Abstract
Several indices of right heart remodeling and function have been associated with survival in pulmonary arterial hypertension (PAH). Outcome analysis and physiological relationships between variables may help develop a consistent grading system.
Patients with Group 1 PAH followed at Stanford Hospital who underwent right heart catheterization and echocardiography within 2 weeks were considered for inclusion. Echocardiographic variables included tricuspid annular plane systolic excursion (TAPSE), right ventricular (RV) fractional area change (RVFAC), free wall strain (RVFWS), RV dimensions, and right atrial volumes. The main outcome consisted of death or lung transplantation at 5 years. Mathematical relationships between variables were determined using weighted linear regression and severity thresholds for were calibrated to a 20% 1‐year mortality risk.
PAH patients (n = 223) had mean (SD) age of 48.1 (14.1) years, most were female (78%), with a mean pulmonary arterial pressure of 51.6 (13.8) mmHg and pulmonary vascular resistance index of 22.5(6.3) WU/m2. Measures of right heart size and function were strongly related to each other particularly RVFWS and RVFAC (R 2 = 0.82, p < 0.001), whereas the relationship between TAPSE and RVFWS was weaker (R 2 = 0.28, p < 0.001). Death or lung transplantation at 5 years occurred in 78 patients (35%). Guided by outcome analysis, we ascertained a uniform set of parameter thresholds for grading the severity of right heart adaptation in PAH. Using these quantitative thresholds, we, then, validated the recently reported REVEAL‐echo score (AUC 0.68, p < 0.001).
This study proposes a consistent echocardiographic grading system for right heart adaptation in PAH guided by outcome analysis.
Keywords: echocardiography, heart failure, pulmonary arterial hypertension, right ventricle function and dysfunction, risk stratification and biomarkers
Abbreviations
- eRAP
Echo Right atrial pressure
- LVEF
left ventricular ejection fraction
- LVID
left ventricle internal diameter
- RHC
right heart catheterization
- rRAP
right heart catheterization measured right atrial pressure
- RVEDA
right ventricular end diastolic area
- RVEDD
right ventricular end diastolic diameter
- RVEF
right ventricular ejection fraction
- RVESA
right ventricular end systolic area
- RVESD
right ventricular end systolic diameter
- RVFAC
RV fractional area change
- RVFWS
myocardial RV free wall strain
- RVSP
right ventricular systolic pressure
- RVTS
right ventricular transverse shortening
- sPAP
systolic pulmonary arterial pressure
- TAPSE
tricuspid annular plane systolic excursion
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a rare disease associated with pulmonary vascular remodeling. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 In patients with PAH, right heart failure (RHF) is the most important cause of mortality. 10 More than 30 years ago, D'Alonzo et al. observed that survival in PAH was closely associated with cardiac index (CI) and right atrial (RA) pressure. 10 Since then, several imaging biomarkers of RHF have predicted event‐free survival in PAH including tricuspid annular plane systolic excursion (TAPSE), 7 , 8 , 10 , 11 , 12 right ventricular (RV) free wall longitudinal strain (RVFWS), RV end systolic dimensions and more recently RA size and function. 6 , 13 , 14 , 15 , 16
With the multiplicity of right heart parameters, ensuring consistency in grading systems becomes even more important. While there is variability in how the right heart adapts to PAH, several right heart metrics are related to each other. 12 , 17 , 18 For example, Evaldsson et al. have demonstrated that RVFWS is strongly associated with RV ejection fraction (RVEF) in patients with PH. 19 In patients with precapillary PH, Kind et al. have also, shown that RV mid‐transverse function is closely associated with RVEF. 20 These relationships are not surprising as the right heart usually adapts to PAH through heterotopic mechanisms (RV dilatation) to compensate for ventriculo‐arterial uncoupling. 21 In addition, RV and RA remodeling and function are often linked to each through diastolic dysfunction, tricuspid regurgitation, and ventriculo‐atrial coupling, (Figure 1a). These physiological relationships can help ensure consistency between thresholds for risk stratification; in addition, they can help guide consistency between grading systems in PAH.
Figure 1.
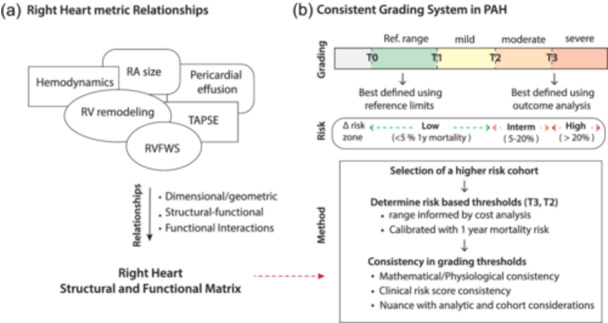
(a) Right heart metric relationships (b) Grading system for chamber quantification. Grading systems are guided by reference limits and outcome analysis. In PAH, high risk is defined as a 1‐year risk of mortality > 20%. To identify consistent thresholds for right chamber quantification, we first selected a high‐risk cohort and then identify thresholds based on outcome analysis with other supporting criteria. PAH, pulmonary arterial hypertension.
To be clinically relevant, thresholds for severity should be first and foremost guided by outcome analysis. For PAH, 20% risk of mortality or lung transplantation at year is often used to define high risk. The 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension (PH), have incorporated several imaging parameters in its risk stratification schema. The cardiac magnetic resonance parameters were mainly guided by the study of Lewis et al. 22 Among the echocardiographic indices also proposed in the ESC/ERS guidelines, RA area size > 26 cm2, the TAPSE/sPAP (systolic pulmonary arterial pressure) ratio < 0.19 mm/mmHg and the presence of moderate pericardial effusion have been incorporated and considered markers of high risk (>20% 1‐year mortality). For other echocardiographic parameters such as RV internal diameters or areas or indexed dimensions, severity thresholds have not been as well established. Deriving these quantitative thresholds would not only be variable for consistency but would provide quantitative criteria for imaging‐based risk scores such as the recently described REVEAL‐ECHO. 23
The main objective of this study is to propose a consistent echocardiographic grading system in PAH anchored on physiological relationships and outcome analysis. Our first objective was to better quantify mathematical and physiological relationships between right heart variables in PAH. The second objective was to identify right heart severity thresholds in PAH based on outcome analysis. Theoretically, these thresholds should follow their physiological relationship and be consistent with validated clinical risk scores. Finally, we aimed to validate the recently proposed REVEAL‐ECHO score using quantitative right heart criteria.
MATERIAL AND METHODS
Study design
The study was a retrospective single center study conducted at the Vera Moulton Wall Center for Pulmonary Vascular Disease. This study was approved by the Stanford University Institutional Review Board and was conducted under the Cardio Share protocol (IRB#25673).
