Abstract
The effects of total and partial inhibition of tumor necrosis factor (TNF) on sensitivity to Mycobacterium bovis BCG infection were investigated by using transgenic mice in which hepatocytes produced different amounts of human soluble TNF receptor 1 (sTNFR1) fused to the Fc fragment of human immunoglobulin G3 that could be detected in the serum. Transgenic mice expressing high serum levels of sTNFR1, neutralizing all circulating TNF, failed to develop differentiated granulomas and bactericidal mechanisms, and they succumbed to BCG infection. sTNFR1 transgenic mice did not activate BCG-induced Th1-type cytokines early in infection, but uncontrolled cytokine release was found late in infection. In this work we also evaluated the effect of partial inhibition of TNF on resistance to BCG infection. Transgenic mice expressing low levels of sTNFR1 were protected against BCG infection, and they developed increased bactericidal mechanisms, such as enhanced inducible nitric oxide synthase activity, increased macrophage activation, and showed higher numbers of liver granulomas early in infection compared to their negative littermates. Our data suggest that while total inhibition of TNF prevented BCG-induced cell-mediated immune responses, partial inhibition of TNF could contribute to macrophage activation, induction of bactericidal mechanisms, and granuloma formation in the early phase of BCG infection.
Tumor necrosis factor (TNF) is a pleiotropic cytokine involved in septic shock and inflammatory and immune responses. TNF is initially synthesized as a transmembrane precursor protein which is biologically active in vivo. The mature soluble TNF is proteolytically cleaved from the plasma membrane by matrix metalloproteinases, including tumor necrosis factor alpha converting enzyme (TACE) (4, 10, 11, 33, 34). The biological activity of TNF is mediated by the binding of TNF to TNF receptor 1 (TNFR1) or TNFR2 on the surface of many cells (43). The extracellular domain of both TNF receptors can be cleaved by metalloproteases belonging to the ADAM family (9, 35). Soluble TNF receptors (sTNFR) bind to both soluble and membrane TNF and therefore can neutralize TNF-mediated activities.
The importance of TNF in host defense mechanisms against infections has been extensively reported. Experimental animal models of TNF inhibition have provided accumulating evidence implicating TNF as a key factor in host defense against mycobacterial infections. Impaired granuloma formation, reductions in bactericidal mechanisms, and alteration of the mycobacterium-induced Th1-type immune response have been observed in animals unable to use TNF (2, 3, 5, 13, 16, 17, 24, 26, 37, 38, 45).
Excess TNF production is one of the causes of the pathogenesis of rheumatoid arthritis (15). Today, TNF inhibitors which are highly effective in the treatment of rheumatoid arthritis are available; however, serious infections, particularly reactivation of tuberculosis, have been reported, and therefore, screening for tuberculosis is essential in patients receiving anti-TNF treatment (25, 28). The appearance of severe infections in patients treated with anti-TNF therapy raised concerns about the complete ablation of TNF-associated functions.
In contrast to the efficacy of TNF inhibitors in rheumatoid arthritis and Crohn's disease treatment, this therapy has not worked in sepsis (1). Strategies to modulate TNF-linked functions would be more suitable than total abrogation depending on the complexity of pathologies. Furthermore, the administration of anti-TNF at the correct time and appropriate dosage has been suggested to be crucial for efficient therapy (20). Therefore, experimental animal models could still increase our understanding of the biological role of TNF inhibitors in infectious diseases.
In this study, we investigated the effect of total and partial inhibition of TNF in cell-mediated immune responses to Mycobacterium bovis BCG infection by using transgenic mice expressing high and low levels of human soluble TNFR fusion protein 1 (sTNFR1) under the control of the liver alpha-1 antitrypsin promoter (18). We report here that BCG infection of transgenic mice expressing high serum levels of sTNFR1 led to impaired granuloma formation, reduced macrophage activation and bactericidal mechanisms, dysregulation of cytokine release, and fatal bacterial growth. Furthermore, we show that transgenic mice expressing low serum levels of sTNFR1 were protected from BCG infection and exhibited enhanced macrophage activation and granuloma formation early in infection.
MATERIALS AND METHODS
Mice.
Four lines of transgenic mice, two lines with the C57BL/6 genetic background and two lines with the BALB/c genetic background, expressing different amounts of the sTNFR1-immunoglobulin G3 (IgG3) fusion protein, were obtained from four individual founders as previously described (18). The sTNFR1 fusion protein contained the extracellular domain of human TNFR1 fused to the hinge region of human FcIgG3. The transgene fusion protein was synthesized in the liver under the control of the alpha-1 antitrypsin promoter. sTNFR1 transgenic mice were maintained as heterozygous animals and were crossed with C57BL/6 or BALB/c mice. Transgenic mice were bred for more than 20 generations with wild-type mice with a corresponding background. Transgenic mouse candidates were bled, and an enzyme-linked immunosorbent assay (ELISA) was performed for identification of positive and negative transgenic mice and quantification of the sTNFR1-IgG3 human protein as previously described (18). All mouse strains were maintained under conventional conditions in the animal facility of the Medical Faculty, University of Geneva. All animal experiments were carried out in accordance with institutional guidelines and were approved by the local ethics committee on animal experimentation.
BCG infection and CFU evaluation in infected organs.
C57BL/6 negative littermates and transgenic mice expressing high (20 to 120 μg/ml) and low (0.1 to 1 μg/ml) serum levels of the sTNFR1-IgG3 fusion protein and BALB/c negative littermates and transgenic mice expressing high (250 to 500 μg/ml) and low (1 to 10 μg/ml) serum levels of the sTNFR1-IgG3 fusion protein (8 to 12 weeks old) were intravenously infected with 107 CFU of M. bovis BCG strain 1173 P2 (G. Marchal, Pasteur Institute, Paris, France). Mice were sacrificed 2, 4, and 8 weeks after BCG infection, and the bacterial loads in the lungs, liver, and spleen were evaluated as previously described (17).
Histological analyses of livers.
Histological analyses of liver tissues were performed 2, 4, and 8 weeks after BCG infection. Livers were fixed in 4% buffered formaldehyde and embedded in paraffin for hematoxylin and eosin staining and Ziehl-Neelsen staining.
Determination of iNOS activity in spleen extracts.
Evaluation of inducible nitric oxide synthase (iNOS) activity was carried out by using crude frozen spleen extracts of mice. Spleens were homogenized in 25 mM Tris-HCl (pH 7.4), 1 mM EDTA, and 1 mM EGTA (125 mg of tissue/ml of buffer). Crude supernatants were obtained by centrifugation of organ homogenates at 10,000 × g for 5 min. iNOS activity was measured by determining the capacity of supernatant to convert radioactive l-[14C]arginine (Amersham Life Sciences) to l-[14C]citrulline as previously described (37).
Northern hybridization.
RNA was isolated from liver, and 5 μg was hybridized with mouse iNOS and TNF probes as previously described (17).
Acid phosphatase activity.
