Abstract
OBJECTIVE
Type 2 diabetes (T2D) increases the risk for major adverse liver outcomes (MALOs), including cirrhosis and its complications. Patients with T2D frequently have other traits of the metabolic syndrome (MetS). It remains uncertain whether there is a synergistic effect of accumulating MetS traits on future MALO risk.
RESEARCH DESIGN AND METHODS
Patients with T2D without a history of liver disease were identified from national registers in Sweden from 1998 to 2021. MetS traits included hypertension, low HDL level, hypertriglyceridemia, obesity, and albuminuria, in addition to T2D. MALO events were identified based on administrative coding from national registers until 31 October 2022. Data were analyzed using Cox regression models.
RESULTS
In total, 230,992 patients were identified (median age 64 years; 58% male), of whom 3,215 (1.39%) developed MALOs over a median follow-up of 9.9 years. Compared with patients with one MetS trait (only T2D) at baseline, those with more than one MetS trait had a higher rate of MALOs (adjusted hazard ratio [aHR] 2.33, 95% CI 1.53–3.54). The rate of MALOs increased progressively with increasing numbers of MetS traits at baseline (aHR 1.28 per added trait, 95% CI 1.23–1.33). During follow-up, patients who acquired additional MetS traits had a progressively higher rate of MALOs. The MetS trait with the largest association with incident MALOs was hypertension (aHR 2.06, 95% CI 1.57–2.71).
CONCLUSIONS
Having or acquiring additional traits of MetS increase the rate of progression to MALOs in patients with T2D. These results could be used to inform screening initiatives for liver disease.
Graphical Abstract
Introduction
The metabolic syndrome (MetS) is a cluster of metabolic abnormalities that may affect the liver. Metabolic dysfunction–associated steatotic liver disease (MASLD) (recently renamed from nonalcoholic fatty liver disease) is considered the hepatic phenotype of the MetS, affecting 38% of the global adult population (1,2) and >55% of patients with type 2 diabetes (T2D) (3). Patients with T2D have more than twice as high a risk of developing cirrhosis or liver cancer compared with the general population (4). Recognizing this high risk for cirrhosis among patients with T2D, several international guidelines now actively recommend screening for MASLD-associated liver fibrosis in patients with T2D. Nevertheless, the implementation of such guidelines in clinical practice is modest at best (5). Identifying subgroups of patients with T2D who have a particularly high risk might help narrow down the target group intended for directed casefinding initiatives and improve the effectiveness of screening efforts.
A more severe metabolic state is associated with a higher rate of progression to cirrhosis, as evidenced by a study involving patients with a diagnosis of MASLD within a U.S. Veterans Affairs cohort (6). This study revealed a gradual increase in cirrhosis and liver cancer risk with the presence of additional metabolic traits, with T2D exhibiting the strongest association. However, for patients with T2D who already are at a high risk, it remains unclear whether a similar pattern is present. Additionally, it is unclear if acquiring additional metabolic traits beyond a T2D diagnosis further amplifies this risk and which specific traits hold the strongest association with liver disease. Such information is important as most patients with MASLD will never develop liver-related outcomes (7). Hence, a risk-based approach to screening or treatment has been advocated for (8,9). That is, instead of solely aiming to diagnose MASLD or fibrosis, one considers the risk a patient has for future liver-related outcomes and tailors appropriate actions accordingly.
Here, we used high-quality Swedish national registers to investigate the association between the number and subtypes of MetS traits and the risk of developing MALOs in Swedish patients with T2D. Our hypothesis was that increasing numbers of MetS traits at or after a diagnosis of T2D would be associated with an increase in the risk of incident liver cirrhosis or complications thereof.
Research Design and Methods
Study Population
We used the Swedish National Diabetes Register (NDR) to identify patients with T2D. In NDR, T2D is defined using the following epidemiological definition: recorded T2D in clinical records, treatment with diet with or without oral antihyperglycemic agents, and treatment with oral antihyperglycemic agents with or without insulin (10). The NDR was initiated in 1996 and contains data for >90% of all patients aged >18 years with T2D in Sweden. This means that for patients diagnosed with T2D after 1996, their first registration in NDR is typically the time of their first diagnosis. The data are collected by trained nurses and physicians and include information on laboratory tests and medication use obtained from primary care and hospital outpatient clinics.
We included all patients recorded in the NDR between 1 January 1998 and 31 October 2021. The first entry date was defined as baseline. Patients were linked to several national registers using their personal identity number, which is assigned to all Swedish citizens (11). The National Patient Register (NPR) contains national coverage on hospital discharges since 1987 and contains data on outpatient visits in specialized care since 2001. It has a validity between 85 and 95%, depending on the diagnosis (12). Specifically, for hepatocellular carcinoma (HCC) and diagnoses related to cirrhosis, the validity rate ranges from 84 to 96% (13).