Population
Adult patients (>18 years) with a diagnosis of PAH followed at Stanford Hospital between January 2002 and 2021 were screened for inclusion. The diagnosis of PAH was established clinically and supported by right heart catheterization (RHC) with evidence of mean pulmonary arterial pressure > 25 mmHg and a pulmonary vascular resistance (PVR) > 3 Wood units. In our practice setting, patients who obtain an echocardiogram and a RHC close by are often higher risk patients with a majority having a REVEAL lite score ≥ 8. Therefore, we selected patients who had RHC and echocardiogram within 2 weeks of each other to ensure inclusion of a high‐risk cohort. We excluded patients with non‐Group 1 PAH and patients with complex congenital heart disease. In addition, for the outcome analysis, we excluded patients with a primary diagnosis of liver cirrhosis as the primary cause of PAH. We also only included who were followed for at least 5 years to allow for sufficient for the combined outcome of death or transplantation to occur.
RHC/hemodynamic assessment
A RHC was performed using when appropriate mild sedation. During the RHC, measures included mean pulmonary artery pressure (mPAP), pulmonary capillary wedge pressure, RA pressure (RAP), cardiac output (CO), PVR as well as vital signs. CO was measured using the Fick method with the oxygen consumption estimated based on the LaFarge table; both CO and PVR were indexed to body surface area using the Dubois formula. 24
Echocardiographic assessment
Echocardiographic images were acquired using Hewlett Packard Sonos 5500 or Philips IE33 ultrasound systems according to the American Society of Echocardiography guidelines for chamber quantification. 25 All measurements were performed using the TomTec platform (TomTec Imaging system) by two certified readers (Supporting Information: e‐Figure 1). Measures were performed on the RV focused views in the Stanford Cardiovascular Institute Biomarker and Phenotypic Core Laboratory by a certified sonographer and verified by a trained cardiologist (B. C.). Measures were conducted at end‐diastole and end‐systole defined by largest and smallest chamber size. Comprehensive measures of the right heart were performed including RV areas, basal and mid‐transverse dimensions and RA areas and volumes. We used the method described by Kind et al. to identify the RV transverse axis allowing consistent transverse shortening measures while antero‐posterior dimensions were measured. 26 Right heart dimensions were indexed using height for diameters and BSA for areas as well as internally scaled to left ventricular or atrial size as appropriate. Functional indices included RV fractional area change (RVFAC), RVFWS, TAPSE as well as transverse and antero‐posterior shortening measures. RVFWS was measured using Lagrangian strain and the lateral wall was measured up to the apico‐septal junction. RVFWS and RVFAC were measured using the same frame to ensure maximal consistency. Since M‐mode were not available in all patients, TAPSE was measured using anatomical 2D plane (in our laboratory, we observed a strong association between anatomical M‐mode and anatomical 2D plane measures r = 0.81, p < 0.001, n = 126). Left ventricular diameters were measured in the parasternal long axis views and LV ejection fraction was quantified using the Simpson's method in the 4‐chamber view. Pericardial effusion was measured in the subcostal view during diastole, we used a semi‐quantitative method to quantify the severity of the pericardial effusion, <10 mm was considered as mild and ≥10 mm was considered as moderate to severe.
Clinical, laboratory, and outcomes data
Demographic data, anthropometric data, etiology of PAH, treatments, New York Heart Association (NYHA) status, 6‐min walk test closest to the RHC and laboratory data (serum creatinine, total bilirubin, sodium, hemoglobin, NT‐terminal pronatriuretic peptide (NT‐proBNP) were collected. We calculated the REVEAL lite score as well as the recently described REVEAL‐echo score for all participants. 23 , 27 Patients were followed for the primary outcome of time to death or transplantation within 5 years. Mortality and lung transplantation outcome were curated through chart review and national death index.
STATISTICS ANALYSIS
Summary statistics include mean ± standard deviation (SD) and number and percentage. Data with skewed a distribution were presented as median and interquartile range. We compared groups using Student T test with adjustment for unequal variance. To quantify relationships between right heart variables, we performed a weighted linear regression which better adjusts for heteroscedasticity; for selected variables such as NT‐proBNP, natural logarithmic transformation was performed before analysis. For variance, we report the average variance terms for the predicted interval. Outcome analysis was based on Cox‐proportional hazard model as well as Kaplan Meier survival analysis. Upset plots were used to analyze combinations between variables used in the multivariable survival analysis.
To determine thresholds for grading severity of PH, we used different methods to ensure consistency between the different metrics (Figure 1b). Three thresholds were used to define severity: T0 or the reference limit threshold, T1 or the intermediate risk thresholds and T2 (high risk threshold). To select T2 thresholds, all metrics were calibrated using a 20% 1‐year risk of mortality or ung transplantation aligning with the most recent ESC/ERS guidelines; 3 the T0 thresholds were selected using the limits of reference based on the ASE recommendations for chamber quantification, the ESC/ERS guidelines and recent WASE values while T1 was calibrated to a lower than 5% 1‐year risk of mortality. The range for T2 was first determined using different classification criterion from cross‐over between sensitivity and sensitivity, Youden index or cost‐analysis. Cost analysis weighs each category of true positive, true negative, false positive and false negative by their respective prevalence and cost values. The cost matrix for the study was 2 for false negative, 1 for false positive and −1 for true positive and negative; we selected a greater cost for false negative associated with the risk of withholding therapy for a patient. In addition, supporting criteria for the selected threshold included the following: first, mathematical consistency anchored on the value of RVFAC, median value of the REVEAL lite score of 8 and 9 (Figure 1b). For statistical analysis, we used R (version 4.2.1) and MedCalc (version 20.218) software.
RESULTS
Patient characteristics
A total of 424 patients were selected from the RHC database with an initial diagnosis of PAH from 2002 to 2021. Among them, 240 patients had RHC and an echocardiography within 2 weeks and 223 had a final diagnosis of PAH with a median REVEAL lite registry score was 8.0 [IQR 6.0−10.0]. A subgroup of 56 patients had a follow‐up echocardiogram and RHC within 2 weeks and were included for longitudinal analysis. After exclusion of the patients with advanced liver disease (n = 14) and patients who did not complete 5 years follow‐up (n = 11), 198 patients remained for survival analysis (Figure 2).
Figure 2.
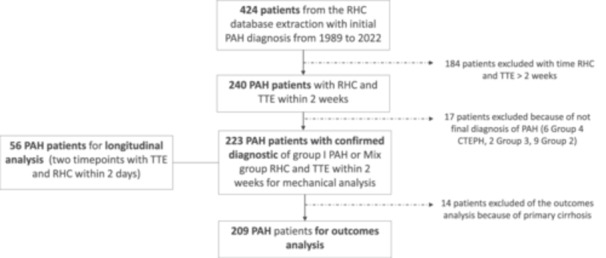
Consort diagram. Four hundred twenty‐four patients were selected from the Stanford Pulmonary Hypertension Registry Mechanical and mathematical analysis was performed in 223 patients and analysis, for the outcome death or transplant within 5 years, in 198 patients.
