Abstract
Plant root systems are highly plastic in their development and can adapt their architecture in response to the prevailing environmental conditions. One important parameter is the availability of phosphate, which is highly immobile in soil such that the arrangement of roots within the soil will profoundly affect the ability of the plant to acquire this essential nutrient. Consistent with this, the availability of phosphate was found to have a marked effect on the root system architecture of Arabidopsis. Low phosphate availability favored lateral root growth over primary root growth, through increased lateral root density and length, and reduced primary root growth mediated by reduced cell elongation. The ability of the root system to respond to phosphate availability was found to be independent of sucrose supply and auxin signaling. In contrast, shoot phosphate status was found to influence the root system architecture response to phosphate availability.
The root systems of plants show highly plastic development. This plasticity is possible because root systems develop by the continual propagation of new meristems. Factors that affect the initiation and activity of the meristems will clearly have a large effect on the three dimensional pattern of roots in space: root system architecture (RSA). RSA is greatly influenced by the soil environment and especially the availability and distribution of nutrients (Thaler and Pages, 1998). For example, the root systems of many species are able to respond to localized regions of high nutrient supply by proliferating or elongating root branches into these nutrient-rich patches (Drew, 1975; Robinson, 1994). Not all plant species respond in the same fashion to heterogeneity in soil nutrient supply (Farley and Fitter, 1999) and not all nutrient ions elicit the response (Drew, 1975). Furthermore, the overall architecture of the root system can be affected by the nutrient status of the plant, such that in nutrient-limiting conditions the RSA may be very different from the RSA in nutrient-rich environments (Robinson, 1994).
The developmental mechanisms by which plants modify their RSA in response to soil nutrients are unknown. However, important advances have been made recently in understanding the mechanism by which nitrate can act in this respect (Zhang and Forde, 1998; Zhang et al., 1999). Growth of Arabidopsis on uniformly high nitrate (10 mm) suppresses lateral root development. In contrast, when plants are grown on low levels of nitrate (10 μm), exposure of a section of the primary root to high nitrate stimulates lateral root production specifically in that area. The main effect of nitrate appears to be on the rate of lateral root elongation rather than on lateral root initiation (Zhang and Forde, 1998). It is striking that the elongation rate of the primary root is identical on 10 μm and 10 mm nitrate and the metabolism of nitrate is not required for the architectural changes because mutants severely deficient in nitrate reductase activity are still able to respond (Zhang and Forde, 1998). This suggests that there are specific nitrate signaling pathways controlling lateral root elongation, a hypothesis supported by the isolation of a MADS box transcription factor called ANR1 that appears to be involved in nitrate signaling. Plants in which ANR1 has been down regulated by antisense mRNA expression or by cosuppression show normal root branching on uniform nitrate but are unable to elongate lateral roots in response to a localized patch of high nitrate (Zhang and Forde, 1998). In addition, the auxin-resistant mutant, axr4, appears to be unable to respond to a localized supply of nitrate, suggesting a role for auxin in mediating the nitrate signal (Zhang et al., 1999). These data have led to a model in which a shoot-derived signal suppresses lateral root growth when nitrate is abundant, but when nitrate is limiting, nitrate perceived at the lateral root tip stimulates elongation (Zhang and Forde, 1998).
Given the high mobility of nitrate in soil it is perhaps surprising that nitrate supply has such a dramatic effect on RSA because a low root density is sufficient to capture all the nitrate in a volume of soil (Robinson, 1996). However, root proliferation in a nitrogen-containing patch has been shown to be advantageous for plants in competition (Hodge et al., 1999). In contrast, phosphate is often the limiting nutrient for plant growth because of its low mobility in soil. Therefore, it is not surprising that phosphate can have a profound effect on RSA. In barley (Hordeum vulgare), phosphate-rich patches have been shown to promote lateral root development in phosphate-starved plants (Drew, 1975). Bean (Phaseolus vulgaris) plants grown on low phosphate change the angle of growth of basal roots in favor of outward rather than downward growth (Bonser et al., 1996). This effect depends on low shoot phosphate, rather than a local root response, because no change in angle was observed in split-root system experiments where half the roots were grown on low phosphate and half on high phosphate (Bonser et al., 1996). Because soil phosphate availability almost invariably decreases with soil depth, the shallow angle of root growth of phosphate-deficient plants can be viewed as an adaptive response allowing increased phosphate uptake. This hypothesis is supported by the observed correlation between the ability of bean cultivars to reduce root angle in low phosphate and yield in phosphate-poor soils (Bonser et al., 1996).
