Highlights
-
•
Most patients with autoimmune diseases can safely adopt moderate exercise training protocols, but the changes in inflammation biomarkers will be modest at best. Exercise interventions may also alleviate the complications of autoimmune diseases.
-
•
Acute and regular exercise interventions have a differential response on inflammation biomarkers in patients with autoimmune diseases. The exercise-induced effect on inflammation depends on the type and severity of the autoimmune diseases.
-
•
From an anti-inflammatory perspective, it is recommended to regularly combine multiple exercise modes, especially aerobic and resistance training, individualized to patients with autoimmune diseases.
-
•
Acute exercise interventions are ineffective or even modestly but transiently pro-inflammatory. Furthermore, acute moderate- to high-intensity exercise even increases inflammatory biomarkers.
Keywords: Autoimmune diseases, Cytokines, Inflammation, Physical activity, Training
Abstract
Background
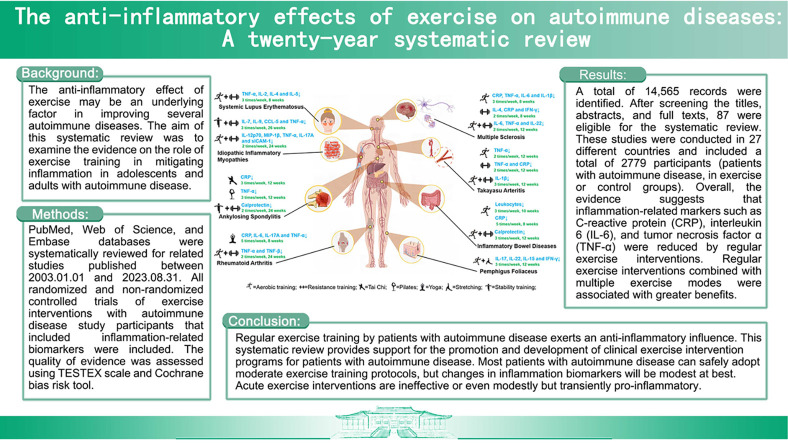
The anti-inflammatory effect of exercise may be an underlying factor in improving several autoimmune diseases. The aim of this systematic review was to examine the evidence on the role of exercise training in mitigating inflammation in adolescents and adults with autoimmune disease.
Methods
PubMed, Web of Science, and Embase databases were systematically reviewed for related studies published between January 1, 2003, and August 31, 2023. All randomized and non-randomized controlled trials of exercise interventions with autoimmune disease study participants that evaluated inflammation-related biomarkers were included. The quality of evidence was assessed using the Tool for the assEssment of Study qualiTy and reporting in EXercise scale and Cochrane bias risk tool.
Results
A total of 14,565 records were identified. After screening the titles, abstracts, and full texts, 87 were eligible for the systematic review. These studies were conducted in 25 different countries and included a total of 2779 participants (patients with autoimmune disease, in exercise or control groups). Overall, the evidence suggests that inflammation-related markers such as C-reactive protein, interleukin 6, and tumor necrosis factor α were reduced by regular exercise interventions. Regular exercise interventions combined with multiple exercise modes were associated with greater benefits.
Conclusion
Regular exercise training by patients with autoimmune disease exerts an anti-inflammatory influence. This systematic review provides support for the promotion and development of clinical exercise intervention programs for patients with autoimmune disease. Most patients with autoimmune disease can safely adopt moderate exercise training protocols, but changes in inflammation biomarkers will be modest at best. Acute exercise interventions are ineffective or even modestly but transiently pro-inflammatory.
Graphical Abstract
1. Introduction
Autoimmune diseases are conditions characterized by immune dysregulation leading to tissue damage in the host. Autoimmune disease rates are increasing rapidly in many parts of the world due to environmental and lifestyle factors.1 Most autoimmune diseases require lifelong treatment and significantly impact affected individuals and their families, society, and healthcare costs.2 Physical inactivity is one of the risk factors for the progression of autoimmune diseases.3 Exercise training is recommended for patients with autoimmune disease in combination with medication and clinical care to improve quality of life, cardiorespiratory capacity, and muscle strength, and to alleviate symptoms such as pain and depression.3, 4, 5
Inflammation palliation is an important goal in the treatment of autoimmune diseases. All autoimmune diseases share common features, including elevated circulating inflammatory markers such as interleukin (IL)-6, tumor necrosis factor α (TNF-α), and C-reactive protein (CRP) under basal or resting conditions.6 Regular exercise training has been linked in numerous studies with an anti-inflammatory effect.7 Possible mechanisms of the anti-inflammatory effects of exercise include increased release of hormones (cortisol and adrenaline) and myokines that downregulate the production of pro-inflammatory cytokines by immune cells. Exercise training also mobilizes regulatory T (Treg) cells, natural killer cells, and other immune cells that release the anti-inflammatory cytokine IL-10. Exercise training is typically associated with decreased visceral adipose tissue volume. This is important because an increase in visceral adipose tissue caused by physical inactivity and overeating leads to an expansion of resident inflammatory innate and adaptive immune cells that undergird systemic inflammation.7,8 Taken together, exercise training is important for overall immune health through its effects on immune cell recruitment and enhanced surveillance, antimicrobial activities, and reduced systemic inflammation.9
In the period from 2003 to 2013, there were few systematic reviews of published papers on exercise training and autoimmune diseases. One systematic review focused on the effects of acute (a single bout) and regular (repeated bouts) exercise on various inflammatory markers in patients with chronic inflammatory disease.6 This review concluded that regular exercise programs reduced chronic inflammation and that single bouts of recommended amounts of moderate-to-vigorous exercise were associated with a variable but modest increase in some inflammation biomarkers. Recent systematic reviews and meta-analyses from 2013 to 2023, most of which included a specific autoimmune disease, reported variable results for the effects of exercise on inflammatory biomarkers. For example, a systematic review for multiple sclerosis (MS) found limited effects of exercise on inflammation,10 whereas a systematic review for rheumatic diseases reported that exercise significantly attenuated some inflammatory-related markers, such as the erythrocyte sedimentation rate, but not CRP.11 In addition, recent systematic reviews favored the selection of exercise programs that could be quantified in terms of intensity, and thus excluded exercise intervention studies using modes such as calisthenics, yoga, Pilates, and Tai Chi.
Despite the existing literatures suggesting that exercise training has an anti-inflammatory effect, no previous investigation has systematically reviewed all types of exercise interventions for the most common autoimmune diseases. Therefore, the aim of this systematic review was to summarize the anti-inflammatory effects of various forms of acute and regular exercise interventions for autoimmune diseases. It assessed the characteristics of exercise programs for specific autoimmune diseases, evaluated the effectiveness of different exercise programs on inflammatory outcomes, identified the types of autoimmune diseases most influenced by exercise programs, and formulated recommendations for further research.
2. Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and was pre-registered in the International Database of Prospectively Registered Systematic Reviews (ID #CRD42023426627). The search strategy was based on the Participants, Intervention, Comparisons, Outcomes, and Study Design worksheet for systematic reviews. The inclusion and exclusion criteria are provided in Table 1.
Table 1.
Selection criteria.
| Category | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Subjects diagnosed with autoimmune diseases, no age or gender restrictions | Subjects had a combination of other non-autoimmune diseases |
| Intervention | Exercise interventions, such as aerobic training, resistance training, high-intensity interval training, yoga, calisthenics, and traditional Chinese exercise, were conducted | Studies included non-exercise interventions or a combined exercise and non-exercise intervention |
| Comparator | Studies had a control group (including healthy or patient control) or self-control | – |
| Outcome | Inflammation-related markers, such as pro- and anti-inflammatory cytokines, C-reactive protein, and immune cell ratios, were included in the results | No analysis or description of inflammatory-related biomarkers was performed |
| Study design | Studies with an experimental design, including randomized controlled trials and non-randomized controlled trials | Protocols, reviews, case reports, pilot studies, follow-up studies, and observational studies were excluded |
2.1. Data sources and searches
This review focused on published studies examining the anti-inflammatory effects of exercise interventions in patients with autoimmune disease. The search strategy was developed through discussions between team members and physicians in the departments of rheumatology, endocrinology, neurology, and gastroenterology. A broad search of 3 databases (PubMed, Web of Science, and Embase) was undertaken for articles containing terms for autoimmune diseases combined with exercise and inflammation. No limitations were applied regarding outcomes. We limited the relevant literatures to English articles published from January 1, 2003 to August 31, 2023 (see Supplementary Table 1 for the details of the search strategy).
2.2. Eligibility criteria
2.2.1. Design
Experimental studies, including both randomized controlled trials (RCTs) and non-RCTs (NRCTs), were considered eligible for inclusion. Studies were included if they investigated the effect of exercise (both acute and regular exercise) on inflammation-related biomarkers in patients with autoimmune disease. Trials were included if the effect was measured following the completion of the exercise program. Case reports and follow-up trials were excluded.
2.2.2. Participants
The patients in the included studies were diagnosed with autoimmune diseases, including MS, rheumatoid arthritis (RA), ankylosing spondylitis (AS), systemic lupus erythematosus (SLE), idiopathic inflammatory myopathies (IIMs), type 1 diabetes (T1D), inflammatory bowel disease (IBD), juvenile idiopathic arthritis (JIA), systemic sclerosis (SSc), Takayasu arteritis (TA), pemphigus foliaceus, Sjogren syndrome, psoriasis, psoriatic arthritis, Hashimoto's disease, Guillain-Barre syndrome, myasthenia gravis, and combinations of same. Studies were excluded if subjects had autoimmune diseases combined with non-autoimmune diseases.
2.2.3. Intervention
The studies included in this review were not limited by the type of exercise intervention. Acute exercise was defined as a single bout or an exercise program. Regular exercise was defined as repeated bouts of exercise.12 If a study assessed the effects of both regular and acute exercise, the study was reviewed twice. Aerobic training (AT) was defined as exercise designed to improve cardiorespiratory fitness. Resistance training (RT) was defined as exercise designed to improve both muscle size and strength. Exercise intensity was systematically assessed in the included studies in accordance with the guidelines of the American College of Sports Medicine.13 Studies were excluded if the protocol contained a combined exercise and non-exercise intervention.
2.2.4. Outcomes
The inflammation-related biomarkers in the included studies were pro- and anti-inflammatory cytokines, such as IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-17, transforming growth factor-β, TNF-α, interferon, granulocyte-macrophage colony-stimulating factor, monocyte chemotactic protein-1, macrophage inflammatory protein-1, calprotectin and CRP, as well as immune cell ratios including leukocytes, lymphocytes, monocytes, neutrophils, natural killer cells, Tregs, B regulatory cells, and dendritic cells.
2.3. Study selection
Results from the literature search were exported to Endnote references manager (Clarivate Analytics, Philadelphia, PA, USA), which was then used to de-duplicate the retrieved articles. After removal of duplicates, 2 review authors (BL and XJ) independently examined titles and abstracts against the eligibility criteria. All articles selected in this process were obtained in full text. All full-text articles were also assessed independently by BL and XJ. Disagreement among review authors regarding eligibility was discussed among the entire group of reviewers until consensus was reached.
2.4. Data extraction
Following the screening process, relevant data from the included articles were extracted into Excel 2019 (Microsoft, Redmond, WA, USA) by BL and XJ. Data were initially extracted by XJ, with BL ensuring the accuracy of the extracted data. Extracted data included study characteristics (design), participant characteristics (sample size, gender, and age), exercise intervention (type, duration, frequency, and intensity), and outcomes (inflammation-related markers and their changes).
