Abstract
Moraxella catarrhalis is an important respiratory tract pathogen, causing otitis media in children and lower respiratory tract infections in adults with chronic obstructive pulmonary disease (COPD). Adults with COPD make antibody responses to M. catarrhalis following infection, but little is known about the identity of the antigens to which these antibodies are directed. In this study, 12 serum samples obtained from adults with COPD who had cleared M. catarrhalis from the respiratory tract following infection and who had developed new serum immunoglobulin G (IgG) to their infecting strain were subjected to a series of assays to identify the antigens to which potentially protective antibodies were directed. Sera were adsorbed with intact bacterial cells, and antibodies were eluted from the surfaces of the bacteria. Analysis by flow cytometry established that adsorption and elution effectively detected antibodies specifically directed to surface-exposed epitopes. Immunoblot assays of adsorbed and eluted serum fractions were performed with purified outer membranes and purified lipooligosaccharide of homologous infecting strains and with a series of mutants deficient in expression of individual outer membrane proteins (OMPs). While heterogeneity in antibody responses among individuals was observed, five major OMPs, UspA1, UspA2, Hag, TbpB, and OMP CD, were identified as targets of antibodies to surface epitopes in the majority of adults with COPD who cleared the organism. These results have important implications in understanding human immune responses to M. catarrhalis and in elucidating the elements of a protective immune response.
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the United States and in the world, and the prevalence of this disease is increasing (16). The course of COPD is characterized by intermittent exacerbations of the disease; approximately half of exacerbations are estimated to be caused by bacteria (19, 23). Bacteria are frequently isolated from the respiratory tracts of adults with COPD (7, 12, 21, 25). Therefore, the role of bacterial infection in the course and pathogenesis of the disease has been the subject of intense interest recently.
Moraxella catarrhalis is isolated from the sputum of 5 to 32% of adults with COPD in point prevalence studies (6, 9, 10, 20). Several lines of evidence implicate M. catarrhalis as a cause of exacerbations of COPD, and these have been reviewed (19). More recently, we have evaluated and characterized the roles of bacterial infection in general and of M. catarrhalis in particular in adults with COPD prospectively (2, 14, 14a, 17). During 81 months of follow-up of 104 patients with COPD, 120 episodes of acquisition and clearance of M. catarrhalis were observed. Interestingly, the organism is cleared efficiently after a relatively short duration of carriage (median, 34 days), and patients appear to develop strain-specific protection following clearance of M. catarrhalis from the respiratory tract (14a).
To assess the immune responses in these patients who cleared M. catarrhalis from their respiratory tracts, whole-cell enzyme-linked immunosorbent assays (ELISAs) were performed with paired serum samples obtained before acquisition and after clearance of M. catarrhalis. Whole-cell ELISAs detect antibodies that bind specifically to the surface of the bacterium. The majority of patients who cleared M. catarrhalis from the respiratory tract developed a new serum immunoglobulin G (IgG) antibody response to their homologous infecting strain of M. catarrhalis. The intensity of the serum IgG response was greater following exacerbations compared to the intensity of the response following asymptomatic colonization (14a). The previous study demonstrated the development of new immune responses to surface-exposed antigens but did not identify the antigens to which these new antibodies were directed. The goal of the present study is to identify the antigens of M. catarrhalis to which these new serum IgG antibodies are directed, with emphasis on identifying immune responses directed specifically against surface-exposed epitopes. These results will identify surface antigens that are important targets of the human immune response following clearance of M. catarrhalis from the respiratory tract and, thus, help guide vaccine development.
MATERIALS AND METHODS
COPD study clinic.
This prospective study at the Buffalo Veterans Affairs Medical Center has been described previously (17). A total of 104 patients were enrolled between March 1994 and December 2000. The inclusion criteria were the presence of chronic bronchitis (1), absence of other lung disease on the basis of a clinical assessment, absence of immunosuppressive or life-threatening disorders, and willingness to make monthly clinic visits. Patients were seen at the Buffalo Veterans Affairs Medical Center monthly and whenever they had symptoms suggestive of an exacerbation. At each clinic visit, clinical information and sputum and serum samples were obtained. A clinical evaluation was performed at each visit to determine whether the patient had stable disease or an exacerbation as previously described (17). This determination was made by one of two examiners (T. F. Murphy and S. Sanjay) before the results of sputum cultures were available.
Bacteriological methods.
Study personnel who processed sputum samples were unaware of the clinical status of patients. Sputum samples that were spontaneously expectorated the morning of the clinic visit were homogenized, diluted, and plated in a quantitative manner as previously described (17). Bacterial pathogens were identified with the use of standard techniques. The identity of an isolate as M. catarrhalis was confirmed by colony morphology and the presence of butyrate esterase.
Bacterial strains.
Isolates of M. catarrhalis were recovered from sputum samples of adults followed in the COPD Study Clinic. Isolates were subjected to molecular typing by pulsed-field gel electrophoresis as part of previously described studies (17). An exacerbation strain was defined as a newly acquired strain isolated from sputum during symptoms of an exacerbation.
The characteristics of M. catarrhalis O35E and its isogenic knockout mutants used in this study are shown in Table 1 and were described previously (11, 22).
TABLE 1.