The work with nitrate has clearly demonstrated the value of Arabidopsis as a model for understanding the control of RSA. The effects of phosphate availability on the RSA of Arabidopsis are largely unknown. The characterization of these effects could be of great value because of the wealth of tools available for studying phosphate uptake and RSA available in Arabidopsis. Two phosphate accumulation mutants have been described: pho1 and pho2. In pho1 mutants, loading of phosphate into the xylem appears to be blocked so that shoot phosphate is 5-fold lower in 21-d-old plants when compared with the wild type (Poirier et al., 1991). In contrast, pho2 mutants overaccumulate phosphate in the shoot so that, when the plants are grown in well-fertilized conditions, shoot phosphate is 4-fold higher in 21-d-old plants when compared with the wild type (Delhaize and Randall, 1995). The concentration of phosphate in 21-d-old roots of both mutants is not significantly different from wild type (Delhaize and Randall, 1995). Therefore, the pho mutants provide an excellent opportunity to study the effects of shoot phosphate levels on RSA. The existence of a large collection of auxin-related mutants similarly can be used to test whether any phosphate-induced RSA changes are mediated by auxin. Addition of exogenous auxin inhibits primary root elongation but promotes lateral root formation. Consistent with this, the aux1, axr4, and axr1 auxin-resistant mutants have all been shown to have long primary roots but a reduced number of lateral roots (Lincoln et al., 1990; Hobbie and Estelle, 1995). These observations illustrate the importance of auxin in the regulation of RSA.
Here, we describe the effects of phosphate availability on Arabidopsis RSA. The involvement of shoot phosphate and auxin in mediating the observed changes are tested using mutants in phosphate uptake and auxin response.
RESULTS
The Effect of Phosphate Availability on RSA
Wild-type Arabidopsis seeds (ecotype Columbia) were germinated on vertically oriented petri dishes containing agar-solidified nutrient solutions with a range of phosphate availibilities. After 14 d their RSA was examined. The effect of increasing phosphate availability was to increase the length of the primary root axis (Fig. 1a), increase the distance from the primary root tip to the first lateral root (Fig. 1b), increase the distance between lateral roots (Fig. 1c), and reduce the length of lateral roots (Fig. 1d). Hence, low phosphate leads to a redistribution of growth from the primary root to lateral roots (Fig. 1f). The dose response relationships for these effects were not identical. In particular, the distance from the tip to first lateral root was not significantly different on 0.5 and 2.5 mm phosphate but was greatly reduced on 0.1 mm phosphate. In contrast, the mean distance between the lateral roots was not significantly different on 0.1 and 0.5 mm phosphate. Although all these trends were reproducible in many experiments, the increases in primary root length and in the distance between the primary root tip and first lateral root were the most robust responses to increasing phosphate, with the other RSA responses being apparently more variable between experiments (data not shown).
Figure 1.
The effect of phosphate availability on RSA. Wild-type Columbia seedlings were grown for 14 d on initial concentrations of 0.1, 0.5, and 2.5 mm phosphate without added Suc on vertically oriented agar dishes. Data are given for the length of the primary root axis (a), the distance from the primary root tip to the first (nearest) lateral root (b), the average distance between the lateral roots (c), the average length of the lateral roots (d), and the distribution of growth between primary and lateral roots (e). Values shown represent mean of six seedlings ± se. The overall effect is of redistribution from primary axis growth to lateral growth as shown in f, a photograph of 10-d-old plants grown on 0.1 mm phosphate (left) and 2.5 mm phosphate (right) on 1% (w/v) Suc. The root systems have been spread to show their architecture.
