Abstract
The zebrafish lateral line mechanosensory system shares considerable morphological and molecular similarities with the inner ear. In particular, mechanosensory hair cells are responsible for transducing sensory stimuli in both structures. The epithelia cell adhesion molecule (EpCAM) is expressed in the cells of the inner ear of mammals and in the lateral lines system of fish. EpCAM regulates the many cellular functions including adhesion, migration, proliferation, and differentiation. In this study, we use the epcam jh79 mutant zebrafish line to determine that EpCAM function is required for proper development and regeneration of posterior lateral line hair cells.
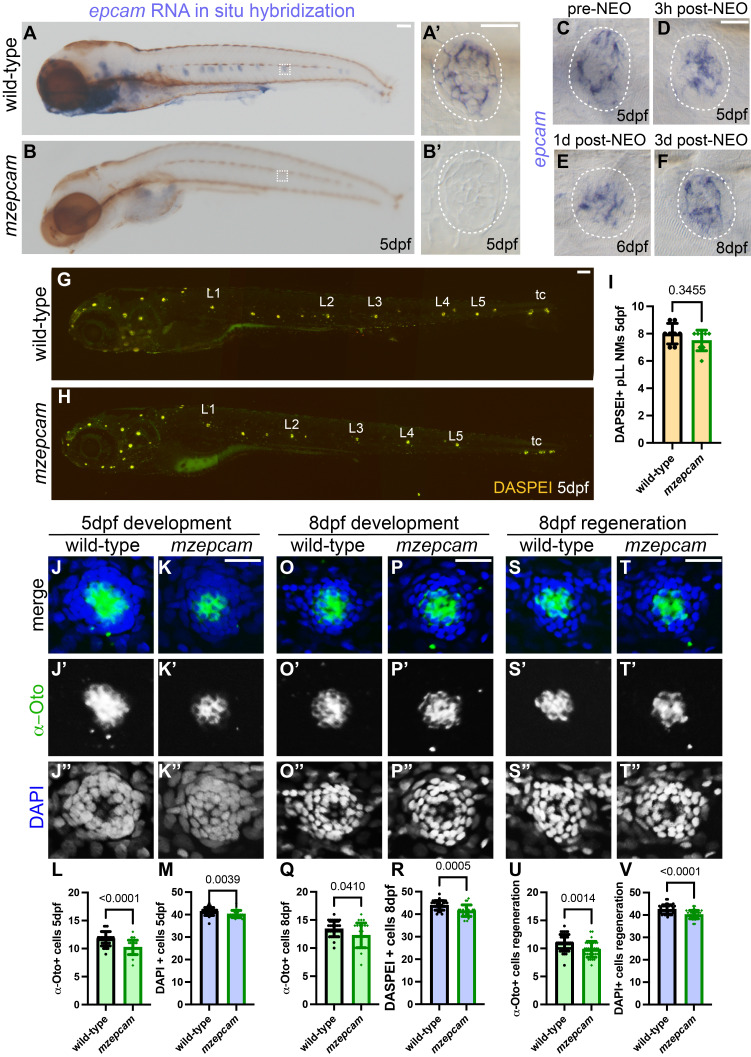
Figure 1.
EpCAM regulates posterior lateral line neuromast development and regeneration.
(A-B) Whole mount images of 5pdf wild-type and mzepcam mutant zebrafish larvae showing expression of epcam mRNA by in situ hybridization. Scale bar=100μm. Dashed squares show area of individual neuromasts in (A’-B’). Scale bar=20μm.
(C-F) RNA in situ hybridization of epcam at 5dpf pre-NEO exposure, 3-hours post-NEO exposure, 1-day post-NEO exposure at 6dpf, and 3-days post-NEO exposure at 8dpf. Scale bar=20μm.
(G-H) Live confocal projections of 5pdf wild-type and mzepcam mutant larvae showing hair cells labeled with DASPEI in the primary posterior lateral line neuromasts (L1-L5) and terminal cluster (tc) neuromasts. Scale bar=100μm.
(I) Quantification of DASPEI-labeled posterior lateral line neuromasts in 5pdf wild-type and mzepcam mutant zebrafish. n=8 larvae per condition. Data presented as ± S.D. Mann-Whitney test, significance at p<0.05.
(J-K’’) Confocal projections of neuromasts in 5pdf wild-type and mzepcam mutant larvae with hair cells labeled with a-Oto antibody and nuclei labeled with DAPI. Scale bar=20μm.