M. catarrhalis mutants used in this study
| Strain | Description | Antibiotic resistance marker(s) |
|---|---|---|
| O35E | Parent strain | None |
| O35E.1 | uspA1 mutant | Kanamycin |
| O35E.2 | uspA2 mutant | Kanamycin |
| O35E.12 | uspA1 uspA2 double mutant | Kanamycin, chloramphenicol |
| O35E.copB | copB mutant | Kanamycin |
| O35E.tbpB | tbpB mutant | Kanamycin |
| O35E.hag | hag mutant | Kanamycin |
| O35E.12-hag | uspA1 uspA2 hag triple mutant | Kanamycin, chloramphenicol, erythromycin |
M. catarrhalis was grown in brain heart infusion (BHI) broth at 37°C with shaking or on BHI agar plates in an atmosphere of 5% CO2. When growing mutants, the medium was supplemented with kanamycin, chloramphenicol, or erythromycin as appropriate at concentrations of 20 mg/liter.
Serum samples.
Preacquisition serum samples were obtained 2 to 6 weeks prior to acquisition of a strain of M. catarrhalis determined by results of monthly sputum cultures. Postclearance serum samples were obtained 4 to 8 weeks following clearance of M. catarrhalis from the respiratory tract. Blood samples were obtained by venipuncture and allowed to clot. Serum samples were prepared by centrifugation and stored at −80°C.
Immunoblot assays and densitometry.
Purified outer membranes of infecting strains of M. catarrhalis were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot assays. Outer membranes of homologous strains were probed with preacquisition and postclearance serum samples at serial twofold dilutions of 1:200 to 1:6,400; selected pairs of serum samples were assayed at higher dilutions as well.
Immunoblot assays were analyzed by densitometry using an Alpha Innotech imaging system. The integrated density values were determined for individual bands in preacquisition and postclearance serum samples run at identical dilutions in adjacent lanes. The results of densitometric analysis were expressed as a percentage based on the relative integrated density value for each band analyzed.
Adsorption and elution assays.
Adsorption and elution assays were performed to identify antibodies that bind to epitopes that are exposed on the bacterial surface (13, 18). Assays were performed with the homologous patient isolate of M. catarrhalis. The bacterial strain was grown to late logarithmic phase (optical density at 600 nm [OD600] of ∼0.75) in BHI broth. Bacteria were harvested by centrifugation at 1,000 × g for 15 min at 4°C. After being washed once by suspension and centrifugation, bacterial cells were suspended in 1 ml of serum diluted 1:10 in phosphate-buffered saline (PBS) containing 0.15 mM CaCl2 and 0.5 mM MgCl2 (PCM) and incubated for 30 min at 4°C. Bacteria were removed by centrifugation at 16,000 × g for 10 min at 4°C. The supernatant was filter sterilized and saved at 4°C. Escherichia coli strain JM109 was subjected to the identical procedure simultaneously as a negative control.
To elute antibodies from the bacterial surface, the bacterial pellet following incubation with serum was washed three times with PCM and resuspended in 0.5 ml of elution buffer (0.2 M glycine, 0.2 M NaCl, pH 2.8). The mixture was incubated for 30 min at room temperature on a nutator mixer and then centrifuged at 16,000 × g for 10 min. The supernatant was transferred to another tube and titrated to a pH of ∼7.4 with 1 M Tris. The elution was performed concurrently with E. coli as a negative control.
Aliquots of unadsorbed serum, M. catarrhalis-adsorbed serum, E. coli-adsorbed serum (negative control), antibodies eluted from the surface of M. catarrhalis, and antibodies eluted from the surface of E. coli (negative control) were subjected to immunoblot assays. The bands to which antibodies were directed were identified by a combination of analysis with monoclonal antibodies of known specificity, amino-terminal sequencing of protein bands, and analysis of well-defined mutants as described below.
Flow cytometry.
Aliquots of serum were subjected to flow cytometry to assess the specificities of the adsorption and elution assays in identifying antibodies that bound epitopes on the surface of the intact bacterial cell (2). The isolate of M. catarrhalis to be studied was grown in broth to mid-logarithmic phase (OD600 of ∼0.2). An aliquot of 0.2 ml was harvested by centrifugation at 16,000 × g for 5 min. The bacteria were resuspended in 0.2 ml of serum diluted in PBS. The mixture was incubated on a nutator mixer for 1 h at 37°C and then centrifuged. The pellet was resuspended in 0.2 ml of a 1:10 dilution of goat anti-human IgG conjugated to fluorescein isothiocyanate (Kirkegaard & Perry Laboratories, Inc.) and mixed on a nutator for 30 min at 37°C. A volume of 1.8 ml of PBS was added, and the cells were subjected to flow cytometry with a fluorescence-activated cell sorter (FACScan; Becton Dickinson). A total of 20,000 cells were counted in a gated region corresponding to unclumped cells. Data were acquired by using an instrument with a logarithmic mode for forward scatter, side scatter, and fluorescence.
Purification of outer membranes.
