Abstract
Cancer is the second most common cause of death in the United States and is a challenging disease to treat. The treatment options for various cancers include but are not limited to surgery, radiation, and chemotherapy. The mechanism behind chemotherapy is intended to promote cellular damage to cells that are proliferating uncontrollably. Unfortunately for the recipients, most chemotherapeutic agents cannot differentiate between malignant cells and healthy cells and tissues. Thus, chemotherapy-induced toxicities are often observed in once-healthy organs. These effects can be acute and self-limiting or chronic, appearing long after chemotherapy is completed. Cancer survivors can then present for non-cancer related surgeries later in life, due to this toxicity. Furthermore, the administration of chemotherapeutic agents can profoundly impact the anesthetic management of patients who are undergoing surgery. This review discusses how chemotherapy-induced organ toxicity can occur in multiple organ systems and what drugs should be avoided if prior toxicity exists in these organ systems.
Keywords: Anesthesia, Cancer, Chemotherapy, Toxicity, Perioperative
1. Introduction
Cancer is the second leading cause of death globally and in the United States [1]. In 2020, roughly 1.8 million people will be diagnosed with cancer, and an estimated 606,520 people will die of cancer in the United States [2]. More than 16.9 million people with a history of cancer were alive on January 1, 2019, in the United States [3]. Chemotherapeutic agents are the mainstay of treatment for most cancers. Each year, about 650,000 cancer patients receive outpatient chemotherapy in the United States [4]. Generally, these agents work by abolishing rapidly dividing cancer cells; however, there are many classes of agents with distinct mechanisms of action [5]. Most of these agents have toxic side effects at therapeutic dose levels related to the effect on non-malignant dividing cells. Chemotherapeutic toxicity is complicated by the frequent use of multidrug regimens and the potential for toxicities to be additive or more than additive [6].
Chemotherapeutic agent-induced organ toxicity has a profound impact on perioperative patient care (Fig. 1). Common toxicities involve the heart, liver, kidneys, gastrointestinal system, lungs, bone marrow, and nervous system; however, other organs can be affected [1–8]. Pre-operatively, a thorough history and physical examination are necessary to understand the patient’s physical status before surgery. Additionally, specific laboratory tests or imaging studies may be required to assess baseline organ function. Intraoperatively, considerations regarding anesthetic use, fluid management, oxygen requirements, vital signs monitoring, and surgical technique must be made. Interactions between chemotherapeutic agents and anesthetics may occur, and chemotherapeutic agent-induced toxicity may necessitate changes to the surgical plan depending on the organ(s) affected. Postoperatively, chemotherapeutic agent-induced organ toxicity may affect pain management and discharge planning. Overall, chemotherapeutic agent-induced organ toxicity may lead to dangerous perioperative complications and cause negative patient outcomes if not thoroughly understood and evaluated [7,9].
Fig. 1.
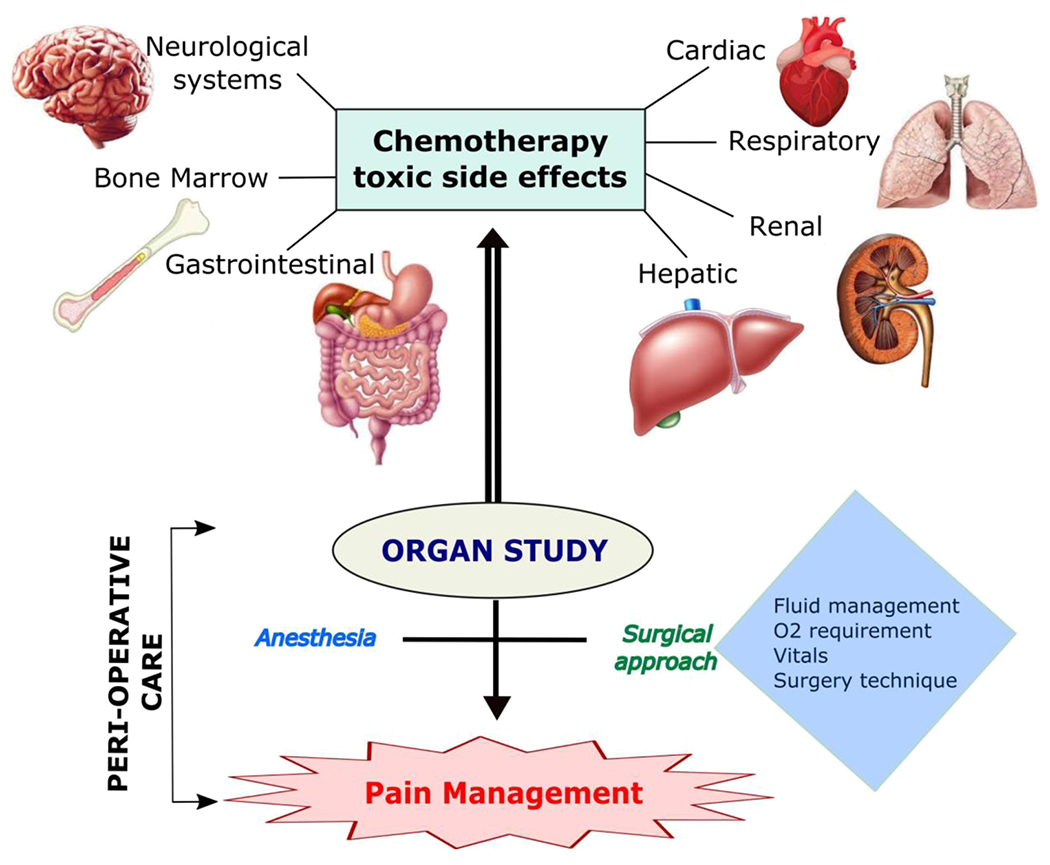
Chemotherapeutic agent-induced organ toxicity impact on perioperative patient care.
2. Preoperative
2.1. Evaluation
In patients with chemotherapeutic agent-induced organ toxicity, a thorough preoperative assessment is paramount (Fig. 2). A pertinent, comprehensive history should be obtained, including past medical history, allergies, medications, family history, baseline functional status, and nutritional status. A review of systems should be conducted to determine symptom control and other general medical issues (Fig. 2). [7,9,10]. Importantly, all information about cancer and cancer treatment should be solicited. This includes the natural history of the cancer, the type of chemotherapeutic agents, the number of treatments, the total amount of agents received, and the date of last treatment [11]. Full knowledge about all cancer treatments received by the patient, including radiation and surgery, is vital to prepare for potential intraoperative complications, such as excessive surgical bleeding [12], and postoperative complications, such as impaired wound healing [10,11]. A complete physical examination should be conducted to identify signs of organ dysfunction and other abnormalities that can complicate intraoperative management [9,12].
Fig. 2.
