Abstract
Streptococcus agalactiae (group B streptococcus [GBS]) is the leading cause of neonatal pneumonia, sepsis, and meningitis. An in silico genome analysis indicated that GBS strain NEM316 encodes 35 proteins containing an LPXTG motif which are thought to be covalently linked to the peptidoglycan by an enzyme called sortase. The role of these cell wall-anchored proteins in GBS pathogenesis was evaluated on a global level by inactivating the srtA gene. This gene encodes the major sortase SrtA that anchors most of the LPXTG-containing proteins. We chose the C5a peptidase (ScpB) and Alp2, an abundant immunogenic protein, as prototypical LPXTG-containing proteins. As expected, the SrtA knockout mutant was unable to anchor the C5a peptidase (ScpB) and Alp2 to the cell wall. Complementation with plasmid-borne srtA inserted into the chromosome restored the correct surface localization of both ScpB and Alp2. Interestingly, the SrtA mutant was impaired for binding to the major extracellular matrix components fibronectin and fibrinogen and displayed a significant reduction in adherence to human (A549, HeLa, and Caco-2) and murine (L2) epithelial cells compared to the parental wild-type strain. Surprisingly, the inactivation of srtA had no effect on the virulence of the type III strain of GBS in a neonatal rat model (measured by the 50% lethal dose and lung colonization) but strongly impaired the capacity of the strain to colonize the intestines of gnotobiotic mice in a competition assay. These results demonstrate that LPXTG-containing proteins are involved in cell adhesion and GBS persistence in vivo.
Gram-positive pathogens express specific surface proteins which can mediate interactions with the components of the host extracellular matrix, adherence to, colonization, and invasion of host cells and tissues, and evasion from the host defenses (39). These bacteria have evolved a variety of different anchoring mechanisms to display proteins to the cell surface, one of which, referred to as “sorting,” results in the covalent attachment of the protein to the peptidoglycan (15, 39). Cell wall-anchored surface proteins contain a characteristic carboxy-terminal sorting signal made of a conserved LPXTG motif followed by a hydrophobic domain and a positively charged tail (19). Following secretion, the sorting signal is cleaved between the threonyl and glycyl residues of the LPXTG motif and the carboxyl group of the threonine is linked to the peptidoglycan by an amide linkage (58). The enzyme that catalyses the protease and transpeptidase activities is a membrane-associated protein called sortase (Srt) (15, 39, 42, 43).
Sortases can be divided into four (classes A, B, C, and D) (17) or five (classes A and B, subfamilies 3, 4, and 5) (13) structural classes depending upon the approach utilized. The class A enzymes, the prototype of which is SrtA of Staphylococcus aureus, anchor most of the LPXTG-containing proteins. They contain a hydrophobic NH2-terminal segment functioning both as a signal peptide and a membrane anchor sequence and a carboxylic catalytic signature sequence (TLXTC) containing an essential cysteyl residue (38). SrtA mutants in S. aureus (37), Listeria monocytogenes (5, 22), Streptococcus gordonii (9), Streptococcus mutans (36), Streptococcus pneumoniae (31), Streptococcus pyogenes (3), and Streptococcus suis (41) are unable to anchor surface proteins and have significantly reduced adherence to epithelial cells (5, 22, 31) and virulence in animal models (5, 9, 22, 30). Genes encoding class A sortases are ubiquitous among gram-positive bacteria, whereas those encoding class B or C enzymes are not present in all sequenced genomes (13, 17). SrtC is a narrow-range enzyme required for anchoring few substrates (13, 17). In Streptococcus agalactiae NEM316, the four class C sortases are clustered by pair, with each pair associated with three LPXTG-containing proteins. It is possible that these accessory C sortases, which are not present in all group B streptococcus (GBS) isolates (data not shown), might specifically anchor the flanking LPXTG-containing proteins.
Lancefield's GBS (33), also referred to as Streptococcus agalactiae, is the leading cause of septicemia, meningitis, and pneumonia in neonates. It is also a serious cause of mortality or morbidity in nonpregnant adults, particularly in elderly persons and those with underlying diseases (18, 40, 51). Colonization of the rectum and vagina of pregnant women with GBS, which causes infection of the amniotic cavity, is correlated with GBS sepsis in newborn infants with early-onset disease. In this case, newborns are colonized intrapartum by the aspiration of contaminated amniotic fluid. The lung is a likely portal entry for GBS into the bloodstream since the bacterium can adhere to and invade alveolar epithelial (47) and endothelial cells (24). The adherence of GBS to the mother's (intestinal and vaginal) and infant's (lung) epithelial cells might therefore be essential for virulence.
A genome analysis of S. agalactiae NEM316 revealed the presence of one class A and four class C sortases (17) and 35 surface proteins bearing a cell wall sorting signal motif (26 proteins had an LPXTG motif, 4 had an IPXTG motif, 2 had an LPXTS motif, 2 had an LPXTN motif, and 1 had an FPXTG motif) (25). For this work, the role of the cell wall anchoring of surface proteins in the virulence of S. agalactiae was envisaged at a global level by inactivating the srtA gene coding for the class A sortase SrtA. The SrtA− mutant of S. agalactiae was unable to properly anchor two prototypes of LPXTG-containing proteins, Alp2 and ScpB, to the cell surface, was impaired for binding to fibronectin, and displayed a significant reduction in adherence to epithelial cell lines compared to the isogenic wild-type strain. Most importantly, the SrtA− mutant displayed a 6-log reduction in its capacity to colonize the intestines of gnotobiotic mice in a competition assay, suggesting a key role for LPXTG-containing proteins in bacterial persistence in vivo.