Livers and spleens were frozen in liquid nitrogen, and 5-μm cryostat tissue sections were used for analysis of acid phosphatase activity as previously described (29). The method used for frozen tissue was modified and adapted for quantification of acid phosphate activity in spleen extracts as described previously (29).
Evaluation of cytokines in serum.
Blood samples were obtained from retroorbital sinuses before and 2, 4, and 8 weeks after BCG infection. Serum levels of interleukin-12p40 (IL-12p40), gamma interferon (IFN-γ), TNF, and IL-18 were evaluated by ELISA with a sensitivity of 2 to 1,000 pg/ml (R&D Systems, Abingdon, United Kingdom).
TNF bioactivity.
The bioactivity of TNF in mouse serum from control littermates and transgenic mice expressing low and high levels of sTNFR1 was measured with WEHI cells (clone 13) compared with the activity of standard murine TNF. WEHI cells (3 × 104/well) were incubated in the presence of actinomycin D (1 μg/ml) with mouse serum (dilution, 1/20 to 1/16,000) for 20 h in a 96-well plate. One picogram of standard TNF killed 50% of the WEHI cells (19).
Statistical analyses.
The unpaired Student's t test was used for analyses. P values of <0.05 were considered statistically significant.
RESULTS
Increased sensitivity to BCG infection of transgenic mice expressing high serum levels of sTNFR1 and resistance of transgenic mice expressing low levels of sTNFR1. C57BL/6 mice expressing high and low levels of sTNFR1 and negative littermate control mice were infected with 107 CFU, and survival was monitored for 12 months. Mice expressing high levels of sTNFR1 (n = 10) were sensitive to this dose of BCG and died between 3 and 10 weeks, whereas no mortality was observed either in transgenic mice expressing low levels of sTNFR1 (n = 10) or in control mice (n = 10) 12 months after infection. We observed that the survival time of mice expressing sTNFR1 depended on the level of transgene expression. Mice expressing the highest serum levels (more than 100 μg/ml; n = 5) survived no longer than 5 weeks (mean survival time, 28 days), and mice expressing 50 to 100 μg/ml (n = 5) survived no longer than 10 weeks (mean survival time, 59 days).
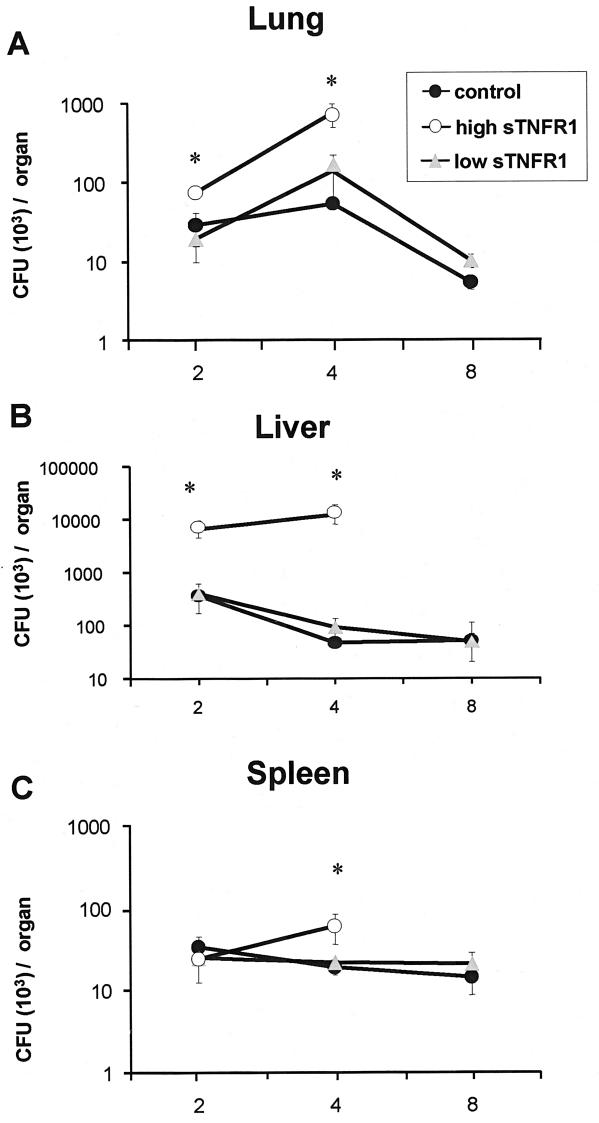
To quantify bacterial loads in infected organs, groups of transgenic mice and their negative littermates (n = 5) were infected intravenously with 107 CFU and killed at different times after infection. The bacterial loads in the lungs, livers, and spleens of transgenic mice expressing high levels of sTNFR1 (30 to 110 μg/ml) were significantly increased compared to the loads of nontransgenic mice (Fig. 1). After 8 weeks of BCG infection, in the group of transgenic mice expressing high levels of sTNFR1, only two mice were still alive, showing important cachexia (15% weight loss). Transgenic mice expressing high levels of sTNFR1 were not able to control bacillus proliferation, whereas transgenic mice expressing low levels of sTNFR1 (0.1 to 1 μg/ml) exhibited bacterial clearance similar to that of their negative littermates, indicating efficient killing of BCG (Fig. 1).
FIG. 1.
Bacterial loads were increased in the lungs, livers, and spleens of BCG-infected mice expressing high levels of sTNFR1 after BCG infection. The numbers of CFU in the lungs (A), liver (B), and spleen (C) were determined 2 weeks (n = 4/group), 4 weeks (n = 5/group), and 8 weeks (n = 4/group) after BCG infection. Bacterial loads were determined in tissue extracts from transgenic mice expressing high levels of sTNFR1 (high sTNFR1) and transgenic mice expressing low levels of sTNFR1 (low sTNFR1) and their negative littermates (control). Data are expressed as means ± standard errors of the means. An asterisk indicates that the P value is <0.02. The results are representative of the results of one of two experiments.
BCG granuloma formation and macrophage activation was reduced in transgenic mice expressing high levels of sTNFR1 and enhanced in transgenic mice expressing low levels of sTNFR1.