A total of 764,944 patients aged >18 years at the time of registration in NDR were eligible for inclusion (see flowchart in Supplementary Fig. 1). For the analysis on MetS traits to be comparable to each other, patients in the study sample needed to have information on the values for all MetS traits in this study. Therefore, 520,605 patients who had missing information on one or more of the MetS traits were excluded. We further excluded patients with preexisting MALOs and liver diseases other than MASLD based on ICD-10 codes and surgical procedure data from NPR. We also excluded patients with preexisting alcohol-related disease to minimize the impact of alcohol misuse. Since the use of ICD-10 codes began in 1997 in Sweden, we applied a look-back period of at least 1 year to identify preexisting diseases, with start of identification and follow-up from 1998. To reduce the potential impact of medications that may induce liver steatosis or fibrosis, we excluded patients who had ever used methotrexate or amiodarone prior to the index date from the Prescribed Drug Register. The Prescribed Drug Register was initiated in July 2005 and includes all drug dispensations at any Swedish pharmacy. Patients who emigrated before baseline were also excluded. Diagnostic, surgical, and Anatomical Therapeutic Chemical codes for all diagnoses and medications used in the study are listed in Supplementary Tables 1–3.
MetS and Other Variables
We used World Health Organization (WHO) 1998 criteria to define the traits of the MetS (14), a definition that has been endorsed by the NDR for identifying the MetS among patients with T2D (15). The WHO definition may be better suited for populations with T2D because unlike other available definitions of the MetS, the WHO definition specifically mandates the presence of T2D as a diagnostic requirement (14), which is central to the pathophysiology of the MetS. In addition to T2D, the traits of MetS in this definition include hypertension, obesity, hypertriglyceridemia, a low level of HDL, and albuminuria. These were defined based on laboratory test data from the NDR, diagnoses from the NPR, or dispensed medications from the Prescribed Drug Register. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, a recorded diagnosis of hypertension, or any dispensed antihypertensive medication. Obesity was determined as BMI ≥30 kg/m2. A high level of triglycerides was identified as plasma triglyceride levels ≥1.7 mmol/L. A low level of HDL was defined as <0.9 mmol/L for men and <1.0 mmol/L for women. Finally, albuminuria was determined as a urinary albumin excretion rate ≥20 μg/min or an albumin-to-creatinine ratio ≥30 mg/mmol. We also calculated the number of MetS traits for each patient, which ranged from one to six.
The following information from the NDR recorded at each clinical visit was also included: time since diabetes diagnosis (years), glycated hemoglobin A1c (HbA1c) (mmol/mol), weight (kg), height (m), total cholesterol (mmol/L), LDL (mmol/L), estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2), active smoking, and physical activity (walking or equivalent at least 30 min/day). Furthermore, the NDR contains information on glucose-lowering medications (defined as any oral medication or insulin), use of glucagon-like peptide 1 receptor agonists (GLP-1RAs), and lipid-lowering drugs but not on specific agents or doses. Besides this, clinical history of cardiovascular disease (CVD) (including myocardial infarct, hemorrhagic or ischemic stroke, and heart failure), chronic kidney disease (CKD), and other diseases included in the Charlson comorbidity index were obtained through linkage to the NPR (16). Hyperlipidemia was determined by recorded diagnosis in the NPR or any lipid-lowering drugs in the NDR or Prescribed Drug Register. Data on other medications, such as sodium–glucose cotransporter 2 (SGLT-2) inhibitors, were obtained from the Prescribed Drug Register.
MALOs
We linked the NDR data set to the NPR, the Swedish Cancer Register (SCR), and the Causes of Death Register (CDR) to identify liver disease outcomes between 1 January 1998 and 31 October 2022. Malignancies are documented in the SCR, which contains ∼96% of all diagnosed cancers in Sweden (17). The CDR contains data on causes of death for all Swedish citizens. It is mandatory for Swedish physicians to report the cause of death and any diagnosis that might have contributed to death as recorded the CDR (18). We used both primary and contributing diagnoses to identify outcomes in the NPR and CDR.
MALOs, as a composite outcome, were defined as having any of the diagnoses defined by ICD-10 codes or surgical procedures in registers. These diagnoses include liver cirrhosis, decompensated cirrhosis, and associated complications (defined as coding for chronic or unspecified hepatic failure, esophageal varices with or without bleeding, portal hypertension, hepatorenal syndromes, ascites, or liver transplant), HCC, or death from any of these (definitions listed in Supplementary Table 1). In general, these diagnoses result in contact with health care or in death, leading to a high capture rate of the outcome in the relevant registries. Since the positive predictive value for hepatic ascites is generally low, we excluded outcomes with ascites because of other diseases, such as heart failure or extrahepatic cancer, defined as present at or before the ascites diagnosis.
Statistical Analyses
Baseline characteristics of the study population are expressed as median and interquartile range (IQR) or frequency and percentage, as appropriate. The follow-up time started at the baseline date, and patients were followed until the first event of MALOs or were censored at development of any other liver disease than MASLD, emigration, death, or the end of the study period (31 October 2022), whichever occurred first. Incidence rates (per 1,000 person-years) were calculated as the number of events divided by total person-time at risk, displayed overall by the number of metabolic traits and by each individual trait in the MetS as measured at baseline. A Cox proportional hazards model was used to estimate hazard ratios (HRs) and 95% CIs of the association between the risk of MALOs and prevalent MetS by numbers and by individual traits, where follow-up time was used as the timescale. We reported results from two models: model 1 was a univariate model with only each respective trait in the MetS as the dependent variable, and model 2 was adjusted for all MetS traits, age at baseline, sex, duration of T2D, HbA1c, CKD, CVD, eGFR, LDL, smoking, hyperlipidemia, and glucose-lowering treatments, all recorded at baseline.