Most patients were female (78.5%), the mean age was 48.1 (14.1) years and 55.6% (124) were Caucasians (Table 1). The majority had either idiopathic (40.4%) or drug and toxin‐associated (24.7%) PAH. The cohort had hemodynamically severe disease, with mPAP of 51.6 (13.8) mmHg and pulmonary vascular resistance index of 22.5 (6.3) WU/m2. Large proportions of the cohort had NYHA functional class IV symptoms (35%) and NT‐proBNP > 1100 pg/mL (48.9%). At baseline, 34.5% of PAH patients were treatment naïve.
Table 1.
Patient characteristics for the pulmonary arterial hypertension cohort.
| Population (n = 223) | |
|---|---|
| Demographics | |
| Age | 48.1 (14.1) |
| Male | 49 (22.0%) |
| White race | 124 (55.6%) |
| Etiology | |
| Idiopathic | 90 (40.4%) |
| Connective tissue disease | 55 (24.7%) |
| IV drug abuse | 7 (3.2%) |
| Simple repaired congenital | 4 (1.8%) |
| Comorbidity | |
| Lung disease | 17 (7.6%) |
| Advanced liver disease | 14 (6.3%) |
| Medications | |
| Treatment naive | 145 (65%) |
| Biological markers | |
| NT‐proBNP (pg/mL) | |
| <300 | 59 (26.5%) |
| 300−1100 | 55 (24.7%) |
| ≥ 1100 | 109 (48.9%) |
| GFR by CKDepi (mL/min/1.73 m2) | 55.0 (22.1) |
| Sodium (mM) | 138 (3.4) |
| Hemoglobin (g/dL) | 13.3 (2.3) |
| Bilirubin (mg/dL) | 0.97 (1.0) |
| RHC parameters | |
| mPAP (mmHg) | 51.6 (13.8) |
| RAP (mmHg) | 9.3 (5.2) |
| PAWP (mmHg) | 11.1 (4.8) |
| CI (L/min/m2) | 2.0 (0.6) |
| PVRi (WU/m2) | 22.5 (6.3) |
| NYHA status | |
| 1 | 6 (2.7%) |
| 2 | 52 (23.3%) |
| 3 | 80 (35.9%) |
| 4 | 78 (35.0%) |
| Reveal lite 2.0 score | |
| ≤5 | 53 (23.8%) |
| 6−7 | 42 (18.8%) |
| ≥8 | 127 (57%) |
| Outcomes | |
| Death or transplant within 5 years | 78 (35%) |
Abbreviations: GFR, glomerular filtration rate; mPAP, mean pulmonary artery pressure; NYHA, New York Heart Association; PAWP, pulmonary capillary wedge pressure; PVRi, pulmonary vascular resistance index; RAP, right atrial pressure; RHC, right heart catheterization.
Relationships between right heart parameters
Right heart metrics in PAH (Table 2) were highly interrelated (Figure 3), with strong linear associations between RVFAC and RVFWS (R 2 = 0.82, p < 0.001), RV mid‐transverse shortening (R 2 = 0.54, p < 0.001) and RVESA index (R 2 = 0.54, p < 0.001). In contrast, only a moderate relationship was observed between TAPSE and RVFAC (R 2 = 0.25, p < 0.001) or RVFWS (R 2 = 0.28, p < 0.001). In the longitudinal cohort, changes in RVFAC were strongly associated with changes in RVFWS (R 2 = 0.76, p < 0.001) but only moderately to TAPSE (R 2 = 0.32, p < 0.001). These relationships and their average variance term are summarized in Table 3 (using RVFAC as the independent variable) and Supporting Information: e‐Table 2 (using RVFWS as the independent variable). For reference, Table 3 also includes selected RVFAC or RVFWS‐RVEF relationships which have been reported in previous studies. 19 , 28
Table 2.
PAH echocardiographic parameters.
| Metrics | PAH, n = 223 mean (SD) |
|---|---|
| RV dimensions | |
| Basal transverse diameter index (mm/m) | |
| diastole | 33.3 (5.9) |
| systole | 29.4 (5.6) |
| Mid transverse diameter index (mm/m) | |
| diastole | 29.6 (6.3) |
| systole | 27.0 (6.9) |
| Mid transverse diameter relative (mm/mm) | |
| Diastole | 1.3 (0.4) |
| RV areas (cm2/m2) | |
| RVEDAi (cm2/m2) | 20.3 (5.8) |
| RVESAi (cm2/m2) | 16.5 (5.6) |
| RV relative areas | |
| RVEDAr | 1.9 (0.8) |
| RVESAr | 2.9 (1.4) |
| RA area(cm2/m2) | 12.6 (4.8) |
| RA relative area | 1.8 (1.1) |
| RA volume(mL/m2) | 44.9 (28.1) |
| RV function | |
| TAPSE (mm) | 14.2 (4.9) |
| TAPSE/RVSP (mm/mmHg) | 0.21 (0.14) |
| RVFAC (%) | 19.8 (6.5) |
| RV free‐wall strain (RVFWS) (%) | 12.6 (4.5) |
| Basal transverse shortening fraction (%) | 12.0 (8.5) |
| Mid transverse shortening fraction (%) | 9.5 (7.2) |
| Anteroposterior shortening fraction (%) | 15.6 (7.3) |
| LV metrics dimensional | |
| LV internal diameter (mm/m) | 23.2 (4.0) |
| LV metrics functional | |
| LVEF Simpson (%) | 64.7 (7.8) |
Abbreviations: PAH, pulmonary arterial hypertension; PAWP, pulmonary capillary wedge pressure; RA, right atrial; RAP, RA pressure; RHC, right heart catheterization; RV, right ventricular; RVFAC, RV fractional area change; RVFWS, RV free wall longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
Figure 3.
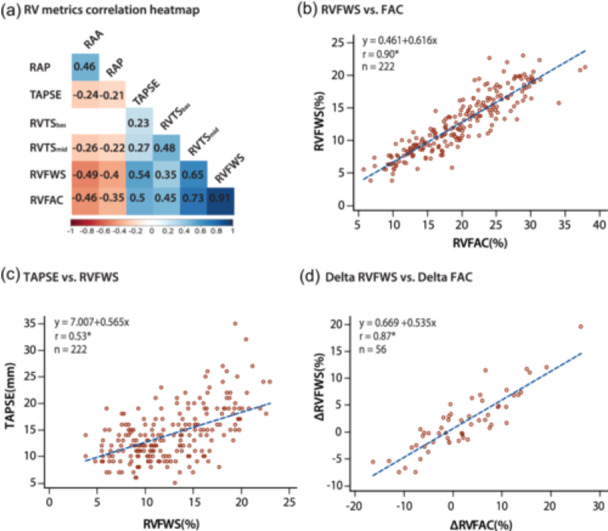
Associations and mathematical relationships between measures of right heart remodeling and function. (a) Correlation heatmap showing association between RV and RA metrics. (b) Mathematical relationship between RVFWS and RVFAC (r = 0.90, p < 0.001). (c) Mathematical relationship between TAPSE and RVFWS (r = 0.53, p < 0.001). (d) Mathematical relationship between delta RVFWS and delta RVFAC showing consistency (r = 0.87, p < 0.001). RA, right atrial; RV, right ventricular; RVFAC, RV fractional area change; RVFWS, RV free wall longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
Table 3.
