Key Points
-
•
Prophylactic Ig is more costly than prophylactic antibiotics in patients with hematological malignancies.
-
•
For many patients, Ig is unlikely to provide sufficient health benefits to warrant the additional cost compared with antibiotics.
Visual Abstract
Abstract
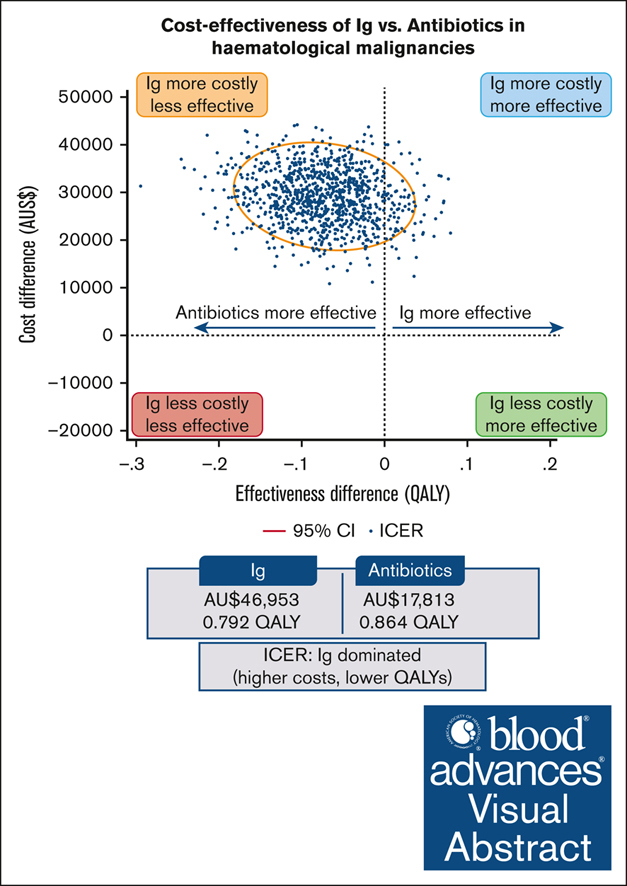
Patients with hematological malignancies are at high risk of developing hypogammaglobulinemia (HGG) and infections. Immunoglobulin (Ig) is one recommended option to prevent these infections, but it is expensive, and its cost-effectiveness compared with other prevention strategies remains unknown. We conducted a trial–based economic evaluation from the Australian health care system perspective to estimate the 12-month cost-effectiveness of prophylactic Ig vs prophylactic antibiotics in 63 adults with HGG and hematological malignancies participating in the RATIONAL feasibility trial. Two analyses were conducted: (1) cost-utility analysis to assess the incremental cost per quality-adjusted life year (QALY) gained; and (2) cost-effectiveness analysis to assess the incremental cost per serious infection prevented (grade ≥3) and per any infection (any grade) prevented. Over 12 months, the total cost per patient was significantly higher in the Ig group than in the antibiotic group (mean difference, AU$29 140; P < .001). Most patients received IVIg, which was the main cost driver; only 2 patients in the intervention arm received subcutaneous Ig. There were nonsignificant differences in health outcomes. Results showed Ig was more costly than antibiotics and associated with fewer QALYs. The incremental cost-effectiveness ratio of Ig vs antibiotics was AU$111 262 per serious infection prevented, but Ig was more costly and associated with more infections when all infections were included. On average and for this patient population, Ig prophylaxis may not be cost-effective compared with prophylactic antibiotics. Further research is needed to confirm these findings in a larger population and considering longer-term outcomes. The trial was registered at the Australian and New Zealand Clinical Trials Registry as #ACTRN12616001723471.
Introduction
Patients with hematological malignancies are at risk of developing hypogammaglobulinemia (HGG), due to disease-related immune deficiencies or immunosuppression caused by systemic anticancer treatments (eg, B-cell–targeted therapies). This increases their risk of infection, hospitalization, and mortality. Individuals with multiple myeloma (MM), chronic lymphocytic leukemia (CLL), and non-Hodgkin lymphoma (NHL) are at particularly high risk of HGG and subsequent infections.1, 2, 3
Immunoglobulin (Ig) is a plasma-derived product manufactured from blood donations, with a complex production process and high cost. Ig can be administered IV (IVIg), with typically monthly infusions provided in hospital, or can be self-administered subcutaneously (SCIg) at home, usually weekly. Prophylactic Ig replacement therapy is 1 recommended option to prevent infections in patients with HGG secondary to hematological malignancies.4, 5, 6 In some countries, a trial of antibiotic prophylaxis is required before initiating subsided Ig replacement therapy.5 Systematic reviews evaluating the effectiveness of Ig vs other prevention strategies in hematological malignancies have indicated a reduction in infections after Ig treatment. Nevertheless, the quality of included studies was considered poor, and most studies were conducted more than 20 years ago.7, 8, 9
Ig use is a key driver of plasma collection and fractionation and, with many competing clinical indications for Ig treatment, demand is putting pressure on the global plasma supply chain. In Europe, ∼60% of the plasma is imported from the United States, and plasma shortages have been identified as a serious risk in some European countries.10 Acquired HGG due to hematological malignancies or hematopoietic stem cell transplant remains the most common indication for which Ig is issued.11,12 Ig demand has been increasing worldwide by 6% to 11% per year in the past decade,11,13,14 and its use in patients with blood cancers may increase further due to the introduction of new B-cell–targeted treatments and chimeric antigen receptor T-cell therapies, which increase the risk of HGG and infections.1 In the United States, the annual cost of Ig treatment in primary immunodeficiencies was estimated at $60 000 per patient.15 In France, the cost of treating a patient with secondary immunodeficiency with Ig was estimated at >€20 000 per year.16 In Australia, 8 million grams of Ig were issued from 2021 to 2022 at a cost of AU$810 million (including the cost of plasma fractionation).17 A similar economic burden was reported in New Zealand in 2021, with annual Ig cost of NZ$57 million.14
Despite increasing costs, the cost-effectiveness of Ig compared with antibiotic prophylaxis or no prophylaxis in patients with hematological malignancies remains unknown. In a recent systematic review,18 we found no economic evaluations comparing Ig vs antibiotics in patients with hematological malignancies and HGG and only 1 cost-utility analysis (CUA)19 published in 1991 that compared Ig (IVIg) with no Ig in patients with CLL. In that study, Weeks et al19 stated that IVIg was not cost-effective compared with no Ig, with an estimated incremental cost of $6 million per quality-adjusted life year (QALY) gained over 1-year time horizon. However, the modeling assumptions and structure were only briefly described, utilities were based on physician’s assessment of the patient’s quality of life (QoL), and no sensitivity analyses were presented. These methodological issues, along with changes in cost and treatment landscape for hematological malignancies in the past 3 decades, limit its generalizability.