As the competing risk of non–liver-related mortality, e.g., cardiovascular death, is high in patients with T2D, we calculated cause-specific hazard rates of MALOs from a Cox regression model, considering non–liver mortality as a competing event. We did not use the Fine and Gray model since the subdistributional hazard derived from this does not have a straightforward interpretation and only gives the direction and not the strength of the association, which was our primary research question (19,20).
Statistical interaction between the main exposures (number and individual metabolic traits) and median age at baseline (age ≥65 or <65 years) and sex was tested separately. The associations between different combinations of baseline MetS traits and the risk of MALOs were estimated using HRs while adjusting for the same set of covariates at baseline. As patients with T2D may develop additional traits of the MetS during follow-up, we further examined the association between MetS and MALOs using time-varying Cox regression, considering both prevalent and incident metabolic traits. In brief, the time-varying Cox regression analyzed time to event with consideration of time-varying exposures by using all available data on MetS traits measured at multiple visits. We fitted two separate models, considering either the number or the different combinations of metabolic traits developed during follow-up as exposures, and reported corresponding HRs after adjusting for covariates at baseline. The cumulative incidence of MALOs by numbers of metabolic traits at baseline were calculated based on the Aalen-Johansen estimator, accounting for the competing risk of non–liver-related death (20).
A two-sided P < 0.05 was considered statistically significant, except in the case of interaction analysis, where P < 0.10 indicated the presence of a significant multiplicative interaction. All statistical analyses were performed using Stata MP 17.0 software (StataCorp).
Ethical Considerations
Ethics approval was granted from the ethical review board in Sweden (registration no. 2021-04422).
Data and Resource Availability
Requests of sharing deidentified data from this article will be considered on a case-by-case basis. A detailed proposal for how the data will be used is required, and a data access agreement must be signed upon request.
Results
We identified 230,996 patents with T2D between 1998 and 2021. The median age of patients was 63.6 (IQR 56.0–72.0) years, and 58.2% were men. Among all patients, 19.6% had albuminuria, 92.4% had hypertension, 49.4% had hypertriglyceridemia, 16.2% had low HDL levels, and 46.5% had obesity. The median BMI was 29.6 (IQR 26.5–33.4) kg/m2, and the median Charlson comorbidity index was 1 (IQR 1–1). There were 22.2% patients with preexisting CVD and 1.2% with CKD. Baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics of study population (N = 230,996)
| Characteristic | Complete case | Median (IQR) or n (%) |
|---|---|---|
| Age at index (years) | 230,996 | 63.6 (56.0–72.0) |
| Male | 230,996 | 134,329 (58.2) |
| Calendar year of the index date | 230,996 | |
| 1998–2003 | 18,547 (8.0) | |
| 2004–2009 | 99,100 (42.9) | |
| 2010–2015 | 56,995 (24.7) | |
| 2016–2021 | 56,354 (24.4) | |
| Metabolic traits | ||
| Albuminuria | 230,996 | 45,378 (19.6) |
| Hypertension | 230,996 | 213,376 (92.4) |
| Hypertriglyceridemia | 230,996 | 114,048 (49.4) |
| Low levels of HDL | 230,996 | 37,352 (16.2) |
| Obesity | 230,996 | 107,449 (46.5) |
| Other lifestyle variables | ||
| BMI (kg/m2) | 230,996 | 29.6 (26.5–33.4) |
| Overweight | 230,996 | 89,051 (38.6) |
| Physical activity | 163,523 | 143,298 (87.6) |
| Smoking | 209,888 | 30,630 (14.6) |
| Clinical information | ||
| Time since T2D diagnosis (years) | 221,018 | 1.0 (0.0–6.0) |
| CVD | 230,996 | 48,547 (21.0) |
| Hyperlipidemia | 230,996 | 120,170 (54.6) |
| CKD | 230,996 | 2,348 (1.0) |