Bacteria were grown on BHI agar overnight and harvested by suspension in 0.01 M HEPES, pH 7.4. Cells were disrupted by sonication on ice with four 15-second periods of sonication at 100 W. Unbroken cells and debris were removed by centrifugation at 10,000 × g for 2 min at 4°C. The suspension was centrifuged at 100,000 × g for 45 min at 4°C to collect cell envelopes. The pellets were suspended in 1% sarcosyl in 0.01 M HEPES and incubated at room temperature for 1 h to solubilize cytoplasmic membranes. The sarcosyl-insoluble fraction was obtained by centrifugation at 100,000 × g for 45 min at 4°C.
Purification of lipooligosaccharide (LOS).
LOS of isolates of M. catarrhalis was prepared using a microphenol method (2). Bacteria were grown on BHI agar overnight and harvested into 10 ml PBS to an OD490 of 0.4. Bacteria were centrifuged at 1,000 × g for 10 min at 4°C. The pellet was suspended in PCM and centrifuged as described above. The pellet was resuspended in 1 ml of 1 mg/ml lysozyme (Ready-Lyse; Epicentre, Madison, WI) in PCM and incubated at room temperature for approximately 45 min or until the suspension became viscous. DNase was added to a concentration of 1 μg/ml, RNase was added to a concentration of 10 μg/ml, and the suspension was incubated for 30 min at 37°C. Proteinase K was added to a final concentration of 46 μg/ml, and the suspension was incubated for 15 min at 65°C. An equal volume of prewarmed 90% phenol was added, followed by incubation with vigorous shaking at 65°C for 15 min. After the suspension was placed on ice for 10 min, the suspension was vortexed and then centrifuged at 16,000 × g for 10 min at 4°C. The aqueous (top) layer was saved. A volume of 1 ml of distilled water was added to the phenol phase, and the incubation and centrifugation steps were repeated. The aqueous phase was added to the previously saved aqueous phase. NaCl was added to a final concentration of 0.5 M, 10 volumes of cold absolute ethanol were added, and the LOS was allowed to precipitate overnight at −20°C. The suspension was centrifuged at 10,000 × g for 15 min at 4°C. The pellet was resuspended in 1 ml H2O and heated in a boiling water bath for 10 min and vortexed to resuspend fully. A volume of 10 μl was added to 10 μl of sample buffer for SDS-PAGE.
RESULTS
Identification of serum samples.
All serum samples were from adults with COPD who were followed in the COPD Study Clinic at the Buffalo VA Medical Center as part of a prospective study. In previous work, analysis of paired serum samples obtained prior to and following episodes of carriage of M. catarrhalis by whole-cell ELISA has revealed that the majority of patients make new antibodies to surface epitopes of their homologous infecting strains of M. catarrhalis (14a). To identify the surface antigens to which these antibodies are directed, 12 pairs of serum samples that yielded the largest increases in the levels of new antibodies from preacquisition of M. catarrhalis to postclearance were chosen for further study. Interestingly, the 12 pairs of sera that yielded the greatest increase in serum IgG by whole-cell ELISA were from patients who experienced exacerbations, consistent with the observation that exacerbations were associated with a greater intensity of immune response compared to asymptomatic colonization. The overall approach to analyzing the serum samples is outlined in Fig. 1.
FIG. 1.
Diagram of strategy used to identify the antigens to which human antibodies are directed.
Immunoblot assays with paired preacquisition and postclearance sera.
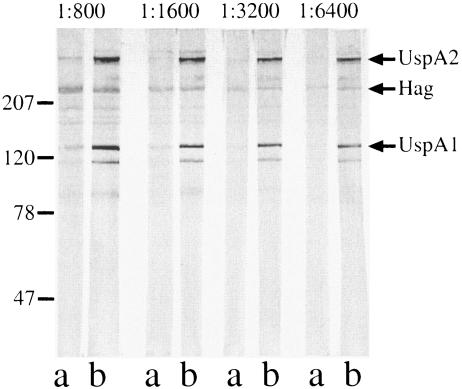
Paired sera were tested in immunoblot assays at multiple dilutions with purified outer membranes of the homologous infecting strains. Figure 2 shows the results with strain 32P6B1 illustrating the development of new antibodies to UspA1 and UspA2. The identities of the bands were determined by the reactivities of the sera with selected mutants lacking expression of individual proteins (see below). In addition, Fig. 2 shows the presence of antibodies to other minor, unidentified protein bands. These immunoblot assays also show the importance of assaying at multiple dilutions, as protein bands from preacquisition sera disappear at higher dilutions than those from postclearance sera, indicating the development of antibodies to those antigens.
FIG. 2.
Immunoblot assay of M. catarrhalis strain 32P6B1. All lanes contain purified outer membrane of M. catarrhalis strain 32P6B1 and were probed with homologous preacquisition serum sample 32E6 (lanes a) or with postclearance serum sample 32E8 (lanes b) with dilutions noted at the top. Antibodies were detected with peroxidase-conjugated anti-human IgG. The positions of molecular mass markers (in kilodaltons) are shown on the left.
