Highlights
-
•
More than 50% of young pediatric cancer survivors present muscle strength deficits.
-
•
Such deficits are associated with lower areal bone mineral density (aBMD) Z-scores at total body (less head), total hip, femoral neck, and lumbar spine.
-
•
Each 1-decile lower in muscle strength was associated with 30%–95% higher odds of having low aBMD at most sites.
-
•
Early detection of muscle strength deficits after pediatric cancer treatment could help survivors, who lack cancer-related treatment exposures to trigger surveillance, to be screened for low aBMD.
Keywords: Bone health, Childhood cancer, DXA, Lean mass, Resistance training
Abstract
Background
Pediatric cancer survivors are at increased risk of muscle weakness and low areal bone mineral density (aBMD). However, the prevalence of muscle strength deficits is not well documented, and the associations of muscle strength with aBMD are unknown in this population. Therefore, this study aimed to investigate the prevalence of upper- and lower-body muscle strength deficits and to examine the associations of upper- and lower-body muscle strength with age-, sex, and race-specific aBMD Z-scores at the total body, total hip, femoral neck, and lumbar spine.
Methods
This cross-sectional study included 116 pediatric cancer survivors (12.1 ± 3.3 years old, mean ± SD; 42.2% female). Upper- and lower-body muscle strength were assessed by handgrip and standing long jump test, respectively. Dual‑energy X‑ray absorptiometry was used to measure aBMD (g/cm2). Associations between muscle strength and aBMD were evaluated in multivariable linear regression models. Logistic regression was used to evaluate the contribution of muscle strength (1-decile lower) to the odds of having low aBMD (Z-score ≤ 1.0). All analyses were adjusted for time from treatment completion, radiotherapy exposure, and body mass index.
Results
More than one-half of survivors were within the 2 lowest deciles for upper- (56.9%) and lower- body muscle strength (60.0%) in comparison to age- and sex-specific reference values. Muscle strength deficits were associated with lower aBMD Z-scores at all sites (B = 0.133–0.258, p = 0.001–0.032). Each 1-decile lower in upper-body muscle strength was associated with 30%–95% higher odds of having low aBMD Z-scores at all sites. Each 1-decile lower in lower-body muscle strength was associated with 35%–70% higher odds of having low aBMD Z-scores at total body, total hip, and femoral neck.
Conclusion
Muscle strength deficits are prevalent in young pediatric cancer survivors, and such deficits are associated with lower aBMD Z-scores at all sites. These results suggest that interventions designed to improve muscle strength in this vulnerable population may have the added benefit of improving aBMD.
Graphical abstract
1. Introduction
Pediatric cancer survival rates have experienced a remarkable increase during recent decades,1 with a current 5-year survivorship rate of 85% in children and 82% in adolescents.2 However, pediatric cancer survivors are at risk of experiencing later health complications.3 Low areal bone mineral density (aBMD), defined by age-, sex-, and race-specific aBMD Z-scores less than –1.0, has been reported in up to two-thirds of survivors.4 Pediatric cancer treatment utilizes DNA-damaging agents and occurs during a critical period of active skeletal growth, thus interfering with accrual of bone mass.5, 6, 7 This is evident by a decrease in bone formation and an increase in bone resorption.8 Chemotherapy and/or radiation not only interfere with bone metabolism but also impact skeletal muscle mass9 and function.10 While the prevalence of muscle strength deficits in young pediatric cancer survivors has not been consistently documented to date, Hoffman et al.11 identified preliminary lower-body muscle strength deficits in 183 young pediatric cancer survivors in comparison to their siblings.
Muscle strength during childhood and adolescence is widely considered a powerful marker of health12 and is strongly associated with higher aBMD during both adolescence13 and later in life.14 In healthy children and adolescents, measured upper- and lower-body muscle strength have been consistently associated with the bone mineral content (BMC) of the total body15,16 and of the upper13,17 and lower13,17 extremities, as well as with total body and femoral neck aBMD.18 Likewise, in adult pediatric cancer survivors, Joyce et al.19 found that upper- (R2 = 0.56) and lower-body (R2 : 0.33–0.40) muscle strength was positively associated with aBMD. However, in younger survivors, the literature describing associations of muscle strength with aBMD is scarce. Physical activity increases muscle strength during growth and, according to the mechanostat theory of Frost HM,20 this creates the stimulus for bone to increase its mass. This is relevant since lower muscle strength following the completion of treatment could anticipate a further decline in aBMD. Early detection of muscle strength deficits could help survivors, who lack cancer-related treatment exposures to trigger surveillance, to be screened for low aBMD. Currently, muscle strength deficits are not considered in pediatric cancer survivor screening guidelines to be a risk factor for low aBMD.21,22
Thus, the aims of this study were to (a) investigate the prevalence of upper- and lower-body muscle strength deficits in young pediatric cancer survivors compared to age- and sex-specific international reference data; and (b) to examine the associations of upper- and lower-body muscle strength with age-, sex-, and race-specific aBMD Z-scores at the total body, total hip, femoral neck, and lumbar spine. We hypothesized that upper- and lower-body muscle strength deficits would be prevalent in young pediatric cancer survivors. We also hypothesized that upper- and lower-body muscle strength deficits would be associated with low aBMD Z-scores.
2. Methods
2.1. Study design and participants
This cross-sectional study included 116 pediatric cancer survivors (12.1 ± 3.3 years old, mean ± SD; 42.2% female) from the iBoneFIT project. A detailed description of the study protocol has been published elsewhere.23 Briefly, iBoneFIT is a multicenter parallel group randomized controlled trial designed to examine the effect of a 9-month online exercise program on bone health in young pediatric cancer survivors. Survivors were recruited from the Units of Pediatric Oncology and Hematology of the “Virgen de las Nieves” (Granada) and “Reina Sofia” (Cordoba) University Hospitals, Spain. Inclusion criteria were aged 6–18 years, not currently receiving treatments for cancer, diagnosed at least 1 year prior to enrolment, and previous exposure to radiotherapy and/or chemotherapy. Data collection occurred in 2 waves due to coronavirus disease 2019 restrictions: (a) October 2020 to February 2021; and (b) December 2021 to March 2022. All parents and survivors provided written informed consent and assent before entering the trial, respectively. The iBoneFIT project was approved by the Ethics Committee on Human Research of Regional Government of Andalusia (Reference: 4500, December 2019) and followed the ethical guidelines of the Declaration of Helsinki (revised Version 2013), and the randomized controlled trial was registered at isrctn.com (Reference: isrctn61195625, 2 April 2020). This study is reported according to the Strengthening the Reporting of OBservational Studies in Epidemiology (STROBE) checklist (Supplementary Table 1).24 Although we recruited 116 young pediatric cancer survivors in total, the sample sizes vary slightly for some variables due to missing data (i.e., some survivors were unable to perform some of the tests, were afraid of being scanned using dual‑energy X‑ray absorptiometry (DXA), or declined a particular test during their assessment).