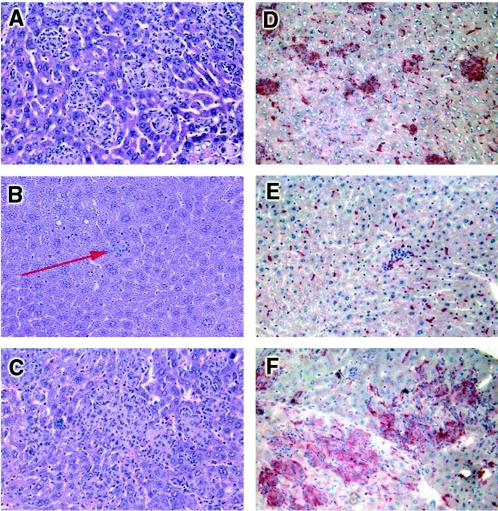
Protective immune responses against mycobacterial infections involve efficient granuloma formation and macrophage activation. Following intravenous BCG infection, a majority of bacilli infected the liver, as shown in Fig. 1B. Therefore, granuloma formation and macrophage activation were analyzed in the livers of mice expressing the transgenic sTNFR1 protein under the control of the hepatic alpha-1 antitrypsin promoter (18). After 2 weeks of infection, liver granulomas from negative littermates were well-defined and numerous (Fig. 2A). In contrast, there were very few liver granulomas in transgenic mice expressing high levels of sTNFR1, and the granulomas contained only a few mononuclear cells and mainly polymorphonuclear cells (the number of the granuloma-like structures was 0.7 ± 0.2 per mm2 of liver section [Fig. 2B]). In contrast, the granulomas of transgenic mice expressing low levels of sTNFR1 were larger than control granulomas, indicating more rapid granuloma formation (Fig. 2C). The numbers of granulomas in transgenic mice expressing low levels of sTNFR1 were higher than the numbers in control mice after 2 weeks of infection (50.7 ± 7.7 granulomas per mm2 in transgenic mice versus 33.8 ± 3.9 granulomas per mm2 in control mice; P < 0.005). The presence and activation of macrophages were evaluated by detection of acid phosphatase activity, which is a marker for activated macrophages (8). Acid phosphatase activity in livers from nontransgenic mice infected for 2 weeks with BCG mice was found on activated macrophages within well-defined granulomas and also on Kupffer cells (Fig. 2D). In contrast, only a few cells from transgenic mice expressing high levels of sTNFR1 were stained for acid phosphatase activity, indicating the absence of activated macrophages on granulomas (Fig. 2E). Liver sections of transgenic mice expressing low levels of sTNFR1 showed greater acid phosphatase staining than negative littermate control mice, indicating enhanced macrophage recruitment and activation (Fig. 2F). Therefore, acid phosphatase staining revealed reduced macrophage activation in transgenic mice expressing high levels of sTNFR1 but enhanced macrophage activation in transgenic mice expressing low levels of sTNFR1.
FIG. 2.
Impaired granuloma formation and reduced macrophage activation in transgenic mice expressing high levels of sTNFR1 and enhanced macrophage activity in transgenic mice expressing low levels of sTNFR1 after 2 weeks of BCG infection. Histological tissue sections were obtained from livers after 2 weeks of BCG infection. Hematoxylin and eosin staining revealed well-defined granulomas in control mice (A), small granuloma-like structures in transgenic mice expressing high levels of sTNFR1 (arrow) (B), and large granulomas in transgenic mice expressing low levels of sTNFR1 (C). (D) Strong staining for acid phosphatase activity (marker of macrophage activation) in control granulomas. (E) Absence of activity in granulomas from transgenic mice expressing high levels of sTNFR1. (F) Increased staining in the large granulomas from transgenic mice expressing low levels of sTNFR1. The results are representative of the results of two independent experiments (five mice per group). Magnification, ×200.
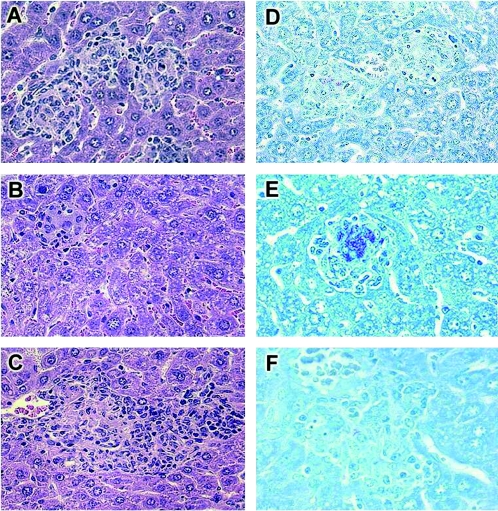
After 4 weeks of BCG infection, at the time of granuloma maturation, liver granulomas from negative littermates were well differentiated (Fig. 3A). In contrast, hepatic granulomas from transgenic mice expressing high levels of sTNFR1 were differentiated, but the numbers were decreased (4.6 ± 1.1 granulomas per mm2), and contained only a few mononuclear cells (Fig. 3B). Liver granulomas from transgenic mice expressing low levels of sTNFR1 were larger and well-differentiated, containing well-formed epithelioid cells, and the number was higher than the number of control liver granulomas (48.4 ± 6.2 granulomas versus 31.1 ± 3.3 granulomas per mm2 in control mice; P < 0.003) (Fig. 3C). Granulomas of negative littermates and transgenic mice expressing low levels of sTNFR1 contained few acid-fast bacilli (AFB), in contrast with the substantial number of AFB contained in granulomas from transgenic mice expressing high levels of sTNFR1 (Fig. 3D, E, and F). Therefore, high levels of sTNFR1 led to impaired granuloma formation and reduced macrophage activity associated with enhanced bacillus proliferation. In contrast, low levels of sTNFR1 resulted in efficient macrophage activation, increased granuloma number, and control of BCG proliferation.
FIG. 3.
Impaired granuloma formation in mice expressing high levels of sTNFR1 and enhanced granuloma formation in mice with low sTNFR1 levels after 4 weeks of BCG infection. Hematoxylin and eosin staining revealed well-differentiated granulomas in control mice (A), very few small granulomas in transgenic mice expressing high levels of sTNFR1 (B), and large well-differentiated granulomas in transgenic mice expressing low levels of sTNFR1 (C). Ziehl-Neelsen staining revealed the presence of few AFB in control mice (D), a higher number of AFB in transgenic mice expressing high levels of sTNFR1 (E), and few AFB in transgenic mice expressing low levels of sTNFR1 (F). The results are representative of the results of two independent experiments with five mice per group. (A to D and F) Magnification, ×344. (E) Magnification, ×400.
iNOS activity is reduced in transgenic mice expressing high levels of sTNFR1 and increased in transgenic mice expressing low levels of sTNFR1.