(L-M) Quantification of α-Oto-labeled hair cells and DAPI total cells in neuromasts of 5pdf wild-type and mzepcam mutant larvae. n=29 neuromasts in 9 wild-type and n=27 neuromasts in 10 mzepcam mutant larvae. Data presented as ± S.D. Mann-Whitney test. Data presented as ± S.D. Mann-Whitney test, significance at p<0.05.
(O-P’’) Confocal projections of neuromasts in 8pdf wild-type and mzepcam mutant larvae with hair cells labeled with α-Oto antibody and nuclei labeled with DAPI. Scale bar=20μm.
(Q-R) Quantification of α-Oto-labeled hair cells and DAPI total cells in neuromasts of 8pdf wild-type and mzepcam mutant larvae. n=32 neuromasts in 10 wild-type and n=27 neuromasts in 10 mzepcam mutant larvae. Data presented as ± S.D. Mann-Whitney test. Data presented as ± S.D. Mann-Whitney test, significance at p<0.05.
(S-T’’) Confocal projections of neuromasts in 8pdf following hair cell ablation with NEO at 5dpf in wild-type and mzepcam mutant larvae with hair cells labeled with α-Oto antibody and nuclei labeled with DAPI. Scale bar=20μm.
(U-V) Quantification of regenerated α-Oto-labeled hair cells and DAPI total cells in neuromasts of 8pdf wild-type and mzepcam mutant larvae. n=33 neuromasts in 11 wild-type and n=32 neuromasts in 11 mzepcam mutant larvae. Data presented as ± S.D. Mann-Whitney test. Data presented as ± S.D. Mann-Whitney test, significance at p<0.05.
Abbreviations: days post fertilization (dpf), neomycin (NEO), standard deviation (S.D.), α-Otoferlin antibody (α-Oto).
Description
The sensations of hearing, balance, and in the case of aquatic vertebrates, water current are transduced by specialized mechanosensory hair cells (Pickett & Raible, 2019) . In mammals, damage to hair cells results in irreversible sensory loss (White, 2020) . In contrast, non-mammalian vertebrates are able to regenerate damaged hair cells (Denans, Baek, & Piotrowski, 2019; Kniss, Jiang, & Piotrowski, 2016; Thomas, Cruz, Hailey, & Raible, 2015) . In particular, the lateral line mechanosensory system of the zebrafish, Danio rerio , has immerged as an excellent model to study the biology of hair cell development and regeneration (Kniss et al., 2016; Thomas et al., 2015) . The lateral line is composed of sensory organs, called neuromasts, which are arrayed along the surface of the body. Within these neuromasts are mechanosensory hair cells and surrounding support cells, which act as stem cells to replace hair cells following damage (Kniss et al., 2016) . Understanding the molecular mechanisms that regulate hair cell regeneration in the zebrafish lateral line could provide clues for potential therapeutic regrowth of hair cells in the human inner ear. In this study, we examine the role of the epithelial cell adhesion molecule (EpCAM) in lateral line development and regeneration. EpCAM is a membrane glycoprotein, which regulates many cellular functions including adhesion, proliferation, stem cell maintenance and differentiation, migration, and invasion of cancer cells (Fagotto, 2020; Fagotto & Aslemarz, 2020; Gires, Pan, Schinke, Canis, & Baeuerle, 2020; Liu et al., 2022) . EpCAM is expressed in the mammalian inner ear and may play a role in specifying hair cell progenitors (Roccio et al., 2018) . In this study, we examine the function of EpCAM in the development and regeneration of zebrafish posterior lateral line neuromast cells.
To investigate the role of EpCAM in the development of posterior lateral line neuromasts, we used homozygous maternal zygotic epcam jh79 ( mzepcam ) (Edelman et al., 2021) mutant zebrafish larvae and compared them to AB wild-type control larvae. We examined epcam expression using RNA in situ hybridization at 5 days post fertilization (dpf), when the lateral line has reached initial functional maturity (Thomas et al., 2015) , and found expression in the neuromasts of the posterior lateral line of wild-type larvae ( Fig. 1A,A ’), but no expression in mzepcam larvae ( Fig. 1B,B ’). This finding agrees with previous reports that the mzepcam mutants show nonsense mediated mRNA decay (Edelman et al., 2021) . As epcam expression is present under homeostatic conditions in lateral line neuromasts, we next asked if expression was altered following hair cell ablation and during regeneration. We used exposure to the aminoglycoside antibiotic neomycin (NEO) (Harris et al., 2003; Owens et al., 2008) to destroy lateral line hair cells in wild-type larvae and assessed epcam expression in neuromasts at 5dpf pre-NEO exposure ( Fig. 1C ), 3-hours post-NEO exposure ( Fig. 1D ), 1-day post-NEO exposure ( Fig. 1E ), and 3-days post-NEO exposure ( Fig. 1F ) when regeneration is functionally complete. We found that epcam mRNA expression is maintained in neuromasts during the course of hair cell regeneration.