2.2. Bone health
Survivors were evaluated using a single DXA scanner (Hologic Series Discovery QDR, Bedford, MA, USA) and analyzed by APEX software (Version 4.0.2; Hologic Series Discovery QDR). The device was calibrated each day using a lumbar spine phantom. Survivors were asked to remain still while being scanned in the supine position, as per guidelines from the International Society of Clinical Densitometry.25 Three regions (total body, right hip, and lumbar spine) were analyzed to characterize aBMD (g/cm2) and BMC of the total body (less head), total hip, femoral neck, and lumbar spine (mean of L1–L4). A single trained researcher analyzed all DXA scans. According to the International Society of Clinical Densitometry,25 DXA assessment should be performed (a) in children and adolescents with diseases that may affect the skeleton, and (b) when they may benefit from interventions to decrease their elevated risk of a clinically significant fracture. These are features of our sample, as described in the literature.4 The DXA coefficient of variation in pediatric population ranges between 1.0% and 2.9%, depending on the region.26 Moreover, using international reference data from the Bone Mineral Density in Childhood Study,27 age-, sex-, and race-specific aBMD and BMC Z-scores at the total body, total hip, femoral neck, and lumbar spine were calculated for all the analyses.
2.3. Muscle strength
Upper-body muscle strength was evaluated using the handgrip test (TKK 5101 Grip D; Takei, Tokyo, Japan). Survivors, keeping the arm straight, squeezed the dynamometer twice with each hand for 5 s at a time; the best score from the right hand and the best score from the left hand were averaged together and reported in kilograms. The handgrip test has shown good validity (intraclass correlation coefficients (ICC): 0.73–0.91) with high reproducibility and excellent test–retest reliability in children (ICC: 0.91–0.93).28,29 Lower-body muscle strength was assessed using the standing long jump test (considering the motor coordination that naturally occurs in human locomotion), which was performed twice after a short warmup; the best score was reported in centimeters. This field-based test has demonstrated good validity (i.e., the strongest association with 1 maximum repetition, p < 0.001) and excellent test–retest reliability (ICC = 0.94) in children.30 To gain an appropriate insight into the status of muscle strength in our sample, test performances were compared to updated age- and sex-specific reference values based on 8 million test results from healthy young populations in nearly 34 countries, which were gathered by the FitBack network.31 Muscle strength deficits were identified as ≤ 2nd decile, which is consistent with previous reports utilizing definitions of fitness deficits based on sex-and age-specific percentiles created by Tomkinson et al.32 and Ortega et al.33
2.4. Anthropometry and somatic maturity
Body mass (kg) was evaluated with an electronic scale (SECA 861; SECA, Hamburg, Germany) with an accuracy of 100 g. Stature (cm) was assessed using a precision stadiometer (SECA 225) to the nearest 0.1 cm. Body mass index (BMI) was calculated as body mass/stature (kg/m2). Additionally, age- and sex-specific body mass index Z-scores and categories were calculated using international reference data for pediatric populations.34 Somatic maturity was measured using the prediction of years before or after peak height velocity using validated algorithms for boys and girls.35
2.5. Clinical data and calcium
Medical record abstraction was used to retrieve diagnosis, time from treatment completion to baseline data collection, and treatment exposures (radiotherapy, chemotherapy and/or surgery, alone or in combination). Diagnosis was not included in analysis as it was colinear with treatment exposure. Time from treatment completion was treated as a continuous variable, and treatment exposure as a dichotomous variable: radiotherapy (yes/no). Finally, daily calcium intake (in mg) was estimated by a validated specific food-frequency questionnaire.36
2.6. Total physical activity
The tri-axial ActiGraph wGT3x-BT accelerometers (GT3X; ActiGraph, Pensacola, FL, USA) were used to measure total physical activity for 7 consecutive days (24 h/day). Young pediatric cancer survivors were instructed to wear devices attached to their non-dominant wrist at all times except during water activities. Accelerometers were initialized at a sampling frequency of 90 Hz, and raw data were processed using the GGIR R open-source package Version 2.8-2 (R Foundation for Statistical Computing, Vienna, Austria).37 Euclidean norm of the raw acceleration minus 1 G with negative values rounded to 0 was calculated along with the angle of the z-axis of the device to estimate physical activity and sleep parameters.38 Non-wear time was detected based on the standard deviation of the raw accelerations recorded in the 3 accelerometer axes as described elsewhere,39 and then it was imputed by means of the acceleration for the rest of the days at the same time window. Appropriate thresholds were used to identify physical activity intensities (i.e., moderate-to-vigorous physical activity: 200 mg, and light physical activity: 35–200 mg).40 We considered a day valid when: (a) the accelerometer registered at least 23 h/day, and (b) survivors wore the accelerometers for at least 16 h/day, since in this study the accelerometers were worn both day and night.41 Survivors having at least 1 valid day were included (sensitivity analyses showed similar results when including participants who had at least 3 valid weekdays and 1 weekend day). Total physical activity was calculated as the sum of the daily averages of moderate-to-vigorous physical activity and light physical activity (the mean of all 7 days).
