Abstract
Elicitors from the plant pathogen Erwinia carotovora trigger coordinate induction of the tryptophan (Trp) biosynthesis pathway and Trp oxidizing genes in Arabidopsis. To elucidate the biological role of such pathogen-induced activation we characterized the production of secondary defense metabolites such as camalexin and indole glucosinolates derived from precursors of this pathway. Elicitor induction was followed by a specific increase in 3-indolylmethylglucosinolate (IGS) content, but only a barely detectable accumulation of the indole-derived phytoalexin camalexin. The response is mediated by jasmonic acid as shown by lack of IGS induction in the jasmonate-insensitive mutant coi1-1. In accordance with this, methyl jasmonate was able to trigger IGS accumulation in Arabidopsis. In contrast, ethylene and salicylic acid seem to play a minor role in the response. They did not trigger alterations in IGS levels, and methyl jasmonate- or elicitor-induced IGS accumulation in NahG and ethylene-insensitive ein2-1 mutant plants was similar as in the wild type. The breakdown products of IGS and other glucosinolates were able to inhibit growth of E. carotovora. The results suggest that IGS is of importance in the defense against bacterial pathogens.
Recognition of the pathogen or pathogen-derived elicitors triggers signal cascades that activate a number of defense responses in plants. These include the hypersensitive response, reinforcement of the cell wall (for review, see De Wit, 1997), production of an oxidative burst (for review, see Bolwell, 1999), induction of pathogenesis-related proteins (PRs; for review, see Kombrink and Somssich, 1995), and other defense related compounds such as secondary metabolites. Here, an enhanced synthesis of already existing defense compounds or a de novo synthesis of antimicrobial phytoalexins is possible (Kuc, 1995; Wink, 1999).
In Arabidopsis, the Trp pathway is important for the production of a series of secondary metabolites (Radwanski and Last, 1995). These include the indole-derived phytoalexin camalexin, which appears after inoculation with virulent or avirulent strains of Pseudomonas syringae or treatment with abiotic elicitors (Hammerschmidt et al., 1993), as well as indole glucosinolates (indole GSs). The GSs are pre-formed amino acid-derived secondary metabolites containing a sulfate and a thio-Glc moiety (Halkier, 1999) of which 23 different derivatives have been described in Arabidopsis (Hogge et al., 1988). The most abundant GSs in the leaves of Arabidopsis ecotype Columbia-0 (Col-0) are the Trp-derived indole GSs as well as aliphatic GSs, derived from chain elongated homologs of Met (Haughn et al., 1991). GSs have been implicated in plant defense against various insects and pathogens, but are also important in host-plant recognition by specialized predators. Upon tissue damage the hydrolysis of the vacuole-located GSs is catalyzed by endogenous β-thioglucosidases (myrosinases), stored in separated compartments. Although GSs themselves are thought to be largely non-toxic, their breakdown products are responsible for their various biological activities (Halkier, 1999; Rask et al., 2000).
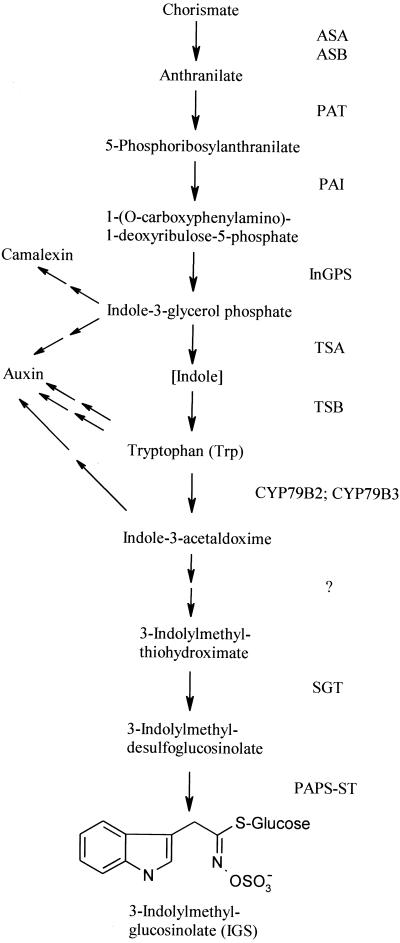
The biosynthetic pathway leading to Trp and subsequently to indole GSs is shown in Figure 1. Although the biosynthesis of Trp in Arabidopsis is well investigated and a series of mutants has been described (Radwanski and Last, 1995; Zhao et al., 1998), much remains to be discovered in the downstream part specific for indole GSs. Two cytochrome P-450 monooxygenases (CYP79B2 and CYP79B3) were recently identified, which are responsible for the specific conversion of Trp to indole-3-acetaldoxime (Hull et al., 2000; Mikkelsen et al., 2000). Indole-3-acetaldoxime is likely to be the intermediate for indole GSs, but may also function as a precursor for auxin formed via the indole-3-acetonitrile pathway (Halkier, 1999; Hull et al., 2000). The genes responsible for the following steps of GS biosynthesis remain to be discovered (Halkier, 1999), except for its penultimate step, the S-glucosylation of a thiohydroximate by an UDPG-thiohydroximate glucosyltransferase (SGT) to produce desulfo-GS (Van Audenhove et al., 1997). SGT has been described in oilseed rape and shows high specificity for thiohydroxymates, but little specificity for the structure of the side chain, so it is probably responsible for the formation of all types of GSs.
Figure 1.
The biosynthetic pathway for Trp and IGS. Enzyme names are shown on the right. Postulated branch points leading to camalexin and auxin are marked with arrows. ASA, Anthranilate synthase α-subunit; ASB, anthranilate synthase β-subunit; PAT, phosphoribosylanthranilate transferase; PAI, phosphoribosyl-anthranilate isomerase; InGPS, indole-3-glycerolphosphate synthase; TSA, Trp synthase α-subunit; TSB, Trp synthase β-subunit; CYP79B2 and CYP79B3, cytochrome P-450 monooxygenases; ?, enzymes between CYP79B and SGT are unknown; SGT, UDPG-thiohydroximate glucosyltransferase; PAPS-ST, 3′-phosphoadenosine 5′-phosphosulfate:desulfogluco-sino- late sulfotransferase.
GSs are thought to form a defense mainly against herbivores. In accordance with this, the GS levels in Brassica napus can be increased by wounding (Bodnaryk, 1992), insect feeding (Hopkins et al., 1998), or treatment with hormones like methyl jasmonate (MeJa) or jasmonic acid (Bodnaryk, 1994; Doughty et al., 1995), but also by fungal infection (Doughty et al., 1991). The inducibility depends on the type of GS: In general, Trp-derived indole GSs are more responsive to induction than aliphatic GSs. Although induction of GSs by bacterial pathogens has not been described, the Trp pathway and, subsequently, camalexin are also induced by P. syringae pv. maculicola and by oxidative stress in Arabidopsis (Zhao and Last, 1996; Zhao et al., 1998).