The aim of this economic evaluation was to evaluate the cost-effectiveness of prophylactic Ig vs prophylactic antibiotics using individual patient data from RATIONAL,20 a randomized controlled feasibility trial comparing prophylactic Ig and prophylactic antibiotics in patients with HGG secondary to hematological malignancies.
Materials and methods
RATIONAL trial
The RATIONAL trial (ACTRN12616001723471) was a phase 2, open-label, multicenter, randomized controlled trial conducted across Australia and New Zealand. The trial was powered to assess the feasibility of prophylactic antibiotics as an alternative to prophylactic Ig to prevent infections in adult patients (≥18 years) with HGG secondary to hematological malignancy and either a history of recurrent/severe infection or IgG level <4 g/L. A total of 63 participants were randomized at a 1:2 ratio to Ig (0.4 g/kg per 4 weekly IV or 0.1 g/kg per week subcutaneously) or antibiotics (trimethoprim-sulfamethoxazole 160 mg/800 mg or doxycycline 100 mg once daily if allergic to trimethoprim-sulfamethoxazole). The protocol intended for the intervention in both arms to be continued for 12 months, although participants randomized to the antibiotic arm who developed a grade ≥3 infection (as per the Common Terminology Criteria for Adverse Events [CTCAE] version 5.0) were allowed to cross over to receive Ig replacement at the discretion of the treating clinician. The primary outcome was the proportion of patients alive and on their assigned treatment 12 months after randomization. Full trial details have been published elsewhere.20
CEA
This was a trial–based post hoc economic evaluation of the RATIONAL trial, which used health care resource use and clinical outcomes from RATIONAL to assess the cost-effectiveness of prophylactic treatment with Ig or antibiotics over 12-month trial period from the Australian health system perspective. The base case was a CUA, in which the incremental cost-effectiveness ratio (ICER) was presented as the measure of additional cost per each unit of improvement in effectiveness and calculated as the difference in total costs per person divided by the difference in total QALYs per person between treatment arms over 12 months of follow-up. Given there is no official willingness to pay (WTP) threshold in Australia, a WTP of AU$50 000 per QALY gained, based on published estimates,21 was used. Although higher thresholds have been applied in Australia to fund some innovative or specialist cancer drugs,22 this threshold was considered appropriate for our base-case analysis. The net monetary benefit was calculated as the average QALY difference multiplied by the WTP threshold, minus the average total cost difference.
A secondary analysis was a cost-effectiveness analysis (CEA) comparing the difference in total costs per serious infection (grade ≥3) or any infection prevented. Sensitivity analyses were also conducted adjusting utilities derived from the 5-level EQ-5D (EQ-5D-5L) instrument by baseline utility values and using utility values derived from the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) data.
Because the time horizon did not exceed the 12-month trial duration, patient outcomes, and costs were not discounted. This study was reported following the Consolidated Health Economic Evaluation Reporting Standards 2022.23
Health outcomes
The base-case analysis used health-related QoL (HRQoL) trial data obtained through the EQ-5D-5L instrument. EQ-5D-5L data were converted into a utility score on a scale in which 0 indicated a health state equivalent to death and 1 indicated full health, using the EQ-5D-5L Australian value set.24 A sensitivity analysis was performed using EORTC QLQ-C30 trial data (supplemental Material), with utility values estimated using Australian utility weights for the EORTC QoL Utility-Core 10 Dimensions,25 a multiattribute utility instrument derived from the EORTC QLQ-C30.
Both instruments were administered at baseline and 3, 6, 9, and 12 months in the RATIONAL trial. Missing utility values were imputed using a linear trend; we estimated the area under the curve between 2 available point measures on either side of the missing data using time-weight averages. Data missing at the first or last observation were imputed with the adjacent value carried forward or backward. Individual QALYs were calculated as the area under the curve, assuming a linear change between HRQoL measurements.
Other outcome data used in the economic evaluation were survival, serious infections, and all infections at 12 months. A blinded outcome adjudication committee reviewed and classified all infection outcomes according to the CTCAE, version 5.0. All infections, including minor infections, were confirmed by the outcome adjudication committee, and those with a CTCAE grade ≥3 were considered serious infections.
Resource use and costs
Patient resource use was collected via patient diaries and as part of the case report forms monthly. Patients had monthly reviews (phone calls) with the trial coordinator and 3-monthly visits (face-to-face) with a clinician. Resource use data included hospital ward and emergency department admissions, intensive care unit stay, general practitioner and specialist consultations, day procedures, investigations, dose of prophylactic Ig and antibiotics, and postinfection antimicrobial use. Unit costs were obtained from Australian sources and presented in supplemental Table 1 (supplemental Materials). Cost of anticancer treatments were not included, given the heterogeneous patient cohort, the small number of patients on treatment during the trial, and the high cost of anticancer drugs in this population, which could have biased the results.
Only direct health care costs were included; indirect costs to the patient (eg, cost of transport to hospital and productivity loss) could not be assessed. Hospital costs were obtained from the National Hospital Cost Data Collection round 24 (2019-2020).26 Ig product costs were obtained from an Australian Government review of Ig in hematological malignancies; these were the same for IVIg and SCIg.27 Ig cost was calculated per gram per patient according to the dosage used. Because small vial sizes are available to minimize wastage, the cost associated with wastage was considered minimal and not included in the analysis. Administration cost of IVIg and SCIg were obtained from a tertiary hospital (Alfred Health, Melbourne, Australia) and a Victorian Government program run in collaboration with Australian Red Cross Lifeblood,28 respectively. SCIg administration costs included in-hospital patient training delivered by a SCIg nurse and consumables. Costs of prophylactic Ig treatment and administration were applied to 9 patients (21.4%) who crossed over from the antibiotic arm to receive Ig. Antibiotic costs (out-of-hospital) were derived from the Australian Pharmaceutical Benefits Schedule, and the number of antibiotic packs used per patient was estimated according to the dose and treatment duration. Wastage was included in the total costs because patients would have to pay for a full pack. Australian Medicare Benefits Schedule item numbers were used to estimate the cost of out-of-hospital investigations and medical consultations. All costs were presented as 2023 Australian dollars. An inflation index was applied to prices published before 2023 using the consumer price index for medical and hospital services published by the Australian Bureau of Statistics (March 2023 quarter).29
Given the small number of patients who died during the trial and that hospitalization costs associated with these deaths were included, additional costs related to death were not applied.
