Key Points
Question
Are kidneys from deceased donors who underwent dialysis prior to kidney donation associated with adverse graft outcomes in kidney transplant recipients compared with kidneys from deceased donors who did not undergo dialysis?
Findings
In an analysis of 1944 kidney transplant recipients (including 954 who received kidneys from deceased donors who underwent dialysis prior to kidney donation), the incidence of delayed graft function was higher in recipients of kidneys from deceased donors who underwent dialysis prior to kidney donation vs recipients of kidneys from deceased donors who did not undergo dialysis (59.2% vs 24.6%, respectively), but there were no significant differences in the incidence of graft failure (adjusted odds ratio, 0.90) or mortality (adjusted hazard ratio, 0.76) at a median follow-up of 34.1 months.
Meaning
Compared with recipients of kidneys from deceased donors who did not undergo dialysis, receiving kidneys from deceased donors who underwent dialysis prior to donation was associated with a higher incidence of delayed graft function, but no difference in graft failure or death at longer-term follow-up.
Abstract
Importance
Recipient outcomes after kidney transplant from deceased donors who received dialysis prior to kidney donation are not well described.
Objective
To compare outcomes of transplant recipients who received kidneys from deceased donors who underwent dialysis prior to kidney donation vs recipients of kidneys from deceased donors who did not undergo dialysis.
Design, Setting, and Participants
A retrospective cohort study was conducted including data from 58 US organ procurement organizations on deceased kidney donors and kidney transplant recipients. From 2010 to 2018, 805 donors who underwent dialysis prior to kidney donation were identified. The donors who underwent dialysis prior to kidney donation were matched 1:1 with donors who did not undergo dialysis using a rank-based distance matrix algorithm; 1944 kidney transplant recipients were evaluated.
Exposure
Kidney transplants from deceased donors who underwent dialysis prior to kidney donation compared with kidney transplants from deceased donors who did not undergo dialysis.
Main Outcomes and Measures
The 4 study outcomes were delayed graft function (defined as receipt of dialysis by the kidney recipient ≤1 week after transplant), all-cause graft failure, death-censored graft failure, and death.
Results
From 2010 to 2018, 1.4% of deceased kidney donors (805 of 58 155) underwent dialysis prior to kidney donation. Of these 805 individuals, 523 (65%) donated at least 1 kidney. A total of 969 kidneys (60%) were transplanted and 641 kidneys (40%) were discarded. Among the donors with kidneys transplanted, 514 (mean age, 33 years [SD, 10.8 years]; 98 had hypertension [19.1%] and 36 had diabetes [7%]) underwent dialysis prior to donation and were matched with 514 (mean age, 33 years [SD, 10.9 years]; 98 had hypertension [19.1%] and 36 had diabetes [7%]) who did not undergo dialysis. Kidney transplants from donors who received dialysis prior to donation (n = 954 kidney recipients) were associated with a higher risk of delayed graft function compared with kidney transplants from donors who did not receive dialysis (n = 990 kidney recipients) (59.2% vs 24.6%, respectively; adjusted odds ratio, 4.17 [95% CI, 3.28-5.29]). The incidence rates did not significantly differ at a median follow-up of 34.1 months for all-cause graft failure (43.1 kidney transplants per 1000 person-years from donors who received dialysis prior to donation vs 46.9 kidney transplants per 1000 person-years from donors who did not receive dialysis; adjusted hazard ratio [HR], 0.90 [95% CI, 0.70-1.15]), for death-censored graft failure (22.5 vs 20.6 per 1000 person-years, respectively; adjusted HR, 1.18 [95% CI, 0.83-1.69]), or for death (24.6 vs 30.8 per 1000 person-years; adjusted HR, 0.76 [95% CI, 0.55-1.04]).
Conclusions and Relevance
Compared with receiving a kidney from a deceased donor who did not undergo dialysis, receiving a kidney from a deceased donor who underwent dialysis prior to kidney donation was associated with a significantly higher incidence of delayed graft function, but no significant difference in graft failure or death at follow-up.
This study compares the outcomes of kidney transplant recipients who received kidneys from deceased donors who underwent dialysis prior to kidney donation vs recipients of kidneys from deceased donors who did not undergo dialysis.
Introduction
In the US, approximately 89 000 patients are on a waiting list for a kidney transplant.1 Fewer than 20 000 patients receive kidney transplants from deceased donors annually.2 Acute kidney injury (AKI) frequently occurs in deceased donors, possibly due to traumatic events leading to donor death, the inflammatory cascade in the setting of brain death, hemodynamic instability, and nephrotoxic damage during terminal hospitalizations.3,4
Due to the concern of incomplete recovery from injury prior to donation, and ischemic-reperfusion injury occurring at transplant, kidneys from deceased donors with AKI are frequently discarded. However, recent evidence suggests that transplanting kidneys from certain deceased donors with AKI (determined either by sensitive markers of tubular injury or by clinical AKI) is associated with similar risk of graft failure compared with transplanting kidneys from deceased donors without AKI.4,5,6 Therefore, careful selection of kidneys from deceased donors with AKI may improve the number of available kidneys and access to kidney transplant.6,7,8
The procurement of kidneys from deceased donors with stage 3 AKI doubled over the past 10 years.9 However, up to 44% of these kidneys are not transplanted due to concerns that kidneys from these donors may lead to more intense acute tubular injury that is less likely to recover and more likely have inferior longer-term graft outcomes.10 Furthermore, prior studies rarely included deceased donors who had the most severe AKI stage and who received dialysis before kidney donation.11 Therefore, recipient outcomes after kidney transplant from these donors are not well described.
This study assessed whether kidney transplants from deceased donors who underwent dialysis prior to donation were associated with worse outcomes in kidney recipients compared with kidney transplants from matched deceased donors who did not undergo dialysis.
