Abstract
Pulmonary alveolar proteinosis (PAP) is a rare disease which involves the accumulation of insoluble lipoproteinaceous material in the alveoli leading to impaired gas exchange and even respiratory failure. Autoimmune PAP is the most common type and is characterized by the presence of anti-granulocyte-monocyte colony stimulating factor (anti GM-CSF) antibody. Whole lung lavage has been traditionally used as first-line management of PAP but there is a lack of clarity especially in the treatment of relapsing cases of PAP. Rituximab is an anti Cluster of Differentiate 20 (CD 20) monoclonal antibody that has been tried as salvage therapy for relapsing cases of PAP. We present a case of 35 years old female patient who was diagnosed as a case of relapsing PAP who was managed initially with neoadjuvant rituximab. This is a retrospective observational report showing novel use of neoadjuvant rituximab in a difficult case of relapsing PAP.
Keywords: Pulmonary alveolar proteinosis, Anti GM-CSF antibodies, Whole lung lavage, Rituximab, Neoadjuvant therapy
Introduction
Pulmonary alveolar proteinosis (PAP) is a rare lung disorder which is characterized by the accumulation of pulmonary surfactant in the alveolar space impairing gas exchange leading to a severe hypoxemia. The most common type of PAP is the autoimmune type. Whole lung lavage (WLL) has been the gold standard therapy in PAP.
Case report
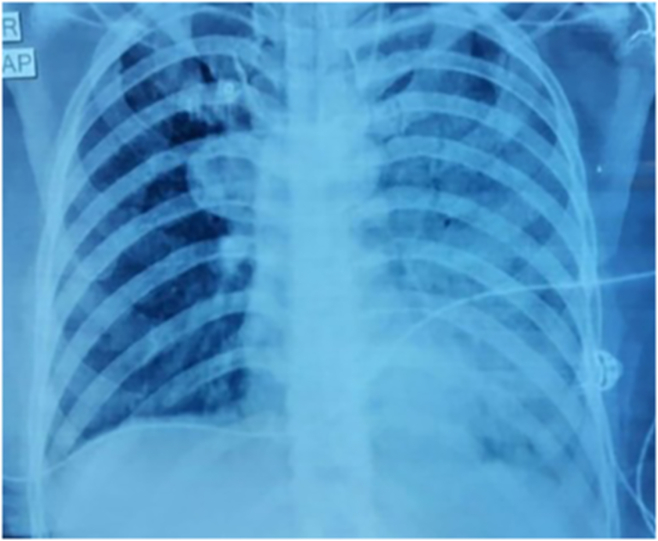
A 35-year-old female patient, non-smoker with no prior comorbidities presented with history of progressive breathlessness (Modified Medical Research Council (mMRC) grade I to mMRC Grade IV) and dry cough of 18 months duration. There was no history of any trigger factors, seasonal variation, haemoptysis, fever, wheeze or chest pain. There were no features of connective tissue disorders or vasculitis. She also gave history of frequent hospital admissions in the past 18 months and had become oxygen-dependant in the past 03 months. On arrival, patient was tachypnoeic (respiratory rate-28/min) and was having tachycardia (104/min). Her saturation at room air was 72% which improved to 94% with supplemental oxygen via nasal prongs at a flow of 6 L/min. Chest auscultation revealed bilateral basal inspiratory crackles. Other systemic examination was unremarkable. Her laboratory parameters showed leucocytosis (white blood cell count of 11,800/μL, neutrophil-59%, lymphoyte-32%), haemoglobin of 13.7 gm/dL and normal platelet count (2.17 lakh/μL). The metabolic and biochemical parameters were within normal limits. Arterial blood gas analysis showed PaO2-48 mmHg, PaCO2-32.9 mmHg and AaDo2 of 60 on room air and her ECG showed sinus tachycardia. Her 2-dimensional echocardiogram showed normal left ventricular ejection fraction (60%). The spirometry showed severe restrictive defect [forced vital capacity (FVC) - 1.17 L (47% of predicted) and forced expiratory volume (FEV) 1-0.88 L (41% predicted)]. The chest radiograph revealed bilateral alveolar infiltrates predominantly in the lower zone (Fig. 1A). High resolution computerised tomogram of chest showed diffuse ground glass haziness and interlobar septal thickening with crazy paving the appearance and dense consolidation of bilateral lower lobes (Fig. 1B). We also performed a videobronchoscopy on the patient which showed milky white lavage fluid and cytology showed dense granular amorphous proteinaceous material at places forming small globules which was periodic acid-Schiff (PAS) positive (Fig. 2A and B). She was suspected to be a case of PAP. To confirm the diagnosis, blood sample for anti-granulocyte-monocyte colony stimulating factor (anti GM-CSF) antibody was sent to Cincinnati Children's Hospital-United States, it showed raised titres of anti GM-CSF antibody [55.6 mcg/mL (normal<3.1 mcg/mL)] confirming the diagnosis of autoimmune PAP.