Statistical analysis
The statistical analysis was conducted on the intention-to-treat population, including all patients according to their originally allocated treatment arm. Mean QALYs and cost per patient were estimated for each treatment arm. Descriptive statistics were presented when appropriate, and unadjusted mean differences in costs and outcomes were compared using t tests. A probabilistic sensitivity analysis (PSA) was conducted using nonparametric bootstrapping with 1000 iterations to explore the uncertainty around the cost-effectiveness results. The 95% confidence intervals (CIs) for costs and QALYs and probabilistic results were plotted on the cost-effectiveness plane. Additional sensitivity analyses of costs and QALYs adjusted by baseline utility values were performed using linear regressions and generalized linear models with a logarithmic link function and gaussian distribution. The Modified Park test was used to determine the generalized linear model family.
The analysis was conducted using Stata version 17 (StataCorp, 2021; Stata Statistical Software: Release 17; College Station, TX)
The RATIONAL trial was registered on the Australian and New Zealand Clinical Trials Registry (ACTRN12616001723471) and was approved by relevant human research ethics committees at participating sites. We obtained informed consent from trial participants.
Results
A total of 63 patients with hematological malignancies were included in the RATIONAL feasibility trial; 21 were randomized to receive prophylactic Ig and 42 to receive prophylactic antibiotics. Baseline characteristics were well balanced between the treatment arms (Table 1). The mean age was 70 years, and 25% were receiving systemic anticancer treatment at randomization. CLL was the most common diagnosis (46%), followed by NHL (32%) and MM (19%). The mean number of infections requiring antibiotics in the year before randomization were 2.5 and 2.8 in the Ig and antibiotic arms, respectively, and the corresponding mean number of infections requiring hospitalizations were 0.4 and 0.7.
Table 1.
Baseline patient characteristics
| Ig | Oral antibiotics | |
|---|---|---|
| n | 21 | 42 |
| Age, mean (SD), y | 71.2 (8.6) | 69.1 (7.8) |
| Female, n (%) | 13 (62%) | 21 (50%) |
| BMI, mean (SD), kg/m2 | 30.3 (7.5) | 28.8 (5.8) |
| IgG, mean (SD), g/L | 5.6 (5.3) | 5.3 (5.5) |
| Diagnosis, n (%) | ||
| CLL | 8 (38%) | 21 (50%) |
| MM | 3 (14%) | 9 (21%) |
| NHL | 8 (38%) | 12 (29%) |
| Other | 2 (10%) | 0 (0%) |
| Disease stage, n (%) | ||
| Treatment-naïve | 4 (19%) | 13 (32%) |
| Remission after treatment | 8 (38%) | 13 (32%) |
| Relapsed/refractory | 3 (14%) | 5 (12%) |
| Currently receiving treatment | 6 (29%) | 9 (22%) |
| Stable disease posttreatment | 0 (0%) | 1 (2%) |
| ECOG performance status, n (%) | ||
| 0 | 8 (38%) | 19 (46%) |
| 1 | 11 (52%) | 15 (37%) |
| 2 | 2 (10%) | 7 (17%) |
| Prior cancer treatments∗, n (%) | ||
| 0 | 4 (19%) | 12 (31%) |
| 1 | 6 (29%) | 11 (28%) |
| 2 | 5 (24%) | 5 (13%) |
| 3 | 4 (19%) | 6 (15%) |
| ≥4 | 2 (10%) | 5 (13%) |
| Prior ASCT, n (%) | ||
| No | 18 (86%) | 37 (88%) |
| Yes | 3 (14%) | 5 (12%) |
| Infections requiring antibiotics in the past year, mean (SD) | 2.5 (1.8) | 2.8 (1.9) |
| Infections requiring hospitalization in the past year, mean (SD) | 0.4 (0.8) | 0.7 (1.1) |
ASCT, autologous stem cell transplant; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; SD, standard deviation.
Including cancer treatments for other prior malignancies.
A summary of health care resource use during the trial and associated costs is presented in Table 2. There were no significant differences between the treatment arms in health care resource use. The mean number of hospital admissions was the same between the 2 groups, but the length of hospital stay was slightly longer in the antibiotic arm, which was the driver of higher in-hospital costs in patients treated with prophylactic antibiotics. On the contrary, there were slightly more emergency department admissions and general practitioner visits in the Ig arm. There was only 1 intensive care unit admission in the Ig arm, due to septic shock. Mean total costs per patient were significantly higher in the Ig than the antibiotic arm (AU$46 953 vs AU$17 813). The main contributor to the higher costs in the Ig arm was the cost of prophylactic Ig treatment, including both Ig product and administration costs, than that of the prophylactic antibiotics arm (AU$37 331 vs AU$5798). Among patients treated with Ig, IVIg was given to most participants, except for 3 who received SCIg (2 of them transitioned from IVIg to SCIg and the other 1 crossed over from the antibiotics arm). In the Ig arm, the mean IVIg and SCIg total costs were similar (AU$39 374 and AU$37 613, respectively); however, given the small number of patients receiving SCIg, these numbers need to be treated with caution.
Table 2.