MATERIALS AND METHODS
Bacterial strains, growth, and media.
S. agalactiae NEM316 was responsible for a fatal septicemia and belongs to capsular serotype III (21). The complete genome sequence of this strain has been determined (25). Escherichia coli DH5α (Gibco-BRL) was used for cloning experiments. S. agalactiae was cultured in Todd-Hewitt (TH) broth or agar (Difco Laboratories, Detroit, Mich.), and E. coli was cultured in Trypticase soy medium. RMPI 1640 (MerckEurolab, Fontenay-sous-Bois, France) was also used as a synthetic medium to study the growth of S. agalactiae strains. Unless otherwise specified, antibiotics were used at the following concentrations: for E. coli, ampicillin was used at 100 μg/ml, erythromycin was used at 150 μg/ml, kanamycin was used at 50 μg/ml, and spectinomycin was used at 60 μg/ml; for S. agalactiae, erythromycin was used at 10 μg/ml, kanamycin was used at 1,000 μg/ml, and spectinomycin was used at 250 μg/ml. S. agalactiae liquid cultures were grown in standing filled flasks. All incubations were performed at 37°C.
General DNA techniques.
Genomic streptococcal DNAs were isolated as previously described (46). Standard recombinant DNA techniques were used for nucleic acid preparation and analysis (48). Plasmid DNA preparations were isolated with a Nucleospin plasmid (Macherey Nagel, Düren, Germany). PCRs were carried out with AmpliTaq Gold polymerase as recommended by the manufacturer (Applied Biosystems, Roissy, France). Amplification products were purified on Sephadex S-400 columns (Pharmacia, Uppsala, Sweden) and sequenced with an ABI 310 automated DNA sequencer by use of an ABI PRISM Dye Terminator cycle sequencing kit (Applied Biosystems). Electrocompetent cells of S. agalactiae were prepared as described previously (16).
Construction of bacterial strains.
To construct an S. agalactiae srtA mutant (NEM2135), we inserted the promoterless and terminatorless kanamycin resistance cassette aphA-3 (59) into srtA in the same direction of transcription. This was done by ligating, after digestion with the appropriate enzymes, the following amplicons: O1-O2 (5′ end of srtA), KanK-KanB (aphA-3 gene), and O3-O4 (3′ end of srtA). The corresponding EcoRI-PstI fragment was cloned into the thermosensitive shuttle plasmid pG+host5, and the resulting recombinant vector, pG+host5ΔsrtA, was introduced by electroporation into NEM316. Transformants were selected by growth on erythromycin agar plates at 30°C. Cells in which pG+host5ΔsrtA had integrated into the chromosome were selected by growth of the transformants at 40°C in the presence of erythromycin. Integrant strains were serially passaged for 5 days in liquid medium at 30°C without erythromycin selection to facilitate the excision of the plasmid pG+host5ΔsrtA, leaving either the desired srtA deletion or the srtA wild-type allele in the chromosome. Dilutions of the serially passaged cultures were plated onto agar, and single colonies were tested for erythromycin sensitivity to identify pG+host5ΔsrtA excisants as previously described (6). Chromosomal DNAs of erythromycin-sensitive/kanamycin-resistant S. agalactiae excisants were tested by Southern blotting and PCR analysis to confirm the insertion of the kanamycin cassette into the srtA gene (data not shown).
For complementation analysis, the promoter region of the kanamycin resistance gene aphA-3 (Kan1-Kan2) and the srtA gene (O5-06) were amplified, digested with the appropriate enzymes, and ligated into the integrative vector pAT113/Sp (10) to give pAT113/SpΩPaphA-3-srtA. This vector was conjugatively transferred from the mobilizing strain HB101/pRK24 to S. agalactiae NEM2135, which harbored pTCV-int, a pAMβ1-derived plasmid directing the synthesis of the integrase Int-Tn required for the integration of pAT113/Sp (44). Following the integration of pAT113/Sp, pTCV-int can be readily lost upon subculture at 41°C in the absence of antibiotic selective pressure. The complemented strain NEM2136, which harbored a single copy of pAT113/SpΩsrtA in its chromosome, was chosen for further analysis.
The insertional inactivation of srtA with the kanamycin resistance cassette by a double crossover constituted a genetically irreversible event, as was the insertion of the functional srtA gene into the chromosome of the SrtA mutant. These strategies were chosen for the construction of genetically stable strains in order to avoid the use of antibiotics during long-term animal experiments.
To carry out in vitro and in vivo bacterial competition experiments, we tagged the wild-type strain NEM316 by inserting the integrative vector pAT113/Sp as described above. The selected strain, NEM2093, was resistant to spectinomycin and contained a single copy of pAT113/Sp, whose insertion site, as characterized by inverted PCR (44), was at coordinate 966,480 of the NEM316 chromosome, i.e., between the 3′ ends of the two convergent genes gbs0295 and gbs0296 (http://genolist.pasteur.fr/SagaList/). All relevant characteristics (growth rate, hemolytic activity, adhesion to various epithelial cells, and virulence in neonatal rats) of NEM2093 were found to be indistinguishable from those of NEM316.
Protein purification.