2.5. Quality and risk of bias assessment
A quality assessment of the included studies using the Tool for the assEssment of Study qualiTy and reporting in EXercise (TESTEX) scale14 was performed independently by BL and XJ. The TESTEX scale was developed specifically for exercise specialists to assess the quality of exercise studies and has been used in other systematic reviews/meta-analyses of exercise studies.5,15 Each TESTEX item was assigned a value of either 0 (absent or inadequately described) or 1 (present or explicitly described) for a maximum score of 15 points (5 for study quality and 10 for reporting). Discrepancies in scoring between reviewers were discussed and resolved when possible. Final scores were determined by consensus among team members when discrepancies could not be resolved. In addition, a Cochrane risk of bias assessment tool was used for the RCTs included in this review. A Cochrane risk of bias assessment evaluates RCTs based on several categories, including sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and “other issues”. These categories were graded as “high risk of bias”, “low risk of bias”, and “unclear risk of bias”. Each article was independently assessed by BL and XJ.
3. Results
3.1. Study selection
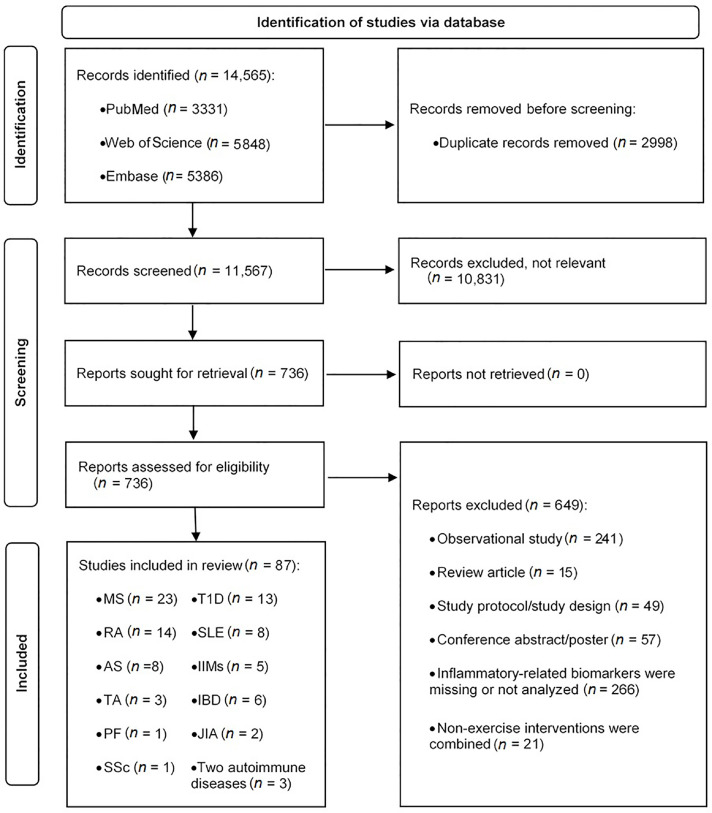
A total of 14,565 records were identified by the searches. Following the removal of duplicates and irrelevant articles, 736 full-text articles were assessed for eligibility. Following the full-text screening process, 87 articles were eligible and were included in the systematic review. The search procedure is summarized in Fig. 1.
Fig. 1.
Flowchart of the search and selection of studies. AS = ankylosing spondylitis; IBD = inflammatory bowel disease; IIMs = idiopathic inflammatory myopathies; JIA = juvenile idiopathic arthritis; MS = multiple sclerosis; PF = pemphigus foliaceus; RA = rheumatoid arthritis; SLE = systemic lupus erythematosus; SSc = systemic sclerosis; T1D = type 1 diabetes; TA = Takayasu arteritis.
3.2. Study characteristics
3.2.1. Participants
A total of 87 studies16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102 published between January 1, 2003, and August 31, 2023, provided results for 2779 patients with autoimmune diseases. The most common autoimmune disease studied was MS,16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 followed by RA,39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 T1D,53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 SLE,66, 67, 68, 69, 70, 71, 72, 73 AS,74, 75, 76, 77, 78, 79, 80, 81 IIM,82, 83, 84, 85, 86 IBD,87, 88, 89, 90, 91, 92 TA,93, 94, 95 JIA,96,97 pemphigus foliaceus,98 and SSc.99 Three studies included patients with more than 1 autoimmune disease (RA and SLE,100 RA and JIA,101 MS and AS,102 respectively). Age and gender, organized in Tables 2 and 3, are significant factors in the effectiveness of exercise interventions as well as in the development of autoimmune diseases.1 There were 12 studies54,55,58, 59, 60, 61, 62,87,91,92,96,97 that enrolled adolescents. Eight studies18,25,35,41,50,54,81,82 reported the median and range of participant age. One study39 did not report the gender. Another study86 did not report the age or gender. These studies were conducted in 25 countries (12 studies from Brazil;16,49,53,67,69, 70, 71,73,75,93,95,98 9 studies from Iran;17,25,26,31, 32, 33,37,58,68 9 studies from the USA;22,38,42,54,55,59, 60, 61, 62 7 studies from Turkey;35,39,43,63,74,77,102 6 studies from Sweden;28,40,50,82, 83, 84 5 studies each from Germany21,27,30,36,89 and Ireland;19,41,56,57,88 4 studies from the UK;51,52,64,100 3 studies each from Czech Republic,76,78,86 the Netherlands,90,92,99 and Norway;66,81,101 2 studies each from Australia,47,85 Belgium,23,34 Canada,24,87 China,79,94 France,96,97 India,44,45 and Switzerland;18,91 and 1 study each from Denmark,29 Israel,20 Republic of Korea,46 Mexico,48 Poland,65 South Africa,80 and Spain72).
Table 2.
Characteristics of the included regular exercise intervention studies.
| Study | Autoimmune disease | Subject (sample size, age, group, gender) | Exercise intervention | Inflammation-related biomarker |
|---|---|---|---|---|
| Alvarenga-Filho et al. (2016)16 | MS | n = 18, 38.5 ± 10.2 years, 8 Ex/10 Con, 3 M/15 F | Combined RT and AT | IL-6↓, TNF-α↓, IL-22↓ |
| Bahmani et al. (2022)17 | MS | n = 20, 27.1 ± 2.9 years, 10 Ex/10 Con, 20 F | AT | CRP↓, TNF-α↓, IL-6↓, IL-1β↓ |
| Bansi et al. (2013)18 | MS | n = 28, 52 years, 28 Ex, 10 M/18 F | AT | — |
| Barry et al. (2019)19 | MS | n = 9, 35.5 ± 2.1 years, 9 Ex, 1 M/8 F | AT | IL-10↑ |
| Briken et al. (2016)21 | MS | n = 42, 50.0 ± 7.5 years, 32 Ex/10 Con, 17 M/25 F | AT | — |
| Castellano et al. (2008)22 | MS | n = 11, 40 ± 10 years, 11 Ex, 3 M/8 F | AT | TNF-α↑, IFN-γ↑ |
| Deckx et al. (2016)23 | MS | n = 45, 48.1 ± 11.2 years, 29 Ex/16 Con, 19 M/26 F | Combined RT and AT | CD80+ pDC↑, CD62L+ pDC↑ |
| Faramarzi et al. (2020)25 | MS | n = 94, 18–50 years, 47 Ex/47 Con, 94 F | Combined RT, AT, stretching, balance training, and Pilates | IL-6↓, hs-CRP↓, PTX3↑, IFN-γ↑ |
| Golzari et al. (2010)26 | MS | n = 20, 33.0 ± 7.7 years, 10 Ex/10 Con, 20 F | Combined RT, AT, and stretching | IL-17↓, IFN-γ↓ |
| Joisten et al. (2021)27 | MS | n = 68, 50.3 ± 10.2 years, 68 Ex (35 HIIT/33 AT), 42 F/26 M | HIIT, AT | NLR↓ |
| Kierkegaard et al. (2016)28 | MS | n = 20, 36.3 ± 7.6 years, 20 Ex, 4 M/16 F | RT | TNF-α↓ |
| Kjølhede et al. (2016)29 | MS | n = 32, 43.4 ± 7.5 years, 16 Ex/16 Con, 8 M/24 F | RT | — |
| Mähler et al. (2018)30 | MS | n = 17, 51 ± 10 years, 17 EX, 6 M/11 F | AT | CD39+ Tregs↑, CD31+ Tregs↓, IL-17A+ CD4+ cells↓ |
| Mokhtarzade et al. (2017)32 | MS | n = 40, 31.7 ± 3.0 years, 22 Ex/18 Con, 40 F | AT | TNF-α↓ |
| Mokhtarzade et al. (2021)33 | MS | n = 42, 35.7 ± 8.6 years, 21 Ex/21 Con, 11 M/31 F | Combined RT and AT | — |
| Nieste et al. (2023)34 | MS | n = 28, 45.4 ± 11.0 years, 28 Ex, 6 M/22 F | AT | CRP↑ |
| Ozkul et al. (2018)35 | MS | n = 36, 34.3 years, 18 Ex/18 Con, 8 M/28 F | Combined AT and Pilates | — |
| Schulz et al. (2004)36 | MS | n = 28, 39.5 ± 9.8 years, 15 Ex/13 Con, 9 M/19F | AT | — |
| Tadayon Zadeh et al. (2020)37 | MS | n = 30, 32.1 ± 2.6 years, 15 Ex/15 Con, 30 F | Combined AT and RT | IL-6↓, CRP↓, IL-10↑ |
| White et al. (2006)38 | MS | n = 10, 47 ± 12 years, 10 Ex, 10 F | RT | IL-4↓, IL-10↓, CRP↓, IFN-γ↓ |
| Acar et al. (2016)39 | RA | n = 28, 55.1 ± 11.8 years, 28 Ex | Combined AT and RT | CRP↓ |
| Andersson et al. (2020)40 | RA | n = 49, 69.5 ± 2.6 years, 24 Ex/25 Con, 9 M/40 F | Combined AT and RT | IL-10↓, Tregs↓, Bregs↓ |
| Azeez et al. (2020)41 | RA | n = 52, 34–74 years, 28 Ex /24 Con, 8 M/44 F | Combined AT and RT | CRP↓ |
| Bartlett et al. (2018)42 | RA | n = 12, 64 ± 7 years, 12 Ex, 1 M/11 F | HIIT | CD16+ monocytes↓ |
| Gautam et al. (2019)44 | RA | n = 72, 43.9 ± 2.4 years, 36 Ex/36 Con, 16 M/56 F | Yoga | CRP↓, IL-6↓, IL-17A↓, TNF-α↓, TGF-β↑ |
| Gautam et al. (2020)45 | RA | n = 66, 44.3 ± 9.0 years, 33 Ex/33 Con, 13 M/53 F | Yoga | IL-6↓, TNF-α↓, TGF-β↑ |
| Joo et al. (2022)46 | RA | n = 37, 49.9 ± 7.9 years, 24 Ex/13 Con, 37 F | RT | — |
| Law et al. (2015)47 | RA | n = 9, 57 ± 14 years, 9 Ex, 1 M/8 F | Combined AT and RT | — |
| Lozada-Mellado et al. (2022)48 | RA | n = 60, 44.4 ± 11.0 years, 30 Ex/30 Con, 60 F | Combined AT and RT | TNF-α↓, TNF-β↓ |
| Sarajlic et al. (2018)50 | RA | n = 29, 60–67 years, 29 Ex | Combined AT and RT | CRP↓ |
| Stavropoulos-Kalinoglou et al. (2013)51 | RA | n = 36, 53.9 ± 9.9 years, 18 Ex/18 Con, 8 M/28 F | Combined AT and RT | CRP↓ |
| Wadley et al. (2014)52 | RA | n = 19, 55.5 ± 10.1 years, 12 Ex/7 Con, 6 M/13 F | AT | — |
| Farinha et al. (2018)53 | T1D | n = 28, 24.5 ± 5.0 years, 28 Ex (9 HIIT/ 9 RT/10 HIIT+RT), 15 M/ 13 F | HIIT, RT, or combined HIIT and RT | — |
| Minnock et al. (2022)57 | T1D | n = 10, 31.6 ± 3.7 years, 10 Ex, 4 M/6 F | Combined AT and RT | — |