2.7. Statistical analyses
The normal distribution of the variables was verified using skewness and kurtosis, the Kolmogorov–Smirnov test, a visual check of histograms, and Q–Q and box plots. Descriptive data were reported as mean ± SD or as frequencies (%). Multivariable linear regression analyses were used to evaluate the associations of upper- and lower-body muscle strength with age-, sex-, and race-specific aBMD Z-scores at each site (the same analyses were carried out for BMC Z-scores). Models were created as follows: Model 0 (no adjustments), Model 1 (adjusted for time from treatment completion to baseline evaluation (years) and radiotherapy exposure (yes/no)), Model 2 (adjusted for covariates in Model 1 plus BMI (kg/m2)), Model 3 (adjusted for covariates in Model 2 plus calcium intake (mg)), and Model 4 (adjusted for covariates in Model 3 plus total physical activity (min/day)). To identify the minimum sufficient adjustment set (MSAS) for the associations of upper- and lower-body muscle strength with age-, sex-, and race-specific aBMD and BMC Z-scores, we built a theoretical causal diagram based on previous associations with muscle strength and/or aBMD and BMC available in the scientific literature.3,11,19,42, 43, 44 We used the online tool DAGitty45 to construct a directed acyclic graph (DAG).46 The covariates age, sex, time from treatment completion, radiotherapy exposure, body mass index, calcium intake, and physical activity were identified as the MSAS (Supplementary Fig. 1). Radiotherapy exposure was the unique oncological treatment variable associated with aBMD and BMC (Supplementary Table 2). Age and sex were already accounted for using international reference data to calculate age-, sex-, and race-specific aBMD and BMC Z-scores. Binary logistic regression was used to evaluate the contribution of muscle strength (1-decile lower) to the odds of having low aBMD (Z-score ≤ 1.0).4 The same analyses were conducted for BMC Z-scores. Results are presented as odds ratios (ORs) with 95% confidence intervals (95%CIs). Similar models were built for logistic regressions. Statistical analyses were performed using the statistical software R Version 4.0.3 (R Foundation for Statistical Computing). B coefficient was presented non-standardized, and p values of <0.05 were considered statistically significant.
3. Results
Of the 196 young pediatric cancer survivors initially screened for participation, 116 were enrolled and included in this study (Supplementary Fig. 2).
3.1. Participant characteristics
Descriptive characteristics of our sample are presented in Table 1. The average age of the total sample was 12.1 ± 3.3 years (mean ± SD), and 42.2% were female. The majority of survivors were diagnosed with acute lymphoblastic leukemia (38.8%), lymphoma (12.1%), and central nervous system tumors (9.5%). Table 2 shows that more than one-half of survivors had muscle strength deficits (upper- (56.9%) and lower- body muscle strength (60.0%) deficits). Regarding bone health (Table 2), the averages were as follows: total body aBMD Z-score = –0.2 ± 1.4, BMC Z-score = –0.5 ± 1.3; total hip aBMD Z-score = 0.1 ± 1.3, BMC Z-score = 0.4 ± 1.4; femoral neck aBMD Z-score = –0.2 ± 1.4, BMC Z-score = –1.3 ± 1.5; lumbar spine aBMD Z-score = –0.1 ± 1.3, BMC Z-score = –0.5 ± 1.3. Participant characteristics by childhood cancer diagnosis (soft/solid tumors) are presented in Supplementary Table 3.
Table 1.
Descriptive characteristics of survivors included in the study.
| Characteristic | Total | n | Female | n | Male | n |
|---|---|---|---|---|---|---|
| Sex (female/male, %) | 42.2/57.8 | 116 | ||||
| Age (year) | 12.1 ± 3.3 | 116 | 12.2 ± 3.5 | 49 | 12.0 ± 3.2 | 67 |
| Body mass (kg) | 46.6 ± 18.0 | 116 | 45.2 ± 18.3 | 49 | 47.6 ± 17.9 | 67 |
| Stature (cm) | 147.5 ± 17.1 | 116 | 145.3 ± 16.0 | 49 | 149.0 ± 17.7 | 67 |
| Body mass index Z-score | 0.9 ± 1.1 | 116 | 0.8 ± 1.1 | 49 | 1.0 ± 1.2 | 67 |
| Body mass index (categories, %) | ||||||
| Underweight | 3.5 | 4 | 6.1 | 3 | 1.5 | 1 |
| Normoweight | 61.2 | 71 | 65.4 | 32 | 58.2 | 39 |
| Overweight | 20.7 | 24 | 16.3 | 8 | 23.9 | 16 |
| Obese | 14.6 | 17 | 12.2 | 6 | 16.4 | 11 |
| Years from peak height velocity | –0.8 ± 2.7 | 116 | 0.0 ± 2.9 | 49 | –1.3 ± 2.5 | 67 |
| Time from treatment completion (year) | 5.0 ± 3.8 | 113 | 5.2 ± 4.1 | 48 | 4.9 ± 3.6 | 65 |
| Radiotherapy exposure (yes/no, %) | 27.6/72.4 | 116 | 24.5/75.5 | 49 | 29.8/70.2 | 67 |
| Cancer type (%) | ||||||
| Acute lymphoblastic leukemia | 38.8 | 45 | 36.7 | 18 | 40.3 | 27 |
| Lymphoma | 12.1 | 14 | 12.2 | 6 | 11.9 | 8 |
| Central nervous system tumors | 9.5 | 11 | 10.2 | 5 | 9.0 | 6 |
| Renal tumors | 7.8 | 9 | 4.1 | 2 | 10.5 | 7 |
| Neuroblastoma | 6.9 | 8 | 12.2 | 6 | 3.0 | 2 |
| Malignant bone tumors | 6.9 | 8 | 8.2 | 4 | 6.0 | 4 |
| Histiocytosis | 5.2 | 6 | 6.1 | 3 | 4.5 | 3 |
| Soft tissue and other extraosseous sarcomas | 4.3 | 5 | 0.0 | 0 | 7.5 | 5 |
| Retinoblastoma | 3.5 | 4 | 4.1 | 2 | 3.0 | 2 |
| Hepatic tumors | 2.6 | 3 | 4.1 | 2 | 1.5 | 1 |
| Other malignant epithelial neoplasms | 1.7 | 2 | 2.0 | 1 | 1.5 | 1 |
| Unspecified malignant neoplasms | 0.9 | 1 | 0.0 | 0 | 1.5 | 1 |
Notes: Data are presented as mean ± SD or as percentage (%), as indicated. Percentage may not add to 100% due to rounding.
Table 2.