Methods
The study was approved by the institutional review board at Johns Hopkins University School of Medicine. This study used data from the Organ Procurement and Transplantation Network (OPTN) provided in July 2023. The OPTN system includes data on all organ donors, wait-listed candidates, and transplant recipients in the US that are entered into the network by members of OPTN and has been described previously.12 The Health Resources and Services Administration within the US Department of Health and Human Services provides oversight to the activities of the OPTN contractor. The current study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
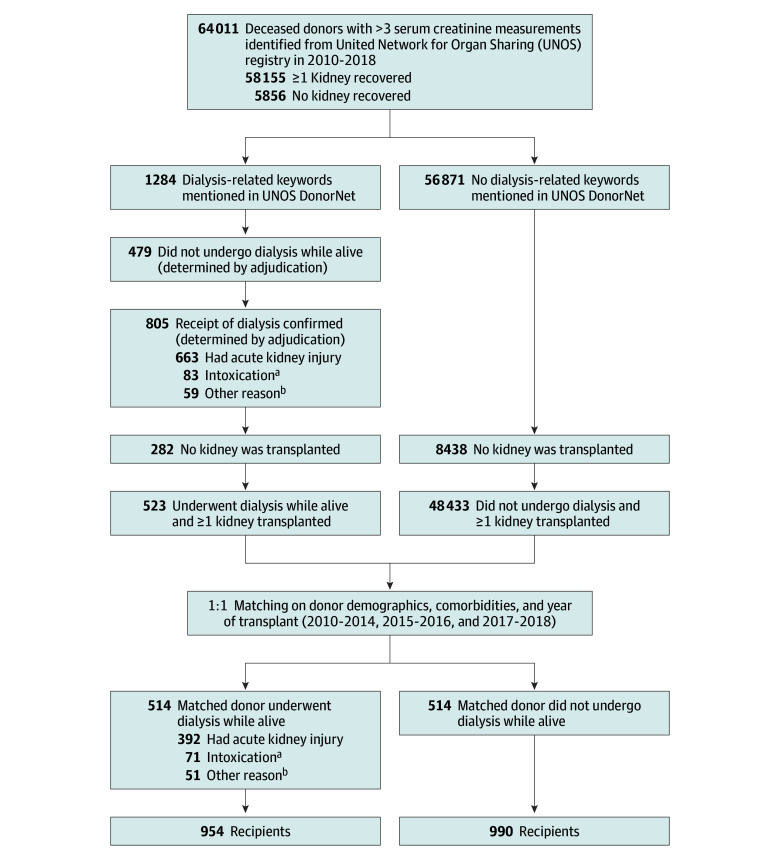
Deceased donors from 2010 to 2018 who received dialysis prior to kidney donation were identified using dialysis-related keywords (dialysis, continuous kidney replacement therapy, hemodialysis, and end-stage kidney disease) from OPTN DonorNet files that contain extensive clinical records from the terminal hospitalizations of all deceased donors in the US (Figure 1). We included all deceased kidney donors who were aged 16 years or older, had 3 or more serum creatinine measurements during hospitalization, had at least 1 procured kidney, and did not donate both kidneys to the same recipients (ie, en-bloc donors). Donor records, including hospital admission course, and the summary of donor medical and social history were queried for text string matches of the dialysis-related keywords and included partial text matches (eg, “dialy” for “dialysis”).
Figure 1. Identification and Adjudication Process for Deceased Donors by Dialysis Status, Matching, and Linkage to Kidney Transplant Recipients.
aIntoxication with methanol, ethylene glycol, lithium, or other substances or medications.
bIncludes dialysis for severe hyperkalemia, acidosis, hypervolemia, hyperammonemia, and other electrolyte abnormalities without laboratory evidence of stage 2 to 3 acute kidney injury.
Donor records and laboratory data from the Standard Transplant Analysis and Research (STAR) deceased donor files were used to adjudicate whether a donor received dialysis at their final hospitalization and the reason (AKI; intoxication with methanol, ethylene glycol, lithium, or other substances or medications; or for other reasons), modality (hemodialysis, continuous kidney replacement therapy, both types of treatment, or unknown), and duration of dialysis (≤3 days, 4-7 days, >7 days, or unknown). Each donor record was adjudicated by 2 nephrologists independently (by Y.W. and S.G.M. or by Y.W. and N.S.), and the discrepancies were resolved by a third nephrologist (by N.S. if adjudicated by Y.W. and S.G.M. or by S.G.M. if adjudicated by Y.W. and N.S.). Adjudication was performed to determine if donors received dialysis prior to organ donation and the indication, duration, and modality of dialysis. The adjudication process primarily relied on OPTN DonorNet text fields. Laboratory data were used if dialysis details were not available in OPTN DonorNet text fields.
Matching Procedure to Identify Comparable Donors
Among deceased donors with any kidney used for transplant, donors who received dialysis prior to kidney donation vs those who did not receive dialysis were 1:1 matched using an iterative, rank-based distance matrix algorithm on sets of 20 pairs.13,14 In evaluating the match ratio, we considered the match efficiency (defined as the total number of donors receiving dialysis who could be matched), generalizability of the included cases, and the sample size. We matched on factors associated with graft outcomes and allocation patterns, including donor age (by 5-year increments), sex, and Black race. Black race was obtained because it is associated with recipient graft outcomes,15 and it was reported primarily by the family members of the donors who responded to open-ended questions or it was obtained from the electronic health record.
Other factors matched on included body mass index (BMI [calculated as weight in kilograms divided by height in meters squared]; ≤18.5, 18.5-<25, 25-<30, 30-40, and >40), hypertension, diabetes, donation after cardiac death (irreversible cessation of circulatory and respiratory functions), stroke as the cause of death, estimated glomerular filtration rate (eGFR) at hospital admission (from 0-120 mL/min/1.73 m2 by increments of 15; calculated using the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] 2021 equation),16 hepatitis C antibody positivity, and year of transplant (2010-2014, 2015-2016, and 2017-2018).5,9 These time points were chosen because the new kidney allocation system was implemented in 2015, and the procurement and use of kidneys from donors with AKI increased recently.9,17
We used hospital admission eGFR as a surrogate for baseline kidney function. Exact matching was applied on clinically important factors and confounders that are associated with recipient graft outcomes.18 These variables included age category, hospital admission eGFR category, diabetes, and donation after cardiac death status. Near-exact matching was used for Black race and fine matching was used for the BMI categories. Matched deceased donors who did not receive dialysis prior to donation were further adjudicated.
Outcome Measures
Follow-up data on recipients of a kidney from donors (who underwent dialysis prior to donation or did not undergo dialysis) were obtained from OPTN; the data were collected by the transplant centers and reported to OPTN. The short-term outcome was delayed graft function (DGF), which was defined as receipt of dialysis by the kidney recipient within 1 week after kidney transplant. Longer-term outcomes consisted of all-cause graft failure (defined as a composite of death and graft failure [return to dialysis or retransplant]), death-censored graft failure, death, eGFR at 6 and 12 months after transplant, and longitudinal decline in eGFR.
Follow-up records for all kidney recipients were administratively censored on January 31, 2020, which was the date of the first US Department of Health and Human Services COVID-19 Emergency Declaration, because of the potential effect of COVID-19 on study outcomes. Follow-up time began immediately after transplant until the earliest outcome of graft failure, death, or last reported follow-up (with the last possible date of January 31, 2020). The eGFR at 6 and 12 months and the longitudinal decline in eGFR were calculated from the serum creatinine values reported in the individual follow-up records (until January 31, 2020), using the CKD-EPI 2021 equation for recipients aged 18 years or older and the Schwartz 2009 equation for recipients younger than 18 years of age at the time of the creatinine measurements.16,19
For the comparison of 6- and 12-month eGFR between kidney recipients (from donors who received dialysis prior to donation vs donors who did not receive dialysis), eGFR was imputed as 10 mL/min/1.73 m2 if the recipients developed graft failure before the 6- and 12-month follow-up time points. For recipients who died before 6- and 12-month follow-up, the last reported eGFR was carried forward for these 2 time points. Only recipients with serum creatinine levels or who developed graft failure reported to OPTN were included in the analysis. Additional sensitivity analyses were performed after imputing eGFR as 1 mL/min/1.73 m2 at the time of death or removing any eGFR imputed at the time of death.
