Key Teaching Points.
-
•
Catheter ablation of premature ventricular contractions originating from the papillary musculature is particularly challenging given the complexity of anatomy and contractile nature of the tissue. Remote magnetic navigation could be used to overcome these challenges.
-
•
Some patients do not have any arrhythmia when presenting for ablation of ventricular arrhythmias, leaving conventional mapping methods redundant. Noninvasive electrocardiographic imaging, such as that employed with View Into Ventricular Onset (Catheter Precision Inc), opens up the possibility of ablating such patients in the absence of arrhythmia in the electrophysiology lab.
-
•
There is a global need for the training of more cardiac electrophysiologists. Telerobotic approaches to catheter ablation would allow current specialists to assist remotely and “tele-proctoring” initiatives are in development.
Introduction
Premature ventricular contractions (PVCs) are a common but complex clinical problem. The general consensus is that catheter ablation is preferred to antiarrhythmic drugs for patients with a high PVC burden or who are symptomatic,1 with ablation success rates of about 85%2 compared to 70% with amiodarone.3
PVCs originating from the papillary muscles are notoriously difficult to localize and ablate4 owing to their complex anatomy, relatively thick musculature, and contractile nature. Additionally, conventional 3-dimensional (3D) mapping systems typically project to a cardiac “shell” as opposed to visualizing an internal structure. The use of remote magnetic navigation to guide PVC ablation has previously been shown to be noninferior to conventional manual catheter ablation.5 Moreover, magnetic navigation may provide an improved catheter stabilization in such a situation. We used the noninvasive mapping tool View Into Ventricular Onset™ (VIVO; Catheter Precision Inc, Fort Mill, SC) to guide the ablation of a papillary muscle PVC origin in a patient with rare ectopy on the day of the ablation procedure.
Case report
A 39-year-old man was referred to the Princess Grace Hospital Centre, Monaco, in February 2022 for a second attempt at catheter ablation of symptomatic PVCs after a previous ablation in 2021 at his local center was unsuccessful. The patient initially had a PVC burden of 20% and after the first ablation this was reduced to 17% on 24-hour Holter. The patient had no other significant past medical or psychosocial history, and examination findings were unremarkable.
The patient had initially been investigated for reversible causes of PVCs, such as electrolytic disorders and thyroid dysfunction; however, these were ruled out. A cardiac magnetic resonance scan showed a structurally normal heart with preserved systolic function, normal left ventricular volume, and no late gadolinium enhancement. A repeat 24-hour Holter recording showed a monomorphic PVC burden of 17%. The patient was diagnosed with idiopathic PVCs and was trialed on bisoprolol, which was discontinued as per patient’s preference.
The initial attempt at catheter ablation had been performed where the PVC origin was localized via electroanatomic mapping (CARTO 3; Biosense Webster, Diamond Bar, CA) with a multielectrode catheter (PentaRay, Biosense Webster) to the free wall of the right ventricle. Despite multiple ablation lesions, suppression but not complete elimination of the clinical PVC was achieved.