In an effort to quantitate the development of antibodies following clearance of M. catarrhalis from the respiratory tract, bands were subjected to densitometry. Table 2 shows the results of densitometric analysis of bands that were shown to bind antibodies to surface-exposed epitopes. All of the postclearance sera that contained antibodies to surface epitopes of UspA1, UspA2, TbpB, CopB, and outer membrane protein (OMP) E showed clear-cut increases in antibody titers by visual inspection and densitometry from preacquisition to postclearance samples indicating seroconversion. Six of the nine sets of sera with antibodies to surface epitopes of Hag showed seroconversion. Serum samples 87E16 and 32E8 showed no difference between the intensity of the Hag band from preacquisition and postclearance sera, and serum sample 46E49 did not detect a band to Hag in the homologous strain in spite of the clear presence of antibodies to surface epitopes of the Hag protein in strain O35E (see below). Densitometry on the OMP CD band in immunoblot assays with paired serum samples did not yield satisfactory results, perhaps because OMP CD is an OMP A-like protein and human serum contains abundant antibodies to cross-reactive determinants that are not on the bacterial surface. In the case of OMP CD, Table 2 shows only the results of assays that specifically detect antibodies to surface epitopes.
TABLE 2.
Summary of outer membrane antigens to which serum IgG is directed following M. catarrhalis carriage in adults with COPDa
| Patient and visit IDb no. | UspA1 | UspA2 | Hag | TbpB | CopB | OMP CD | OMP E | LOS |
|---|---|---|---|---|---|---|---|---|
| 39E34 | + | + | + | + | + | + | ||
| 17.4/82.6 | 26.2/73.8 | 37.8/62.2 | 27.9/72.1 | 42.8/57.2 | 42.7/57.3 | |||
| 11E31 | + | + | + | |||||
| 36.1/63.9 | 18.4/81.6 | 27.8/72.2 | ||||||
| 46E49 | + | + | + | + | ||||
| 11.2/88.8 | 29.4/70.6 | |||||||
| 76E11 | + | + | + | |||||
| 42.5/57.5 | 38.1/61.9 | 17.9/82.1 | ||||||
| 87E16 | + | + | + | |||||
| 41.1/58.9 | 52.8/47.2 | |||||||
| 80E22 | + | + | + | |||||
| 29.9/70.1 | 34.0/66.0 | 12.3/87.7 | ||||||
| 10E67 | + | + | + | + | + | |||
| 24.4/75.6 | 12.6/87.4 | 30.2/69.8 | 17.1/82.9 | |||||
| 52E4 | + | + | + | + | ||||
| 35.3/64.7 | 40.9/59.1 | 36.4/63.6 | ||||||
| 32E8 | + | + | + | |||||
| 15.9/84.1 | 37.8/62.2 | 46.1/53.9 | ||||||
| 19E55 | + | + | + | |||||
| 41.2/58.8 | 20.9/79.1 | |||||||
| 7E95 | + | + | + | + | ||||
| 25.7/74.3 | 29.2/70.8 | |||||||
| 63E25 | + | + | + | + | ||||
| 7.7/92.3 | 35.9/64.1 | 12.4/87.6 |
The presence (+) of antibodies to surface epitopes in the postclearance serum sample demonstrated by adsorption and elution with the homologous infecting strain of M. catarrhalis is indicated. The antigens to which antibodies were directed were identified by a combination of analysis with monoclonal antibodies of known specificity, amino-terminal sequencing of protein bands, and analysis of well-defined mutants. The numbers indicate the relative densities of bands in immunoblot assay based on the integrated density values of preacquisition/postclearance serum samples assayed with the homologous infecting strain.
ID, identification.
Adsorption and elution assays.
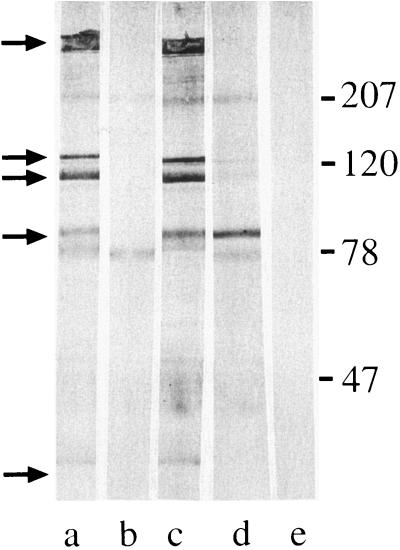
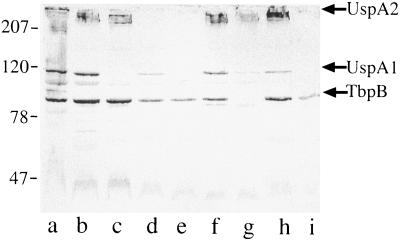
Because immunoblot assays do not distinguish antibodies to surface-exposed antigens from antibodies to antigens buried in the outer membrane, a series of adsorption and elution experiments was performed with the postexacerbation sera that showed the development of new serum IgG in whole-cell ELISA. An aliquot of serum was adsorbed with logarithmic-phase cells of the homologous infecting strain. In the example shown in Fig. 3, the adsorbed serum fraction (lane b) was tested in immunoblot assays with purified outer membrane along with unadsorbed serum (lane a). The disappearance or reduced intensity of a band in the adsorbed sample compared to the unadsorbed sample indicates that antibodies to that antigen bind epitopes on the bacterial surface. Figure 3, lane b, shows that five bands disappeared with adsorption compared to the unadsorbed sample in lane a, indicating that the serum sample contains antibodies to surface epitopes of these antigens.
FIG. 3.