We have employed the non-specific gram-negative bacterium Erwinia carotovora as a model to probe plant defense responses (Vidal et al., 1998; Norman-Setterblad et al., 2000). This pathogen causes soft-rot disease in a wide variety of plant species with serious damage to crops in the field and during storage (Pérombelon and Kelman, 1980). The pathogenicity of E. carotovora is dependent on its ability to secrete a series of plant cell wall-degrading enzymes, including pectinases and cellulases, which are thought to be the principal virulence factors (Pirhonen et al., 1991). The enzymes are necessary for virulence, but will also release plant cell wall elicitors sufficient to trigger plant defense. Acellular preparations of these enzymes (culture filtrate, CF) of E. carotovora trigger local and systemic induction of defense-related genes in tobacco and in Arabidopsis, as well as enhanced systemic resistance to the pathogen (Palva et al., 1993; Vidal et al., 1997, 1998; Norman-Setterblad et al., 2000). In Arabidopsis, CF triggers two distinct salicylic acid- (SA) independent systemic responses. One is ethylene as well as jasmonic acid-dependent and it has been proposed (Norman-Setterblad et al., 2000) that these hormones act in concert to induce the defense-related genes for hevein-like protein (HEL; Potter et al., 1993), basic chitinase (CHIB; Samac et al., 1990), and a defensin (PDF1.2; Penninckx et al., 1996). The second response related to wound induction is strictly jasmonic acid-mediated (Norman-Setterblad et al., 2000), inhibited by ethylene, and induces vegetative storage protein acid phosphatase (Berger et al., 1995; AtVSP). The involvement of the jasmonate signaling in Arabidopsis defense against E. carotovora was indicated by the enhanced susceptibility of the jasmonate-insensitive coi1-1 mutant to this pathogen (Norman-Setterblad et al., 2000). This fact and the induction of jasmonate-responsive genes suggested that E. carotovora might provide a good model for probing possible jasmonate-controlled responses in plants such as GS induction. This would also give more insight into the general role of secondary metabolites toward bacterial pathogens including E. carotovora. The induction of 3-indolylmethylglucosinolate (IGS) and camalexin in other plant-pathogen interactions suggested that the Trp pathway-derived metabolites in particular might be important in the defense response against E. carotovora.
Here we report that elicitors of E. carotovora trigger specific induction of certain indole GSs in Arabidopsis, which correlates with the induction of Trp and GS pathway transcripts. Furthermore, we show that this induction is mainly jasmonic acid-dependent, whereas ethylene and SA are of minor importance. We suggest that GSs play a role in resistance to E. carotovora.
RESULTS
Induction of the Trp and GS Pathway in Arabidopsis by Elicitors from E. carotovora
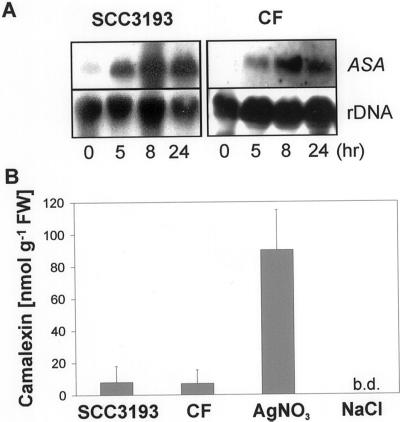
To gain insight into the plant defense mechanisms triggered by E. carotovora and the role of secondary metabolites in this response we screened a subtracted cDNA library of Arabidopsis for CF-induced genes. Our previous work has established that CF of E. carotovora containing secreted plant cell wall-degrading enzymes elicit a response similar to the pathogen in Arabidopsis and tobacco (Vidal et al., 1997, 1998; Norman-Setterblad et al., 2000). From over 200 CF-induced genes of the subtracted cDNA library (G. Brader, E. Tas, and E.T. Palva, unpublished data), several were potentially involved in the production of Trp-derived secondary metabolites. These include the committing step of the Trp pathway ASA, as well as indole-3-glycerolphosphate synthase, TSA, and TSB. RNA gel-blot analyses with ASA show a clear induction of the transcripts in axenic Arabidopsis Col-0 wild-type plants after treatment with CF, as well as after bacterial infection (Fig. 2A).
Figure 2.
Induction of anthranilate synthase α (ASA) and camalexin by E. carotovora. Three-week-old axenic Arabidopsis Col-0 wild-type plants were treated on leaves with 2 μL of CF of E. carotovora subsp. carotovora SCC3193 or the bacteria itself (SCC3193; 2–3 × 106 CFU). A, Material harvested after 0, 5, 8, and 24 h as indicated on the bottom of the figure. Gel RNA blots were hybridized with the probes indicated on the right. B, Camalexin accumulation 48 h after CF and bacterial treatment. As a positive control, camalexin levels 48 h after treatment with 2 μL of silver nitrate (AgNO3; 10 mm) are shown. Mock treatment was performed with 0.9% (w/v) NaCl. b.d., Amounts below detection limit. Values represent averages ± se of three measurements. FW, Fresh weight of tissue.
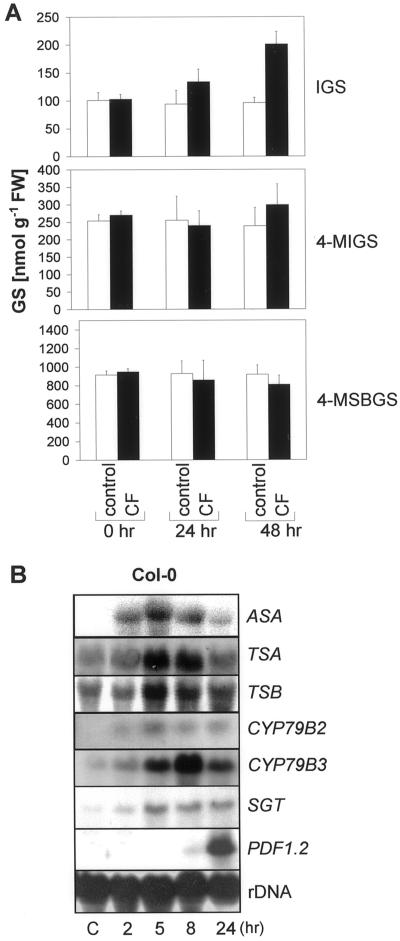
The accumulation of the indolic phytoalexin camalexin in Arabidopsis is coordinately regulated with ASA after P. syringae pv. maculicola infection (Zhao and Last, 1996). Thus, we expected a similar response after E. carotovora infection or CF treatment. However, within 48 h these treatments caused only a very slight accumulation of camalexin in comparison with silver nitrate-treated positive controls (Fig. 2B). The induction of the Trp pathway, but lack of camalexin induction by CF suggested that other Trp-derived secondary metabolites like indole GSs may be affected. To examine this we determined the levels of IGS and 4-methoxy-IGS (4-MIGS) in CF- and mock-treated Col-0 plants. After 48 h, IGS levels in the leaves of the CF-treated plants increased clearly, and the levels of 4-MIGS increased slightly in comparison with mock-treated control plants (Fig. 3A). The content of the most abundant aliphatic GS 4-MSBGS, which does not derive from Trp but Met, did not change significantly within 48 h of CF treatment.
Figure 3.
Induction of GSs and Trp/IGS pathway genes by CF of E. carotovora. Three-week-old axenic Col-0 wild-type plants were treated on leaves with 2 μL of CF of E. carotovora subsp. carotovora SCC3193 or mock treated with 0.9% (w/v) NaCl (control). A, The GSs IGS, 4-MIGS, and 4-methylsulphinylbutylglucosinolate (4-MSBGS) were quantified by HPLC. The accumulation of the GSs 0, 24, and 48 h after treatment is indicated. The data shown are the means ±se of five measurements. In each case the GS content was determined within five pooled plants. FW, Fresh weight of tissue. B, RNA gel blots were probed with the IGS synthesis-related genes ASA, TSA, TSB, CYP79B2, CYP79B3, and SGT as well as the defense related PDF1.2. at different time points after the addition of CF or 5 h after mock treatment (C). Membranes were reprobed with a ribosomal probe to ensure equal loading.