For the comparison of longitudinal decline in eGFR between kidney recipients (from donors who received dialysis prior to donation vs donors who did not receive dialysis), eGFR was imputed as 10 mL/min/1.73 m2 at day 0 if recipients developed primary nonfunction (defined as kidney graft removal or as dependent on dialysis within the first 90 days after the first week). For recipients who developed graft failure, eGFR was imputed as 10 mL/min/1.73 m2 at the time of graft failure. Recipients without reported serum creatinine levels and who did not develop graft failure up to January 31, 2020, were excluded from the analysis.
Statistical Analysis
Descriptive statistics are presented using mean (SD), median (IQR), and proportions. The kidney recipient outcomes (by donors who received dialysis prior to donation vs donors who did not receive dialysis) are presented as cumulative incidence and incidence rates. The donor characteristics (by dialysis status) and the kidney recipient characteristics (by donor dialysis status) were compared using standardized mean differences.20
The donor characteristics were compared using t tests and χ2 tests for all donors who had their kidneys procured and for those who did (vs did not) have their kidneys used for transplant. To evaluate the geographic variation in transplanting kidneys from deceased donors who received dialysis, the number of donors who received dialysis and had kidneys transplanted by organ procurement organizations across the US was mapped after normalizing to the number of eligible and imminent deaths reported by the organ procurement organizations.
Multiple logistic regression was used to determine the association between kidney transplant from deceased donors who received dialysis prior to donation vs those who did not receive dialysis and recipient DGF. Cox proportional hazard regression was used to determine associations with time to all-cause graft failure, death-censored graft failure, and death. Proportional hazard assumptions were not violated according to the Grambsch-Therneau tests.
Multiple linear regression was used to determine the association between kidney transplant from donors who received dialysis prior to donation vs those who did not receive dialysis and recipient eGFR at 6 and 12 months after the transplant. Linear mixed-effects models with random intercepts, random slopes, and the restricted maximum likelihood estimator were used to determine the associations between recipients of kidneys from donors who received dialysis prior to donation vs those who did not receive dialysis and annual decline in eGFR in recipients. In the mixed-effects models, fixed-effects coefficients were used (1) for time to estimate the annual decline in eGFR in recipients of a kidney from deceased donors who did not receive dialysis and (2) for interactions between time and donor receiving dialysis to compare the rate (ie, slope) of decline in eGFR between groups of recipients. In these models, eGFR was log2 transformed and the coefficients were transformed to percentage changes for interpretation.
In all statistical models, we adjusted for cold ischemic time (defined as the period during the transplant from the cessation of circulation to the beginning of vascular anastomosis in the kidney graft), recipient age, BMI, diabetes, preemptive transplant (defined as receipt of a kidney transplant before requiring chronic kidney replacement therapy), previous kidney transplant, human leukocyte antigen mismatch, and panel-reactive antibody categories (0%, 1%-20%, 21%-80%, and >80%). These factors were selected because they are statistically significantly associated with kidney recipient graft outcomes in univariate analysis. Because we only matched on categories of donors’ hospital admission eGFR, we also adjusted for donor hospital admission eGFR as a continuous variable to address residual confounding. Sandwich estimators were used to account for pairs of donor kidneys in all multivariable analyses.
Subgroup analyses were performed for the recipients of a kidney from donors who received dialysis prior to transplant for different reasons, modalities, and durations vs the recipients of a kidney from donors who did not receive dialysis. In a sensitivity analysis, recipient outcomes were compared without any matching on donor variables. The donor characteristics used for matching were adjusted for (the characteristics included donor age, sex, Black race, BMI, hypertension, diabetes, donation after cardiac death status, stroke as the cause of death, and year of transplant).
We considered P < .05 statistically significant and performed all analyses using R version 4.1.3 (R Foundation for Statistical Computing).
Results
Among 58 155 deceased donors with any kidney procured from 2010 to 2018, 805 (1.4%) received dialysis prior to transplant, including 663 for AKI, 83 for intoxication (methanol, ethylene glycol, lithium, or other toxic substances or medications), and 59 for other reasons (electrolyte, acid base, fluid imbalance, or hyperammonemia without laboratory evidence of stage 2-3 AKI) (Figure 1). Donors who received dialysis prior to transplant were younger vs donors who did not receive dialysis (mean age, 35.2 years [SD, 12.2 years] vs 41.5 years [SD, 14.7 years], respectively), had higher serum creatinine levels (at hospital admission, peak level, and terminal level [terminal creatinine is the last creatinine before deceased donor nephrectomy]), had lower hospital admission eGFRs (mean, 63.6 [SD, 31.1] mL/min/1.73 m2 vs 85.8 [SD, 25.6] mL/min/1.73 m2), had a lower prevalence of hypertension (25.6% vs 33.6%), had lower rates of stroke as the cause of death (11.3% vs 31.3%), and had lower rates of donation after cardiac death (7% vs 14.7%) (eTable 1 in Supplement 1).
A total of 969 kidneys (60%) were transplanted and 641 kidneys (40%) were discarded. Among 805 deceased donors who received dialysis prior to transplant, 523 had at least 1 of their kidneys transplanted; those who donated at least 1 kidney were younger than the 282 donors for which both kidneys were discarded (mean age, 32.9 years [SD, 10.8 years] vs 39.6 years [SD, 13.3 years], respectively), had lower serum creatinine levels (at hospital admission, peak level, and terminal level), had higher eGFRs (mean, 65.9 [SD, 31] mL/min/1.73 m2 vs 59.3 [SD, 30.8] mL/min/1.73 m2), had a lower prevalence of hypertension (19.1% vs 37.6%) and diabetes (7.5% vs 16.3%), and lower rates of stroke as the cause of death (7.1% vs 19.1%) (eTable 2 in Supplement 1). Kidney transplants from donors who received dialysis came from multiple geographic regions and higher proportions were from the Mid-Atlantic, Midwest, and Southwest regions (Figure 2).
Figure 2. Geographic Variation in Kidney Transplants From Deceased Donors Who Underwent Dialysis Across 58 Donation Service Areas in the US From 2010 to 2018.