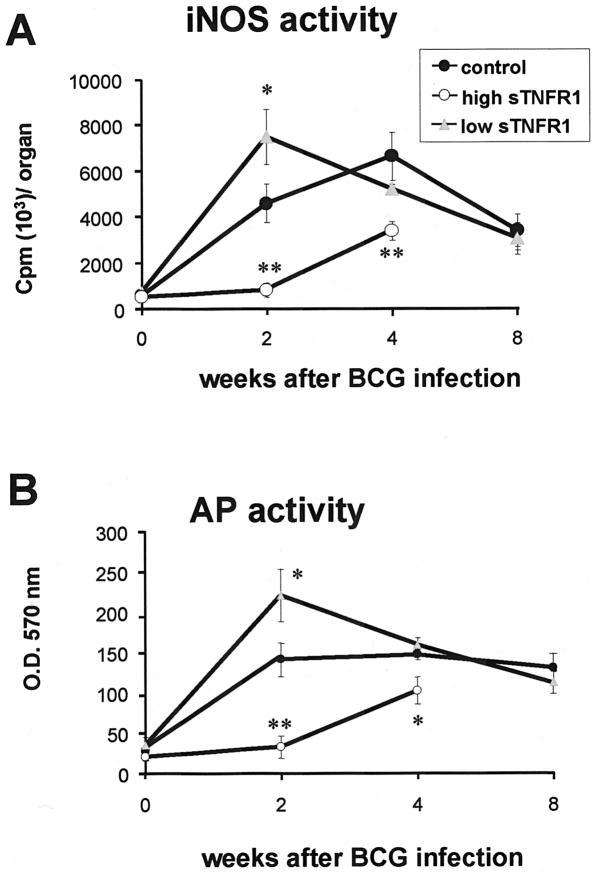
Bactericidal mechanisms, such as activation of iNOS, are important steps in the protective immune responses against mycobacterial infections (6, 7, 19, 30). iNOS activity was measured in crude spleen extracts. Transgenic mice expressing high levels of sTNFR1 exhibited no or decreased iNOS activity compared to control mice 2 and 4 weeks after BCG infection. In contrast, iNOS activity was increased in transgenic mice expressing low levels of sTNFR1 compared to negative littermates after 2 weeks of infection (Fig. 4A). Acid phosphatase activity assessed in spleens also correlated with iNOS expression, as shown in Fig. 4B. Thus, high levels of sTNFR1 inhibited both iNOS and acid phosphatase activation, whereas low levels enhanced these macrophage activities early in a BCG infection. Since technical reasons did not allow us to detect iNOS activity in the liver, expression of iNOS was assessed in liver tissues by Northern hybridization. iNOS mRNA levels were increased threefold in transgenic mice expressing low levels of sTNFR1 (n = 4) after 2 weeks of infection compared to negative littermates. In transgenic mice expressing high levels of sTNFR1 (n = 4), iNOS mRNA was undetectable after 2 and 4 weeks of BCG infection.
FIG. 4.
Decreased iNOS activity in spleens from transgenic mice expressing high levels of sTNFR1 and enhanced iNOS activity in transgenic mice expressing low levels of sTNFR1 after BCG infection. (A) iNOS activity was determined in crude spleen extracts from control mice, transgenic mice expressing high levels of sTNFR1 (high sTNFR1), and transgenic mice expressing low levels of sTNFR1 (low sTNFR1) before BCG infection (n = 3) and 2 weeks (n = 4), 4 weeks (n = 4 to 5), and 8 weeks (n = 4) after BCG infection. The data are expressed in cpm of 14C per organ and are means ± standard errors of the means. One asterisk indicates that the P value is <0.05, and two asterisks indicate that the P value is <0.01. The results are representative of the results of two independent experiments. (B) Reduced acid phosphatase (AP) activity in transgenic mice expressing high levels of sTNFR1 and enhanced activity in transgenic mice expressing low levels of sTNFR1 after BCG infection. Acid phosphatase activity was quantified in crude spleen extracts from control mice, transgenic mice expressing high levels of sTNFR1, and transgenic mice expressing low levels of sTNFR1 before BCG infection (n = 3) and 2 weeks (n = 4), 4 weeks (n = 4 to 5), and 8 weeks (n = 4) after BCG infection. The data are expressed as optical density at 570 nm (O.D.570 nm) in the organ and are means ± standard errors of the means. One asterisk indicates that the P value is <0.04, and two asterisks indicate that the P value is <0.008). The results are representative of the results of two independent experiments.
Partial or total TNF neutralization does not prevent systemic TNF release upon BCG infection.
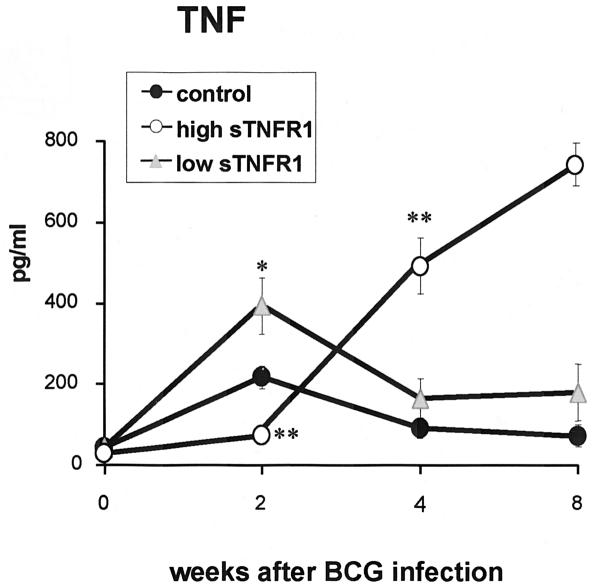
Serum amounts of TNF were measured in nontransgenic and transgenic mice expressing low and high serum levels of sTNFR1 (Fig. 5). The amounts of TNF in serum in transgenic mice expressing low levels of sTNFR1 were increased after 2 weeks of BCG infection, whereas TNF in transgenic mice expressing high levels of sTNFR1 was not activated compared to control mice. In contrast, after 4 weeks of infection, the amounts of TNF in serum in transgenic mice expressing high levels of sTNFR1 were significantly higher than those in nontransgenic mice. At 8 weeks postinfection, only two transgenic mice expressing high levels of sTNFR1 were alive, which showed excessive amounts of systemic TNF. However, the abundant serum TNF (600 to 800 pg/ml) was neutralized by human sTNFR1 (>60 μg/ml, which represents a 105-fold excess of receptors compared to TNF), as confirmed by a TNF bioassay activity with WEHI cells. Serum from transgenic mice expressing low levels of sTNFR1, as well as control mice, did not showed TNF cytotoxic activity or bioactivity with sensitive WEHI clone 13 cells. As previously reported, not only does murine BCG infection activate TNF production, but the shedding of murine soluble TNF receptors also is induced, and this can be monitored in the serum. Indeed, murine sTNFR1 and sTNFR2 are found at high concentrations in serum at 2 and 4 weeks after BCG infection (10-fold for sTNFR1 and 100-fold for sTNFR2) (19). Thus, TNF in serum of infected mice is neutralized by endogenous or transgenic sTNFR, but this does not eliminate the possibility that TNF can be effective in organs. To explore if the liver played a role in TNF release in mice expressing high levels of sTNFR1, a Northern blot analysis of liver RNA was performed. The levels of TNF mRNA in transgenic mice expressing high levels of sTNFR1 mice were 4.2-fold lower at 2 weeks and 3.1-fold lower at 4 weeks than the TNF mRNA levels in control mice. This demonstrated that sTNFR1 generated by hepatocytes efficiently blocked TNF production in the liver.
FIG. 5.
TNF serum levels after BCG infection. Serum TNF levels were evaluated in the three groups of mice at different times after BCG infection. TNF amounts were determined by ELISA in serum from transgenic mice expressing low levels of sTNFR1 (low sTNFR1) and transgenic mice expressing high levels of sTNFR1 (high sTNFR1) and their control littermates before BCG infection (n = 3) and 2 weeks (n = 4), 4 weeks (n = 4 to 5), and 8 weeks (n = 4) after BCG infection (except for transgenic mice expressing high levels of sTNFR1, since only two mice were alive at 8 weeks). The data are expressed in picograms of protein per milliliter of serum and are means ± standard errors of the means. One asterisk indicates that the P value is <0.03, and two asterisks indicates that the P value is <0.001. The experiment was repeated twice with similar results.