The accumulation of indole GSs was accompanied by a preceding and transient induction of the Trp-pathway genes ASA, TSA, and TSB (Fig. 3B). It was previously shown that TSA and TSB, although induced by P. syringae pv. maculicola, are not involved in camalexin synthesis (Zhao and Last, 1996; Zook, 1998), but in biosynthesis of Trp and indole GSs (Zhao et al., 1998; Halkier, 1999; Fig. 1). The conversion of Trp into the intermediate indole-3-acetaldoxime subsequently leading to GS biosynthesis is catalyzed by at least two cytochrome P450s, encoded by the CYP79B2 and CYP79B3 genes (Hull et al., 2000). One of these, CYP79B3, was clearly induced after CF treatment (Fig. 3B), whereas only weaker induction of CYP79B2 could be detected. In oilseed rape, a UDP-Glc: thiohydroximate SGT responsible for the penultimate step in the GS biosynthesis has been identified (Van Audenhove et al., 1997). It shows 88% homology on the protein level to a putative glucosyltransferase of Arabidopsis (accession no. AC002396). As a consequence, the putative Arabidopsis SGT is a likely part of the GS biosynthetic pathway and the Arabidopsis homologue was used to assess the expression pattern. After CF application, this SGT showed a weak but clear induction in Col-0 ecotype with a similar temporal pattern of expression as the other biosynthetic genes (Fig. 3B).
The Increase of the IGS Content Is Dependent on Jasmonate Signaling
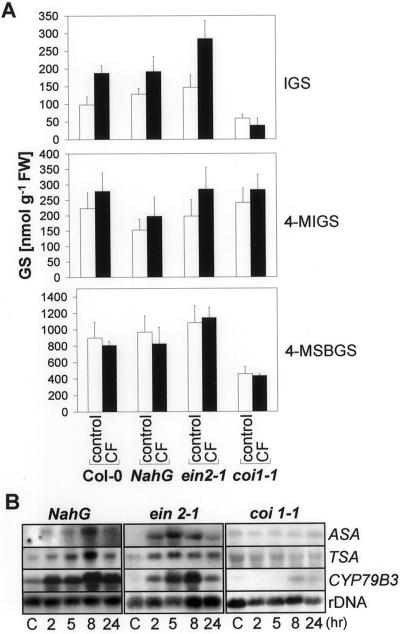
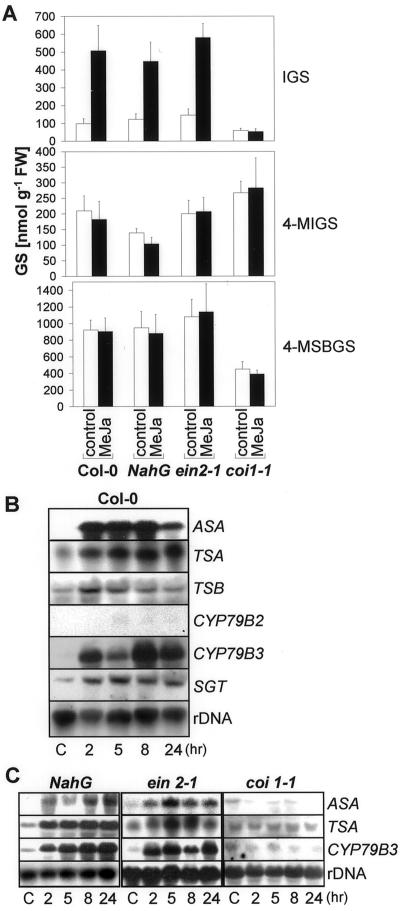
Wounding and MeJa treatment have been shown to induce indole GSs in B. napus (Bodnaryk, 1992, 1994; Doughty et al., 1995). To examine the role of MeJa and other defense-related hormones in regulation of GS levels we characterized CF-induced GS accumulation in various mutants and transgenic plants affected in different defense-response pathways. The requirement for jasmonate signaling was determined by assessing GS amounts in the coronatine and jasmonate-insensitive mutant coi1-1 (Feys et al., 1994), and for ethylene using the ethylene-insensitive mutant ein2-1 (Guzmán and Ecker, 1990). The role of SA was characterized by using NahG-expressing Arabidopsis plants, which are unable to accumulate SA after pathogen attack (Delaney et al., 1994).
CF-induced increase in IGS was not mediated by SA or ethylene signaling. After CF application IGS levels in NahG plants rose to a similar extent as in the wild type, whereas those in ein2-1 rose to even higher levels. In contrast, the jasmonate-insensitive coi1-1 mutant does not show any detectable response (Fig. 4A), suggesting that the response is jasmonate mediated. The other GSs, 4-MIGS and 4-MSBGS, were less affected. However, CF slightly increases 4-MIGS levels in all plants no matter in which pathway they are affected (Fig. 4A). Moreover, already basal levels of IGS differ between Col-0 wild-type plants and these mutants and transgenic plants. Although the ein2-1 and NahG plants had slightly higher levels of IGS than the wild type, coi1-1 plants showed drastically lowered amounts. A similar pattern was also found for the aliphatic GS, 4-MSBGS. On the other hand, amounts of 4-MIGS are slightly higher in the coi1-1 mutant and lower in plants carrying the NahG construct.
Figure 4.
CF-Induction of GSs and Trp/IGS pathway genes in signal transduction mutants. Three-week-old NahG, ein2-1, and coi1-1 axenic plants were treated on leaves with 2 μL of CF or mock treated with 0.9% (w/v) NaCl (control). A, Forty-eight hours after treatment, leaves were harvested and the GSs IGS, 4-MIGS, and 4-MSBGS were quantified by HPLC. The data shown are the means ± se of four measurements. In each case the GS content was determined within five pooled plants. FW, Fresh weight of tissue. B, RNA gel blots were probed with ASA, TSA, and CYP79B3 at different time points after the addition of CF or 5 h after mock treatment (C). Membranes were reprobed with a ribosomal probe to ensure equal loading.
The genes involved in IGS synthesis that are most responsive to CF in wild-type plants include ASA, TSA, and CYP79B3 (Fig. 3B). To correlate the CF-induced expression of these genes to IGS synthesis we characterized the accumulation of the corresponding transcripts in mutant and transgenic plants by RNA gel blots. Consistent with the IGS induction, these genes are also clearly induced by CF in wild-type as well as NahG and ein2-1 plants, as shown in Figure 4B. In contrast, no induction of ASA, TSA, and CYP79B3 could be detected in coi1-1 plants, where no IGS induction could be observed.
The lack of CF induction of IGS and the reduced expression of genes involved in Trp/IGS biosynthesis in the coi1-1 mutant indicated that the jasmonic acid pathway played an important role in the regulation of the GS content. We could detect a clear induction of IGS levels in the leaves of Col-0 plants 24 (data not shown) to 48 h after MeJa application (Fig. 5A). Here, IGS induction was accompanied by enhanced accumulation (up to 42 nmol g−1 fresh weight) of another indole GS, 1-methoxy-3-indolylmethylglucosinolate. The concentration of this GS was lower than 10 nmol g−1 fresh weight in uninduced plants. IGS induction by MeJa was also observed in NahG and ein2-1 plants (Fig. 5A). The coi1-1 mutant did not respond to MeJa and its IGS content remained very low. In contrast to the MeJa induction of IGS, the amounts of the third indole GS, 4-MIGS, and the aliphatic GS, 4-MSBGS, were not up-regulated by MeJa treatment in the mutants or in the wild type (Fig. 5A).
Figure 5.
Induction of GSs and Trp/IGS pathway genes by MeJa. Three-week-old Col-0 wild-type as well as NahG, ein 2-1, and coi 1-1 plants were treated on leaves with 2 μL of MeJa (500 μm) or 0.1% (v/v) Tween 20 (control). A, Forty-eight h after treatment, leaves were harvested and the GSs IGS, 4-MIGS, and 4-MSBGS were quantified by HPLC. The data shown are the means ± se of four measurements. In each case the GS content was determined within five pooled plants. FW, Fresh weight of tissue. B, In experiments with Col-0 wild type, RNA gel blots were probed with ASA, TSA, TSB, CYP79B2, CYP79B3, and SGT at different time points after the addition of MeJa or 5 h after addition of 0.1% (v/v) Tween 20 as control (C). Membranes were reprobed with a ribosomal probe to ensure equal loading. C, RNA gel blots from NahG, ein2-1, and coi1-1 plants were probed with ASA, TSA, and CYP79B3 at different time points after the addition of MeJa or 5 h after mock treatment (C). Membranes were reprobed with a ribosomal probe to ensure equal loading.