| Charlson comorbidity index | 230,996 | 1 (1–1) |
| Pharmaceutical treatment | ||
| Any glucose-lowering drugs | 230,996 | 152,898 (66.2) |
| Metformin | 230,996 | 124,105 (53.7) |
| Insulin | 230,996 | 40,890 (17.7) |
| GLP-1RAs | 230,996 | 1,888 (0.8) |
| SGLT-2 inhibitors | 230,996 | 1,721 (0.7) |
| Laboratory values | ||
| eGFR (mL/min/1.73 m2) | 224,755 | 81.6 (68.1–96.6) |
| HbA1c (mmol/mol) | 228,460 | 50.0 (44.0–59.0) |
| HDL (mg/dL) | 230,996 | 1.2 (1.0–1.4) |
| LDL (mg/dL) | 226,943 | 2.9 (2.3–3.6) |
| Triglycerides (mg/dL) | 230,996 | 1.6 (1.2–2.3) |
| Urine albumin-to-creatinine ratio (mg/mmol) | 12,471 | 0.9 (0.4–2.8) |
Number of MetS Traits and Rate of MALOs
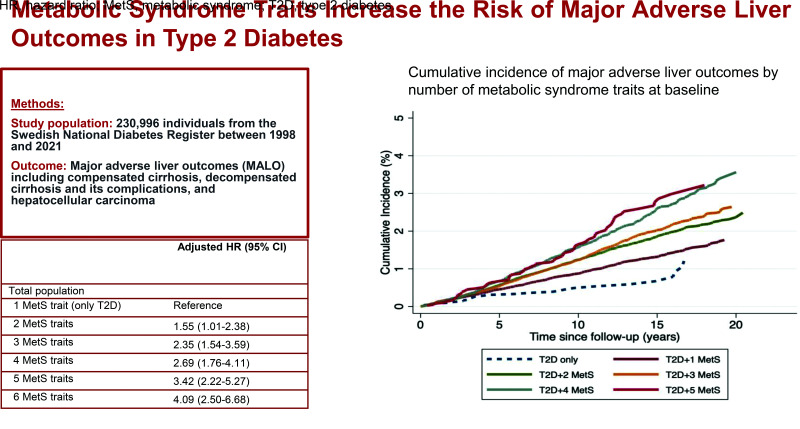
During a median follow-up of 9.9 years (corresponding to 2,235,356 person-years), 3,215 (1.39%; incidence rate 1.44/1,000 person-years) patients developed MALOs (Table 2). Within these outcomes, 1,344 cases of cirrhosis, 2,000 cases of decompensated cirrhosis and its complications, and 839 cases of HCC were identified. Patients with additional metabolic traits had a higher incidence of MALOs (1.46/1,000 person-years for two or more MetS traits vs. 0.56/1,000 person-years for those with only T2D). Furthermore, patients with a higher total number of metabolic traits at baseline had a higher incidence rate of MALOs, and the rate progressively increased as the number of metabolic traits increased, also after adjusting for other covariates (adjusted HR [aHR] 1.28, 95% CI 1.23–1.33, Ptrend < 0.001) (Table 2A). Compared with patients with only T2D at baseline, having more than one MetS trait was associated with a 2.3-fold increased rate of MALOs (95% CI 1.53–3.54); for those with all MetS traits at baseline, the aHR was 4.09 (95% CI 2.50–6.68).
Table 2.
Incidence rate and HRs of MALOs associated with numbers and individual traits of the MetS present at baseline in patients with T2D
| Events/patients, n | Incidence rate per 1,000 person-years | Crude HR (95% CI) | aHR (95% CI) | |
|---|---|---|---|---|
| Total population | 3,215/230,996 | 1.44 | ||
| A: Number of MetS traits | ||||
| 1 (only T2D) | 29/5,794 | 0.56 | Reference | Reference |
| ≥2 | 3,186/225,202 | 1.46 | 2.55 (1.77–3.68) | 2.33 (1.53–3.54) |
| 2 | 609/56,664 | 1.07 | 1.86 (1.28–2.70) | 1.55 (1.01–2.38) |
| 3 | 1,109/77,706 | 1.47 | 2.56 (1.77–3.70) | 2.35 (1.54–3.59) |
| 4 | 926/61,632 | 1.57 | 2.74 (1.89–3.97) | 2.69 (1.76–4.11) |
| 5 | 471/25,369 | 2.01 | 3.56 (2.44–5.18) | 3.42 (2.22–5.27) |
| 6 | 71/3,831 | 2.12 | 3.78 (2.45–5.83) | 4.09 (2.50–6.68) |
| Continuous MetS | 1.22 (1.18–1.25) | 1.28 (1.23–1.33) | ||
| B: Individual MetS traits | ||||
| No albuminuria | 2,421/185,618 | 1.33 | Reference | Reference |
| Albuminuria | 794/45,378 | 1.92 | 1.46 (1.34–1.58) | 1.23 (1.13–1.35) |
| No hypertension | 68/17,620 | 0.48 | Reference | Reference |
| Hypertension | 3,147/213,376 | 1.50 | 3.02 (2.37–3.83) | 2.06 (1.57–2.71) |
| No hypertriglyceridemia | 1,569/116,948 | 1.36 | Reference | Reference |
| Hypertriglyceridemia | 1,646/114,048 | 1.51 | 1.11 (1.03–1.19) | 1.11 (1.02–1.20) |
| No low HDL level | 2,589 /193,644 | 1.36 | Reference | Reference |
| Low HDL level | 626/37,352 | 1.86 | 1.38 (1.26–1.51) | 1.37 (1.23–1.51) |
| No obesity | 1,584/123,547 | 1.31 | Reference | Reference |
| Obesity | 1,631/107,449 | 1.59 | 1.23 (1.14–1.31) | 1.38 (1.28–1.50) |
A: The model was adjusted for numbers of MetS traits (continuous), age, sex, smoking status, diabetes duration, HbA1c level, CKD, CVD, eGFR, LDL level, hyperlipidemia, any diabetes treatment use, insulin, GLP-1RAs, and SGLT-2 inhibitors. B: The model was adjusted for albuminuria, hypertension, hypertriglyceridemia, low HDL levels, obesity, age, sex, smoking status, diabetes duration, HbA1c level, CKD, CVD, eGFR, LDL level, hyperlipidemia, any diabetes treatment use, insulin, GLP-1RAs, and SGLT-2 inhibitors.