| Nazari et al. (2023)58 | T1D | n = 40, 11.1 ± 2.3 years, 20 EX/ 20 Con, 19 M/21 F | Combined AT and RT | — |
| Turner et al. (2014)64 | T1D | N = 8, 38 ± 17 years, 8 Ex, 7 M/1 F | RT | IL-6↑ |
| Clarke-Jenssen et al. (2005)66 | SLE | n = 6, 47.0 ± 3.8 years, 6 Ex, 6F | AT | — |
| Hashemi et al. (2022)68 | SLE | n = 24, 25.9 ± 5.8 years, 14 Ex/10 Con, 24 F | Combined AT and RT | TNF-α↓, IL2↓, IL-4↓, IL-5↓ |
| Perandini et al. (2014)69 | SLE | n = 8, 35.8 ± 6.5 years, 8 Ex, 8 F | AT | sTNFR2↓, IL-10↓ |
| Soriano-Maldonado et al. (2018)72 | SLE | n = 58, 44.0 ± 13.9 years, 26 Ex/32 Con, 58 F | AT | — |
| Timóteo et al. (2018)73 | SLE | n = 14, 37.4 ± 12.6 years, 5 Ex/9 Con, 14 F | Combined RT, AT, and stretching | — |
| Aydin et al. (2016)74 | AS | n = 37, 34.6 ± 7.9 years, 37 Ex, 20 M/17 F | Calisthenic exercise | — |
| de Souza et al. (2017)75 | AS | n = 60, 44.4 ± 9.9 years, 30 Ex/30 Con, 44 M/16 F | RT | — |
| Hulejová et al. (2012)76 | AS | n = 26, 36.0 ± 6.5 years, 26 Ex, 18 M/8 F | Combined RT, postural corrections, and stretching | — |
| Kisacik et al. (2016)77 | AS | n = 24, 39.9 ± 10.0 years, 24 Ex, 24 F | Combined dance and Pilates | TNF-α↓ |
| Levitova et al. (2016)78 | AS | n = 40, 36.8 ± 1.1 years, 40 Ex, 27 M/13 F | Combined core spinal traction and RT | Calprotectin↓ |
| Ma et al. (2020)79 | AS | n = 84, 36.1 ± 5.2 years, 42 Ex/42 Con, 59 M/25 F | Tai Chi | CRP↓ |
| Nolte et al. (2021)80 | AS | n = 29, 39.2 ± 13.7 years, 16 Ex/13 Con, 15 M/14 F | Combined AT and RT | — |
| Sveaas et al. (2020)81 | AS | n = 100, 47.2 years, 50 EX/50 Con, 47 M/53 F | Combined HIIT and RT | — |
| Alexanderson et al. (2007)82 | IIMs | n = 9, 53 years, 9 Ex, 4 M/5 F | RT | — |
| Alexanderson et al. (2014)83 | IIMs | n = 19, 60.0 ± 3.8 years, 10 Ex/9 Con, 5 M/14 F | RT | — |
| Arnardottir et al. (2003)84 | IIMs | n = 7, 60.4 ± 12.5 years, 7 Ex, 7 M | Combined AT and RT | — |
| Coudert et al. (2022)85 | IIMs | n = 14, 65 ± 14 years, 14 Ex, 14 M | Combined AT and RT | IL-12p70↓, MIP-1β↓, TNF-α↓, IL-17A↓, sICAM-1↓ |
| Švec et al. (2022)86 | IIMs | n = 23, 23 Ex | Combined RT and stability training | IL-7↓, IL-9↓, CCL-5↓, TNF-α↓ |
| Bjelica et al. (2023)87 | IBD | n = 10, 15.4 ± 1.2 years, 10 EX, 9 M/1 F | Combined AT and RT | — |
| Cronin et al. et al. (2019)88 | IBD | n = 20, 25.0 ± 6.5 years, 13 Ex/7 Con, 15 M/5 F | Combined AT and RT | — |
| Klare et.al. (2015)89 | IBD | n = 30, 41.1 ± 14.1 years, 15 Ex/15 Con, 8 M/22 F | AT | Leukocytes↓ |
| Lamers et al. (2021)90 | IBD | n = 37, 54.0 ± 11.9 years, 18 Ex/19 Con, 15 M/22 F | AT | IL-6↑, IL-8↑, IL-10↑ |
| Legeret et al. (2019)91 | IBD | n = 21, 13.3 ± 3.0 years, 21 Ex, 11 M/10 F | Exercise games (dance) | CRP↓ |
| Scheffers et al. (2023)92 | IBD | n = 15, 9.5 ± 4.0 years, 8 Ex/7 Con, 9 M/6 F | Combined AT and RT | Calprotectin↓ |
| Astley et al. (2021)93 | TA | n = 14, 18.3 ± 3.8 years, 5 Ex/9 Con, 4 M/10 F | Combined AT and RT | IL-1β↓ |
| Li et al. (2020)94 | TA | n = 278, 36.8 ± 8.6 years, 140 Ex/138 Con, 145 M/ 133 F | RT | TNF-α↓, CRP↓ |
| Oliveira et al. (2017)95 | TA | n = 6, 35.3 ± 6.6 years, 6 Ex, 6 F | AT | TNF-α↓ |
| Timóteo et al. (2019)98 | PF | n = 19, 34.11 ± 15.70 years, 9 Ex/10 Con, 8 M/11 F | Combined RT and stretching | IL-17↓, IL-22↓, IL-15↓, IFN-γ↓ |
| Sandstad et al. (2015)101 | RA and JIA | n = 18, 32.9 ± 8.2 years, 9 Ex/9 Con, 18 F | HIIT | — |
| Taspinar et al. (2015)102 | AS and MS | n = 73, 34.7 ± 7.9 years, 73 Ex, 39 M/34 F | Calisthenic exercise | — |
Notes: Ages are presented as mean ± SD, median, or range. ↑, increase or obtain a higher value; ↓, impair or obtain a lower value; —, unchanged.
Abbreviations: AS = ankylosing spondylitis; AT = aerobic training; Bregs = B regulatory cells; CCL = chemokine ligand; CD = cluster of differentiation; Con = control group; CRP = C-reactive protein; Ex = exercise group; F = female; HIIT = high-intensity interval training; hs-CRP = high-sensitivity CRP; IBD = inflammatory bowel disease; IFN-γ = interferon-γ; IIMs = idiopathic inflammatory myopathies; IL = interleukin; JIA = juvenile idiopathic arthritis; M = male; MIP = macrophage inflammatory protein; MS = multiple sclerosis; NLR = neutrophil-to-lymphocyte ratio; pDC = plasmacytoid dendritic cells; PF = pemphigus foliaceus; PTX = pentraxins; RA = rheumatoid arthritis; RT = resistance training; sICAM = soluble intercellular adhesion molecule; sIL-6R = soluble IL-6 receptor; SLE = systemic lupus erythematosus; sTNFR = soluble TNF receptor; T1D = type 1 diabetes; TA = Takayasu arteritis; TGF = transforming growth factor; TNF-α = tumor necrosis factor α; Tregs = T regulatory cells.
Table 3.
Characteristics of the included acute exercise intervention studies.
| Study | Autoimmune disease | Subject (sample size, age, group, gender) | Exercise intervention | Inflammation-related biomarker |
|---|---|---|---|---|
| Berkowitz et al. (2019)20 | MS | n = 15, 33.8 ± 7.8 years, 15 Ex, 15 F | AT (moderate) | IL-6↑ |
| AT (vigorous) | IL-10↓ | |||
| Briken et al. (2016)21 | MS | n = 42, 50.0 ± 7.5 years, 32 Ex/10 Con, 17 M/25 F | AT | — |
| Castellano et al. (2008)22 | MS | n = 11, 40 ± 10 years, 11 Ex, 3 M/8 F | AT | — |
| Devasahayam et al. (2021)24 | MS | n = 14, 54.1 ± 8.5 years, 14 Ex, 4 M/10 F | AT | IL-6↑ |
| Joisten et al. (2021)27 | MS | n = 68, 50.3 ± 10.2 years, 68 Ex (35 HIIT/33 AT), 42 F/26 M | HIIT or AT | PLR↑ (HIIT) |
| Kjølhede et al. (2016)29 | MS | n = 32, 43.4 ± 7.5 years, 16 Ex/16 Con, 8 M/24 F | RT | — |
| Majdinasab et al. (2018)31 | MS | n = 35, 28.5 ± 4.0 years, 35 Ex, 35 F | AT | TNF-α↓, IL-6↑ (immediately after AT), TNF-α↑ (1-h post) |
| Ercan et al. (2023)43 | RA | n = 40, 45.3 ± 9.6 years, 40 Ex, 11 M/29 F | AT | IL-6↑ |
| Law et al. (2015)47 | RA | n = 8, 60 ± 12 years, 8 Ex, 2 M/6 F | Combined AT and RT | — |
| Pereira Nunes Pinto et al. (2017)49 | RA | n = 17, 55.6 ± 6.4 years, 17 Ex, 17 F | RT | IL-1ra↑, IL-10↑, IL-6↑, IL-1β↓ |
| Galassetti et al. (2006)54 | T1D | n = 12, 13.7 years, 12 Ex, 7 M/5 F | AT | IL-6↑ |
| Galassetti et al. (2006)55 | T1D | n = 20, 13.7 ± 0.3 years, 20 Ex, 12 M/8 F | AT | IL-6↑ |
| Minnock et al. (2020)56 | T1D | n = 12, 31.8 ± 5.3 years, 12 Ex, 6 M/6 F | AT RT Combined AT and RT |
IL6, TNFa, and MCP1 mRNA↑ IL6, TNFa, and MCP1 mRNA↑ IL6, TNFa, and MCP1 mRNA↑ |
| Rosa et al. (2008)59 | T1D | n = 21, 13.0 ± 0.3 years , 21 Ex, 13 M/8 F | AT | IL-6↑, TNF-α↑, IL-4↑, IL-12p70↑, GM-CSF↑, MCP-1↑, MIP-1α↑ |
| Rosa et al. (2010)60 | T1D | n = 47, 13.8 ± 0.4 years, 47 Ex, 22 M/25 F | AT | IL-6↑ |
| Rosa et al. (2011)61 | T1D | n = 49, 13.9 ± 0.2 years, 49 Ex, 29 M/20 F | AT | IL-6↑ |
| Rosa et al. (2011)62 | T1D | n = 23, 13.9 ± 0.3 years, 23 Ex, 13 M/10 F | AT | — |
| Salman et al. (2008)63 | T1D | n = 19, 22.1 ± 2.8 years, 19 Ex, 19 M | AT | CD3+CD4+ T cells ↓, NK cells ↑ |
| Żebrowska et al. (2018)65 | T1D | n = 14, 26.0 ± 5.9 years, 14 Ex, 7 M/7 F | AT HIIT |
TGF-β↑ TGF-β↑,TNF-α↓ |
| da Silva et al. (2013)67 | SLE | n = 27, 29.4 ± 2.2 years, 27 Ex, 27 F | AT | — |
| Perandini et al. (2014)69 | SLE | n = 8, 35.8 ± 6.5 years, 8 Ex, 8 F | AT | IL-10↓ |
| Perandini et al. (2015)70 | SLE | n = 23, 33.0 ± 5.6 years, 23 Ex, 23 F | AT (moderate) AT (vigorous) |
IL-6↓ sTNFR1↓, TNF-α↑ |
| Perandini et al. (2016)71 | SLE | n = 8, 33.5 ± 3.3 years, 8 Ex, 8F | AT | TLR3, IFNG, GATA3, FOXP3, STAT4 mRNA↓ |
| Oliveira et al. (2017)95 | TA | n = 11, 33.5 ± 5.7 years, 11 Ex, 11 F | AT | — |
| Rochette et al. (2018)96 | JIA | n = 12, 12.3 ± 2.8 years, 12 Ex, 4 M/8 F | AT | Calprotectin↑ |
| Rochette et.al (2018)97 | JIA | n = 12, 12.3 ± 2.8 years, 12 Ex, 4 M/8 F | AT | IL-6↓, sIL-6R↓ (24-h post) |
| Hargardóttir et al. (2010)99 | SSc | n = 11, 59 ± 3 years, 11 Ex, 3 M/8 F | AT | Leukocytes↑, IL-6↑ |
| Pool et al. (2004)100 | RA and SLE | n = 13, 34.1 ± 5.0 years, 13 Ex, 13 F | AT | CD4+ T lymphocytes↓ (RA and SLE) CD4+/CD8+ T lymphocytes↓(RA) |
Notes: Ages are presented as mean ± SD or as median. ↑, increase or obtain a higher value; ↓, impair or obtain a lower value; —, unchanged.