Fig. 1.
(A) Chest radiograph showing bilateral alveolar infiltrates in all zones. B) High resolution computed tomogram images showing dense bilateral consolidation with interlobar septal thickening with crazy paving appearance.
Fig. 2.
(A) H & E stained section at 200X showing alveoli which are dilated and are occupied by eosinophilic, amorphous material (arrow) along with mild lymphocytic inflammatory infiltrate. The alveolar walls appear thickened due to inflammatory infiltrate and type 2 pneumocyte hyperplasia (B) The same material was PAS (periodic acid-Schiff) positive.
She underwent WLL of left lung under general anaesthesia. The non-lavaged right lung was mechanically ventilated. We lavaged the lung by instilling 12 L of warm normal saline (37 °C). The fluid was then collected by gravity with manual chest compression. The lung lavage fluid was positive for periodic acid-Schiff stain. Post procedure, patient was weaned off to non-invasive ventilation and later to low flow oxygen device. We performed lavage of other lung after 3 weeks with 10.5 L of normal saline. The patient had improvement in oxygenation and was later discharged to her home on minimal oxygen @1–2 L/min.
The patient reported for review after 3 weeks, and on admission, she was symptomatic with exercise desaturation and was maintaining a saturation of 92% @ 8–10 L/min. The chest radiograph showed worsening of bilateral alveolar opacities with reticulation in bilateral mid and lower zones. A diagnosis of relapse of PAP was made and decision of starting anti CD20 therapy in form of Inj Rituximab was taken. The patient was given 2 cycles of rituximab of 1 gm intravenously in each cycle 2 weeks apart as salvage therapy.
The patient underwent repeat computed tomography of chest which showed bilateral interlobular and intralobular septal thickening with crazy paving (Right > Left). In view of aggressive disease and relapse, we planned a repeat WLL. The patient underwent 3rd cycle of WLL with total of 12 L of warmed saline of right lung. The repeat chest radiograph showed significant clearing on the right side and is presently maintaining saturation with minimal oxygen supply of 1 L/min via nasal prongs (Fig. 3). The repeat spirometry after 4 weeks showed significant improvement in the FVC (1.4 L and 60%). Her repeat Arterial Blood Gas (ABG) on room air showed significant improvement in PaO2 and AaDo2 (55 mmHg and 24). The patient is presently under follow-up and is presently asymptomatic. Patient consent was obtained for use of images and inclusion in the study.
Fig. 3.
Repeat chest radiograph showing significant clearing (right > left) post 3rd cycle of WLL. WLL, whole lung lavage.