Altered serum levels of Th1-type cytokines in transgenic mice expressing sTNFR1.
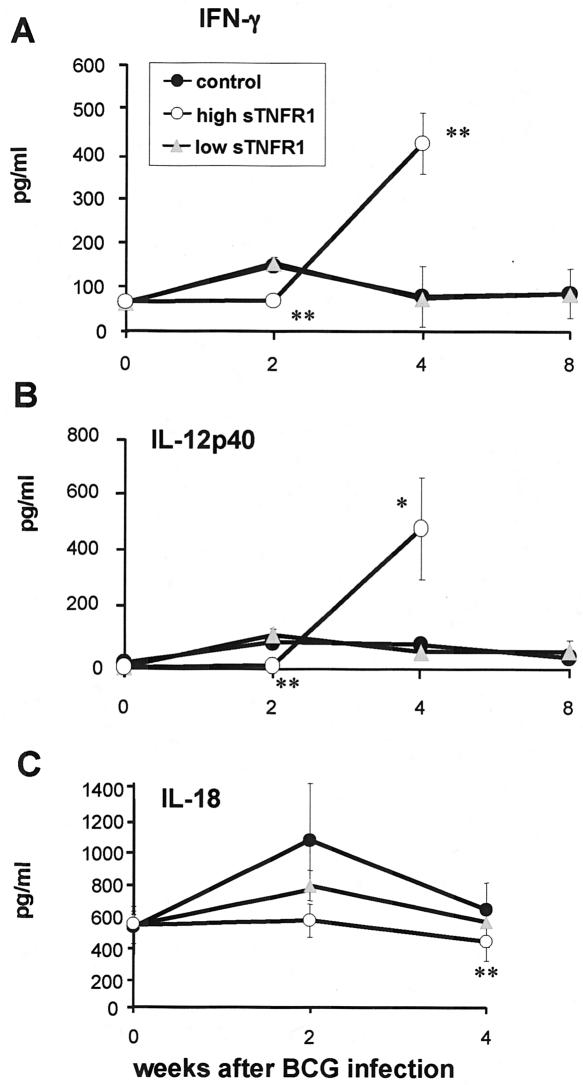
BCG infection induces a Th1 type of immune response, characterized by secretion of IFN-γ, IL-12, and also IL-18. Therefore, the amounts of these cytokines in serum were measured in the three groups of mice at different times after BCG infection (Fig. 6). IFN-γ was not activated in transgenic mice expressing high levels of sTNFR1 at 2 weeks, but after 4 weeks of BCG infection, the amounts of IFN-γ in serum were increased compared to those in control mice. The serum levels of IL-12p40 were also low at 2 weeks, but this was followed by increased release at 4 weeks after BCG infection. Furthermore, the amounts of IL-18 in serum were decreased in transgenic mice expressing high levels of sTNFR1 after BCG infection, indicating that IFN-γ release was independent of IL-18. Transgenic mice expressing low levels of sTNFR1 had amounts of IFN-γ, IL-12p40, and IL-18 in serum similar to those of control mice after BCG infection. Therefore, the presence of high levels of sTNFR1 led to reduced BCG-induced release of IFN-γ and IL-12p40 in the acute phase of infection, but later in infection the serum amounts of these cytokines were abnormally increased.
FIG. 6.
Altered serum levels of Th1-type cytokines in transgenic mice expressing high levels of sTNFR1. The levels of IFN-γ (A), IL-12p40 (B), and IL-18 (C) were determined by ELISA in serum from transgenic mice expressing high levels of sTNFR1 (high sTNFR1) and transgenic mice expressing low levels of sTNFR1 (low sTNFR1) and their control littermates before BCG infection (n = 3) and 2 weeks (n = 4), 4 weeks (n = 4 to 5), and 8 weeks (n = 4) after BCG infection. The data are expressed in picograms of protein per milliliter of serum and are means ± standard errors of the means. One asterisk indicates that the P value is <0.03, and two asterisks indicate that the P value is <0.003. The results are representative of the results of two independent experiments.
Transgenic mice with a BALB/c background expressing high levels of sTNFR1 were susceptible to BCG infection, but BALB/c transgenic mice expressing low levels of sTNFR1 were resistant to BCG infection.
Studies of sensitivity to BCG infection were also performed with mice with a BALB/c background. Two lines of transgenic mice expressing high levels (50 to 300 μg/ml) and low levels (0.5 to 10 μg/ml) of sTNFR1 and their corresponding negative littermates were used in this study. Groups of these mice (n = 10) were infected with BCG and sacrificed 2 and 4 weeks later. Histological examinations of liver granulomas after 2 weeks of infection showed well-defined granulomas in nontransgenic mice, very rare granuloma-like structures in transgenic mice expressing high levels of sTNFR1, and well-differentiated and large granulomas in transgenic mice expressing low levels of sTNFR1. After 4 weeks of infection, the number of granulomas in transgenic mice expressing high levels of sTNFR1 was about 10-fold lower than the number in the negative littermates. In contrast, the number of granulomas in mice expressing low levels of sTNFR1 was increased twofold, and the size was also larger than that in nontransgenic mice. Granulomas from transgenic mice expressing high levels of sTNFR1contained a high number of AFB, whereas in transgenic mice expressing low levels of sTNFR1 the number was similar to that in nontransgenic mice (data not shown). The bacterial loads in livers of infected mice after 2 and 4 weeks of infection showed similar numbers of CFU in transgenic mice expressing low levels of sTNFR1 and control mice but a more-than-10-fold increase in transgenic mice expressing high levels of sTNFR1.
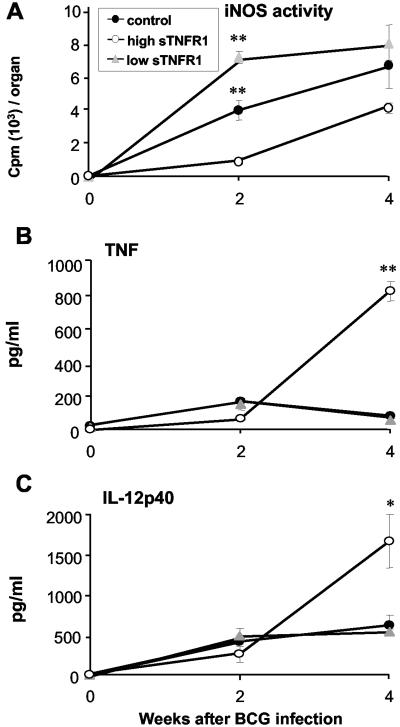
The activity of iNOS was assessed in spleens at different times of infection. Mice expressing high levels of sTNFR1 were not able to activate the iNOS, whereas transgenic mice expressing low levels of sTNFR1 induced higher levels of iNOS than nontransgenic mice (Fig. 7). The serum TNF levels, as well as the serum IL-12p40 levels, were evaluated in the three groups of mice. The differences observed between C57BL/6 transgenic and nontransgenic mice after 2 weeks of infection were attenuated in BALB/c mice, but at 4 weeks the TNF and IL-12p40 levels were abnormally increased in transgenic mice expressing high levels of sTNFR1. These results demonstrated that the granuloma formation and iNOS activation observed in transgenic mice expressing low and high levels of sTNFR1 were independent of the mouse background.