Weighted regression between measures of Right heart metrics versus RVFAC.
| Variable (y) | Equation | R2a | Variance term |
|---|---|---|---|
| RV function | |||
| RVFWS (%) | 0.54 + 0.61 (FAC) | 0.82 | SD = 12.1% ŷ |
| RVTSmid (%) | −5.6 + 0.76 (FAC) | 0.54 | SD = 33% ŷ |
| TAPSE (mm) | 7.06 + 0.36 (FAC) | 0.25 | SD = 24.7% ŷ |
| TAPSE (mm) | 7.0 + 0.57 (RVFWS) | 0.28 | SD = 22% ŷ |
| RV size | |||
| RVEDAi (cm2/m2) | 29.3 – 0.46 (FAC) | 0.35 | SD = 18% ŷ |
| RVESAi (cm2/m2) | 26.5 – 0.51 (FAC) | 0.54 | SD = 18.5% ŷ |
| RVEDAr | 3.4 – 0.076 (FAC) | 0.44 | SD = 25% ŷ |
| RVESAr | 5.5 – 0.13 (FAC) | 0.48 | SD = 28.6% ŷ |
| RVESA (cm2) | −4.7 + 0.94 (RVEDA) | 0.97 | SD = 5.4% ŷ |
| RA size | |||
| RAA (cm2) | 34.3 – 0.56 (FAC) | 0.19 | SD = 27.5% ŷ |
| RAAi (cm2/m2) | 18.1 – 0.28 (FAC) | 0.16 | SD = 26.7% ŷ |
| RAAr | 3.0 −0.063 (FAC) | 0.27 | SD = 33.9% ŷ |
| RAVi (mL/m2) | 75− 1.5 (FAC) | 0.17 | SD = 42.6% ŷ |
| RAVi (mL/m2) | −19.1 + 5.05 (RAAi) | 0.92 | SD = 8.7% ŷ |
| RVFAC (Echo) 28 | 3.9 + 0.7 (RVEF CMR) | 0.71 | SD = 17% ŷ |
| RVFAC (Echo) 19 | 1.8 + 0.7 (RVEF CMR) | 0.46 | NA |
| RVFWS | 4.3 + 0.3 (RVEF CMR) | 0.61 | NA |
Abbreviations: RA, right atrial; RV, right ventricular; RVFAC, RV fractional area change; RVFWS, RV free wall longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
All relationships presented are significant p < 0.001; variance terms determined after exclusion of outlier values by Tukey method; PP indicates percent predicted value.
Outcome analysis and guidelines imaging based criteria for risk stratification
Death or transplantation occurred in 78 individuals (35%) at 5 years. Before comprehensive outcome analysis for right heart metrics, we first assessed whether out cohort was representative usinf the REVEAL‐lite score and echocardiographic metrics for risk stratification including in the ESC/ERS guidelines for PH. 3 In our cohort, a REVEAL lite score of ≥8 was associated with a 20% risk of death or transplantation at 1 year and a 50% risk at 5 years (Figure 4a). Similarly, the echocardiographic parameters of TAPSE/sPAP, RA area and pericardial effusion were strongly associated with event‐free survival with thresholds suggested by the ESC/ERS guidelines consistent with a 20% risk of death or transplantation at 1 year (Figure 4b−d).
Figure 4.
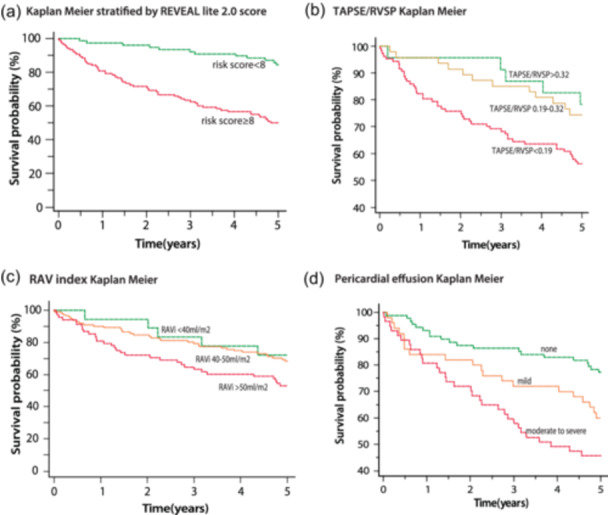
Cox regression analysis based on risk score and echocardiographic parameters (a) Kaplan−Meier stratified by Reveal lite score < 8 and ≥ 8. (b) Kaplan−Meier stratified by three groups of TAPSE/RVSP (c) Kaplan Meier stratified by three groups of RAV index. (d) Kaplan−Meir based on the presence of absence of pericardial effusion. RVSP, right ventricular systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
Table 4 summarized the high‐risk severity thresholds (20% 1 year mortality risk) for significant echocardiographic parameters related to outcome; the table outlines the receiver operating characteristic (ROC) value, the calibrated threshold, the value of the metric derived using the mathematical relationships in Table 3 anchors to the RVFAC values and the median value of the REVEAL score 8−9. (Supporting Information: e‐Table 3) presents the criterion obtained using sensitivity/specificity cross‐over, Youden index or cost analysis (Supporting Information: e‐Figure 2). In general, the classifier thresholds using cost analysis and Youden index were consistent with each other but higher than cross‐over values. Example of Youden index and Cost analysis are presented in Supporting Information: Figure 2 while Kaplan−Meier curves are presented in Supporting Information: e‐Figure 3. Of note, using classifier criterion won't necessarily yield a calibrated threshold to risk and therefore selection was guided by the nearest unit. Supporting Information: e‐Table 4 presents the scaled hazard ratios (per SD) for comprehensive clinical and echocardiographic metrics. Among echocardiographic parameters, TAPSE was borderline significant as were antero‐posterior RV dimensions and LVEF while RV antero‐posterior shortening was not associated with outcome. Among laboratory data, the strongest predictors were hemoglobin concentration, NT‐proBNP and glomerular filtration rate. Table 5 summarizes the thresholds obtained by our analysis with limits of reference informed by ASE and ESC/ERS guidelines, recent WASE initiative studies and selected studies. 29 , 30 , 31
Table 4.
