Highlights
-
•
Exercise and hypoxia induce distinct and overlapping immune responses.
-
•
Physiological responses to both exercise and hypoxia regulate metabolism, resilience and effector functions of immune cells.
-
•
The controlled combination of exercise and hypoxia could be used to harness synergistic/complementary immune effects.
-
•
Altitude acclimatization, appropriate exercise training and nutritional strategies mitigate increased immune risks of exercising in hypoxia.
Keywords: Altitude, Exercise, Hypoxia, Immune response, Training
Abstract
Immune outcomes are key mediators of many health benefits of exercise and are determined by exercise type, dose (frequency/duration, intensity), and individual characteristics. Similarly, reduced availability of ambient oxygen (hypoxia) modulates immune functions depending on the hypoxic dose and the individual capacity to respond to hypoxia. How combined exercise and hypoxia (e.g., high-altitude training) sculpts immune responses is not well understood, although such combinations are becoming increasingly popular. Therefore, in this paper, we summarize the impact on immune responses of exercise and of hypoxia, both independently and together, with a focus on specialized cells in the innate and adaptive immune system. We review the regulation of the immune system by tissue oxygen levels and the overlapping and distinct immune responses related to exercise and hypoxia, then we discuss how they may be modulated by nutritional strategies. Mitochondrial, antioxidant, and anti-inflammatory mechanisms underlie many of the adaptations that can lead to improved cellular metabolism, resilience, and overall immune functions by regulating the survival, differentiation, activation, and migration of immune cells. This review shows that exercise and hypoxia can impair or complement/synergize with each other while regulating immune system functions. Appropriate acclimatization, training, and nutritional strategies can be used to avoid risks and tap into the synergistic potentials of the poorly studied immune consequences of exercising in a hypoxic state.
Graphical Abstract
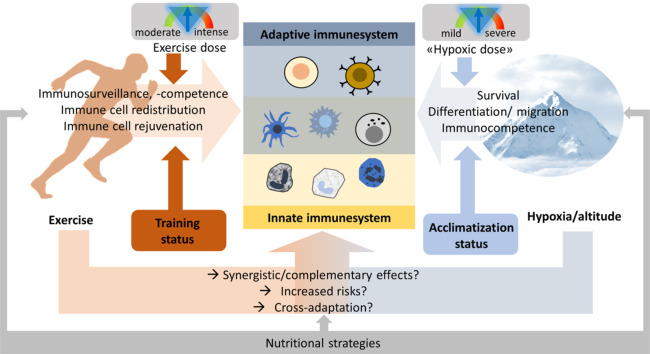
1. Introduction
Regular physical exercise of moderate intensity and duration can improve immune function.1,2 In contrast, strenuous exercise as performed by athletes may transiently compromise the immune system and promote infection.1,3 The threshold between beneficial and detrimental amounts of exercise varies individually. It not only depends on exercise intensity and volume but also on genetic and medical preconditions, sleep, and dietary habits, as well as psychological and environmental stressors like extreme temperatures and high-altitude hypoxia.4,5 The interplay among all these factors is complex and poorly understood, but it also influences the immunological outcomes of exercise. Performing exercise in a hypoxic state, such as when trekking or climbing high mountains and training or competing at terrestrial moderate or high altitude, but also in normobaric or hypobaric hypoxia rooms (i.e., for preparatory, performance-enhancing or therapeutic reasons) has become extremely popular.6, 7, 8 In contrast to the well-described modulation of immune function by exercise, the evidence for immune effects of hypoxia is rather scarce, and the combination of exercise and hypoxic conditions is, in particular, poorly understood. Therefore, the present narrative review aims at advancing our understanding of and developing theories around the immune consequences of exercising in hypoxia in order to pave the way for the future data-acquisition required for meaningful systematic assessment of this topic. This narrative review presents the current knowledge on the potential immune consequences of exercising in a normobaric or hypobaric hypoxic state as well as the modulatory effects of different exercise characteristics, hypoxia levels, and nutritional measures. We compare the different and overlapping immune system components, along with their distinct activation mechanisms, through exercise and exposure to hypoxia; we derive preliminary exercise recommendations and identify knowledge gaps.
2. The immune system and hypoxia
2.1. Innate and adaptive immunity
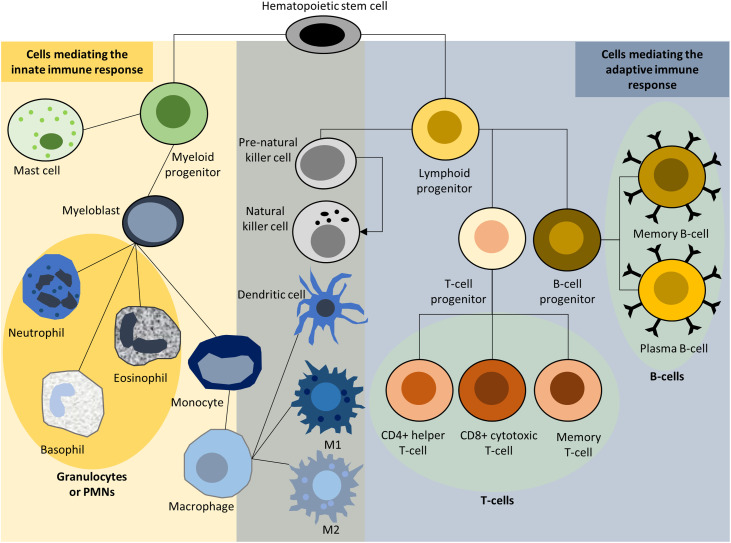
The immune system is composed of a network of cells and organs, including primary lymphoid organs (e.g., thymus and bone marrow), secondary lymphoid organs (e.g., lymph nodes, spleen, and mucosal-associated lymphoid tissue), blood and the lymphatic system, and cells of the myeloid and the lymphoid lineage (i.e., leukocytes).9 Innate and adaptive immunity form the 2 major arms of the immune system, which work together to clear pathogens to maintain normal body physiology and to protect the body from infection. Innate immunity consists of germline-encoded molecular and cellular defense mechanisms and includes physical and chemical barriers against pathogens. It provides the first line of defense (destroying pathogens, controlling the adaptive immune response) against infection by resident (epithelial and endothelial) cells, blood components, antimicrobial peptides, and proteins, and it is responsible for the activation of neutrophils, macrophages, mast cells, dendritic cells, and natural killer cells (Fig. 1). These mediate a fast but non-specific immune response. In contrast, adaptive immunity evolves and adapts to improve during the course of action. Adaptive immunity relies on B and T lymphocytes, antigen-presenting cells, antibodies, and cytokines, including interferon gamma and tumor necrosis factor alpha (TNF-α). Antigen-activated B and T lymphocytes can differentiate into effector cells and memory cells and induce humoral and cell-mediated immune responses. Effector B cells facilitate the antibody-mediated humoral immune response, while effector CD4+ helper T cells and CD8+ cytotoxic T cells facilitate the cell-mediated immune response by either mobilizing other immune cells via cytokines or by killing target cells. Lastly, memory cells, produced during the primary adaptive immune response, enable a faster and more effective secondary immune response to subsequent infections by the same pathogen.10 Several leukocytes (natural killer cells, dendritic cells, macrophages) play roles in both innate and adaptive immunity.
Fig. 1.
Innate and adaptive immune responses. M1 and M2 describe the 2 primary activities of macrophages; pro-inflammatory M1 activity damages tissue, while M2 activity increases cell proliferation and tissue repair. PMNs = polymorphonuclear cells.
Immune system activation is not only a consequence of pathogens or pathogen-associated molecular patterns. Independent factors leading to inflammation can be reactive oxygen species (ROS) or other damage-associated molecular patterns following cellular injury. Pathogen-associated molecular patterns and damage-associated molecular patterns induce immune responses and inflammation. Mitochondria, which are the primary energy producers in cells, make important contributions to this process; that is, various mitochondrial components (e.g., ROS, mitochondrial DNA, or membrane components) trigger immune responses when released into the cytoplasm. However, mitochondria also play a role in regulating and modulating immune responses.11, 12, 13
Another crucial factor in innate and adaptive immune system activation is the cell–environmental oxygen level.14
2.2. Hypoxia and immune responses
Physiological oxygen concentrations are required for the normal functioning of cells. The term hypoxia denotes a state of low oxygen. Ambient hypoxia refers to reduced oxygen availability due to low barometric pressure (hypobaric hypoxia, which occurs, for example, at high altitudes) or low oxygen concentrations under normal barometric pressure conditions (normobaric hypoxia, which is frequently used in hypoxic chambers or in cell culture incubators), both of which result in the lower partial pressure of oxygen (pO2). The pO2 also determines oxygen availability on the tissue and cellular levels.
Acute ambient hypoxia has been defined as the initial phase of a hypoxia exposure, encompassing the most pronounced (patho) physiological responses (duration from minutes to days), and chronic hypoxia refers to a prolonged (continuous or intermittent) hypoxia exposure following the acute phase (days to years).
Physiological oxygen levels are highly tissue-dependent and influence specialized cell functions.15 Changes in ambient oxygen levels, increasing oxygen demand (e.g., by skeletal muscles during exercise16), or pathological changes, such as those due to ischemia, inflammation,14 or cancer,17 can reduce cellular oxygen availability below normal levels.
Hypoxia responses involve many biochemical and molecular processes,18 with hypoxia-inducible factors (HIFs) being master transcriptional regulators of cellular adaptations to hypoxia.19,20 HIFs are primarily regulated by oxygen availability. HIF α-subunits are continuously degraded under normoxic conditions after their hydroxylation by prolyl hydroxylases and ubiquitination by the Van Hippel Lindau protein.18,21, 22, 23, 24, 25, 26, Three α-subunit isoforms heterodimerize with a constitutive β subunit under hypoxic conditions. The ubiquitous HIF-1 and cell-specific HIF-2 isoforms are better understood than HIF-3 and regulate the expression of many genes27 and pathways, including cell proliferation and survival, glycolysis, metabolism, angiogenesis, metastasis, erythropoiesis, apoptosis, and autophagy.