Resource use and cost (AU$) per patient per year
| Ig (n = 21), mean (SD) |
Antibiotics (n = 42), mean (SD) |
Mean difference (95% CI) | |
|---|---|---|---|
| Resource use per patient per year (12-mo follow-up) | |||
| Hospital ward admissions | 0.48 (0.68) | 0.48 (0.92) | 0 (–0.45 to 0.45) |
| ED admissions | 0.24 (0.62) | 0.17 (0.44) | 0.07 (–0.20 to 0.34) |
| ICU admissions | 0.05 (0.22) | 0 (0) | 0.05 (–0.02 to 0.11) |
| Length of hospital stay,∗ d | 3.24 (4.10) | 4.24 (7.10) | –1 (–4.35 to 2.35) |
| Day procedures | 0.09 (0.44) | 0.07 (0.35) | 0.02 (–0.18 to 0.22) |
| Specialist consultations | 0.05 (0.22) | 0.05 (0.31) | 0 (–0.15 to 0.15) |
| GP visits | 1.90 (1.48) | 1.71 (1.07) | 0.19 (–0.46 to 0.84) |
| Investigations (out-of-hospital) | 0.57 (0.87) | 0.50 (1.13) | 0.07 (–0.49 to 0.63) |
| Antimicrobials (excluding IV) | 2.95 (2.13) | 2.07 (2.57) | 0.88 (–0.42 to 2.18) |
| Cost per patient per year (12-mo follow-up) | |||
| In-hospital costs | |||
| Hospital ward | $8 637 (10 984) | $11 473 (19 225) | –$2 836 (–11 905 to 6 233) |
| ED | $221 (581) | $155 (406) | $66 (–185 to 318) |
| ICU | $279 (1 277) | $0 (0) | $279 (–112 to 669) |
| Day procedures | $217 (993) | $162 (777) | $54 (–402 to 511) |
| Total in-hospital costs | $9 354 (11 436) | $11 791 (19 159) | –$2 436 (–11 532 to 6 657) |
| Out-of-hospital costs | |||
| Specialist consultations | $8 (35) | $8 (50) | $0 (–24 to 24) |
| GP visits | $111 (86) | $100 (62) | $11 (–27 to 49) |
| Investigations (out-of-hospital) | $21 (32) | $19 (42) | $2 (–18 to 23) |
| Antimicrobials (excluding IV) | $128 (161) | $98 (190) | $30 (–67 to 127) |
| Total out-of-hospital costs | $268 (211) | $224 (224) | $44 (–74 to 161) |
| Prophylactic treatment (Ig or antibiotics) | $37 331 (16 493) | $5 798 (11 635) | $31 533 (24 360-38 707) |
| Total cost | $46 953 (18 626) | $17 813 (27 173) | $29 140 (15 940-42 334) |
All costs in AU$ (2023).
ED, emergency department; GP, general practitioner; ICU, intensive care unit.
ICU and ward.
There were no significant differences between the treatment groups in the mean number of infections (difference, 0.76; 95% CI, –0.33 to 1.86) and serious infections (difference, –0.26; 95% CI, –0.74 to 0.21). The effect of infections on QoL could not be fully assessed due to the small patient numbers. Three patients died, 2 in the Ig arm and 1 in the antibiotic arm, of which 1 death in the Ig group occurred within 7 days of diagnosis of infection, and another death in the antibiotic arm occurred within 1 month of a serious infection.
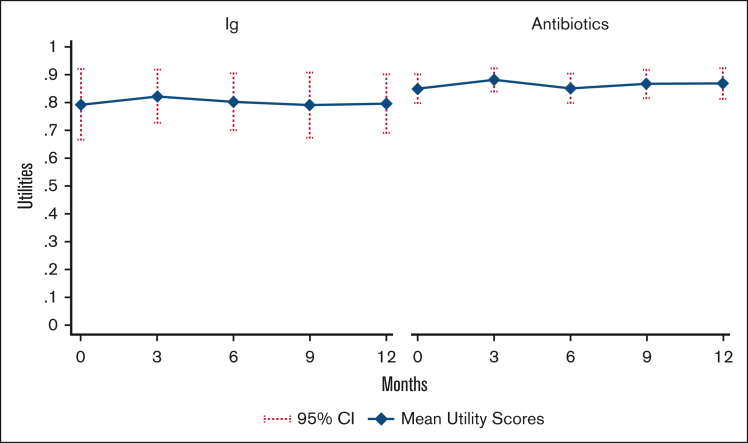
Figure 1 presents mean utility scores derived from the EQ-5D-5L through the 12-month trial duration. Baseline mean utility values were lower in the Ig arm than in the antibiotic arm and did not change considerably during the trial, but CIs in the Ig arm were large and overlapped with those in the antibiotic arm. Of the 61 patients with EQ-5D-5L data available, 6 (9.8%) had missing data at baseline. Imputed utility values were used to calculate QALYs in the base-case analysis (mean Ig, 0.800; mean antibiotics, 0.863); mean unimputed utilities (Ig, 0.796; antibiotics, 0.864) were similar to imputed utility values.
Figure 1.
Mean utility scores by treatment group. Mean imputed utility scores by treatment group, derived from EQ5D-5L trial data. Higher utility values reflect better HRQoL.
The cost-effectiveness results are presented in Table 3. The base-case CUA using unadjusted total costs and QALYs (derived from the EQ-5D-5L) indicated that Ig was dominated by prophylactic antibiotics; that is, Ig was associated with fewer QALYs (0.792 vs 0.864) and higher costs (AU$46 953 vs AU$17 813) than antibiotics. At a WTP of AU$50 000 per QALY, the incremental net monetary benefit of prophylactic Ig vs antibiotic treatment was –AU$32 740. In the CEA, the ICER of Ig vs antibiotics was AU$111 262 per serious infection prevented, whereas Ig was dominated by antibiotics (ie, Ig was associated with more infections and higher costs) when all infections were considered.
Table 3.
Cost-effectiveness results over the 12-month trial period (costs in AU$)
| Trial–based 12-mo results | Prophylactic Ig, mean (95% CI) |
Prophylactic antibiotics, mean (95% CI) |
Difference, mean (95% CI) |
|---|---|---|---|
| Total costs per person | $46 953 (38 474-55 431) | $17 813 (9 345-26 280) | $29 140 (15 941-42 340) |
| QALY (unadjusted) per person | 0.792 (0.687-0.897) | 0.864 (0.820-0.910) | –0.072 (–0.167 to 0.023) |
| Serious infections per person | 0.24 (0.04-0.44) | 0.50 (0.17-0.82) | –0.26 (–0.74 to 0.21) |
| All infections per person | 2.76 (1.78-3.75) | 2.00 (1.38-2.62) | 0.76 (–0.33 to 1.86) |
| ICER (cost/QALY) | Ig dominated (higher costs, lower QALYs) | ||
| ICER (cost/serious infection) | $111 262 per serious infection prevented | ||
| ICER (cost/any infection) | Ig dominated (higher costs, more infections) | ||
| Incremental net benefit | –$32 740 (–45 678 to –19 958) | ||
Utilities were derived from EQ-5D-5L trial data. All costs are in AU$ for the year 2023.