Identification of thresholds using outcome cost‐based analysis and mathematical and risk score consistency.
| Outcome based ROC | Thresholds (calibrated to 1 y survival) | Physiological Consistency (centered on RVFAC relat.) | REVEAL lite (centered on 8/9 score) | |
|---|---|---|---|---|
| REVEAL lite | 0.74 (0.04) | ≥8 | 8.0 | ‐ |
| NT‐proBNP | 0.65 (0.04) | >1100 | 1031 | 1626 |
| RV function | ||||
| RVFAC (%) | 0.63 (0.04) | <19 | 19† | 18 |
| RVFWS (%) | 0.64 (0.04) | <10 | 12 | 10 |
| TAPSE/RVSP (mm/mmHg) | 0.59 (0.04) | <0.19 | 0.18 | 0.16 |
| RV base (end‐diastolic) | ||||
| RVEDD base (mm) | 0.62 (0.04) | >55 | 55 | 56 |
| RVEDDi (ht) (mm/m) | 0.63 (0.04) | >33 | 34 | 34 |
| RVEDD/LVID | 0.60 (0.04) | >1.5 | 1.5 | 1.5 |
| RV mid (end‐diastolic) | ||||
| RVEDD mid (mm) | 0.64 (0.04) | >50 | 49 | 51 |
| RVEDDi mid (ht) (mm/m) | 0.64 (0.04) | >30 | 30 | 31 |
| RVEDD mid/LVID | 0.62 (0.04) | >1.4 | 1.3 | 1.4 |
| RV areas (end‐diastolic) | ||||
| RVEDAi (bsa)(cm2) | 0.62 (0.04) | >21 | 21 | 21 |
| RVESAi (bsa) | 0.63 (0.04) | >17 | 17 | 18 |
| RVEDAr | 0.59 (0.04) | >2 | 2 | 2.0 |
| RVESAr | 0.62 (0.04) | >3 | 3 | 3.0 |
| RA size (maximal) | ||||
| RAA (cm2) | 0.58 (0.04) | >26 | 24 | 24 |
| RAAi (cm2/m2) | 0.60 (0.04) | >14 | 13 | 13 |
| RAVi (mL/m2) | 0.60 (0.04) | >50 | 47 | 43 |
| RAAr | 0.58 (0.04) | >1.9 | 1.8 | 1.7 |
| TR severity (grade) | 0.57 (0.04) | >2 | >2 | 2 |
| eRAP (mmHg) | 0.61 (0.04) | >8 | 8 | 15 |
| rRAP (RHC) | 0.60 (0.01) | >14 | >14 | 11 |
Abbreviations: PAWP, pulmonary capillary wedge pressure; RA, right atrial; RAP, RA pressure; RHC, right heart catheterization; ROC, receiver operating characteristic; RV, right ventricular; RVFAC, RV fractional area change; RVFWS, RV free wall longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
Table 5.
Grading severity of right heart remodeling, dysfunction based on outcome analysis.
| Limits of reference based on ASE, ESC, WASE or other | Mild | Moderate | Severe | |
|---|---|---|---|---|
| RV function | ||||
| RVFAC (%) 31 | 35 | 28−34 | 19−27 | <19 |
| RVFWS (%) (absolute) 31 | 20 | 16−19 | 10−15 | <10 |
| TAPSE/RVSP (mm/mmHg) | 0.55 | 0.33−0.55 | 0.19−0.32 | <0.19 |
| RV base diameters | ||||
| RVEDD base (mm) 31 , 32 | 45/40 | UL‐48 | 49−54 | >55 |
| RVEDDI (ht) (mm/m) 31 | 25.7/24.5 | 26−28 | 29−33 | >34 |
| RVEDD/LVID 3 | 0.8 | 0.9−1.1 | 1.2−1.4 | >1.5 |
| RV mid diameters | ||||
| RVEDD mid (mm) 31 , 32 | 41/34 | UL‐43 | 44−49 | >50 |
| RVEDDI mid (ht) (mm/m) | 23.7/21.5 | UL‐25 | 25−29 | >30 |
| RV areas | ||||
| RVEDAi (bsa) (cm2/m2) 31 | 13.6/12.2 | UL‐17 | 18−21 | >21 |
| RVESAi (bsa) (cm2/m2) 31 | 7.9/7.1 | UL‐13 | 14−17 | >17 |
| RVEDAr | 0.9 | 1.0−1.3 | 1.4−2.0 | >2.0 |
| RVESAr | 0.9 | 1.0−2 | 2.0−3.0 | >3.0 |
| RA size | ||||
| RA area (cm2) 3 , 32 | 18 | UL‐21 | 22−26 | >26 |
| RAVI (BSA) (mL/m2) 32 | 32/28 | UL‐40 | 40−50 | >50 |
Abbreviations: PAWP, pulmonary capillary wedge pressure; RA, right atrial; RAP, RA pressure; RHC, right heart catheterization; RV, right ventricular; RVFAC, RV fractional area change; RVFWS, RV free wall longitudinal strain; TAPSE, tricuspid annular plane systolic excursion.
On multivariable Cox analysis, REVEAL lite score ≥ 8, RVFWS < 10% and the presence of anemia (Hb < 13 g/dL) were independently associated with outcome with a small improvement in AUC of 0.763 (p < 0.001) compared to REVEAL lite score with 0.741 (p < 0.001) (Supporting Information: e‐Figure 4) with the upset plot showing the combination of metrics.
Incorporating parameter thresholds into the REVEAL‐ECHO framework
We tested whether the thresholds identified in the current study could be applied to the recently described REVEAL‐ECHO score. 23 In our cohort, the median REVEAL‐ECHO score was 5 [3–6]. The REVEAL‐ECHO was associated with survival and risk categories were associated with survival on Kaplan−Meier analysis (Figure 5).
Figure 5.
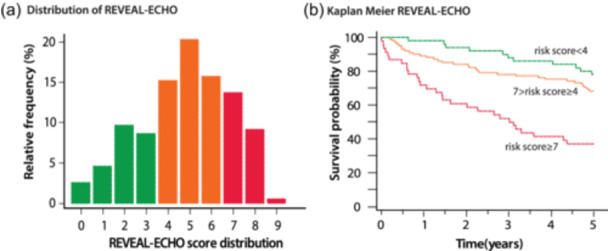
REVEAL lite 2.0 and REVEAL‐ECHO Score with quantitative echocardiographic criteria. (a) Distribution of the REVEAL‐ECHO score in our population. (b) Kaplan−Meier stratified by three groups of REVEAL‐ECHO (risk score < 4, risk score between 4 and 7 and risk score ≥ 7.