Abbreviations: AT = aerobic training; CCL = chemokine ligand; CD = cluster of differentiation; Con = control group; Ex = exercise group; F = female; FOXP3 = Forkhead box p3; GATA3 = GATA binding protein 3; GM-CSF = granulocyte-macrophage colony-stimulating factor; HIIT = high-intensity interval training; IFNG = interferon gamma; IL = interleukin; JIA = juvenile idiopathic arthritis; M = male; MCP = monocyte chemotactic protein; MIP = macrophage inflammatory protein; mRNA = messenger RNA; MS = multiple sclerosis; NK = natural killer; NLR = neutrophil-to-lymphocyte ratio; PLR = platelet-to-lymphocyte ratio; RA = rheumatoid arthritis; RT = resistance training; sIL-6R = soluble IL-6 receptor; SLE = systemic lupus erythematosus; SSc = systemic sclerosis; STAT = signal transducer and activator of transcription; sTNFR = soluble TNF receptor; T1D = type 1 diabetes; TA = Takayasu arteritis; TGF = transforming growth factor; TLR = Toll-like receptor; TNF-α = tumor necrosis factor α.
3.2.2. Exercise interventions
In these studies, 66 studies16, 17, 18, 19,21, 22, 23,25, 26, 27, 28, 29, 30,32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42,44, 45, 46, 47, 48,50, 51, 52, 53,57,58,64,66,68,69,72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95,98,101,102 were conducted regular exercise interventions, 28 studies20, 21, 22,24,27,29,31,43,47,49,54, 55, 56,59, 60, 61, 62, 63,65,67,69, 70, 71,95, 96, 97,99,100 were acute, and 7 studies21,22,27,29,47,69,95 investigated the effects of both acute and regular (reviewed twice). For regular exercise interventions, 32 trials16,23,25,26,33,35,37,39, 40, 41,47,48,50,51,53,57,58,68,73,76, 77, 78,80,81,84, 85, 86, 87, 88,92,93,98 used a combination of exercise, 17 trials used AT,17, 18, 19,21,22,27,30,32,34,36,52,66,69,72,89,90,95 9 trials used RT,28,29,38,46,64,75,82,83,94 while 4 studies27,42,53,101 used high-intensity interval training (HIIT). The frequency of exercise was 3–5 times per week. For acute exercise interventions, 22 trials20, 21, 22,24,31,43,54,55,59, 60, 61, 62, 63,67,69, 70, 71,95, 96, 97,99,100 used AT, 2 trials29,49 used RT, 2 trials27,65 used HIIT and AT, and 2 trials47,56 used combined exercise. Other interventions were yoga,44,45 Pilates,25,35,77 Tai Chi,79 stretching,25,26,73,76,98 and calisthenic exercise.74,102 Detailed exercise protocols were summarized in Tables 2, 3, and Supplementary Table 2. Safety related information was extracted from participants who withdrew from the exercise intervention, including reasons for dropping out and any adverse events that occurred during the exercise intervention. The relevant information is available in Supplementary Table 2.
3.3. Main findings of the reviewed studies
3.3.1. MS
In the regular exercise intervention trials for people with MS, the intervention programs included AT, RT, HIIT, and combined exercise. Nine intervention protocols17, 18, 19,21,22,30,32,34,36 were AT, 3 studies28,29,38 were RT, 1 study27 was HIIT and AT, and 7 studies16,23,25,26,33,35,37 were a combination of exercises. Exercise interventions generally ranged from 8 to 12 weeks, with a minimum of 4 days and a maximum of 6 months. The frequency of exercise interventions was 2–5 times per week. Seven of 20 studies found that exercise had anti-inflammatory effects with reductions in CRP, IL-6, and TNF-α in the peripheral circulation and increases in IL-10 (3 AT, 1 RT, and 3 combined exercise).16,17,19,25,28,33,37 Two exercise interventions reported changes in the number of dendritic cells23 and the ratio of Tregs.30 Two studies26,38 reported an exercise-induced decrease in interferon-γ, but another study25 showed an increase. Seven studies18,21,27,29,32,35,36 reported no effects of exercise interventions on inflammation-related markers (4 AT, 1 RT, and 2 combined exercise). Among the acute exercise intervention studies for MS, 5 studies20, 21, 22,24,31 were AT, 1 study29 was RT, and 1 study27 was HIIT and AT. Only one of these studies showed a decrease in TNF-α immediately after AT in patients with MS.31 Three studies21,22,29 found no anti-inflammatory effects (2 AT and 1 RT). In contrast, 4 acute exercise interventions20,24,27,31 (3 AT and 1 HIIT) reported increases in IL-6 and TNF-α (1-h post AT) in the peripheral circulation and decreases in IL-10. Patients with MS exhibited improvements in clinical disability,22,26 fatigue,16,28,33,35,38 mood,28,30,32 cognition,28 quality of life,28,33,36 exercise capacity,22,25,28,29,33,35 physical fitness,17,36 cardiorespiratory fitness,18 muscle strength,26,28,29,38 and balance26 after exercise interventions.
3.3.2. RA
In the regular exercise intervention trials for people with RA, the intervention programs included AT, RT, yoga, HIIT, and combined exercise. Seven intervention protocols39,40,43,47,48,50,51 were combined exercise, 2 studies44,45 were yoga, 1 study52 was AT, 1 study46 was RT, and 1 study42 was HIIT. Exercise interventions generally ranged from 8 to 24 weeks, with a maximum of 2 years. The frequency of exercise interventions was 2–5 times per week. Seven of 12 studies found that regular exercise had anti-inflammatory effects with decreases in CRP, IL-6, and TNF-α in the peripheral circulation and increases in IL-10 (5 combined exercise and 2 yoga).39,41,44,45,48,50,51 One study40 with combined exercise reported changes in the ratios of both Tregs and B regulatory cells. However, 4 studies42,46,47,52 found no effect of exercise interventions on inflammation-related markers (1 AT, 1 RT, 1 HIIT, and 1 combined exercise). Two acute exercise intervention studies47,49 found no anti-inflammatory effects (1 combined exercise and 1 RT), and 1 acute AT intervention study43 reported an increase in IL-6. Patients with RA exhibited improvements in disease activity39,42,45,52 and fatigue40,41 following exercise. Additionally, patients demonstrated significant enhancements in cardiorespiratory fitness,42,47,51,52 exercise capacity,40, 41, 42 and muscle strength40,46,47 after engaging in exercise.
3.3.3. T1D
Three regular exercise intervention trials53,57,58 (10–12 weeks, 3 sessions per week) for people with T1D reported no changes in inflammation biomarkers. One regular RT exercise intervention trial64 increased IL-6. Of the 9 acute exercise intervention trials in T1D, 7 studies54,55,59, 60, 61, 62, 63 were AT, 1 study65 was AT and HIIT, and 1 study56 included AT, RT, and combined exercise interventions. One study reported that acute AT exercise intervention increased the ratio of natural killer cells,63 and another study 65 showed a decrease in TNF-α. Seven studies showed pro-inflammatory effects after acute exercise interventions, including the elevation of IL-6 in the peripheral circulation54,55,59, 60, 61,64 and in the muscle.56 It is noteworthy that the subjects in 6 of these studies were adolescents.54,55,59, 60, 61, 62 After combined AT and RT exercise intervention, patients with T1D experienced a decrease in blood glucose levels and an improvement in glycemic control.56,57 Additionally, patients showed significant improvements in muscle strength and cardiorespiratory fitness.57
3.3.4. SLE
In 5 regular (2–4 months, 2–3 sessions per week) exercise intervention trials (3 trials66,69,72 used AT, 2 trials68,73 used combined exercise) with female patients with SLE, only 1 study68 showed a reduction of TNF-α, IL-2, IL-4, and IL-5 after 8-week combined exercise. Four studies66,69,72,73 reported no changes in inflammation biomarkers. Leukocyte gene expression in patients with SLE compared to healthy controls following an acute 30-min bout of exercise (70% peak O2 consumption (VO2peak)) was less organized, which suggests a deficiency in the normal exercise-induced immune transcriptional response.71 Exercise intervention improves quality of life and physical function,66 cardiorespiratory fitness,72 flexibility, strength, and pain73 in patients with SLE.
3.3.5. AS
Five regular (2–6 months, 2–5 sessions per week) exercise intervention trials74, 75, 76,80,81 for patients with AS generally reported no changes in inflammation biomarkers. One Tai Chi intervention study79 found a decrease in CRP, and 2 investigating combined exercise reported a reduction in TNF-α77 and calprotectin78 in the peripheral circulation. Patients with AS exhibited improvements in disease activity,76,78, 79, 80, 81 quality of life,77,81 mood,74,77,81 as well as reduced pain78,79,81 after exercise. Furthermore, patients demonstrated a greater improvement in functional capacity,74,76,77,79,80 cardiorespiratory fitness, and muscle strength75,80,81 after exercise intervention.
3.3.6. IIM
Of 5 regular (7–26 weeks, 2–5 sessions per week) exercise intervention (2 RT,82,83 3 combined exercise84, 85, 86) trials for people with IIM, 2 studies85,86 reported reductions in plasma TNF-α, IL-7, IL-9, IL-17A, IL-12p70, macrophage inflammatory protein-1β, chemokine ligand-5, and soluble intercellular adhesion molecule-1. The other studies reported no changes in inflammation biomarkers.82, 83, 84 Exercise intervention also improved disability, stability, aerobic capacity, and muscle strength in patients with IIM.83,86
3.3.7. IBD
Five regular (4 days–16 weeks, 3–5 sessions per week) exercise intervention trials (AT, combined exercise, active video games) for patients with IBD reported a decrease in the blood leukocyte count,89 serum CRP,91 calprotectin,92 or no change in inflammation biomarkers.87,88 However, plasma cytokine IL-6, IL-8, and IL-10 increases following 4 bouts of prolonged walking were modest and similar in study participants with or without IBD, and both groups experienced no changes in fecal calprotectin.90 Patients with IBD exhibited an improved quality of life and reduced fatigue following exercise.92 Additionally, they exhibit a decrease in body fat percentage88 and improved cardiorespiratory fitness and muscle strength.87,88 The acute exercise intervention did not change IBD disease activity in patients with ulcerative colitis, but it did increase in patients with Crohn's disease.90
3.3.8. TA
AT, RT, and combined AT and RT regular (each 12 weeks, 2–3 sessions per week) exercise intervention trials for patients with TA showed reductions in serum CRP and plasma TNF-α94,95 and IL-1β.93 After exercise, patients with TA experience alleviated TA symptoms,94 reduced visceral adiposity,93 and significant improvements in physical activity levels as well as muscle strength and function.93,95
3.3.9. Other autoimmune diseases
One 12-week RT exercise intervention NRCT study98 (3 sessions per week, 70% 1 repetition maximum) with patients with pemphigus foliaceus showed reductions in plasma IL-17, IL-22, IL-15, and IFN-γ. Most patients with SSc were unable to complete a maximal cardiopulmonary exercise test, and thus acute changes in inflammation biomarkers were difficult to interpret relative to healthy controls.99 The normal increase in blood CD8+ concentrations following an acute, maximal incremental cycling bout was blunted in patients with both RA and SLE.100 Two exercise intervention studies101,102 in patients with combined autoimmune diseases (RA and JIA, MS and AS, respectively) showed no changes in inflammation biomarkers.