This MeJa-induced increase of IGS levels in wild-type plants was accompanied by an induction of the Trp and GS pathway genes ASA, TSA, CYP79B3, and to a smaller extent TSB, as well as SGT (Fig. 5B). No clear induction of transcripts of the second Trp-oxidizing enzyme, CYP79B2, could be observed. The clear induction of Trp transcripts is most remarkable since the used amounts of MeJa were not sufficient to raise camalexin levels to detectable amounts (<1 μg/g fresh weight). The ASA, TSA, and CYP79B3 transcripts were induced by MeJa in ein2-1 and NahG plants in a similar way to the wild type (Fig. 5C). In contrast, no MeJa induction of these transcripts could be observed in the coi1-1 mutant. The induction of IGS by MeJa appears specific. The other signal molecules investigated have less effect on the induction of GSs in Arabidopsis. Treatment with ethylene and with SA was not sufficient to induce significant changes in IGS levels in Col-0 plants within 24 to 48 h. The same is true for 4-MIGS and 4-MSBGS (data not shown).
The GS content varies between plants grown under in vitro conditions and soil plants (data not shown). The IGS concentrations were approximately 3-fold higher in soil-grown than in MS2-grown axenic plants in the Col-0 wild type and in the mutants. In Col-0, CF treatment led in both cases to a 2-fold induction of IGS content. However, after MeJa treatment, amounts of IGS increased 4- to 5-fold in axenic plants compared with a 2- to 3-fold induction in soil-grown plants (data not shown).
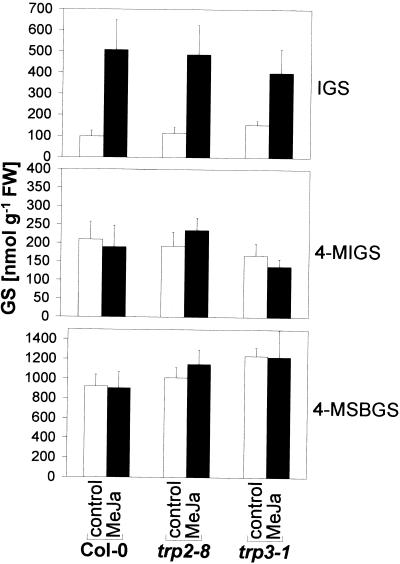
GS Contents in Trp Pathway Mutants
How does a mutation in the Trp pathway affect the synthesis of Trp-derived indole GSs? To answer this question we determined GS levels in the Trp pathway mutants trp3-1 (Radwanski et al., 1996) and trp2-8 (Barczak et al., 1995) with decreased steady-state levels of TSA and TSB proteins, respectively. As shown in Figure 6, these mutations did not have a strong effect on GS regulation. As it has been shown previously for trp3-1 (Müller and Weiler, 2000), both Trp mutants exhibited increased basal IGS levels, but reacted to the MeJa treatment in a similar way as the wild type. However, a slight decrease in MeJa induction of IGS was observed in the trp3-1 mutant. No clear difference in 4-MIGS and 4-MSBGS levels between Col-0 and the mutants were detected.
Figure 6.
GS Induction by MeJa in Trp biosynthesis mutants. Three-week-old axenic trp2-8 and trp3-1 Trp mutant as well as Col-0 wild-type plants were treated on leaves with 2 μL MeJa (500 μm) or 0.1% (v/v) Tween 20 (control). Forty-eight h after treatment, leaves were harvested and the GSs IGS, 4-MIGS, and 4-MSBGS were quantified by HPLC. The data shown are the means ± se of three measurements. In each case the GS content was determined within five pooled plants.
Breakdown Products of IGS Inhibit the Growth of E. carotovora
Could CF-induced IGS or its degradation products help Arabidopsis to protect itself against invading bacterial pathogens such as E. carotovora? To answer this question we determined the concentrations of Col-0, ein2-1, and coi1-1 extracts, which induce 50% inhibition of bacterial growth (IC50) with or without exogenously added myrosinase. The endogenous myrosinase activity was inactivated by heat treatment during the extraction. As shown in Table I, the activity of the extracts correlates with the IGS and 4-MSBGS content. Extracts from ein2-1 and wild type were more potent inhibitors of the growth of E. carotovora than coi1-1 extracts with lower GS content. Only extracts with added myrosinase showed any growth inhibiting activity. Together with the high substrate specificity of myrosinase (Halkier, 1999), this demonstrates that GSs and their breakdown products, respectively, but no other compounds are responsible for the observed effects.
Table I.
Effect of GS-containing Arabidopsis extracts on the growth of E. carotovora subsp. carotovora SCC3193
| Extract | Myrosinase | IC50a | FLb |
|---|---|---|---|
| Col-0 | − | >60 | |
| Col-0 | + | 3.3 | (2.7–4.2) |
| ein2-1 | − | >60 | |
| ein2-1 | + | 1.6 | (0.8–3.5) |
| coi1-1 | − | >60 | |
| coi1-1 | + | 12.7 | (9.2–17.3) |
| Myrosinase | No inhibitory effect up to 10 units mL−1 | ||
Arabidopsis extracts from Col-0 wild type, as well as ein 2-1 and coi 1-1 mutant plants, were dried and resuspended in Luria-Bertani (LB) medium. During extraction, endogenous myrosinases were heat inactivated. Serial dilutions of the extracts were added to bacterial cultures suspended in 50 mm phosphate buffer (−) or in 50 mm phosphate buffer containing 1 unit mL−1 myrosinase (+).
The IC50 is the concentration, which resulted in 50% inhibition of bacterial growth after 15 h of incubation at 28°C. Values are in milligrams of extract (dry wt) per milliliter of LB medium.
FL, 95% Fiducial limits.
Similar results were obtained testing purified IGS, as shown in Table II. Only IGS with added myrosinase showed bacterial growth retarding activity, but not IGS alone. It is interesting that except for indole-3-acetonitrile, the other possible degradation products of IGS, indole-3-carbinol, potassium thiocyanate, 3,3′-diindolylmethane, and 3-indolylmethyl-ascorbate (Mithen et al., 1986; Agerbirk et al., 1998) showed no activity at tested concentrations.
Table II.
Effect of IGS and its degradation products on the growth of E. carotovora subsp. carotovora SCC3193
| Compound | IC50a | FLb |
|---|---|---|
| IGS | >1.2 | |
| IGS + myrosinase | 0.095 | (0.053–0.191) |
| Indole-3-acetonitrile | 0.246 | (0.190–0.335) |
| Indole-3-carbinol | >1.2 | |
| Potassium thiocyanate | >1.2 | |
| 3-Indolylmethylascorbate | >1.2 | |
| 3,3′-Diindolylmethane | >1.2 |
Test compounds were dissolved in LB medium containing 0.5% ethanol and 0.1% Tween 20. Serial dilutions were added to bacterial cultures suspended in 50 mm phosphate buffer or in 50 mm phosphate buffer containing 1 unit mL−1 myrosinase.
The IC50 is the concentration, which resulted in 50% inhibition of bacterial growth after 15 h of incubation at 28°C. Values are in milligrams per milliliter of LB medium.
FL = 95% Fiducial limits.