Outer surface proteins of S. agalactiae grown overnight in TH broth at 37°C were prepared essentially as described previously (54). The bacteria in a 200-ml overnight culture were spun down, washed twice with 10 ml Tris-HCl buffer (50 mM, pH 7.3), and resuspended in 1.5 ml of osmotic digestion buffer (20% sucrose-2.5 μM phenylmethylsulfonyl fluoride in 50 mM Tris-HCl, pH 7.3). Mutanolysin (Sigma Chemical Co., St. Louis, Mo.), dissolved to 5,000 U/ml in potassium phosphate buffer (10 mM, pH 6.2), was then added to the bacterial suspension to give a final concentration of 350 U/ml. The digestion reaction was allowed to proceed for 18 h at 37°C with gentle shaking. After the mutanolysin treatment, the reaction mixture was centrifuged twice (15,000 rpm, 4°C, 15 min; Sigma 2K15) to remove cell debris and remaining protoplasts, and the supernatant containing the proteins released from the cell wall was kept frozen at −20°C for subsequent analysis.
The proteins present in the culture medium were prepared as follows. The supernatant of a 200-ml overnight culture was filter sterilized, and the proteins present in the filtrate were precipitated with 2% trichloroacetic acid (TCA) for 2 h at 4°C. Following centrifugation, the pellet was washed twice for 30 min in acetone at −20°C, solubilized in 1 ml of Tris-HCl buffer (10 mM, pH 8.0), and kept frozen at −20°C.
Protein solubility in hot SDS.
Cell wall-anchored proteins are insoluble in hot sodium dodecyl sulfate (SDS) unless the peptidoglycan has first been digested enzymatically with mutanolysin. In contrast, membrane-anchored proteins are generally extractable in hot SDS without any prior treatment. An assay described by Garandeau et al. (22) was used to study the solubility of SpcB and Alp2 in NEM316 and its derivatives. The bacteria in a 2-ml overnight culture were collected by centrifugation (6,000 rpm, 4°C, 10 min). The pellet was washed with phosphate-buffered saline (PBS), centrifuged, and resuspended in 100 μl of 4% SDS-0.5 M Tris-HCl, pH 8. The bacterial suspension was boiled for 10 min and then centrifuged at 10,000 rpm for 5 min. The supernatant fractions were further analyzed by immunoblotting.
Western blot analysis of proteins and immunofluorescence staining.
A 594-bp DNA fragment encoding the NH2 moiety of ScpB (C5a peptidase) was amplified by using the primers O7 and O8 and was cloned into pET28a+ (Novagen, Madison, Wis.) that had been digested with NdeI and BamHI. The corresponding recombinant protein, which was devoid of a signal peptide but contained an NH2-terminal histidyl tag, was expressed in E. coli BL21(DE3) and purified by affinity chromatography on Ni-nitrilotriacetic acid columns according to the manufacturer's recommendations (Novagen). This purified truncated ScpB protein was injected into a rabbit to produce polyclonal anti-ScpB antibodies. A rabbit antiserum raised to the protein R28/Alp2 was kindly provided by G. Lindhal (University of Lund, Lund, Sweden) and was described previously (53). Following electrophoresis under denaturing conditions, the proteins of S. agalactiae were transferred onto a nylon membrane and revealed as described previously (23) by using a rabbit anti-ScpB or anti-R28 antiserum (diluted 1:1,000 in PBS). Immunofluorescence staining of R28/Alp2 was performed as described previously (34), using specific rabbit polyclonal antibodies, and was revealed with anti-immunoglobulin G (anti-IgG) coupled to Alexa 488 (Molecular Probes, Eugene, Oreg.). Images were scanned on a Zeiss LSM 510 microscope.
Cell culture techniques and adherence assays.
The human cell lines A549 (ATCC CCL-185; from an alveolar epithelial carcinoma), HeLa (ATCC CCL-2; from a cervical carcinoma), and Caco-2 (ATCC HTB-37; from a colorectal adenocarcinoma) and the rat epithelial cell line L2 (ATCC CCL-149; from a lung) were cultured in Dulbecco's modified Eagle medium containing Glutamax (Biochrom AG, Berlin, Germany) and supplemented with 10% fetal calf serum (Biochrom AG). Cells were incubated in 10% CO2 at 37°C and were seeded at a density of 0.5 × 105 to 1 × 105 cells per well in 24-well tissue culture plates. Monolayers were used after 24 to 48 h of incubation.
Bacteria were grown to mid-log phase in TH broth to an optical density at 600 nm of 0.4 (approximately 108 CFU/ml), washed once with PBS, and resuspended in Dulbecco's modified Eagle medium. Cells were infected at a multiplicity of infection of 10 bacteria per cell for 2 h at 37°C in 10% CO2. The monolayers were then washed four to five times with PBS, and the cells were disrupted by the addition of 1 ml of sterile deionized ice-cold water and repeated pipetting. Serial dilutions of the lysate were plated onto TH agar for counts of viable bacteria. The percent adherence was calculated as follows: (CFU on plate/CFU in original inoculum) × 100. Assays were performed in duplicate and were repeated at least three times.
Binding of S. agalactiae to human fibronectin.