Immunoblot assay of M. catarrhalis strain 87P15B1. All lanes contain purified outer membrane of M. catarrhalis strain 87P15B1. Lanes were probed with aliquots of homologous postclearance serum sample 87E16 as follows: a, unadsorbed; b, adsorbed with M. catarrhalis strain 87P15B1; c, adsorbed with E. coli; d, serum fraction eluted from the surface of M. catarrhalis strain 87P15B1; e, serum fraction eluted from E. coli. Antibodies were detected with peroxidase-conjugated anti-human IgG. The positions of molecular mass markers (in kilodaltons) are shown on the right. The arrows indicate bands to which antibodies were adsorbed (lane b) and/or eluted (lane d).
To control for nonspecific adsorption, an aliquot of the same serum sample was adsorbed simultaneously with a strain of E. coli (an irrelevant bacterium for the purpose of this study) and tested in an immunoblot assay. Figure 3 shows that all of the bands present in the unadsorbed aliquot (lane a) are present in the E. coli-adsorbed aliquot (lane c), excluding nonspecific loss of antibodies with adsorption.
To further characterize antibodies to surface epitopes, antibodies that bound to the bacterial surface following incubation of serum with whole bacterial cells were eluted from the bacterial surface and tested in immunoblot assays. Figure 3, lane d, shows that antibodies to selected bands were eluted from the surface of the homologous strain of M. catarrhalis. To assess the specificities of the elution assays, E. coli cells were subjected to the identical elution method; Fig. 3, lane e, shows the absence of detectable bands eluted from the surface of E. coli, providing evidence for the specificity of the elution assays for identifying antibodies to surface epitopes.
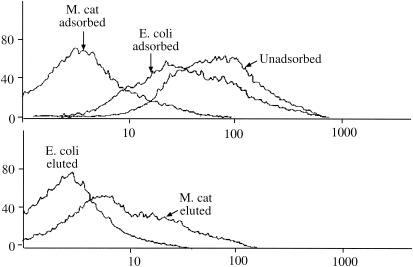
To further evaluate the adsorption and elution assays to detect antibodies specifically to surface-exposed epitopes, serum fractions were subjected to flow cytometry. Flow cytometry has previously been shown to effectively detect antibodies to surface-exposed epitopes of M. catarrhalis (2). Figure 4 (top) shows that adsorption with M. catarrhalis caused a prominent reduction in fluorescence as detected by a shift to the left of the adsorbed serum compared to unadsorbed serum. The minor shift observed on adsorption with E. coli is due to either nonspecific loss of antibody with adsorption, cross-reactive surface epitopes between M. catarrhalis and E. coli, or a combination of both. Figure 4 (bottom) shows that eluted antibodies recognize epitopes on the surface of M. catarrhalis. We conclude from these experiments that adsorption and elution effectively identify human antibodies that bind to surface-exposed epitopes.
FIG. 4.
Results of flow cytometry with M. catarrhalis strain 87P15B1. Fluorescence is shown on the x axis (in arbitrary units), and the number of cells is shown on the y axis. Serum fractions of the homologous postclearance serum sample 87E16 are noted. (Top) Adsorption with M. catarrhalis (M. cat) caused a prominent reduction in fluorescence as detected by a shift to the left of the adsorbed serum compared to unadsorbed serum. (Bottom) Eluted antibodies recognize epitopes on the surface of M. catarrhalis as indicated by prominent staining of M. catarrhalis cells by the eluted antibodies.
The postexacerbation sera from all 12 episodes of infection were subjected to adsorption and elution assays as described above and shown in Fig. 3 for serum sample 87E16. Bands that showed adsorption and elution were identified by assaying adjacent lanes in immunoblot assays with monoclonal antibodies with well-defined binding specificity and/or by cutting bands of interest from gels and determining N-terminal amino acid sequences. The overall results are summarized in Table 2, which contains data from multiple approaches to identify antigens detected in adsorption and elution assays (Fig. 1).
Analysis of eluted serum fractions with mutants.
As another approach to determining the identities of antigens to which human serum IgG antibodies were directed, a series of isogenic knockout mutants of M. catarrhalis were studied in immunoblot assays with eluted antibodies. The characteristics of the mutants are noted in Table 1 and have been described previously (22). In Fig. 5, postexacerbation serum sample 80E22 was incubated with the homologous infecting strain, and antibodies were eluted from the bacterial surface. The eluted antibodies were subjected to immunoblot assays with purified outer membranes of the homologous strain (Fig. 5, lane a) along with the mutants described in Table 1. Figure 5 clearly shows that antibodies recognize epitopes on UspA2, UspA1, and TbpB on the basis of the patterns of bands recognized. In the case of UspA1, lanes c, e, and i contain mutants that are deficient in expression of UspA1. The eluted antibodies bind to the corresponding UspA1 band in all lanes except for those three, allowing the conclusion that antibodies recognize UspA1. Similarly, the eluted antibodies bind to the broad UspA2 band at the top of the gel in all lanes except those that contain mutants deficient in UspA2 (lanes d, e, and i), supporting the conclusion that the serum sample contains antibodies to surface epitopes of UspA2. Aliquots of all 12 postexacerbation sera were subjected to elution from the homologous strain and analysis with mutants. The results are summarized in Table 2, which includes data from multiple approaches to identify the target of new serum IgG antibodies as outlined in Fig. 1.