Distribution of upper- and lower-body muscle strength deciles and age-, sex-, and race-specific aBMD and BMC Z-scores.
| Characteristic | Total | n | Female | n | Male | n |
|---|---|---|---|---|---|---|
| Muscle strength | ||||||
| Upper-body reference decile (%) | ||||||
| 1 | 32.8 | 38 | 32.7 | 16 | 32.8 | 22 |
| 2 | 24.1 | 28 | 26.5 | 13 | 22.4 | 15 |
| 3 | 12.1 | 14 | 12.2 | 6 | 11.9 | 8 |
| 4 | 6.0 | 7 | 6.1 | 3 | 6.0 | 4 |
| 5 | 5.2 | 6 | 6.1 | 3 | 4.5 | 3 |
| 6 | 7.8 | 9 | 8.2 | 4 | 7.5 | 5 |
| 7 | 6.9 | 8 | 4.2 | 2 | 8.9 | 6 |
| 8 | 2.6 | 3 | 2.0 | 1 | 3.0 | 2 |
| 9 | 1.6 | 2 | 0 | 0 | 3.0 | 2 |
| 10 | 0.9 | 1 | 2.0 | 1 | 0 | 0 |
| Lower-body reference decile (%) | ||||||
| 1 | 40.9 | 47 | 40.8 | 20 | 40.9 | 27 |
| 2 | 19.1 | 22 | 22.5 | 11 | 16.7 | 11 |
| 3 | 9.6 | 11 | 6.1 | 3 | 12.1 | 8 |
| 4 | 12.2 | 14 | 18.4 | 9 | 7.6 | 5 |
| 5 | 7.0 | 8 | 6.2 | 3 | 7.6 | 5 |
| 6 | 5.2 | 6 | 2.0 | 1 | 7.6 | 5 |
| 7 | 2.6 | 3 | 2.0 | 1 | 3.0 | 2 |
| 8 | 0.9 | 1 | 0 | 0 | 1.5 | 1 |
| 9 | 1.7 | 2 | 0 | 0 | 3.0 | 2 |
| 10 | 0.9 | 1 | 2.0 | 1 | 0 | 0 |
| aBMD Z-score | ||||||
| Total body (less head) | –0.2 ± 1.4 | 116 | –0.2 ± 1.2 | 49 | –0.2 ± 1.5 | 67 |
| Total hip | 0.1 ± 1.3 | 115 | 0.2 ± 1.2 | 48 | 0.1 ± 1.3 | 67 |
| Femoral neck | –0.2 ± 1.4 | 115 | 0.1 ± 1.5 | 48 | –0.4 ± 1.3 | 67 |
| Lumbar spine | –0.1 ± 1.3 | 116 | –0.1 ± 1.2 | 49 | –0.1 ± 1.5 | 67 |
| BMC Z-score | ||||||
| Total body (less head) | –0.5 ± 1.3 | 116 | –0.5 ± 1.1 | 49 | –0.5 ± 1.4 | 67 |
| Total hip | 0.4 ± 1.4 | 115 | 0.3 ± 1.2 | 48 | 0.5 ± 1.6 | 67 |
| Femoral neck | –1.3 ± 1.5 | 115 | –1.4 ± 1.5 | 48 | –1.2 ± 1.5 | 67 |
| Lumbar spine | –0.5 ± 1.3 | 116 | –0.4 ± 1.1 | 49 | –0.5 ± 1.4 | 67 |
Notes: Data are presented as mean ± SD or as percentage (%), as indicated. Upper- and lower-body muscle strength reference deciles are shown using FitBack reference values. Age-, sex-, and race-specific aBMD and BMC Z-scores at each site are presented using international reference data from the Bone Mineral Density in Childhood Study. Percentage may not add to 100% due to rounding.
Abbreviations: aBMD = areal bone mineral density; BMC = bone mineral content.
3.2. Associations of muscle strength with aBMD Z-scores at each site
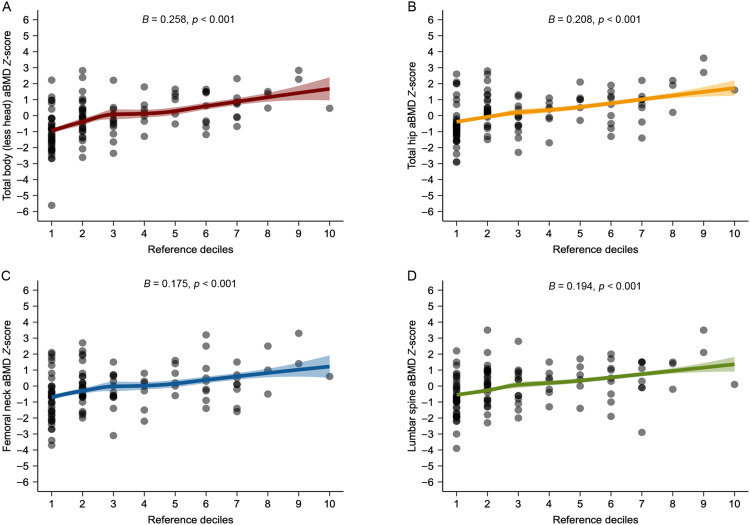
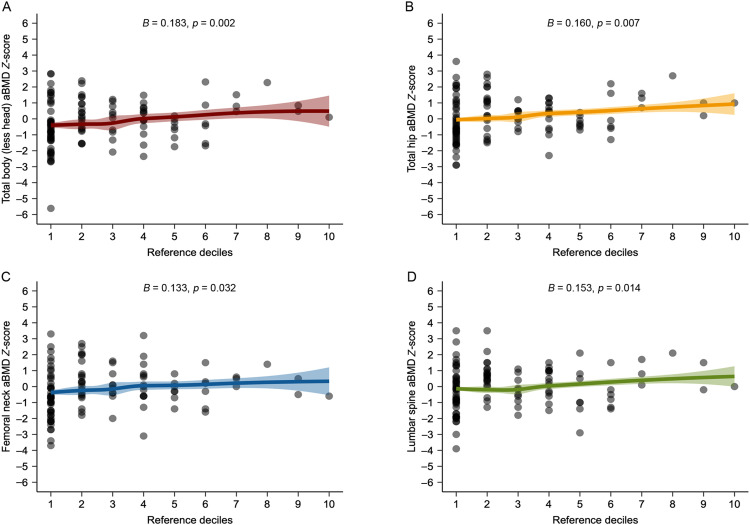
All associations of upper- and lower-body muscle strength with aBMD Z-scores at the total body, total hip, femoral neck, and lumbar spine examined by multivariable linear regression are shown in Fig. 1, Fig. 2. We observed that upper-body muscle strength deficits were associated with lower aBMD Z-scores at total body (B = 0.258, 95%CI: 0.169–0.346, p < 0.001), total hip (B = 0.208, 95%CI: 0.116–0.301, p < 0.001), femoral neck (B = 0.175, 95%CI: 0.076–0.275, p < 0.001), and lumbar spine (B = 0.194, 95%CI: 0.095–0.294, p < 0.001). Concerning lower-body muscle strength deficits, we found significant associations with lower aBMD Z-scores at total body (B = 0.183, 95%CI: 0.068–0.298, p = 0.002), total hip (B = 0.160, 95%CI: 0.045–0.275, p = 0.007), femoral neck (B = 0.133, 95%CI: 0.011–0.254, p = 0.032), and lumbar spine (B = 0.153, 95%CI: 0.031–0.275, p = 0.014). After adjusting for calcium intake (mg) and total physical activity (min/day), results were similar (Supplementary Tables 4 and 5). Likewise, when examining the same analyses for BMC Z-scores, the results were consistent (Supplementary Fig. 3 and Tables 6 and 7).