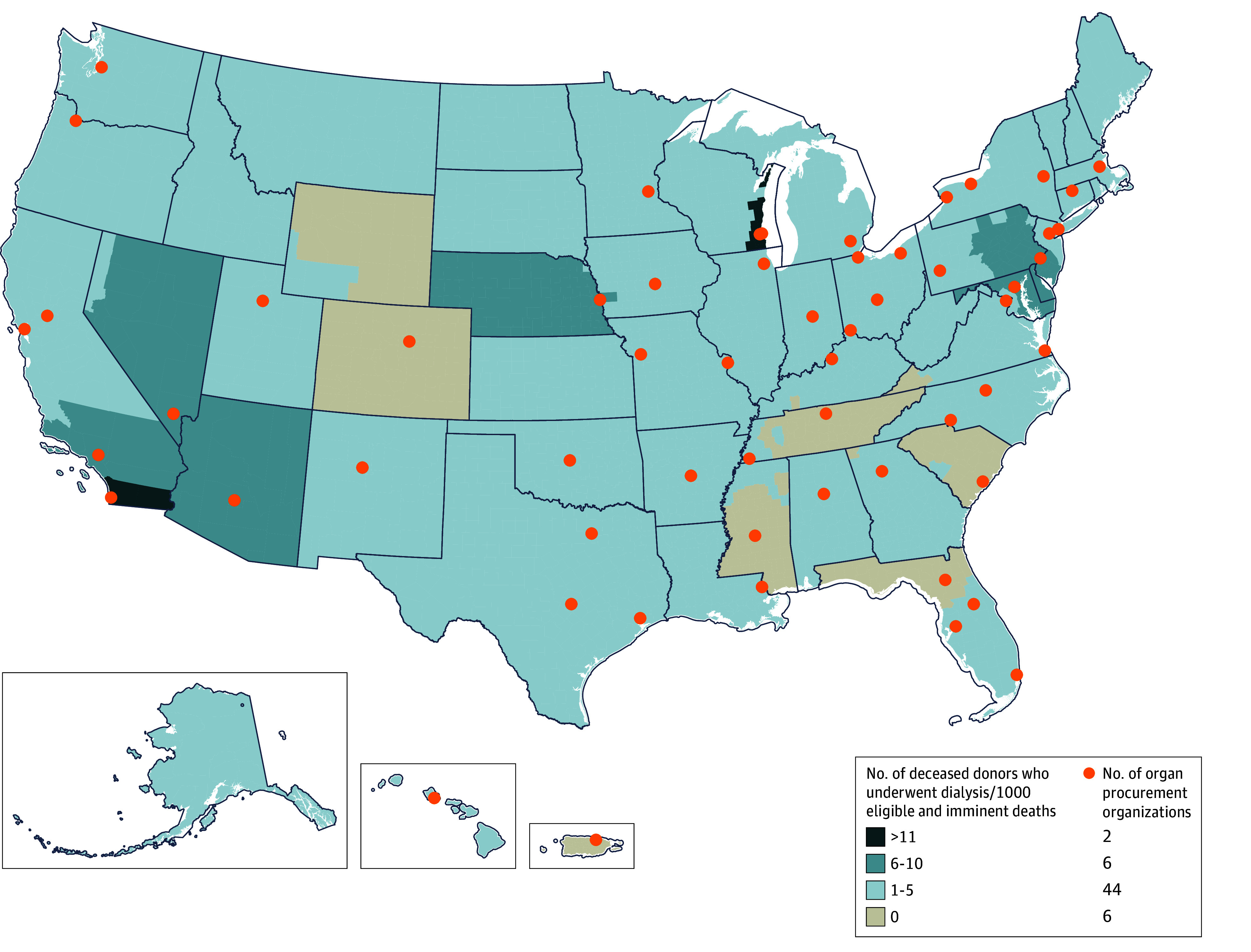
Geographical heat map depicting rates and locations of kidneys transplanted from deceased donors who underwent dialysis during their final hospitalization.
There were 514 deceased donors who received dialysis prior to donation matched to 514 deceased donors who did not receive dialysis. Of the 514 donors who received dialysis, 392 underwent dialysis for AKI, 71 for intoxication, and 51 for other reasons (Table 1). The 2 donor groups were comparable after matching except that fewer of the donors who received dialysis prior to donation had both kidneys transplanted (Table 1).
Table 1. Characteristics of Matched Deceased Donors.
| Donor characteristics | Matched donorsa | Standardized mean difference | |
|---|---|---|---|
| Underwent dialysis (n = 514) | Did not undergo dialysis (n = 514) | ||
| Age, mean (SD), y | 33 (10.8) | 33.1 (10.9) | −0.014 |
| Sex | |||
| Male | 313 (60.9) | 314 (61.1) | −0.004 |
| Female | 201 (39.1) | 200 (38.9) | 0.004 |
| Raceb | |||
| Blackc | 76 (14.8) | 76 (14.8) | 0 |
| Otherd | 438 (85.2) | 438 (85.2) | 0 |
| Body mass index, mean (SD)e | 29.5 (7.1) | 29.5 (7.2) | 0.0046 |
| Estimated glomerular filtration rate at hospital admission, mean (SD), mL/min/1.73 m2 | 66.8 (30.6) | 68.2 (30.1) | −0.048 |
| Serum creatinine, median (IQR), mg/dL | |||
| Level at hospital admission | 1.4 (1 to 1.9) | 1.4 (1 to 1.8) | 0.077 |
| Peak level | 4.2 (2.1 to 6.2) | 1.8 (1.3 to 2.6) | 0.96 |
| Terminal level | 2.3 (1.2 to 4) | 1.1 (0.8 to 1.8) | 0.67 |
| Reason for dialysis | |||
| Acute kidney injury | 392 (76.3) | ||
| Intoxicationf | 71 (13.8) | ||
| Other reasong | 51 (9.9) | ||
| Dialysis modality, No./total (%) | |||
| Continuous kidney replacement therapy | 289/505 (57.2) | ||
| Hemodialysis | 201/505 (39.8) | ||
| Both | 15/505 (3.0) | ||
| Duration of dialysis, No./total (%) | |||
| ≤3 d | 374/495 (75.6) | ||
| 4-7 d | 100/495 (20.2) | ||
| >7 d | 21/495 (4.2) | ||
| No. of vasopressors and inotropes used, median (IQR) | 0 (0 to 1) | 0 (0 to 1) | 0.17 |
| Hypertension | 98 (19.1) | 98 (19.1) | 0 |
| Diabetes | 36 (7) | 36 (7) | 0 |
| Donation after cardiac death | 35 (6.8) | 35 (6.8) | 0 |
| Stroke as cause of death | 37 (7.2) | 37 (7.2) | 0 |
| Hepatitis C status | 11 (2.1) | 11 (2.1) | 0 |
| No. of kidneys transplanted | |||
| 1 | 74 (14.4) | 38 (7.4) | 0.23 |
| 2 | 440 (85.6) | 476 (92.6) | −0.23 |
| Transplant year range | |||
| 2010-2014 | 144 (28) | 143 (27.8) | −0.0043 |
| 2015-2016 | 147 (28.6) | 148 (28.8) | 0.0043 |
| 2017-2018 | 223 (43.4) | 223 (43.4) | 0 |
SI conversion factor: To convert creatinine to μmol/L, multiply by 88.4.
Data are expressed as No. (%) unless otherwise indicated. The following variables were used for matching deceased donors by dialysis status: age (by 5-year increments), sex, and Black race, body mass index categories (≤18.5, 18.5-<25, 25-<30, 30-40, and >40), hypertension, diabetes, donation after cardiac death status, stroke as the cause of death, estimated glomerular filtration rate at hospital admission (range, 0-120 mL/min/1.73 m2 by increments of 15), hepatitis C antibody positivity, and year of transplant (2010-2014, 2015-2016, or 2017-2018).
Primarily reported by the donor’s family or collected by clinical staff from the organ procurement organizations and recorded in the electronic health record.
Specifically reported by the Organ Procurement and Transplantation Network because it is associated with recipient graft outcomes.15
Includes American Indian, Asian, Pacific Islander, White, and multiple races.