Bacteria were grown to stationary phase in TH broth (approximately 3 × 108 CFU/ml), washed once with PBS, and resuspended in PBS containing 1% bovine serum albumin (BSA; fraction V; Sigma) and 0.75% Tween 20 (Sigma) to give 0.5 × 108 to 1.0 × 108 CFU/ml. A standard enzyme-linked immunosorbent assay (ELISA) was performed by using microtiter plates (MaxiSorb; Nunc) that had been coated overnight at 4°C with 100 μl of fibronectin (Roche Diagnostics GmbH, Mannheim, Germany) diluted in 0.1 M carbonate buffer, pH 9.6, in the range of 0.5 to 8 μg/ml. The wells were rinsed four times with 0.75% Tween 20 in PBS, blocked with 100 μl of 3% BSA in PBS for 1 h at 37°C, and rinsed once with 0.75% Tween 20 in PBS. One hundred microliters of the bacterial suspension was then added to each well, and the plates were incubated for 2 h at 37°C. After four washes with 100 μl of 0.75% Tween 20 in PBS to remove the unbound bacteria, 100 μl of a rabbit polyclonal anti-GBS antibody in PBS containing 1% BSA and 0.75% Tween 20 was added to each well. The plates were then incubated for 30 min at room temperature and washed four times with 0.75% Tween 20 in PBS, and 100 μl of horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (TEBU, Le Perray-en-Yvelines, France) at a dilution of 1:3,000 was added to each well. After 30 min of incubation at room temperature, the plates were washed four times with 0.75% Tween 20 in PBS and one time with 0.05 M citrate buffer, pH 5.0. One hundred microliters of O-phenylenediamine dihydrochloride (Sigma) (0.4 mg/ml in citrate buffer containing 0.015% H2O2) was added to each well, and after 30 min at room temperature, the yellow color that developed was read as the A450 with an ELISA plate reader (Multiskan RC; ThermoLife Sciences, Cergy-Pontoise, France). Assays were performed in triplicate and were repeated three times.
In vivo virulence studies.
Neonatal Sprague-Dawley rat pups (48 h old) were used for 50% lethal dose (LD50) studies. Randomized groups of five rat pups were inoculated intraperitoneally (i.p.) with serial log dilutions of mid-log-phase bacteria (0.1 ml of each strain in 0.9% NaCl). The LD50 was calculated after 72 h by the probit method, and the LD50 experiment was performed in duplicate.
Groups of 20 rat pups were also inoculated i.p. with 5 × 106 bacteria, and mortality was observed over a 7-day period. Statistical analysis of the mortality data was performed with the Mann-Whitney test as calculated with GraphPad Instat (version 3.0).
To study lung colonization, we delivered 50 μl of mid-log-phase bacterial suspensions (approximately 108 CFU in 0.9% NaCl) intranasally to groups of five neonatal rat pups that had been lightly anesthetized with isoflurane. Bacterial numbers in lung homogenates were determined at various intervals by plating on TH agar plates. Animals were deeply anesthetized with ketamine (10 μg/g) and xylazine (13 μg/g) administered by intramuscular injection or were killed by cervical dislocation in accordance with the policies of the Animal Welfare Committee of the Faculté Necker (Paris). The lung colonization experiment was performed in triplicate.
In vitro and in vivo analysis of bacterial interactions.
Twenty milliliters of TH broth was inoculated with 106 CFU of the tagged wild-type strain NEM2093 (Spr SrtA+) and NEM2135 (Kmr SrtA−) and incubated at 37°C without agitation. The bacterial mixture was subcultivated over a 23-day period by inoculating 20 ml of fresh TH daily with 0.2 ml of the previous night's bacterial growth. The two bacterial populations were enumerated daily on agar plates containing the appropriate antibiotic. This experiment was repeated three times.
Five germfree consanguineous C3H mice, supplied by Unité d’Ecologie et Physiologie du Système Digestif, INRA-CRJ (Jouy-en-Josas, France) and maintained in isolators (J.C.E. Biotechnology, Vichy, France), were fed ad libitum with a commercial diet sterilized by gamma irradiation (4 megarads). The germfree status of the mice was ensured before the beginning of the experiment by testing fecal samples for aerobic and anaerobic growth of bacteria and yeast. Every mouse received intragastrically 108 CFU of each of the two bacterial strains in a volume of 300 μl. Since the number of isolates in mouse feces reflects the number of bacteria in the cecum (20), fecal samples were collected directly from the anuses of mice at various periods to monitor the levels of bacterial populations. At the end of the experiment, the mice were killed, their intestinal tracts were removed from the pylori to the rectum, weighed, and diluted 10-fold in PBS, and serial dilutions of homogenates were plated to enumerate the two bacterial populations. At each time point, the mean counts of both strains were calculated for the five mice.
In these experiments, the competitive indices were calculated by dividing the number of NEM2093 (SrtA+) CFU by the number of NEM2135 (SrtA−) CFU. The in vitro competitive index was the average of values from three experiments, whereas the in vivo competition index was the average of values from five individual mouse experiments.
Oligonucleotides.
The sequences (5′ to 3′) of the relevant oligonucleotides used for this study are listed below. For construction of the srtA mutant, the primers were as follows: O1, AACGAATTCGCAATGCTTTCATAGCTCATC; O2, CCTTCATGGTACCTGCACCATAACTCAACTC; O3, TACGGATCCAGAAGCCACAGAAC; O4, ATTCTGCAGTTCTATCTTGTACGCTTCCA; KanK, GGGGTACCTTTAAATACTGTAG; and KanB, TCTGGATCCTAAAACAATTCATCC. For construction of the complemented strain, the primers were as follows: Kan1, CCGGAATTCCCAGCGAACCATTTGAGG; Kan2, CGGGGTACCGATTTTGAAACCACAAT; O5, CGGGTACCGATAAGTCTAATATAGAGGATAC; and O6, CCCAAGCTTTAATTTCATAATTCTAACTACC. For cloning of the 5′ end of spcB, the primers were as follows: O7, AAATCATATGACAGAAGACACTCCTGC; and O8, GTCCGGATCCAATTTCGACACGCATC. The sequences of the restriction sites added for molecular cloning are shown in bold.
RESULTS
The srtA gene of S. agalactiae NEM316.