3.4. Quality and risk of bias
In the studies that were included in this review, 39 studies were RCTs17,18,21,23,25, 26, 27,29,30,32, 33, 34, 35, 36, 37,40,41,44,45,48,52,53,56,58,68,74,75,79, 80, 81,83,85,88,89,92, 93, 94,101,102 and 48 were NRCTs.16,19,20,22,24,28,31,39,39,42,43,46,47,49, 50, 51,54,55,57,59, 60, 61, 62, 63, 64, 65, 66, 67,69, 70, 71, 72, 73,76, 77, 78,82,84,86,87,90,91,95, 96, 97, 98, 99, 100 The quality assessments of RCTs and NRCTs were in Supplementary Table 3. The quality of the included studies ranged from 3 to 13 (maximum 15), with an average quality score of 7.39 points. The average quality score of included RCT studies was 9.13 points. The risk of bias for randomization was low in 28 of 39 studies17,21,23,27,29,30,32,34,35,40,41,44,45,48,53,58,74,79, 80, 81,83,85,88,89,92, 93, 94,102 and unclear in 11 studies.18,25,26,33,36,37,52,56,68,75,101 For allocation concealment, 11 of 39 studies had a low risk,21,23,25,29,32,34,40,48,56,81,88 24 studies17,18,26,27,30,33,35, 36, 37,41,44,45,52,53,68,74,75,79,80,89,93,94,101,102 were unclear, and 4 studies58,83,85,92 had a high risk. Due to the nature of the exercise interventions, it was difficult to blind participants to the intervention programs. For blinding of participants and personnel, only 1 study30 was low risk, 17 studies17,18,21,26,27,36,37,40,41,48,52,53,56,74,79,85,94 were equivocal, and 21 studies23,25,29,32, 33, 34, 35,44,45,58,68,75,80,81,83,88,89,92,93,101,102 were high risk. For blinding of outcome assessment, 17 of 39 achieved low risk,18,23,25,32,35,40,44,45,48,53,75,81,83,85,93,94,102 14 of 39 were unclear,17,26,29,33,36,37,41,52,56,68,74,79,89,101 and 8 of 39 had high risk.21,27,30,34,58,80,88,92 For risk of bias on incomplete outcome data, 31 studies18,21,23,25,26,29,32, 33, 34, 35,37,39,41,44,45,48,52,53,58,74,75,80,81,83,88,89,92, 93, 94,101,102 were low risk and 8 studies17,27,36,40,56,68,79,85 were unclear. Risk of bias was low in regard to selective reporting and other related areas in all studies. The graph and summary on risk of bias for the included RCTs are shown in Supplementary Figs. 1 and 2.
4. Discussion
4.1. Exercise characteristics and main findings
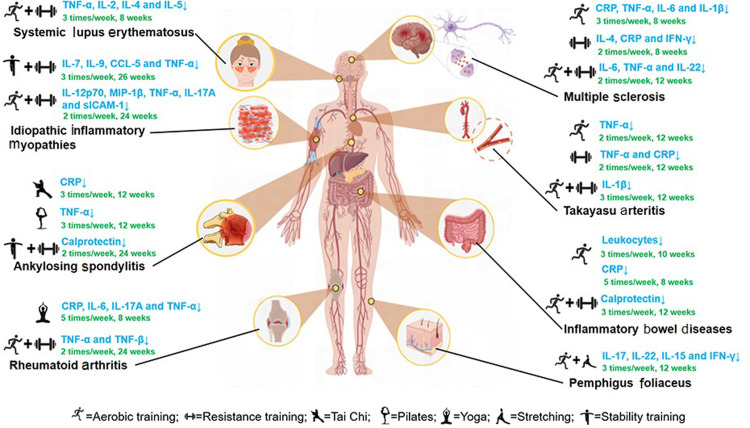
This systematic review showed that acute and regular exercise interventions have a differential response on inflammation biomarkers in patients with autoimmune diseases. The exercise-induced effect on inflammation is also dependent on the type and severity of the autoimmune diseases (as summarized in Fig. 2). According to the literatures we reviewed, changes in inflammatory markers and disease-related symptoms were not synchronized. For example, exercise interventions with similar protocols had inconsistent effects on inflammatory markers and disease symptoms in patients with AS and MS. Consistent with other studies, acute exercise modestly and transiently increases the inflammatory response and regular exercise has an anti-inflammatory effect.6,7 Most of the regular exercise intervention studies employed moderate exercise training protocols due to safety concerns and patient limitations. Acute moderate- to high-intensity exercise increases inflammatory biomarkers, possibly due to exercise-induced muscle micro injury. This finding is consistent with the study conducted by Contrepois et al.103 The review also analyzed the effects of exercise interventions on inflammatory markers in various autoimmune disease symptoms. Furthermore, exercise interventions can alleviate the complications of autoimmune diseases. For instance, exercise can enhance joint mobility and improve the quality of life for patients with RA. However, the pathogenesis of autoimmune disease is complex, and for many of them it remains unclear.
Fig. 2.
Anti-inflammatory effects of regular exercise on autoimmune diseases. ↑ = increase; ↓ = decrease; CCL = chemokine ligand; CRP = C-reactive protein; IFN-γ = interferon-γ; IL = interleukin; MIP = macrophage inflammatory protein; sICAM = soluble intercellular adhesion molecule; TNF-α = tumor necrosis factor α.
4.1.1. MS and RA
Thirty-seven of the 87 papers included in this systematic review were exercise interventions for MS and RA. The inflammation response to exercise interventions for these 2 types of autoimmune diseases has been investigated extensively and systematically reviewed.10,11 The reviews related to exercise interventions for these 2 types of diseases focused on exercise intervention programs that could be quantified in terms of intensity and workload (e.g., AT and RT). The systematic review of studies in patients with MS concluded that despite exercise-induced improvements in quality of life, no significant changes were measured for inflammation.10 A review of studies in patients with RA showed regular exercise-related improvements in disease activity scores and small beneficial effects on the erythrocyte sedimentation rate but no effect on CRP.11 The current review employed a broader inclusion of exercise training protocols for patients with autoimmune diseases and showed that the best effects on inflammation were from studies that utilized combined exercise interventions. The combined exercise interventions for MS combined AT and RT, and some studies added Pilates, stretching, and balance training.25,26,35 Notably, exercise interventions for RA demonstrating anti-inflammatory effects were usually in the form of individual and group classes with high participation rates.39,42,44,45,48 These combined exercise training studies support an individualized and clinical team-based approach. In general, most regular combined exercise interventions in patients with RA report no worsening of or improvements in disease activity scores with variable and modest anti-inflammatory effects. More meaningful improvements in inflammation biomarkers may require higher exercise workload volumes and significant weight loss that exceed the capabilities of patients with both MS and RA.
4.1.2. SLE
The leukocytes of SLE patients, regardless of disease activity, exhibited a decrease in inflammatory gene expression immediately after acute aerobic exercise, followed by an increase during recovery.71 Additionally, less organized gene networks were observed in SLE patients, indicating a potential deficiency in triggering a normal exercise-induced immune transcriptional response. SLE patients may have high levels of inflammation-related transcripts, which could result in lower exercise-induced changes in transcript levels compared to disease-induced changes. Furthermore, the immunosuppressive medications used by SLE patients may make them less sensitive to exercise-induced immune-related transcriptional responses. Exercise may also alleviate disease-related inflammatory responses by reducing body weight and body fat percentage in patients with SLE.104
4.1.3. T1D
Although exercise intervention has been established as effective for type 2 diabetes, fewer studies have been reported for T1D, and the effects on inflammatory factors were not significant. However, exercise interventions can improve glycemic homeostasis and increase muscle strength in patients with T1D. Combined resistance and AT at a moderate intensity improved blood glucose homeostasis and growth hormone in children with T1D.58 In addition, in patients with T1D, a recent study suggests that exercise combined with dietary interventions may be more effective for alleviating symptoms.105
4.1.4. Other autoimmune diseases
The anti-inflammatory effects of acute and regular exercise interventions were studied in other autoimmune disease groups, including those with AS, IIM, IBD, TA, FS, JIA, and SSc (Tables 2 and 3). Acute exercise bouts generally did not evoke clinically important changes in inflammation biomarkers, and this is in part due to the moderate exercise workloads used in these studies. The effect of regular exercise training on inflammation biomarkers in other autoimmune disease groups is variable, and any improvements are modest at best. Combined exercise interventions may have some effects on inflammation in patients with autoimmune diseases. The effective exercise intervention for patients with AS was Tai Chi, dance therapy, and Pilates or core muscle stabilization and balance training;77, 78, 79 and for IIM, it was strength and stability training.86 Pathways related to aerobic metabolism are altered in patients with IIM after endurance exercise.106 Thus, study results may be due in part to the type of autoimmune diseases and related effects on physiological function. For example, RT may not be appropriate for patients with IBD because RT increases intra-abdominal pressure, which may worsen symptoms of diarrhea or abdominal pain. In contrast, 1 study has shown that breathing exercises combined with meditation, which help regulate intra-abdominal pressure, may relieve IBD symptoms.107 The metabolic modulating effect of exercise is one of the ways in which exercise interventions are thought to alleviate symptoms in patients with autoimmune diseases.
4.1.5. Immune homeostasis in exercise
Acute exercise induces inflammation by modifying factors such as adrenaline, IL-6, and TNF-α in the microenvironment. This, in turn, improves the recognition and response to abnormalities such as foreign pathogens and internal malignant cells.108 Exercise not only enhances positive immune function but also enhances regulatory modalities such as IL-1 receptor antagonist, vascular growth factor D, and immune checkpoints. These facilitate a compensatory anti-inflammatory response resulting in a more robust immune homeostasis.103 However, the findings regarding the effect of exercise on immune function are still controversial. For instance, cytotoxic T lymphocyte-associated antigen-4 is a crucial immune checkpoint that negatively regulates T cells. The agonist is clinically used to treat RA and juvenile RA. Nevertheless, Gautam et al.45 found that T lymphocyte-associated antigen-4 was reduced after yoga exercise in patients with RA. The mechanism and effect of this change are unclear. Autoimmune diseases occur when the immune system fails to regulate itself, leading to an abnormal immune response by T cells or antibodies against normal cells and tissues. The mechanisms behind this are complex and require further research to be elucidated.
4.2. Limitations and implications
RCTs of exercise interventions were not available for all types of autoimmune diseases, and there were fewer high-quality RCTs from 2003 to 2013. The quality of the literatures included in this systematic review was variable. There was only 1 exercise intervention trial each for both FS and SSc. There were no acute exercise intervention trials for AS or IIMs, and fewer regular exercise interventions for T1D. Some of the included trials had imprecise definitions of the duration and intensity of exercise interventions and did not describe how exercise intensity was monitored. Some studies did not adequately address the characteristics of the exercise intervention (periodicity, incremental load) and used acute exercise or a small number of sessions. Therefore, more well-controlled and organized RCTs are needed to investigate the effects of regular exercise interventions.