FIG. 7.
BCG infection in BALB/c sTNFR1 transgenic mice expressing high and low levels of the transgene. (A) Activity of iNOS in spleens of control mice, transgenic mice expressing low levels of sTNFR1 (high sTNFR1), and transgenic mice expressing high levels of sTNFR1 (low sTNFR1) before infection and 2 and 4 weeks after BCG infection (n = 5). (B) Serum levels of TNF before BCG infection and 2 and 4 weeks after infection (n = 5). (C) Serum levels of IL-12p40 before and 2 and 4 weeks after BCG infection (n = 5). The data are expressed in picograms of protein per milliliter of serum and are means ± standard errors of the means. One asterisk indicates that the P value is <0.03, and two asterisks indicate that the P value is <0.001. The results are representative the results of two independent experiments.
DISCUSSION
The importance of TNF in mycobacterial infections has been extensively reported. TNF was shown to contribute to granuloma development and maintenance, to the regulation of chemokine expression mediating the recruitment of leukocytes into functional granulomas, and to induction of bactericidal mechanisms, such as activation of iNOS for efficient killing of invading mycobacteria (2, 3, 5, 16, 17, 24, 26, 37, 38, 40, 41, 42). For long time TNF has been considered an essential cytokine contributing to the induction of the Th1 type of immune responses required for cell-mediated resistance against mycobacteria. More recent studies have reconsidered this notion in view of new observations made with mice unable to use TNF. A study has identified TNF as a regulatory cytokine for BCG-induced Th1-type immune responses which can mediate immune suppression to restrain a detrimental Th1-type immune response. Animals unable to produce TNF showed overproduction of Th1-type cytokines in the lung and peripheral blood and hyperreactive CD4 and CD8 T-cell responses upon BCG infection, and depletion of CD4 and CD8 T cells prevented tissue destruction in TNF−/− infected mice (45). This study supported previous reports suggesting an anti-inflammatory role for TNF involving its capacity to regulate Th1-type cytokines (22, 31). A dual role for TNF was previously observed depending on different pathological situations. TNF was shown to induce either proinflammatory cytokines or anti-inflammatory mediators (15). The complex roles of TNF in immunity have to be considered when therapies to abrogate TNF activities are used. In this context, we have shown that the transmembrane form of TNF expressed in TNF/lymphotoxin α (LT-α)−/− mice was able to induce an efficient cell-mediated immune response against BCG infection and to prevent the abnormal Th1-type immune responses elicited in TNF/LT-α-deficient mice (37). This suggests that maintenance of a transmembrane TNF by TACE inhibitors or another strategy is a way to avoid release of TNF in excessive amounts. Indeed, administration of a TACE inhibitor in healthy humans reduced TNF release after a single lipopolysaccharide dose (12, 32).
The present study showed the effects of total and partial inhibition of TNF on BCG infection. Complete inhibition of TNF in mice expressing high levels of sTNFR1 led to increased susceptibility to BCG infection and consequently to the absence of differentiated granulomas, decreased macrophage activation, reduced iNOS activity, dysregulation of TNF release, and abnormal Th1-type cytokine production, resulting in bacterial overgrowth and rapid death. We observed that granuloma formation requires sequential activation of cells and factors which are altered when TNF is neutralized. Blockade of TNF resulted in inhibition of BCG-induced macrophage functions, such as acid phosphatase and iNOS activities. In addition, the Th1 type of immune responses was abrogated early in infection, whereas in the late phase of infection, hyperrelease of Th1-type cytokines and TNF was found. Systemic TNF in transgenic mice expressing high levels of sTNFR1 was blocked by human soluble receptors, as shown by the absence of bioactivity on sensitive WEHI cells. This can be explained by the fact that the level of human sTNFR1 was 5-fold higher than the level of TNF in mouse serum, which obviously prevented any TNF bioactivity. In addition, BCG infection also induces the shedding of murine TNFR that can be detected in the serum of infected mice (19). BCG-induced murine sTNFR also blocks the bioactivity of circulating TNF but does not inhibit its expression and function in specific cells, such as macrophages forming granulomas (37). Furthermore, the presence of circulating sTNFR has been considered a marker of disease activity of tuberculosis (23). Since sTNFR1 neutralizes both TNF and LT-α, we cannot exclude the contribution of LT-α, which is important for the control of mycobacterial growth (39). However, our data on LT-α-deficient mice show that the inactivity of LT-α leads to both a reduction in BCG-induced TNF and a reduction in the Th1 type of immune responses (5).
In this work we also evaluated the effects of low levels of sTNFR1 on the sensitivity to BCG infection, which led to higher numbers of differentiated granulomas and increased bactericidal mechanisms early in infection, as shown by enhanced acid phosphatase and iNOS activities. These data suggested that the presence of circulating low levels of sTNFR1 favored macrophage activation required for granuloma differentiation.
Our previous data showed that transgenic mice expressing low levels of sTNFR1 had increased TNF-associated activities. In the previous study transgenic mice expressing low levels of sTNFR1 were more sensitive to lethal septic shock and cerebral malaria than nontransgenic mice (18). The underlying cellular mechanisms are still unknown; however, several possibilities can be considered. It may be the case that reverse signaling of TNF plays a role in modulating the immune response to BCG infection through activation of adhesion molecules and cytokine expression or regulation of bactericidal mechanisms in transgenic mice expressing low levels of sTNFR1. We cannot exclude from our data the finding that the Fc fragment of the sTNFR1 fusion protein could also lead to Fc-mediated effects, such as opsonization, complement activation, and antibody-dependent cellular cytotoxicity (20). However, in vitro studies using human fibroblasts, which do not express Fc receptors, showed that incubation with the sTNFR1-IgG3 fusion protein at a low concentration resulted in the activation of prostaglandin E2 and collagenase but that incubation with a high concentration inhibited these activities (36). This observation, which is comparable to observations made in our work, argues against Fc receptor mediation.
Previously, biological roles for sTNFR have been defined purely on the assumption that these are neutralizing agents and carriers for soluble TNF, inactivating TNF-associated cellular functions. sTNFR may also act as ligands for membrane-bound TNF and probably activate the cell. It has been found that a dimeric form of sTNFR1 dephosphorylates transmembrane TNF and induces reverse signaling of TNF (44). This effect could be prevented by treatment with phosphatase inhibitors. Binding of sTNFR1 to membrane-bound TNF induced an increase in the intracellular calcium level in a mouse macrophage cell line. Reverse signaling through transmembrane TNF with soluble TNF receptors (sTNFR1 and sTNFR2) as ligands mediated lipopolysaccharide resistance in human monocytes and macrophages (14). In addition, activation of membrane TNF on human T cells by TNFR2 induced expression of the adhesion molecule E-selectin (21). Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase signaling pathway has been reported on macrophages by reverse signaling through transmembrane TNF (27). These studies predicted the presence of associated signaling pathways by binding of soluble TNF receptors to membrane-bound TNF.