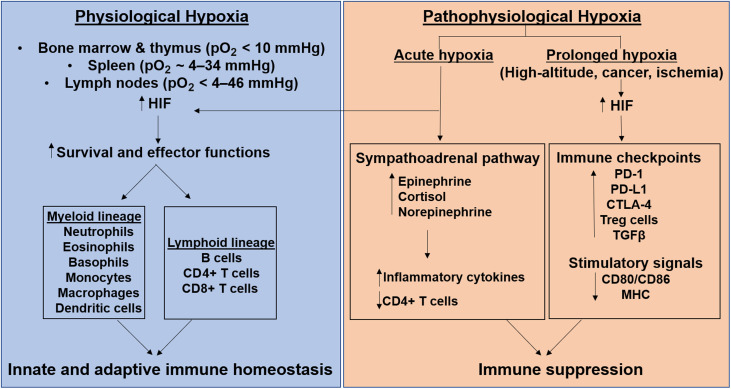
Tissue-specific oxygen level variations affect immune cells.28 Physiological hypoxia exists in several primary and secondary lymphoid organs. In the bone marrow and thymus, pO2 is < 10 mmHg; in the spleen, it is about 4–34 mmHg, and in the lymph nodes, it is <4–46 mmHg. Compare this to a pO2 of 80–100 mmHg in arterial blood.14 Such spatial hypoxic lymphoid regions are functionally important because they maintain the turnover of hematopoietic stem cells and regulate the antigen-specific antibody production by germinal centers, thus modulating both innate and adaptive immune responses.29, 30, 31
Recent advances in immunometabolism research indicate that the metabolic state of immune cells determines their immune function; thus, their function depends on cell-environmental nutrient and oxygen availability, on the one hand, and on internal redox and metabolic states, on the other.32,33 Hypoxia is an important regulator of the immune system and inflammation, with HIFs representing key mediators primarily due to their role in regulating cellular metabolism. However, inflammation is also associated with high energy and thus oxygen—demand (e.g., by activated immune cells), and vascular damage and can lead to tissue hypoxia.34, 35, 36
2.3. Hypoxia and HIFs regulate the innate and adaptive immune system
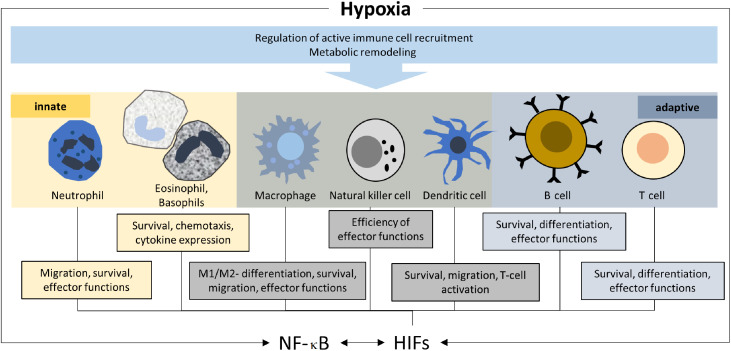
Hypoxia regulates both resident and infiltrating mediators of the immune response (Fig. 2). The immune barrier function of resident cells is strongly modulated by hypoxic stress. While hypoxia-related injury and reduced energy availability jeopardize barrier functions, the induction of HIFs has been linked to protective effects. For example, hypoxia-induced intestinal permeability is counteracted by the HIF-1 regulation of human intestinal trefoil factor37 and surface-expressed ecto-5′-nucleotidase (CD73),38 while epithelial and endothelial cell resilience is enhanced by HIF-1-mediated increased expression of the multidrug resistance gene MDR1.39 HIFs are further involved in the regulation of mucin and antimicrobial peptide production.35 In addition, local states of hypoxia lead to the recruitment of a variety of leukocytes, including macrophages.40 HIFs importantly contribute to the differentiation and activation of these cells, primarily by preserving energy in a hypoxic environment, allowing leukocytes to translocate to target tissue and execute their immune functions.36
Fig. 2.
Regulation of immune cells by hypoxia. M1 and M2 describe the 2 primary activities of macrophages; pro-inflammatory M1 activity damages tissue, while M2 activity increases cell proliferation and tissue repair. HIFs = hypoxia inducible factors; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells.
HIF-1 maintains innate and adaptive immune homeostasis under physiological and acute hypoxic conditions,14,41 while chronic pathophysiological hypoxic conditions result in immunosuppression.42,43 HIF-1 regulates metabolism and transcription in immune cells of both the myeloid lineage (e.g., neutrophils, eosinophils, basophils, monocytes, macrophages, dendritic cells) and the lymphoid lineage (e.g., B and T cells).14,35 Of these, neutrophils, constituting a significant 50%–70% of circulating leukocytes, are the first responders recruited in response to a stimulus.44 In the acutely hypoxic micro-environment at the site of pathogen infiltration, HIFs enhance neutrophil migration and survival by inhibiting apoptosis and their associated-effector functions, including phagocytosis and the production of antimicrobial peptides.45 Acute exposure to high-altitude/hypoxia has been suggested to cause an initial priming of the innate immune system combined with a synergistic toll-like receptor 4 (TLR4)-induced sensitization to the ensuing inflammatory stimuli, as well as the activation and recruitment of neutrophils.46 Thus, attenuation of the inflammatory response is required to prevent the development of hypoxia-induced pathologies; this attenuation usually occurs during acclimatization. The rarer populations of other granulocytes (eosinophils and basophils) and mast cells are key mediators in allergic and anti-parasitic infiltration.47 Like neutrophils, HIFs also enhance their survival and chemotactic ability, and increase their cytokine profile expression, especially that of interleukin 8 (IL-8), TNF-α, vascular endothelial growth factor, and IL-4.48
Phagocytic myeloid antigen-presenting cells, including macrophages and dendritic cells, form cellular bridges between the innate and adaptive immune systems.49 Of the 2 HIF isoforms, HIF-1 specifically enhances the pro-inflammatory cytokine profile (including TNF-α, IL-1, IL-12, and IL-6 among the classically activated inflammatory M1 macrophages), thereby contributing to their antimicrobial activity. HIFs also promote the survival and migration of dendritic cells as well as dendritic cell-mediated T cell activation.50 As with the myeloid lineage in the innate immune response, HIFs also regulate the adaptive immune response of lymphoid lineage cells. HIFs enhance the survival, differentiation, and effector functions of both CD4+/CD8+ T cells and B cells. Of the several sub-populations of CD4+ T cells, HIFs specifically upregulate Th1 and Th17 subtypes, both of which are involved in inflammation and cell-mediated immunity.51 Although HIFs seem to downregulate regulatory T cells, preventing the repression of immune responses, their role in controlling regulatory T cells is still controversial.52 Like CD4+ cells, HIFs also enhance the survival of cytotoxic CD8+ T cells, one of the key players in cell-mediated immunity. HIFs upregulate the production and release of their lytic proteins, perforin and granzyme, thereby facilitating target-cell death.53 In addition to controlling cell-mediated immunity, HIFs regulate humoral immunity by regulating the activation, survival, proliferation, and development of B cells. HIFs also orchestrate B cell antibody class-switching in germinal centers as well as their cytokine profile.54
2.4. Crosstalk between hypoxia/HIFs and the immune system/inflammation
Hypoxic conditions can lead to inflammation, and inflammation can cause hypoxia.14 This is due to the increased oxygen consumption induced by pathogens, infiltrating immune cells and inflamed cells, and the reduced oxygen supply due to vascular pathology.36 The resulting inflammatory hypoxia leads to HIF stabilization. Low oxygen levels and the canonical stabilization via inhibited prolyl hydroxylases contribute to HIF accumulation and/or increased activity, as do various oxygen-independent factors. Notably, these include inflammatory cytokines such as IL-1β and TNF-α, which increase HIF-1 protein levels (IL-1β) or HIF-1 binding to DNA (IL-1β, TNF-α).55 Moreover, adenosine (which prevents cullin-2-based HIF-α degradation56) and various microbe-derived products influence HIF activity and stability.35 Thus, the microbiome can stabilize HIF and enhance its transcriptional activity through diverse mechanisms, including specific microbial toxins. In the adaptive immune system, T helper 17 cell (TH-17) cell-specific polarizing cytokines, IL-6, and transforming growth factor β (TGF-β) upregulate signal transducer and activator of transcription 3 (STAT3)-dependent HIF-1ɑ expression.57 HIF-1α, in turn, upregulates miR-210 expression, thereby stimulating TH-17 differentiation. In both CD4+ T (especially TH-17 cell type) and CD8+ T cells, T cell antigen receptor engagement upregulates and stabilizes HIF-1α, resulting in its accumulation.58
While HIF is considered to be a master regulator of transcriptional responses to hypoxia, other transcription factors are also involved. One of these is the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which is activated by hypoxia as well.59 NF-κB, the main transcriptional regulator of inflammation, regulates HIF transcription and its DNA interactions,60 while HIFs regulate NF-κB transcription and the expression of TLRs.36 The hypoxia-induced upregulation of TLRs further stimulates innate immune responses.61
2.5. Pathophysiological hypoxia-associated immune response
While physiological hypoxia is important for normal immune system function, pathophysiological hypoxia, such as occurs in high-altitude associated illnesses, pulmonary hypertension, ischemia, and cancer, favors immune suppression.62,63 To maintain homeostasis, the acute and chronic hypoxia experienced at high altitudes influence sympathoadrenal pathway-associated physiologic and metabolic adaptations. The sympathetic nervous and endocrine systems contribute to restoring the disturbed homeostasis at high altitudes by first increasing epinephrine and cortisol, then norepinephrine, under prolonged hypoxic conditions.64 These sympathoadrenal pathways also affect the immune system and decrease lymphocyte numbers, particularly CD4+ T cells, impair T cell activation and proliferation, and increase the numbers of immunoglobulins, neutrophils, natural killer cells, and inflammatory cytokines, including IL-6, IL-1, and C-reactive protein. Hypoxia together with hypoxia-induced adenosine upregulate the expression of immune checkpoints, including programmed cell death-1, programmed cell death-1-ligand, cytotoxic T-lymphocyte associated protein-4, immunosuppressive cytokines (transforming growth factor-β), and regulatory T cells, all of which function as physiological negative feedback mechanisms of immune activities, ultimately leading to immunosuppression.65 Hypoxia also impairs T cell activation by down-regulating the required vital signals, including the major histocompatibility complex and the positive co-stimulatory molecules CD80/CD86 on the antigen-presenting cells.66 Thus, although acute hypoxia-induced inflammation plays a protective role and mounts an adaptive response, chronic hypoxia-associated inflammation could aggravate the disease, especially in several high-altitude pathologies (e.g., acute mountain sickness, high-altitude pulmonary edema, high-altitude cerebral edema, high-altitude pulmonary hypertension, erythrocytosis).67 The varied HIF-mediated immune responses to both physiological and pathophysiological hypoxia are depicted in Fig. 3. In addition to the HIF-pathway genes EGLN1 (encoding prolyl hydroxylase 2) and EPAS1 (encoding HIF-2α), several inflammatory pathway genes such as IL-1α, IL-1β, IL-6, TNF, nitric oxide synthase 1, and Nitric oxide synthase 2 (NOS2) also display positive selection in high-altitude native populations;68, 69, 70 thus, evidence suggests that epigenetic mechanisms could regulate immune response at high altitudes.68,71 Moreover, increased frequencies of the major histocompatibility complex class II alleles serotype subgroup human histocompatibility leucocyte antigen DR6 (HLA-DR6) and/or serotype subgroup human histocompatibility leucocyte antigen DQ4 (HLA-DQ4) are associated with susceptibility to high-altitude pulmonary edema.72 Since class II major histocompatibility complex molecules present antigens to CD4+ T cells to regulate cell-mediated and humoral immune responses, they may contribute to immunosuppression at high altitudes.73
Fig. 3.