Discussion
Primary PAP constitutes more than 90% of cases and is caused due to neutralizing auto-antibodies against GM-CSF receptors. The presence of raised titer of anti-GM-CSF antibodies clinches the diagnosis of autoimmune PAP.1,3
The gold standard of treatment of PAP is WLL which has been used since 1960. It has an efficiency of 60–80% with a relapse rate upto 15%. WLL is an invasive procedure and is done under general anaesthesia.1, 2, 3, 4 GM-CSF therapy in form of subcutaneous of nebulised form has also been described with mixed results in patients not responding to WLL.2, 3, 4 Rituximab is an anti CD20 monoclonal antibodies which has been rarely used with scarce experience. It causes reduction in population of B cells and the mode of action includes complement mediated cytotoxicity, antibody dependant cell-mediated cytotoxicity or antibody mediated apoptosis. It has been used mainly as salvage therapy in refractory patients in dose of 1000 mg which is given 2 weeks apart.5, 6, 7, 8, 9, 10
Our patient had recurrence of PAP within few months of WLL, repeating the procedure in the presence of high antibody titres might not have helped as she had aggressive disease. The patient had worsening of her clinico–radiological parameters, so we decided to give Rituximab as a neoadjuvant therapy before planning repeat WLL. Rituximab has shown response in earlier studies in decreasing anti GM-CSF antibodies, but it has never been tried as a neoadjuvant therapy. The patient showed a significant response and after the third WLL she has not shown any signs of recurrence of disease. This is a very rare case of relapsing PAP which was managed with repeated WLL with neoadjuvant rituximab before the third WLL. This is a novel report where neoadjuvant rituximab has been used before WLL in management of relapsing PAP with favourable response. The treating physician dealing with such kind of difficult case of PAP can plan anti CD20 antibodies before subsequent WLL to prevent recurrence.
Disclosure of competing interest
The authors have no conflicts of interest to declare.
References
- 1.Kumar A., Abdelmalak B., Inoue Y., Culver D.A. Pulmonary alveolar proteinosis in adults: pathophysiology and clinical approach. Lancet Respir Med. 2018 Jul;6(7):554–565. doi: 10.1016/S2213-2600(18)30043-2. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez Portal J.A. Treatment of adult primary alveolar proteinosis. Arch Bronconeumol (English Ed) 2015;51(7):344–349. doi: 10.1016/j.arbres.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Ariel Boris M., Dvorakovskaya Ivetta V., Ludmila N., Novikova M.M.I. Pulmonary alveolar proteinosis: own experience of diagnosis and treatment. J Rare Dis Res Treat [Internet] 2019;4(2):1–6. [Google Scholar]
- 4.Campo I., Kadija Z., Zorzetto M., Mariani F., Paracchini E., Luisetti M. Novel treatment options for autoimmune pulmonary alveolar proteinosis. Cit EMJ Respir. 2013;1(October:122–128. [Google Scholar]
- 5.Kavuru M.S., Malur A., Marshall I., et al. An open-label trial of rituximab therapy in pulmonary alveolar proteinosis. Eur Respir J [Internet] 2011 Dec 1;38(6):1361–1367. doi: 10.1183/09031936.00197710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malur A., Kavuru M.S., Marshall I., et al. Rituximab therapy in pulmonary alveolar proteinosis improves alveolar macrophage lipid homeostasis. Respir Res. 2012 Dec 14;13(1):46–53. doi: 10.1186/1465-9921-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soyez B., Borie R., Menard C., et al. Rituximab for auto-immune alveolar proteinosis, a real life cohort study. Respir Res. 2018;19(1):74–81. doi: 10.1186/s12931-018-0780-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meybodi F.A., Fard S.K., Zarch M.B., Babai M. Rituximab therapy in pulmonary alveolar proteinosis: a rare case report. J Clin Diagn Res. 2018;12(4):7–8. [Google Scholar]
- 9.Jin S.Y., Yun H.R., Choi Y.J., et al. A pediatric case of relapsed pulmonary alveolar proteinosis despite successful whole lung lavage. Korean J Pediatr [Internet] 2017;60(7):232–236. doi: 10.3345/kjp.2017.60.7.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amital A., Dux S., Shitrit D., Shpilberg O., Kramer M.R. Therapeutic effectiveness of rituximab in a patient with unresponsive autoimmune pulmonary alveolar proteinosis. Thorax. 2010 Nov 1;65(11):1025–1026. doi: 10.1136/thx.2010.140673. [DOI] [PubMed] [Google Scholar]