Calculated as weight in kilograms divided by height in meters squared.
For poisoning from ethylene glycol, methanol, lithium, or another toxic substance or medication.
Severe hyperkalemia, acidosis, hypervolemia, hyperammonemia, and other electrolyte abnormalities without laboratory evidence of stage 2 or 3 acute kidney injury.
There were 954 kidney transplant recipients identified from the donors who received dialysis prior to donation and there were 990 kidney transplant recipients identified from the donors who did not receive dialysis. Recipients of a kidney from donors who received dialysis were older vs recipients of a kidney from donors who did not receive dialysis (mean, 52.7 years [SD, 13.8 years] vs 49.2 years [15.2 years], respectively), had lower rates of panel reactive antibody greater than 80% (11.2% vs 16.8%) or receiving a previous transplant (8.1% vs 11.5%), and had higher rates of receiving corticosteroids (72% vs 64.8%) and antithymocyte globulin for induction immunosuppression therapy (62.2% vs 57%) (Table 2). Recipients of a kidney from donors who received dialysis prior to donation had longer cold ischemic times vs kidney transplants from donors who did not receive dialysis (median, 19 hours [IQR, 13.7-25 hours] vs 15 hours [IQR, 9.8-21.4 hours], respectively).
Table 2. Characteristics of Kidney Transplant Recipients.
| Recipient characteristics | Kidney transplant recipientsa | Standardized mean difference | |
|---|---|---|---|
| Matched donor underwent dialysis (n = 954) | Matched donor did not undergo dialysis (n = 990) | ||
| Cold ischemic time, median (IQR), h | 19 (13.7 to 25) | 15 (9.8 to 21.4) | 0.41 |
| Age, mean (SD), y | 52.7 (13.8) | 49.2 (15.2) | 0.24 |
| Sex | |||
| Male | 580 (60.8) | 605 (61.1) | −0.0064 |
| Female | 374 (39.2) | 385 (38.9) | 0.0064 |
| Self-identified race | |||
| Black | 279 (29.2) | 299 (30.2) | −0.021 |
| Otherb | 675 (70.8) | 691 (69.8) | 0.021 |
| Body mass index, mean (SD)c | 28 (5.4) | 28 (5.7) | −0.0045 |
| End-stage kidney disease caused by diabetes | 273 (28.6) | 294 (29.7) | −0.024 |
| Level of human leukocyte antigen mismatch, mean (SD) | 4.3 (1.4) | 4.1 (1.5) | 0.12 |
| Undergoing dialysis prior to transplant, median (IQR), y | 4.1 (2.3 to 6.6) | 3.8 (1.8 to 6.2) | 0.11 |
| Preemptive transplantd | 97 (10.2) | 131 (13.2) | −0.095 |
| Previous transplant | 77 (8.1) | 114 (11.5) | −0.12 |
| Panel reactive antibody category, % | |||
| 0 | 640 (67.1) | 591 (59.7) | 0.15 |
| 1-20 | 63 (6.6) | 93 (9.4) | −0.1 |
| 21-80 | 144 (15.1) | 140 (14.1) | −0.027 |
| >80 | 107 (11.2) | 166 (16.8) | −0.016 |
| Induction immunosuppression therapye | |||
| Corticosteroid | 687 (72) | 642 (64.8) | 0.15 |
| Antithymocyte globulin | 593 (62.2) | 564 (57) | 0.11 |
| Basiliximab | 181 (19) | 198 (20) | −0.026 |
| Alemtuzumab | 147 (15.4) | 132 (13.3) | 0.059 |
| Other therapyf | 23 (2.4) | 35 (3.5) | −0.066 |
| Maintenance immunosuppression therapy at discharge from index hospitalization for transplante | |||
| Mycophenolate | 915 (95.9) | 946 (95.6) | 0.018 |
| Tacrolimus | 901 (94.4) | 935 (94.4) | 0 |
| Corticosteroid | 681 (71.4) | 696 (70.3) | 0.024 |
| Cyclosporine | 19 (2) | 21 (2.1) | −0.0091 |
| Belatacept | 19 (2) | 17 (1.7) | 0.02 |
| Other therapyg | 15 (1.6) | 24 (2.4) | −0.061 |
Data are expressed as No. (%) unless otherwise indicated.
Includes American Indian, Asian, Pacific Islander, White, and multiple races.
Calculated as weight in kilograms divided by height in meters squared.
Defined as receipt of a kidney transplant before requiring chronic kidney replacement therapy.
The sum of percentages exceeds 100% because patients may have required the use of multiple immunosuppressive agents.
Included belatacept, antithymocyte globulin, muromonab, rituximab, sirolimus, intravenous immunoglobulin, or bortezomib.
Included azathioprine, everolimus, sirolimus, rituximab, or intravenous immunoglobulin.
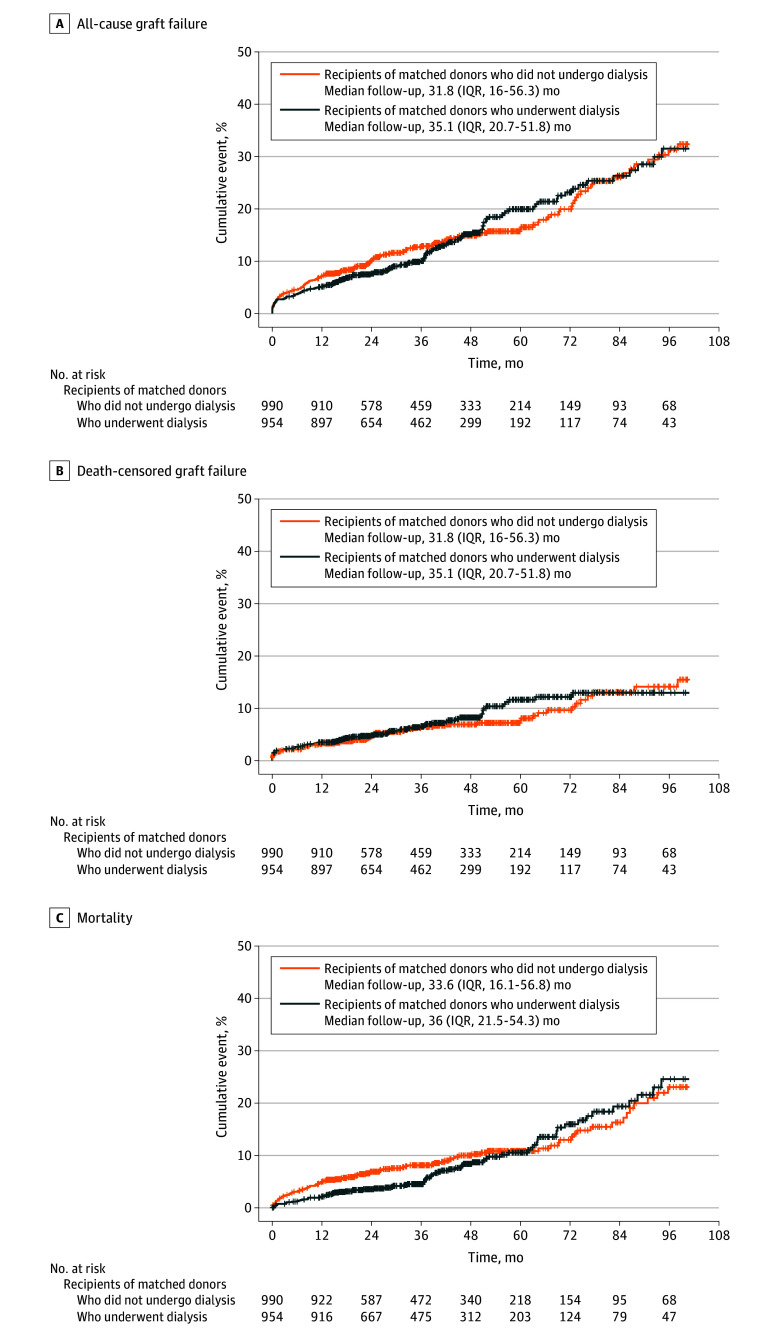
Kidney transplants from donors who received dialysis prior to donation were associated with a higher risk of DGF vs kidney transplants from donors who did not receive dialysis (565 [59.2%] vs 244 [24.6%], respectively; adjusted odds ratio [OR], 4.17 [95% CI, 3.28-5.29]; Figure 3). After a median follow-up of 34.1 months (IQR, 17.8-54.8 months), recipients of a kidney from donors who received dialysis prior to donation had similar risks of all-cause graft failure vs recipients of a kidney from donors who did not receive dialysis (incident rate, 43.1 vs 46.9 per 1000 person-years, respectively; adjusted hazard ratio [HR], 0.90 [95% CI, 0.70-1.15], Figures 3 and 4), death-censored graft failure (incident rate, 22.5 vs 20.6 per 1000 person-years; adjusted HR, 1.18 [95% CI, 0.83-1.69]), and death (incident rate, 24.6 vs 30.8 per 1000 person-years; adjusted HR, 0.76 [95% CI, 0.55-1.04]).