Therefore, the decision was made to proceed with a second ablation attempt using remote magnetic navigation and the noninvasive electrocardiogram imaging (ECGi) platform VIVO was used. VIVO combines the raw data from a 12-lead electrocardiogram (ECG) or Holter monitor recording with either computed tomography or cardiac magnetic resonance imaging and a 3D photograph of the patient’s thorax to produce a patient-specific model of activation—an “electrical roadmap”—that has been previously shown to accurately predict the site of origin of PVCs.6
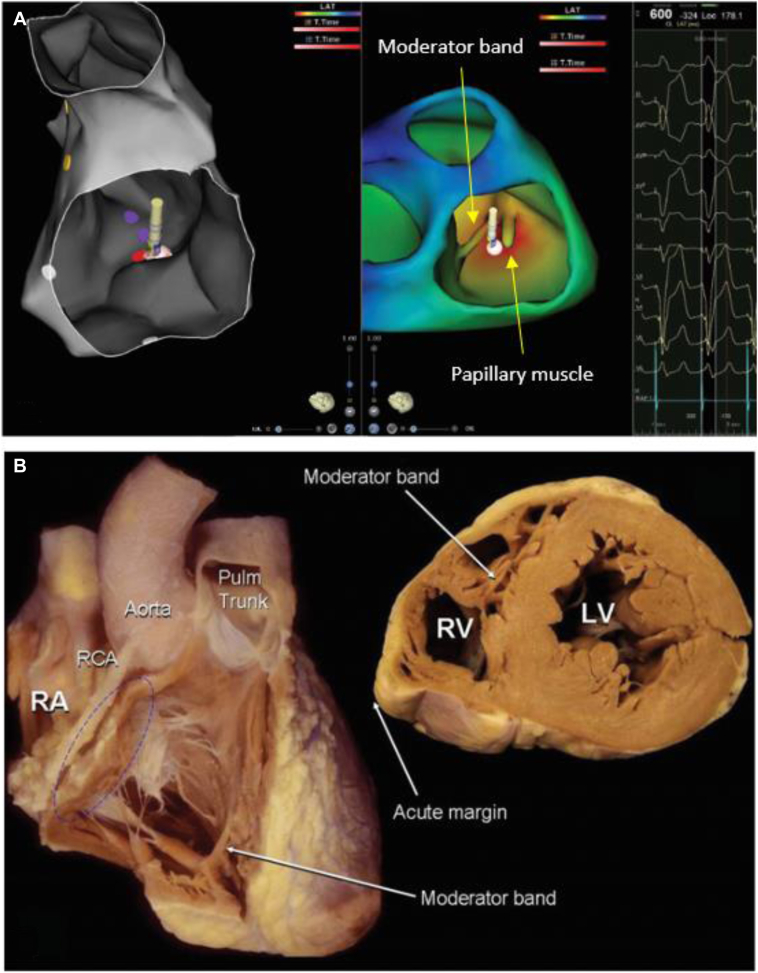
The patient was admitted a day before the procedure to allow for 12-lead Holter monitoring to assess PVC burden and morphology of the PVC(s). Despite discontinuation of antiarrhythmic medication, only 1 PVC was captured during overnight Holter monitoring. The 12-lead ECG (Figure 1A) had a left bundle branch block morphology with a positive QRS in leads I and aVL demonstrating a left-superior axis. The QRS transition point was between V4 and V5, consistent with a relatively apical origin. Inferior lead morphology suggested a right-sided origin. This single PVC was uploaded into the ECGi software, which localized the site of origin to be at the base of insertion of the anterior papillary muscle of the tricuspid valve (Figure 2). A discussion was held with the patient and the decision was jointly made to proceed with ablation.
Figure 1.
A: Premature ventricular contraction (PVC) seen on 12-lead Holter monitoring showing a negative V1, a superior axis, and QRS transition at V4–V5. B: “Warm-up” PVCs seen on the patient’s 12-lead electrocardiogram at the time of radiofrequency delivery.
Figure 2.
A: Reconstruction of the right ventricle (RV) in CARTO (left) and in VIVO (right) showing site of origin arising from the base of the anterior papillary muscle of the tricuspid valve adjacent to the moderator band. B: Relevant cardiac anatomy demonstrated by gross anatomical specimen (used with permission from Anatomy for Cardiac Electrophysiologists: A Practical Handbook7). LV = left ventricle; Pulm = pulmonary; RA = right atrium; RCA = right circumflex artery; RV = right ventricle.