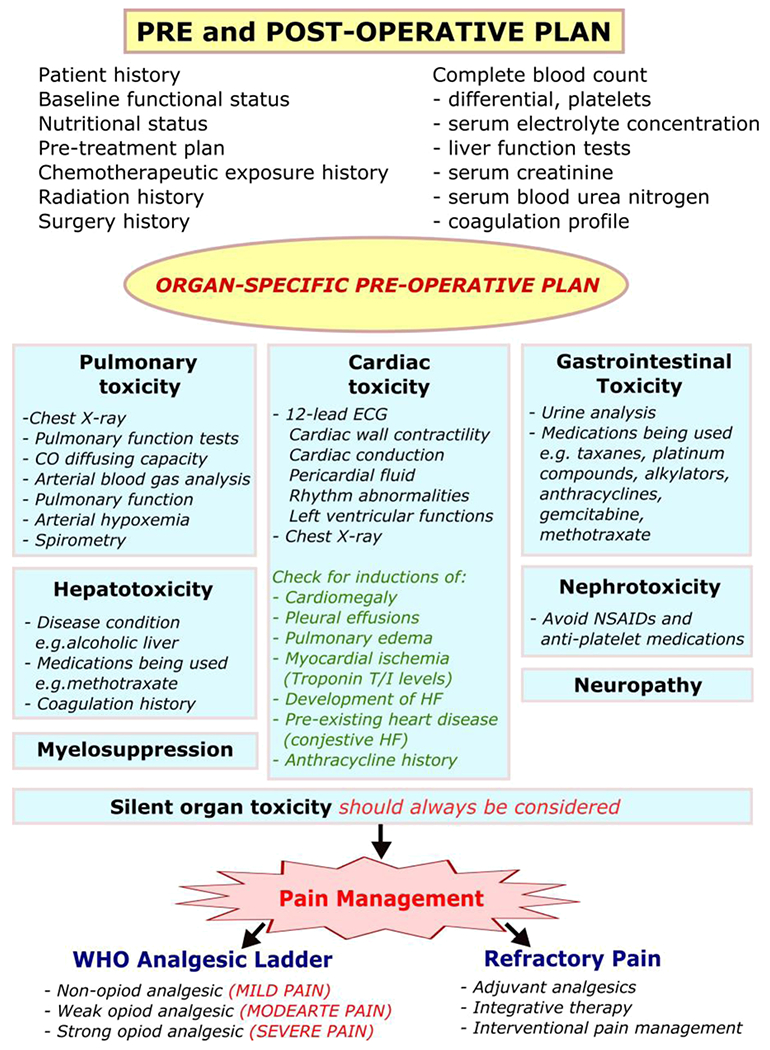
Preoperative plan.
All patients with a history of chemotherapeutic agents should undergo laboratory studies to include a complete blood count with differential and platelets, serum electrolyte concentrations, liver function tests, serum creatinine, serum blood urea nitrogen, and a coagulation profile. Additional preoperative testing should be tailored to the specific organ toxicity. Patients with chemotherapeutic agent induced-pulmonary toxicity should receive a chest X-ray and may also require pulmonary function tests, carbon monoxide diffusing capacity, and arterial blood gas analysis [9]. Patients with cardiotoxicity should receive a 12-lead electrocardiogram to assess for cardiac conduction and rhythm abnormalities along with a chest X-ray to identify evidence of cardiomegaly, pleural effusions, or pulmonary edema [10,11,13].
Additionally, select patients experiencing cardiotoxicity from chemotherapeutic agent use may require echocardiography to assess left ventricular function, cardiac wall contractility, and pericardial fluid [14]. One study found that measuring troponin T or I in patients with chemotherapy-induced cardiotoxicity detected early myocardial ischemia and predicted the development of heart failure; however, this is not yet standard of care [15]. Urine studies may provide useful information for managing fluid losses in patients with chemotherapeutic agent-induced gastrointestinal toxicity [10]. Despite comprehensive preoperative evaluation, the perioperative team must be aware that clinically silent organ toxicity may exist and should carefully monitor organ function intraoperatively and postoperatively [13].
2.2. Protocols for Care
For patients with chemotherapy-induced organ toxicity, specific protocols for care do not exist; instead, general guidelines have been developed to optimize the patient’s physical status before surgery. Those with chemotherapy-induced cardiotoxicity are at high risk for potential cardiac events intraoperatively. Patients exposed to cardiotoxic agents with minimal or no symptoms should have aggressive attenuation of their cardiovascular risk factors [10,16]. Studies have demonstrated positive results in countering the cardiotoxic effects of chemotherapeutic agents, particularly anthracyclines, when patients are prescribed angiotensin-converting enzyme (ACE) inhibitors or beta-blockers [17,18]. Even in patients with chemotherapy-induced heart failure or hypertension, ACE inhibitors, beta-blockers, and statins have improved cardiac function and overall functional status of the patient [19].
Considerations for patients with chemotherapy-induced pulmonary toxicity include treatment with corticosteroids before surgery and the use of the lowest possible inspiratory oxygen fraction. Corticosteroid treatment before surgery has demonstrated positive results in improving carbon monoxide diffusing capacity and vital capacity and reducing postoperative complications [20]. Recommendations for inspired oxygen concentration are to use less than 30% or the lowest concentration possible to ensure adequate oxygenation. Previous chemotherapeutic agent use, particularly bleomycin, sensitizes the lungs to oxygen concentrations that normally are not harmful [10,21].
Other considerations include those for patients with chemotherapy-induced nephrotoxicity and myelosuppression. Before surgery, abnormal electrolyte levels, such as sodium, potassium, and calcium, should be normalized and carefully monitored. Non-steroidal anti-inflammatory drugs (NSAIDs) should be avoided in patients who have received nephrotoxic chemotherapy due to the risk of precipitating acute renal failure [16]. Additionally, NSAIDs and other antiplatelet medications can interfere with platelet function, further necessitating the need to stop these medications before surgery. Patients with myelosuppression should undergo infection control practices with strict aseptic technique when placing peripheral intravenous lines or other lines due to potential neutropenia. Standard protocols for care, vigilant monitoring, and appropriate intraoperative precautions should be employed [10].
2.3. Candidacy and Timing of Surgery
Generally, there are no specific recommendations about candidacy or timing of surgery for patients with chemotherapy-induced organ toxicity. Nonetheless, certain organ toxicities necessitate the employment of guidelines to determine surgical risk and decide whether medical optimization needs to occur before surgery can proceed [22]. For patients with chemotherapy-induced cardiotoxicity, the 2014 American College of Cardiology/American Heart Association guidelines are used to determine cardiac risk before noncardiac surgery [22,23]. These guidelines dictate that each patient’s functional status should be assessed before surgery, especially those with cardiovascular risk factors [23]. Patients with adequate functional status are ideal surgical candidates and can proceed to surgery; however, patients with low functional status should undergo further testing and medical optimization before surgery [22]. Patients with pulmonary toxicity may proceed to surgery as long as their pulmonary function tests and carbon monoxide diffusing capacity are in an optimal range. Those with baseline shortness of breath at rest may not be ideal candidates for surgery and will require medical optimization before undergoing surgery [10,16]. Surgery also may need to be postponed if high concentrations of inspired oxygen are required for patients with recent bleomycin treatment as case reports have shown hyperoxia (inspiratory oxygen fraction > 30%) in these patients may increase the risk of acute respiratory failure [24,25].
Patients with chemotherapy-induced myelosuppression are ideal candidates for all surgeries if their platelet count exceeds 100,000/mm3 and neutropenia is absent. If platelet counts are low, surgery should be delayed two weeks until bone marrow function returns and new platelets are produced. The specific surgical procedure and platelet function determine the minimum platelet count required [26]. Nonemergency surgery in neutropenic patients should be postponed whenever possible. Neutropenia typically lasts 8–10 days but may vary depending on the chemotherapeutic treatment [27]. Patients with hepatotoxicity and other organ toxicities may undergo surgery assuming preoperative testing is adequate [10].