Individual MetS Traits and Rates of MALOs
Patients with individual MetS traits had a higher incidence rate of MALOs compared with those who did not display such traits (e.g., 1.50/1,000 person-years for patients with hypertension vs. 0.48/1,000 person-years for those without hypertension) (Table 2B). The strongest association with incident MALOs was found for patients who had hypertension (aHR 2.06, 95% CI 1.57–2.71), followed by obesity (aHR 1.38, 95% CI 1.28–1.50), low HDL levels (aHR 1.37, 95% CI 1.23–1.51), albuminuria (aHR 1.23, 95% CI 1.13–1.35), and hypertriglyceridemia (aHR 1.11, 95% CI 1.02–1.20) (Table 2B). We did not detect any statistical interaction between the number or individual traits of MetS and age or sex on the rate of MALOs (Pinteraction > 0.1 for all). Among the other covariates, CKD (aHR 2.29, 95% CI 1.61–3.25), smoking (aHR 1.36, 95% CI 1.23–1.52), CVD (aHR 1.17, 95% CI 1.06–1.29), and eGFR (aHR 1.01, 95% CI 1.00–1.01) were associated with a higher rate of MALOs, while LDL (aHR 0.81, 95% CI 0.78–0.85) and hyperlipidemia (aHR 0.69, 95% CI 0.64–0.75) were related to a lower rate of MALOs in the fully adjusted models (Supplementary Table 4).
Combinations of MetS Traits and Rates of MALOs
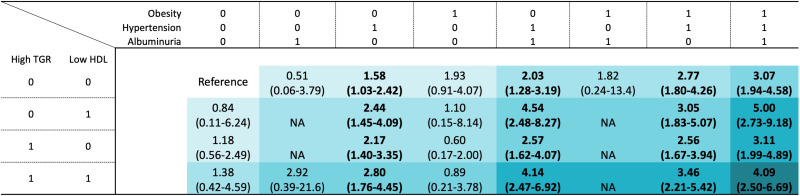
The associations of combinations of baseline MetS traits with MALOs are shown in Fig. 1. For the different combinations, hypertension consistently had the strongest association with incident MALOs. For instance, patients with hypertension had a higher rate of MALOs (aHR 1.58, 95% CI 1.03–2.42) compared with those with only T2D. For patients with two additional MetS traits, those with comorbid hypertension and obesity had the highest rate of MALOs (aHR 2.77, 95% CI 1.80–4.26), followed by those with hypertension and low HDL (aHR 2.44, 95% CI 1.45–4.09). We consistently observed higher event rates in any combination of hypertension and other MetS traits with regard to all three and four combinations.
Figure 1.
HRs and 95% CIs of baseline combinations of metabolic traits with MALOs in patients with T2D. The reference group comprises patients with T2D without any metabolic traits. The numbers of baseline metabolic traits are displayed as a shade of colored squares. NA, not applicable; TGR, triglycerides.
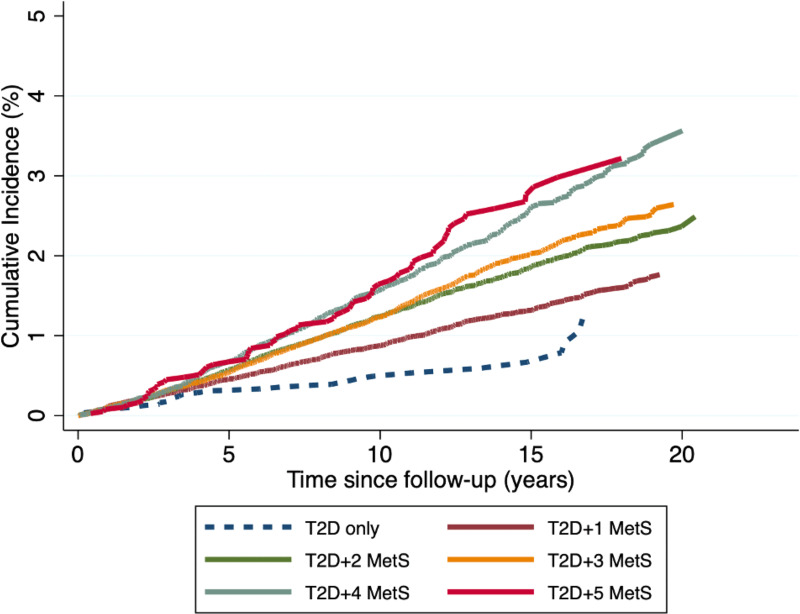
Cumulative incidences of MALOs by the total number of MetS traits over time are shown in Fig. 2. Overall, patients with additional MetS traits had a higher probability of MALOs than those with only T2D. The highest probability was observed in patients with all traits of the MetS. For instance, the 10-year cumulative incidence of MALOs was 0.49, 0.87, 1.24, 1.23, 1.57, and 1.64% for patients with only T2D, two, three, four, five, and six MetS traits, respectively (Supplementary Table 5). Regarding individual metabolic traits, there was a clear distinction in the probability of MALOs between patients with hypertension and those without. Similarly, there was a difference in the likelihood of outcome development among patients with obesity and without, and among patients with a low HDL level and without (Supplementary Fig. 2). When considering incident traits of the MetS occurring after baseline using time-varying models, we observed slightly higher HRs with regard to both number and different combinations of MetS traits, providing additional validity to our findings (Supplementary Fig. 3).
Figure 2.
Cumulative incidence of MALOs by number of MetS traits at baseline.