DISCUSSION
Our study leveraged physiological relationships and outcome analysis to identify consistent echocardiographic thresholds for risk stratification in PAH. We first quantified the relationships between right heart metrics in PAH highlighting the strong relationship between RVFAC and RVFWS. In contrast, a weaker relationship was found between TAPSE and RVFAC or RVFWS. Second, we validated in an independent cohort, the echocardiographic criteria proposed by the ESC/ESR guidelines for TAPSE/sPAP, RA area and pericardial effusion. We further proposed using calibrated outcome analysis consistent echocardiographic thresholds for risk stratification and grading the severity of right heart maladaptation. The different thresholds were also concordant with their mathematical relationship as well as with median REVEAL lite score of 8. Finally, we validated the recently described REVEAL‐ECHO score using quantitative criteria identified in our study. In will be interesting to compare our outcome driven criteria with the upcoming right heart chamber quantification thresholds proposed by the ASE.
Previous studies have analyzed the relationship between right heart variables using echocardiography and CMR. In patients with precapillary PH, Kind et al. showed than mid transverse fractional shortening was more closely related to RVEF and functional status than measures of tricuspid annular displacements. 20 In a recent 3D echocardiography study on 151 consecutive patients with chronic thromboembolic disease, Tao et al. 32 highlighted the strong relationship between RVEF and RVFWS (r = −0.70). In multimodality CMR and echocardiographic cohorts, Evaldsson et al. 19 as well as Shiran et al. 28 have also shown the relationships between RVEF and RVFAC or RVFWS. Our study builds on these previous studies by quantifying these relationships (mean function and variance terms) in a larger cohort of patients with PAH. The fact that we observed a stronger relationship between RVFAC, RVFWS and RV areas than with TAPSE is not surprising. In fact, as PAH progress, the right heart often dilates, a process known as heterometric adaptation. 33 The strong association between RVEF, RVFAC, RVFWS, RVTS (all fractional spatial change metrics) can also be explained in part by the dimensional dependency between 3D (volume), 2D (area) and axial (shortening) fractional changes as the right ventricle dilates. In contrast, TAPSE reflects an excursion rather than a relative change and per se will not be as strongly associated with other metrics. Our study also highlights as did the previous work of Kind et al. 26 that both transverse and longitudinal contribute to right heart function in PAH. This is consistent with the architecture of the right heart showing both longitudinal orientated subendocardial myofibers and mid‐wall oriented myofibers. 17 , 34 The observed mathematical relationships have implications for clinical practice. For example, the presence of highly discordant values between RVFAC and RVFWS both cross‐sectionally and longitudinally should prompt repeated measures to ensure no error of measurement occurred. In addition, the strong association between many variables will likely simplify the number of features needed for phenomapping and profiling. Finally, these mathematical relationships may further verify consistency in grading between metrics.
Echocardiography and CMR based markers have been recently incorporated to the 2022 ESC/ERS guidelines for the diagnosis and treatment of PH. 3 For CMR variables, the risk stratification three strata model has included for severe thresholds: RVEF (37%), RVES volume index (54 mL/m2) and stroke volume index (<26 mL/m2) in large part based on the study of Lewis et al. 22 For echocardiographic variables, the presence of moderate pericardial effusion, TAPSE/RVSP < 0.19 mm/mmHg and RA area (> 26cm2) were chosen. 3 Different methods have been used in the literature to evaluate thresholds of severity including 1 pre‐selection guided by reference limits, or hemodynamically guided thresholds (based on stroke volume or PVR), 2 guided by outcome analysis using a Youden index criteria or 3 based on quantile‐risk assessment. In the study of Forfia et al. 11 the threshold of 18 mm for TAPSE was based on ROC analysis of stroke volume index of 29 mL/m2. The threshold for RV relative area of Goda et al. 35 of 0.93 was based on a ROC analysis for a PVR threshold of 3 Wood units. In the study of Fine et al. 6 lower quintiles of RVFWS corresponding to −15% was based on quintile risk association. 6 For RA area indexed values, the study of Raymond et al. 8 and Murata et al. 36 both used median values yielding threshold of 19 cm2/m2 and 12 cm2/m2, respectively. In the study of Kind et al. 20 an RVEF threshold of 35% was based on ROC analysis (Youden index) for the combined end‐point of death or transplantation in a cohort 110 patients with incident PAH. The CMR study of Lewis et al. was comprehensive with a derivation and validation cohort and used quintile‐risk associated as well as nonlinear threshold modeling; of note in their study high risk was defined using the previous 10% risk thresholds which now correspond more to the moderate range. 22
Original to our study, we used multiple criteria to ensure a consistent grading system including calibrated outcome analysis, median REVEAL score values and physiological relationships. The fact that the values for RA area, TAPSE/RVSP and pericardial effusion coincided with the guideline thresholds adds confidence to the representative nature of our cohort (for the high‐risk strata). In addition to these variables, other consistent thresholds for other commonly used variables especially right heart enlargement were derived. Right to left chamber indexing also provided internal scaling measures that emerged valuable in the analysis. Using our quantitative criteria, we were also able to validate the REVEAL‐ECHO score in an independent cohort. Finally, our multivariable outcome analysis highlights that right heart imaging parameters and other markers of end‐organ dysfunction (anemia) can be complementary to the REVEAL lite score.
Our study also has limitations that should be mentioned. First, the selection of patients with echocardiography and RHC close by favors a higher risk population that may not be representative to a treatment naïve cohort. This has, however, allowed the selection of a cohort that would allow high risk strata analysis. Second, the measures were performed in a core laboratory setting dedicated to right heart measures and may not reflect usual clinical practice. Finally, criterion selection should always be nuanced by methodologic considerations and each laboratory should test its transferability. For example, RVFWS measured in our studies was performed at the mid‐myocardial levels and includes the apical region which is likely several percentages lower than speckle tacking based values.
In conclusion, using outcome‐based analysis and physiological relationships we propose a consistent grading system for right heart function and remodeling in PAH.
AUTHOR CONTRIBUTIONS
Bettia Celestin and Francois Haddad contributed to the study design. Bettia Celestin and Shadi Bagherzadeh acquired the data. Bettia Celestin, Francois Haddad, Kenzo Ichimura and Everton J. Santana analyzed the data. Bettia Celestin and Francois Haddad conceptualized the study conception, interpreted the data, and participated in drafting the manuscript. Shadi Bagherzadeh, Kenzo Ichimura, Pablo Amador Sanchez, Michael Salerno, Roham T. Zamanian, Andrew J. Sweatt, Anna R. Hemnes, Anton Vonk Noordegraaf, Tobore Tobore and Everton J. Santana revised it critically for important intellectual content. All authors approved the final version for publication and take responsibility for appropriate portions of the content.