3. Intraoperative
3.1. Cardiac
The cardiovascular status of patients in the peri-operative period is important to anesthesiologists who create a comprehensive anesthetic plan to manage the patient’s surgery. The toxic effects of chemotherapy drugs on the cardio-pulmonary system directly impact the anesthetic treatment. The group of anti-cancer drugs that most commonly poison the cardiac system are the Anthracyclines. The anthracycline antibiotics form superoxide anion radicals through a series of highly destructive reactions to the cardiac myocytes, resulting in decreased myocardial contractility and even congestive heart failure [28].
Doxorubicin (adriamycin), daunorubicin, and epirubicin are anthracyclines known to weaken myocardial contractility [29]. Mitoxantrone at doses more than 140 mg/m2 can cause congestive heart failure and anthracycline-induced cardiomyopathy [29]. Another drug shown to cause cardiac tissue injury is cyclophosphamide. Over two days, a cyclophosphamide dose of more than 120 mg/kg−1 could produce severe congestive heart failure, pericarditis, necrosis, and hemorrhagic myocarditis [30]. Patients receiving an antineoplastic agent called busulfan in traditional oral daily usage may result in endocardial fibrosis and constrictive cardiomyopathy.
This cardiac toxicity may occur immediately after a single dose, or it may present months to years after completion of chemotherapy. Acute cardiac toxicity normally presents as non-specific ECG changes, but various arrhythmias and bundle branch blocks have also been reported [28]. These side effects can occur at all doses but normally resolve 1–2 months after chemotherapy completion. There are reported cases of grave complications, including sudden death from ventricular fibrillation, acute failure of the left ventricle, and fatal-pericarditis-myocarditis syndrome [31]. Chronic cardiotoxicity from anthracyclines normally presents as cardiomyopathy, revealed on chest X-rays (CXR) as cardiomegaly. Chronic use may also include the same non-specific ECG changes and arrhythmias seen with acute toxicity. Anthracycline cardiotoxicity is a cumulative dose-related incidence that may manifest as congestive heart failure. It has been reported that increases in CHF incidence occur after a doxorubicin dose of 550 mg/m2 [32].
Patients, usually elderly, with preexisting heart disease like congestive heart failure are more susceptible to these cardiac toxicities, but the effects are generally transient and respond to normal medical management. Previous therapy with anthracyclines in patients with normal resting cardiac function can boost the myocardial depressive effect of certain anesthetics [33]. To properly assess patients that have received some of the chemotherapy mentioned above agents, a 2D-echocardiogram or nuclear medicine study is permitted to measure left ventricular ejection fraction and to detect any regional and global myocardial dysfunction that may be present [29]. Diastolic dysfunction on the echocardiogram may be a sign of early anthracycline toxicity. A decrease in left ventricular ejection fraction (shown by radionuclide angiocardiography) to less than 45% is considered to suggest anthracycline-induced cardiotoxicity. The new non-invasive test to show actual myocardial damage uses imaging with monoclonal indium–111–antimyosin antibodies. The antibodies bind to the exposed myosin when necrosis of myocardial cells occurs. Diffuse uptake of the antibodies on imaging indicates acute anthracycline cardiomyopathy, while a focal uptake on imaging suggests local processes such as a myocardial infarction [34].
Anesthesiologists must be aware of patients previously treated with anthracyclines because these patients can develop acute intraoperative left ventricular failure refractory to β- adrenergic receptor agonists while under general anesthesia.
4. Pulmonary
Along with the cardiovascular status, the pulmonary fortitude of each patient directly affects the anesthetic management. Cancer patients are very susceptible to respiratory complications, a majority of which are due to infection. When cancer patients succumb to respiratory failure that requires mechanical ventilation, the associated mortality rate is 75% [35]. Busulfan, cyclophosphamide, and paclitaxel are anticancer drugs that can lead to pulmonary complications, with bleomycin producing most lung damage.
Bleomycin has been reported to cause dose-dependent interstitial pneumonitis, which may progress to chronic fibrosis, hypersensitivity pneumonitis with peripheral eosinophilia, acute chest pain syndrome, pulmonary veno-occlusive disease, and bronchitis obliterans [36]. The most common toxicities seen with bleomycin are progressive interstitial pneumonitis and fibrosis, with symptoms usually presenting between 4 and 10 weeks after chemotherapy.
The mechanism of pulmonary toxicity seen with the use of bleomycin is thought to be direct cytotoxicity in which type I pneumocytes are replaced by type II pneumocytes. Continued use leads to metaplasia of these type II pneumocytes into the cuboidal epithelium. Fibroblasts and macrophages travel to the interstitial tissue and alveoli, eventually leading to pulmonary fibrosis [37]. Another theory for bleomycin toxicity describes the production of superoxide free radicals, which cleave nuclear DNA and might be increased with the inspiration of fortified oxygen concentrations.
Clinically, the CXR may show bilateral basal and peri-hilar infiltrates with fibrosis in the lower lobes and sub-pleural areas. The first symptoms of toxicity patients may show include fever, cough, dyspnea and rhonchi, and rales that mild X-ray changes may accompany. Patients may exhibit a normal resting PaO2 or present with severe hypoxia at rest.
Respiratory toxicity may be detected by measuring carbon monoxide diffusion capacity (DLCO), arterial hypoxemia, and spirometry, revealing decreased lung volumes commonly seen in types of restrictive lung disease. Immediate cessation of the chemotherapeutic agent may result in toxicity regression, but steroids effectively ameliorate lung damage. Pulmonary disease is generally seen around the dose ranges of 400–50 mg, but there have been reported cases of fatal pulmonary fibrosis with doses as low as 50 mg [38].
There is debate about the amount of oxygen given to a patient after receiving bleomycin. Recent studies show that only 25% of patients developed minor pulmonary complications, and none of them developed ARDS or died when receiving inspired oxygen > 40% [39]. They concluded that perioperative oxygen restriction is unnecessary and that careful perioperative fluid balance is a better predictor of postoperative pulmonary morbidity. Furthermore, the duration of surgery and post-chemotherapy forced vital capacity were significant predictive variables of procedure-related pulmonary morbidity. Anesthesiologists can use intraoperative PEEP to enhance oxygenation and arterial blood gas analysis with indwelling arterial cannulas.
5. Renal
Cisplatinum is an anti-neoplastic agent commonly used to fight many different types of cancers. Of note, it has been shown to have toxic side effects such as nephrotoxicity, myelosuppression, neuropathy, and even auditory and visual disturbances. The renal damage occurs when cisplatinum induces coagulation necrosis in the renal tubular epithelial cells and collecting ducts, leading to a reduction in the renal blood flow and glomerular filtration rate (GFR). 30% of patients receiving cisplatinum will develop nephrotoxicity with a single dose of 2 mg/kg or 50–75 mg/m2’ especially when properly hydrated [40]. Acute kidney damage can present within 24 h of a single dose of cisplatinum. Chronic damage was reported 16–52 months after administration in patients who were noted to have a 12.5% reduction in GFR [41].
It is important to properly hydrate patients with forced diuresis because it reduces the concentration of the drugs in the renal tubules, not allowing them to cause damage. Normal saline has proven beneficial because the higher chloride concentrations inhibit the hydrolysis of cisplatinum in the renal tubules. The renal toxicity seen with cisplatinum can be accentuated when patients receive antibiotics such as aminoglycosides concurrently. Today, newer analogs of cisplatinum like carboplatinum and oxaloplatinum are favored because they are less nephrotoxic while demonstrating an equal efficacy against malignancy.