Sensitivity Analysis
As the association might be driven by the development of HCC, we conducted a sensitivity analysis distinguishing between patients who developed HCC and those who did not. The results indicated that both the individual and the total number of metabolic traits are associated with HCC and non-HCC events; therefore, our estimates are robust (Supplementary Table 6). We also found that each metabolic trait is associated with a higher risk of decompensated cirrhosis and complications and that hypertension, obesity, and low HDL level consistently demonstrate a higher risk across the spectrum of cirrhosis (Supplementary Table 7).
Conclusions
In this population-based cohort study of >230,000 patients with T2D, we found that an increasing number of metabolic traits is associated with an increased risk of MALOs in patients with T2D. Each additional metabolic trait increased the risk for liver-related outcomes in a stepwise manner. We expand on previous knowledge by also showing that acquiring additional traits of the MetS after T2D diagnosis further increases the risk of liver-related outcomes, suggesting a strong association between poor metabolic health and liver disease risk.
Additionally, we investigated which individual parameters included in the MetS, as well as other traits of poor metabolic health that had differing associations with incident liver disease. The strongest association was seen for hypertension and CKD, whereas hyperlipidemia was negatively associated with incident liver disease. This may possibly be explained by a protective effect of statin treatment (which was included to define presence of hyperlipidemia), which has been suggested to have hepatoprotective effects and is associated with a reduced incidence of primarily HCC in patients with T2D (21). The other plausible explanation could be that patients with mutations in lipid-modifying genes, such as PNPLA3, may have lower blood lipid levels but a higher risk for development of cirrhosis and HCC (22,23). An alternate explanation might involve hepatic synthetic failure during the early stages of cirrhosis, leading to a decrease in serum lipoprotein levels.
In the absence of primary care diagnoses, similarly to hyperlipidemia, hypertension was also defined by use of antihypertensive treatments in addition to having a recorded diagnosis in specialist care or an elevated blood pressure. As such, the risk increase associated with hypertension in this study may also warrant additional studies that can disentangle the effects associated with hypertension and the associated medical treatment.
In summary, our findings suggest that individualized risk prediction for incident MALOs is possible in patients with T2D. Future studies are needed to examine whether established noninvasive tests, such as the fibrosis 4 score or vibration-controlled transient elastography, can further increase prediction specifically in patients with T2D.
Comparison With Previous Studies
Our findings are in accordance with those of previous studies in this field in that each additional metabolic trait increased the risk of MALOs, regardless of alcohol intake (24–26). Indeed, the complex relationship between alcohol consumption and MetS complicates its individual impact of the MetS on liver outcomes. We investigated this by excluding all patients who had alcohol-related disease and found that even in the absence of known alcohol overconsumption with health care contacts, metabolic factors are independently related to liver-related outcomes.
The traits of the MetS seemingly have additive effects on the progression to cirrhosis, with hypertension demonstrating the strongest association among the individual traits. Indeed, any combination of hypertension with other MetS traits was associated with a higher risk of MALOs compared with only T2D, while such risk was not observed in the absence of hypertension. The presence of hypertension may indicate the occurrence of MASLD, as suggested by a meta-analysis that showed a bidirectional association between hypertension and MASLD independent of other cardiometabolic risk factors (27). As >90% of patients in this cohort had hypertension, we examined which combination of MetS traits, in addition to hypertension, conveyed the highest risk of MALOs in our cohort. We found that, in addition to hypertension, patients with obesity had the highest risk of MALOs, followed by those with low HDL, among all the two possible combinations of MetS traits. The risk increased substantially when patients had hypertension, low HDL, and comorbid albuminuria or obesity. This supports previous data from a large U.S. Veterans Affairs cohort of patients with a diagnosis of MASLD, where those with diabetes and coexisting hypertension and obesity or dyslipidemia had the highest risk for cirrhosis or HCC (6). That study also found a stepwise increase in risk for cirrhosis and HCC with each additional metabolic trait. Our findings extend this by including a less selected population of patients, all of whom have T2D. This is important since current guidelines promote screening for MASLD in all patients with T2D (28,29), aiming to identify patients on the path to cirrhosis. Our findings are important since they are more generalizable to the larger T2D population compared with previous data. A systematic review and meta-analysis found that in >22.8 million individuals with T2D, the overall risk of cirrhosis was 2.25-fold higher compared with people without diabetes (26). We extend this finding by showing that in patients with T2D, there are distinct risk profiles, possibly allowing for personalization of clinical management and follow-up.
Previous studies of the association between T2D and cirrhosis (30–33) often used mortality from cirrhosis as the outcome rather than incidence of nonfatal cirrhosis (31,33). Other studies reported an association between diabetes and more severe forms of liver disease, such as occurrence of hepatic failure (28,32). However, these studies did not differentiate between type 1 diabetes and T2D and were unable to study risk factors for development of liver-related outcomes.