Immune response to physiological and pathophysiological hypoxia. HIF mediates innate and adaptive immune homeostasis in the immune-system-associated physiological hypoxia state, while it mediates immunosuppression in the pathophysiological hypoxia state associated with high altitudes, cancer, and ischemia. CD = cluster of differentiation; CTLA-4 = cytotoxic T-lymphocyte associated protein-4; HIF = hypoxia-inducible factor; MHC = major histocompatibility complex; PD = programmed cell-death; PD-L = programmed cell-death ligand; pO2 = partial pressure of oxygen; TGF = transforming growth factor; Treg = T regulatory cells.
Manella and colleagues74 recently demonstrated that living at a high altitude (5100 m) substantially affects the blood transcriptome. This included the downregulation of innate immune system-related genes, for example, those involved in myeloblast differentiation and neutrophil activation. Such changes are expected to compromise innate immunity.74 These results are therefore a further indication of the potential negative effects of severe hypoxia on immune functions, comparable to excessive exercise (see Section 3).
3. The immune system and exercise
Many of the health benefits of regular exercise, such as improved quality of life or reduced mortality from chronic diseases,75 may be mediated by its protective effects on immune function1,2 and depend on the characteristics of exercise programs.
3.1. Immune responses depend on exercise characteristics
Exercise benefits for the human immune system decline or even become detrimental when the volume and/or intensity of exercise exceeds a certain threshold of the individual's stress tolerance.1,4,76, 77, 78 An excessive training load/intensity hampers immune functions and increases the risk of infection and disease development.1,2 Thus, understanding the immune consequences of different forms of exercise (i.e., type, volume, and intensity) is crucial. Based on the knowledge available a decade ago, Walsh and colleagues79 concluded in their position statement that acute intensive exercise of any type causes the transient depression of several aspects of the acquired immune function, but also that the affected cell numbers and functions usually recover within 24 h. They also expressed concerns about the chronic suppression of acquired immunity observed after prolonged periods of intense exercise training with insufficient recovery time.79
3.2. Endurance exercise: Volume and intensity, acute and chronic effects
Many studies indicate that regular moderate-intensity continuous training with a duration below 60 min is associated with improved immune defense.2
Moreover, regular exercise enhances mitochondrial integrity and reduces oxidative stress, thereby reducing inflammation.80 Immune system-enhancing and anti-inflammatory effects, therefore, are believed to be important components of exercise benefits, which reduce the risk of developing inflammatory diseases.81
In contrast, acute bouts of high-intensity or high-volume aerobic exercises seem to temporarily reduce immune cell counts and compromise immune function for periods of 3–72 h depending on the subject's characteristics, type of exercise, and the markers analyzed.5 Acute exercise activates the immune system via, for example, the production of ROS and activation of beta-2-adrenergic receptors on lymphocytes.82 This leads to improved immunocompetence by increasing the immune cell availability in peripheral tissues,83 such as cytotoxic natural killer cell subtypes and CD8+ T cells,84 possibly primarily immature B cells,85 and preferentially plasmacytoid dendritic cells86 and macrophages.87 Granulocyte levels also rise in an exercise dose-dependent manner.88 The hours directly following intensive acute aerobic exercise are often associated with lymphopenia (i.e., a reduction in the numbers of circulating lymphocytes below baseline levels), which is believed to reflect a cell redistribution, including of cytotoxic T and B cells.82,89,90
Hill et al.91 found elevated gut barrier permeability and increased pro- and anti-inflammatory cytokine concentrations after 1 h of treadmill exercise at 65% of individual maximum rate of oxygen consumption in normobaric hypoxia (fraction of inspired oxygen = 13.5%), which could promote an “open window” of susceptibility for infections following the workout. These authors also demonstrated a state of immunosuppression caused by this type of acute hypoxic exercise.92
A recent meta-analysis on immune function following interval training (IT), defined as an aerobic training strategy including high-intensity efforts, confirmed transitory leukocytosis (for up to 6 h) and lymphocytosis followed by transitory lymphopenia.93
Regarding T cells, this decrease is also due to apoptosis, with different T cell subtypes being differentially affected depending on whether IT or continuous training had been performed.94 This observed apoptosis after exercise may later facilitate immune progenitor cell mobilization from bone marrow.95 Lymphocyte counts usually recover to baseline levels after about 24 h of aerobic exercise82 and can then sometimes exceed baseline levels, as observed for regulatory T cells.96
Transient negative effects are often compensated for by the long-term health benefits of regular exercise mediated by anti-inflammatory effects, which are elicited by exercise-provoked cytokines and/or the downregulation of TLR expression.97 Favorable functional adaptations of lymphocytes, monocytes, and neutrophils were seen after chronic IT.91 Regular endurance training may promote lymphocyte homeostasis and improve lymphocyte resistance to apoptosis, thereby counteracting transient immune suppression following an acute exercise bout.96 Regular aerobic exercise also favors the development of more anti-inflammatory (M2-like) vs. pro-inflammatory (M1-like) macrophage subtypes in adipose tissue,98 thereby attenuating systemic inflammation. Macrophages87,99 and neutrophils100 also exhibited better metabolic and phagocytic capacities after chronic exercise. This has been shown to improve dendritic cell activity against tumor cells in rats101 and to attenuate excessive dendritic cell maturation and activation in a rodent model of asthma.102 The availability and functions of immune cells, including natural killer cells, neutrophils, T cells, and B cells, were shown to be less strongly affected by age-related changes with regular exercise.103
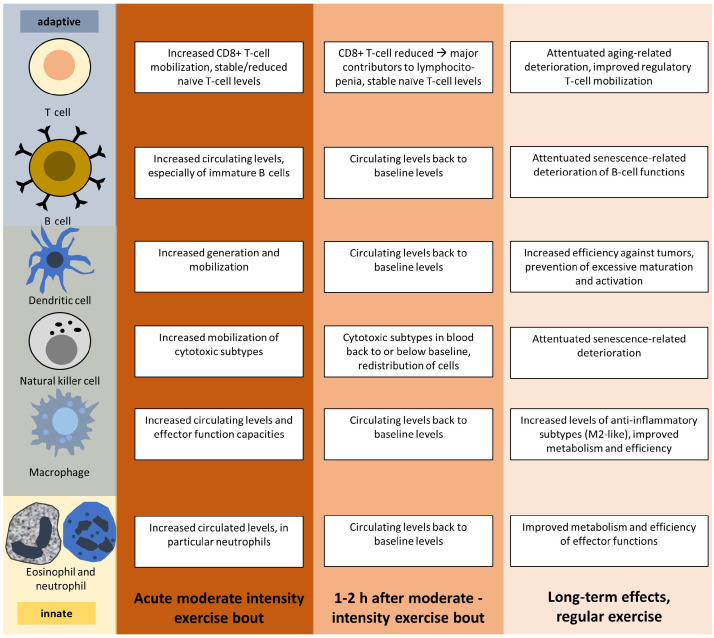
Depending on the exercise intensity and type and the individual immune status, exercise can have diverse effects on the immune system. The potential beneficial outcomes of moderate endurance exercise on specialized immune cells are summarized in Fig. 4.
Fig. 4.
Immune responses to moderate acute and regular aerobic exercise.
Sufficiently intense acute exercise bouts trigger immune responses that depend on the intensity, duration, and type of exercise, with excessively intense or long acute exercise sessions having detrimental effects on immune functions. Moderate intensity exercise bouts are depicted.