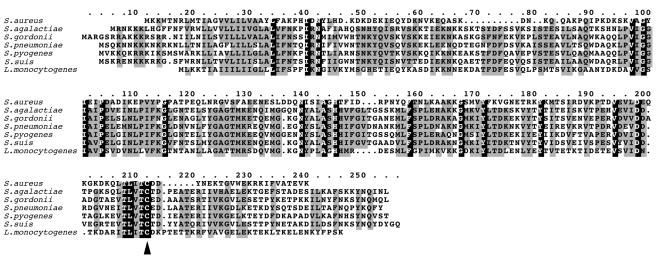
The SrtA protein (GBS 0929) of NEM316 was previously identified by its similarity with other sortase proteins (25). This 248-amino-acid (aa) protein possesses a molecular mass of 27.5 kDa and displays significant sequence identity with the SrtA proteins of S. pyogenes (60%), S. gordonii (54.4%), S. suis (54%), S. pneumoniae (53%), L. monocytogenes (35.5%), and S. aureus (27.3%) (Fig. 1). This protein contains an NH2-terminal hydrophobic signal peptide that could also serve for membrane anchoring (37) and, at the COOH terminus, the critical cysteyl residue within the catalytic TLXTC signature sequence (Fig. 1). As is the case for other streptococcal species (S. gordonii, S. mutans, S. suis, S. pneumoniae, and S. pyogenes) (41), the srtA gene of S. agalactiae NEM316 is located downstream of the housekeeping gene gyrA encoding the DNA gyrase subunit A (http://genolist.pasteur.fr/SagaList/). The ATG translational start site of srtA is preceded by a poorly conserved ribosome binding site (ATAGGaagttATG) which includes the TAG stop codon of gyrA (the RBS sequence and ATG start site are in uppercase letters). This genetic organization may result in translational coupling of both genes to provide coordinated synthesis of the corresponding proteins. A 411-bp long open reading frame, orfX, was located 15 bp downstream from srtA, but the function of the corresponding putative cytoplasmic protein is not known. An ortholog of orfX is present downstream from srtA in S. pyogenes, Streptococcus equi, and S. mutans but not in S. gordonii, Streptococcus mitis, S. pneumoniae, and S. suis (data not shown). A palindromic sequence forming a possible stem-loop transcriptional terminator (ΔG = −16.9 kcal/mol) was detected immediately downstream of orfX (http://genolist.pasteur.fr/SagaList/). It is therefore likely that gyrA, srtA, and orfX are transcribed from the gyrA promoter and that the corresponding transcript ends at this palindrome.
FIG. 1.
Amino acid sequence comparisons of SrtA proteins from various gram-positive bacteria. Identical residues which are present in the seven sequences are marked by white letters on a black background. Conserved amino acid residues which are present in five of the seven sequences are written in black on a gray background. The critical cysteyl residue within the catalytic TLXTC motif is indicated with a black arrow. The single letter code is that recommended by IUPAC/IUB. Dots represent gaps introduced into the sequences to ensure optimal homology.
Inactivation of S. agalactiae srtA.
To characterize the function of SrtA, we constructed the S. agalactiae mutant NEM2135 by replacing the internal fragment of srtA, encoding amino acid residues 121 to 208 of SrtA, with an 841-bp DNA fragment containing the promoterless kanamycin resistance gene aphA-3 (data not shown). In NEM2135 (NEM316ΔsrtA), the aphA-3 cassette is thought to be transcribed from the promoter directing srtA transcription in NEM316. To exclude the possibility that the phenotypes associated with srtA inactivation were due to a polar effect on the expression of the downstream gene orfX, we constructed the complemented strain NEM2136 by reinserting a functional copy of srtA into the chromosome of NEM2135 (see Materials and Methods). A phenotypic comparison (morphology, growth rate, hemolysis, and antibiotic resistance) of NEM316 (wild-type strain), NEM2135 (SrtA−), and NEM2136 (SrtA− SrtA+) cultivated either in TH broth or RPMI 1640 at 37°C did not reveal any significant differences, although we repeatedly observed that the SrtA mutant strain exhibited a 1-h decrease of the lag phase of growth (data not shown).
Role of S. agalactiae SrtA in sorting of cell wall proteins.