In conclusion, total neutralization of TNF led to increased susceptibility, whereas partial TNF inhibition resulted in enhanced granuloma formation and macrophage activities. Therefore, high levels of sTNFR1 neutralizing TNF reduced protective immune functions but did not prevent release of TNF and Th1-type cytokines in severe pathological conditions, while low levels of sTNFR1 increased BCG-induced macrophage functions.
Acknowledgments
This work was supported by grant 3200B0-105914 (to I.G.) from the Swiss National Foundation for Scientific Research and by grants from Roche, E&L Schmidheiny, The Sir Jules Thorn Charitable Overseas Trust, and the E&L Schmidheiny Foundation.
We thank G. Levraz, J. Stalder, and T. Le Minh for histological analyses.
Editor: A. D. O'Brien
REFERENCES
- 1.Abraham, E. 1999. Why immunomodulatory therapies have not worked in sepsis. Intensive Care Med. 5:556-566. [DOI] [PubMed] [Google Scholar]
- 2.Adams, L. B., C. M. Mason, J. K. Kolls, D. Scollard, J. L. Kraehenbuhl, and S. Nelson. 1995. Exarcebation of acute and chronic murine tuberculosis by administration of tumor necrosis factor receptor-expressing adenovirus. J. Infect. Dis. 171:400-405. [DOI] [PubMed] [Google Scholar]
- 3.Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. [PubMed] [Google Scholar]
- 4.Black, R. A., C. T. Rauch, C. J. Kozlosky, J. J. Peschon, J. L. Slack, M. F. Wolfson, B. J. Castner, K. L. Stocking, P. Reddy, S. Srinivasan, N. Nelson, N. Boiani, K. A. Schooley, M. Gerhart, R. Davis, J. N. Fitzner, R. S. Johnson, R. J. Paxton, C. J. March, and D. P. Cerretti. 1997. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385:729-733. [DOI] [PubMed] [Google Scholar]
- 5.Bopst, M., I. Garcia, R. Guler, M. L. Olleros, T. Rulicke, M. Muller, S. Wyss, K. Frei, M. Le Hir, and H. P. Eugster. 2001. Differential effects of TNF and LTalpha in the host defense against M. bovis BCG. Eur. J. Immunol. 31:1935-1943. [DOI] [PubMed] [Google Scholar]
- 6.Chan, J., Y. Xing, R. S. Magliozzo, and B. R. Bloom. 1992. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, J., K. Tanaka, D. Carroll, J. L. Flynn, and B. R. Bloom. 1995. Effect of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect. Immun. 63:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandrasekhar, S. 1978. Lysosomial enzymes and microbicidal capacity of activated macrophage. Microbios 22:27-34. [PubMed] [Google Scholar]
- 9.Crowe, P. D., B. N. Walter, K. M. Mohler, C. Otten-Evans, R. A. Black, and C. F. Ware. 1995. A metalloprotease inhibitor blocks shedding of the 80-kD TNF receptor and TNF processing in T lymphocytes. J. Exp. Med. 181:1205-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cseh, K., and B. Beutler. 1989. Alternative cleavage of the cachectin/tumor necrosis factor propeptide results in a larger, inactive form of secreted protein. J. Biol. Chem. 264:16256-16260. [PubMed] [Google Scholar]
- 11.Decoster, E., B. Vanhaesebroeck, P. Vandenabeele, J. Grooten, and W. Fiers. 1995. Generation and biological characterization of membrane-bound, uncleavable murine tumor necrosis factor. J. Biol. Chem. 270:18473-18478. [DOI] [PubMed] [Google Scholar]
- 12.Dekkers, P. E., F. N. Lauw, T. ten Hove, A. A. te Velde, P. Lumley, D. Becherer, S. J. van Deventer, and T. van der Poll. 1999. The effect of a metalloproteinase inhibitor (GI5402) on tumor necrosis factor-alpha (TNF-alpha) receptors during human endotoxemia. Blood 94:2252-2258. [PubMed] [Google Scholar]
- 13.Ehlers, S. 2003. Role of tumor necrosis factor (TNF) in host defence against tuberculosis: implications for immunotherapies targeting TNF. Ann. Rheum. Dis. 62:ii37-ii42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eissner, G., S, Kirchner, H. Lindner, W. Kolch, P. Janosch, M. Grell, P. Scheurich, R. Andreesen, and E. Holler. 2000. Reverse signaling through transmembrane TNF confers resistance to lipopolysaccharide in human monocytes and macrophages. J. Immunol. 164:6193-6198. [DOI] [PubMed] [Google Scholar]
- 15.Feldmann, M., and R. N. Maini. 2003. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat. Med. 9:1245-1250. [DOI] [PubMed] [Google Scholar]
- 16.Flynn, J. L., M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 17.Garcia, I., Y. Miyazaki, G. Marchal, W. Lesslauer, and P. Vassalli. 1997. High sensitivity of transgenic mice expressing soluble TNFR1 fusion protein to mycobacterial infections; synergistic action of TNF and IFN-γ in the differentiation of protective granulomas. Eur. J. Immunol. 27:3182-3190. [DOI] [PubMed] [Google Scholar]
- 18.Garcia, I., Y. Miyazaki, K. Araki, M. Araki, R. Lucas, G. E. Grau, G. Milon, Y. Belkaid, C. Montixi, W. Lesslauer, and P. Vassalli. 1995. Transgenic mice expressing high levels of soluble TNF-R1 fusion protein are protected from lethal septic shock and cerebral malaria, and are highly sensitive to Listeria monocytogenes and Leishmania major infections. Eur. J. Immunol. 25:2401-2407. [DOI] [PubMed] [Google Scholar]
- 19.Garcia, I., R. Guler, D. Vesin, M. L. Olleros, P. Vassalli, Y. Chvatchko, M. Jacobs, and B. Ryffel. 2000. Lethal Mycobacterium bovis Bacillus Calmette Guerin infection in nitric oxide synthase 2-deficient mice: cell-mediated immunity requires nitric oxide synthase 2. Lab. Investig. 80:1385-1397. [DOI] [PubMed] [Google Scholar]
- 20.Grau, G. E., and D. N. Maennel. 1997. TNF inhibition and sepsis—sounding a cautionary note. Nat. Med. 3:1193-1195. [DOI] [PubMed] [Google Scholar]
- 21.Harashima, S., T. Horiuchi, N. Hatta, C. Morita, M. Higuchi, T. Sawabe, H. Tsukamoto, T. Tahira, K. Hayashi, S. Fujita, and Y. Niho. 2001. Outside-to-inside signal through the membrane TNF-alpha induces E-selectin (CD62E) expression on activated human CD4+ T cells. J. Immunol. 166:130-136. [DOI] [PubMed] [Google Scholar]