6. Central Nervous System
Vinca alkaloids are a class of anticancer drugs that have been found to inflict neurotoxic side effects. Vincristine is one of these drugs that can cause toxicities in the central, peripheral, and autonomic nervous systems. Peripheral nervous system toxicities typically present as peripheral paresthesias with decreased deep tendon reflexes [42]. The nerve dysfunction can progress to motor and gait disorders as well. Vincristine, vinblastine, procarbazine, and cisplatinum can all cause neuropathies with accompanying paresthesias, loss of deep tendon reflexes, and muscle weakness [43]. Autonomic neuropathy with subsequent orthostatic hypotension, although rare, is a side effect of cancer. Cranial nerve toxicities may present as ophthalmoplegia and facial palsies, while other autonomic toxic effects may include erectile dysfunction, constipation, difficulty in micturition, and bladder atony.
As mentioned above, cisplatinum, along with its renal toxicity, may affect the nervous system. Generally seen as paresthesias, progression and continued treatment can result in loss of deep tendon reflexes, vibration sense, and sensory ataxia. Nervous system toxicities are particularly important when regional anesthesia is considered for pre-op patients. Patients previously treated with cisplatinum chemotherapy often possess a sub-clinical neuropathy that may interfere with nerve blocks. A full, detailed neurological exam is warranted in this subset of patients before any anesthetic plan is administered.
7. Hepatic
Hepatocellular damage from chemotherapeutic agents presents as increased or abnormal liver enzymes and fatty infiltration and cholestasis due to either the direct toxic effect of the chemotherapy drug or one of its metabolites. Two anti-neoplastic drugs commonly used that inflict hepatocellular dysfunction are L-asparginase and cytarabine. When the liver’s synthetic function is decreased, low protein production can progress to coagulation abnormalities, ascites, and encephalopathy.
In addition to anti-cancer drugs that cause hepatocellular dysfunction, other conditions concomitantly damage the liver, such as alcoholic liver disease and some hepatotoxic medications like Methotrexate. Methotrexate has been reported to cause hepatic cirrhosis and fibrosis, while cyclophosphamide is associated with diffuse hepatocellular [44]. There are many drugs, chemotherapy, and routine metabolized by the hepatic system and will directly impact the dosage of other medications if the metabolic function is impaired. Routine liver function tests can be formed as well as imaging to view any gross abnormalities. Of note, anesthesiologists must be aware of any liver dysfunction before administering regional anesthetic due to the chance of associated coagulopathy that may occur.
8. Gastrointestinal
It is very common for chemotherapeutic drugs to cause gastrointestinal toxicity. Chemotherapy agents target rapidly dividing cells, both normal and malignant, but cannot differentiate between the two types of tissues. These agents damage the intestinal mucosa resulting in nausea, vomiting, mucositis, diarrhea, abdominal pain, and sometimes gastric reflux. These symptoms are well documented and known for causing dose reductions, medication changes, delays, and cessation of chemotherapy. The list of chemotherapy drugs that cause GI toxicity is extensive and includes but is not limited to taxanes, platinum compounds, anthracyclines, gemcitabine, methotrexate, and alkylators.
The proposed mechanism of chemotherapy-induced nausea results from the release of serotonin from the enterochromaffin cells, which line the GI tract. Serotonin then stimulates 5-HT3 receptors located in the gastrointestinal tract, the nucleus tractus solitarius of the medulla oblongata, and the chemoreceptor trigger zone, sending impulses to the vomiting center [45]. Ondansetron, granisetron, and dolasetron are available anti-emetics used to battle this nausea. Continued vomiting and diarrhea can lead to dehydration and electrolyte imbalances. Chemotherapy patients with mucositis may also bleed easier during direct laryngoscopy, causing a difficult view of the airway. Anesthesiologists must always be aware of the risk of aspiration and take precautionary steps to prevent it.
Cancer patients treated with chemotherapy deserve special consideration when formulating an anesthetic plan for general surgeries and regional procedures. It’s important to understand the toxic effects certain anti-cancer agents may inflict and know the perioperative tests to avoid further complications. See Table 1.
Table 1.
Chemotherapeutic Agents and their toxic effects.
| Drug | Effect |
|---|---|
| Cardiac | |
| Doxorubicin (adriamycin) | weaken myocardial contractility |
| Daunorubicin | weaken myocardial contractility |
| Epirubicin | weaken myocardial contractility |
| Mitoxantrone | at doses more than 140 mg/m2 can cause congestive heart failure and anthracycline-induced cardiomyopathy |
| Cyclophosphamide | cause cardiac tissue injury. Over two days, a cyclophosphamide dose of more than 120 mg/kg− 1 could produce severe congestive heart failure, pericarditis, necrosis, and hemorrhagic myocarditis |
| Busulfan | traditional oral daily usage may result in endocardial fibrosis and constrictive cardiomyopathy |
| Pulmonary | |
| Bleomycin | dose-dependent interstitial pneumonitis, which may progress to chronic fibrosis, hypersensitivity pneumonitis with peripheral eosinophilia, acute chest pain syndrome, pulmonary veno-occlusive disease, and bronchitis obliterans |
| Cyclophosphamide | pulmonary complications |
| Paclitaxel | pulmonary complications |
| Renal | |
| Cisplatinum | nephrotoxicity, myelosuppression, neuropathy, and even auditory and visual disturbances |
| Central Nervous System | |
| Vincristine | toxicities in the central, peripheral, and autonomic nervous systems |
| Vinblastine | neuropathies with accompanying paresthesias, loss of deep tendon reflexes, and muscle weakness |
| Procarbazine | neuropathies with accompanying paresthesias, loss of deep tendon reflexes, and muscle weakness |
| Cisplatinum | neuropathies with accompanying paresthesias, loss of deep tendon reflexes, and muscle weakness |
| Hepatic | |
| L-asparginase | hepatocellular dysfunction |
| cytarabine | hepatocellular dysfunction |
| Methotrexate | hepatic cirrhosis and fibrosis, while cyclophosphamide is associated with diffuse hepatocellular |
9. New Chemotherapeutic agents
Avapritinib (Ayvakit, Blueprint Medicines), a once-daily tyrosine kinase inhibitor (TKI) indicated for gastrointestinal stromal tumor (GIST) with a platelet-derived growth factor receptor alpha exon 18 mutation. Adverse reactions include edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, hair color changes, increased lacrimation, abdominal pain, constipation, rash and dizziness. Avapritinib has a risk of CNS effects (58% of patients). CNS effects related to cognitive impairment occurred in 41% of patients, but only 3.6% of patients experienced Grade ≥ 3 cognitive impairments. Avapritinib has a risk of intracranial hemorrhage, which occurred in 1% of patients with GIST and 3% of patients with any malignancy [46].
Ripretinib (Qinlock, Deciphere), an oral TKI for the fourth-line treatment of advanced GIST, also was approved in 2020. Cardiovascular ischemic events (cardiac arrest, acute coronary syndrome, and myocardial infarction) occurred in 1.1% of patients in the pooled safety group. Among these, cardiac arrest and myocardial infarction were deadly [47].
Tazemetostat, (Tazverik, Epizyme) oral dimethyltransferase inhibitor, approved to treat inoperable epithelioid sarcoma. Tazemetostat increases the risk of secondary malignancy. In 729 people receiving TAZVERIK 800 mg/ 2x day, 0.7% developed myelodysplastic syndrome or acute myeloidleukemia. The most common adverse reactions (≥20%) were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Serious adverse reactions include hemorrhage, pleural effusion, skin infection, dyspnea, pain, and respiratory distress [48].
