trial of preoperative short-course radiotherapy followed by chemotherapy and camrelizumab in locally advanced rectal cancer. J Immunother Cancer 2021;9:e003554. 10.1136/jitc-2021-003554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cercek A, Lumish M, Sinopoli J, et al. PD-1 blockade in mismatch repair-deficient, locally advanced Rectal cancer. N Engl J Med 2022;386:2363–76. 10.1056/NEJMoa2201445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen G, Jin Y, Guan W-L, et al. Neoadjuvant PD-1 blockade with sintilimab in mismatch-repair deficient, locally advanced rectal cancer: an open-label, single-centre phase 2 study. Lancet Gastroenterol Hepatol 2023;8:422–31. 10.1016/S2468-1253(22)00439-3 [DOI] [PubMed] [Google Scholar]
- 34. Xiao B-Y, Zhang X, Cao T-Y, et al. Neoadjuvant Immunotherapy leads to major response and low recurrence in localized mismatch repair-deficient colorectal cancer. J Natl Compr Canc Netw 2023;21:60–6. 10.6004/jnccn.2022.7060 [DOI] [PubMed] [Google Scholar]
- 35. Ciardiello D, Vitiello PP, Cardone C, et al. Immunotherapy of colorectal cancer: challenges for therapeutic efficacy. Cancer Treat Rev 2019;76:22–32. 10.1016/j.ctrv.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 36. MOLE RH. Whole body irradiation; radiobiology or medicine Br J Radiol 1953;26:234–41. 10.1259/0007-1285-26-305-234 [DOI] [PubMed] [Google Scholar]
- 37. Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy: using Immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015;41:503–10. 10.1016/j.ctrv.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shamseddine A, Zeidan Y, Bouferraa Y, et al. SO-30 efficacy and safety of neoadjuvant short-course radiation followed by mFOLFOX-6 plus avelumab for locally-advanced rectal adenocarcinoma: averectal study. Annals of Oncology 2021;32:S215. 10.1016/j.annonc.2021.05.054 [DOI] [Google Scholar]
- 39. Wang Y, Xia F, Shen L, et al. Short-course radiotherapy combined with CAPOX and Toripalimab for the total Neoadjuvant therapy of locally advanced Rectal cancer: preliminary findings from a randomized, prospective, multicenter, double-arm, phase II trial (TORCH). JCO 2022;40:e15602. 10.1200/JCO.2022.40.16_suppl.e15602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Salvatore L, Bensi M, Corallo S, et al. Phase II study of preoperative (PREOP) chemoradiotherapy (CTRT) plus Avelumab (AVE) in patients (PTS) with locally advanced Rectal cancer (LARC): the AVANA study. JCO 2021;39:3511. 10.1200/JCO.2021.39.15_suppl.3511 [DOI] [Google Scholar]
- 41. Tamberi S, Grassi E, Zingaretti C, et al. A phase II study of Capecitabine plus concomitant radiation therapy followed by Durvalumab (Medi4736) as preoperative treatment in Rectal cancer: PANDORA study final results. JCO 2022;40:LBA3513. 10.1200/JCO.2022.40.17_suppl.LBA3513 [DOI] [Google Scholar]
- 42. Rahma OE, Yothers G, Hong TS, et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol 2021;7:1225–30. 10.1001/jamaoncol.2021.1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Argilés G, Tabernero J, Labianca R, et al. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020;31:1291–305. 10.1016/j.annonc.2020.06.022 [DOI] [PubMed] [Google Scholar]
- 44. Morton D, Seymour M, Magill L, et al. Preoperative chemotherapy for operable colon cancer: mature results of an international randomized controlled trial. J Clin Oncol 2023;41:1541–52. 10.1200/JCO.22.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant Immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020;26:566–76. 10.1038/s41591-020-0805-8 [DOI] [PubMed] [Google Scholar]