A remotely located second expert operator joined the invasive team on the day of the procedure to provide support for the use of ECGi in combination with remote magnetic navigation. Despite multiple attempts, including isoprenaline infusion and pacing maneuvers in the electrophysiology laboratory, no PVCs could be induced, so it was decided that an ablation attempt would be performed using the ECGi activation map to determine the ablation target. The right ventricle was mapped in sinus rhythm using the CARTO 3 system, by fast anatomical mapping and point-by-point acquisition of electrograms using an irrigated tip catheter (NaviStar ThermoCool RMT; Biosense Webster, Irvine, CA). The RV electroanatomical map was manually merged with the biventricular VIVO reconstruction. Using a side-by-side view, the ECGi image was used to guide pace mapping of the anterior papillary muscle of the tricuspid valve. A near-match was found between the morphology of the pace-mapped beat and the single PVC captured prior to ablation and radiofrequency was delivered in this region. During radiofrequency delivery, a “warm-up” occurred whereby PVCs were seen with the same morphology as the single beat seen on the original Holter monitoring (Figure 1B). As the initial radiofrequency delivery resulted in “warming up” of the PVC with identical morphology, the local operator elected to ablate the same region tagged during the first delivery with a significant impedance drop. Various magnetic vectors were applied to achieve different tissue/tip orientations to create a large enough lesion. There were no PVCs in the 30-minute postablation waiting period and the procedure was considered complete. The total procedure time was 120 minutes with only 1 minute of fluoroscopy exposure and 403 seconds of radiofrequency delivery.
The patient was followed up by the referring team and a 7-day Holter recording at 14 months showed no PVCs.
Discussion
Mapping the site of origin of ventricular arrhythmias with focal propagation conventionally requires the presence of the arrhythmia during the electrophysiology study to allow for localization via electroanatomic mapping. Many factors, including the use of antiarrhythmic drugs, sedation, or general anesthesia, can result in suppression of these arrhythmias and hinders the likelihood of a successful ablation. PVC frequency is often variable, in which circumstance the use of noninvasive ECGi may act as an alternative mapping method or may even be used to guide ablation in the first instance for patients with paucity of PVCs at the time of the procedure.
Arrhythmias arising from the papillary muscles are challenging to ablate. Their anatomy and thick musculature, along with difficulties in achieving good catheter stability and contact force owing to papillary muscle contractions, make catheter ablation difficult.7 There are multiple algorithms that attempt to localize ventricular arrhythmias using the 12-lead ECG; however, such tools are more prone to human error and have limited specificity.8,9 Additionally, one of the most common approaches to ablation of PVCs is to map the arrhythmia via pace mapping; however, this requires the presence of many spontaneous PVCs to occur during the procedure.10
The concept of the VIVO mapping system has been previously described in detail.6 In brief, it calculates vector cardiograms from the ECG using the anatomical positions of the electrodes from a 3D photograph of the patient’s thorax. The PVC burden, number of morphologies, and total recording time were recorded. Templates of the PVC morphologies were imported into the VIVO system. Using either computed tomography or cardiac magnetic resonance imaging, 3D reconstructions (an “electrical roadmap”) were created for each PVC morphology and imported as VTK files into our 3D electroanatomic mapping system (CARTO; Biosense Webster).
A recent study by Bassil and colleagues11 demonstrated similar acute success rates between manual ablation and robotic magnetic navigation (RMN) ablation of ventricular arrhythmias arising from the papillary muscles.11 Unlike the rigid catheters used in manual ablation, the catheters used for RMN ablation are flexible and allow for greater catheter stability when navigating the papillary musculature.12 In a meta-analysis of manual ablation vs RMN for ablation of ventricular tachycardia, RMN resulted in reduced fluoroscopy, lower procedure times, and fewer complications.13 However, the fact that this meta-analysis did not include tachycardia arising from the papillary musculature should be considered.
Patients commonly travel great distances to be seen by specialists, or expert operators will travel to other countries to perform complex procedures to train local teams in novel technologies such as ECGi. With the advent of the COVID-19 pandemic, traveling became difficult if not impossible at times, and the development of a robust telerobotic approach to catheter ablation foregoes the need for this travel. Shinoda and colleagues14 report on an initial experience of a more experienced “advisor” assisting with radiofrequency catheter ablation cases using an audiovisual telesupport system.