Serious infections are grade ≥3, as confirmed by an independent committee.
We do not report the 95% CI around a negative ICER because it can be misleading. A negative ICER can either represent a very cost-effective result (lower costs, better health outcomes) or a not cost-effective result (higher costs, worse health outcomes). Please see a visual presentation of the 95% CI in Figure 2, which allows an easier interpretation.
Sensitivity analyses
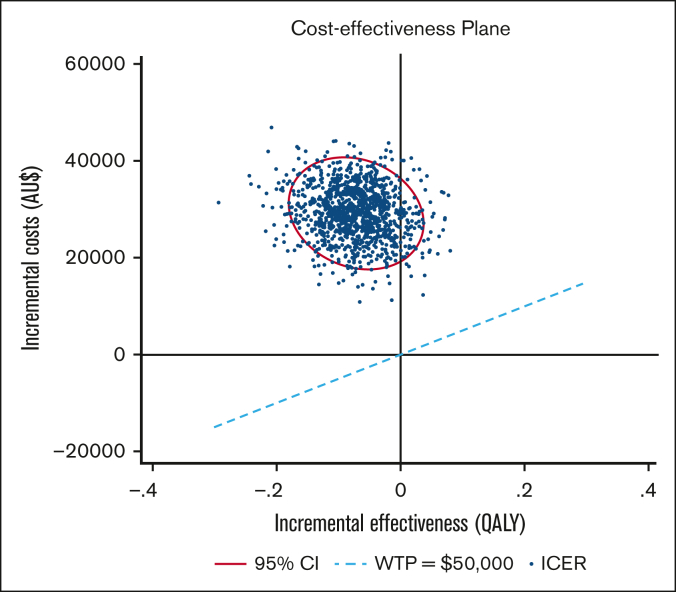
A PSA using 1000 bootstrapped estimates of incremental costs and QALYs was used to analyze the uncertainty around the ICER (Figure 2). The majority of the ICER pairs were in the “north-western” quadrant, above the AU$50 000 WTP threshold, indicating that Ig was more costly and less effective than antibiotics. Under this WTP threshold, Ig had a 0% probability of being cost-effective and remained as such up to a WTP of ∼AU$1 000 000 per QALY.
Figure 2.
Cost-effectiveness plane Ig vs antibiotics. PSA. The ellipse represents the 95% CI around the ICER points, calculated via bootstrap (1000 iterations). The ICER scatter lies over the WTP line of AU$50 000 per QALY, indicating prophylactic Ig is less effective and more costly than antibiotics in this population and at that WTP threshold.
Ig remained dominated after adjusting for baseline utilities (supplemental Table 2; supplemental Materials). The ICER in the CEA was reduced to AU$104 744 per serious infection prevented after adjusting for baseline utility, but the impact on the incremental costs was minimal. Additional sensitivity analyses were conducted assuming 16% and 50% of patients in the Ig arm received SCIg (supplemental Table 2), with minimal impact on incremental costs and ICER.
A PSA of incremental costs and QALYs adjusted by baseline utilities led to similar results to those in the base case (supplemental Figure 1; supplemental Materials). A sensitivity analysis was also conducted using EORTC QLQ-C30 to derive utility values (supplemental Table 2; supplemental Materials). Both utilities and QALYs were lower using this instrument than EQ-5D-5L, but the QALY difference between treatment arms was similar to the base-case analysis, and Ig remained dominated by antibiotics in the CUA.
Discussion
To our knowledge, this is the first trial–based economic evaluation to assess the cost-effectiveness of prophylactic Ig compared with prophylactic antibiotics in patients with HGG secondary to hematological malignancy. Our results indicated that, over the 12-month trial period, Ig was more costly than antibiotics and did not lead to significant QALY gains. Similarly, there were no significant differences in annual infection rates between the treatment arms. There was a trend toward a lower occurrence of serious infections in the Ig arm, which was reversed when all infections were considered. The incremental cost per serious infection prevented for Ig vs antibiotics was AU$111 262, whereas Ig was dominated by antibiotics when all infections were included.
There was a high level of uncertainty around the HRQoL and infection outcomes. Mean utilities in the Ig arm were highly uncertain, as indicated by large CIs that overlapped with those in the antibiotic arm. Baseline utility values were lower in patients randomized to Ig than in those receiving prophylactic antibiotics and did not vary substantially throughout the trial. Adjustment for baseline utilities decreased QALY differences between the treatment arms, but mean QALYs in the Ig group remained lower than that in the antibiotic group. These QALY differences were maintained when EORTC QLQ-C30 data were used to derive utility scores, although QALYs in both treatment arms were lower than that in the base-case analysis, indicating the EORTC QLQ-C30 may be more sensitive to detect HRQoL changes in patients with hematological malignancies than the EQ-5D-5L.
Our utility values were consistent with published estimates.30,31 The systematic review of EQ-5D–derived utilities by Golicki et al30 reported a wide range of utility values across different hematological malignancies, which varied depending on disease stage, severity, and treatment. The large CIs in the Ig arm may be in part due to the small number of patients but also reflects the heterogeneity of our patient cohort. Golicki et al also noted that, overall, patients with CLL had higher health state utilities (range, 0.75-0.90) than those with MM or NHL.30 Other studies in patients with CLL have reported decreasing utility values as treatment intensifies, ranging from 0.82 in those treatment-free during progression-free survival to 0.42 after relapsed lines of treatment for CLL.31 This might explain why utility scores in our study were relatively high, because CLL was the largest diagnostic group (29 of 63 patients), and the majority of trial participants had only received a maximum of 2 lines of treatment.