The plants growing on high phosphate were more vigorous than those on lower phosphate. The total number of root branches (Fig. 2a) and total root system length (Fig. 2b) were greater on higher phosphate and an increase in shoot dry weight was observed (Fig. 2c). It could be argued then that the observed architectural differences simply reflect differences between smaller and larger root systems. This also could explain the variability between experiments mentioned above because in some experiments the plants were more vigorous than in others. To test this hypothesis we examined the relationship between shoot dry weight and total root length, primary root length, lateral root length, and lateral root density (Fig. 3). Data from four independent experiments, in which plants were grown as described above on 2.5 mm and 0.1 mm phosphate, were pooled. As expected, all the RSA parameters vary with shoot dry weight (Fig. 3). Total root length (Fig. 3a), primary root length (Fig. 3b), and lateral root length (Fig. 3d) all increase as shoot dry weight increases. In contrast, the mean distance between lateral roots decreases with increasing shoot dry weight (Fig. 3c). However, these graphs clearly demonstrate that although the relationship between shoot dry weight and total root length is unaffected by phosphate availability, growth on reduced phosphate results in reduced primary root length, reduced internode length, and increased lateral root length for any one shoot dry weight. Therefore, these data confirm that growth on reduced phosphate results in a redistribution of root growth from the primary root to lateral roots.
Figure 2.
The effect of phosphate availability on the whole plant root and shoot. Data are presented for 14-d-old plants for the total number of readily visible root meristems (a) and the total root system length (b). Shoot dry weight results (c) are given for 28-d-old plants. Values shown represent mean of six seedlings ± se.
Figure 3.
Comparison of the effect of different phosphate availabilities on the relationship between RSA and shoot dry weight. Log:log plots are presented for total root length against shoot dry weight (a), primary root length against shoot dry weight (b), internode length against shoot dry weight (c), and lateral root length against shoot dry weight (d). Analysis of covariance shows that log shoot dry weight varies significantly with each RSA parameter (degrees of freedom 1, 86; P < 0.001). Phosphate availability has no significant effect on the relationship between shoot dry weight and total root length (a: F1,86 = 3.25 and P > 0.075), but a highly significant effect on the relationship between shoot dry weight and all the other RSA parameters (b: F1,86 = 266 and P < 0.001; c: F1,86 = 72.0 and P < 0.001; and d: F1,86 = 43.5.0 and P < 0.001).
The Effect of Phosphate Availability on Cell Length at the Primary Root Tip
To investigate the cellular basis for the reduction in primary root length, the primary root tips of plants grown on a range of phosphate availabilities were examined. The root tip is classically divided into zones (Dolan et al., 1993). Immediately behind the root cap and root cell initials is a zone of cell division, followed by a zone of elongation and then a zone of differentiation, which is most clearly defined by the differentiation of root hairs. The total length from the tip of the root cap to the first root hair was found to be significantly longer in plants grown on 2.5 mm phosphate compared with either 0.5 or 0.1 mm phosphate (Fig. 4a). The lengths of mature cortical cells from immediately above the differentiation zone were found to be significantly longer in roots grown on 2.5 mm phosphate compared with those grown on the lower phosphate availabilities (Fig. 4b). This difference, which involves a 30% increase in mature cortical cell length between 0.1 and 2.5 mm phosphate, mirrors the change in primary root length (Fig. 4d) across those two phosphate levels (26%). Cell files were traced toward the tip from the first root hair and the number of cells in the elongation zone was determined. Cells were deemed to have entered the elongation zone when their length exceeded 35 μm because this length appeared to mark the beginning of the elongation phase. Roots growing on the highest phosphate availability had significantly more cells in the elongation zone (Fig. 4c).
Figure 4.
The effect of phosphate availability on cell length at the primary root tip. Data are presented for the length from the tip of the root cap to the first root hair (a), the mean length of six mature cortical cells from above the differentiation zone (b), the number of cells in the elongation zone (c), and total primary root length (d). Values shown represent mean of six seedlings ± se.
Photosynthate Availability and RSA Responses to Phosphate
The observed changes in RSA might be controlled by a variety of factors. One hypothesis is that on low phosphate, photosynthate is redistributed away from the primary root and into lateral roots. To test this hypothesis, 1% (w/v) Suc was added to the medium so that all root tips would receive a ready supply of photosynthate. This had no effect on the root system responses to phosphate (Fig. 5). Primary root length decreased with decreasing phosphate availability (Fig. 5a) without any significant change in total root system length (Fig. 5b), illustrating the redistribution of resources from primary to lateral roots.
Figure 5.
The effect of phosphate availability on RSA in plants grown on 1% (w/v) Suc. The graphs show primary root length over time (a) and total root system length over time (b). Values shown represent the mean of nine to 10 seedlings ± se.