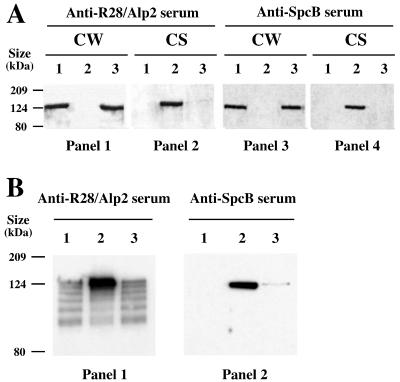
To test if the SrtA mutant was defective in cell wall anchoring of LPXTG-containing proteins, we focused our studies on two unrelated surface proteins that were previously extensively characterized, namely, Alp2 (GBS 0470) and ScpB (GBS1308). Alp2 (1,126 aa) is an α-C-like protein which contains an internal series of tandem repeats and a carboxylic sorting signal including a canonical LPXTG motif (32). This protein, which likely contributes to the genesis of antigenic diversity, is highly related (99.3% identity between the first 400 aa constituting their NH2-terminal extremities) to the R28 protein originally described for S. pyogenes (53). Therefore, antibodies directed against R28 cross-react with Alp2. The cell wall-anchored C5a peptidase ScpB (1,150 aa) cleaves the complement factor C5a, the major neutrophil chemoattractant produced by activation of the complement cascade (12, 55). More recent studies have shown that ScpB is also a fibronectin binding protein (4) which contributes to epithelial cell invasion by GBS, although it is surprisingly not involved in bacterial adhesion (11). ScpB and its homolog ScpA in S. pyogenes both contain a carboxylic sorting signal with an LPXTN motif, and the cell wall anchoring of ScpA was shown to be SrtA dependent (3). The presence of Alp2 and ScpB at the bacterial surface or in the culture supernatant was studied by immunoblotting using specific anti-R28/Alp2 and anti-ScpB polyclonal antibodies. As shown in Fig. 2A (panel 1), a band of approximately 124 kDa corresponding to Alp2 (122 kDa) was detected in the cell wall extracts of the wild-type and complemented strains but not in the cell wall extract of the SrtA− mutant. In contrast, Alp2 was not detected in culture supernatants of the wild-type and complemented strains but was present in a large amount in the culture supernatant of the SrtA− mutant (Fig. 2A, panel 2). Similarly, a band of about 105 kDa corresponding to ScpB was detected in the cell wall extracts from the wild-type and complemented strains but not in the extract from the SrtA− mutant (Fig. 2A, panel 3). We also observed that this protein was only present in the culture supernatant of the mutant strain (Fig. 2A, panel 4) and not in the wild-type and complemented strains. In a SrtA− mutant, the LPXTG-containing proteins conserve their carboxylic extremities containing the hydrophobic segment and might be transiently retained at the bacterial surface through hydrophobic interactions with the membrane. Consistent with this hypothesis, solubilization of Alp2 and ScpB was observed with the SrtA− mutant but not with the wild-type and complemented strains following incubation with hot SDS (Fig. 2B). The multiple band pattern detected with the R28/Alp2 antibodies was likely due to partial protein degradation (Fig. 2B, panel 1). The presence of Alp2 on the surfaces of bacteria incubated with SDS at room temperature was further analyzed by immunofluorescence using specific anti-R28/Alp2 polyclonal antibodies. As shown in Fig. 3, Alp2 was not detected on the surfaces of SrtA− mutant cells, whereas it was clearly detected on the surfaces of cells of the wild-type and complemented strains. Taken together, these results suggest that S. agalactiae SrtA is required for cell wall anchoring of proteins bearing an LPXTG signature sequence.
FIG. 2.
Western blot analysis of GBS proteins with anti-R28/Alp2 and anti-ScpB sera. (A) Proteins attached to the cell wall (CW) and culture supernatant proteins (CS) were purified from the wild-type strain NEM316 (lanes 1), the SrtA− mutant NEM2135 (lanes 2), and the SrtA−/SrtA+ complemented strain NEM2136 (lanes 3). (B) Proteins were extracted by a hot SDS treatment from NEM316 (lanes 1), NEM2135 (lanes 2), and the complemented strain NEM2136 (lanes 3). Note that Alp2 and ScpB were massively released by SDS treatment of the SrtA− mutant. In contrast, only a fraction of Alp2 was solubilized in the wild-type and complemented strains, and only a faint release of ScpB was observed for the complemented strain.
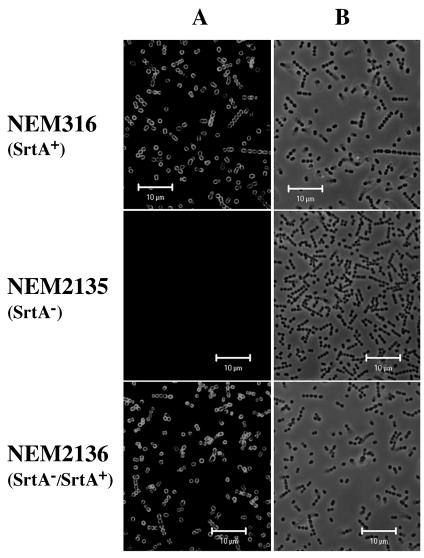
FIG. 3.
Display of the LPXTG-containing protein Alp2 on the cell surfaces of S. agalactiae NEM316 (wild-type strain), NEM2135 (SrtA− mutant), and NEM2136 (SrtA−/SrtA+ complemented strain) cells. Bacteria were grown in TH broth at 37°C to an optical density at 600 nm of 0.5, washed twice with PBS, and incubated for 20 min at room temperature in PBS containing 2% SDS. The cells were analyzed by immunofluorescence with affinity-purified polyclonal anti-R28/Alp2 antibodies and revealed with anti-IgG coupled to Alexa 488 (A), and the same samples were observed by phase-contrast microscopy (B).
SrtA contributes to adherence of S. agalactiae to cultured epithelial cells and to fibronectin.