Fig. 1.
Associations of upper-body muscle strength (reference deciles using FitBack reference values) with age-, sex-, and race-specific areal bone mineral density (aBMD) Z-score at each site. Multivariable linear regression models were adjusted for time from treatment completion (years), radiotherapy exposure (yes/no) and body mass index. Age-, sex-, and race-specific aBMD Z-score at each site is presented using international reference data from the Bone Mineral Density in Childhood Study.27
Fig. 2.
Associations of lower-body muscle strength (Reference deciles using FitBack reference values) with age-, sex-, and race-specific areal bone mineral density (aBMD) Z-score at each site. Multivariable linear regression models were adjusted for time from treatment completion (years), radiotherapy exposure (yes/no) and body mass index. Age-, sex-, and race-specific aBMD Z-score at each site is presented using international reference data from the Bone Mineral Density in Childhood Study.27
3.3. ORs of low aBMD Z-scores at each site
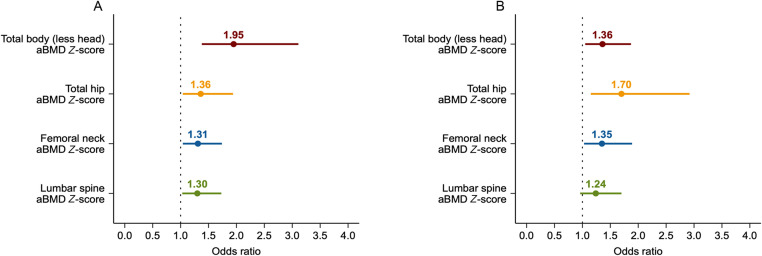
Fig. 3 presents the risk of low aBMD Z-scores associated with 1-decile lower in upper- and lower-body muscle strength. Each 1-decile lower in upper-body muscle strength was associated with higher odds of having aBMD Z-scores less than –1.0 at the total body (OR = 1.95, 95%CI: 1.38–3.11), total hip (OR = 1.36, 95%CI: 1.04–1.95), femoral neck (OR = 1.31, 95%CI: 1.04–1.74), and lumbar spine (OR = 1.30, 95%CI: 1.03–1.73). Regarding lower-body muscle strength, each 1-decile lower was associated with higher odds of having aBMD Z-scores less than –1.0 at the total body (OR = 1.36, 95%CI: 1.05–1.87), total hip (OR = 1.70, 95%CI: 1.15–2.92), and femoral neck (OR = 1.35, 95%CI: 1.03–1.89). These results did not change after controlling for calcium intake (mg) and total physical activity (min/day) (Supplementary Table 8). Similarly, findings were consistent when examining the same analyses for BMC Z-scores (Supplementary Fig. 4 and Table 8).
Fig. 3.
Odds ratios of low age-, sex-, and race-specific areal bone mineral density (aBMD) Z-score at each site per one-decile lower in (A) upper- and (B) lower-body muscle strength (Reference deciles using FitBack reference values). Binary logistic regression (low aBMD identified as Z-score less than ‒1.0, according to according to van Atteveld et al.4 and normal aBMD identified as Z-score higher than ‒1.0) was used to estimate odds ratios with 95% confidence intervals. Adjusted models included time from treatment completion (years), radiotherapy exposure (yes/no) and body mass index. Age-, sex-, and race-specific aBMD Z-score at each site is presented using international reference data from the Bone Mineral Density in Childhood Study.27
4. Discussion
4.1. Main findings
More than one-half of young pediatric cancer survivors enrolled in a clinical trial to improve bone health had upper- and lower-body muscle strength deficits when compared to geographically diverse updated age- and sex-specific reference values.31 Importantly, we found that muscle strength deficits were consistently associated with lower aBMD Z-scores at the total body, total hip, femoral neck, and lumbar spine.27 Each 1-decile lower in muscle strength was associated with 30%–95% higher odds of having low aBMD Z-scores. These results suggest that interventions designed to improve muscle strength in pediatric cancer survivors may potentially improve aBMD.
The literature describing associations of muscle strength with aBMD in young pediatric cancer survivors is scarce. Objectively measured upper- and lower-body muscle strength have been strongly associated with BMC of total body15,16 and of upper13,17 and lower13,17 extremities, as well as with total body and femoral neck aBMD18 in healthy children and adolescents. Our results indicate that these associations could be even stronger in young pediatric cancer survivors (6–18 years), who may never recover from these early deficits. Previous data from Joyce et al.,19 where muscle strength deficits and aBMD were positively correlated among 493 adult survivors of pediatric onset acute lymphoblastic leukemia (33.3 ± 7.1 years), suggest that loss of muscle strength early in life may precipitate further decline in aBMD.
Our findings regarding the associations of upper-body muscle strength with aBMD at multiple sites are consistent with data from reports among healthy children and adolescents. Vicente-Rodríguez et al.15 reported that upper-body muscle strength was consistently the strongest fitness variable to positively correlate with total body BMC in 278 adolescents (13.0–18.5 years); Gracia-Marco et al.13 showed that among 234 non-active adolescents (14.8 ± 1.2 years), those with reduced upper-body muscle strength also had lower BMC at total body and upper extremities; Saint-Maurice et al.16 reported positive associations between upper-body muscle strength and height-adjusted total BMC in 433 children and adolescents (14.1 ± 2.3 years); and Wang et al.17 reported positive correlations between maximal voluntary contraction of the elbow flexors and upper-extremity BMC among 258 pubertal girls (mean age = 11.2; 95%CI: 9.8–12.6 years).