RSA Responses in the pho2 Phosphate Uptake Mutant
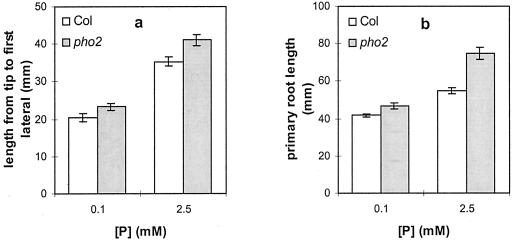
Phosphate-induced changes in RSA could be affected by the phosphate status of the shoot. To test whether phosphate accumulation in the shoot influences root RSA, the RSA of the phosphate overaccumulating mutant, pho2, was examined on low and high phosphate. Phosphate overaccumulation in pho2 shoots was previously only verified in plants grown on agar in the phosphate range between 0.2 and 10 mm (Delhaize and Randall, 1995). Phosphate levels in the roots of pho2 mutants are wild type (Delhaize and Randall, 1995). At our highest phosphate level, which lies within this range, the RSA of pho2 and wild-type roots differs significantly, with pho2 showing an exaggerated response (Fig. 6). At high (2.5 mm) phosphate, compared with wild type, the pho2 plants have a longer primary root and a longer distance from the primary root tip to the first lateral root. These differences are greatly reduced at 0.1 mm phosphate.
Figure 6.
Comparison of the root architectures of wild-type Columbia (Col) and the pho2 mutant under two different phosphate availabilities and 1% (w/v) Suc. The graphs show the distance from the primary root tip to the first (nearest) lateral root (a) and primary root length (b). Values shown represent the mean of seven to 10 seedlings ± se.
RSA Responses in Auxin Signaling Mutants
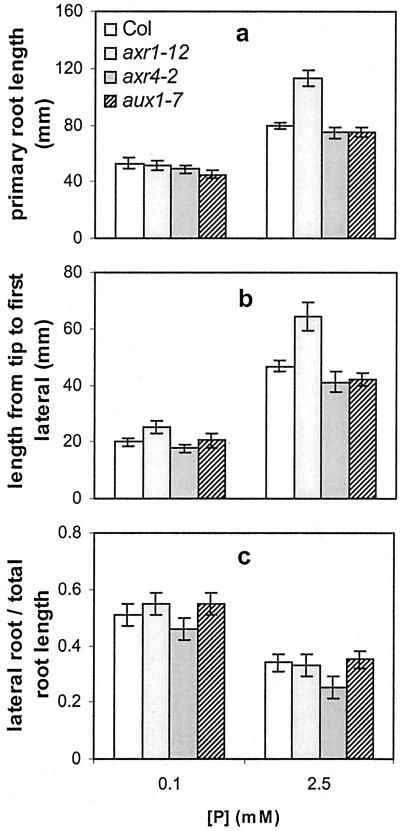
The architecture of plants growing on low phosphate is reminiscent of plants treated with the hormone auxin, which stimulates lateral root formation and inhibits primary root growth. To test whether auxin is involved in mediating the RSA changes in response to phosphate levels, the RSA of auxin-resistant mutants was studied on low and high phosphate. The axr1–12, axr4–2, and aux1–7 mutants were used because these mutations have previously been shown to affect RSA (Lincoln et al., 1990; Hobbie and Estelle, 1995). In our growth conditions RSA differences were most striking for axr1–12, which compared with the other genotypes showed longer primary roots and a longer distance from the root tip to the first lateral root when grown on 2.5 mm phosphate (Fig. 7). At this phosphate level, consistent with previous reports, all the mutant genotypes showed a reduced lateral root density compared with wild type (data not shown; Lincoln et al., 1990; Hobbie and Estelle, 1995). Despite these differences in RSA, when the auxin mutant plants grown on low phosphate were compared with those grown on high phosphate, the changes in RSA observed were similar to those observed in wild-type plants (Fig. 7). At the lower phosphate level, all genotypes showed a similar increase in the ratio of lateral to primary root, indicating that in all genotypes, low phosphate favors lateral root growth over primary root growth.
Figure 7.
RSA for wild-type Columbia (Col) and the auxin-resistant mutants axr1–12, axr4–2, and aux1–7, at two different phosphate availabilities and 1% (w/v) Suc. The graphs show primary root length (a), the distance from the primary root tip to the first (nearest) lateral root (b), and the ratio of lateral root length to total root length (c). Values shown represent the mean of 14 to 20 seedlings ± se.