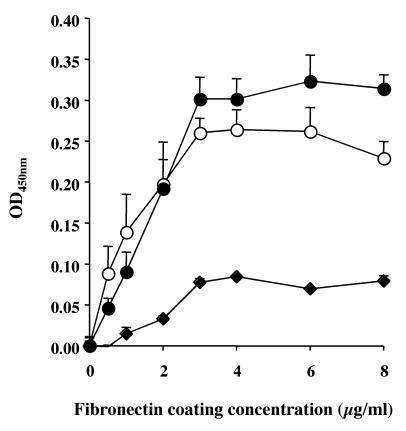
We investigated whether the SrtA− mutant displayed a defect in the ability to adhere to various tissue-cultured epithelial cell lines of human (A549, Caco-2, and HeLa) and murine (L2) origins. As shown in Table 1, the adherence of the SrtA− mutant was reduced approximately 10-fold compared to that of the parental strain in the four cell lines tested. Complementation of the SrtA− mutant fully restored the defect in adherence in human epithelial A549, Caco-2, and HeLa cells but only partially restored the defect in the rat cell line L2 (Table 1). Many bacteria bind to host tissues by adhering to extracellular matrix proteins, and Tamura and Rubens have clearly established that GBS binds to immobilized human fibronectin (56). Up to now, ScpB was the only fibronectin binding protein identified for this bacterial species (4). To determine if fibronectin binding was affected in the SrtA− mutant strain, we used a simple binding assay (ELISA) to compare its binding properties to those of the wild-type and complemented strains. As shown in Fig. 4, the S. agalactiae SrtA-expressing strains (NEM316 and NEM2136) bound in significantly larger numbers to fibronectin than did the isogenic SrtA− mutant strain (NEM2135). In a similar assay, we observed that the SrtA− mutant displayed reduced binding to fibrinogen compared to the wild-type and complemented strains (data not shown). It is thus conceivable that the major GBS fibrinogen-binding protein FbsA (GBS1087) is a SrtA-dependent LPXTG-containing protein (49, 50).
TABLE 1.
Comparison of capacities of S. agalactiae NEM316 (wild-type strain), NEM2135 (SrtA− mutant), and NEM2136 (SrtA−/SrtA+ complemented strain) to adhere to various epithelial cell lines
| Strain | Relative % adherence to cell linea
|
|||
|---|---|---|---|---|
| A549 | HeLa | Caco-2 | L2 | |
| NEM316 | 100 | 100 | 100 | 100 |
| NEM2135 | 13.4 ± 4.2 | 13.7 ± 2.5 | 10.9 ± 4.5 | 13.7 ± 2.4 |
| NEM2136 | 91 ± 10 | 84.4 ± 7.5 | 114.1 ± 19 | 76.3 ± 4.2 |
| Lactococcus lactis MG1363 | 3.1 ± 0.8 | 3 ± 1.2 | NT | NT |
Cells were infected at a multiplicity of infection of 10 bacteria per cell for 2 h at 37°C, and adherence frequencies were calculated from the numbers of bacteria remaining attached to cells after the incubation period with respect to the total number of inoculated bacteria. The level of adherence of the wild-type strain is arbitrarily reported as 100, and the levels of adherence of the derivative strains are relative values. The results are presented as mean values (± SD) from at least three experiments performed in duplicate. For these experiments, L. lactis MG363 was used as a negative control for bacterial adherence. NT, not tested.
FIG. 4.
Adherence of S. agalactiae strains to immobilized fibronectin. Microtiter wells were coated with various concentrations of fibronectin, and 107 CFU of the wild-type strain NEM316 (○), the SrtA− mutant NEM2135 (♦), or the complemented strain NEM2136 (•) was added. The wells were washed and bound bacteria were assayed as described in Materials and Methods. The results are presented as mean values (± standard deviations [SD]) of one experiment performed in triplicate. The curves are representative of three independent experiments.
Taken together, these results indicate that SrtA activity in S. agalactiae is essential for bacterial adherence to eucaryotic cells and for binding to fibronectin and fibrinogen.
Virulence of the S. agalactiae SrtA mutant in neonatal rats.
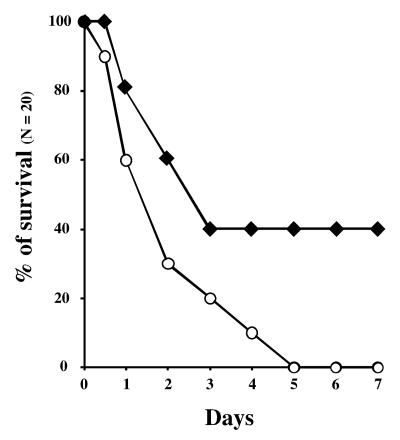
The role of SrtA in the virulence of S. agalactiae was first studied by determining the LD50 of the wild-type strain NEM316 and the SrtA mutant NEM2135 in an intraperitoneal injection model using neonatal rats. In two separate experiments, the LD50 of NEM316 (wild type) (6.8 × 105 and 5.8 × 105 CFU/animal; mean = 6.3 × 105 CFU/animal) was found to be slightly inferior (fourfold increase) to that of NEM2135 (SrtA−) (2.9 × 106 and 2.1 × 106 CFU/animal; mean = 2.5 × 106 CFU/animal). The virulence of these strains was more accurately compared by following, over a period of 7 days, the mortality of rat pups infected i.p. with 5 × 106 bacteria (Fig. 5). On day 3 postinfection, 80% and 60% of the rats infected with NEM316 and NEM2135, respectively, were dead (Fig. 5). No additional death was subsequently recorded for the SrtA− mutant, whereas all pups infected with NEM316 died by day 5. However, this slight difference in virulence was not considered statistically significant by the Mann-Whitney test (P = 0.0849).
FIG. 5.
Mortality curves for rat pups infected with the wild-type strain NEM316 (○) and the SrtA− mutant NEM2135 (♦). Groups of 20 neonatal Sprague-Dawley rat pups (48 h old) were inoculated i.p. with 5 × 106 bacteria, and mortality was observed over a 7-day period. The difference in virulence of the two strains was considered not quite significant, with a two-tailed P value of 0.0849 by the Mann-Whitney test, as calculated with GraphPad Instat (version 3.0).