Isatuximab-irfc, (Sarclisa, Sanofi), approved as an IV infusion given once weekly for four weeks, then every other week for patients with relapsed/refractory multiple myeloma. It binds to the CD38 receptor on multiple myeloma cells, is used with pomalidomide (Pomalyst, Bristol Myers Squibb) and dexamethasone (pom-dex) to treat adults who have received at least two prior therapies. Infusion-related reactions occur in 39% of patients receiving the first dose, but were resolved on the same day in 98% of patients. Infusion-related reactions include cough, chills, dyspnea, and nausea. Severe symptoms include hypertension and dyspnea [49].
Tucatinib (Tukysa, Seattle Genetics) was approved with trastuzumab and capecitabine (Xeloda, Genentech) to treat patients with advanced HER2-positive breast cancer, including those with brain metastases, after failing at least one prior HER2 regimen. TUKYSA can cause severe diarrhea including dehydration, hypotension, acute kidney injury, and death. In HER2CLIMB, 81% of patients who received TUKYSA experienced diarrhea, including 12% with Grade 3 diarrhea and 0.5% with Grade 4 diarrhea. Both patients who developed Grade 4 diarrhea subsequently died, with diarrhea as a contributor to death. TUKYSA can cause severe hepatotoxicity [50].
Sacituzumab govitecan-hziy (Trodelvy, Immunomedics), an antibody drug conjugate for the third-line treatment of metastatic triplenegative breast cancer. Severe, life-threatening, or fatal neutropenia can occur in patients treated with TRODELVY. Neutropenia occurred in 61% of patients treated with TRODELVY. Grade 3–4 neutropenia occurred in 47% of patients. Febrile neutropenia occurred in 7% of patients. TRODELVY can cause severe diarrhea. Diarrhea occurred in 65% of all patients treated with TRODELVY. Grade 3–4 diarrhea occurred in 12% of all patients treated with TRODELVY. One patient had intestinal perforation following diarrhea. Neutropenic colitis occurred in 0.5% of patients [51].
Pemigatinib (Pemazyre, Incyte) is an oral fibroblast growth factor receptor 2 (FGFR2) indicated to treat cholangiocarcinoma that has spread and cannot be resected. The most common adverse reactions (incidence ≥ 20%) are hyperphosphatemia, alopecia, diarrhea, nail toxicity, fatigue, dysgeusia, nausea, decreased appetite, vomiting, arthralgia, abdominal pain, hypophosphatemia, back pain, constipation, stomatitis, dry eye, dry mouth, and dry skin [52].
Capmatinib (Tabrecta, Novartis) is an oral TKI approved for adults with metastatic or inoperable non-small cell lung cancer (NSCLC) with a mesenchymal-epithelial transition (MET) exon 14 skipping mutation. The most common adverse reactions (≥ 20%) are peripheral edema, vomiting, dyspnea, nausea, fatigue, and decreased appetite. ILD/pneumonitis can occur and be fatal. ILD/pneumonitis occurred in 4.5% of patients with 1.8% of patients experiencing Grade 3 ILD/pneumonitis and one patient experiencing death (0.3%). Hepatotoxicity can also occur. Increased alanine aminotransferase (ALT)/aspartate aminotransferase (AST) occurred in 13% of patients. Grade 3 or 4 increased ALT/AST occurred in 6% of patients [53].
10. Postoperative
Adequate pain control in patients with chemotherapy-induced organ toxicity is a key element in successful recovery after surgery. Currently, no specific protocols exist to guide pain management in patients with chemotherapy-induced organ toxicity. However, general guidelines have been developed to manage pain in patients with cancer. In contrast, several studies have elucidated important considerations for analgesic regimen options in patients with specific organ diseases. Together, these findings can help guide pain treatment in cancer patients affected by chemotherapy-induced organ toxicity.
10.1. Current guidelines for cancer pain management
In 1989, the World Health Organization (WHO) established guidelines for medical management of cancer pain: the three-step analgesic ladder [54]. This is the mainstay framework for the achievement of satisfactory cancer pain relief [54]. “Step 1” of the WHO three-step analgesic ladder endorses nonopioid, over-the-counter analgesic use such as acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs) for mild pain; “Step 2” incorporates weak opioids such as codeine, oxycodone, dihydrocodeine, hydrocodone, and tramadol for moderate pain; and “Step 3” recommends the use of strong opioids including morphine, hydromorphone, methadone, and fentanyl for severe pain [55]. A review of the overall effectiveness of the WHO cancer relief guidelines found that it provides adequate pain relief in 20–100% of patients [54]. However, up to 10% of cancer patients may continue to experience cancer pain refractory to analgesic drug management [56]. Cancer patients with refractory pain may benefit from adjuvant analgesics, integrative therapies, and interventional pain management techniques [55,56]. Adjuvant analgesics include antidepressants (e.g., duloxetine, venlafaxine, and amitriptyline) and anticonvulsants (e.g., gabapentin, pregabalin), integrative therapies include medical marijuana, acupuncture, and mindfulness practice, and interventional pain management strategies include radiation therapy, epidural/intrathecal analgesics, local nerve blocks, neuraxial analgesia techniques, neuro-destructive procedures, and neurolytic sympathetic blocks [55–57].
10.2. Pain management considerations in cancer patients with organ toxicity
While all chemotherapy-induced organ toxicities should be considered when designing a cancer patient’s pain regimen, the renal, hepatic, and cardiac systems are the most important organ systems to pay attention to. Toxicity in these organs may cause structural and functional damage, which impacts anesthesia providers’ analgesic choices to optimize the patient’s cancer pain management plan. While the WHO analgesic ladder should still be followed when designing the patient’s pain management plan, special considerations are warranted for the pharmacological nonopioid and opioid treatments commonly used in these patients. Interventional pain management techniques should be implemented when organ toxicity-safe systemic analgesics are exhausted [57].
Chemotherapeutic agents can cause renal toxicity by directly injuring the glomerulus, tubules, or interstitium, leading to acute kidney injury, toxic acute tubular necrosis, crystal nephropathy, proteinuria/nephrotic syndrome, and chronic kidney disease [58]. Therefore, analgesic management in cancer patients with chemotherapy-induced renal toxicity should be managed as if they have kidney disease and reduced kidney function. These patients are at risk of further renal injury and metabolic derangements from analgesic drug usage [59]. Medications should be dosed renally, and nephrotoxic drugs should be avoided. Of the nonopioid pharmacologic options, acetaminophen is preferred over NSAIDs. NSAIDs are likely to adversely affect renal hemodynamics and cause interstitial renal damage [59]. Of the weak opioids, tramadol and methadone may be considered. However, codeine is not recommended because this drug is 90% renally cleared, and its accumulation can lead to serious adverse effects [59]. Strong opioids such as fentanyl may be acceptable, but this medication should be renally dosed [59]. Morphine should be avoided due to the accumulation of active neurotoxic metabolites [59]. Furthermore, opioids are primarily renally excreted, and therefore, in patients with kidney dysfunction, toxic parent and metabolite opioid compounds may accumulate and lead to adverse complications [59].