Previous research on the function of EpCAM in the zebrafish lateral line suggests that knocking down function with antisense morpholino oligonucleotides (MO) against epcam results in delayed and disrupted formation (Neelathi, Dalle Nogare, & Chitnis, 2018; Villablanca et al., 2006) . To determine if EpCAM is required for formation of posterior lateral line, we compared live wild-type ( Fig. 1G ) and mzepcam (Fig.1H) larvae at 5dpf in which hair cells have been labeled with the vital dye DASPEI (Harris et al., 2003) and found that primary neuromast numbers are not significantly different between control and mutant larvae ( Fig. 1I ). These results suggest that EpCAM function is not required for neuromast number in the posterior lateral line.
We next asked if EpCAM is required for hair cell development in primary posterior lateral line neuromasts. We used an α-Otoferlin antibody (α-Oto) to specifically label hair cells and DAPI to label nuclei in the posterior lateral line neuromasts of 5dpf wild-type ( Fig. 1J -J’’) and mzepcam (Fig.1K-K’’) larvae and found a significant reduction in hair cells ( Fig. 1L ) and total DAPI+ cells ( Fig. 1M ) in mutant larvae. To determine if the decrease in cell numbers is due to delay, we also examined neuromasts at 8dpf and once again found the mzepcam mutant larvae have fewer hair cells and total cells in their neuromasts ( Fig. 1P -P’’, Q,R) as compared to wild-type controls ( Fig. 1O -O’’, Q,R). These data suggest that EpCAM function is required for the proper development of the full complement of hair cells and total cells in posterior lateral line neuromasts.
Finally, we asked if there is a change in the ability of hair cells to regenerate following damage in the absence of EpCAM function. We ablated hair cells at 5dpf using exposure to NEO in wild-type and mzepcam mutant larvae and then allowed them to regenerate for 72-hours and examined α-Oto-labeled hair cells and DAPI-labeled nuclei at 8dpf. We found that hair cells regenerated in our wild-type ( Fig. 1S -S’’) and mzepcam mutant neuromasts, but overall, there were fewer hair cells and total cells in mutant larva as compared to controls ( Fig. 1U,V ). Together, these results suggest that EpCAM function regulates the development and regeneration of posterior lateral line neuromast cells.
In conclusion, this work shows that epcam is expressed in the developing and regenerating posterior lateral line neuromasts of zebrafish larvae. In the absence of EpCAM function, the full complement of neuromasts form by 5dpf, suggesting that EpCAM may be dispensable for this process. When we specifically examined hair development and regeneration in neuromasts, we find the loss of EpCAM function results in reduced cell numbers. These results suggest that EpCAM plays a role in regulating the formation and regrowth of cells within posterior lateral line neuromasts. Future work is needed to determine the mechanism of EpCAM activity. In other tissues and model systems, EpCAM interacts with canonical Wnt signaling pathway (Gires et al., 2020) . Previous work has shown that Wnt signaling regulates the development and regeneration of the posterior lateral line in zebrafish (Megerson et al., 2024; Romero-Carvajal et al., 2015) and might interact with EpCAM in neuromasts. EpCAM regulates the development of the ear in mammals and in fish (Edelman et al., 2021; Han et al., 2011; Roccio et al., 2018; Ueda et al., 2023) ; moreover, future work is required to determine if it might be a target for clinical intervention in hearing loss.