CONFLICTS OF INTEREST STATEMENT
Bettia Celestin, Shadi P. Bagherzadeh, Kenzo Ichimura, Everton J. Santana, Andrew John Sweatt and Pablo Amador Sanchez, have no conflicts to report. Francois Haddad has received funding from Janssen Inc. to conduct the research. Tobore Tobore is a senior director of Medical Affairs at Janssen. Anton Vonk Noordegraaf is supported by the Netherlands CardioVascular Research Initiative (CVON‐2012‐08 PHAEDRA, CVON‐2017‐10 DOLPHIN‐GENESIS) and the Netherlands Organization for Scientific Research (NWO‐VICI: 918.16.610). In addition, his institute received speakers money from Johnson & Johnson, MSD, Actelion, Bayer and Ferrer in the past 3 years. Finally, he served as a member of the scientific advisory board of Morphogen‐X, Ferrer, Gosammer Bio Services Inc, Altavant, MSD and Johnson & Johnson. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
Ethical approval for this study was obtained from Stanford University Institutional Review Board and was conducted under the Cardio Share protocol (IRB#25673) and all patients gave written informed consent.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
This study was supported by Actelion Pharmaceuticals, Janssen Company of Johnson & Johnson (Investigator Initiated Study IIS—Computational Approaches to Surrogate end‐point research in pulmonary hypertension and Actelion has reviewed this publication. In addition, the work was also supported by PO1 research grant, Stanford Cardiovascular Institute, Vera Moulton Wall Center for Pulmonary Vascular Disease. This work has been funded through research grants from Stanford Cardiovascular Institute and Janssen Investigator initiated study (Computational Approaches to surrogate end‐point research in pulmonary hypertension). Bettia Celestin is the guarantor of the content of the manuscript, including data and analysis.
Celestin BE, Bagherzadeh SP, Ichimura K, Santana EJ, Sanchez PA, Tobore T, Hemnes AR, Vonk Noordegraaf A, Salerno M, Zamanian RT, Sweatt AJ, Haddad F. Identifying consistent echocardiographic thresholds for risk stratification in pulmonary arterial hypertension. Pulm Circ. 2024;14:e12361. 10.1002/pul2.12361
Contributor Information
Bettia E. Celestin, Email: bcelest@stanford.edu.
Francois Haddad, Email: fhaddad@stanford.edu.
REFERENCES
- 1. Frost AE, Badesch DB, Barst RJ, Benza RL, Elliott CG, Farber HW, Krichman A, Liou TG, Raskob GE, Wason P, Feldkircher K, Turner M, McGoon MD. The changing picture of patients with pulmonary arterial hypertension in the United States. Chest. 2011;139(1):128–137. [DOI] [PubMed] [Google Scholar]
- 2. Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa‐Hahnle K, Jing ZC, Gibbs JSR. A global view of pulmonary hypertension. The Lancet Respiratory Medicine. 2016;4(4):306–322. [DOI] [PubMed] [Google Scholar]
- 3. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S, Schwerzmann M, Dinh‐Xuan AT, Bush A, Abdelhamid M, Aboyans V, Arbustini E, Asteggiano R, Barberà JA, Beghetti M, Čelutkienė J, Cikes M, Condliffe R, de Man F, Falk V, Fauchier L, Gaine S, Galié N, Gin‐Sing W, Granton J, Grünig E, Hassoun PM, Hellemons M, Jaarsma T, Kjellström B, Klok FA, Konradi A, Koskinas KC, Kotecha D, Lang I, Lewis BS, Linhart A, Lip GYH, Løchen ML, Mathioudakis AG, Mindham R, Moledina S, Naeije R, Nielsen JC, Olschewski H, Opitz I, Petersen SE, Prescott E, Rakisheva A, Reis A, Ristić AD, Roche N, Rodrigues R, Selton‐Suty C, Souza R, Swift AJ, Touyz RM, Ulrich S, Wilkins MR, Wort SJ. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–3731. [DOI] [PubMed] [Google Scholar]
- 4. Brierre G, Blot‐Souletie N, Degano B, Tetu L, Bongard V, Carrie D. New echocardiographic prognostic factors for mortality in pulmonary arterial hypertension †. Eur J Echocardiogr. 2010;11(6):516–522. [DOI] [PubMed] [Google Scholar]
- 5. Cassady SJ, Ramani GV. Right heart failure in pulmonary hypertension. Cardiol Clin. 2020;38(2):243–255. [DOI] [PubMed] [Google Scholar]
- 6. Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, Kane GC. Outcome prediction by quantitative right ventricular function assessment in 575 subjects evaluated for pulmonary hypertension. Circ Cardiovas Imag. 2013;6(5):711–721. [DOI] [PubMed] [Google Scholar]
- 7. Ghio S, Klersy C, Magrini G, D'Armini AM, Scelsi L, Raineri C, Pasotti M, Serio A, Campana C, Viganò M. Prognostic relevance of the echocardiographic assessment of right ventricular function in patients with idiopathic pulmonary arterial hypertension. Int J Cardiol. 2010;140(3):272–278. [DOI] [PubMed] [Google Scholar]
- 8. Raymond RJ, Hinderliter AL, Willis PW, Ralph D, Caldwell EJ, Williams W, Ettinger NA, Hill NS, Summer WR, de Boisblanc B, Schwartz T, Koch G, Clayton LM, Jöbsis MM, Crow JW, Long W. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. JACC. 2002;39(7):1214–1219. [DOI] [PubMed] [Google Scholar]
- 9. Ryan JJ, Huston J, Kutty S, Hatton ND, Bowman L, Tian L, Herr JE, Johri AM, Archer SL. Right ventricular adaptation and failure in pulmonary arterial hypertension. Can J Cardiol. 2015;31(4):391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, Levy PS, Pietra GG, Reid LM, Reeves JT, Rich S, Vreim CE, Williams GW, Wu M. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115(5):343–349. [DOI] [PubMed] [Google Scholar]
- 11. Forfia PR, Fisher MR, Mathai SC, Housten‐Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174(9):1034–1041. [DOI] [PubMed] [Google Scholar]
- 12. Haddad F, Contrepois K, Amsallem M, Denault AY, Bernardo RJ, Jha A, Taylor S, Arthur Ataam J, Mercier O, Kuznetsova T, Vonk Noordegraaf A, Zamanian RT, Sweatt AJ. The right heart network and risk stratification in pulmonary arterial hypertension. Chest. 