Figure 3. Recipient Short- and Longer-Term Outcomes After Kidney Transplant From Matched Deceased Donors.
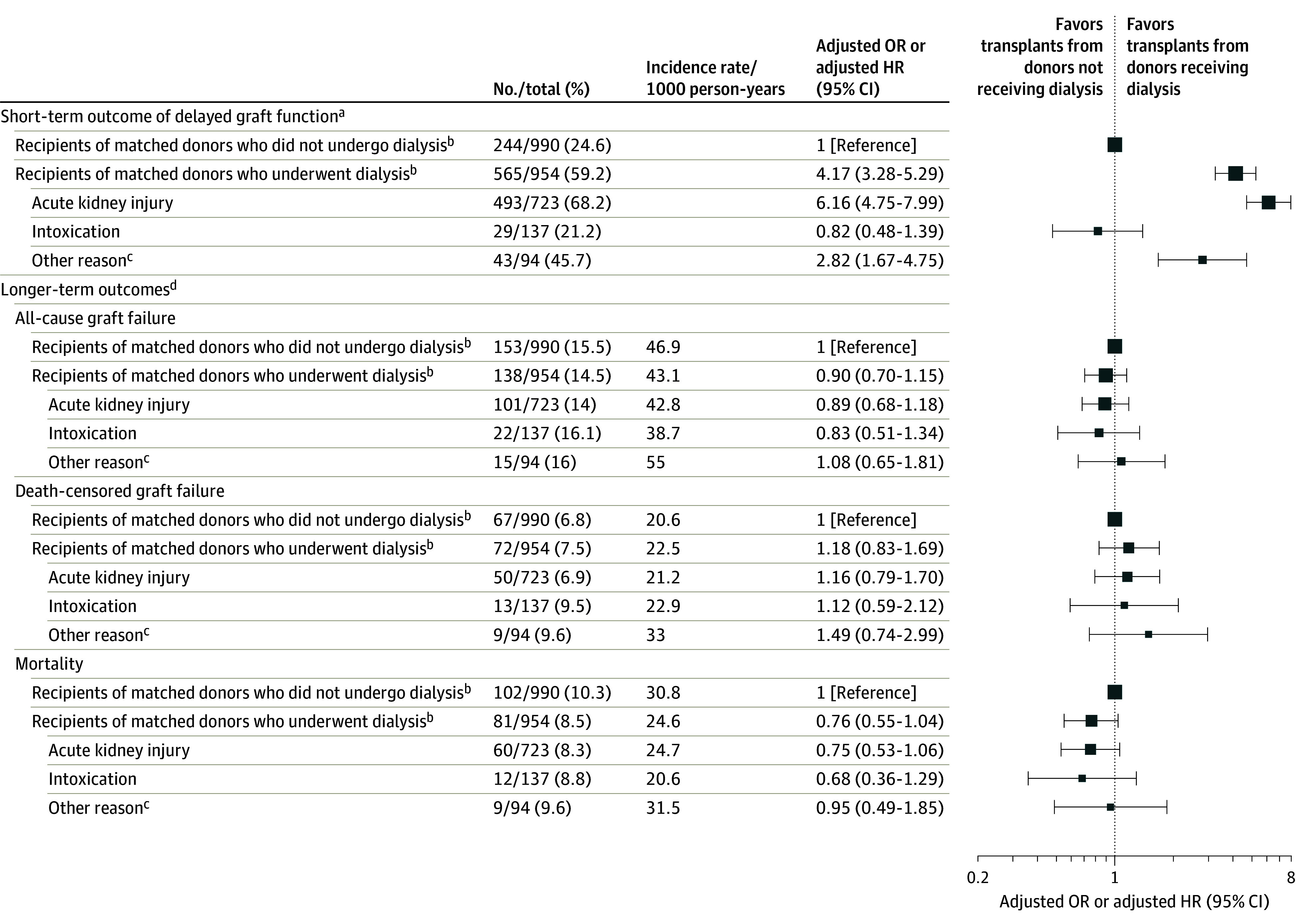
HR indicates hazard ratio; OR, odds ratio.
aThe data in column 4 are adjusted ORs (95% CIs). Short-term is defined as within 7 days after kidney transplant.
bFor the kidney recipient comparisons by matched donor dialysis status (underwent dialysis vs did not undergo dialysis), logistic and Cox proportional hazard regression models were used and adjusted for the following covariates: cold ischemic time, recipient age, body mass index, diabetes as the cause of recipient end-stage kidney disease, preemptive transplant status, previous kidney transplant, level of human leukocyte antigen mismatch, panel reactive antibody category (0%, 1%-20%, 21%-80%, and >80%), and donor estimated glomerular filtration rate at hospital admission. Sandwich estimators were used to account for outcome dependency when both donor kidneys were explanted.
cIncludes dialysis for severe hyperkalemia, acidosis, hypervolemia, hyperammonemia, and other electrolyte abnormalities without laboratory evidence of stage 2 or 3 acute kidney injury.
dThe data in column 4 are adjusted HRs (95% CIs).