Chemotherapy-induced hepatotoxicity can lead to hepatitis, cirrhosis, and eventually liver failure [60]. Like renal toxicity, hepatotoxicity impairs analgesic metabolism and excretion, which increases the risk of toxic metabolite accumulation and increased drug bioavailability [61]. Thus, drug doses must be carefully managed to avoid the risk of adverse effects. For mild pain, acetaminophen is preferred and should be carefully managed. Patients should be properly educated on safe acetaminophen use. NSAIDs should be avoided due to their ability to cause multisystem damage in the kidneys, GI, bone marrow, and heart in patients with hepatic derangements [62]. For moderate pain, methadone may be used without dose modification because it does not produce toxic metabolites, while codeine should be avoided due to limited conversion to the active metabolite, causing a reduction of analgesic effects [63]. For severe pain, morphine and hydromorphone may be considered with careful dosing [63]. Lastly, fentanyl’s pharmacokinetics appear to be unaffected in hepatic disease and may be used within regular guidelines [64].
Chemotherapy-induced cardiotoxicity is described as cardiac dysfunction that may lead to reversible or irreversible heart failure [65]. Clinicians must be vigilant about avoiding analgesic medications that may cause arrhythmias and further cardiac dysfunction. For mild pain, acetaminophen is a first-line choice, and NSAIDs should be avoided [66]. NSAIDs have been documented to increase the risk of cardiotoxicity and cause sodium and fluid retention [66,67]. For moderate to severe pain, opioid management is generally acceptable, with a few notable considerations for arrhythmias. Methadone is not recommended due to its association with prolonged QT interval and ventricular arrhythmias [68]. Fentanyl and morphine may be considered as their use appear to have minimal effects on the cardiovascular system [68]. Furthermore, adjuvant medications like tricyclic antidepressants (TCAs) and anticonvulsants are often useful in treating neuropathic pain associated with cancer. However, in patients with chemotherapy-induced cardiotoxicity, TCAs should be avoided as they can cause orthostatic hypotension and arrhythmias [66].
Pain management in cancer patients with chemotherapy-induced organ toxicity should follow the WHO stepwise analgesia ladder with special considerations and modifications made in regard to the patient’s specific organ toxicities. Medications that may cause further organ system dysfunction or increase the risk of toxic metabolite accumulation should be avoided or carefully dosed. Integrative therapies (e.g., medical marijuana, acupuncture, mindfulness) and interventional pain management techniques (e.g., epidural/intrathecal analgesics, local nerve blocks, neuraxial analgesia) should be strategically employed to decrease the need for pharmacologic pain management and the risk for adverse effects.
11. Overall anesthetic considerations
Anesthesiologists should monitor patients receiving chemotherapy for organ system adverse effects. Anthracyclines are cardiotoxic, whereas platinum-based chemotherapeutic drugs are nephrotoxic. Many chemotherapeutic drugs cause liver damage, vomiting, and diarrhea. Furthermore, surgery may raise the risk of cancer metastasis and recurrence. To minimize perioperative immunosuppression, which increases the risk of cancer recurrence, cancer patients should be given regional or general anesthesia with propofol. Propofol is the most often used intravenous induction and maintenance medication. Additionally, preclinical research shows propofol has anticancer properties. Propofol shows anticancer effects in lab tests by directly altering ribonucleic acid pathways and signaling in cancer cells [69]. Propofol also has antiinflammatory and antioxidative effects, which can protect against perioperative immune suppression [70]. Palliative pain treatment developments for cancer patients are still ongoing. Cooperation of oncologists and anesthesiologists allows for the best possible patient outcome.
12. Conclusion
The cancer patient with a history of chemotherapy deserves special anesthetic considerations and perioperative care when undergoing surgery. While effective at destroying rapidly dividing cancer cells, chemotherapeutic agents can produce serious toxic side effects impacting multiple organ systems, including cardiac, pulmonary, renal, hepatic, gastrointestinal, bone marrow, and neurological systems. The presence of these organ toxicities directly impacts perioperative patient care, giving rise to various unpredictable and possibly life-threatening complications pre-, intra-, and post-operatively. As such, a detailed and thorough pre-operative assessment of chemotherapy-treated cancer patients is required to evaluate for multisystemic dysfunction of organs. A complete history and physical examination, along with laboratory studies and additional pre-operative testing tailored to specific organ toxicities, are necessary to assess risk and management. Intraoperatively, careful anesthesia planning regarding interactions between anesthetic use, surgical approach, and organ dysfunction is required for patients with chemotherapy-induced organ toxicity. Postoperatively, the cancer patient’s pain management plan should be optimized according to the WHO stepwise analgesic ladder to provide satisfactory relief while carefully considering organ toxicity-safe analgesia choices and incorporating interventional pain management techniques when appropriate. Understanding the implications of chemotherapy-induced organ toxicity on perioperative care is paramount to providing effective, individualized pain management for the cancer patient.
Funding
Diana Cruz-Topete, PhD, Heart, Lung, and Blood Institute (NHBLI) 5K01HL144882-0 (D.C.-T.); Center for Redox Biology and Cardiovascular Disease (D.C.-T. and H.A.D.).
Footnotes
CRediT authorship contribution statement
Justin Zeien, Elyse M. Cornett: Conceptualization, Hemangini A. Dhaibar, Wendy Qiu: Methodology, Mason Triay: Software, Hemangini A. Dhaibar: Data curation, Elyse M. Cornett, Wendy Qiu: Writing – original draft preparation. Diana Cruz-Topete: Visualization, Elyse M. Cornett, Diana Cruz-Topete: Investigation. Elyse M. Cornett, Alan David Kaye: Supervision. Ivan Urits: Software, Validation. Omar Viswanath, Alan David Kaye: Writing – review & editing.
Conflict of interest statement
None.
References
- [1].World Health Organization. Cancer [Internet]. 2018. [cited 2020 Sep 8]. Available from: 〈https://www.who.int/news-room/fact-sheets/detail/cancer〉.
- [2].National Cancer Institute Surveillance E and ERP. Common Cancer Sites — Cancer Stat Facts [Internet]. [cited 2020 Sep 8]. Available from: 〈https://seer.cancer.gov/statfacts/html/common.html〉.