- 22.Hodge-Dufour, J., M. W. Marino, M. R. Horton, A. Jungbluth, M. D. Burdick, R. M. Strieter, P. W. Noble, C. A. Hunter, and E. Pure. 1998. Inhibition of interferon γ induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc. Natl. Acad. Sci. USA 95:13806-13811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juffermans, N. P., A. Verbon, S. J. van Deventer, H. van Deutekom, P. Speelman, and T. van der Poll. 1998. Tumor necrosis factor and interleukin-1 inhibitors as markers of disease activity of tuberculosis. Am. J. Respir. Crit. Care Med. 157:1328-1331. [DOI] [PubMed] [Google Scholar]
- 24.Kaneko, H., H. Yamada, S. Mizuno, T. Udagawa, Y. Kazumi, K. Sekikawa, and I. Sugawara. 1999. Role of tumor necrosis factor-alpha in Mycobacterium-induced granuloma formation in tumor necrosis factor-alpha-deficient mice. Lab. Investig. 79:379-386. [PubMed] [Google Scholar]
- 25.Keane, J., S. Gershon, R. P. Wise, E. Mirabile-Levens, J. Kasznica, W. D. Schwieterman, J. N. Siegel, and M. M. Braun. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098-1104. [DOI] [PubMed] [Google Scholar]
- 26.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56:731-740. [DOI] [PubMed] [Google Scholar]
- 27.Kirchner, S., S. Boldt, W. Kolch, S. Haffner, S. Kazak, P. Janosch, E. Holler, R. Andreesen, and G. Eissner. 2004. LPS resistance in monocytic cells caused by reverse signalling through transmembrane TNF (mTNF) is mediated by MAP/ERK pathway. J. Leukoc. Biol. 75:324-331. [DOI] [PubMed] [Google Scholar]
- 28.Long, R., and M. Gardam. 2003. Tumour necrosis factor-alpha inhibitors and the reactivation of latent tuberculosis infection. Can. Med. Assoc. J. 168:1153-1156. [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas, R., F. Tacchini-Cottier, R. Guler, D. Vesin, S. Jemelin, M. L. Olleros, G. Marchal, J. L. Browning, P. Vassalli, and I. Garcia. 1999. A role for lymphotoxin beta receptor in host defense against Mycobacterium bovis BCG infection. Eur. J. Immunol. 29:4002-4010. [DOI] [PubMed] [Google Scholar]
- 30.MacMicking, J. D., R. J. North, R. LaCourse, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marino, M. W., A. Dunn, D. Grail, M. Inglese, Y. Noguchi, E. Richards, A. Jungbluth, H. Wada, M. Moore, B. Williamson, S. Basu, and L. J. Old. 1997. Characterization of tumor necrosis factor-deficient mice. Proc. Natl. Acad. Sci. USA 94:8093-8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohler, K. M., P. R. Sleath, J. N. Fitzner, D. P. Cerretti, M. Alderson, et al. 1994. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature 370:218-222. [DOI] [PubMed] [Google Scholar]
- 33.Moss, M. L., S. L. Jin, M. E. Milla, D. M. Bickett, W. Burkhart, H. L. Carter, et al. 1997. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature 385:733-736. [DOI] [PubMed] [Google Scholar]
- 34.Moss, M. L., and M. H. Lambert. 2002. Shedding of membrane proteins by ADAM family proteases. Essays Biochem. 38:141-153. [DOI] [PubMed] [Google Scholar]
- 35.Mullberg, J., F. H. Durie, C. Otten-Evans, M. R. Alderson, S. Rose-John, D. Cosman, R. A. Black, and K. M. Mohler. 1995. A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J. Immunol. 155:5198-5205. [PubMed] [Google Scholar]
- 36.Nicod, L. P., P. Isler, R. Chicheportiche, F. Songeon, and J.-M. Dayer. 1996. Production of prostaglandine E2 and collagenase is inhibited by the recombinant soluble tumor necrosis factor p55-human γ3 fusion protein at concentrations a hundred-fold lower than those decreasing T cell activation. Eur. Cytokine Netw. 7:757-763. [PubMed] [Google Scholar]
- 37.Olleros, M. L., R. Guler, N. Corazza, D. Vesin, H. P. Eugster, G. Marchal, P. Chavarot, C. Mueller, and I. Garcia. 2002. Transmembrane TNF induces an efficient cell-mediated immunity and resistance to Mycobacterium bovis bacillus Calmette-Guerin infection in the absence of secreted TNF and lymphotoxin-alpha. J. Immunol. 68:3394-3401. [DOI] [PubMed] [Google Scholar]
- 38.Roach, D. R., A. G. Bean, C. Demangel, M. P. France, H. Briscoe, and W. J. Britton. 2002. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J. Immunol. 168:4620-4627. [DOI] [PubMed] [Google Scholar]
- 39.Roach, D. R., H. Briscoe, B. Saunders, M. P. France, S. Riminton, and W. J. Britton. 2001. Secreted lymphotoxin-alpha is essential for the control of an intracellular bacterial infection. J. Exp. Med. 193:239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roach, D. R., H. Briscoe, K. Baumgart, D. A. Rathjen, and W. J. Britton. 1999. Tumor necrosis factor (TNF) and a TNF-mimetic peptide modulate the granulomatous response to Mycobacterium bovis BCG infection in vivo. Infect. Immun. 67:5473-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saito, S., and M. Nakano. 1996. Nitric oxide production by peritoneal macrophages of Mycobacterium bovis BCG-infected or non-infected mice: regulatory role of T lymphocytes and cytokines. J. Leukoc. Biol. 59:908-915. [DOI] [PubMed] [Google Scholar]
- 42.Senaldi, G., S. Yin, C. L. Shaklee, P. F. Piguet, T. M. Mak, and T. R. Ulich. 1996. Corynebacterium parvum- and Mycobacterium bovis Bacillus Calmette-Guérin-induced granuloma formation is inhibited in TNF receptor I (TNF-RI) knockout mice and by treatment with soluble TNF-RI. J. Immunol. 157:5022-5026. [PubMed] [Google Scholar]
- 43.Vandenabeele, P., W. Declercq, R. Beyaert, and W. Fiers. 1995. Two tumor necrosis factor receptors: structure and function. Trends Cell Biol. 5:392-399. [DOI] [PubMed] [Google Scholar]
- 44.Watts, A. D., N. H. Hunt, Y. Wanigasekara, G. Bloomfield, D. Wallach, B. D. Roufogalis, and G. Chaudhri. 1999. A casein kinase I motif present in the cytoplasmic domain of members of the tumour necrosis factor ligand family is implicated in 'reverse signaling.' EMBO J. 18:2119-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zganiacz, A., M. Santosuosso, J. Wang, T. Yang, L. Chen, M. Anzulovic, S. Alexander, B. Gicquel, Y. Wan, J. Bramson, M. Inman, and Z. Xing. 2004. TNF-alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J. Clin. Investig. 113:401-413. [DOI] [PMC free article] [PubMed] [Google Scholar]