Thus, a single session of high-intensity or high-volume endurance exercise may challenge cell homeostasis and immune function, while endurance exercise of moderate intensity and volume will acutely improve immunosurveillance and immunocompetence. When performed on a regular basis, such moderate endurance exercise improves immune functions while reducing chronic inflammation.83
3.3. Resistance exercise: Volume and intensity, acute and chronic effects
In comparison to endurance exercise, the immune effects of resistance exercise have been less thoroughly investigated. Petridou and colleagues104 suggested that moderate- to high-intensity resistance exercises (3 sets of 10 exercises with 10–12 repetitions at 70%–75% of the 1-repetition maximum) did not exert considerable negative effects on immune function in healthy men based on the unchanged serum concentrations of cell adhesion molecules. Allsopp et al.105 evaluated effects on the leukocyte responses in healthy adults (18–35 years) following a bout of resistance exercise performed in normobaric hypoxia (fraction of inspired oxygen = 14.4%). These authors showed that neutrophils were amplified following resistance exercise, but all other leukocyte subsets remained unaffected, indicating non-detrimental responses to exercise.105 In contrast, in older adults (60–70 years), a bout of resistance exercise in normobaric hypoxia (14.4%) elicited an increased lymphocyte response but did not affect other leukocyte populations.106
Results of a systematic review indicate that even a single acute bout of resistance exercise can activate the NF-κB signaling pathway in peripheral blood mononuclear cells in both younger and older individuals.107 Notably, the activity of natural killer cells after an exercise bout was increased in older but reduced in younger subjects. Moreover, the cytosolic oxidative stress response to acute resistance training increased and the antioxidant enzyme expression improved when regular resistance training was continued.107 Not surprisingly, acute neutrophilia, lymphocytosis, and monocytosis seem to occur more rapidly and extensively after a single bout of high-dose as compared to low-dose resistance exercises.108 Lower intensity seems to benefit immune function more than higher intensity resistance training, also in the long term. Accordingly, 6 weeks of strength endurance training at 40% of the 1-repetition maximum, but not intensive strength training (80% of 1-repetition maximum), was associated with a reduction in the relative and absolute counts of senescence-prone T cells.109
Taken together, these findings indicate that resistance training modulates immune function and, especially if practiced moderately and regularly, may enhance long-term immune system function. In comparison to research on endurance training, research on the relationship between resistance training and immune functions is scarce. The roles of resistance training characteristics (e.g., intensity, frequency, training session composition), individual predispositions, and mechanisms (e.g., interactions of molecular consequences of resistance training with immune system components) deserve further scrutiny.
3.4. Comparing and combining endurance and resistance exercise: Volume and intensity, acute and chronic effects
When comparing the acute effects of endurance (cycling at 60% of peak power output) and resistance (70% of the 1-repetition maximum) exercise, the results reveal a more pronounced mobilization of immune cells and an altered systemic immune inflammation index (neutrophil × platelet counts/lymphocyte counts) after the endurance exercise session.110 Both submaximal endurance and resistance exercise performed at 85% of the individual maximum rate of oxygen consumption or 1-repetition maximum, respectively, until exhaustion induced temporary (30 min post exercise) immune dysfunction, indicated by a decline in the CD4+/CD8+ T cell ratio and an increase in intra-cellular ROS levels.111 A reduced CD4+/CD8+ T cell ratio is indicative of a weaker immune system, characterized by the lower availability of CD4+ helper T cells in comparison to cytotoxic CD8+ T cells. In contrast, short-term and low-threshold combined resistance and endurance training reduced the number of hallmarks of immune aging in older people.112 A 6-week training intervention elevated the CD4+/CD8+ T cell ratio and reduced both systemic IL (IL-6, IL-8, and IL -10) and vascular endothelial growth factor levels.112 The findings of a recent literature review suggest that secretory salivary immunoglobulin A is elevated after acute endurance exercise in older subjects, while the plasma/muscle levels of IL-6, IL-8, and TNF-α were elevated in this population after acute bouts of muscle strengthening exercises (e.g., isokinetic, eccentric, or knee extensor exercises).113 Recently, the role of the inflammasome nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3), an important mitochondria-associated pattern recognition receptor in the innate immune system, in the immunomodulation of exercise has been highlighted.110 NLRP3 activation upregulates several pro-inflammatory cytokines, including IL-1β and IL-18.114 These authors suggested that acute exercise bouts activate the NLRP3 inflammasome, while chronic moderate-intensity endurance training, high-intensity IT, and resistance training may inhibit it.114
While our understanding of the effects of combined endurance and resistance exercise on immune outcomes is limited (due in part to the difficulties inherent in comparing endurance and resistance exercise intensities), several risks and potential complementary effects can be identified. At high intensity, both exercise types reduce immune functions; thus, sufficient recovery periods (at least 24 h) between intense exercise sessions of any kind are recommended. Although molecular overlaps and specificities of distinct exercise types still need to be defined in more detail, different aspects of the immune system can potentially be modulated more efficiently by practicing different exercise types. Therefore, general physical activity recommendations (e.g., from the World Health Organization115) that suggest minimum amounts of both endurance (at least 75 min of intensive training or 150 min of moderate training per week) and resistance training (2–3 times per week) are believed to effectively activate different immune pathways thus taking advantage of the potential synergistic or complementary immune system reinforcements.
4. Combined effects of exercise and hypoxia on immune function
Like severe hypoxia, intense exercise bouts may compromise immune function and increase infection risk.73 Exercising in hypoxic environments (i.e., in hypoxic chambers or at high altitudes), depending on the individual hypoxic responses and tolerance, may aggravate the risks of high intensity exercise. Like exercise does, moderate states of hypoxia may also exert favorable effects on the immune system.
The immune-suppressive potential of exercise in a hypoxic state are firmly established, and both factors seem to be additive,64,116 requiring the modification of exercise characteristics depending on the severity of the hypoxia.116 The α/β-adrenergic system is believed to play an important role, since it increases inflammatory cytokine levels and activates immune cells, as well as being activated by and involved in adaptations to both exercise117 and hypoxia.118 When the absolute workload is appropriately reduced under hypoxic conditions (applying the same relative intensity as in normoxia, e.g., via heart rate and/or blood lactate monitoring), the immune function does not seem to be more adversely affected by acute exercise in the hypoxic (normobaric or hypobaric) as compared to the normoxic state.119 The more pronounced immunological changes observed when exercising in hypoxia may partially result from the increased work intensity, but hypoxemia per se also directly regulates various immune functions, such as macrophage migration and phagocytosis by polymorphonuclear cells.120 As previously discussed, oxygen signaling mechanisms (e.g., via HIF) also contribute to hypoxia-induced immune alterations.40,121 Since HIFs can be activated by exercise,16 immune responses to exercise in hypoxia may also be amplified. Similar overlaps of the molecular consequences of exercise and hypoxia that have direct consequences on immune function and inflammation occur at the level of mitochondrial adaptations and oxidative stress/antioxidant mechanisms. While hypoxia is acutely associated with increased oxidative stress and reduced mitochondrial respiration, cellular adaptations can eventually lead to improved antioxidative capacities and increase the mitochondrial and cellular resilience and metabolic reprogramming that allows the maintenance of cellular energy levels.118 Similarly, while excessive exercise may result in mitochondrial dysfunction,122 regular moderate exercise improves mitochondrial integrity and functions and leads to a better cellular antioxidant defense.80
As is well known from high-altitude climbing, acclimatization to high altitude/hypoxia largely decreases the risk of adverse effects associated with acute hypoxia exposure.123 The acclimatization process is mediated by systemic physiological (e.g., hyperventilation and hemoconcentration) and molecular responses. The latter are orchestrated by transcription factors (including HIFs) and biochemical alterations, together affecting cellular functions/components like energy metabolism and mitochondria, reduction-oxidation (REDOX)-regulation, and inflammation.123 Comparable to the increased exercise tolerance following exercise training, the exposure to hypoxia (acclimatization or conditioning) improves resilience to subsequent hypoxic stress. The co-occurrence of 2 stressors (exercise and hypoxia), with partially overlapping adaptations required for both exercise and hypoxia conditioning, means increased physical strain. This renders the avoidance of overreaching and overtraining key aspects of exercising in hypoxia. Therefore, the coordination and incremental elevation of exercise and hypoxia levels together with regular monitoring of individual physiological responses are crucial for the optimal execution of exercise in hypoxia.
5. Immune-modulatory effects of diet when exercising in hypoxia
The risks of exercising in a hypoxic state120 are the disrupted homeostasis of several physiologic systems, including immune function.124 Immunosuppression and related risks, such as an increased risk of illness and respiratory infection,77 seem to result from reduced mucosal immunity and inflammation and a predominance of the humoral immune response.124 These adverse effects may be partly counteracted by appropriately modifying the exercise volume and intensity under hypoxic conditions, but also by applying nutritional strategies (e.g., meeting the demand for more iron, carbohydrates and fluids, and antioxidant-rich foods to maintain robust immunity).124, 125, 126
The micronutrient iron is especially important at high altitudes as hypoxia increases hemoglobin mass gains, which depend on adequate iron availability. Iron is necessary for oxygen transport, energy metabolism, and immune function, with iron deficiency primarily impacting cell-mediated immunity.127,128 A suboptimal iron status (i.e., ferritin <30 ng/mL) may result from limited energy and iron intake, poor bioavailability, or increased iron demands due to high training loads and environmental factors (e.g., hypoxia-induced erythropoiesis or exercise-induced hemolysis).129,130 Therefore, the iron status should be high (e.g., ferritin >50 ng/mL) when exercising under hypoxic conditions.131 An inadequate iron status can be corrected through supplementation in several ways. First, athletes should increase their dietary iron intake, and particularly from haem iron sources like red meat and seafood with the addition of legumes and green vegetables.131 Second, athletes with iron deficiency should use oral iron supplements for an 8- to 12-week period at doses between 40 and 60 mg per day in conjunction with 500–1000 mg of vitamin C; this has proven to be the most effective approach.131,132 Recent research has shown that providing iron supplements on alternate days over a longer time period and in single doses optimizes iron absorption and might be a preferable dosing regimen in iron-depleted athletes.133 Moreover, excessive supplementation of iron is thought to increase oxidative stress and the production of free radicals.134 This is of utmost importance, as an elevated level of oxidative stress is already present at high altitudes, and acute exercise leads to oxidative stress.