Our findings regarding the associations of lower-body muscle strength with aBMD are not completely consistent with previous findings in healthy young populations since lower-body lean mass seemed to be a better predictor of aBMD than muscle strength.47 This could be because the lower extremities are subject to higher mechanical loadings than the upper extremities, with more opportunity for bone regeneration and formation,48 or because our measure of lower-body strength required not only strength but also balance and coordination. Nevertheless, our lower-body muscle strength and aBMD results are consistent with results in non-cancer populations. Baptista et al.18 evaluated 114 healthy younger children (8.5 ± 0.4 years) and found positive associations between lower-body muscle strength (vertical jump test) and height-adjusted total body and femoral neck aBMD; Gracia-Marco et al.13 evaluated non-active adolescents and found that those with reduced lower-body muscle strength (standing long jump test) presented decreased BMC at total body and lower extremities; and Wang et al.17 evaluated pubertal girls (mean age = 11.2; 95%CI: 9.8–12.6 years) and found that maximal isometric voluntary extension of the left knee was positively correlated with lower-extremity BMC. Altogether, our findings could be explained in terms of the functional muscle bone unit49 based on the mechanostat theory of Frost,20 which predicts that the increasing muscle strength during growth creates the stimulus for bone to increase its mass. Given the high risk of muscle strength deficits and low aBMD Z-scores in young pediatric cancer survivors, these associations tend to be stronger in comparison to studies done in healthy children and adolescents.
4.2. Limitations
Our study results should be considered in the context of some potential limitations. First, the cross-sectional design does not allow us to examine the temporal associations between reduced muscle strength and aBMD. Second, included survivors were those who elected to enroll in an exercise intervention to improve aBMD. They may not be representative of all young pediatric cancer survivors, making our prevalence estimates particularly vulnerable to selection bias. Third, although we adjusted the analyses for some major potential confounders identified through the DAG method (i.e., age, sex, time from treatment completion, radiotherapy exposure, BMI, physical activity, and calcium intake), residual confounding cannot be eliminated. Fourth, given that bone depth is not factored into DXA results, reliance on aBMD may systematically underestimate bone density in shorter individuals. Fifth, although standing long jump has been proven valid and reliable in children, other tests might be more appropriate to assess muscle strength specifically.
4.3. Public health implications
The previous literature documents preliminary lower-body muscle strength deficits and low aBMD Z-scores in young pediatric cancer survivors. However, our study indicates that not only lower- but also upper-body muscle strength deficits are prevalent and associated with low aBMD soon after the completion of treatment, even among survivors without known risk factors for low aBMD. For instance, a 10-year-old girl performing 7.3 kg on the handgrip strength test (within Decile 1, using FitBack reference values) has an aBMD Z-score of –2.2, which is considered low. However, a girl of the same age performing 16.8 kg on the same test (within Decile 6) has an aBMD Z-score of 1.4, which is not considered low. Our data indicate that children and adolescents who present muscle strength deficits should be screened for low aBMD and suggest that interventions to improve muscle strength could also improve aBMD.50 However, a very recent meta-analysis has found that previous interventions aimed at improving muscle strength and/or aBMD were inappropriate (i.e., performed in microgravity environments such as swimming pools,51 short durations of 3 months,51,52 types of exercises not including weight-bearing impact exercises of high intensity53) and, hence, were ineffective at illustrating any beneficial effect in this population.54 These findings warrant further research.
5. Conclusion
This study identified both upper- and lower-body muscle strength deficits and associations of such deficits with lower aBMD in a sample of young pediatric cancer survivors who electively enrolled in an intervention study to improve bone health. Further research in cohort studies is needed to validate these findings so they can be incorporated into surveillance guidelines that will provide a foundation for the development of individualized exercise-oncology plans adapted to the unique needs of each patient.
Acknowledgments
Acknowledgments
We would love to thank the dispose and consideration of all families involved in the investigation. The corresponding author affirms that all authors have contributed significantly to the work. The authors also thank the financial support by the Spanish Ministry of Science and Innovation (Ref: PID2020-117302RA-I00), La Caixa Foundation (Ref: LCF/BQ/PR19/11700007), the University of Granada Plan Propio de Investigación 2021-Excellence actions: Unit of Excellence on Exercise, Nutrition, and Health (UCEENS) and by CIBEROBN, Centro de Investigación Biomédica en Red (CB22/3/00058), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea - European Regional Development Fund. AMP was also recipient of a predoctoral fellowship (FPU20/05530) by the Spanish Ministry of Education, Culture and Sport, and EUG was supported by the Maria Zambrano fellowship by the Ministerio de Universidades y la Unión Europea-NextGenerationEU.
Authors’ contributions
AMP had full access to all the data and took responsibility for the integrity and accuracy of the data analysis and wrote the manuscript drafts under the supervision of JJGC and LGM; JJGC, EUG, FJLC, JFPG, KKN, VMV, JRR, and LGM reviewed and edited the final manuscript; JJGC, VMV, JRR, and LGM also contributed helping with the data curation, statistical analysis, visualization, and interpretation of the findings. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2024.01.003.