Prophylactic Ig treatment has previously been found to reduce the risk of clinically documented infections in patients with hematological malignancies and HGG, although it did not appear to affect overall survival.7,9 The efficacy of prophylactic antibiotics in this population is more unclear; the systematic review by Chai et al7 did not find any significant reduction in the risk of infections after prophylactic antibiotics compared with placebo. In the clinical evaluation of RATIONAL,20 time to serious infection was similar in the Ig and antibiotic arms (11.1 vs 9.7 months). In our analysis, the rate of serious infections in the Ig group was consistent with annual rates previously described in patients with secondary immunodeficiencies during SCIg treatment.32
We could not examine the impact of antibiotic prophylaxis on antibiotic resistance and associated clinical outcomes and costs. Antibiotics are known to induce changes in the intestinal microbiome that can lead to antibiotic resistance, but the effect on the microbiome depends on the antibiotic class.33 Beyond the impact of antibiotic resistance on morbidity and mortality,34 the associated economic burden is considerable, leading to higher overall health care costs, length of hospital stay, and readmission rates.35 Long-term antibiotic use is not uncommon; a recent survey of infectious disease physicians identified that 88% had previously prescribed long-term antibiotics.36 The need for further research on the full impact of long-term antibiotic prophylaxis has been highlighted, in particular regarding changes in microbiome and development of resistance, long-term effectiveness, and chronic side effects.37
In our analysis, mean total costs per patient were significantly higher in the prophylactic Ig arm than the prophylactic antibiotic arm, mainly driven by higher product and administration costs. Annual hospitalization rates were similar in both treatment arms, but mean length of hospital stay was slightly longer in patients receiving prophylactic antibiotics. Only 3 patients received SCIg in RATIONAL. Uptake of SCIg in Australia remains low across all approved clinical indications, with ∼16% of patients eligible for Ig treatment currently receiving SCIg.38 Some of the identified barriers to SCIg uptake are lack of funding and resourcing, clinician and hospital preferences, low awareness of SCIg and its benefits, limited access, and patient’s preference for IVIg.38 Cost-minimization analyses in primary immunodeficiency diseases have indicated that SCIg is less costly than IVIg to the health system.39,40 Similarly, 2 Canadian studies41,42 in patients with primary and secondary immunodeficiencies estimated that switching from IVIg to SCIg would decrease nurse shortages and health care costs, with ∼CA$31 million in cost savings to the health system if 80% of individuals switched from clinic-administered IVIg to SCIg.42 Our sensitivity analysis assuming that 50% of patients in the Ig arm received SCIg did not significantly affect the incremental costs and ICER. Nevertheless, given the upfront costs of training and key consumables associated with SCIg, further research over a longer time horizon may be needed to evaluate the potential for lower incremental costs with more widespread use of SCIg.
Our evaluation followed a health care system perspective, and only direct costs to the health system were included. Including costs to the patient may lead to higher overall costs in those treated with Ig, particularly for patients receiving IVIg in hospital. The cost-minimization analysis by Perraudin et al40 in primary immunodeficiency diseases indicated that indirect costs (ie, transport and productivity loss) accounted for ∼9% and ∼4% of the total costs in patients treated with IVIg and SCIg, respectively.
The main limitation of our study was the small sample size, particularly in the Ig arm. RATIONAL was a phase 2 feasibility trial that was not powered for noninferiority nor superiority, and therefore, we cannot discard the possibility that important differences in clinical outcomes may not have been detected. Our patient population was heterogeneous, with 3 main diagnoses, varying lines of treatment, and disease stages. Given the small sample size, we could not assess the impact of infections, hematological malignancy diagnosis, disease stage, stem cell transplantation, or anticancer treatment on utilities and QALYs. Small differences in these factors could have affected QALY gained due to the small number of patients in the study. Nevertheless, our adjustment for baseline utility values would at least partially account for any imbalances in baseline characteristics, because these would be reflected on baseline utilities. Moreover, we conducted a PSA to test the robustness of the model, which indicated that a very large increase in QALYs would be needed for Ig to become cost-effective, given its high cost. Crossover was allowed from the antibiotic arm to Ig arm, which may have led to an increase in the cost of prophylactic treatment in the antibiotic arm and potentially affected infection rates. However, this approach is more reflective of the real-world setting in which patients would likely receive Ig treatment after a serious infection. Our trial–based economic evaluation only included costs and benefits over the 12-month trial duration. Many of these patients can have prolonged HGG, and our 12-month time horizon did not allow for us to account for the longer-term costs, benefits, and/or harms of treatment with Ig or antibiotics, including the potential risk of antibiotic resistance.
Despite these limitations, RATIONAL is one of the largest trials of Ig in this patient population and, to our knowledge, the only trial to date comparing prophylactic Ig with prophylactic antibiotics. This is also, to our knowledge, the only trial–based economic evaluation to date comparing prophylactic Ig and prophylactic antibiotics and the only CEA comparing Ig with any other therapeutic strategy since the appearance of targeted anticancer treatments for hematological malignancies. This evaluation highlights the high costs and uncertainty regarding the effectiveness of Ig compared with prophylactic antibiotic treatment in this patient population. Either a significant improvement in clinical outcomes, a significant reduction in costs, or more likely both would be required for Ig to become cost-effective compared with antibiotics at standard cost-effectiveness thresholds. Our results challenge previously reported physician expectations on Ig replacement therapy,2 in which the majority of respondents anticipated improvements in QoL, prevention of severe and moderate infections, and reduction in hospitalization rates. Further research is warranted to assess the full health economic impact of Ig according to HGG severity, previous infections, hematological diagnoses, and different stages of the treatment pathway, including a larger number of patients and longer follow-up. A follow-on phase 2/3 trial is currently open for recruitment and will provide an opportunity to address these questions (ACTRN12622000359730).
Conflict-of-interest disclosure: R.W. reports advisory board and speaker fees from Janssen and AbbVie; and research funding to institution from Janssen, BioOra, and Wellington Zhaotai Therapies Limited. J.R. is an equity holder in the publicly traded company Novartis and Alcon. J.T. reports research funding to institution from Janssen, BeiGene, Roche, Bristol Myers Squibb, and Cellectar Biosciences. A.J. participated in advisory boards and received honoraria from Roche, Link, MSD, BeiGene, Sanofi, EUSA Pharma, and Novartis; and provided medical education for Takeda. E.M.W. and Z.K.M. received grant funding from CSL Behring not related to this study; and research funding to institution from AbbVie, Amgen, AstraZeneca, BeiGene, Celgene, Janssen, New Zealand Blood Service, Novartis, Sanofi, and Takeda. The remaining authors declare no competing financial interests.
Acknowledgments
The RATIONAL trial was funded by a grant from the Australian National Blood Authority. This study is supported by the National Health and Medical Research Council (NHMRC) Blood Synergy Grant (#1189490) (S.C.d.A.); an NHMRC Emerging Leader Fellowship (GNT2008447) (A.M.H.); the Medical Research Future Fund Value-Ig Study (MRF2017480) (D.P.); a Health Research Council of New Zealand Clinical Practitioner Research Fellowship (19/139) (R.W.); an NHMRC Investigator grant (GNT1177784) (E.M.W.); and an NHMRC Emerging Leader Investigator grant (GNT1194811) (Z.K.M.).