In addition, the inflammatory markers selected for the included studies were inconsistent. For example, some studies assessed the inflammatory response using CRP and cytokines, whereas others measured the degree of inflammation using white blood cell counts or the proportion of certain immune cells. In addition, there was a paucity of literatures using inflammation as a primary outcome in autoimmune diseases exercise intervention studies.
Third, the language of the included studies was English, so fewer studies of traditional Chinese exercise, such as Tai Chi, were included. Only 3 databases were selected for the study, and no other databases were thoroughly searched for studies related to autoimmune diseases exercise interventions. This systematic review did not include direct analysis of trial data, and the authors of the included trials were not contacted to provide data for statistical analysis.
5. Conclusion
From an anti-inflammatory perspective, regular interventions that combine multiple exercise modes individualized to patients with autoimmune diseases are recommended. Most patients with autoimmune disease can safely adopt moderate exercise training protocols, but changes in inflammation biomarkers will be modest at best. Regular exercise training, especially when combined with AT and RT, is an effective countermeasure to autoimmune diseases. Acute exercise interventions are ineffective or even modestly but transiently pro-inflammatory.
Acknowledgments
Acknowledgments
This study was supported by the National Natural Science Foundation of China (NO. 31801003 for DX, NO. 31701040 for BL) and Shanghai Key Lab of Human Performance (Shanghai University of Sport) (NO. 11DZ2261100). We appreciate the medical advice and proofreading by Dr. Li Guo from the Department of Rheumatology, Renji Hospital, and Dr. Bo Li from the Department of Endocrinology, Xinhua Hospital, Shanghai Jiao Tong University. We also express gratitude to Prof. Yan Gu from National Key Laboratory of Immunity and Inflammation, Department of Immunology, Naval Medical University, for useful comments and discussions; to Xin Xiao from the library, Shanghai University of Sport, for reviewing and verifying the search strategy; and to Shuangyu Gu of the School of Exercise and Health, Shanghai University of Sport, for conducting the initial screening of literatures. We also thank Figdraw platform for visualization.
Authors’ contributions
BL made contributions to the conception and design, study selection, data extraction, risk of bias assessment, data analysis, and drafting of the manuscript; DX contributed to the literature search, study selection, data extraction, risk of bias assessment, data analysis, drafting of the manuscript, and participated in the conception and design of the study and contributed to the revision of the manuscript; XJ contributed to the literature search, study selection, data extraction, risk of bias assessment, data analysis, and drafting of the manuscript; XC, RL, and SZ contributed to the study selection, data extraction, risk of bias assessment, data analysis, and drafting of the manuscript; YM contributed to the literature search; DCN participated in the conception and design of the study and contributed to the revision of the manuscript; PC made contributions to the conception and design. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2024.02.002.
Contributor Information
David C. Nieman, Email: niemandc@appstate.edu.
Peijie Chen, Email: chenpeijie@sus.edu.cn.
Supplementary materials
References
- 1.Conrad N, Misra S, Verbakel JY, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: A population-based cohort study of 22 million individuals in the UK. The Lancet. 2023;401:1878–1890. doi: 10.1016/S0140-6736(23)00457-9. [DOI] [PubMed] [Google Scholar]
- 2.Miller FW. The increasing prevalence of autoimmunity and autoimmune diseases: An urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr Opin Immunol. 2023;80 doi: 10.1016/j.coi.2022.102266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharif K, Watad A, Bragazzi NL, Lichtbroun M, Amital H, Shoenfeld Y. Physical activity and autoimmune diseases: Get moving and manage the disease. Autoimmun Rev. 2018;17:53–72. doi: 10.1016/j.autrev.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Einstein O, Katz A, Ben-Hur T. Physical exercise therapy for autoimmune neuroinflammation: Application of knowledge from animal models to patient care. Autoimmun Rev. 2022;21 doi: 10.1016/j.autrev.2022.103033. [DOI] [PubMed] [Google Scholar]
- 5.Edwards T, Michelsen AS, Fakolade AO, Dalgas U, Pilutti LA. Exercise training improves participation in persons with multiple sclerosis: A systematic review and meta-analysis. J Sport Health Sci. 2022;11:393–402. doi: 10.1016/j.jshs.2021.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ploeger HE, Takken T, de Greef MHG, Timmons BW. The effects of acute and chronic exercise on inflammatory markers in children and adults with a chronic inflammatory disease: A systematic review. Exerc Immunol Rev. 2009;15:6–41. [PubMed] [Google Scholar]
- 7.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 8.Valenzuela PL, Carrera-Bastos P, Castillo-García A, Lieberman DE, Santos-Lozano A, Lucia A. Obesity and the risk of cardiometabolic diseases. Nat Rev Cardiol. 2023;20:475–494. doi: 10.1038/s41569-023-00847-5. [DOI] [PubMed] [Google Scholar]
- 9.Nieman DC, Sakaguchi CA. Physical activity lowers the risk for acute respiratory infections: Time for recognition. J Sport Health Sci. 2022;11:648–655. doi: 10.1016/j.jshs.2022.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong VL, Holahan MR. A systematic review of aerobic and resistance exercise and inflammatory markers in people with multiple sclerosis. Behav Pharmacol. 2019;30:653–660. doi: 10.1097/FBP.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 11.Sveaas SH, Smedslund G, Hagen KB, Dagfinrud H. Effect of cardiorespiratory and strength exercises on disease activity in patients with inflammatory rheumatic diseases: A systematic review and meta-analysis. Br J Sports Med. 2017;51:1065–1072. doi: 10.1136/bjsports-2016-097149. [DOI] [PubMed] [Google Scholar]
- 12.Fiuza-Luces C, Valenzuela PL, Gálvez BG, et al. The effect of physical exercise on anticancer immunity. Nat Rev Immunol. 2024;24:282–293. doi: 10.1038/s41577-023-00943-0. [DOI] [PubMed] [Google Scholar]
- 13.Garber CE, Blissmer B, Deschenes MR, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 14.Smart NA, Waldron M, Ismail H, et al. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc. 2015;13:9–18. doi: 10.1097/XEB.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 15.Cuthbert M, Ripley N, McMahon JJ, Evans M, Haff GG, Comfort P. The effect of Nordic hamstring exercise intervention volume on eccentric strength and muscle architecture adaptations: A systematic review and meta-analyses. Sports Med. 2020;50:83–99. doi: 10.1007/s40279-019-01178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarenga-Filho H, Sacramento PM, Ferreira TB, et al. Combined exercise training reduces fatigue and modulates the cytokine profile of T-cells from multiple sclerosis patients in response to neuromediators. J Neuroimmunol. 2016;293:91–99. doi: 10.1016/j.jneuroim.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Bahmani E, Hoseini R, Amiri E. Home-based aerobic training and vitamin D improve neurotrophins and inflammatory biomarkers in MS patients. Mult Scler Relat Disord. 2022;60 doi: 10.1016/j.msard.2022.103693. [DOI] [PubMed] [Google Scholar]
- 18.Bansi J, Bloch W, Gamper U, Kesselring J. Training in MS: Influence of two different endurance training protocols (aquatic versus overland) on cytokine and neurotrophin concentrations during three week randomized controlled trial. Mult Scler. 2013;19:613–621. doi: 10.1177/1352458512458605. [DOI] [PubMed] [Google Scholar]
- 19.Barry A, Cronin O, Ryan AM, et al. Cycle ergometer training enhances plasma interleukin-10 in multiple sclerosis. Neurol Sci. 2019;40:1933–1936. doi: 10.1007/s10072-019-03915-2. [DOI] [PubMed] [Google Scholar]
- 20.Berkowitz S, Achiron A, Gurevich M, Sonis P, Kalron A. Acute effects of aerobic intensities on the cytokine response in women with mild multiple sclerosis. Mult Scler Relat Disord. 2019;31:82–86. doi: 10.1016/j.msard.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Briken S, Rosenkranz SC, Keminer O, et al. Effects of exercise on Irisin, BDNF and IL-6 serum levels in patients with progressive multiple sclerosis. J Neuroimmunol. 2016;299:53–58. doi: 10.1016/j.jneuroim.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Castellano V, Patel DI, White LJ. Cytokine responses to acute and chronic exercise in multiple sclerosis. J Appl Physiol (1985) 2008;104:1697–1702. doi: 10.1152/japplphysiol.00954.2007. [DOI] [PubMed] [Google Scholar]
- 23.Deckx N, Wens I, Nuyts AH, et al. 12 weeks of combined endurance and resistance training reduces innate markers of inflammation in a randomized controlled clinical trial in patients with multiple sclerosis. Mediators Inflamm. 2016;2016 doi: 10.1155/2016/6789276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devasahayam AJ, Kelly LP, Williams JB, Moore CS, Ploughman M. Fitness shifts the balance of BDNF and IL-6 from inflammation to repair among people with progressive multiple sclerosis. Biomolecules. 2021;11:504. doi: 10.3390/biom11040504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faramarzi M, Banitalebi E, Raisi Z, et al. Effect of combined exercise training on pentraxins and pro- inflammatory cytokines in people with multiple sclerosis as a function of disability status. Cytokine. 2020;134 doi: 10.1016/j.cyto.2020.155196. [DOI] [PubMed] [Google Scholar]
- 26.Golzari Z, Shabkhiz F, Soudi S, Kordi MR, Hashemi SM. Combined exercise training reduces IFN-γ and IL-17 levels in the plasma and the supernatant of peripheral blood mononuclear cells in women with multiple sclerosis. Int Immunopharmacol. 2010;10:1415–1419. doi: 10.1016/j.intimp.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Joisten N, Proschinger S, Rademacher A, et al. High-intensity interval training reduces neutrophil-to-lymphocyte ratio in persons with multiple sclerosis during inpatient rehabilitation. Mult Scler. 2021;27:1136–1139. doi: 10.1177/1352458520951382. [DOI] [PubMed] [Google Scholar]
- 28.Kierkegaard M, Lundberg IE, Olsson T, et al. High-intensity resistance training in multiple sclerosis—An exploratory study of effects on immune markers in blood and cerebrospinal fluid, and on mood, fatigue, health-related quality of life, muscle strength, walking and cognition. J Neurol Sci. 2016;362:251–257. doi: 10.1016/j.jns.2016.01.063. [DOI] [PubMed] [Google Scholar]
- 29.Kjølhede T, Dalgas U, Gade AB, et al. Acute and chronic cytokine responses to resistance exercise and training in people with multiple sclerosis. Scand J Med Sci Sports. 2016;26:824–834. doi: 10.1111/sms.12504. [DOI] [PubMed] [Google Scholar]