DISCUSSION
We have shown that phosphate availability clearly affects RSA in Arabidopsis. Lower phosphate favors lateral root growth over primary root growth by reducing primary root elongation, increasing lateral root elongation, and increasing lateral root density. It is interesting to compare this redistribution of growth with the changes in angle of growth observed in bean plants grown on low phosphate (Bonser et al., 1996). In both cases, the changes in growth pattern result in root biomass being concentrated near the soil surface. Because phosphate availability decreases with soil depth, these changes are likely to improve phosphate acquisition.
The phosphate-dependent changes we observed in Arabidopsis RSA are different from those observed with nitrate. Varying nitrate availability over several orders of magnitude apparently has no effect on primary root elongation or on lateral root spacing (Zhang et al., 1999). The effects of nitrate observed by Zhang et al. (1999) are entirely on lateral root elongation, with uniformly applied high nitrate levels being inhibitory. In contrast, phosphate appears to influence all these variables. The differences in response may reflect different adaptive strategies for nitrate foraging because nitrate is much more mobile than phosphate. Therefore, RSA responses to phosphate may reflect a phosphate-foraging strategy, whereas RSA responses to nitrate distribution may reflect improved nitrate acquisition in competition with neighboring plants (Hodge et al., 1999).
The mechanisms regulating the redistribution of growth in response to phosphate availability are unknown. We have shown that redistribution occurs when plants are grown on 1% (w/v) Suc, indicating that redistribution of photosynthate is unlikely to direct RSA changes. Auxin similarly does not appear to be directly involved because the RSAs of three different auxin resistant mutants, axr1, aux1, and axr4, appear to respond normally to changes in phosphate availability. This is in contrast to the role of the AXR4 gene in the root response to a patch of high nitrate because the axr4 mutant is apparently unable to increase the growth of lateral roots in such nitrate patches (Zhang et al., 1999).
Shoot phosphate homeostasis appears to have a role in regulating RSA because RSA and changes in RSA are affected in the shoot phosphate overaccumulator pho2. pho2 mutant plants have been previously shown to overaccumulate phosphate in the shoot when phosphate is plentiful. Shoot phosphate was not measured in this study so the correlation between shoot phosphate and RSA can only be implied from the observations of Delhaize and Randall (1995). However, there is good evidence for a similar role for shoot nitrate concentration (Scheible et al., 1997; Zhang et al., 1999). In tobacco (Nicotiana tabacum) plants, growth on low nitrate promotes root growth and there is a strong inverse correlation between shoot nitrate levels and root growth (Scheible et al., 1997). If tobacco plants are grown in a split-root system with half the roots exposed to high nitrate and half to low nitrate, then the stimulatory effect of low nitrate is not observed in the low nitrate-treated roots, despite the fact they accumulate only low levels on nitrate (Scheible et al., 1997). This suggests that shoot nitrate status regulates root growth responses to exogenous nitrate. It is interesting that the suppression of root growth by high nitrate can be overridden by growth on low phosphate (Scheible et al., 1997).
As well as the evidence for signaling from the shoot, it is possible that the phosphate-dependent changes in growth rate of the primary and lateral roots are regulated locally at the root tips. Measurements of mature cortical cells at the primary root tip indicate that cell elongation decreases with decreasing external phosphate availability. This correlates with a decrease in the number of cells in the elongation zone and a corresponding decrease in the distance from the root tip to the first root hair. Not only is root hair differentiation accelerated on low phosphate, but also root hair elongation is stimulated (Bates and Lynch, 1996). Hence, at a cellular level, as for the whole root system, lateral growth is favored over elongation of the primary axis. In the case of root hair elongation, however, there is some evidence that auxin is involved in mediating the phosphate effect because auxin antagonists were found to inhibit low phosphate-induced root hair elongation (Bates and Lynch, 1996).
As mentioned above, varying the level of nitrate appears to have little effect on the rate of primary root growth, but nitrate can affect the rate of lateral root elongation. Cellular level measurements indicate that this response to locally high nitrate is accompanied by an increase in size of the division zone of the meristem, whereas cell length is not greatly affected (Zhang et al., 1999). This contrasts with the effects of varying phosphate levels reported here, which indicate changing external phosphate availability can significantly change cell elongation at the primary root tip.