- 46. Chalabi M, Verschoor YL, van den Berg J, et al. Lba7 Neoadjuvant immune checkpoint inhibition in locally advanced MMR-deficient colon cancer: the NICHE-2 study. Ann Oncol 2022;33:S1389. 10.1016/j.annonc.2022.08.016 [DOI] [Google Scholar]
- 47. Hu H, Kang L, Zhang J, et al. Neoadjuvant PD-1 blockade with Toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol Hepatol 2022;7:38–48. 10.1016/S2468-1253(21)00348-4 [DOI] [PubMed] [Google Scholar]
- 48. Avallone A, De Stefano A, Pace U, et al. 491p Neoadjuvant Nivolumab in early stage colorectal cancer. Ann Oncol 2020;31:S449. 10.1016/j.annonc.2020.08.602 [DOI] [Google Scholar]
- 49. Kasi PM, Hidalgo M, Jafari MD, et al. Neoadjuvant botensilimab plus balstilimab response pattern in locally advanced mismatch repair proficient colorectal cancer [published online ahead of print]. Oncogene 2023;42:3252–9. 10.1038/s41388-023-02835-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jiang M, Hu Y, Lin G, et al. Radiotherapy combined with immune checkpoint inhibitors in locally advanced/metastatic esophageal squamous cell carcinoma: clinical trials, efficacy and future directions. Front Immunol 2023;14:1177085. 10.3389/fimmu.2023.1177085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Al-Batran S-E, Lorenzen S, Homann N, et al. 1429P pathological regression in patients with microsatellite instability (MSI) receiving perioperative Atezolizumab in combination with FLOT vs. FLOT alone for Resectable Esophagogastric adenocarcinoma: results from the DANTE trial of the German gastric group at the AIO and SAKK. Ann Oncol 2021;32:S1069. 10.1016/j.annonc.2021.08.1538 [DOI] [Google Scholar]
- 52. Cohen R, Taieb J, Fiskum J, et al. Microsatellite instability in patients with stage III colon cancer receiving fluoropyrimidine with or without oxaliplatin: an ACCENT pooled analysis of 12 adjuvant trials. J Clin Oncol 2021;39:642–51. 10.1200/JCO.20.01600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. André T, Tougeron D, Piessen G, et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the gercor neonipiga phase II study. JCO 2023;41:255–65. 10.1200/JCO.22.00686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pietrantonio F, Raimondi A, Lonardi S, et al. INFINITY: a multicentre, single-arm, multi-cohort, phase II trial of tremelimumab and durvalumab as neoadjuvant treatment of patients with microsatellite instability-high (MSI) resectable gastric or gastroesophageal junction adenocarcinoma (GAC/GEJAC). JCO 2023;41:358. 10.1200/JCO.2023.41.4_suppl.358 [DOI] [Google Scholar]
- 55. Coutzac C, Bibeau F, Ben Abdelghani M, et al. Immunotherapy in MSI/dMMR tumors in the perioperative setting: the IMHOTEP trial. Dig Liver Dis 2022;54:1335–41. 10.1016/j.dld.2022.07.008 [DOI] [PubMed] [Google Scholar]
- 56. Al-Batran S-E, Lorenzen S, Thuss-Patience PC, et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German gastric cancer group and Swiss SAKK. JCO 2022;40:4003. 10.1200/JCO.2022.40.16_suppl.4003 [DOI] [Google Scholar]
- 57. Bang Y-J, Van Cutsem E, Fuchs CS, et al. KEYNOTE-585: phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol 2019;15:943–52. 10.2217/fon-2018-0581 [DOI] [PubMed] [Google Scholar]
- 58. Janjigian YY, Van Cutsem E, Muro K, et al. MATTERHORN: phase III study of durvalumab plus FLOT chemotherapy in resectable gastric/gastroesophageal junction cancer. Future Oncol 2022;18:2465–73. 10.2217/fon-2022-0093 [DOI] [PubMed] [Google Scholar]
- 59. Zhang R, Song D sheng, Liu W, et al. Efficacy and safety of Camrelizumab combined with chemotherapy versus chemotherapy alone as preoperative Neoadjuvant therapy for Resectable locally advanced Esophageal squamous cell carcinoma: preliminary results from a multicenter, prospective, randomized controlled study. JCO 2023;41:4064. 10.1200/JCO.2023.41.16_suppl.4064 [DOI] [Google Scholar]