- [3].Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL, Cancer treatment and survivorship statistics, 2019, CA Cancer J. Clin 69 (5) (2019) 363–385 (Sep 11). [DOI] [PubMed] [Google Scholar]
- [4].Division of Cancer Prevention and Control C for DC and P. Information for Health Care Providers | Preventing Infections in Cancer Patients | CDC; [Internet]. [cited 2020 Sep 8]. Available from: 〈https://www.cdc.gov/cancer/preventinfections/providers.html〉. [Google Scholar]
- [5].Livshits Z, Rao RB, Smith SW, An approach to chemotherapy-associated toxicity, Emerg. Med Clin. North Am 32 (1) (2014) 167–203. [DOI] [PubMed] [Google Scholar]
- [6].Hale KE, Chapter 95: toxicities of chemotherapy, in: Hall JB, Schmidt GA, Kress JP (Eds.), Principles of Critical Care, 4th ed., McGraw-Hill Education / Medical, New York City, 2015. [Google Scholar]
- [7].Gudaitytė J, Dvylys D, šimeliūnaitė I, Anaesthetic challenges in cancer patients: current therapies and pain management, Acta Med. Litu 24 (2) (2017) 121–127 (Jul 17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Common Toxicities of Chemotherapy [Internet]. [cited 2020 Sep 8]. Available from: 〈https://www1.cgmh.org.tw/intr/intr5/c6700/OBGYN/f/web/ChemotherapyToxicity/index.htm〉. [Google Scholar]
- [9].Gehdoo RP, Anticancer chemotherapy and it’s anaesthetic implications (current concepts), Indian J. Anaesth 53 (1) (2009) 18–29 (Feb). [PMC free article] [PubMed] [Google Scholar]
- [10].Maracic L, Van Nostrand J, Anesthetic implications for cancer chemotherapy, AANA J. 75 (3) (2007) 219–226 (Jun). [PubMed] [Google Scholar]
- [11].Lefor AT, Perioperative management of the patient with cancer, Chest 115 (5 SUPPL.) (1999) 165S–171S (May). [DOI] [PubMed] [Google Scholar]
- [12].McDowall RH, Anesthesia considerations for pediatric cancer, Semin Surg. Oncol 9 (6) (1993) 478–488. [DOI] [PubMed] [Google Scholar]
- [13].Kvolik S, Glavas-Obrovac L, Sakic K, Margaretic D, Karner I, Anaesthetic implications of anticancer chemotherapy, Eur. J. Anaesthesiol 20 (2003) 859–871. [DOI] [PubMed] [Google Scholar]
- [14].Khouri MG, Douglas PS, Mackey JR, Martin M, Scott JM, Scherrer-Crosbie M, Jones LW, Cancer therapy-induced cardiac toxicity in early breast cancer addressing the unresolved issues, Circulation 126 (23) (2012) 2749–2763 (Dec 4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Colombo A, Cipolla C, Beggiato M, Cardinale D, Cardiac toxicity of anticancer agents, Curr. Cardiol. Rep 15 (5) (2013) 362. [DOI] [PubMed] [Google Scholar]
- [16].Huettemann E, Sakka SG, Anaesthesia and anti-cancer chemotherapeutic drugs, Curr. Opin. Anaesthesiol 18 (3) (2005) 307–314 (Jun). [DOI] [PubMed] [Google Scholar]
- [17].Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM, Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensinconverting enzyme inhibition, Circulation 114 (23) (2006) 2474–2481 (Dec). [DOI] [PubMed] [Google Scholar]
- [18].Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, Inane T, Oguzhan A, Eryol NK, Topsakal R, Ergin A, Protective effects of carvedilol against anthracycline-induced cardiomyopathy, J. Am. Coll. Cardiol 48 (11) (2006) 2258–2262 (Dec 5). [DOI] [PubMed] [Google Scholar]
- [19].Armenian SH, Gelehrter SK, Chow EJ, Strategies to prevent anthracydine-related congestive heart failure in survivors of childhood cancer, Cardiol. Res Pract 2012 (2012), 713294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mathes DD, Bleomycin and hyperoxia exposure in the operating room, Anesth. Analg 81 (3) (1995) 624–629. [DOI] [PubMed] [Google Scholar]
- [21].Luis M, Ayuso A, Martinez G, Souto M, Ortells J, Intraoperative respiratory failure in a patient after treatment with bleomycin: previous and current intraoperative exposure to 50% oxygen, Eur. J. Anaesthesiol 16 (1) (1999) 66–68. [DOI] [PubMed] [Google Scholar]
- [22].Ai D, Banchs J, Owusu-Agyemang P, Cata J, Chemotherapy induced cardiovascular toxicity: beyond anthracyclines, Minerva Anestesiol. 80 (5) (2014) 586–594. [PubMed] [Google Scholar]
- [23].Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila-Roman VG, Gerhard-Herman MD, Holly TA, Kane GC, Marine JE, Nelson MT, Spencer CC, Thompson A, Ting HH, Uretsky BF, Wijeysundera DN, American College of C, American Heart A, ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American college of cardiology/American heart association task force on practice guidelines, J. Am. Coll. Cardiol 64 (22) (2014) e77–e137, 2014 Dec 9. [DOI] [PubMed] [Google Scholar]
- [24].Hulbert JC, Grossman JE, Cummings KB, Risk factors of anesthesia and surgery in bleomycin-treated patients, J. Urol 130 (1) (1983) 163–164. [DOI] [PubMed] [Google Scholar]
- [25].Ingrassia TS, Ryu JH, Trastek VF, Rosenow EC, Oxygen-exacerbated bleomycin pulmonary toxicity, Mayo Clin. Proc 66 (2) (1991) 173–178. [DOI] [PubMed] [Google Scholar]
- [26].Rubin R, Anesthetic implications for chemotherapy, Curr. Rev. Nurse Anesth 9 (1987) 182–192. [Google Scholar]
- [27].Caggiano V, Weiss RV, Rickert TS, Linde-Zwirble WT, Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy, Cancer 103 (9) (2005) 1916–1924 (May 1). [DOI] [PubMed] [Google Scholar]
- [28].Burrows FA, Hickey PR, Colan S, Perioperative complications in patients with anthracycline chemotherapeutic agents, Can. Anaesth. Soc. J 32 (2) (1985) 149–157 (Mar). [DOI] [PubMed] [Google Scholar]
- [29].Weesner KM, Bledsoe M, Chauvenet A, Wofford M, Exercise echocardiography in the detection of anthracycline cardiotoxicity, Cancer 68 (2) (1991) 435–438 (Jul 15). [DOI] [PubMed] [Google Scholar]
- [30].Lewkow LM, Hooker JL, Movahed A, Cardiac complications of intensive dose mitoxantrone and cyclophosphamide with autologous bone marrow transplantation in metastatic breast cancer, Int. J. Cardiol 34 (3) (1992) 273–276 (Mar). [DOI] [PubMed] [Google Scholar]
- [31].Bristow MR, Billingham ME, Mason JW, Daniels JR, Clinical spectrum of anthracycline antibiotic cardiotoxicity, Cancer Treat. Rep 62 (6) (1978) 873–879 (Jun). [PubMed] [Google Scholar]
- [32].Von Hoff DD, Layard MW, Basa P Jr Davis HL, Von Hoff AL, Rozencweig M, Muggia FM, Risk factors for doxorubicin-induced congestive heart failure, Ann. Intern. Med 91 (5) (1979) 710–717 (Nov). [DOI] [PubMed] [Google Scholar]
- [33].Huettemann E, Junker T, Chatzinikolaou KP, Petrat G, Sakka SG, Vogt L, Reinhart K, The influence of anthracycline therapy on cardiac function during anesthesia, Anesth. Analg 98 (4) (2004) 941–947 (Apr) (table of contents). [DOI] [PubMed] [Google Scholar]
- [34].Ganz WI, Sridhar KS, Ganz SS, Gonzalez R, Chakko S, Serafini A, Review of tests for monitoring doxorubicin-induced cardiomyopathy, Oncology 53 (6) (1996) 461–470 (Dec). [DOI] [PubMed] [Google Scholar]
- [35].Dumont P, Wihlm JM, Hentz JG, Roeslin N, Lion R, Morand G, Respiratory complications after surgical treatment of esophageal cancer. A study of 309 patients according to the type of resection, Eur. J. Cardio Thorac. Surg. J. Eur. Assoc. Cardio Thorac. Surg 9 (10) (1995) 539–543. [DOI] [PubMed] [Google Scholar]
- [36].Waid-Jones MI, Coursin DB, Perioperative considerations for patients treated with bleomycin, Chest 99 (4) (1991) 993–999 (Apr). [DOI] [PubMed] [Google Scholar]
- [37].Goldiner PL, Carlon GC, Cvitkovic E, Schweizer O, Howland WS, Factors influencing postoperative morbidity and mortality in patients treated with bleomycin, Br. Med. J 1 (6128) (1978) 1664–1667 (Jun 24). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Goldiner PL, Schweizer O, The hazards of anesthesia and surgery in bleomycin-treated patients, Semin Oncol. 6 (1) (1979) 121–124 (Mar). [PubMed] [Google Scholar]
- [39].Machele Donat S, David A Levy, Bleomycin associated pulmonary toxicity: is perioperative oxygen restriction necessary? J. Urol 160 (4) (1998) 1347–1352 (Oct 1). [DOI] [PubMed] [Google Scholar]
- [40].Madias NE, Harrington JT, Platinum nephrotoxicity, Am. J. Med 65 (2) (1978) 307–314 (Aug). [DOI] [PubMed] [Google Scholar]
- [41].Fjeldborg P, Sørensen J, Helkjaer PE, The long-term effect of cisplatin on renal function, Cancer 58 (10) (1986) 2214–2217 (Nov 15). [DOI] [PubMed] [Google Scholar]
- [42].Weiss HD, Walker MD, Wiernik PH, Neurotoxicity of commonly used antineoplastic agents (first of two parts), N. Engl. J. Med 291 (2) (1974) 75–81 (Jul 11). [DOI] [PubMed] [Google Scholar]
- [43].Kedar A, Cohen ME, Freeman AI, Peripheral neuropathy as a complication of cis-dichlorodiammineplatinum(II) treatment: a case report, Cancer Treat. Rep 62 (5) (1978) 819–821 (May). [PubMed] [Google Scholar]
- [44].Perry MC, Chemotherapeutic agents and hepatotoxicity, Semin. Oncol 19 (5) (1992) 551–565 (Oct). [PubMed] [Google Scholar]
- [45].Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, Rubenstein EB, The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis, Cancer 98 (7) (2003) 1531–1539 (Oct 1). [DOI] [PubMed] [Google Scholar]
- [46].NDA/BLA Multi-disciplinary Review and Evaluation NDA 212608 AYVAKIT (avapritinib) [Internet]. 2018. [cited 2021 Nov 29] p. 186. Available from: 〈https://www.accessdata.fda.gov/drugsatfda_docs/nda/2020/212608Orig1s000MultidisciplineR.pdf〉.