In addition to the contrasting effects on cell behavior, phosphate and nitrate availabilities affect the primary root and lateral roots completely differently. Low nitrate has no effect on primary root growth but promotes lateral root growth (Zhang and Forde, 1998). Therefore, it seems likely that the primary site for nitrate action is at the lateral root tips. In contrast, low phosphate inhibits primary root growth and promotes lateral root growth. It is possible that the elongation of the lateral roots is a secondary response to the reduction in primary root growth. In this case, the primary site for phosphate action would be the primary root tip. However, this inhibition of primary root growth by low phosphate cannot be caused by direct phosphate starvation because the levels of phosphate are clearly sufficient to allow vigorous growth of the lateral roots.
In conclusion, the data presented here suggest that the cells of root tips are highly sensitive to external phosphate availability and to shoot phosphate status. They alter their growth rate in a cell type-specific manner, which may improve phosphate acquisition. However, both the adaptive significance of these changes and the mechanism by which they are achieved remain to be established.
MATERIALS AND METHODS
Seed
Seed from the pho2 mutant was kindly provided by Dr. Emmanuel Delhaize (Commonwealth Scientific and Industrial Research Organization, Canberra, Australia). All the lines used are in the Columbia genetic background.
Plant Growth Conditions
Arabidopsis seedlings were grown under sterile conditions on vertical 10-cm2 square petri dishes (Sterilin, Stone, UK) containing 75 mL of Arabidopsis salts (ATS) agar medium (Wilson et al., 1990). ATS agar contains 5 mm KNO3, 2.5 mm KH2PO4 buffered with 2.5 mm K2HPO4 to pH5.5, 2 mm MgSO4, 2 mm Ca(NO3)2, 70 μm H3BO4, 50 μm FeEDTA, 14 μm MnCl2, 10 μm NaCl, 1 μm ZnSO4, 0.5 μm CuSO4, 0.2 μm NaMoO4, 0.01 μm CoCl2, and 0.8% (w/v) agar (plant cell culture tested, Sigma, St. Louis). Seedlings grown with Suc were grown on medium containing 1% (w/v) Suc. Where seedlings were grown on lower phosphate levels, the KH2PO4/K2HPO4 was replaced with KCl to maintain the potassium ion concentration in the medium. The phosphate levels chosen represent a range from plentiful phosphate to growth-limiting phosphate (Fig. 2). Although the concentrations at the start of the experiment are above those usually found in soil (Farley and Fitter, 1999), soil phosphate concentration is well buffered, whereas in this agar plate system, phosphate is depleted over the course of the experiment.
Seeds were surface sterilized for 15 min in 10% (v/v) bleach and 0.01% (v/v) Triton X-100 solution, washed briefly in 70% (v/v) ethanol, and rinsed four to five times in sterile distilled water. After cold treatment for 2 d at 4°C, seeds were individually pipetted out in a single row at the top of the petri dishes. Plants grown on Suc-free medium were grown for either 8 d (in the cell length experiments), 14 d (for RSA and dry weight measurements), or 28 d (for dry weight measurements). Plants grown on medium containing Suc were grown for 10 d. All plants were grown at 22°C under a 16-h-light/8-h-dark regime and a light intensity of approximately 65 μmol m−2 s−2.
RSA and Dry Weight Measurements
Seedlings Grown on Suc-Free Medium
For each phosphate level in an experiment (2.5, 0.5, and 0.1 mm phosphate), six ATS agar plates with eight wild-type seeds per plate were used (18 plates in all). After 14 d, three healthy seedlings on each plate were kept and the rest discarded. One seedling per plate was used for RSA measurements. Arabidopsis root systems were viewed with a MAGISCAN image analysis system (Joyce-Loebl, now Applied Imaging, Newcastle, UK), measurements made using the TRACKROOT program (written by A.H. Fitter and T. Stickland), and statistics from the measurements (e.g. average lateral root length) compiled using SMART 4.1. The same seedling subsequently was dried at 70°C and the shoots and roots weighed separately. The remaining two seedlings were left to grow until d 28 when they were dried and the shoots and roots weighed separately.