- 60. Zheng Y, Liu X-B, Sun H-B, et al. A phase III study on Neoadjuvant chemotherapy versus Neoadjuvant Toripalimab plus chemotherapy for locally advanced Esophageal squamous cell carcinoma: Henan cancer hospital Thoracic oncology group 1909 (Hchtog1909). Ann Transl Med 2021;9:73. 10.21037/atm-20-5404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang Q, Cao G, Fan Z. Two cycles versus four cycles of neoadjuvant camrelizumab plus chemotherapy in patients with locally advanced Esophageal squamous cell carcinoma (ESCC): a prospective, multicenter and randomized study. JCO 2023;41:350. 10.1200/JCO.2023.41.4_suppl.350 [DOI] [Google Scholar]
- 62. Shah MA, Bennouna J, Doi T, et al. KEYNOTE-975 study design: a phase III study of definitive chemoradiotherapy plus pembrolizumab in patients with Esophageal carcinoma. Future Oncol 2021;17:1143–53. 10.2217/fon-2020-0969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eads JR, Weitz M, Catalano PJ, et al. A phase II/III study of perioperative nivolumab and ipilimumab in patients (Pts) with Locoregional Esophageal (E) and gastroesophageal junction (GEJ) adenocarcinoma: results of a safety run-in—A trial of the ECOG-ACRIN cancer research group (Ea2174). JCO 2021;39:4064. 10.1200/JCO.2021.39.15_suppl.4064 [DOI] [Google Scholar]
- 64. McGranahan N, Furness AJS, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016;351:1463–9. 10.1126/science.aaf1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamamoto H, Imai K. Microsatellite instability: an update. Arch Toxicol 2015;89:899–921. 10.1007/s00204-015-1474-0 [DOI] [PubMed] [Google Scholar]
- 66. Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch repair deficiency, Microsatellite instability, and survival: an exploratory analysis of the medical research Council adjuvant gastric Infusional chemotherapy (MAGIC) trial [published correction appears in JAMA Oncol. 2022 Sep 1;8(9):1359]. JAMA Oncol 2017;3:1197–203. 10.1001/jamaoncol.2016.6762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gervaso L, Pellicori S, Cella CA, et al. Biomarker evaluation in radically resectable locally advanced gastric cancer treated with neoadjuvant chemotherapy: an evidence reappraisal. Ther Adv Med Oncol 2021;13:17588359211029559. 10.1177/17588359211029559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chida K, Kawazoe A, Kawazu M, et al. A low tumor mutational burden and PTEN mutations are predictors of a negative response to PD-1 blockade in MSI-H/dMMR gastrointestinal tumors. Clin Cancer Res 2021;27:3714–24. 10.1158/1078-0432.CCR-21-0401 [DOI] [PubMed] [Google Scholar]
- 69. Gervaso L, Bottiglieri L, Meneses-Medina MI, et al. Role of microsatellite instability and Her2 positivity in locally advanced Esophago-gastric cancer patients treated with peri-operative chemotherapy. Clin Transl Oncol 2023;25:3287–95. 10.1007/s12094-023-03179-5 [DOI] [PubMed] [Google Scholar]
- 70. Li K, Luo H, Huang L, et al. Microsatellite instability: a review of what the oncologist should know. Cancer Cell Int 2020;20:16. 10.1186/s12935-019-1091-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li S, Xu Q, Dai X, et al. Neoadjuvant therapy with immune checkpoint inhibitors in gastric cancer: a systematic review and meta-analysis. Ann Surg Oncol 2023;30:3594–602. 10.1245/s10434-023-13143-w [DOI] [PubMed] [Google Scholar]
- 72. Li S, Yu W, Xie F, et al. A prospective, phase II, single-arm study of neoadjuvant/conversion therapy with camrelizumab, apatinib, S-1 ± Oxaliplatin for locally advanced Ct4A/bN+ gastric cancer. JCO 2021;39:4061. 10.1200/JCO.2021.39.15_suppl.4061 [DOI] [Google Scholar]