- [47].QINLOCKTM (ripretinib) tablets, for oral use [Internet]. 2020. [cited 2021 Nov 29]. Report No.: Reference ID: 4609421. Available from: 〈https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213973s000lbl.pdf〉. [Google Scholar]
- [48].TAZVERIK (tazemetostat) tablets, for oral use [Internet]. 2020. Report No.: Reference ID: 4627347. Available from: 〈https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213400s000lbl.pdf〉. [Google Scholar]
- [49].SARCLISA® (isatuximab-irfc) injection, for intravenous use [Internet]. 2020. Report No.: Reference ID: 4568826. Available from: 〈https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761113s000lbl.pdf〉. [Google Scholar]
- [50].TUKYSATM (tucatinib) tablets, for oral use [Internet]. 2020. Report No.: Reference ID: 4593756. Available from: 〈https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213411s000lbl.pdf〉. [Google Scholar]
- [51].TRODELVY® (sacituzumab govitecan-hziy) for injection, for intravenous use [Internet]. 2020. Report No.: Reference ID: 4778226. Available from: 〈https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761115s009lbl.pdf〉. [Google Scholar]
- [52].PEMAZYRETM (pemigatinib) tablets, for oral use [Internet]. 2020. Report No.: Reference ID: 4591160. Available from: 〈https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213736s000lbl.pdf〉. [Google Scholar]
- [53].TABRECTATM (capmatinib) tablets, for oral use [Internet]. Report No.: Reference ID: 4603951. Available from: 〈https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213591s000lbl.pdf〉. [Google Scholar]
- [54].Carlson CL, Effectiveness of the World Health Organization cancer pain relief guidelines: an integrative review, J. Pain. Res. Dove Med. Press Ltd 9 (2016) 515–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Scarborough BM, Smith CB, Optimal pain management for patients with cancer in the modern era, CA Cancer J. Clin 68 (3) (2018) 182–196 (May). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gudaitytė J, Dvylys D, šimeliūnaitė I, Anaesthetic challenges in cancer patients: current therapies and pain management, Acta Med. Litu 24 (2) (2017) 121–127 (Jul 17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kurita GP, Sjøgren P, Klepstad P, Mercadante S, Interventional techniques to management of cancer-related pain: clinical and critical aspects, Cancers 11 (4) (2019) 443 (Apr 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Małyszko J, Kozłowska K, Kozłowski L, Małyszko J, Nephrotoxicity of anticancer treatment, Nephrol. Dial. Transpl 32 (6) (2017) 924–936. [DOI] [PubMed] [Google Scholar]
- [59].Pham PC, Khaing K, Sievers TM, Pham PM, Miller JM, Pham SV, Pham PA, Pham PT, Update on pain management in patients with chronic kidney disease, Clin. Kidney J 10 (5) (2017) 688–697 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sharma A, Houshyar R, Bhosale P, Il Choi J, Gulati R, Lall C, Chemotherapy induced liver abnormalities: an imaging perspective, Clin. Mol. Hepatol 20 (3) (2014) 317–326 (Sep 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Bosilkovska M, Walder B, Besson M, Daali Y, Desmeules J, Analgesics in patients with hepatic impairment: pharmacology and clinical implications, Drugs 72 (2012) 1645–1669. [DOI] [PubMed] [Google Scholar]
- [62].Rakoski M, Goyal P, Spencer-Safier M, Weissman J, Mohr G, Volk M, Pain management in patients with cirrhosis, in: Clinical Liver Disease, vol. 11, John Wiley and Sons Inc., 2018, pp. 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Soleimanpour H, Safari S, Nia KS, Sanaie S, Alavian SM, Opioid drugs in patients with liver disease: a systematic review, Hepat. Mon. Kowsar Med. Publ. Co. Vol 16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Murphy EJ, Acute pain management pharmacology for the patient with concurrent renal or hepatic disease, Anaesth. Intensive Care 33 (3) (2005) 311–322. [DOI] [PubMed] [Google Scholar]
- [65].Walls GM, Lyon AR, Harbinson MT, Hanna GG, Cardiotoxicity following cancer treatment, Ulst. Med. J. Ulst. Med. Soc 86 (2017) 3–9. [PMC free article] [PubMed] [Google Scholar]
- [66].Wingate S, Wheeler MS, Pain in heart failure patients, support care, Heart Fail 19 (6) (2011) 75–83. [Google Scholar]
- [67].Singh BK, Haque SE, Pillai KK, Assessment of nonsteroidal anti-inflammatory drug-induced cardiotoxicity, Expert Opin. Drug Metab. Toxicol. Expert Opin. Drug Metab. Toxicol 10 (2014) 143–156. [DOI] [PubMed] [Google Scholar]
- [68].Behzadi M, Joukar S, Beik A, Opioids and cardiac arrhythmia: a literature review, Med. Princ. Pract 27 (2018) 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Jiang S, Liu Y, Huang L, Zhang F, Kang R, Effects of propofol on cancer development and chemotherapy: potential mechanisms, Eur. J. Pharm 831 (2018) 46–51 (Jul 15). [DOI] [PubMed] [Google Scholar]
- [70].Kim R., Anesthetic technique and cancer recurrence in oncologic surgery: unraveling the puzzle, Cancer Metastasis Rev. 36 (1) (2017) 159–177 (Mar). [DOI] [PubMed] [Google Scholar]