Seedlings Grown on Medium Containing Suc
The more uniform growth of plants grown on 1% (w/v) Suc allowed all germinated seeds to be used for the RSA measurements. Measurements were made at 10 d for wild-type Columbia (Col), axr1–12, axr4–2, and aux1–7 seedlings using the MAGISCAN image analysis system described above. pho2 seedlings and their wild-type controls were viewed using a desktop scanner (ScanJet 6100C, Hewlett-Packard, Palo Alto, CA) connected to a PC, and root systems analyzed with WinRhizo software (Régent Instruments, Quebec).
Cell Length Measurements
Three ATS agar plates were used for each phosphate level in the experiment (2.5, 0.5, and 0.1 mm phosphate). Eight seeds were plated out per dish, and two healthy seedlings were selected from each plate for cell length measurements. Root cortical cells were viewed under phase contrast microscopy (optiphot-2, Nikon, Tokyo) connected to a video camera (TK-1070E, JVC, Yokohama, Japan), and cell lengths measured using LUCIA G. software (v.3.5, Laboratory Imaging Ltd., Prague). Measurements started 385 μm from the root tip until the first root hair was reached. Measurements of mature cortical cell lengths were taken from cells 11 through 16 behind the first root hair.
Statistics
Except for the data in Figures 3 and 5, data were analyzed in MINITAB using one-way ANOVAs with Fisher's LSD test (threshold P = 0.05). Analysis of covariance was used to analyze the data in Figure 3 (threshold P = 0.05). Two sample Student's t tests were used for the data in Figure 5 (threshold P = 0.05).
ACKNOWLEDGMENTS
We would like to thank the horticultural team for expert plant care and Birgit Linkohr, Hugh Williamson, and Stephen Day for critical reading of the manuscript.
Footnotes
This work was supported by the Nature and Environment Research Council and by the Biotechnology and Biological Science Research Council of the United Kingdom.
LITERATURE CITED
- Bates TR, Lynch JP. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant Cell Environ. 1996;19:529–538. [Google Scholar]
- Bonser AM, Lynch J, Snapp S. Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytol. 1996;132:281–288. doi: 10.1111/j.1469-8137.1996.tb01847.x. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Randall PJ. Characterization of a phosphate-accumulator mutant of Arabidopsis thaliana. Plant Physiol. 1995;107:207–213. doi: 10.1104/pp.107.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. Cellular organization of the Arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- Drew MC. Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol. 1975;75:479–490. [Google Scholar]
- Farley RA, Fitter AH. Temporal and spatial variation in soil resources in a deciduous woodland. J Ecol. 1999;87:688–696. [Google Scholar]
- Hobbie L, Estelle M. The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J. 1995;7:211–220. doi: 10.1046/j.1365-313x.1995.7020211.x. [DOI] [PubMed] [Google Scholar]
- Hodge A, Robinson D, Griffiths BS, Fitter AH. Why plants bother: root proliferation results in increased nitrogen capture from an organic patch when two grasses compete. Plant Cell Environ. 1999;22:811–820. [Google Scholar]
- Lincoln C, Britton JH, Estelle M. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell. 1990;2:1071–1080. doi: 10.1105/tpc.2.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier Y, Thoma S, Somerville C, Schiefelbein J. A mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol. 1991;97:1087–1093. doi: 10.1104/pp.97.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. The responses of plants to non-uniform supplies of nutrients. New Phytol. 1994;127:635–674. doi: 10.1111/j.1469-8137.1994.tb02969.x. [DOI] [PubMed] [Google Scholar]
- Robinson D. Resource capture by localized root proliferation: why do plants bother? Ann Bot. 1996;77:179–185. [Google Scholar]
- Scheible WR, Lauerer M, Schulze ED, Caboche M, Stitt M. Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J. 1997;11:671–691. [Google Scholar]
- Thaler P, Pages L. Modeling the influence of assimilate availability on root growth and architecture. Plant Soil. 1998;201:307–320. [Google Scholar]
- Wilson AK, Pickett FB, Turner JC, Estelle M. A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol Gen Genet. 1990;222:377–383. doi: 10.1007/BF00633843. [DOI] [PubMed] [Google Scholar]
- Zhang H, Forde B. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science. 1998;279:407–409. doi: 10.1126/science.279.5349.407. [DOI] [PubMed] [Google Scholar]
- Zhang H, Jennings A, Barlow PW, Forde B. Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA. 1999;96:6529–6534. doi: 10.1073/pnas.96.11.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]