- 30.Mähler A, Balogh A, Csizmadia I, et al. Metabolic, mental and immunological effects of normoxic and hypoxic training in multiple sclerosis patients: A pilot study. Front Immunol. 2018;9:2819. doi: 10.3389/fimmu.2018.02819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Majdinasab N, Motl RW, Mokhtarzade M, et al. Acute responses of cytokines and adipokines to aerobic exercise in relapsing vs. remitting women with multiple sclerosis. Complement Ther Clin Pract. 2018;31:295–301. doi: 10.1016/j.ctcp.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Mokhtarzade M, Ranjbar R, Majdinasab N, Patel D, Molanouri Shamsi M. Effect of aerobic interval training on serum IL-10, tnfalpha, and adipokines levels in women with multiple sclerosis: Possible relations with fatigue and quality of life. Endocrine. 2017;57:262–271. doi: 10.1007/s12020-017-1337-y. [DOI] [PubMed] [Google Scholar]
- 33.Mokhtarzade M, Molanouri Shamsi M, Abolhasani M, et al. Home-based exercise training influences gut bacterial levels in multiple sclerosis. Complement Ther Clin Pract. 2021;45 doi: 10.1016/j.ctcp.2021.101463. [DOI] [PubMed] [Google Scholar]
- 34.Nieste I, Franssen WMA, Duvivier BMFM, Spaas J, Savelberg HHCM, Eijnde BO. Replacing sitting with light-intensity physical activity throughout the day versus 1 bout of vigorous-intensity exercise: Similar cardiometabolic health effects in multiple sclerosis. A randomised cross-over study. Disabil Rehabil. 2023;45:3293–3302. doi: 10.1080/09638288.2022.2122601. [DOI] [PubMed] [Google Scholar]
- 35.Ozkul C, Guclu-Gunduz A, Irkec C, et al. Effect of combined exercise training on serum brain-derived neurotrophic factor, suppressors of cytokine signaling 1 and 3 in patients with multiple sclerosis. J Neuroimmunol. 2018;316:121–129. doi: 10.1016/j.jneuroim.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Schulz KH, Gold SM, Witte J, et al. Impact of aerobic training on immune-endocrine parameters, neurotrophic factors, quality of life and coordinative function in multiple sclerosis. J Neurol Sci. 2004;225:11–18. doi: 10.1016/j.jns.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Tadayon Zadeh F, Amini H, Habibi S, Shahedi V, Isanejad A, Akbarpour M. The effects of 8-week combined exercise training on inflammatory markers in women with multiple sclerosis. Neurodegener Dis. 2020;20:212–216. doi: 10.1159/000518580. [DOI] [PubMed] [Google Scholar]
- 38.White LJ, Castellano V, Mc Coy SC. Cytokine responses to resistance training in people with multiple sclerosis. J Sports Sci. 2006;24:911–914. doi: 10.1080/02640410500357036. [DOI] [PubMed] [Google Scholar]
- 39.Acar A, Guzel S, Sarifakioglu B, et al. Calprotectin levels in patients with rheumatoid arthritis to assess and association with exercise treatment. Clin Rheumatol. 2016;35:2685–2692. doi: 10.1007/s10067-016-3240-y. [DOI] [PubMed] [Google Scholar]
- 40.Andersson SEM, Lange E, Kucharski D, et al. Moderate- to high intensity aerobic and resistance exercise reduces peripheral blood regulatory cell populations in older adults with rheumatoid arthritis. Immun Ageing. 2020;17:12. doi: 10.1186/s12979-020-00184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azeez M, Clancy C, O'Dwyer T, Lahiff C, Wilson F, Cunnane G. Benefits of exercise in patients with rheumatoid arthritis: A randomized controlled trial of a patient-specific exercise programme. Clin Rheumatol. 2020;39:1783–1792. doi: 10.1007/s10067-020-04937-4. [DOI] [PubMed] [Google Scholar]
- 42.Bartlett DB, Willis LH, Slentz CA, et al. Ten weeks of high-intensity interval walk training is associated with reduced disease activity and improved innate immune function in older adults with rheumatoid arthritis: A pilot study. Arthritis Res Ther. 2018;20:127. doi: 10.1186/s13075-018-1624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ercan Z, Deniz G, Yentur SB, et al. Effects of acute aerobic exercise on cytokines, Klotho, irisin, and vascular endothelial growth factor responses in rheumatoid arthritis patients. Ir J Med Sci. 2023;192:491–497. doi: 10.1007/s11845-022-02970-7. [DOI] [PubMed] [Google Scholar]
- 44.Gautam S, Tolahunase M, Kumar U, Dada R. Impact of yoga based mind-body intervention on systemic inflammatory markers and co-morbid depression in active rheumatoid arthritis patients: A randomized controlled trial. Restor Neurol Neurosci. 2019;37:41–59. doi: 10.3233/RNN-180875. [DOI] [PubMed] [Google Scholar]
- 45.Gautam S, Kumar M, Kumar U, Dada R. Effect of an 8-week yoga-based lifestyle intervention on psycho-neuro-immune axis, disease activity, and perceived quality of life in rheumatoid arthritis patients: A randomized controlled trial. Front Psychol. 2020;11:2259. doi: 10.3389/fpsyg.2020.02259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joo YB, Lee KB, Sul B, Lee HS, Lim SH, Park YJ. Effect of resistance exercise on serum leptin levels in a prospective longitudinal study of women patients with rheumatoid arthritis. Arthritis Res Ther. 2022;24:76. doi: 10.1186/s13075-022-02765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Law RJ, Saynor ZL, Gabbitas J, et al. The effects of aerobic and resistance exercise on markers of large joint health in stable rheumatoid arthritis patients: A pilot study. Musculoskeletal Care. 2015;13:222–235. doi: 10.1002/msc.1103. [DOI] [PubMed] [Google Scholar]
- 48.Lozada-Mellado M, Llorente L, Hinojosa-Azaola A, et al. Comparison of the impacts of a dynamic exercise program vs. a Mediterranean diet on serum cytokine concentrations in women with rheumatoid arthritis. A secondary analysis of a randomized clinical trial. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.834824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pereira Nunes Pinto AC, Natour J, de Moura Castro CH, Eloi M, Lombardi I., Jr Acute effect of a resistance exercise session on markers of cartilage breakdown and inflammation in women with rheumatoid arthritis. Int J Rheum Dis. 2017;20:1704–1713. doi: 10.1111/1756-185X.13204. [DOI] [PubMed] [Google Scholar]
- 50.Sarajlic P, Fridén C, Lund LH, et al. Enhanced ventricular-arterial coupling during a 2-year physical activity programme in patients with rheumatoid arthritis: A prospective substudy of the physical activity in rheumatoid arthritis 2010 trial. J Intern Med. 2018;284:664–673. doi: 10.1111/joim.12715. [DOI] [PubMed] [Google Scholar]
- 51.Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, Nightingale P, Kitas GD, Koutedakis Y. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819–1825. doi: 10.1136/annrheumdis-2012-202075. [DOI] [PubMed] [Google Scholar]
- 52.Wadley AJ, Veldhuijzen van Zanten JJ, Stavropoulos-Kalinoglou A, et al. Three months of moderate-intensity exercise reduced plasma 3-nitrotyrosine in rheumatoid arthritis patients. Eur J Appl Physiol. 2014;114:1483–1492. doi: 10.1007/s00421-014-2877-y. [DOI] [PubMed] [Google Scholar]
- 53.Farinha JB, Ramis TR, Vieira AF, et al. Glycemic, inflammatory and oxidative stress responses to different high-intensity training protocols in type 1 diabetes: A randomized clinical trial. J Diabetes Complications. 2018;32:1124–1132. doi: 10.1016/j.jdiacomp.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Galassetti PR, Iwanaga K, Crisostomo M, Zaldivar FP, Larson J, Pescatello A. Inflammatory cytokine, growth factor and counterregulatory responses to exercise in children with type 1 diabetes and healthy controls. Pediatr Diabetes. 2006;7:16–24. doi: 10.1111/j.1399-543X.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 55.Galassetti PR, Iwanaga K, Pontello AM, Zaldivar FP, Flores RL, Larson JK. Effect of prior hyperglycemia on IL-6 responses to exercise in children with type 1 diabetes. Am J Physiol Endocrinol Metab. 2006;290:E833–E839. doi: 10.1152/ajpendo.00445.2005. [DOI] [PubMed] [Google Scholar]
- 56.Minnock D, Annibalini G, Le Roux CW, et al. Effects of acute aerobic, resistance and combined exercises on 24-h glucose variability and skeletal muscle signalling responses in type 1 diabetics. Eur J Appl Physiol. 2020;120:2677–2691. doi: 10.1007/s00421-020-04491-6. [DOI] [PubMed] [Google Scholar]
- 57.Minnock D, Annibalini G, Valli G, et al. Altered muscle mitochondrial, inflammatory and trophic markers, and reduced exercise training adaptations in type 1 diabetes. J Physiol. 2022;600:1405–1418. doi: 10.1113/JP282433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nazari M, Shabani R, Hassanzadeh-Rad A, Esfandiari MA, Dalili S. Effect of concurrent resistance-aerobic training on inflammatory factors and growth hormones in children with type 1 diabetes: A randomized controlled clinical trial. Trials. 2023;24:519. doi: 10.1186/s13063-023-07553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rosa JS, Oliver SR, Mitsuhashi M, et al. Altered kinetics of interleukin-6 and other inflammatory mediators during exercise in children with type 1 diabetes. J Investig Med. 2008;56:701–713. doi: 10.2310/JIM.0b013e31816c0fba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosa JS, Flores RL, Oliver SR, Pontello AM, Zaldivar FP, Galassetti PR. Resting and exercise-induced IL-6 levels in children with type 1 diabetes reflect hyperglycemic profiles during the previous 3 days. J Appl Physiol (1985) 2010;108:334–342. doi: 10.1152/japplphysiol.01083.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosa JS, Oliver SR, Flores RL, et al. Altered inflammatory, oxidative, and metabolic responses to exercise in pediatric obesity and type 1 diabetes. Pediatr Diabetes. 2011;12:464–472. doi: 10.1111/j.1399-5448.2010.00724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosa JS, Heydari S, Oliver SR, et al. Inflammatory cytokine profiles during exercise in obese, diabetic, and healthy children. J Clin Res Pediatr Endocrinol. 2011;3:115–121. doi: 10.4274/jcrpe.v3i3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salman F, Erten G, Unal M, et al. Effect of acute maximal exercise on lymphocyte subgroups in type 1 diabetes. Acta Physiol Hung. 2008;95:77–86. doi: 10.1556/APhysiol.95.2008.1.5. [DOI] [PubMed] [Google Scholar]
- 64.Turner D, Luzio S, Kilduff LP, et al. Reductions in resistance exercise-induced hyperglycaemic episodes are associated with circulating interleukin-6 in type 1 diabetes. Diabet Med. 2014;31:1009–1013. doi: 10.1111/dme.12462. [DOI] [PubMed] [Google Scholar]
- 65.Żebrowska A, Hall B, Maszczyk A, Banaś R, Urban J. Brain-derived neurotrophic factor, insulin like growth factor-1 and inflammatory cytokine responses to continuous and intermittent exercise in patients with type 1 diabetes. Diabetes Res Clin Pract. 2018;144:126–136. doi: 10.1016/j.diabres.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 66.Clarke-Jenssen AC, Fredriksen PM, Lilleby V, Mengshoel AM. Effects of supervised aerobic exercise in patients with systemic lupus erythematosus: A pilot study. Arthritis Rheum. 2005;53:308–312. doi: 10.1002/art.21082. [DOI] [PubMed] [Google Scholar]
- 67.da Silva AE, dos Reis-Neto ET, da Silva NP, Sato EI. The effect of acute physical exercise on cytokine levels in patients with systemic lupus erythematosus. Lupus. 2013;22:1479–1483. doi: 10.1177/0961203313508832. [DOI] [PubMed] [Google Scholar]
- 68.Hashemi S, Habibagahi Z, Heidari M, Abdollahpour-Alitappeh M, Karimi MH. Effects of combined aerobic and anaerobic exercise training on cytokine profiles in patients with systemic lupus erythematosus (SLE): A randomized controlled trial. Transpl Immunol. 2022;70 doi: 10.1016/j.trim.2021.101516. [DOI] [PubMed] [Google Scholar]