2022;161(5):1347–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amsallem M, Sweatt AJ, Aymami MC, Kuznetsova T, Selej M, Lu H, Mercier O, Fadel E, Schnittger I, McConnell MV, Rabinovitch M, Zamanian RT, Haddad F. Right heart End‐Systolic remodeling index strongly predicts outcomes in pulmonary arterial hypertension: comparison with validated models. Circ Cardiovasc Imag. 2017;10(6):e005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Swift AJ, Capener D, Johns C, Hamilton N, Rothman A, Elliot C, Condliffe R, Charalampopoulos A, Rajaram S, Lawrie A, Campbell MJ, Wild JM, Kiely DG. Magnetic resonance imaging in the prognostic evaluation of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;196(2):228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu K, Zhang C, Chen B, Li M, Zhang P. Association between right atrial area measured by echocardiography and prognosis among pulmonary arterial hypertension: a systematic review and meta‐analysis. BMJ Open. 2020;10(9):e031316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Querejeta Roca G, Campbell P, Claggett B, Solomon SD, Shah AM. Right atrial function in pulmonary arterial hypertension. Circ Cardiovas Imag. 2015;8(11):e003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bidviene J, Muraru D, Maffessanti F, Ereminiene E, Kovács A, Lakatos B, Vaskelyte JJ, Zaliunas R, Surkova E, Parati G, Badano LP. Regional shape, global function and mechanics in right ventricular volume and pressure overload conditions: a three‐dimensional echocardiography study. Int J Cardiovasc Imaging. 2021;37(4):1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kovács A, Lakatos B, Tokodi M, Merkely B. Right ventricular mechanical pattern in health and disease: beyond longitudinal shortening. Heart Fail Rev. 2019;24(4):511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Evaldsson AW, Lindholm A, Jumatate R, Ingvarsson A, Smith GJ, Waktare J, Rådegran G, Roijer A, Meurling C, Ostenfeld E. Right ventricular function parameters in pulmonary hypertension: echocardiography vs. cardiac magnetic resonance. BMC Cardiovasc Disord. 2020;20(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kind T, Mauritz GJ, Marcus JT, van de Veerdonk M, Westerhof N, Vonk‐Noordegraaf A. Right ventricular ejection fraction is better reflected by transverse rather than longitudinal wall motion in pulmonary hypertension. J Cardiovasc Magn Reson. 2010;12(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. JACC. 2017;69(2):236–243. [DOI] [PubMed] [Google Scholar]
- 22. Lewis RA, Johns CS, Cogliano M, Capener D, Tubman E, Elliot CA, Charalampopoulos A, Sabroe I, Thompson AAR, Billings CG, Hamilton N, Baster K, Laud PJ, Hickey PM, Middleton J, Armstrong IJ, Hurdman JA, Lawrie A, Rothman AMK, Wild JM, Condliffe R, Swift AJ, Kiely DG. Identification of cardiac magnetic resonance imaging thresholds for risk stratification in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2020;201(4):458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. El‐Kersh K, Zhao C, Elliott G, Farber HW, Gomberg‐Maitland M, Selej M, Garcia‐Ferrer J, Benza R. Derivation of a risk score (REVEAL‐ECHO) based on echocardiographic parameters of patients with pulmonary arterial hypertension. Chest. 2023;163(5):1232–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Du Bois D. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;XVII(6_2):863–871. [Google Scholar]
- 25. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European Association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28(1):1–39 e14. [DOI] [PubMed] [Google Scholar]
- 26. Kind T, Marcus JT, Westerhof N, Vonk‐Noordegraaf A. Longitudinal and transverse movements of the right ventricle. Chest. 2011;140(2):556–557. [DOI] [PubMed] [Google Scholar]
- 27. Benza RL, Kanwar MK, Raina A, Scott JV, Zhao CL, Selej M, Elliott CG, Farber HW. Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL lite 2, for use in patients with pulmonary arterial hypertension. Chest. 2021;159(1):337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shiran H, Zamanian RT, McConnell MV, Liang DH, Dash R, Heidary S, Sudini NL, Wu JC, Haddad F, Yang PC. Relationship between echocardiographic and magnetic resonance derived measures of right ventricular size and function in patients with pulmonary hypertension. J Am Soc Echocardiogr. 2014;27(4):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Addetia K, Miyoshi T, Citro R, Daimon M, Gutierrez Fajardo P, Kasliwal RR, Kirkpatrick JN, Monaghan MJ, Muraru D, Ogunyankin KO, Park SW, Ronderos RE, Sadeghpour A, Scalia GM, Takeuchi M, Tsang W, Tucay ES, Tude Rodrigues AC, Vivekanandan A, Zhang Y, Schreckenberg M, Mor‐Avi V, Asch FM, Lang RM. Two‐dimensional echocardiographic right ventricular size and systolic function measurements stratified by sex, age, and ethnicity: results of the world alliance of societies of echocardiography study. J Am Soc Echocardiogr. 2021;34(11):1148–1157.e1. [DOI] [PubMed] [Google Scholar]
- 30. Carvalho Singulane C, Singh A, Miyoshi T, Addetia K, Soulat‐Dufour L, Schreckenberg M, Blankenhagen M, Hitschrich N, Amuthan V, Citro R, Daimon M, Gutiérrez‐Fajardo P, Kasliwal R, Kirkpatrick JN, Monaghan MJ, Muraru D, Ogunyankin KO, Park SW, Tude Rodrigues AC, Ronderos R, Sadeghpour A, Scalia GM, Takeuchi M, Tsang W, Tucay ES, Zhang Y, Mor‐Avi V, Asch FM, Lang RM. Sex‐, age‐, and Race‐Related normal values of right ventricular diastolic function parameters: data from the world alliance societies of echocardiography study. J Am Soc Echocardiogr. 2022;35(4):426–434. [DOI] [PubMed] [Google Scholar]
- 31. Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American society of echocardiography endorsed by the European Association of echocardiography, a registered branch of the european society of cardiology, and the Canadian society of echocardiography. J American Soc Echocardio. 2010;23(7):685–713. [DOI] [PubMed] [Google Scholar]
- 32. Tao X, Liu M, Liu W, Xie W, Wan J, Zhai Z, Wang C. CMR‐based heart deformation analysis for quantification of hemodynamics and right ventricular dysfunction in patients with CTEPH. Clin Respirat J. 2020;14(3):277–284. [DOI] [PubMed] [Google Scholar]
- 33. Naeije R, Brimioulle S, Dewachter L. Biomechanics of the right ventricle in health and disease (2013 Grover Conference series). Pulm Circ. 2014;4(3):395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haber I, Metaxas DN, Geva T, Axel L. Three‐dimensional systolic kinematics of the right ventricle. American J Physiol Heart Circu Physiol. 2005;289(5):H1826–H1833. [DOI] [PubMed] [Google Scholar]
- 35. Goda A, Ryo K, Delgado‐Montero A, Tayal B, Handa R, Simon MA, Gorcsan J. The prognostic utility of a simplified biventricular echocardiographic index of cardiac remodeling in patients with pulmonary hypertension. J Am Soc Echocardiogr. 2016;29(6):554–560. [DOI] [PubMed] [Google Scholar]
- 36. Murata M, Tsugu T, Kawakami T, Kataoka M, Minakata Y, Endo J, Tsuruta H, Itabashi Y, Maekawa Y, Fukuda K. Right ventricular dyssynchrony predicts clinical outcomes in patients with pulmonary hypertension. Int J Cardiol. 2017;228:912–918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