DISCUSSION
We have shown that E. carotovora CF triggers jasmonate-dependent production of IGS in Arabidopsis and we have indicates that IGS might form part of the defense response against bacterial pathogens. The role of IGS in defense is supported by the data showing that Arabidopsis extracts containing breakdown products of GSs display in vitro activity against E. carotovora. Also, purified IGS treated with myrosinase is capable of inhibiting bacterial growth. The formation and the toxicity of the unstable intermediate 3-indolylmethylisothiocyanate can probably explain this effect. This compound is thought to be formed during IGS-hydrolysis, but disintegrates quickly into indole-3-carbinol and thiocyanate (Hanley et al., 1990; Agerbirk et al., 1998). It is not very likely that indole-3-acetonitrile is responsible for the observed activity of IGS + myrosinase, since at the pH values used in the bioassay (pH 7), only traces of this compound are formed (Agerbirk et al., 1998). Similar in vitro activities of indole GS breakdown products have been described previously against the fungus Leptosphaeria maculans (Mithen et al., 1986). However, the activity of the GS extracts (Table I) can only be partially explained by the toxicity of IGS breakdown products alone, because even diluted plant extracts containing less calculated IGS than its IC50 (determined by testing isolated IGS as shown in Table II) exhibited clear bacterial inhibition. This may be due to additional or synergistic effects of the breakdown products of other GSs.
However, our studies suggest that there is a correlation between GS content and plant defense against bacterial pathogens such as E. carotovora. This is supported by our previous studies demonstrating that the jasmonate-insensitive mutant coi1-1 exhibits increased susceptibility to E. carotovora infection (Norman-Setterblad et al., 2000) and has a low GS content, as shown here (Fig. 4A). Moreover, pre-treatment of Col-0 plants with MeJa, which leads to increase of IGS content (Fig. 5A), triggers efficient protection against invading bacteria (G. Brader, E. Tas, and E.T. Palva, unpublished data), but does not result in strong induction of the defense-related genes PDF1.2, CHIB, and HEL in axenic plants (Penninckx et al., 1998; Norman-Setterblad et al., 2000).
The regulation of IGS accumulation by MeJa and the low GS content in the coi1-1 mutant may also help to explain the jasmonate-induced resistance toward some fungi and insects (Reymond and Farmer, 1998). Thomma et al. (1998) showed that the Arabidopsis coi1-1 mutant is more susceptible to the fungus Alternaria brassicicola than the wild type. Moreover, Thomma et al. (1999b) showed that MeJa can boost the level of resistance of the camalexin deficient mutant pad3-1 as well as the wild type, but not of the coi1-1 mutant. The response is independent of ethylene, because ein2-1 can also be protected by pre-treatment with MeJa (Thomma et al., 1999a). Thomma et al. (1999b) predicted MeJa-dependent effector molecules that contribute (together with camalexin) to the resistance of Arabidopsis against A. brassicola. IGS (probably together with other GSs) fulfill the criteria for these effectors and may take part in resistance of Arabidopsis against A. brassicola.
The definite proof for the extent of contribution of indole GSs in vivo to plant protection against pathogens has to await generation of mutants or transgenic plants affected in IGS biosynthesis (Fig. 1). Such a mutant would help to clarify the biological importance of these compounds in plant defense. So far, no biosynthetic mutants downstream of Trp have been described. Most of the known GS mutants (Haughn et al., 1991) have an altered aliphatic GS content. Only Tu8 has reduced indole GS levels in leaves, but enhanced indole GS levels in seeds and root tissue. The altered growth morphology of Tu8 suggests that it is a pleiotropic signal transduction rather than a GS biosynthesis mutant (Haughn et al., 1991; Ludwig-Müller et al., 1999). Other candidates for mutants with low IGS contents are the mutants deficient in Trp biosynthesis such as trp2-8 and trp3-1 with decreased TSB and TSA enzyme activity, respectively. However, these mutants turned out to have slightly enhanced basal levels of IGS and responded to MeJa almost to wild-type levels (Fig. 6; Müller and Weiler, 2000). Similar results were obtained when measuring camalexin levels in Trp pathway mutants (Zhao and Last, 1996). The mutant trp1-100 and the Trp auxotroph double mutant trp1-100 trp4-1 show only slight reduction in camalexin accumulation after infection with P. syringae pv. maculicola. This suggests that the flux through the Trp pathway in the mutants is obviously sufficient for the production of the secondary metabolites camalexin and IGS.
The CF- and MeJa-induced accumulation of IGS is preceded by an induction of several of the Trp and GS biosynthetic pathway genes including ASA, TSA, TSB, CYP79B3, and SGT in Arabidopsis (Figs. 3 and 5). It has been previously demonstrated that infection by P. syringae pv. maculicola as well as oxidative stress induce, coordinately, camalexin and Trp pathway gene expression and enzymes (Zhao and Last, 1996; Zhao et al., 1998). In contrast to these studies, treatment with CF from E. carotovora or MeJa does not lead to camalexin accumulation. Because the Trp pathway intermediate indole-3-glycerol phosphate is thought to be the branching point in camalexin and Trp biosynthesis (Fig. 1; Zook, 1998), the up-regulation of TSA and TSB downstream of indole-3-glycerol phosphate cannot be explained by further need of Trp for camalexin biosynthesis. However, it agrees with the observed IGS accumulation. Also, the observed induction of CYP79B3 agrees with IGS accumulation, since this gene is responsible for oxidization of Trp in the first step in indole GS biosynthesis downstream of Trp (Fig. 1; Hull et al., 2000; Mikkelsen et al., 2000). It is interesting that CYP79B2, the second gene shown to be responsible for Trp oxidation (Hull et al., 2000), does not respond to MeJa in the same way (Fig. 5). Our demonstration of MeJa and CF induction of Trp/IGS pathway gene expression and IGS accumulation is in agreement with and explains earlier induction data. It has been demonstrated that MeJa treatment leads to an increase of IGS levels in oilseed rape and Indian mustard (Bodnaryk, 1994; Doughty et al., 1995) and that ASA1 mRNA levels increase after wounding in Arabidopsis (Radwanski and Last, 1995).
Our results show that the induction of IGS requires jasmonate signaling. First, IGS is induced strongly by MeJa and second, the induction of IGS is blocked in the jasmonate signal mutant coi1-1, but not in the ethylene signal mutant ein2-1 or in NahG plants unable to accumulate SA. Unlike induction in the wild-type plants, IGS levels and Trp/IGS pathway transcripts do not increase in the coi1-1 mutant after induction by MeJa or CF. Moreover, already basal IGS levels are lower in the coi1-1 mutant, demonstrating the requirement for functional COI in IGS regulation. However, production of IGS does not rely completely on COI, since coi1-1 plants produce IGS, albeit at lower levels. The stronger induction of IGS with MeJa than with CF may have various reasons. One likely explanation would be that other signal pathways triggered by CF involving ethylene, SA, or reactive oxygen species may interfere with the MeJa response. Such interference is suggested by results with the ein2-1 mutant blocked in ethylene signaling. This mutant shows elevated IGS induction compared with the wild type (Fig. 4A), suggesting that ethylene down-regulates the jasmonate response. Similar results have been observed previously for jasmonate-induced expression of AtVSP (Norman-Setterblad et al., 2000). In contrast to IGS, 4-MIGS levels were even slightly higher in coi1-1 than in the wild-type plants (Fig. 4A). Moreover, 4-MIGS was not induced by MeJa treatment of Col-0. This is unexpected, since it has been implicated that 4-MIGS is also derived from Trp (Halkier, 1999). If this is the case, the separating steps in the biosynthesis of IGS and 4-MIGS are regulated in a different way.
Ethylene signaling does not appear to be necessary for CF-induced gene expression of the Trp/IGS pathway. The induction of IGS was even slightly elevated in the ein2-1 mutant in comparison with the wild type. Moreover, ethylene at concentrations known to induce defense-related genes like PDF1.2 (Penninckx et al., 1998) is not sufficient to induce accumulation of any of the tested GSs (data not shown). The slightly higher basal IGS levels in ein2-1 may be due to the higher values of free jasmonic acid in this mutant (Penninckx et al., 1998). Like ethylene, SA seems to have a minor role in GS regulation. SA at concentrations known to induce PR-1 (Norman-Setterblad et al., 2000) is not sufficient to induce changes of IGS, 4-MIGS, and 4-MSBGS (data not shown). Furthermore, the induction of the Trp/IGS pathway was similar in NahG and wild-type plants. However, in oilseed rape, SA is able to induce changes in the content of phenolic GSs (Kiddle et al., 1994), which are not present in detectable amounts in Arabidopsis Col-0.