Implications
Several important implications can be made based on these data. Although this study cannot show causality, our findings suggest the importance of adequately treating not only hyperglycemia in patients with T2D to reduce diabetes-related complications but also other comorbidities to reduce the risk for liver-related outcomes. Treating and preventing hypertension and obesity are particularly crucial to prevent the development of cirrhosis in this population. In addition to its impact on liver-related outcomes, a more severe metabolic profile in patients with T2D also increases the risk for cardiovascular events and overall mortality (34). By extension, this suggests that upcoming treatments for MASLD might be holistically more effective if they also improve the full metabolic profile of the patient. Data from randomized controlled trials are needed to corroborate this. In the absence of approved therapies to treat hepatic fibrosis due to MASLD, screening for liver fibrosis in all patients with T2D is currently controversial. The findings from this study may aid in identifying subgroups at high risk of developing future liver disease, which would be particularly efficient for targeted screening initiatives. To determine the cost-effectiveness of different interventions, such as screening for MASLD or advanced fibrosis, health economic evaluation is necessary. Our results suggest that patients with T2D have different metabolic risk factors, with a differing risk of progression to MALOs. Recognizing such distinct risk groups may be important in future clinical guidelines to tailor appropriate interventions and enhance patient outcomes.
Strengths and Limitations
The major strengths of this study are the use of high-quality registers with high coverage, minimal loss to follow-up, and an unprecedented sample size. Hence, estimates are more precise, accurate, and generalizable to the target population than that of previous studies. While large studies are often limited to register-based coding of metabolic traits, we used direct laboratory tests, in combination with medication use and register coding, to define metabolic traits. We further assessed the associations with repeated exposures to investigate whether newly developed metabolic traits also confer a higher risk of outcomes. We used hard outcomes that are validated and unlikely to be misclassified and that are important to patients (13). Several studies have shown that waist circumference may be a better predictor of central obesity than BMI and may also predict progression to MALO better than BMI; unfortunately, such data are not available in the used data sources (35,36). Additionally, to combine different MetS traits, we used categorized predictors. However, continuous versions of each MetS trait may provide an even more granular understanding. For instance, glycemic control is a better predictor for this purpose than diabetes status as a binary parameter (37). Combining continuous versions of MetS traits should be a focus for future studies. Another limitation is the lack of direct data on the presence and severity of MASLD, such as radiology or liver-related blood biochemistry, which are not available in the NDR. To address this limitation and further explore the severity of each MetS component, we plan to conduct future linkages to data sources that provide such detailed information. Furthermore, we excluded 486,377 patients because of missing data on at least one MetS trait. These patients tended to be older and more likely to have MetS, CVD, CKD, and severe diabetes as indicated by insulin use compared with those included in the study. Therefore, the estimated associations would have been stronger if we had included all eligible patients in the analysis.
In conclusion, in this large study of patients with T2D in Sweden, we found that an increasing number of metabolic comorbidities is associated with a higher rate of progression to MALOs, most likely attributable to MASLD. Hypertension and CKD were the parameters with the strongest association, whereas no positive association was seen for hyperlipidemia. This finding helps in narrowing down the specific group intended for directed casefinding among patients with T2D, potentially enhancing screening efficiency.
This article contains supplementary material online at https://doi.org/10.2337/figshare.25280482.
Article Information
Acknowledgments. The authors thank the staff of the National Diabetes Register and all the study participants.
Funding. Y.S. was supported by Mag-Tarm Fonden, Swedish Gastroenterology Society, Karolinska Institutet research funding, and Region Stockholm. H.H. was supported by grants from Stockholm City County, The Swedish Cancer Society, and The Swedish Research Council. This study received financial support from Pfizer.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.S., E.T.G., A.M., H.S., O.S., F.N., S.G., and H.H. contributed to the data interpretation. Y.S., E.T.G., A.M., and H.H. contributed to the study concept and design. Y.S., E.T.G., H.S., and H.H. contributed to the statistical analysis. Y.S. and H.H. drafted the manuscript. E.T.G., O.S., and H.H. contributed to the data acquisition. All authors contributed to the critical revision of the manuscript. Y.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Handling Editors. The journal editors responsible for overseeing the review of the manuscript were Elizabeth Selvin and Amalia Gastaldelli.
Funding Statement
Y.S. was supported by Mag-Tarm Fonden, Swedish Gastroenterology Society, Karolinska Institutet research funding, and Region Stockholm. H.H. was supported by grants from Stockholm City County, The Swedish Cancer Society, and The Swedish Research Council. This study received financial support from Pfizer.