Authorship
Contribution: Regarding economic evaluation, S.C.d.A., A.M.H., D.P., and Z.K.M. designed the health economic analysis; S.C.d.A. drafted the health economics protocol, analyzed the data, and wrote this manuscript draft; A.M.H., D.P., L.F., and A.I. also contributed to the data analysis; and all authors revised the manuscript; regarding RATIONAL trial, Z.K.M., E.M.W., R.W., and C.O.M. conceived and designed the study; Z.K.M. drafted the protocol; R.W., P.C., C.D., M.G., A.J., A.K., D.P., H.P., J.R., T.v.T., J.T., N.W., C.W., H.W., C.O.M., E.M.W., Z.K.M. performed the research; J.R. and Z.K.M. wrote the statistical analysis plan; and E.M.W. is the grant holder.
Footnotes
Deidentified participant data and study protocol will be made available with the publication upon request to the RATIONAL Principal Investigators by emailing the corresponding author, Sara Carrillo de Albornoz (sara.carrillo@monash.edu).
Investigators whose proposal has been reviewed and approved by the RATIONAL investigators and relevant ethical review committees will be able to undertake analyses to achieve the aims specified in the approved proposal by accessing data through a web-based data portal safe-haven based at Monash University, Australia.
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Patel SY, Carbone J, Jolles S. The expanding field of secondary antibody deficiency: causes, diagnosis, and management. Front Immunol. 2019;10:33. doi: 10.3389/fimmu.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benbrahim O, Viallard JF, Choquet S, et al. A French observational study describing the use of human polyvalent immunoglobulins in hematological malignancy-associated secondary immunodeficiency. Eur J Haematol. 2018;101(1):48–56. doi: 10.1111/ejh.13078. [DOI] [PubMed] [Google Scholar]
- 3.Friman V, Winqvist O, Blimark C, Langerbeins P, Chapel H, Dhalla F. Secondary immunodeficiency in lymphoproliferative malignancies. Hematol Oncol. 2016;34(3):121–132. doi: 10.1002/hon.2323. [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Agency Guideline on Core SmPC for Human Normal Immunoglobulin for Intravenous Administration (IVIg) 2018. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-core-smpc-human-normal-immunoglobulin-intravenous-administration-ivig-rev-5_en.pdf
- 5.UK Department of Health Clinical Guidelines for Immunoglobulin Use, Update to 2nd Edition. 2011. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/216671/dh_131107.pdf
- 6.BloodStar Criteria for Clinical Use of Immunoglobulin in Australia: Acquired Hypogammaglobulinaemia Secondary to Haematological Malignancies, or Post-Haemopoietic Stem Cell Transplantation (HSCT) https://www.criteria.blood.gov.au/MedicalCondition/View/2621
- 7.Chai KL, Wong JWK, Weinkove R, et al. Interventions to reduce infections in patients with hematological malignancies: a systematic review and meta-analysis. Blood Adv. 2023;7(1):20–31. doi: 10.1182/bloodadvances.2022008073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monleón Bonet C, Waser N, Cheng K, Tzivelekis S, Edgar JDM, Sánchez-Ramón S. A systematic literature review of the effects of immunoglobulin replacement therapy on the burden of secondary immunodeficiency diseases associated with hematological malignancies and stem cell transplants. Expert Rev Clin Immunol. 2020;16(9):911–921. doi: 10.1080/1744666X.2020.1807328. [DOI] [PubMed] [Google Scholar]
- 9.Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. Immunoglobulin prophylaxis in hematological malignancies and hematopoietic stem cell transplantation. Cochrane Database Syst Rev. 2008;2008(4) doi: 10.1002/14651858.CD006501.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brand A, De Angelis V, Vuk T, Garraud O, Lozano M, Politis D, European Mediterranean Initiative for Transfusion Medicine Review of indications for immunoglobulin (IG) use: narrowing the gap between supply and demand. Transfus Clin Biol. 2021;28(1):96–122. doi: 10.1016/j.tracli.2020.12.005. [DOI] [PubMed] [Google Scholar]
- 11.National Blood Authority National Blood Authority National Report on the Issue and Use of Immunoglobulin (Ig) Annual Report 2017-18. 2018. https://www.blood.gov.au/data-analysis-reporting#Report-on-the-Issue-and-Use-of-Immunoglobulin
- 12.National Blood Authority Ig Usage Data and Statistics – May 2023. https://www.blood.gov.au/ig-usage-data-and-statistics
- 13.Prevot J, Jolles S. Global immunoglobulin supply: steaming towards the iceberg? Curr Opin Allergy Clin Immunol. 2020;20(6):557–564. doi: 10.1097/ACI.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misra UK, Mani VE, Kalita J. A cost-effective approach to the diagnosis and management of acute infectious encephalitis. Eur Neurol. 2017;77(1-2):66–74. doi: 10.1159/000453662. [DOI] [PubMed] [Google Scholar]
- 15.Sun D, Heimall JR, Greenhawt MJ, Bunin NJ, Shaker MS, Romberg N. Cost utility of lifelong immunoglobulin replacement therapy vs hematopoietic stem cell transplant to treat agammaglobulinemia. JAMA Pediatr. 2022;176(2):176–184. doi: 10.1001/jamapediatrics.2021.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legendre P, Chahwan D, Marjanovic Z, et al. Utilization of intravenous or subcutaneous immunoglobulins in secondary immune deficiency (ULTIMATE): a retrospective multicenter study. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108419. [DOI] [PubMed] [Google Scholar]
- 17.National Blood Authority Annual Report 2021-2022. 2022. https://blood.gov.au/sites/default/files/NBA0861%20%20NBA%20Annual%20Report%20202122%20-%20WCAG.pdf
- 18.De Albornoz SC, Chai KL, Higgins AM, Petrie D, Wood EM, McQuilten ZK. OP95 a systematic review of the cost and cost effectiveness of immunoglobulin treatment in patients with hematological malignancies. Int J Technol Assess Health Care. 2023;39(S1):S26–S27. doi: 10.1017/S026646232400028X. [DOI] [PubMed] [Google Scholar]