Taken together, our data clearly demonstrates that jasmonate signaling is required for the induction of certain defense compounds such as IGS. This is in agreement with previous studies on jasmonate-dependent induction of camalexin and thionins by certain pathogens (Reymond and Farmer, 1998; Zhou et al., 1999) and underlines the importance of jasmonate signaling in plant defense response.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of Arabidopsis ecotype Col-0 were vernalized for 2 d at 4°C, surface sterilized, sown on Murashige-Skoog-2 medium plates (Murashige and Skoog, 1962), and replanted in 300-mL jars (three plants per jar) after germination. Sterile plants were grown at 22°C with a light/dark regime of 12 h/12 h at 40 μmol m−2 s−1. Soil-grown plants were cultivated at the same conditions on a 1:1 mixture of vermiculite and peat (Finnpeat, B2). The Arabidopsis mutants trp2-8 (Barczak et al., 1995), trp3-1 (Radwanski et al., 1996), and ein2-1 (Guzmán and Ecker, 1990) were obtained from the Biological Resource Center (Columbus, OH; accession nos. CS8328, CS8331, and CS3071) and the coi1-1 mutant (Feys et al., 1994) was obtained from J. Turner (University of East Anglia, Norwich, UK). The transgenic line expressing the NahG gene (Delaney et al., 1994) was provided by Dr. John Ryals (Ciba Geigy, Research Triangle Park, NC). All plants were derived from the Col-0 ecotype.
Application of Bacteria, Extracellular Enzyme Preparations, and Chemicals
Erwinia carotovora subsp. carotovora strain SCC3193 (Pirhonen et al., 1988) was cultured overnight at 28°C in LB medium. Bacteria were harvested by centrifugation (15 min at 4,000g), resuspended in 0.9% (w/v) NaCl at 2 to 3 × 108 CFU per mL, and applied as 2-μL droplets on leaves to inoculate plants. CF was prepared from E. carotovora subsp. carotovora SCC3193 as described previously and (Vidal et al., 1998) CF, silver nitrate (10 mm), MeJa (500 μm), SA (5 mm), 0.9% (w/v) NaCl, and 0.1% (v/v) Tween 20 were applied as 2-μL droplets on five leaves of 3-week-old plants as one drop on each leaf for axenic plants and five drops for soil-grown plants. Ethylene treatment was done for 24 h by placing jars in an airtight chamber containing air only or 50 μL L−1 ethylene. Ethylene concentrations were measured by a photoionizer (PI 101 trace gas analyzer, HNU Systems, Newton, MA).
Antibacterial Assay
The concentrations of the test compounds inducing 50% bacterial growth inhibition (IC50) were determined by monitoring the proliferation of E. carotovora subsp. carotovora SCC3193 at 28°C with optical density measurements at 600 nm as described by Feder et al. (2000). The inhibition of bacterial growth caused by the test compounds and the Arabidopsis extracts was determined in microtiter plates 15 h after inoculation with 105 CFU mL−1 E. carotovora subsp. carotovora SCC3193 resuspended in 50 mm sodium phosphate buffer, pH 7, with or without myrosinase (1 unit mL−1). IC50 values and the error range (95% fiducial limits) were calculated by probit analysis. Arabidopsis extracts containing intact GSs were achieved by extracting 1 g of leaves from 12 pooled, 3-week-old soil-grown plants for 15 min in boiling 70% (v/v) ethanol to denature myrosinases. Extracts were evaporated, resuspended in 500 μL of LB medium, and serially diluted. IGS was purified from 50 g of brussel sprouts by the method described by Thies (1988) and Agerbirk et al. (1998) to give a yield of 6.1 μmol pure IGS as potassium salt. 3,3′-diindolylmethane and 3-indolylmethylascorbate were synthesized from 3-indolylcarbinol and ascorbic acid, as described (Agerbirk et al., 1996, 1998). Spectroscopic data agree with Agerbirk et al. (1998). Ascorbic acid, indole-3-acetonitrile, indole-3-carbinol, and myrosinase (thioglucosidase, EC 3.2.3.1 from white mustard) were purchased from Sigma-Aldrich (St. Louis) and potassium thiocyanate was purchased from ICN Biomedicals (Aurora, OH). The test compounds were dissolved in LB medium with 0.1% (v/v) Tween 20 and 0.5% (v/v) ethanol at a concentration of 1 mg mL−1 and were then serially diluted.
GS and Camalexin Analysis
After removal of the two oldest leaves, the leaf material (200–400 mg) of five pooled plants was rapidly frozen and ground in liquid nitrogen and extracted twice into 20 times their fresh weight of boiling 70% (v/v) ethanol for 10 min. After cooling on ice, a lead acetate solution (0.5 m) was added to a final concentration of 0.05 m. The extract was vortexed, allowed to stand on ice for 15 min, and was centrifuged for 5 min at 2,500g. GSs in the supernatant were concentrated by adsorption onto diethylaminoethyl Sephadex A-25 (pyridine acetate form). Bound GSs were enzymatically desulfated with 100 μL of aryl sulfatase (Type H-1, Sigma) at 28°C and were eluted with three times 330 μL of water after 15 h (Shaw et al., 1989), of which 100 μL was used for HPLC analysis. The desulfo-GSs were determined and quantified by the use of response factors according to Hogge et al. (1988) and Haughn et al. (1991). Twenty micrograms of an internal standard (benzylglucosinolate; Merck, Rahway, NJ) was added at the beginning of the extraction procedure to calculate losses during the extraction and separation. HPLC analysis was performed on a Perkin-Elmer Series 200 (Foster City, CA) with a Photodiode Array Detector 2356 on a Lichrospher (Merck) C18 column (125 × 4 mm i.d., 5-μm particle size; UV diode-array detection: 225 nm; mobile phase: 1% [v/v] acetonitrile in water for 5 min, a linear gradient from 1%–22.5% [v/v] acetonitrile over the next 15 min, constant at 22.5% [v/v] acetonitrile for 5 min, followed by washing with pure acetonitrile and reconditioning of the column at 1% [v/v] acetonitrile for 10 min; flow rate was 1 mL min−1). Camalexin was determined using the protocol of Hammerschmidt et al. (1993).
Extraction of RNA and Northern Hybridization Analyses
Total RNA from each plant was separately prepared by SDS/phenol/chloroform extraction and LiCl precipitation (Kingston, 1997). Ten micrograms of RNA was denatured in formamide, separated by electrophoresis through formaldehyde agarose gels, and blotted onto a positively charged nylon membrane (Boehringer Mannheim, Basel) via capillary transfer with 20× SSC (Sambrook et al., 1989). The membrane was hybridized overnight at 50°C with digoxigenin-dUTP- (Boehringer Mannheim) labeled probes described below, followed by two washes for 15 min in 2× SSC, 0.1% (w/v) SDS and 0.1× SSC, 0.1% (w/v) SDS at 50°C. Chemiluminescent detection was done with disodium 3-(4-methoxyspiro[1,2-dioxetane-3,2′-{5′-chloro}tricyclo{3.3.1.13,7}decan]4-yl) phenyl phosphate, according to the instructions of the supplier (Boehringer Mannheim). DNA probes were amplified from the cDNA of the enzymes ASA1 (Niyogi and Fink, 1992), TSA1 (Radwanski et al., 1995), and TSB1 (Berlyn et al., 1989). The probes for PDF 1.2 and CYP79B2 contain the expressed sequence tag with GenBank accession nos. T04323 and T42902, respectively, both obtained from the Arabidopsis Biological Resource Center. The fragments of the gene for the putative Arabidopsis UDP-Glc: thiohydroximate SGT and the cytochrome P450 CYP79B3 were amplified from genomic Arabidopsis Col-0 DNA by PCR and were cloned into the pCR 2.1 vector (Invitrogen, San Diego). The primers for SGT correspond to the bases 4,891 to 4,911 and 5,548 to 5,568 of the bacterial artificial chromosome F316 genomic sequence with the GenBank accession no. AC002396. The primers for CYP79B3 correspond to the bases 1,249 to 1,268 and 2,237 to 2,256 of the sequence with the GenBank accession no. AC006592.4. To verify equal loading and transfer, the RNA loading buffer was supplemented with ethidium bromide and the blots were reprobed with a ribosomal probe.