FIG. 5.
Immunoblot assay with postexacerbation serum sample 80E22 eluted from the surface of the homologous strain 80P20B1. Lanes contain purified outer membrane preparations of strains a) 80P20B1, b) O35E, c) UspA1 mutant, d) UspA2 mutant, e) UspA1 UspA2 double mutant, f) CopB mutant, g) TbpB mutant, h) Hag mutant, and i) UspA1 UspA2 Hag triple mutant. The positions of molecular mass markers (in kilodaltons) are shown on the left. The positions of UspA2, UspA1, and TbpB are indicated by arrows on the right.
Antibodies to surface epitopes of lipooligosaccharide.
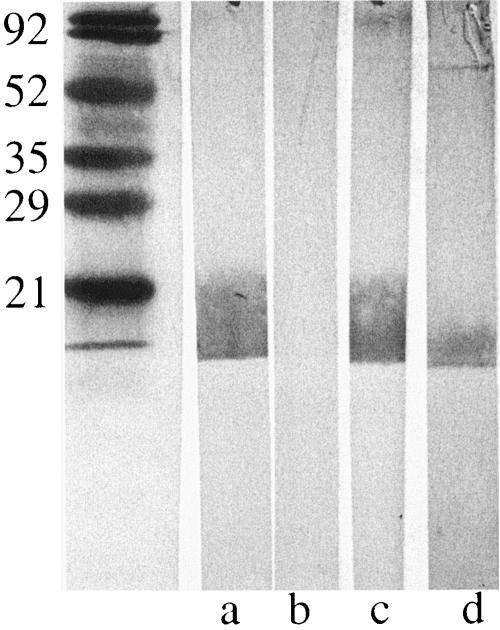
Analysis of immunoblot assays with purified outer membranes of homologous strains revealed a broad band of reactivity near the bottom of the gel in selected samples, suggesting the possibility of antibodies to LOS. To more carefully evaluate the serum samples for this possibility, purified LOS from each of the 12 homologous isolates was subjected to immunoblot assays with preacquisition and postclearance serum samples. Two postclearance serum samples (87E16 and 7E95) revealed the development of a new band to LOS compared to preacquisition serum samples. These serum samples were adsorbed with the homologous infecting strains, and antibodies were eluted from the bacterial surface. Figure 6 depicts the results of an immunoblot assay showing the adsorbed and eluted fractions tested with homologous LOS. These assays show that serum samples from both patients contain antibodies specifically to surface-exposed epitopes on LOS.
FIG. 6.
Immunoblot assay of purified LOS of M. catarrhalis strain 87P15B1 (all lanes). Lanes were probed with aliquots of homologous postclearance serum sample 87E16 as follows: a, unadsorbed; b, adsorbed with M. catarrhalis strain 87P15B1; c, adsorbed with E. coli; d, serum fraction eluted from the surface of M. catarrhalis strain 87P15B1. Antibodies were detected with peroxidase-conjugated anti-human IgG. The positions of molecular mass markers (in kilodaltons) are shown on the left.
DISCUSSION
In this study, well-characterized serum samples from adults with M. catarrhalis infection were used to identify the major outer membrane antigens to which the human serum antibody response is directed. Serum samples from adults with COPD who cleared M. catarrhalis from the respiratory tract and showed new antibody responses by whole-cell ELISAs were studied. The serum samples were subjected to immunoassays designed to detect specifically antibodies to epitopes on the bacterial surface. Although heterogeneity among individuals with regard to the antigens to which antibodies were directed was observed, several surface antigens were prominent targets of the human immune response. Five surface proteins were identified as targets of antibodies in the majority of the patients studied; these included UspA1, UspA2, Hag, TbpB, and OMP CD (Table 2). In addition, CopB, OMP E, and LOS were the targets of human antibodies of a smaller proportion of the patients studied. Heterogeneity is a hallmark of human immune responses to bacteria in adults with COPD (19).
Two elements of the design of the present study should be emphasized. The approach of using adsorption and elution assays detects antibodies specifically directed at surface-exposed epitopes. Bacterial outer membrane proteins contain a large proportion of epitopes that are buried in the outer membrane and are thus not accessible to antibodies that would be capable of binding to the intact bacterium. Immunoblot assays of purified outer membranes, for example, do not distinguish between antibodies to surface-exposed and non-surface-exposed epitopes. Antibodies that are likely to mediate protection bind to surface-exposed epitopes. Such antibodies would include, for example, bactericidal antibodies, opsonizing antibodies, and antibodies that block adherence to host cell receptors. The antibodies detected in the present study bind epitopes on the bacterial surface.
A second important feature of the present study is that serum samples were obtained from patients followed prospectively with monthly sputum cultures (17). Long-term follow-up established that patients cleared M. catarrhalis from the respiratory tract and did not reacquire the strain, indicating that a protective response occurred (14a). The protective responses appear to be strain specific because while reacquisition of the same strain did not occur, some patients acquired new strains of M. catarrhalis after clearing previous strains. These serum samples are valuable with regard to identifying antibodies that have the potential to mediate protective immune responses; however, one cannot yet conclude that the antibodies detected in the present study are mediating protection.