The low availability of energy can impair hematological adaptations to higher altitudes, as energy status interacts with iron metabolism.135 At high altitudes, energy and fluid requirements are higher due to increased respiratory water loss caused by increased breathing rates coupled with a more than 3-fold increase in the resting metabolic rate as compared to the rate at sea level. Weight loss frequently occurs at high altitudes due to hypoxia-induced appetite suppression, which can negatively influence lean body mass retention and systemic immune function and related risks (e.g., hypoglycemia injury and illness).136
At high altitudes, the stress response to exercise is enhanced; thus, carbohydrate requirements are higher than at sea level when exercise of the same relative intensity is performed. Since reduced blood glucose levels are linked to increased immune activation, ingesting carbohydrates during and immediately after exercise is critical, especially for athletes training at altitude.137 Experts recommend 8–12 g of carbohydrates per kilogram of body mass per day for daily fuel needs and recovery,138 and additionally, as an ergogenic aid, 30–70 g of carbohydrates per hour of exercise depending upon the intensity and duration.137,139 Several studies have been published on the value of carbohydrate intake as a countermeasure to exercise-induced immune changes. One consistent finding is that the carbohydrate intake during prolonged and intense exercise attenuates post-exercise inflammation by 30%–40% and results in higher plasma glucose and insulin levels and lower plasma stress hormones (epinephrine and cortisol).2 Recent evidence also indicates that sufficient supplementation with carbohydrates (8% maltodextrin, 200 mL every 20 min during exercise) and glutamine (20 g/day for 6 days) can effectively mitigate immunosuppression, with carbohydrate supplementation inducing more anti-inflammatory responses (changes in IL-10 and TNF-α concentrations) than glutamine.140 This does not prevent the high rating of perceived exertion during intense exercise under hypoxic conditions (equivalent to an altitude of 4500 m), but it can attenuate fatigue or tiredness in people who exercise under these conditions.141
Immunonutrition can extend beyond carbohydrates to include polyphenols from fruits and vegetables, which are metabolized by gut microbial species and exert a variety of bioactive effects (e.g., anti-inflammatory, antiviral, antioxidative, and immune cell signaling effects).142 A polyphenol-rich dietary pattern can also improve the functionality of intestinal microbiota and intestinal permeability, which is strictly associated with chronic activation of the inflammatory response.143 These findings are relevant, since some evidence suggests that acute exposures to high altitudes damage the intestinal barrier through hypoxic and oxidative stress.144 In addition, chronic hypoxic exposures may lead to atrophy of the mucosal layers, further compromising the intestinal barrier. Gut microbiota, however, may contribute to a variability of host responses to high altitudes, regardless of the macronutrient composition of the diet.145 Certain probiotics may help to protect the intestinal barrier during exposure to hypoxic conditions and to lower the risk of upper respiratory tract infections by modulating the gut microbiota (enhanced intestinal barrier function and protection from pathogens), the mucosal immune system (enhanced bioactive metabolite production, such as short chain fatty acids), and lung macrophage and T lymphocyte functions.146 Some of these effects appear to be connected with alterations in tryptophan metabolism,147 which may play a role in the onset of central fatigue through the gut-brain axis.148 However, as with any dietary supplementation, probiotics should be considered in the overall context of a balanced dietary intake by taking a “food first” approach; namely, by consuming whole foods instead of supplements. For example, some food-based probiotic products (e.g., kefir and yogurt) contain energy, carbohydrates, protein, and other nutrients that can form an important part of an athlete's overall nutrition plan.
Finally, recent findings suggest a reconsideration of antioxidant use when exercising under hypoxic conditions. Antioxidant supplementation did not affect markers of oxidative stress associated with increased energy expenditure at high altitudes.149 An analysis of recent data also showed that more than doubling the daily antioxidant intake from natural food sources during a 3-week altitude camp (2320 m), first, did not negatively influence the adaptive response to altitude training in elite endurance athletes (measured as hemoglobin mass and maximal oxygen uptake)150 and, second, enhanced the antioxidant capacity and attenuated the altitude-induced increases in systematic inflammatory biomarkers (micro-C-reactive protein, IL-13, IL-6) but, again, had no impact on altitude-induced oxidative stress.151 Thus, although antioxidant supplementation may hinder ventilatory acclimatization, recent studies show that increasing the intake of natural antioxidant-rich foods (e.g., fruit-berry-vegetable smoothies, nuts, dark chocolate, and dried fruits/berries) is a sensible addition to elite athletes’ dietary routines that causes no harm while training at moderate altitudes. Indeed, it has been suggested that antioxidant supplementation may interfere with adaptations to exercise at sea level,152,153 although a recent meta-analysis indicated that little evidence supports this.154 Overall, only moderate evidence shows that vitamins C and E benefit recovery. However, under hypoxic conditions equivalent to those present at an altitude of 4200 m, providing an environment associated with greater ROS production and vitamin E supplementation (250 mg) 1 h before exercise (60 min at 70% maximum rate of oxygen consumption) reduced concentrations of blood markers for muscle damage (creatine kinase, myocardial band version of creatine kinase, and lactate dehydrogenase) and of inflammation markers (IL-6, TNF-α, IL-1ra, and IL-10).126
In summary, immune consequences that occur when exercising under hypoxic conditions can be positively influenced by ensuring appropriate nutrition. Nevertheless, few studies have been conducted under hypoxic conditions to investigate immune responses. Future studies should examine caloric intakes that optimize hypoxic adaptations and prevent exercise-induced immune changes. The micronutrient iron is especially important at high altitudes to optimize hemoglobin mass gains. Both iron deficiency and excessive iron supplementation can negatively affect a healthy immune system. The higher demand for specific nutrients (e.g., antioxidant-rich foods, polyphenols from fruits, and probiotics) should also be considered in athletes, as these improve mucosal and systemic immunity, enhance resistance towards illness and respiratory infections and, last but not least, preserve mental well-being, all of which also help boost athletes’ immune systems.
6. Conclusions
Both exercise and hypoxia substantially modulate the immune system. While tissue-specific physiological hypoxia continuously regulates the function of immunological niches, exposure to ambient hypoxia or exercising rapidly induces the activation of immune cells or immunosuppresion. The outcome is determined by the exercise intensity and/or hypoxic dose, exercise type, and interplay among individual characteristics (e.g., genetics, gut microbiome composition), experiences (e.g., training status, acclimatization), environmental conditions (e.g., temperature), mood, diet, and behavior (e.g., hydration).
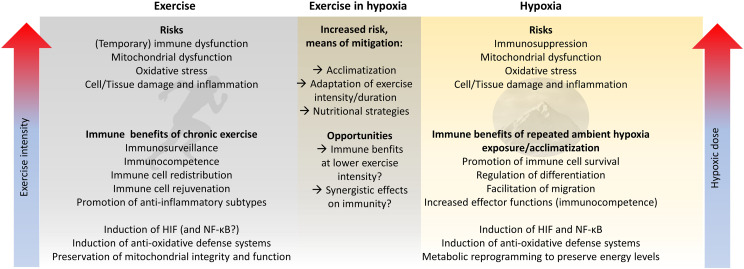
On the one hand, the overlapping effects of exercise and hypoxia are potentially deterimental to the immune system. Even low exercise intensities may increase oxidative stress and cellular/tissue damage and eventually compromise immune function if performed under hypoxic conditions. Adequate acclimatization strategies, adjustment of exercise parameters to the hypoxic conditions, and nutritional strategies can buffer these detrimental effects (Fig. 5). On the other hand, the potentially complementary immune consequences of moderate exercise and hypoxia could enhance the immune benefits of exercise when combined with hypoxic exposure, like we see with the proposed complementary effects of endurance and strength training. The immune outcomes of exercise and hypoxia can interfere with or complement each other. To better understand the complexity of these effects, and how their interplay changes due to the amount, intensity, and type of both exercise and hypoxia, more interdisciplinary research needs to be carried out in traditional fields such as exercise physiology and immunology by applying modern approaches to molecular/computational biology.
Fig. 5.
Risks and opportunities to improve immune system function by exercise in hypoxia. HIF = hypoxia inducible factor; NF-κB = nuclear factor kappa-light-chain-enhancer of activated B cells.