Figure 4. Kaplan-Meier Survival Curves of Longer-Term Outcomes for Recipients After Kidney Transplant From Matched Donors by Dialysis Status.
By 6 months, 41 recipients had died (11 [1.2%] recipients of kidneys from donors who received dialysis prior to donation vs 30 [3%] recipients of kidneys from donors who did not receive dialysis). By 12 months, 69 recipients had died (20 [2.1%] recipients of kidneys from donors who received dialysis vs 49 [4.9%] recipients of kidneys from donors who did not receive dialysis). Among the recipients alive at 6 months (943 [98.8%] recipients of kidneys from donors who received dialysis vs 960 [97%] recipients of kidneys from donors who did not receive dialysis), eGFR data were missing for 115 (6%) recipients.
Among the recipients alive at 12 months (934 [97.9%] recipients of kidneys from donors who received dialysis prior to donation vs 941 [95.1%] recipients of kidneys from donors who did not receive dialysis), eGFR data were missing for 144 (7.7%). The remaining recipients had similar 6-month eGFRs (mean, 63.3 [SD, 21.4] mL/min/1.73 m2 for recipients of kidneys from donors who received dialysis vs 64.4 [SD, 21.8] mL/min/1.73 m2 for recipients of kidneys from donors who did not receive dialysis; between-group difference, −0.33% [95% CI, −4.02% to 3.51%]) and 12-month eGFRs (mean, 64.7 [SD, 21] mL/min/1.73 m2 vs 65.1 [SD, 22.2] mL/min/1.73 m2, respectively; between-group difference, 0.51% [95% CI, −3.26% to 4.42%]) (eTables 3-4 in Supplement 1).
There were 83 (4.3%) recipients who had no follow-up data on eGFR reported to the United Network for Organ Sharing through January 31, 2020. The remaining recipients had comparable rates of decline in eGFR (−3.2% per year for recipients of kidneys from donors who received dialysis prior to donation vs −4.48% per year for recipients of kidneys from donors who did not receive dialysis; between-group difference, 1.28% [95% CI, −0.66% to 3.25%]) (eTable 5 in Supplement 1) after a median of 3 serum creatinine measurements over 2.2 years of follow-up.
In the subgroup analyses, the risk for DGF was higher when donors received dialysis for AKI prior to donation (n = 493; adjusted OR, 6.16 [95% CI, 4.75-7.99]) and for other reasons (acid base, volume, electrolyte abnormalities) (n = 43; adjusted OR, 2.82 [95% CI, 1.67-4.75]), but not for intoxication (n = 29; adjusted OR, 0.82 [95% CI, 0.48-1.39]) (Figure 3). There were no statistically significant differences in longer-term outcomes by reasons for dialysis. The finding of increased risk of DGF and of no increased risk for longer-term outcomes held across dialysis modalities and durations (eTable 6 in Supplement 1), and without matching when comparing all recipients of kidneys from donors who received dialysis prior to donation vs those who did not receive dialysis (eTables 7-8 in Supplement 1).
Discussion
In this cohort study, kidney transplants from deceased donors who received dialysis prior to donation were associated with a statistically significantly higher risk of DGF, but no increased risk of adverse longer-term outcomes compared with kidney transplants from deceased donors who did not receive dialysis.
Given the severe shortages of organs and the increases in the rates of discarded kidneys from potential donors, there is growing interest in using less than ideal donor kidneys (such as kidneys from deceased donors with AKI).21,22 Prior work showed that the risk of DGF was approximately 1.5 to 3 times higher in recipients of kidneys from deceased donors with AKI compared with recipients of kidneys from donors without AKI.6,11,23,24,25,26 In a prospective cohort of deceased donors and their kidney transplant recipients, risks for DGF increased with AKI severity.18
In the current study, the risk of DGF was approximately 6-fold higher when donors received dialysis for AKI. Despite the higher risk of DGF, kidney recipients from donors who received dialysis prior to donation had longer-term graft function that was comparable with kidney transplants from donors who did not receive dialysis. This finding is consistent with observations made in a smaller cohort of recipients of kidneys from deceased donors who had severe AKI and received dialysis,11 as well as in other cohorts of recipients of kidneys from deceased donors who had less severe AKI.5,6,27,28 A study from the UK demonstrated slightly poorer kidney function after 1 year among recipients of kidneys from donors with stage 3 AKI compared with those without AKI.10 However, the overall acceptance rate of kidneys was higher in the UK (90%) than in the US (75%),6,10 suggesting that further research is needed to identify the small subgroup of donors with suboptimal kidney repair capacity.
Delayed graft function is an important outcome because it has been associated with increased costs, length of stay, and adverse outcomes.29,30,31,32 Considering the high incidence of DGF after receiving kidneys from donors who underwent dialysis, kidneys from these donors should be considered for recipients who may be sufficiently healthy to tolerate repeated hemodialysis sessions after the transplant if DGF occurs. Transplant centers may also benefit from proactive planning to mitigate the longer hospitalization and the higher resource use associated with DGF.31 Currently, transplant centers review OPTN DonorNet information within the time constraints of organ allocation when making decisions about potential kidney donor opportunities.
However, predonation dialysis is not reported in a standardized manner. The information about receipt of dialysis is currently reported as free text and different terminologies are used rather than documentation in a discrete field. Documenting standardized donor dialysis information in the donor forms as a separate field, including the reason, modality, and duration, may facilitate better clinical decision-making with appropriate consideration to improve use of available kidneys for transplant. This would be particularly important given the increasing use of dialysis modalities for volume and electrolyte management in donors with AKI over time, as demonstrated in the current study.
Although only a small proportion of deceased donors received dialysis, there was large geographic variation across the US in procuring and accepting kidneys from donors who had received dialysis. This suggests that more kidneys might become available if organ procurement organizations and transplant centers carefully expand their threshold of offering dialysis to stabilize donors with severe AKI, as well as expand their kidney procurement and acceptance criteria to donors receiving dialysis but not currently considered eligible because of severe AKI. Future research should investigate whether the higher procurement and acceptance rates of kidneys from deceased donors who received dialysis are associated with shorter waiting times and better outcomes for patients.
Limitations
First, this retrospective study was subject to confounding and selection bias. Younger and healthier donors were more likely to have kidneys procured by organ procurement organizations and accepted by transplant centers even when they were receiving dialysis.
Second, the number of donors who received dialysis, particularly those who were identified as Black, was relatively small. Third, the study was limited by the lack of specific information on donor demographics such as race, recipient comorbidities, and changes in immunosuppression over time in the OPTN registry.