Supplementary materials
References
- 1.Miller KD, Fidler-Benaoudia M, Keegan TH, Hipp HS, Jemal A, Siegel RL. Cancer statistics for adolescents and young adults, 2020. CA Cancer J Clin. 2020;70:443–459. doi: 10.3322/caac.21637. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 3.Marcucci G, Beltrami G, Tamburini A, et al. Bone health in childhood cancer: Review of the literature and recommendations for the management of bone health in childhood cancer survivors. Ann Oncol. 2019;30:908–920. doi: 10.1093/annonc/mdz120. [DOI] [PubMed] [Google Scholar]
- 4.van Atteveld JE, Pluijm MF, Ness KK, et al. Prediction of low and very low bone mineral density among adult survivors of childhood cancer. J Clin Oncol. 2019;37:2217–2225. doi: 10.1200/JCO.18.01917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzoli R, Bianchi ML, Garabédian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Chemaitilly W, Cohen LE, Mostoufi-Moab S, et al. Endocrine late effects in childhood cancer survivors. J Clin Oncol. 2018;36:2153–2159. doi: 10.1200/JCO.2017.76.3268. [DOI] [PubMed] [Google Scholar]
- 7.van Santen HM, Chemaitilly W, Meacham LR, Tonorezos ES, Mostoufi-Moab S. Endocrine health in childhood cancer survivors. Pediatr Clin North Am. 2020;67:1171–1186. doi: 10.1016/j.pcl.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Kelly PM, Pottenger E. Bone health issues in the pediatric oncology patient. Semin Oncol Nurs. 2022;38:151275. doi: 10.1016/j.soncn.2022.151275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rayar M, Webber CE, Nayiager T, Sala A, Barr RD. Sarcopenia in children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2013;35:98–102. doi: 10.1097/MPH.0b013e318279eea2. [DOI] [PubMed] [Google Scholar]
- 10.Goodenough CG, Partin RE, Ness KK. Skeletal muscle and childhood cancer: Where are we now and where we go from here. Aging Cancer. 2021;2:13–35. doi: 10.1002/aac2.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman MC, Mulrooney DA, Steinberger J, Lee J, Baker KS, Ness KK. Deficits in physical function among young childhood cancer survivors. J Clin Oncol. 2013;31:2799–2805. doi: 10.1200/JCO.2012.47.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortega FB, Ruiz JR, Castillo MJ, Sjöström M. Physical fitness in childhood and adolescence: A powerful marker of health. Int J Obes (Lond) 2008;32:1–11. doi: 10.1038/sj.ijo.0803774. [DOI] [PubMed] [Google Scholar]
- 13.Gracia-Marco L, Vicente-Rodríguez G, Casajús JA, Molnar D, Castillo MJ, Moreno LA. Effect of fitness and physical activity on bone mass in adolescents: The HELENA Study. Eur J Appl Physiol. 2011;111:2671–2680. doi: 10.1007/s00421-011-1897-0. [DOI] [PubMed] [Google Scholar]
- 14.García-Hermoso A, Ramírez-Campillo R, Izquierdo M. Is muscular fitness associated with future health benefits in children and adolescents? A systematic review and meta-analysis of longitudinal studies. Sports Med. 2019;49:1079–1094. doi: 10.1007/s40279-019-01098-6. [DOI] [PubMed] [Google Scholar]
- 15.Vicente-Rodríguez G, Urzanqui A, Mesana MI, et al. Physical fitness effect on bone mass is mediated by the independent association between lean mass and bone mass through adolescence: A cross-sectional study. J Bone Miner Metab. 2008;26:288–294. doi: 10.1007/s00774-007-0818-0. [DOI] [PubMed] [Google Scholar]
- 16.Saint-Maurice PF, Laurson K, Welk GJ, et al. Grip strength cutpoints for youth based on a clinically relevant bone health outcome. Arch Osteoporos. 2018;13:92. doi: 10.1007/s11657-018-0502-0. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Alén M, Nicholson P, et al. Weight-bearing, muscle loading and bone mineral accrual in pubertal girls—A 2-year longitudinal study. Bone. 2007;40:1196–1202. doi: 10.1016/j.bone.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 18.Baptista F, Mil-Homens P, Carita AI, Janz K, Sardinha LB. Peak vertical jump power as a marker of bone health in children. Int J Sports Med. 2016;37:653–658. doi: 10.1055/s-0042-105290. [DOI] [PubMed] [Google Scholar]
- 19.Joyce ED, Nolan VG, Ness KK, et al. Association of muscle strength and bone mineral density in adult survivors of childhood acute lymphoblastic leukemia. Arch Phys Med Rehabil. 2011;92:873–879. doi: 10.1016/j.apmr.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frost HM. Bone “mass” and the “mechanostat”: A proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 21.van Atteveld JE, Mulder RL, van den Heuvel-Eibrink MM, et al. Bone mineral density surveillance for childhood, adolescent, and young adult cancer survivors: Evidence-based recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Diabetes Endocrinol. 2021;9:622–637. doi: 10.1016/S2213-8587(21)00173-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Atteveld JE, de Winter DTC, Pluimakers VG, et al. Risk and determinants of low and very low bone mineral density and fractures in a national cohort of Dutch adult childhood cancer survivors (DCCSS-LATER): A cross-sectional study. Lancet Diabetes Endocrinol. 2023;11:21–32. doi: 10.1016/S2213-8587(22)00286-8. [DOI] [PubMed] [Google Scholar]
- 23.Gil-Cosano JJ, Ubago-Guisado E, Sánchez MJ, et al. The effect of an online exercise programme on bone health in paediatric cancer survivors (iBoneFIT): Study protocol of a multi-centre randomized controlled trial. BMC Public Health. 2020;20:1520. doi: 10.1186/s12889-020-09607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007;147:W163–W194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 25.Shuhart CR, Yeap SS, Anderson PA, et al. Executive summary of the 2019 ISCD Position Development Conference on monitoring treatment, DXA cross-calibration and least significant change, spinal cord injury, peri-prosthetic and orthopedic bone health, transgender medicine, and pediatrics. J Clin Densitom. 2019;22:453–471. doi: 10.1016/j.jocd.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Johnson J, Dawson-Hughes B. Precision and stability of dual-energy X-ray absorptiometry measurements. Calcif Tissue Int. 1991;49:174–178. doi: 10.1007/BF02556113. [DOI] [PubMed] [Google Scholar]
- 27.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: Results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96:3160–3169. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gąsior JS, Pawłowski M, Jeleń PJ, et al. Test–retest reliability of handgrip strength measurement in children and preadolescents. Int J Environ Res Public Health. 2020;17:8026. doi: 10.3390/ijerph17218026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Beld WA, van der Sanden GAC, Sengers RCA, Verbeek ALM, Gabreëls FJM. Validity and reproducibility of hand-held dynamometry in children aged 4‒11 years. J Rehabil Med. 2006;38:57–64. doi: 10.1080/16501970510044043. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Santos JR, Ruiz JR, Cohen DD, Gonzalez-Montesinos JL, Castro-Piñero J. Reliability and validity of tests to assess lower-body muscular power in children. J Strength Cond Res. 2015;29:2277–2285. doi: 10.1519/JSC.0000000000000864. [DOI] [PubMed] [Google Scholar]