- 19.Weeks JC, Tierney MR, Weinstein MC. Cost effectiveness of prophylactic intravenous immune globulin in chronic lymphocytic leukemia. N Engl J Med. 1991;325(2):81–86. doi: 10.1056/NEJM199107113250202. [DOI] [PubMed] [Google Scholar]
- 20.McQuilten ZK, Weinkove R, Thao LTP, et al. Immunoglobulin replacement vs prophylactic antibiotics for hypogammaglobulinemia secondary to haematological malignancy. Blood Adv. Published online 15 November 2023 doi: 10.1182/bloodadvances.2023011231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang L, Frijters P, Dalziel K, Clarke P. Life satisfaction, QALYs, and the monetary value of health. Soc Sci Med. 2018;211:131–136. doi: 10.1016/j.socscimed.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Commonwealth of Australia Availability of New, Innovative and Specialist Cancer Drugs in Australia. Chapter 3. Affordable Access-Assessing Cost-Effectiveness. 2015. https://www.aph.gov.au/Parliamentary_Business/Committees/Senate/Community_Affairs/Cancer_Drugs/Report
- 23.Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;376 doi: 10.1136/bmj-2021-067975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norman R, Mulhern B, Lancsar E, et al. The use of a discrete choice experiment including both duration and dead for the development of an EQ-5D-5L value set for Australia. Pharmacoeconomics. 2023;41(4):427–438. doi: 10.1007/s40273-023-01243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King MT, Viney R, Simon Pickard A, et al. Australian utility weights for the EORTC QLU-C10D, a multi-attribute utility instrument derived from the cancer-specific quality of life questionnaire, EORTC QLQ-C30. Pharmacoeconomics. 2018;36(2):225–238. doi: 10.1007/s40273-017-0582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Independent Health and Aged Care Pricing Authority National Hospital Cost Data Collection (NHCDC) Public Hospitals Report 2019-20. 2022. https://www.ihacpa.gov.au/resources/national-hospital-cost-data-collection-nhcdc-public-hospitals-report-2019-20
- 27.Wyndham A, Vogan A, Newton S, Schubert C. Immunoglobulin for Acquired Hypogammaglobulinaemia Secondary to Haematological Malignancies, or Post-Haemopoietic Stem Cell Transplantation (HSCT). MSAC. 2019. http://www.msac.gov.au/internet/msac/publishing.nsf/Content/1565-public
- 28.Blood Matters Consultation Paper on the Pricing Framework for Australian Public Hospital Services 2023-24. 2022. https://www.ihacpa.gov.au/sites/default/files/2022-09/blood_matters_program_national_bloody_authority.pdf
- 29.Australian Bureau of Statistics Consumer Price Index, Australia. https://www.abs.gov.au/statistics/economy/price-indexes-and-inflation/consumer-price-index-australia/mar-quarter-2023
- 30.Golicki D, Jaśkowiak K, Wójcik A, et al. EQ-5D-derived health state utility values in hematologic malignancies: a catalog of 796 utilities based on a systematic review. Value Health. 2020;23(7):953–968. doi: 10.1016/j.jval.2020.04.1825. [DOI] [PubMed] [Google Scholar]
- 31.Kosmas CE, Shingler SL, Samanta K, et al. Health state utilities for chronic lymphocytic leukemia: importance of prolonging progression-free survival. Leuk Lymphoma. 2015;56(5):1320–1326. doi: 10.3109/10428194.2014.961012. [DOI] [PubMed] [Google Scholar]
- 32.Cinetto F, Neri R, Vianello F, et al. Subcutaneous immunoglobulins replacement therapy in secondary antibody deficiencies: real life evidence as compared to primary antibody deficiencies. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0247717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lange K, Buerger M, Stallmach A, Bruns T. Effects of antibiotics on gut microbiota. Dig Dis. 2016;34(3):260–268. doi: 10.1159/000443360. [DOI] [PubMed] [Google Scholar]
- 34.Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poudel AN, Zhu S, Cooper N, et al. The economic burden of antibiotic resistance: a systematic review and meta-analysis. PLoS One. 2023;18(5) doi: 10.1371/journal.pone.0285170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiss CR, Lau JSY, Yeung A, Woolley I. Infectious diseases physician attitudes to long-term antibiotic use. Int J Clin Pharm. 2019;41(1):18–21. doi: 10.1007/s11096-018-00781-4. [DOI] [PubMed] [Google Scholar]
- 37.Lau JSY, Korman TM, Woolley I. Life-long antimicrobial therapy: where is the evidence? J Antimicrob Chemother. 2018;73(10):2601–2612. doi: 10.1093/jac/dky174. [DOI] [PubMed] [Google Scholar]
- 38.National Blood Authority Evaluate and Develop Options to Improve Access to Subcutaneous Immunoglobulin (SCIg). Final Report. 2023. https://blood.gov.au/system/files/documents/HealthConsult%20NBA%20SCIg%20Service%20Model%20FINAL%20Report.pdf
- 39.Martin A, Lavoie L, Goetghebeur M, Schellenberg R. Economic benefits of subcutaneous rapid push versus intravenous immunoglobulin infusion therapy in adult patients with primary immune deficiency. Transfus Med. 2013;23(1):55–60. doi: 10.1111/j.1365-3148.2012.01201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perraudin C, Bourdin A, Spertini F, Berger J, Bugnon O. Switching patients to home-based subcutaneous immunoglobulin: an economic evaluation of an Interprofessional Drug Therapy Management Program. J Clin Immunol. 2016;36(5):502–510. doi: 10.1007/s10875-016-0288-z. [DOI] [PubMed] [Google Scholar]
- 41.Gerth WC, Betschel SD, Zbrozek AS. Implications to payers of switch from hospital-based intravenous immunoglobulin to home-based subcutaneous immunoglobulin therapy in patients with primary and secondary immunodeficiencies in Canada. Allergy Asthma Clin Immunol. 2014;10(1):23. doi: 10.1186/1710-1492-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritchie B, Martins KJB, Tran DT, Blain H, Richer L, Klarenbach SW. Economic impact of self-administered subcutaneous versus clinic-administered intravenous immunoglobulin G therapy in Alberta, Canada: a population-based cohort study. Allergy Asthma Clin Immunol. 2022;18(1):99. doi: 10.1186/s13223-022-00735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.