In summary, this case highlights the benefits that noninvasive 3D mapping with ECGi can bring to the ablation of ventricular arrhythmias such as (rare) PVCs.15 The ECGi tool we have used in this case report can reconstruct patient-specific intracardiac structures and integrate the resulting simulated activation model into CARTO. The ability to successfully ablate these patients using data from a single heartbeat means that ablation is possible for an entire cohort of patients that otherwise could not be treated. The data on the applications of the VIVO technology is still limited; however, we are excited to see its future potential.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgments
Funding Sources
None.
References
- 1.Priori S., Blomström-Lundqvist C., Mazzanti A., et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 2.Latchamsetty R., Bogun F. Premature ventricular complexes and premature ventricular complex induced cardiomyopathy. Curr Prob Cardiol. 2015;40:379–422. doi: 10.1016/j.cpcardiol.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Singh S., Fletcher R., Fisher S., et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med. 1995;333:77–82. doi: 10.1056/NEJM199507133330201. [DOI] [PubMed] [Google Scholar]
- 4.Naksuk N., Kapa S., Asirvatham S. Spectrum of ventricular arrhythmias arising from papillary muscle in the structurally normal heart. Card Electrophysiol Clin. 2016;8:555–565. doi: 10.1016/j.ccep.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Qiu X., Zhang N., Luo Q., et al. Remote magnetic navigation facilitates the ablations of frequent ventricular premature complexes originating from the outflow tract and the valve annulus as compared to manual control navigation. Int J Cardiol. 2018;267:94–99. doi: 10.1016/j.ijcard.2018.03.105. [DOI] [PubMed] [Google Scholar]
- 6.Misra S., van Dam P., Chrispin J., et al. Initial validation of a novel ECGI system for localization of premature ventricular contractions and ventricular tachycardia in structurally normal and abnormal hearts. J Electrocardiol. 2018;51:801–808. doi: 10.1016/j.jelectrocard.2018.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Ho S., Ernst S. 1st ed. Cardiotext Publishing; 2012. Anatomy for Cardiac Electrophysiologists: A Practical Handbook. [Google Scholar]
- 8.Efremidis M., Vlachos K., Kyriakopoulou M., et al. The RV 1-V 3 transition ratio: a novel electrocardiographic criterion for the differentiation of right versus left outflow tract premature ventricular complexes. Heart Rhythm O2. 2021;2:521–528. doi: 10.1016/j.hroo.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson R., Kumar S., Parameswaran R., et al. Differentiating right- and left-sided outflow tract ventricular arrhythmias: classical ECG signatures and prediction algorithms. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007392. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson W.G., Delacretaz E. Mapping techniques in the diagnosis and ablation of ventricular tachycardia. Heart. 1998;79:207–213. [Google Scholar]
- 11.Bassil G., Liu C.F., Markowitz S.M., et al. Comparison of robotic magnetic navigation-guided and manual catheter ablation of ventricular arrhythmias arising from the papillary muscles. Europace. 2018;20:ii5–ii10. doi: 10.1093/europace/eux374. [DOI] [PubMed] [Google Scholar]
- 12.Turagam M.K., Atkins D., Tung R., et al. A meta-analysis of manual versus remote magnetic navigation for ventricular tachycardia ablation. J Interv Card Electrophysiol. 2017;49:227–235. doi: 10.1007/s10840-017-0257-3. [DOI] [PubMed] [Google Scholar]
- 13.Davis D.R., Tang A.S.L., Golob M.H., et al. Remote magnetic navigation-assisted catheter ablation enhances catheter stability and ablation success with lower catheter temperatures. Pacing Clin Electrophysiol. 2008;31:893–898. doi: 10.1111/j.1540-8159.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 14.Shinoda Y., Sato A., Adach T., et al. Early clinical experience of radiofrequency catheter ablation using an audiovisual telesupport system. Heart Rhythm. 2020;17:870–875. doi: 10.1016/j.hrthm.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths J.R., Cazzzoli I., Ailoaei S., et al. Non-invasive electrocardiographic mapping on the ward to guide ablation of premature ventricular contractions. J Electrocardiol. 2023;78:65–68. doi: 10.1016/j.jelectrocard.2023.01.015. [DOI] [PubMed] [Google Scholar]