The adsorption and elution assays are complementary assays, yielding somewhat different results. For example, Fig. 3, lane b, illustrates adsorption of antibodies to five bands (noted by arrows); by contrast, the corresponding elution assay (lane d) detected exclusively antibodies to a band of ∼80 kDa. Several explanations likely account for the differences in the assays. Antibodies may bind with an affinity high enough to result in adsorption but may be removed from the bacterial surface by washing prior to elution. High-affinity antibodies may bind to the bacterial surface and resist elution. Some antibodies may undergo denaturation and loss of binding during the conditions of elution. If a serum sample were to contain a combination of antibodies to surface and nonsurface epitopes, one would expect the elution assays to be positive (by virtue of the presence of antibodies to surface epitopes) but the adsorption assay to be negative (by virtue of the presence of antibodies to non-surface-exposed epitopes). The results of adsorption and elution assays should be interpreted with these considerations in mind.
Limitations of the approach used in this study should be considered. The current study relies on immunoblot assays to identify antigens to which human antibodies bind. Therefore, antibodies to conformational epitopes that are denatured in SDS-PAGE and immunoblot assay will not be detected. The methods used in this study will detect antibodies to major surface proteins expressed during growth of bacteria in the laboratory. However, the method will not detect antibodies to minor surface antigens and will not detect antibodies to antigens that are expressed exclusively in vivo. Therefore, the results of this study are highly specific, demonstrating that the antigens noted in Table 2 are unequivocally the targets of a human immune response. However, the sensitivity of the approach is limited by the methods, indicating that antibodies to other antigens are likely present and alternative experimental approaches will be necessary to characterize these antibodies. Finally, while the focus of the present study is the serum IgG response, analysis of mucosal immune responses to M. catarrhalis will be important in characterizing potentially protective immune responses as well.
Previous work characterizing the human antibody response to M. catarrhalis following infection with laboratory strains and immunoblot assays revealed the presence of antibodies to multiple proteins, including UspA1, UspA2, TbpB, CopB, and a 60-kDa band that is probably OMP CD (4, 5, 8). TbpB (previously called OMP B1) is a target of serum IgG antibody responses in adults with respiratory tract infections and children with otitis media (3, 15, 18, 24). Analysis of paired serum samples from adults with COPD by ELISA using recombinant purified protein has demonstrated that some patients make new serum IgG to OMP CD (14). More recently, analysis of mucosal IgA antibodies in saliva from healthy adults and from children with otitis media using the mutants used in the present study demonstrated the presence of antibodies to similar antigens identified here as important targets of serum IgG (11, 22). The observation that both salivary IgA of healthy adults and serum IgG following infection in adults with COPD are directed at similar major antigens suggests conservation of the human immune response.
The present study extends the previous work in several ways. (i) The current study utilizes serum samples from adults with COPD who have cleared M. catarrhalis from the respiratory tract based on monthly sputum cultures, thus providing the opportunity to identify potentially protective antibodies. (ii) Antibodies that bind specifically to surface epitopes were measured by eluting antibodies from the bacterial surface. (iii) The homologous infecting strains of M. catarrhalis were used in adsorption and elution assays, enhancing the capability to detect strain-specific antibody responses. (iv) Complementary methods to identify surface antigens were used, including analysis with a set of well-defined mutants, analysis with monoclonal antibodies of known specificity, and amino-terminal sequence determination of antigens. (v) Detection of antibodies to surface epitopes of LOS by analysis of adsorbed and eluted serum fractions with purified LOS from homologous infecting strains.
In summary, analysis of carefully chosen serum samples from adults with COPD who have cleared M. catarrhalis from the respiratory tract and who have developed new antibodies to surface epitopes were studied to identify the surface antigens to which antibodies were directed. Most patients made serum IgG to surface-exposed epitopes of UspA1, UspA2, Hag, TbpB, and OMP CD. In addition, a smaller proportion made antibodies to CopB, OMP E, and LOS. These results contribute important new data on elucidating the surface antigens to which human antibody responses are directed following infection due to M. catarrhalis. Future work will address the potential protective capacity of antibodies to these major surface antigens.
Acknowledgments
This work was supported by NIH grants AI 46422 and AI 28304 and by the Department of Veterans Affairs.