Methods
Zebrafish lines and maintenance
The following zebrafish lines were used: wild-type*AB (ZIRC;http://zebrafish.org) and epcam jh79 (Edelman et al., 2021) . Adult zebrafish were maintained in a facility with 14 hour-light/10 hour-dark and water temperature at 28°C. Larvae were kept at 28°C and staged according to standard protocols (Kimmel, Ballard, Kimmel, Ullmann, & Schilling, 1995) . This study used larvae at stages between 5-8dpf. epcam jh79 fish were maintained as homozygous mutant adults and progeny used for experiments were maternal zygotic homozygous mutants ( mzepcam ). Experimental larvae were kept in E3 embryo medium (14.97 mM NaCl, 500 μM KCl, 42 μM Na 2 HPO 4 , 150 μM KH 2 PO 4 , 1 mM CaCl 2 dihydrate, 1 mM MgSO 4 , 0.714 mM NaHCO 3 , pH 7.2). For all experiments, larvae were treated with tricaine (Syndel) prior to fixation in 4% paraformaldehyde/PBS (Thermo Fisher). All work was performed in accordance with the McGraw laboratory protocol #45344 approved by the UMKC IACUC committee.
RNA in situ hybridization
RNA in situ hybridization was carried out using established protocols (Thisse & Thisse, 2008) , modified with a 5-minute Proteinase K (Thermo Fisher) treatment to preserve neuromast integrity. Following coloration, the larvae were cleared in methanol and stored in 50% glycerol/PBS for imaging . Antisense probe for epcam was generated using a PCR-based protocol (Logel, Dill, & Leonard, 1992) in which the cDNA of interest is amplified by PCR with a reverse primer containing a T7 RNA binding sequence (CCAAGCTTCTAATACGACTCACTATAGGGAGA). Following PCR amplification of probe template, antisense RNA probes were generated with T7 RNA polymerase and digoxygenin RNA labeling mix.
Immunohistochemistry and DASPEI labeling.
Whole mount immunolabeling was performed using established protocols (Ungos, Karlstrom, & Raible, 2003) . The α-Otoferlin primary antibody (α-Oto; mouse monoclonal, 1:200, DSHB, University of Iowa) was diluted in blocking solution and larvae were incubated overnight at 4°C, then washed in 1x PBS-0.1% Triton-X 100 and incubated overnight in goat anti-mouse Alexa-647 (1:1000, Invitrogen) secondary antibody diluted to 1:1000. Larvae were stored in 50% glycerol/PBS for imaging. For live imaging of neuromasts, 5dpf larvae were incubated in 0.005% (2-(4-(Dimethylamino)styryl)-N-ethylpyridinium iodide) (DASPEI) in E3 embryo medium for 15 minutes and then rinsed in clean E3 (Harris et al., 2003) . Larvae were anesthetized in tricaine for imaging.
Neomycin exposure and regeneration
For hair cell ablation, 5 dpf larvae were incubated in 400 μM neomycin (NEO, Millipore-Sigma) in E3 embryo medium for 0.5 hours and then washed 3x in fresh E3 embryo medium (Harris et al., 2003) . In experiments analyzing complete regeneration, larvae were collected 3 days following NEO exposure. For RNA in situ hybridization, larvae were collected 3-hours, 1-day, or 3-days post NEO exposure.
Image collection
For imaging of RNA in situ hybridization and immunohistochemistry, processed larvae were placed in 50% glycerol/PBS and mounted on slides. Images were collected using a Zeiss Imager.D2 compound microscope with an Axiocan 506 color camera and Zen software. Fluorescent images were collected using a Zeiss 510 meta confocal microscope using Zen 2009 software. Images were processed using Fiji software (Schindelin et al., 2012) , and brightness and contrast were adjusted using Photoshop (Adobe). Neuromasts L2-L4 were analyzed for each fish and cells were manually counted under blinded conditions.
Quantification and statistical analysis
All statistical analysis was carried out using Graphpad Prism 10 (GraphPad Prism version 10.0.0 for Mac, GraphPad Software, San Diego, California USA, www.graphpad.com). A Mann-Whitney nonparametric test was used for all pair-wise comparisons. Significance was set at p<0.05. All data is presented as ± standard deviation (S.D.).