Conclusions
Compared with receiving a kidney from a deceased donor who did not undergo dialysis, receiving a kidney from a deceased donor who underwent dialysis prior to kidney donation was associated with a significantly higher incidence of DGF, but no significant difference in graft failure or death at follow-up.
eTable 1. Characteristics of all deceased donors receiving vs. not receiving dialysis with kidney procured
eTable 2. Characteristics of deceased donors receiving dialysis with vs. without kidney transplanted
eTable 3. Recipient kidney function at 6 and 12 months after kidney transplant from matched donors receiving vs. not receiving dialysis
eTable 4. Recipient kidney function at 6 and 12 months after kidney transplant from matched donors receiving vs. not receiving dialysis using alternative imputation approaches
eTable 5. Recipient kidney function decline after kidney transplantation from matched donors receiving vs. not receiving dialysis
eTable 6. Recipient outcomes after kidney transplantation from matched donors receiving vs. not receiving dialysis stratified by donor dialysis modality and duration
eTable 7. Recipient outcomes after kidney transplantation from all donors receiving and not receiving dialysis
eTable 8. Recipient kidney function decline after kidney transplantation from all donors receiving vs. not receiving dialysis
Data sharing statement
Reference
- 1.US Department of Health and Human Services . OPTN/SRTR 2020 annual data report. Accessed May 6, 2024. https://srtr.transplant.hrsa.gov/annual_reports/2020_ADR_Preview.aspx
- 2.US Department of Health and Human Services . OPTN: national data. Accessed January 18, 2022. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/
- 3.Mansour SG, Khoury N, Kodali R, et al. Clinically adjudicated deceased donor acute kidney injury and graft outcomes. PLoS One. 2022;17(3):e0264329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reese PP, Hall IE, Weng FL, et al. Associations between deceased-donor urine injury biomarkers and kidney transplant outcomes. J Am Soc Nephrol. 2016;27(5):1534-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall IE, Akalin E, Bromberg JS, et al. Deceased-donor acute kidney injury is not associated with kidney allograft failure. Kidney Int. 2019;95(1):199-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Hall IE, Mansour S, et al. Association of deceased donor acute kidney injury with recipient graft survival. JAMA Netw Open. 2020;3(1):e1918634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu K, King K, Husain SA, et al. Kidney nonprocurement in solid organ donors in the United States. Am J Transplant. 2020;20(12):3413-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu K, Husain SA, King K, et al. Kidney nonprocurement in deceased donors with acute kidney injury. Clin Transplant. 2022;36(11):e14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Alasfar S, Reese PP, et al. Trends in the procurement and discard of kidneys from deceased donors with acute kidney injury. Am J Transplant. 2022;22(3):898-908. [DOI] [PubMed] [Google Scholar]
- 10.Boffa C, van de Leemkolk F, Curnow E, et al. Transplantation of kidneys from donors with acute kidney injury. Am J Transplant. 2017;17(2):411-419. [DOI] [PubMed] [Google Scholar]
- 11.Heilman RL, Smith ML, Kurian SM, et al. Transplanting kidneys from deceased donors with severe acute kidney injury. Am J Transplant. 2015;15(8):2143-2151. [DOI] [PubMed] [Google Scholar]
- 12.Lentine KL, Smith JM, Miller JM, et al. OPTN/SRTR 2021 annual data report. Am J Transplant. 2023;23(2)(suppl 1):S21-S120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zubizarret JR, Kilcioglu C, Vielma JP, Cohn ER. designmatch: matched samples that are balanced and representative by design. Published 2022. Accessed November 11, 2023. https://cran.r-project.org/web/packages/designmatch/index.html
- 14.Hart A, Smith JM, Skeans MA, et al. Kidney. Am J Transplant. 2016;16(suppl 2):11-46. doi: 10.1111/ajt.13666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locke JE, Warren DS, Dominici F, et al. Donor ethnicity influences outcomes following deceased-donor kidney transplantation in Black recipients. J Am Soc Nephrol. 2008;19(10):2011-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Israni AK, Salkowski N, Gustafson S, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol. 2014;25(8):1842-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall IE, Schröppel B, Doshi MD, et al. Associations of deceased donor kidney injury with kidney discard and function after transplantation. Am J Transplant. 2015;15(6):1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228-1234. doi: 10.1080/03610910902859574 [DOI] [Google Scholar]
- 21.Mohan S, Yu M, King KL, Husain SA. Increasing discards as an unintended consequence of recent changes in United States kidney allocation policy. Kidney Int Rep. 2023;8(5):1109-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puttarajappa CM, Hariharan S, Zhang X, et al. Early effect of the circular model of kidney allocation in the United States. J Am Soc Nephrol. 2023;34(1):26-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Y, Song T, Liu J, et al. Single kidney transplantation from donors with acute kidney injury. Pediatr Transplant. 2019;23(3):e13326. [DOI] [PubMed] [Google Scholar]
- 24.Koyawala N, Parikh CR. A review of donor acute kidney injury and posttransplant outcomes. Transplantation. 2020;104(8):1553-1559. [DOI] [PubMed] [Google Scholar]
- 25.Molina M, Apaza J, González Monte E, et al. Results of kidney transplantation from deceased donors with acute kidney injury. Transplant Proc. 2015;47(1):42-44. [DOI] [PubMed] [Google Scholar]
- 26.Domagala P, Gorski L, Wszola M, et al. Successful transplantation of kidneys from deceased donors with terminal acute kidney injury. Ren Fail. 2019;41(1):167-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anil Kumar MS, Khan SM, Jaglan S, et al. Successful transplantation of kidneys from deceased donors with acute renal failure. Transplantation. 2006;82(12):1640-1645. [DOI] [PubMed] [Google Scholar]
- 28.Farney AC, Rogers J, Orlando G, et al. Evolving experience using kidneys from deceased donors with terminal acute kidney injury. J Am Coll Surg. 2013;216(4):645-655. [DOI] [PubMed] [Google Scholar]
- 29.Xu L, Guo J, Moledina DG, Cantley LG. Immune-mediated tubule atrophy promotes acute kidney injury to chronic kidney disease transition. Nat Commun. 2022;13(1):4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper M, Wiseman AC, Doshi MD, et al. Understanding delayed graft function to improve organ utilization and patient outcomes. Am J Kidney Dis. 2024;83(3):360-369. doi: 10.1053/j.ajkd.2023.08.018 [DOI] [PubMed] [Google Scholar]
- 31.Kim DW, Tsapepas D, King KL, et al. Financial impact of delayed graft function in kidney transplantation. Clin Transplant. 2020;34(10):e14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper M, Formica R, Friedewald J, et al. Report of National Kidney Foundation consensus conference to decrease kidney discards. Clin Transplant. 2019;33(1):e13419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of all deceased donors receiving vs. not receiving dialysis with kidney procured
eTable 2. Characteristics of deceased donors receiving dialysis with vs. without kidney transplanted
eTable 3. Recipient kidney function at 6 and 12 months after kidney transplant from matched donors receiving vs. not receiving dialysis
eTable 4. Recipient kidney function at 6 and 12 months after kidney transplant from matched donors receiving vs. not receiving dialysis using alternative imputation approaches
eTable 5. Recipient kidney function decline after kidney transplantation from matched donors receiving vs. not receiving dialysis
eTable 6. Recipient outcomes after kidney transplantation from matched donors receiving vs. not receiving dialysis stratified by donor dialysis modality and duration
eTable 7. Recipient outcomes after kidney transplantation from all donors receiving and not receiving dialysis
eTable 8. Recipient kidney function decline after kidney transplantation from all donors receiving vs. not receiving dialysis
Data sharing statement