References
- 1. Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2022;7:851–861 [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 2023;77:1335–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol 2019;71:793–801 [DOI] [PubMed] [Google Scholar]
- 4. Björkström K, Franzén S, Eliasson B, et al. Risk factors for severe liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol 2019;17:2769–2775.e4 [DOI] [PubMed] [Google Scholar]
- 5. Anstee QM, Hallsworth K, Lynch N, et al. Real-world management of non-alcoholic steatohepatitis differs from clinical practice guideline recommendations and across regions. JHEP Rep 2021;4:100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kanwal F, Kramer JR, Li L, et al. Effect of metabolic traits on the risk of cirrhosis and hepatocellular cancer in nonalcoholic fatty liver disease. Hepatology 2020;71:808–819 [DOI] [PubMed] [Google Scholar]
- 7. Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol 2017;67:1265–1273 [DOI] [PubMed] [Google Scholar]
- 8. Abeysekera KWM, Macpherson I, Glyn-Owen K, et al. Community pathways for the early detection and risk stratification of chronic liver disease: a narrative systematic review. Lancet Gastroenterol Hepatol 2022;7:770–780 [DOI] [PubMed] [Google Scholar]
- 9. Rowe IA, D’Amico G. Taking a risk-based approach to testing for liver disease in primary care, a step in the right direction. J Hepatol 2022;77:293–295 [DOI] [PubMed] [Google Scholar]
- 10. Eliasson B, Gudbjörnsdottir S. Diabetes care--improvement through measurement. Diabetes Res Clin Pract 2014;106(Suppl. 2):S291–S294 [DOI] [PubMed] [Google Scholar]
- 11. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009;24:659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bengtsson B, Askling J, Ludvigsson JF, Hagström H. Validity of administrative codes associated with cirrhosis in Sweden. Scand J Gastroenterol 2020;55:1205–1210 [DOI] [PubMed] [Google Scholar]
- 14. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 15. Eliasson B, Cederholm J, Nilsson P; Steering Committee of the Swedish National Diabetes Register . The gap between guidelines and reality: type 2 diabetes in a National Diabetes Register 1996–2003. Diabet Med 2005;22:1420–1426 [DOI] [PubMed] [Google Scholar]
- 16. Ludvigsson JF, Appelros P, Askling J, et al. Adaptation of the Charlson comorbidity index for register-based research in Sweden. Clin Epidemiol 2021;13:21–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barlow L, Westergren K, Holmberg L, Talbäck M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol 2009;48:27–33 [DOI] [PubMed] [Google Scholar]
- 18. Brooke HL, Talbäck M, Hörnblad J, et al. The Swedish Cause of Death Register. Eur J Epidemiol 2017;32:765–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics 1995;51:524–532 [PubMed] [Google Scholar]
- 20. Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 2012;41:861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim G, Jang SY, Han E, et al. Effect of statin on hepatocellular carcinoma in patients with type 2 diabetes: a nationwide nested case-control study. Int J Cancer 2017;140:798–806 [DOI] [PubMed] [Google Scholar]
- 22. Krarup NT, Grarup N, Banasik K, et al. The PNPLA3 rs738409 G-allele associates with reduced fasting serum triglyceride and serum cholesterol in Danes with impaired glucose regulation. PLoS One 2012;7:e40376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trépo E, Nahon P, Bontempi G, et al. Association between the PNPLA3 (rs738409 C>G) variant and hepatocellular carcinoma: evidence from a meta-analysis of individual participant data. Hepatology 2014;59:2170–2177 [DOI] [PubMed] [Google Scholar]
- 24. Åberg F, Byrne CD, Pirola CJ, Männistö V, Sookoian S. Alcohol consumption and metabolic syndrome: clinical and epidemiological impact on liver disease. J Hepatol 2023;78:191–206 [DOI] [PubMed] [Google Scholar]
- 25. Pose E, Pera G, Torán P, et al. Interaction between metabolic syndrome and alcohol consumption, risk factors of liver fibrosis: a population-based study. Liver Int 2021;41:1556–1564 [DOI] [PubMed] [Google Scholar]
- 26. Jarvis H, Craig D, Barker R, et al. Metabolic risk factors and incident advanced liver disease in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of population-based observational studies. PLoS Med 2020;17:e1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li G, Peng Y, Chen Z, Li H, Liu D, Ye X. Bidirectional association between hypertension and NAFLD: a systematic review and meta-analysis of observational studies. Int J Endocrinol 2022;2022:8463640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El-Serag HB, Everhart JE. Diabetes increases the risk of acute hepatic failure. Gastroenterology 2002;122:1822–1828 [DOI] [PubMed] [Google Scholar]
- 29. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD) ; European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 2016;59:1121–1140 [DOI] [PubMed] [Google Scholar]
- 30. Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology 1990;12:1106–1110 [DOI] [PubMed] [Google Scholar]
- 31. de Marco R, Locatelli F, Zoppini G, Verlato G, Bonora E, Muggeo M. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes Care 1999;22:756–761 [DOI] [PubMed] [Google Scholar]
- 32. Liu TL, Trogdon J, Weinberger M, Fried B, Barritt AS 4th. Diabetes is associated with clinical decompensation events in patients with cirrhosis. Dig Dis Sci 2016;61:3335–3345 [DOI] [PubMed] [Google Scholar]
- 33. Zoppini G, Fedeli U, Gennaro N, Saugo M, Targher G, Bonora E. Mortality from chronic liver diseases in diabetes. Am J Gastroenterol 2014;109:1020–1025 [DOI] [PubMed] [Google Scholar]
- 34. Guzder RN, Gatling W, Mullee MA, Byrne CD. Impact of metabolic syndrome criteria on cardiovascular disease risk in people with newly diagnosed type 2 diabetes. Diabetologia 2006;49:49–55 [DOI] [PubMed] [Google Scholar]
- 35. Alberti KGMM, Eckel RH, Grundy SM, et al.; International Diabetes Federation Task Force on Epidemiology and Prevention ; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 36. Andreasson A, Carlsson AC, Önnerhag K, Hagström H. Waist/hip ratio better predicts development of severe liver disease within 20 years than body mass index: a population-based cohort study. Clin Gastroenterol Hepatol 2017;15:1294–1301.e2 [DOI] [PubMed] [Google Scholar]
- 37. Alexopoulos AS, Crowley MJ, Wang Y, et al. Glycemic control predicts severity of hepatocyte ballooning and hepatic fibrosis in nonalcoholic fatty liver disease. Hepatology 2021;74:1220–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]