Authors' contributions
JB conceived and planned the manuscript, wrote the first draft of the article, and created the figures; MB and BS conceived and planned the manuscript and wrote the first draft of the article; QP wrote the first draft of the article; NC created the figures and critically reviewed the manuscript draft and contributed important intellectual inputs; GPM critically reviewed the manuscript draft and contributed important intellectual inputs. All authors read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Simpson RJ, Campbell JP, Gleeson M, et al. Can exercise affect immune function to increase susceptibility to infection? Exerc Immunol Rev. 2020;26:8–22. [PubMed] [Google Scholar]
- 2.Nieman DC, Wentz LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019;8:201–217. doi: 10.1016/j.jshs.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurowski M, Seys S, Bonini M, et al. Physical exercise, immune response, and susceptibility to infections-current knowledge and growing research areas. Allergy. 2022;77:2653–2664. doi: 10.1111/all.15328. [DOI] [PubMed] [Google Scholar]
- 4.Gleeson M, Nieman DC, Pedersen BK. Exercise, nutrition and immune function. J Sports Sci. 2004;22:115–125. doi: 10.1080/0264041031000140590. [DOI] [PubMed] [Google Scholar]
- 5.Gonçalves CAM, Dantas PMS, Dos Santos IK, et al. Effect of acute and chronic aerobic exercise on immunological markers: A systematic review. Front Physiol. 2019;10:1602. doi: 10.3389/fphys.2019.01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burtscher M, Niedermeier M, Burtscher J, Pesta D, Suchy J, Strasser B. Preparation for endurance competitions at altitude: Physiological, psychological, dietary and coaching aspects. A narrative review. Front Physiol. 2018;9:1504. doi: 10.3389/fphys.2018.01504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burtscher M, Hefti U, Hefti JP. High-altitude illnesses: Old stories and new insights into the pathophysiology, treatment and prevention. Sports Med Health Sci. 2021;3:59–69. doi: 10.1016/j.smhs.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Millet GP, Debevec T, Brocherie F, Malatesta D, Girard O. Therapeutic use of exercising in hypoxia: Promises and limitations. Front Physiol. 2016;7:224. doi: 10.3389/fphys.2016.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodin P, Davis MM. Human immune system variation. Nat Rev Immunol. 2017;17:21–29. doi: 10.1038/nri.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sonnenberg GF, Hepworth MR. Functional interactions between innate lymphoid cells and adaptive immunity. Nat Rev Immunol. 2019;19:599–613. doi: 10.1038/s41577-019-0194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banoth B, Cassel SL. Mitochondria in innate immune signaling. Transl Res. 2018;202:52–68. doi: 10.1016/j.trsl.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burtscher J, Cappellano G, Omori A, Koshiba T, Millet GP. Mitochondria: In the crossfire of SARS-CoV-2 and immunity. iScience. 2020;23 doi: 10.1016/j.isci.2020.101631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor CT, Scholz CC. The effect of HIF on metabolism and immunity. Nat Rev Nephrol. 2022;18:573–587. doi: 10.1038/s41581-022-00587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeley TP, Mann GE. Defining physiological normoxia for improved translation of cell physiology to animal models and humans. Physiol Rev. 2019;99:161–234. doi: 10.1152/physrev.00041.2017. [DOI] [PubMed] [Google Scholar]
- 16.Ameln H, Gustafsson T, Sundberg CJ, et al. Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J. 2005;19:1009–1011. doi: 10.1096/fj.04-2304fje. [DOI] [PubMed] [Google Scholar]
- 17.Schito L, Semenza GL. Hypoxia-inducible factors: Master regulators of cancer progression. Trends Cancer. 2016;2:758–770. doi: 10.1016/j.trecan.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21:268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 20.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 22.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 23.Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 24.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 25.Lendahl U, Lee KL, Yang H, Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet. 2009;10:821–832. doi: 10.1038/nrg2665. [DOI] [PubMed] [Google Scholar]
- 26.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors–Similar but not identical. Mol Cell. 2010;29:435–442. doi: 10.1007/s10059-010-0067-2. [DOI] [PubMed] [Google Scholar]
- 28.Zenewicz LA. Oxygen levels and immunological studies. Front Immunol. 2017;8:324. doi: 10.3389/fimmu.2017.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7:380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollard PJ, Kranc KR. Hypoxia signaling in hematopoietic stem cells: A double-edged sword. Cell Stem Cell. 2010;7:276–278. doi: 10.1016/j.stem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Cho SH, Raybuck AL, Stengel K, et al. Germinal centre hypoxia and regulation of antibody qualities by a hypoxia response system. Nature. 2016;537:234–238. doi: 10.1038/nature19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic instruction of immunity. Cell. 2017;169:570–586. doi: 10.1016/j.cell.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buck MD, O'Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell EL, Bruyninckx WJ, Kelly CJ, et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity. 2014;40:66–77. doi: 10.1016/j.immuni.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colgan SP, Furuta GT, Taylor CT. Hypoxia and innate immunity: Keeping up with the HIFsters. Annu Rev Immunol. 2020;38:341–363. doi: 10.1146/annurev-immunol-100819-121537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furuta GT, Turner JR, Taylor CT, et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- 40.Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: Relevance to disease mechanisms. J Leukoc Biol. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- 41.McGettrick AF, O'Neill LA. The role of HIF in immunity and inflammation. Cell Metab. 2020;32:524–536. doi: 10.1016/j.cmet.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Mo Z, Liu D, Rong D, Zhang S. Hypoxic characteristic in the immunosuppressive microenvironment of hepatocellular carcinoma. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.611058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnaiz E, Harris AL. Role of hypoxia in the interferon response. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.821816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fine N, Tasevski N, McCulloch CA, Tenenbaum HC, Glogauer M. The neutrophil: Constant defender and first responder. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.571085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walmsley SR, Print C, Farahi N, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pham K, Frost S, Parikh K, Puvvula N, Oeung B, Heinrich EC. Inflammatory gene expression during acute high-altitude exposure. J Physiol. 2022;600:4169–4186. doi: 10.1113/JP282772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(Suppl. 2):S73–S80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crotty Alexander LE, Akong-Moore K, Feldstein S, et al. Myeloid cell HIF-1α regulates asthma airway resistance and eosinophil function. J Mol Med (Berl) 2013;91:637–644. doi: 10.1007/s00109-012-0986-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paul WE. Bridging innate and adaptive immunity. Cell. 2011;147:1212–1215. doi: 10.1016/j.cell.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 50.Takeda N, O'Dea EL, Doedens A, et al. Differential activation and antagonistic function of HIF-α isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cho SH, Raybuck AL, Blagih J, et al. Hypoxia-inducible factors in CD4(+) T cells promote metabolism, switch cytokine secretion, and T cell help in humoral immunity. Proc Natl Acad Sci U S A. 2019;116:8975–8984. doi: 10.1073/pnas.1811702116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clambey ET, McNamee EN, Westrich JA, et al. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A. 2012;109:E2784–E2793. doi: 10.1073/pnas.1202366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gubser PM, Bantug GR, Razik L, et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol. 2013;14:1064–1072. doi: 10.1038/ni.2687. [DOI] [PubMed] [Google Scholar]
- 54.Meng X, Grötsch B, Luo Y, et al. Hypoxia-inducible factor-1α is a critical transcription factor for IL-10-producing B cells in autoimmune disease. Nat Commun. 2018;9:251. doi: 10.1038/s41467-017-02683-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hellwig-Bürgel T, Rutkowski K, Metzen E, Fandrey J, Jelkmann W. Interleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1. Blood. 1999;94:1561–1567. [PubMed] [Google Scholar]
- 56.Khoury J, Ibla JC, Neish AS, Colgan SP. Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. J Clin Invest. 2007;117:703–711. doi: 10.1172/JCI30049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dang EV, Barbi J, Yang HY, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doedens AL, Phan AT, Stradner MH, et al. Hypoxia-inducible factors enhance the effector responses of CD8(+) T cells to persistent antigen. Nat Immunol. 2013;14:1173–1182. doi: 10.1038/ni.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pham K, Parikh K, Heinrich EC. Hypoxia and inflammation: Insights from high-altitude physiology. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.676782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rius J, Guma M, Schachtrup C, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuhlicke J, Frick JS, Morote-Garcia JC, Rosenberger P, Eltzschig HK. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS One. 2007;2:e1364. doi: 10.1371/journal.pone.0001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corzo CA, Condamine T, Lu L, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie W, Simats A, Guo Y, et al. Perspective review of myeloid immune cell responses and poststroke immunosuppression. Stroke. 2023;54:1920–1929. doi: 10.1161/STROKEAHA.122.042075. [DOI] [PubMed] [Google Scholar]
- 64.Mazzeo RS. Altitude, exercise and immune function. Exerc Immunol Rev. 2005;11:6–16. [PubMed] [Google Scholar]
- 65.Wu Q, You L, Nepovimova E, et al. Hypoxia-inducible factors: Master regulators of hypoxic tumor immune escape. J Hematol Oncol. 2022;15:77. doi: 10.1186/s13045-022-01292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mancino A, Schioppa T, Larghi P, et al. Divergent effects of hypoxia on dendritic cell functions. Blood. 2008;112:3723–3734. doi: 10.1182/blood-2008-02-142091. [DOI] [PubMed] [Google Scholar]