- 31.Ortega FB, Leskošek B, Blagus R, et al. European fitness landscape for children and adolescents: Updated reference values, fitness maps and country rankings based on nearly 8 million test results from 34 countries gathered by the FitBack network. Br J Sports Med. 2023;57:299–310. doi: 10.1136/bjsports-2022-106176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomkinson GR, Lang JJ, Tremblay MS, et al. International normative 20 m shuttle run values from 1,142,026 children and youth representing 50 countries. Br J Sports Med. 2017;51:1545–1554. doi: 10.1136/bjsports-2016-095987. [DOI] [PubMed] [Google Scholar]
- 33.Ortega FB, Lavie CJ, Blair SN. Obesity and cardiovascular disease. Circ Res. 2016;118:1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
- 34.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7:284–294. doi: 10.1111/j.2047-6310.2012.00064.x. [DOI] [PubMed] [Google Scholar]
- 35.Moore SA, McKay HA, Macdonald H, et al. Enhancing a somatic maturity prediction model. Med Sci Sports Exerc. 2015;47:1755–1764. doi: 10.1249/MSS.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 36.Julián Almárcegui C, Huybrechts I, Gómez Bruton A, et al. Validity of a food-frequency questionnaire for estimating calcium intake in adolescent swimmers. Nutr Hosp. 2015;32:1773–1779. doi: 10.3305/nh.2015.32.4.9490. [DOI] [PubMed] [Google Scholar]
- 37.Migueles JH, Rowlands AV, Huber F, Sabia S, Van Hees VT. GGIR: A research community-driven open source R package for generating physical activity and sleep outcomes from multi-day raw accelerometer data. J Meas Phys Behav. 2019;2:188–196. [Google Scholar]
- 38.van Hees VT, Sabia S, Anderson KN, et al. A novel, open access method to assess sleep duration using a wrist-worn accelerometer. PLoS One. 2015;10:e0142533. doi: 10.1371/journal.pone.0142533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Hees VT, Renström F, Wright A, et al. Estimation of daily energy expenditure in pregnant and non-pregnant women using a wrist-worn tri-axial accelerometer. PLoS One. 2011;6:e22922. doi: 10.1371/journal.pone.0022922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hildebrand M, Hansen BH, van Hees VT, Ekelund U. Evaluation of raw acceleration sedentary thresholds in children and adults. Scand J Med Sci Sports. 2017;27:1814–1823. doi: 10.1111/sms.12795. [DOI] [PubMed] [Google Scholar]
- 41.Rowlands AV, Mirkes EM, Yates T, Clemes S, et al. Accelerometer-assessed physical activity in epidemiology: Are monitors equivalent? Med Sci Sports Exerc. 2018;50:257–265. doi: 10.1249/MSS.0000000000001435. [DOI] [PubMed] [Google Scholar]
- 42.Marmol-Perez A, Ubago-Guisado E, Llorente-Cantarero FJ, et al. Determinants of bone parameters in young paediatric cancer survivors: The iBoneFIT project. Pediatr Res. 2023;4:1538–1546. doi: 10.1038/s41390-023-02645-8. [DOI] [PubMed] [Google Scholar]
- 43.Ness KK, DeLany JP, Kaste SC, et al. Energy balance and fitness in adult survivors of childhood acute lymphoblastic leukemia. Blood. 2015;125:3411–3419. doi: 10.1182/blood-2015-01-621680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heidemann M, Mølgaard C, Husby S, et al. The intensity of physical activity influences bone mineral accrual in childhood: The Childhood Health, Activity and Motor Performance School (the CHAMPS) study, Denmark. BMC Pediatr. 2013;13:32. doi: 10.1186/1471-2431-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Textor J, van der Zander B, Gilthorpe MS, Liśkiewicz M, Ellison GT. Robust causal inference using directed acyclic graphs: The R package “dagitty”. Int J Epidemiol. 2016;45:1887–1894. doi: 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- 46.Tennant PWG, Murray EJ, Arnold KF, et al. Use of directed acyclic graphs (DAGs) to identify confounders in applied health research: Review and recommendations. Int J Epidemiol. 2021;50:620–632. doi: 10.1093/ije/dyaa213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattila VM, Tallroth K, Marttinen M, Pihlajamäki H. Physical fitness and performance. Body composition by DEXA and its association with physical fitness in 140 conscripts. Med Sci Sports Exerc. 2007;39:2242–2247. doi: 10.1249/mss.0b013e318155a813. [DOI] [PubMed] [Google Scholar]
- 48.Hart NH, Nimphius S, Rantalainen T, Ireland A, Siafarikas A, Newton RU. Mechanical basis of bone strength: Influence of bone material, bone structure and muscle action. J Musculoskelet Neuronal Interact. 2017;17:114–139. [PMC free article] [PubMed] [Google Scholar]
- 49.Schoenau E. From mechanostat theory to development of the “Functional Muscle–Bone-Unit”. J Musculoskelet Neuronal Interact. 2005;5:232–238. [PubMed] [Google Scholar]
- 50.Braam KI, Van Dijk-Lokkart EM, Kaspers GJL, et al. Effects of a combined physical and psychosocial training for children with cancer: A randomized controlled trial. BMC Cancer. 2018;18:1289. doi: 10.1186/s12885-018-5181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elnaggar RK, Mohamed RR. Aqua-plyometric exercises: Potential implications for bone mineral density, functional capacity, and quality of life in survivors of childhood acute lymphoblastic leukemia. Semin Oncol Nurs. 2021;37 doi: 10.1016/j.soncn.2021.151225. [DOI] [PubMed] [Google Scholar]
- 52.Braam KI, van Dijk-Lokkart EM, Kaspers GJL, et al. Effects of a combined physical and psychosocial training for children with cancer: A randomized controlled trial. BMC Cancer. 2018;18:1289. doi: 10.1186/s12885-018-5181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dubnov-Raz G, Azar M, Reuveny R, Katz U, Weintraub M, Constantini NW. Changes in fitness are associated with changes in body composition and bone health in children after cancer. Acta Paediatr. 2015;104:1055–1061. doi: 10.1111/apa.13052. [DOI] [PubMed] [Google Scholar]
- 54.Marmol-Perez A, Ubago-Guisado E, Rodriguez-Solana A, et al. Effect of exercise on bone health in children and adolescents with cancer during and after oncological treatment: A systematic review and meta-analysis. Front Physiol. 2023;14 doi: 10.3389/fphys.2023.1088740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.