Reagents
|
REAGENT or RESOURCE |
SOURCE |
IDENTIFIER |
|
Antibodies | ||
|
Mouse monoclonal anti-Otoferlin antibody (HCS-1) |
DSHB |
Cat.#HCS-1; RRID:AB_10804296 |
|
Alexa fluor-674 goat anti-mouse antibody |
Thermo Fisher |
Cat.# A-21236; RRID:AB_2535805 |
|
Anti-Digoxigenin-AP, Fab Fragments |
Millipore Sigma |
Cat.# 11093274910; RRID:AB_2734716 |
|
Chemicals, peptides, and recombinant proteins | ||
|
(2-(4-(Dimethylamino)styryl)-N-ethylpyridinium iodide) (DASPEI) |
BIOTIUM |
70018 |
|
DAPI |
Thermo Fisher |
D1306 |
|
Neomycin sulphate (NEO) |
Millipore Sigma |
N0400000 |
|
Digoxygenin RNA labeling mix |
Millipore Sigma |
11277073901 |
|
Experimental models: Organisms/strains | ||
|
Zebrafish: Wildtype *AB |
ZIRC http://zebrafish.org |
ZFIN:ZDB-GENO-960809-7 |
|
Zebrafish: epcam jh79 |
ZFIN:ZDB-ALT220225-4 | |
|
Oligonucleotides | ||
|
epcam in situ hybridization probe template primers Forward:5’GGTTCCCAAGTGCTTCCTCA3’ Reverse:5’CCAAGCTTCTAATACGACTCACTATAGGGAGACAGCACAACTGCCCCAATTT3’ |
IDT |
ZFIN:ZDB-GENE-040426-2209 |
|
Software and algorithms | ||
|
FIJI |
https://imagej.nih.gov/ij/ |
|
|
PRISM |
GraphPad |
www.graphpad.co |
Acknowledgments
Acknowledgments
The authors thank Dr. Michael J Parsons for the epcam jh79 zebrafish line. The authors also thank zfin.org for zebrafish database curation.
Funding Statement
<p>This work was supported by funding provided to HFM by NIGMS (R16GM146690; <a href="https://www.nigms.nih.gov/">https://www.nigms.nih.gov/</a>).</p>
References
- Denans, N., Baek, S., & Piotrowski, T. (2019). Comparing Sensory Organs to Define the Path for Hair Cell Regeneration. Annu Rev Cell Dev Biol, 35 , 567-589. doi:10.1146/annurev-cellbio-100818-125503 [DOI] [PubMed]
- Edelman, H. E., McClymont, S. A., Tucker, T. R., Pineda, S., Beer, R. L., McCallion, A. S., & Parsons, M. J. (2021). SOX9 modulates cancer biomarker and cilia genes in pancreatic cancer. Hum Mol Genet, 30 (6), 485-499. doi:10.1093/hmg/ddab064 [DOI] [PMC free article] [PubMed]
- Fagotto, F. (2020). EpCAM as Modulator of Tissue Plasticity. Cells, 9 (9). doi:10.3390/cells9092128 [DOI] [PMC free article] [PubMed]
- Fagotto, F., & Aslemarz, A. (2020). EpCAM cellular functions in adhesion and migration, and potential impact on invasion: A critical review. Biochim Biophys Acta Rev Cancer, 1874 (2), 188436. doi:10.1016/j.bbcan.2020.188436 [DOI] [PubMed]
- Gires, O., Pan, M., Schinke, H., Canis, M., & Baeuerle, P. A. (2020). Expression and function of epithelial cell adhesion molecule EpCAM: where are we after 40 years? Cancer Metastasis Rev, 39 (3), 969-987. doi:10.1007/s10555-020-09898-3 [DOI] [PMC free article] [PubMed]
- Han, Y., Mu, Y., Li, X., Xu, P., Tong, J., Liu, Z., . . . Meng, A. (2011). Grhl2 deficiency impairs otic development and hearing ability in a zebrafish model of the progressive dominant hearing loss DFNA28. Hum Mol Genet, 20 (16), 3213-3226. doi:10.1093/hmg/ddr234 [DOI] [PubMed]
- Harris, J. A., Cheng, A. G., Cunningham, L. L., MacDonald, G., Raible, D. W., & Rubel, E. W. (2003). Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J Assoc Res Otolaryngol, 4 (2), 219-234. doi:10.1007/s10162-002-3022-x [DOI] [PMC free article] [PubMed]
- Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B., & Schilling, T. F. (1995). Stages of embryonic development of the zebrafish. Dev Dyn, 203 (3), 253-310. doi:10.1002/aja.1002030302 [DOI] [PubMed]
- Kniss, J. S., Jiang, L., & Piotrowski, T. (2016). Insights into sensory hair cell regeneration from the zebrafish lateral line. Curr Opin Genet Dev, 40 , 32-40. doi:10.1016/j.gde.2016.05.012 [DOI] [PubMed]