- 67.Grocott M, Montgomery H, Vercueil A. High-altitude physiology and pathophysiology: Implications and relevance for intensive care medicine. Crit Care. 2007;11:203. doi: 10.1186/cc5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mishra A, Mohammad G, Norboo T, Newman JH, Pasha MAQ. Lungs at high-altitude: Genomic insights into hypoxic responses. J Appl Physiol (1985) 2015;119:1–15. doi: 10.1152/japplphysiol.00513.2014. [DOI] [PubMed] [Google Scholar]
- 69.Yang J, Jin ZB, Chen J, et al. Genetic signatures of high-altitude adaptation in Tibetans. Proc Natl Acad Sci U S A. 2017;114:4189–4194. doi: 10.1073/pnas.1617042114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foll M, Gaggiotti OE, Daub JT, Vatsiou A, Excoffier L. Widespread signals of convergent adaptation to high altitude in Asia and America. Am J Hum Genet. 2014;95:394–407. doi: 10.1016/j.ajhg.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davis FM, Gallagher KA. Epigenetic mechanisms in monocytes/macrophages regulate inflammation in cardiometabolic and vascular disease. Arterioscler Thromb Vasc Biol. 2019;39:623–634. doi: 10.1161/ATVBAHA.118.312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanaoka M, Kubo K, Yamazaki Y, et al. Association of high-altitude pulmonary edema with the major histocompatibility complex. Circulation. 1998;97:1124–1128. doi: 10.1161/01.cir.97.12.1124. [DOI] [PubMed] [Google Scholar]
- 73.Walsh NP, Whitham M. Exercising in environmental extremes: A greater threat to immune function? Sports Med. 2006;36:941–976. doi: 10.2165/00007256-200636110-00003. [DOI] [PubMed] [Google Scholar]
- 74.Manella G, Ezagouri S, Champigneulle B, et al. The human blood transcriptome exhibits time-of-day-dependent response to hypoxia: Lessons from the highest city in the world. Cell Rep. 2022;40 doi: 10.1016/j.celrep.2022.111213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burtscher J, Burtscher M. Run for your life: Tweaking the weekly physical activity volume for longevity. Br J Sports Med. 2020;54:759–760. doi: 10.1136/bjsports-2019-101350. [DOI] [PubMed] [Google Scholar]
- 76.Bermon S, Castell LM, Calder PC, et al. Consensus statement immunonutrition and exercise. Exerc Immunol Rev. 2017;23:8–50. [PubMed] [Google Scholar]
- 77.Walsh NP, Oliver SJ. Exercise, immune function and respiratory infection: An update on the influence of training and environmental stress. Immunol Cell Biol. 2016;94:132–139. doi: 10.1038/icb.2015.99. [DOI] [PubMed] [Google Scholar]
- 78.Walsh NP. Recommendations to maintain immune health in athletes. Eur J Sport Sci. 2018;18:820–831. doi: 10.1080/17461391.2018.1449895. [DOI] [PubMed] [Google Scholar]
- 79.Walsh NP, Gleeson M, Shephard RJ, et al. Position westatement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 80.Burtscher J, Burtscher M, Millet GP. The central role of mitochondrial fitness on antiviral defenses: An advocacy for physical activity during the covid-19 pandemic. Redox Biol. 2021;43 doi: 10.1016/j.redox.2021.101976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985) 2005;98:1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 82.Campbell JP, Turner JE. Debunking the myth of exercise-induced immune suppression: Redefining the impact of exercise on immunological health across the lifespan. Front Immunol. 2018;9:648. doi: 10.3389/fimmu.2018.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scheffer DDL, Latini A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim Biophys Acta Mol Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campbell JP, Riddell NE, Burns VE, et al. Acute exercise mobilises CD8+ T lymphocytes exhibiting an effector-memory phenotype. Brain Behav Immun. 2009;23:767–775. doi: 10.1016/j.bbi.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 85.Turner JE, Spielmann G, Wadley AJ, Aldred S, Simpson RJ, Campbell JP. Exercise-induced b cell mobilisation: Preliminary evidence for an influx of immature cells into the bloodstream. Physiol Behav. 2016;164:376–382. doi: 10.1016/j.physbeh.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 86.Brown FF, Campbell JP, Wadley AJ, Fisher JP, Aldred S, Turner JE. Acute aerobic exercise induces a preferential mobilisation of plasmacytoid dendritic cells into the peripheral blood in man. Physiol Behav. 2018;194:191–198. doi: 10.1016/j.physbeh.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 87.Woods JA. Exercise and neuroendocrine modulation of macrophage function. Int J Sports Med. 2000;21(Suppl. 1):S24–S30. doi: 10.1055/s-2000-1448. [DOI] [PubMed] [Google Scholar]
- 88.Hansen JB, Wilsgård L, Osterud B. Biphasic changes in leukocytes induced by strenuous exercise. Eur J Appl Physiol Occup Physiol. 1991;62:157–161. doi: 10.1007/BF00643735. [DOI] [PubMed] [Google Scholar]
- 89.Krüger K, Lechtermann A, Fobker M, Völker K, Mooren FC. Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav Immun. 2008;22:324–338. doi: 10.1016/j.bbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 90.Bigley AB, Rezvani K, Pistillo M, et al. Acute exercise preferentially redeploys NK-cells with a highly-differentiated phenotype and augments cytotoxicity against lymphoma and multiple myeloma target cells. Part II: Impact of latent cytomegalovirus infection and catecholamine sensitivity. Brain Behav Immun. 2015;49:59–65. doi: 10.1016/j.bbi.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 91.Hill GW, Gillum TL, Lee BJ, et al. Prolonged treadmill running in normobaric hypoxia causes gastrointestinal barrier permeability and elevates circulating levels of pro- and anti-inflammatory cytokines. Appl Physiol Nutr Metab. 2020;45:376–386. doi: 10.1139/apnm-2019-0378. [DOI] [PubMed] [Google Scholar]
- 92.Hill GW, Gillum TL, Lee BJ, Romano PA, Schall ZJ, Kuennen MR. Reduced inflammatory and phagocytotic responses following normobaric hypoxia exercise despite evidence supporting greater immune challenge. Appl Physiol Nutr Metab. 2020;45:628–640. doi: 10.1139/apnm-2019-0657. [DOI] [PubMed] [Google Scholar]
- 93.Souza D, Vale AF, Silva A, et al. Acute and chronic effects of interval training on the immune system: A systematic review with meta-analysis. Biology (Basel) 2021;10:868. doi: 10.3390/biology10090868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Krüger K, Alack K, Ringseis R, et al. Apoptosis of T-cell subsets after acute high-intensity interval exercise. Med Sci Sports Exerc. 2016;48:2021–2029. doi: 10.1249/MSS.0000000000000979. [DOI] [PubMed] [Google Scholar]
- 95.Mooren FC, Krüger K. Apoptotic lymphocytes induce progenitor cell mobilization after exercise. J Appl Physiol (1985) 2015;119:135–139. doi: 10.1152/japplphysiol.00287.2015. [DOI] [PubMed] [Google Scholar]
- 96.Clifford T, Wood MJ, Stocks P, Howatson G, Stevenson EJ, Hilkens CMU. T-regulatory cells exhibit a biphasic response to prolonged endurance exercise in humans. Eur J Appl Physiol. 2017;117:1727–1737. doi: 10.1007/s00421-017-3667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gleeson M. Immune function in sport and exercise. J Appl Physiol (1985) 2007;103:693–699. doi: 10.1152/japplphysiol.00008.2007. [DOI] [PubMed] [Google Scholar]
- 98.Alack K, Krüger K, Weiss A, et al. Aerobic endurance training status affects lymphocyte apoptosis sensitivity by induction of molecular genetic adaptations. Brain Behav Immun. 2019;75:251–257. doi: 10.1016/j.bbi.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 99.Goh J, Goh KP, Abbasi A. Exercise and adipose tissue macrophages: New frontiers in obesity research? Front Endocrinol (Lausanne) 2016;7:65. doi: 10.3389/fendo.2016.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Syu GD, Chen HI, Jen CJ. Differential effects of acute and chronic exercise on human neutrophil functions. Med Sci Sports Exerc. 2012;44:1021–1027. doi: 10.1249/MSS.0b013e3182408639. [DOI] [PubMed] [Google Scholar]
- 101.Liao HF, Chiang LM, Yen CC, et al. Effect of a periodized exercise training and active recovery program on antitumor activity and development of dendritic cells. J Sports Med Phys Fitness. 2006;46:307–314. [PubMed] [Google Scholar]
- 102.Mackenzie B, Andrade-Sousa AS, Oliveira-Junior MC, et al. Dendritic cells are involved in the effects of exercise in a model of asthma. Med Sci Sports Exerc. 2016;48:1459–1467. doi: 10.1249/MSS.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 103.Yan H, Kuroiwa A, Tanaka H, Shindo M, Kiyonaga A, Nagayama A. Effect of moderate exercise on immune senescence in men. Eur J Appl Physiol. 2001;86:105–111. doi: 10.1007/s004210100521. [DOI] [PubMed] [Google Scholar]
- 104.Petridou A, Chatzinikolaou A, Fatouros I, et al. Resistance exercise does not affect the serum concentrations of cell adhesion molecules. Br J Sports Med. 2007;41:76–79. doi: 10.1136/bjsm.2006.031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Allsopp G, Barnard J, Goodear S, et al. The effects of normobaric hypoxia on the leukocyte responses to resistance exercise. Biol Sport. 2023;40:101–109. doi: 10.5114/biolsport.2023.112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Allsopp GL, Addinsall AB, Stephenson G, et al. The acute leukocyte and cytokine response of older adults to resistance exercise in normobaric hypoxia. Biol Sport. 2023;40:425–438. doi: 10.5114/biolsport.2023.116005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salimans L, Liberman K, Njemini R, Kortekaas Krohn I, Gutermuth J, Bautmans I. The effect of resistance exercise on the immune cell function in humans: A systematic review. Exp Gerontol. 2022;164 doi: 10.1016/j.exger.2022.111822. [DOI] [PubMed] [Google Scholar]
- 108.Szlezak AM, Szlezak SL, Keane J, Tajouri L, Minahan C. Establishing a dose-response relationship between acute resistance-exercise and the immune system: Protocol for a systematic review. Immunol Lett. 2016;180:54–65. doi: 10.1016/j.imlet.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 109.Cao Dinh H, Njemini R, Onyema OO, et al. Strength endurance training but not intensive strength training reduces senescence-prone T cells in peripheral blood in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2019;74:1870–1878. doi: 10.1093/gerona/gly229. [DOI] [PubMed] [Google Scholar]
- 110.Schlagheck ML, Walzik D, Joisten N, et al. Cellular immune response to acute exercise: Comparison of endurance and resistance exercise. Eur J Haematol. 2020;105:75–84. doi: 10.1111/ejh.13412. [DOI] [PubMed] [Google Scholar]