- 69.Perandini LA, Sales-de-Oliveira D, Mello SB, et al. Exercise training can attenuate the inflammatory milieu in women with systemic lupus erythematosus. J Appl Physiol (1985) 2014;117:639–647. doi: 10.1152/japplphysiol.00486.2014. [DOI] [PubMed] [Google Scholar]
- 70.Perandini LA, Sales-de-Oliveira D, Mello S, et al. Inflammatory cytokine kinetics to single bouts of acute moderate and intense aerobic exercise in women with active and inactive systemic lupus erythematosus. Exerc Immunol Rev. 2015;21:174–185. [PubMed] [Google Scholar]
- 71.Perandini LA, Sales-de-Oliveira D, Almeida DC, et al. Effects of acute aerobic exercise on leukocyte inflammatory gene expression in systemic lupus erythematosus. Exerc Immunol Rev. 2016;22:64–81. [PubMed] [Google Scholar]
- 72.Soriano-Maldonado A, Morillas-de-Laguno P, Sabio JM, et al. Effects of 12-week aerobic exercise on arterial stiffness, inflammation, and cardiorespiratory fitness in women with systemic lupus erythematosus: Non-randomized controlled trial. J Clin Med. 2018;7:477. doi: 10.3390/jcm7120477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Timóteo RP, Silva AF, Micheli DC, et al. Increased flexibility, pain reduction and unaltered levels of IL-10 and CD11b+ lymphocytes in patients with systemic lupus erythematosus were associated with kinesiotherapy. Lupus. 2018;27:1159–1168. doi: 10.1177/0961203318768880. [DOI] [PubMed] [Google Scholar]
- 74.Aydin T, Taspinar O, Sariyildiz MA, et al. Evaluation of the effectiveness of home based or hospital based calisthenic exercises in patients with ankylosing spondylitis. J Back Musculoskelet Rehabil. 2016;29:723–730. doi: 10.3233/BMR-160677. [DOI] [PubMed] [Google Scholar]
- 75.de Souza MC, Jennings F, Morimoto H, Natour J. Swiss ball exercises improve muscle strength and walking performance in ankylosing spondylitis: A randomized controlled trial. Rev Bras Reumatol Engl Ed. 2017;57:45–55. doi: 10.1016/j.rbre.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 76.Hulejová H, Levitová A, Kuklová M, et al. No effect of physiotherapy on the serum levels of adipocytokines in patients with ankylosing spondylitis. Clin Rheumatol. 2012;31:67–71. doi: 10.1007/s10067-011-1773-7. [DOI] [PubMed] [Google Scholar]
- 77.Kisacik P, Unal E, Akman U, Yapali G, Karabulut E, Akdogan A. Investigating the effects of a multidimensional exercise program on symptoms and antiinflammatory status in female patients with ankylosing spondylitis. Complement Ther Clin Pract. 2016;22:38–43. doi: 10.1016/j.ctcp.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Levitova A, Hulejova H, Spiritovic M, Pavelka K, Senolt L, Husakova M. Clinical improvement and reduction in serum calprotectin levels after an intensive exercise programme for patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Arthritis Res Ther. 2016;18:275. doi: 10.1186/s13075-016-1180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma C, Qu K, Wen B, et al. Clinical effect of “Tai Chi spinal exercise” on spinal motor function in patients with axial spondyloarthritis. Int J Clin Exp Med. 2020;13:673–681. [Google Scholar]
- 80.Nolte K, van Rensburg DCJ, Fletcher L. Effects of a 6-month exercise programme on disease activity, physical and functional parameters in patients with ankylosing spondylitis: Randomised controlled trial. S Afr J Physiother. 2021;77:1546. doi: 10.4102/sajp.v77i1.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sveaas SH, Bilberg A, Berg IJ, et al. High intensity exercise for 3 months reduces disease activity in axial spondyloarthritis (axSpA): A multicentre randomised trial of 100 patients. Br J Sports Med. 2020;54:292–297. doi: 10.1136/bjsports-2018-099943. [DOI] [PubMed] [Google Scholar]
- 82.Alexanderson H, Dastmalchi M, Esbjornssön-Liljedahl M, Opava CH, Lundberg IE. Benefits of intensive resistance training in patients with chronic polymyositis or dermatomyositis. Arthritis Rheum. 2007;57:768–777. doi: 10.1002/art.22780. [DOI] [PubMed] [Google Scholar]
- 83.Alexanderson H, Munters LA, Dastmalchi M, et al. Resistive home exercise in patients with recent-onset polymyositis and dermatomyositis–A randomized controlled single-blinded study with a 2-year follow up. J Rheumatol. 2014;41:1124–1132. doi: 10.3899/jrheum.131145. [DOI] [PubMed] [Google Scholar]
- 84.Arnardottir S, Alexanderson H, Lundberg IE, Borg K. Sporadic inclusion body myositis: Pilot study on the effects of a home exercise program on muscle function, histopathology and inflammatory reaction. J Rehabil Med. 2003;35:31–35. doi: 10.1080/16501970306110. [DOI] [PubMed] [Google Scholar]
- 85.Coudert JD, Slater N, Sooda A, et al. Immunoregulatory effects of testosterone supplementation combined with exercise training in men with inclusion body myositis: A double-blind, placebo-controlled, cross-over trial. Clin Transl Immunology. 2022;11:e1416. doi: 10.1002/cti2.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Švec X, Storkanova H, Spiritovic M, et al. Hsp90 as a myokine: Its association with systemic inflammation after exercise interventions in patients with myositis and healthy subjects. Int J Mol Sci. 2022;23:11451. doi: 10.3390/ijms231911451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bjelica M, Walker RG, Obeid J, Issenman RM, Timmons BW. A pilot study of exercise training for children and adolescents with inflammatory bowel disease: An evaluation of feasibility, safety, satisfaction, and efficacy. Pediatr Exerc Sci. 2023;35:239–248. doi: 10.1123/pes.2022-0012. [DOI] [PubMed] [Google Scholar]
- 88.Cronin O, Barton W, Moran C, et al. Moderate-intensity aerobic and resistance exercise is safe and favorably influences body composition in patients with quiescent inflammatory bowel disease: A randomized controlled cross-over trial. BMC Gastroenterol. 2019;19:29. doi: 10.1186/s12876-019-0952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Klare P, Nigg J, Nold J, et al. The impact of a ten-week physical exercise program on health-related quality of life in patients with inflammatory bowel disease: A prospective randomized controlled trial. Digestion. 2015;91:239–247. doi: 10.1159/000371795. [DOI] [PubMed] [Google Scholar]
- 90.Lamers CR, de Roos NM, Bongers CCWG, et al. Repeated prolonged moderate-intensity walking exercise does not appear to have harmful effects on inflammatory markers in patients with inflammatory bowel disease. Scand J Gastroenterol. 2021;56:30–37. doi: 10.1080/00365521.2020.1845791. [DOI] [PubMed] [Google Scholar]
- 91.Legeret C, Mahlmann L, Gerber M, et al. Favorable impact of long-term exercise on disease symptoms in pediatric patients with inflammatory bowel disease. BMC Pediatr. 2019;19:297. doi: 10.1186/s12887-019-1680-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Scheffers LE, Vos IK, Utens EMWJ, et al. Physical training and healthy diet improved bowel symptoms, quality of life, and fatigue in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2023;77:214–221. doi: 10.1097/MPG.0000000000003816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Astley C, Clemente G, Terreri MT, et al. Home-based exercise training in childhood-onset Takayasu arteritis: A multicenter, randomized, controlled trial. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.705250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li G, Liu F, Wang Y, Zhao M, Song Y, Zhang L. Effects of resistance exercise on treatment outcome and laboratory parameters of Takayasu arteritis with magnetic resonance imaging diagnosis: A randomized parallel controlled clinical trial. Clin Cardiol. 2020;43:1273–1278. doi: 10.1002/clc.23439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oliveira DS, Shinjo SK, Silva MG, et al. Exercise in Takayasu arteritis: Effects on inflammatory and angiogenic factors and disease-related symptoms. Arthritis Care Res (Hoboken) 2017;69:892–902. doi: 10.1002/acr.23011. [DOI] [PubMed] [Google Scholar]
- 96.Rochette E, Merlin E, Hourdé C, et al. Inflammatory response 24 h post-exercise in youth with juvenile idiopathic arthritis. Int J Sports Med. 2018;39:867–874. doi: 10.1055/a-0640-9063. [DOI] [PubMed] [Google Scholar]
- 97.Rochette E, Pascale D, Hourdé C, et al. Single bout exercise in children with juvenile idiopathic arthritis: Impact on inflammatory markers. Mediators Inflamm. 2018;2018 doi: 10.1155/2018/9365745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Timóteo RP, Sales-Campos H, Silva MV, et al. Pemphigus foliaceus patients (Fogo Selvagem) treated with kinesiotherapy presented lower levels of proinflammatory cytokines. J Exerc Rehabil. 2019;15:460–467. doi: 10.12965/jer.1938146.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hargardóttir H, van Helvoort HAC, Vonk MC, van den Hoogen FHJ, Dekhuijzen PNR, Heijdra YF. Exercise in systemic sclerosis intensifies systemic inflammation and oxidative stress. Scand J Rheumatol. 2010;39:63–70. doi: 10.3109/03009740903124416. [DOI] [PubMed] [Google Scholar]
- 100.Pool AJ, Whipp BJ, Skasick AJ, Alavi A, Bland JM, Axford JS. Serum cortisol reduction and abnormal prolactin and CD4+/CD8+ T-cell response as a result of controlled exercise in patients with rheumatoid arthritis and systemic lupus erythematosus despite unaltered muscle energetics. Rheumatology (Oxford) 2004;43:43–48. doi: 10.1093/rheumatology/keg425. [DOI] [PubMed] [Google Scholar]
- 101.Sandstad J, Stensvold D, Hoff M, Nes BM, Arbo I, Bye A. The effects of high intensity interval training in women with rheumatic disease: A pilot study. Eur J Appl Physiol. 2015;115:2081–2089. doi: 10.1007/s00421-015-3186-9. [DOI] [PubMed] [Google Scholar]
- 102.Taspinar O, Aydin T, Celebi A, et al. Psychological effects of calisthenic exercises on neuroinflammatory and rheumatic diseases. Z Rheumatol. 2015;74:722–727. doi: 10.1007/s00393-015-1570-9. [DOI] [PubMed] [Google Scholar]
- 103.Contrepois K, Wu S, Moneghetti KJ, et al. Molecular choreography of acute exercise. Cell. 2020;181:1112–1130. doi: 10.1016/j.cell.2020.04.043. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sola-Rodríguez S, Vargas-Hitos JA, Gavilán-Carrera B, et al. Physical fitness attenuates the impact of higher body mass and adiposity on inflammation in women with systemic lupus erythematosus. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.729672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kristensen KB, Ranjan AG, McCarthy OM, et al. Effects of a low-carbohydrate-high-protein pre-exercise meal in type 1 diabetes – A randomised, crossover trial. J Clin Endocrinol Metab. 2023;109:208–216. doi: 10.1210/clinem/dgad427. [DOI] [PubMed] [Google Scholar]
- 106.Munters LA, Loell I, Ossipova E, et al. Endurance exercise improves molecular pathways of aerobic metabolism in patients with myositis. Arthritis Rheumatol. 2016;68:1738–1750. doi: 10.1002/art.39624. [DOI] [PubMed] [Google Scholar]
- 107.Gerbarg PL, Jacob VE, Stevens L, et al. The effect of breathing, movement, and meditation on psychological and physical symptoms and inflammatory biomarkers in inflammatory bowel disease: A randomized controlled trial. Inflamm Bowel Dis. 2015;21:2886–2896. doi: 10.1097/MIB.0000000000000568. [DOI] [PubMed] [Google Scholar]
- 108.Pedersen L, Idorn M, Olofsson GH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016;23:554–562. doi: 10.1016/j.cmet.2016.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.