Editor: D. L. Burns
REFERENCES
- 1.American Thoracic Society. 1995. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 152:S77-S121. [PubMed] [Google Scholar]
- 2.Bakri, F., A. L. Brauer, S. Sethi, and T. F. Murphy. 2002. Systemic and mucosal antibody response to Moraxella catarrhalis following exacerbations of chronic obstructive pulmonary disease. J. Infect. Dis. 185:632-640. [DOI] [PubMed] [Google Scholar]
- 3.Campagnari, A. A., T. F. Ducey, and C. A. Rebmann. 1996. Outer membrane protein B1, an iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect. Immun. 64:3920-3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, D., V. Barniak, K. R. van der Meid, and J. C. McMichael. 1999. The levels and bactericidal capacity of antibodies directed against the UspA1 and UspA2 outer membrane proteins of Moraxella (Branhamella) catarrhalis in adults and children. Infect. Immun. 67:1310-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen, J. J., N. Q. Hansen, and B. Bruun. 1996. Serum antibody response to outer membrane proteins of Moraxella (Branhamella) catarrhalis in patients with bronchopulmonary infection. Clin. Diagn. Lab. Immunol. 3:717-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gump, D. W., C. A. Phillips, B. R. Forsyth, F. K. McIntosh, K. R. Lamborn, and W. H. Stouch. 1976. Role of infection in chronic bronchitis. Am. Rev. Respir. Dis. 113:465-473. [DOI] [PubMed] [Google Scholar]
- 7.Hill, A. T., E. J. Campbell, S. L. Hill, D. L. Bayley, and R. A. Stockley. 2000. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am. J. Med. 109:288-295. [DOI] [PubMed] [Google Scholar]
- 8.Mathers, K., M. Leinonen, and D. Goldblatt. 1999. Antibody response to outer membrane proteins of Moraxella catarrhalis in children with otitis media. Pediatr. Infect. Dis. J. 18:982-988. [DOI] [PubMed] [Google Scholar]
- 9.May, J. R. 1953. The bacteriology of chronic bronchitis. Lancet 12:534-537. [DOI] [PubMed] [Google Scholar]
- 10.McHardy, V. U., J. M. Inglis, M. A. Calder, and J. W. Crofton. 1980. A study of infective and other factors in exacerbations of chronic bronchitis. Br. J. Dis. Chest 74:228-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier, P. S., S. Freiburghaus, A. Martin, N. Heiniger, R. Troller, and C. Aebi. 2003. Mucosal immune response to specific outer membrane proteins of Moraxella catarrhalis in young children. Pediatr. Infect. Dis. J. 22:256-262. [DOI] [PubMed] [Google Scholar]
- 12.Monso, E., J. Ruiz, A. Rosell, J. Manterola, J. Fiz, J. Morera, and V. Ausina. 1995. Bacterial infection in chronic obstructive pulmonary disease. A study of stable and exacerbated outpatients using the protected specimen brush. Am. J. Respir. Crit. Care Med. 152:1316-1320. [DOI] [PubMed] [Google Scholar]
- 13.Murphy, T. F., and L. C. Bartos. 1989. Surface-exposed and antigenically conserved determinants of outer membrane proteins of Branhamella catarrhalis. Infect. Immun. 57:2938-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy, T. F., C. Kirkham, D. F. Liu, and S. Sethi. 2003. Human immune response to outer membrane protein CD of Moraxella catarrhalis in adults with chronic obstructive pulmonary disease. Infect. Immun. 71:1288-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a.Murphy, T. F., A. L. Brauer, B. J. B. Grant, and S. Sethi. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am. J. Respir. Crit. Care Med., in press. [DOI] [PMC free article] [PubMed]
- 15.Myers, L. E., Y.-P. Yang, R.-P. Du, Q. Wang, R. E. Harkness, A. B. Schryvers, M. H. Klein, and S. M. Loosmore. 1998. The transferrin binding protein B of Moraxella catarrhalis elicits bactericidal antibodies and is a potential vaccine antigen. Infect. Immun. 66:4183-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pauwels, R. A., A. S. Buist, P. M. Calverley, C. R. Jenkins, and S. S. Hurd. 2001. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am. J. Respir. Crit. Care Med. 163:1256-1276. [DOI] [PubMed] [Google Scholar]
- 17.Sethi, S., N. Evans, B. J. B. Grant, and T. F. Murphy. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 347:465-471. [DOI] [PubMed] [Google Scholar]
- 18.Sethi, S., S. L. Hill, and T. F. Murphy. 1995. Serum antibodies to outer membrane proteins (OMPs) of Moraxella (Branhamella) catarrhalis in patients with bronchiectasis: identification of OMP B1 as an important antigen. Infect. Immun. 63:1516-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sethi, S., and T. F. Murphy. 2001. Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin. Microbiol. Rev. 14:336-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith, C. B., C. Golden, M. R. Klauber, R. Kanner, and A. Renzetti. 1976. Interactions between viruses and bacteria in patients with chronic bronchitis. J. Infect. Dis. 134:552-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soler, N., S. Ewig, A. Torres, X. Filella, J. Gonzalez, and A. Zaubet. 1999. Airway inflammation and bronchial microbial patterns with stable chronic obstructive pulmonary disease. Eur. Respir. J. 14:1015-1022. [DOI] [PubMed] [Google Scholar]
- 22.Stutzmann Meier, P., N. Heiniger, R. Troller, and C. Aebi. 2003. Salivary antibodies directed against outer membrane proteins of Moraxella catarrhalis in healthy adults. Infect. Immun. 71o:6793-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson, R. 1998. The role of infection in COPD. Chest 113:242S-248S. [DOI] [PubMed] [Google Scholar]
- 24.Yu, R.-H., R. A. Bonnah, S. Ainsworth, and A. B. Schryvers. 1999. Analysis of the immunological responses to transferrin and lactoferrin receptor proteins from Moraxella catarrhalis. Infect. Immun. 67:3793-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zalacain, R., V. Sobradillo, J. Amilibia, J. Barron, V. Achotegui, J. I. Pijoan, and J. L. Llorente. 1999. Predisposing factors to bacterial colonization in chronic obstructive pulmonary disease. Eur. Respir. J. 13:343-348. [DOI] [PubMed] [Google Scholar]