- 111.Jin CH, Paik IY, Kwak YS, Jee YS, Kim JY. Exhaustive submaximal endurance and resistance exercises induce temporary immunosuppression via physical and oxidative stress. J Exerc Rehabil. 2015;11:198–203. doi: 10.12965/jer.150221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Despeghel M, Reichel T, Zander J, Krüger K, Weyh C. Effects of a 6 week low-dose combined resistance and endurance training on T cells and systemic inflammation in the elderly. Cells. 2021;10:843. doi: 10.3390/cells10040843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sellami M, Bragazzi NL, Aboghaba B, Elrayess MA. The impact of acute and chronic exercise on immunoglobulins and cytokines in elderly: Insights from a critical review of the literature. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.631873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang T, Ding S, Wang R. Research progress of mitochondrial mechanism in NLRP3 inflammasome activation and exercise regulation of NLRP3 inflammasome. Int J Mol Sci. 2021;22:10866. doi: 10.3390/ijms221910866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.World Health Organization . 2020. WHO guidelines on physical activity and sedentary behaviour.https://apps.who.int/iris/rest/bitstreams/1315866/retrieveinternal-pdf://3829176424/9789240015128-eng.pdf Available at: [accessed 16.05.2023] [PubMed] [Google Scholar]
- 116.Bailey DM, Davies B. Physiological implications of altitude training for endurance performance at sea level: A review. Br J Sports Med. 1997;31:183–190. doi: 10.1136/bjsm.31.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008;38:401–423. doi: 10.2165/00007256-200838050-00004. [DOI] [PubMed] [Google Scholar]
- 118.Burtscher J, Mallet RT, Pialoux V, Millet GP, Burtscher M. Adaptive responses to hypoxia and/or hyperoxia in humans. Antioxid Redox Signal. 2022;37:887–912. doi: 10.1089/ars.2021.0280. [DOI] [PubMed] [Google Scholar]
- 119.Svendsen IS, Hem E, Gleeson M. Effect of acute exercise and hypoxia on markers of systemic and mucosal immunity. Eur J Appl Physiol. 2016;116:1219–1229. doi: 10.1007/s00421-016-3380-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Knowles R, Keeping H, Graeber T, et al. Cytokine control of PMN phagocytosis: Regulatory effects of hypoxemia and hypoxemia-reoxygenation. Am J Physiol. 1997;272:C1352–C1364. doi: 10.1152/ajpcell.1997.272.4.C1352. [DOI] [PubMed] [Google Scholar]
- 121.Pedersen BK, Steensberg A. Exercise and hypoxia: Effects on leukocytes and interleukin-6-shared mechanisms? Med Sci Sports Exerc. 2002;34:2004–2013. doi: 10.1097/00005768-200212000-00022. [DOI] [PubMed] [Google Scholar]
- 122.Flockhart M, Nilsson LC, Tais S, Ekblom B, Apró W, Larsen FJ. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021;33:957–970. doi: 10.1016/j.cmet.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 123.Mallet RT, Burtscher J, Pialoux V, et al. Molecular mechanisms of high-altitude acclimatization. Int J Mol Sci. 2023;24:1698. doi: 10.3390/ijms24021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Caris AV, Santos RVT. Performance and altitude: Ways that nutrition can help. Nutrition. 2019;60:35–40. doi: 10.1016/j.nut.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 125.Michalczyk M, Czuba M, Zydek G, Zając A, Langfort J. Dietary recommendations for cyclists during altitude training. Nutrients. 2016;8:377. doi: 10.3390/nu8060377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Santos SA, Silva ET, Caris AV, Lira FS, Tufik S, Dos Santos RVT. Vitamin E supplementation inhibits muscle damage and inflammation after moderate exercise in hypoxia. J Hum Nutr Diet. 2016;29:516–522. doi: 10.1111/jhn.12361. [DOI] [PubMed] [Google Scholar]
- 127.Sim M, Garvican-Lewis LA, Cox GR, et al. Iron considerations for the athlete: A narrative review. Eur J Appl Physiol. 2019;119:1463–1478. doi: 10.1007/s00421-019-04157-y. [DOI] [PubMed] [Google Scholar]
- 128.Castell LM, Nieman DC, Bermon S, Peeling P. Exercise-induced illness and inflammation: Can immunonutrition and iron help? Int J Sport Nutr Exerc Metab. 2019;29:181–188. doi: 10.1123/ijsnem.2018-0288. [DOI] [PubMed] [Google Scholar]
- 129.Peeling P, Dawson B, Goodman C, Landers G, Trinder D. Athletic induced iron deficiency: New insights into the role of inflammation, cytokines and hormones. Eur J Appl Physiol. 2008;103:381–391. doi: 10.1007/s00421-008-0726-6. [DOI] [PubMed] [Google Scholar]
- 130.Pedlar CR, Brugnara C, Bruinvels G, Burden R. Iron balance and iron supplementation for the female athlete: A practical approach. Eur J Sport Sci. 2018;18:295–305. doi: 10.1080/17461391.2017.1416178. [DOI] [PubMed] [Google Scholar]
- 131.Clénin G, Cordes M, Huber A, et al. Iron deficiency in sports - definition, influence on performance and therapy. Swiss Med Wkly. 2015;145:w14196. doi: 10.4414/smw.2015.14196. [DOI] [PubMed] [Google Scholar]
- 132.Constantini K, Wilhite DP, Chapman RF. A clinician guide to altitude training for optimal endurance exercise performance at sea level. High Alt Med Biol. 2017;18:93–101. doi: 10.1089/ham.2017.0020. [DOI] [PubMed] [Google Scholar]
- 133.Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: Two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524–e533. doi: 10.1016/S2352-3026(17)30182-5. [DOI] [PubMed] [Google Scholar]
- 134.Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013;65:1174–1194. doi: 10.1016/j.freeradbiomed.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 135.Petkus DL, Murray-Kolb LE, De Souza MJ. The unexplored crossroads of the female athlete triad and iron deficiency: A narrative review. Sports Med. 2017;47:1721–1737. doi: 10.1007/s40279-017-0706-2. [DOI] [PubMed] [Google Scholar]
- 136.Stellingwerff T, Peeling P, Garvican-Lewis LA, et al. Nutrition and altitude: Strategies to enhance adaptation, improve performance and maintain health: A narrative review. Sports Med. 2019;49:169–184. doi: 10.1007/s40279-019-01159-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nieman DC, Mitmesser SH. Potential impact of nutrition on immune system recovery from heavy exertion: A metabolomics perspective. Nutrients. 2017;9:513. doi: 10.3390/nu9050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Burke LM, Hawley JA, Wong SHS, Jeukendrup AE. Carbohydrates for training and competition. J Sports Sci. 2011;29(Suppl. 1):S17–S27. doi: 10.1080/02640414.2011.585473. [DOI] [PubMed] [Google Scholar]
- 139.Cermak NM, van Loon LJ. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 2013;43:1139–1155. doi: 10.1007/s40279-013-0079-0. [DOI] [PubMed] [Google Scholar]
- 140.Caris AV, Da Silva ET, Dos Santos SA, Tufik S, Dos Santos RVT. Effects of carbohydrate and glutamine supplementation on oral mucosa immunity after strenuous exercise at high altitude: A double-blind randomized trial. Nutrients. 2017;9:692. doi: 10.3390/nu9070692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Caris AV, Thomatieli-Santos RV. Carbohydrate and glutamine supplementation attenuates the increase in rating of perceived exertion during intense exercise in hypoxia similar to 4200 m. Nutrients. 2020;12:3797. doi: 10.3390/nu12123797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nieman DC, Lila MA, Gillitt ND. Immunometabolism: A multi-omics approach to interpreting the influence of exercise and diet on the immune system. Annu Rev Food Sci Technol. 2019;10:341–363. doi: 10.1146/annurev-food-032818-121316. [DOI] [PubMed] [Google Scholar]
- 143.Peron G, Hidalgo-Liberona N, González-Domínguez R, et al. Exploring the molecular pathways behind the effects of nutrients and dietary polyphenols on gut microbiota and intestinal permeability: A perspective on the potential of metabolomics and future clinical applications. J Agric Food Chem. 2020;68:1780–1789. doi: 10.1021/acs.jafc.9b01687. [DOI] [PubMed] [Google Scholar]
- 144.McKenna ZJ, Gorini Pereira F, Gillum TL, Amorim FT, Deyhle MR, Mermier CM. High-altitude exposures and intestinal barrier dysfunction. Am J Physiol Regul Integr Comp Physiol. 2022;322:R192–R203. doi: 10.1152/ajpregu.00270.2021. [DOI] [PubMed] [Google Scholar]
- 145.Karl JP, Berryman CE, Young AJ, et al. Associations between the gut microbiota and host responses to high altitude. Am J Physiol Gastrointest Liver Physiol. 2018;315:G1003–G1015. doi: 10.1152/ajpgi.00253.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Colbey C, Cox AJ, Pyne DB, Zhang P, Cripps AW, West NP. Upper respiratory symptoms, gut health and mucosal immunity in athletes. Sports Med. 2018;48:65–77. doi: 10.1007/s40279-017-0846-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Strasser B, Geiger D, Schauer M, et al. Probiotic supplements beneficially affect tryptophan-kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: A randomized, double-blinded, placebo-controlled trial. Nutrients. 2016;8:752. doi: 10.3390/nu8110752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ribeiro G, Ferri A, Clarke G, Cryan JF. Diet and the microbiota - gut - brain-axis: A primer for clinical nutrition. Curr Opin Clin Nutr Metab Care. 2022;25:443–450. doi: 10.1097/MCO.0000000000000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Subudhi AW, Jacobs KA, Hagobian TA, et al. Antioxidant supplementation does not attenuate oxidative stress at high altitude. Aviat Space Environ Med. 2004;75:881–888. [PubMed] [Google Scholar]
- 150.Koivisto AE, Paulsen G, Paur I, et al. Antioxidant-rich foods and response to altitude training: A randomized controlled trial in elite endurance athletes. Scand J Med Sci Sports. 2018;28:1982–1995. doi: 10.1111/sms.13212. [DOI] [PubMed] [Google Scholar]
- 151.Koivisto AE, Olsen T, Paur I, et al. Effects of antioxidant-rich foods on altitude-induced oxidative stress and inflammation in elite endurance athletes: A randomized controlled trial. PLoS One. 2019;14 doi: 10.1371/journal.pone.0217895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gomez-Cabrera MC, Domenech E, Romagnoli M, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 153.Paulsen G, Hamarsland H, Cumming KT, et al. Vitamin c and e supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J Physiol. 2014;592:5391–5408. doi: 10.1113/jphysiol.2014.279950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Clifford T, Jeffries O, Stevenson EJ, Davies KAB. The effects of vitamin c and e on exercise-induced physiological adaptations: A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2020;60:3669–3679. doi: 10.1080/10408398.2019.1703642. [DOI] [PubMed] [Google Scholar]