Subtractive Library
Three-week-old axenic Arabidopsis Col-0 were sprayed with CF of E. carotovora subsp. carotovora strain SCC3193 or with LB media. Samples were collected after 2, 4, and 6 h, pooled, and total RNA was extracted as noted above. Poly(A) RNA was purified from total RNA with DynaBeads (Dynal A.S., Oslo). cDNA synthesis and the cDNA subtraction between CF and control-treated plants was performed using the PCR-Select cDNA Subtraction Kit (CLONTECH Laboratories, Palo Alto, CA) following the instruction of the manufacturer. The subtracted library was cloned into the vector pCR2.1 (Invitrogen).
ACKNOWLEDGMENTS
We thank John G. Turner for coi1-1 mutant seeds, Dr. John Ryals for seeds of transgenic Arabidopsis carrying the NahG gene, and the Arabidopsis Biological Resource Center (Columbus, OH) for providing the ein2-1 and the Trp pathway mutants. We want to acknowledge R. Hiltunen for providing HPLC and spectroscopic facilities.
Footnotes
This work was supported by the Academy of Finland (Finnish Centre of Excellence program), Biocentrum Helsinki, and the European Union (contract no. ERBIC15–CT96–0908).
LITERATURE CITED
- Agerbirk N, Bjergegaard C, Olsen CE, Sørensen H. Kinetic investigation of the transformations of indol-3-ylcarbinol into oligomeric indolyl compounds based on micellar elektrokinetic capillary chromatography. J Chromatography A. 1996;745:239–248. [Google Scholar]
- Agerbirk N, Olsen CE, Sørensen H. Initial and final products, nitriles, and ascorbigens produced in myrosinase-catalyzed hydrolysis of indole glucosinolates. J Agric Food Chem. 1998;46:1563–1571. [Google Scholar]
- Barczak AJ, Zhao J, Pruitt KD, Last RL. 5-Fluoroindole resistance identifies tryptophan synthase beta subunit mutants in Arabidopsis thaliana. Genetics. 1995;140:303–313. doi: 10.1093/genetics/140.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Bell E, Sadka A, Mullet JE. Arabidopsis thaliana Atvsp is homologous to soybean VspA and VspB, genes encoding vegetative storage protein acid phosphatases, and is regulated similarly by methyl jasmonate, wounding, sugars, light and phosphate. Plant Mol Biol. 1995;27:933–942. doi: 10.1007/BF00037021. [DOI] [PubMed] [Google Scholar]
- Berlyn MB, Last RL, Fink GR. A gene encoding the tryptophan synthase beta subunit of Arabidopsis thaliana. Proc Natl Acad Sci USA. 1989;86:4604–4608. doi: 10.1073/pnas.86.12.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnaryk RP. Effects of wounding on glucosinolates in the cotyledons of oilseed rape and mustard. Phytochemistry. 1992;31:2671–2677. [Google Scholar]
- Bodnaryk RP. Potent effect of jasmonates on indole glucosinolates in oilseed rape and mustard. Phytochemistry. 1994;35:301–305. [Google Scholar]
- Bolwell GP. Role of active oxygen species and NO in plant defense responses. Curr Opin Plant Biol. 1999;2:287–294. doi: 10.1016/S1369-5266(99)80051-X. [DOI] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weyman K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- De Wit PJGM. Pathogen avirulence and plant resistance: a key role for recognition. Trends Plant Sci. 1997;2:452–458. [Google Scholar]
- Doughty KJ, Kiddle GA, Pye BJ, Wallsgrove RM, Pickett JA. Selective induction of glucosinolates in oilseed rape leaves by methyl jasmonate. Phytochemistry. 1995;38:347–350. [Google Scholar]
- Doughty KJ, Porter AJR, Morton AM, Kiddle GA, Bock CH, Wallsgrove R. Variation in the glucosinolate content of oilseed rape (Brassica napus L.) leaves: II. Response to infection by Alternaria brassicae (Berk.) Sacc. Ann Appl Biol. 1991;118:469–478. [Google Scholar]
- Feder R, Dagan A, Mor A. Structure-activity relationship study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J Biol Chem. 2000;275:4230–4238. doi: 10.1074/jbc.275.6.4230. [DOI] [PubMed] [Google Scholar]
- Feys BJF, Benedetti CE, Penfold CN, Turner JG. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell. 1994;6:751–759. doi: 10.1105/tpc.6.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkier BA. Glucosinolates. In: Ikan R, editor. Naturally Occurring Glycosides: Chemistry, Distribution, and Biological Properties. New York: John Wiley & Sons; 1999. pp. 193–223. [Google Scholar]
- Hammerschmidt R, Tsuji J, Zook M, Somerville SA. A phytoalexin from Arabidopsis thaliana and its relationship to other phytoalexins of crucifers. In: Davis KR, Hammerschmidt R, editors. Arabidopsis thaliana As a Model for Plant-Pathogen Interactions. APS Press, St. Paul. 1993. pp. 73–84. [Google Scholar]
- Hanley BA, Parsley KR, Lewis JA, Fenwick RG (1990) Chemistry of indole glucosinolates: Intermediacy of indole-3-ylmethyl isothiocyanates in the enzyme hydrolysis of indole glucosinolates. J Chem Soc Perkin Trans I: 2273–2276
- Haughn GW, Davin L, Giblin M, Underhill EW. Biochemical genetics of plant secondary metabolites in Arabidopsis thaliana: the glucosinolates. Plant Physiol. 1991;97:217–226. doi: 10.1104/pp.97.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogge LR, Reed DW, Underhill EW, Haughn GW. HPLC separation of glucosinolates from leaves and seeds of Arabidopsis thaliana and their identification using thermospray liquid chromatography-mass spectrometry. J Chromatogr Sci. 1988;26:551–556. [Google Scholar]
- Hopkins RJ, Griffiths DW, Birch ANE, McKinlay RG. Influence of increasing herbivore pressure on modification of glucosinolate content of swedes (Brassica napus spp. rapifera) J Chem Ecol. 1998;24:2003–2019. [Google Scholar]
- Hull AK, Vij R, Celenza JL. Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc Natl Acad Sci USA. 2000;97:2379–2384. doi: 10.1073/pnas.040569997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiddle GA, Doughty KJ, Wallsgrove RM. Salicylic acid-induced accumulation of glucosinolates in oilseed rape (Brassica napus L.) leaves. J Exp Bot. 1994;45:1343–1346. [Google Scholar]
- Kingston RF. Preparation and analysis of RNA. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1997. pp. 4.3.1–4.3.4. [Google Scholar]
- Kombrink E, Somssich IE. Defense responses of plants to pathogens. Adv Bot Res. 1995;21:1–34. [Google Scholar]
- Kuc J. Phytoalexins, stress metabolism, and disease resistance in plants. Annu Rev Phytopathol. 1995;33:275–297. doi: 10.1146/annurev.py.33.090195.001423. [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J, Pieper K, Ruppel M, Cohen JD, Epstein E, Kiddle G, Bennett R. Indole glucosinolate and auxin biosynthesis in Arabidopsis thaliana (L.) Heynh: glucosinolate mutants and the development of clubroot disease. Planta. 1999;208:409–419. doi: 10.1007/s004250050576. [DOI] [PubMed] [Google Scholar]
- Mikkelsen MD, Hansen CH, Wittstock U, Halkier BA. Cytochrome P450 CYP79B2 from Arabidopsis catalyzes the conversion of tryptophan to indole-3-acetaldoxime, a precursor of indole glucosinolates and indole-3-acetic acid. J Biol Chem. 2000;275:33712–33717. doi: 10.1074/jbc.M001667200. [DOI] [PubMed] [Google Scholar]