- Liu, Y., Wang, Y., Sun, S., Chen, Z., Xiang, S., Ding, Z., . . . Zhang, B. (2022). Understanding the versatile roles and applications of EpCAM in cancers: from bench to bedside. Exp Hematol Oncol, 11 (1), 97. doi:10.1186/s40164-022-00352-4 [DOI] [PMC free article] [PubMed]
- Logel, J., Dill, D., & Leonard, S. (1992). Synthesis of cRNA probes from PCR-generated DNA. Biotechniques, 13 (4), 604-610. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1476730 [PubMed]
- Megerson, E., Kuehn, M., Leifer, B., Bell, J. M., Snyder, J. L., & McGraw, H. F. (2024). Kremen1 regulates the regenerative capacity of support cells and mechanosensory hair cells in the zebrafish lateral line. iScience, 27 (1), 108678. doi:10.1016/j.isci.2023.108678 [DOI] [PMC free article] [PubMed]
- Neelathi, U. M., Dalle Nogare, D., & Chitnis, A. B. (2018). Cxcl12a induces snail1b expression to initiate collective migration and sequential Fgf-dependent neuromast formation in the zebrafish posterior lateral line primordium. Development, 145 (14). doi:10.1242/dev.162453 [DOI] [PMC free article] [PubMed]
- Owens, K. N., Santos, F., Roberts, B., Linbo, T., Coffin, A. B., Knisely, A. J., . . . Raible, D. W. (2008). Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet, 4 (2), e1000020. doi:10.1371/journal.pgen.1000020 [DOI] [PMC free article] [PubMed]
- Pickett, S. B., & Raible, D. W. (2019). Water Waves to Sound Waves: Using Zebrafish to Explore Hair Cell Biology. J Assoc Res Otolaryngol, 20 (1), 1-19. doi:10.1007/s10162-018-00711-1 [DOI] [PMC free article] [PubMed]
- Roccio, M., Perny, M., Ealy, M., Widmer, H. R., Heller, S., & Senn, P. (2018). Molecular characterization and prospective isolation of human fetal cochlear hair cell progenitors. Nat Commun, 9 (1), 4027. doi:10.1038/s41467-018-06334-7 [DOI] [PMC free article] [PubMed]
- Romero-Carvajal, A., Navajas Acedo, J., Jiang, L., Kozlovskaja-Gumbrienė, A., Alexander, R., Li, H., & Piotrowski, T. (2015). Regeneration of Sensory Hair Cells Requires Localized Interactions between the Notch and Wnt Pathways. Dev Cell, 34 (3), 267-282. doi:10.1016/j.devcel.2015.05.025 [DOI] [PMC free article] [PubMed]
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., . . . Cardona, A. (2012). Fiji: an open-source platform for biological-image analysis. Nat Methods, 9 (7), 676-682. doi:10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed]
- Thisse, C., & Thisse, B. (2008). High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc, 3 (1), 59-69. doi:10.1038/nprot.2007.514 [DOI] [PubMed]
- Thomas, E. D., Cruz, I. A., Hailey, D. W., & Raible, D. W. (2015). There and back again: development and regeneration of the zebrafish lateral line system. Wiley Interdiscip Rev Dev Biol, 4 (1), 1-16. doi:10.1002/wdev.160 [DOI] [PMC free article] [PubMed]
- Ueda, Y., Nakamura, T., Nie, J., Solivais, A. J., Hoffman, J. R., Daye, B. J., & Hashino, E. (2023). Defining developmental trajectories of prosensory cells in human inner ear organoids at single-cell resolution. Development, 150 (12). doi:10.1242/dev.201071 [DOI] [PMC free article] [PubMed]
- Ungos, J. M., Karlstrom, R. O., & Raible, D. W. (2003). Hedgehog signaling is directly required for the development of zebrafish dorsal root ganglia neurons. Development, 130 (22), 5351-5362. doi:10.1242/dev.00722 [DOI] [PubMed]
- Villablanca, E. J., Renucci, A., Sapede, D., Lec, V., Soubiran, F., Sandoval, P. C., . . . Allende, M. L. (2006). Control of cell migration in the zebrafish lateral line: implication of the gene "tumour-associated calcium signal transducer," tacstd. Dev Dyn, 235 (6), 1578-1588. doi:10.1002/dvdy.20743 [DOI] [PubMed]
- White, P. M. (2020). Perspectives on Human Hearing Loss, Cochlear Regeneration, and the Potential for Hearing Restoration Therapies. Brain Sci, 10 (10). doi:10.3390/brainsci10100756 [DOI] [PMC free article] [PubMed]