- Mithen RF, Lewis BG, Fenwick GR. In vitro activity of glucosinolates and their products against Leptosphaeria maculans. Trans Br Mycol Soc. 1986;87:433–440. [Google Scholar]
- Müller A, Weiler EW. Indolic constituents and indole-3-acetic acid biosynthesis in the wild-type and a tryptophan auxotroph mutant of Arabidopsis thaliana. Planta. 2000;211:855–863. doi: 10.1007/s004250000353. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Niyogi KK, Fink GR. Two anthranilate synthase genes in Arabidopsis: defense-related regulation of the tryptophan pathway. Plant Cell. 1992;4:721–733. doi: 10.1105/tpc.4.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman-Setterblad C, Vidal S, Palva ET. Interacting signal pathways control defense gene expression in Arabidopsis in response to the plant pathogen Erwinia carotovora. Mol Plant-Microbe Interact. 2000;13:430–438. doi: 10.1094/MPMI.2000.13.4.430. [DOI] [PubMed] [Google Scholar]
- Palva TK, Holmström KO, Heino P, Palva ET. Induction of plant defense response by exoenzymes of Erwinia carotovora ssp. carotovora. Mol Plant-Microbe Interact. 1993;6:190–196. [Google Scholar]
- Penninckx IAMA, Eggermont K, Terras FRG, Thomma BPHJ, De Samblanx GW, Buchala A, Métraux JP, Manners JM, Broekart WF. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell. 1996;8:2309–2323. doi: 10.1105/tpc.8.12.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx IAMA, Thomma BPHJ, Buchala A, Métraux JP, Broekaert WF. Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell. 1998;10:2103–2113. doi: 10.1105/tpc.10.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérombelon MCM, Kelman A. Ecology of the soft rot Erwinia. Annu Rev Phytopathol. 1980;18:361–387. [Google Scholar]
- Pirhonen M, Heino P, Helander I, Harju P, Palva ET. Bacteriophage T4-resistant mutants of the plant pathogen Erwinia carotovora. Microbe Pathog. 1988;4:359–367. doi: 10.1016/0882-4010(88)90063-0. [DOI] [PubMed] [Google Scholar]
- Pirhonen M, Saarilahti H, Karlsson MB, Palva ET. Identification of pathogenicity determinants of Erwinia carotovora ssp. carotovora by transposon mutagenesis. Mol Plant-Microbe Interact. 1991;4:276–283. [Google Scholar]
- Potter S, Uknes S, Lawton K, Winter AM, Chandler D, Di Maio J, Novitzky R, Ward E, Ryals J. Regulation of a hevein-like gene in Arabidopsis. Mol Plant-Microbe Interact. 1993;6:680–685. doi: 10.1094/mpmi-6-680. [DOI] [PubMed] [Google Scholar]
- Radwanski ER, Barczak AJ, Last RL. Characterization of tryptophan synthase alpha subunit mutants of Arabidopsis thaliana. Mol Gen Genet. 1996;253:353–361. doi: 10.1007/pl00008602. [DOI] [PubMed] [Google Scholar]
- Radwanski ER, Last RL. Tryptophan biosynthesis and metabolism: biochemical and molecular genetics. Plant Cell. 1995;7:921–934. doi: 10.1105/tpc.7.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanski ER, Zhao J, Last RL. Arabidopsis thaliana tryptophan synthase alpha: gene cloning, expression, and subunit interaction. Mol Gen Genet. 1995;248:657–667. doi: 10.1007/BF02191705. [DOI] [PubMed] [Google Scholar]
- Rask L, Andréasson E, Ekbom B, Eriksson S, Pontoppidan B, Meijer J. Myrosinase: gene family evolution and herbivore defense in Brassicaceae. Plant Mol Biol. 2000;42:93–113. [PubMed] [Google Scholar]
- Reymond P, Farmer EE. Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol. 1998;1:404–411. doi: 10.1016/s1369-5266(98)80264-1. [DOI] [PubMed] [Google Scholar]
- Samac DA, Hironaka CM, Yallaly PE, Shah DM. Isolation and characterization of the genes encoding basic and acidic chitinase in Arabidopsis thaliana. Plant Physiol. 1990;93:907–914. doi: 10.1104/pp.93.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Shaw GJ, Andrzejewski D, Roach JAG, Sphon JA. Separation and identification of glucosinolates from Brassica vegetables using high-performance capillary gas chromatography (GC)-positive ion chemical ionization mass spectrometry (PICIMS) and GC-PICIMS/MS. J Agric Food Chem. 1989;37:372–378. [Google Scholar]
- Thies W. Isolation of sinigrin and glucotropaeolin from cruciferous seeds. Fat Sci Technol. 1988;90:311–314. [Google Scholar]
- Thomma BPHJ, Eggermont K, Penninckx IAMA, Mauch-Mani B, Vogelsang R, Cammue BPA, Broekart WF. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc Natl Acad Sci USA. 1998;95:15107–15111. doi: 10.1073/pnas.95.25.15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Eggermont K, Tierens KFM-J, Broekart WF. Requirement of functional EIN2 (ethylene insensitive 2) gene for efficient resistance of Arabidopsis thaliana to infection by Botrytis cinerea. Plant Physiol. 1999a;121:1093–1101. doi: 10.1104/pp.121.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BPHJ, Nelissen I, Eggermont K, Broekart WF. Deficiency in phytoalexin production causes enhanced susceptibility of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J. 1999b;19:163–171. doi: 10.1046/j.1365-313x.1999.00513.x. [DOI] [PubMed] [Google Scholar]
- Van Audenhove K, Marillia E, Peferoen M, Grootwassink JWD, Reed DW, Hemmingsen SM, Kolenovsky AD, Underhill EW, Macpherson JM, inventors May 9, 1997. Plants with reduced glucosinolate content. Patent No. WO 97/16559
- Vidal S, de León IP, Denecke J, Palva ET. Salicylic acid and the plant pathogen Erwinia carotovora induce defense genes via antagonistic pathways. Plant J. 1997;11:115–123. [Google Scholar]
- Vidal S, Eriksson ARB, Montesano M, Denecke J, Palva ET. Cell wall-degrading enzymes from Erwinia carotovora cooperate in the salicylic acid-independent induction of a plant defense response. Mol Plant-Microbe Interact. 1998;11:23–32. [Google Scholar]
- Wink M. Biochemistry, role and biotechnology of secondary metabolites. In: Wink M, editor. Functions of Plant Secondary Metabolites and Their Exploitation in Biotechnology. Sheffield, UK: CRC Press; 1999. pp. 1–16. [Google Scholar]
- Zhao J, Last RL. Coordinate regulation of the tryp-tophan biosynthetic pathway and indolic phytoalexin accumulation in Arabidopsis. Plant Cell. 1996;8:2235–2244. doi: 10.1105/tpc.8.12.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Williams CC, Last RL. Induction of Arabidopsis tryptophan pathway enzymes and camalexin by amino acid starvation, oxidative stress, and an abiotic elicitor. Plant Cell. 1998;10:359–370. doi: 10.1105/tpc.10.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Tootle TL, Glazebrook J. Arabidopsis PAD3, a gene required for camalexin biosynthesis, encodes a putative cytochrome P450 monooxygenase. Plant Cell. 1999;11:2419–2428. doi: 10.1105/tpc.11.12.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zook M. Biosynthesis of camalexin from tryptophan pathway intermediates in cell- suspension cultures of Arabidopsis. Plant Physiol. 1998;118:1389–1393. doi: 10.1104/pp.118.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]