We hypothesized that SrtA might be required during early stages of infection such as during the colonization of the lung. To test this possibility, we inoculated rat pups intranasally with approximately 108 CFU of NEM316 and NEM2135 and monitored bacterial clearance in the lung over a 72-h period. The initial colonization levels, determined 6 h after inoculation, were identical with both strains and their clearance from the lungs occurred at similar rates (data not shown). We therefore concluded that S. agalactiae SrtA does not play a major role in virulence in neonatal rats.
Role of SrtA in colonization of the mouse gut.
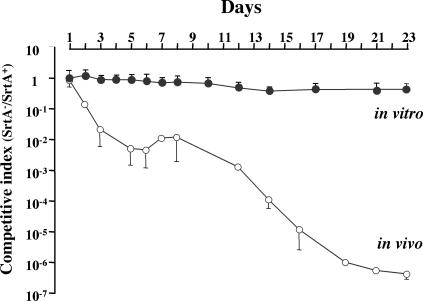
Colonization of the intestine and vagina of the mother by GBS constitutes an essential step for the development of disease in neonates. To address whether SrtA-dependent cell surface adhesins might be required for colonization of the intestines, we monitored the implantation of NEM2093 (a SrtA+ spectinomycin resistance derivative of NEM316) and of the SrtA− kanamycin-resistant mutant NEM2135 in the intestinal tracts of axenic mice. Preliminary experiments revealed that the oral inoculation of 108 CFU of either strain singly enabled their stable establishment at an average level of 9.0 log10 CFU/g of feces throughout a 10-day experiment (data not shown). A competitive colonization assay in which 108 CFU of each strain were simultaneously inoculated into axenic mice was then carried out. On day 1 of the experiment, the SrtA+ and SrtA− strains were established at similar levels, of 9.2 and 9.0 log10 CFU/g of feces, respectively, to give a competitive index (CI) of 0.98 (SrtA− CFU/SrtA+ CFU) (Fig. 6). For 23 days, the SrtA+ population was maintained at the same level, whereas from day 2 of the experiment, the SrtA− population regularly declined to reach 3.1 log10 CFU/g of feces at the end of the experiment to give a CI of 3.9 × 10−7 (Fig. 6). Mice were sacrificed, and their intestines were collected for bacterial counts 3 weeks after inoculation. The mean log counts of NEM2093 (SrtA+) and of NEM2135 (SrtA−) were 9.1 (± 0.2) and 1.9 (± 0.3) log10 CFU/g of intestine, respectively, confirming that the number of bacteria in mouse feces reflects the number of bacteria in the cecum. In contrast, when NEM2093 (SrtA+) and NEM2135 (SrtA−) were serially cocultivated over a 23-day period in TH broth, the CI remained approximately constant throughout the experiment, varying from 0.92 (day 1) to 0.4 (day 23) (Fig. 6). We therefore concluded that in a competitive situation the SrtA− mutant is unable to stably colonize the intestines of mice.
FIG. 6.
Competitive index analysis of mixed cultures and colonizations with the SrtA+ strain NEM2093 (Spr SrtA+) and the SrtA− mutant NEM2135 (Kmr SrtA−). For in vitro experiments (•), 20 ml of TH broth was inoculated with 106 CFU each of NEM2093 and NEM2135, and the bacterial mixture was subcultivated over a 23-day period. The two bacterial populations were enumerated daily on agar plates containing spectinomycin (NEM2093) or kanamycin (NEM2135). For in vivo experiments (○), germfree mice were inoculated on day zero with 108 CFU each of the SrtA+ strain NEM2093 and the SrtA− mutant NEM2135. The bacterial numbers in homogenates were determined at various intervals by plating on TH agar plates containing the appropriate antibiotic. The competitive indices were calculated by dividing the CFU of NEM2093 by the CFU of NEM2135. The in vitro competitive index was the average of values from three independent experiments, whereas the in vivo competitive index was the average of values from five individual mouse experiments. The vertical bars represent one SD.
DISCUSSION
The availability of the whole genome sequence of the human pathogen S. agalactiae has provided insights into the repertoire of the cell surface proteins present in this bacterial species (25, 57). Thirty-five cell wall-associated proteins that may play a role in adhesion, invasion, the inhibition of phagocytosis, and the evasion of the host immune defense mechanisms have been annotated. Since most of these proteins are thought to be substrates of the universal sortase SrtA, evaluations of the role of their cell wall anchoring in virulence can be tested globally by inactivating the srtA gene. Previous studies have shown that SrtA is required for the virulence of the intracellular pathogen L. monocytogenes (5, 22) and the extracellular pathogen S. aureus (30). Among the streptococci, a SrtA-deficient mutant of S. gordonii was shown to exhibit reduced adhesive properties in vitro and a decreased ability to colonize the oral mucosa of mice (9). Similarly, a SrtA− mutant of S. mutans was also found to display a decrease in colonization of the oral mucosae and teeth of rats (36). The inactivation of srtA in S. pneumoniae decreased its adherence to human pharyngeal cells but had no effect on the virulence of a capsular type III strain in an intraperitoneally inoculated mouse model (31).
In this study, we showed that an SrtA− mutant of S. agalactiae is unable to anchor two unrelated LPXTG-containing proteins, Alp2 and ScpB, possessing different sorting signals (LPXTG and LPXTN, respectively). We also showed that this mutant displays a defect in adherence to human fibronectin, fibrinogen, and various epithelial cells. These observations are consistent with the finding that the SrtA-dependent LPXTG-containing protein ScpB mediates fibronectin binding (4). Our results also suggest that the major GBS fibrinogen binding protein, FbsA, which promotes adherence to human epithelial cells (27, 49), is also anchored to the cell wall by SrtA. Restoration of the wild-type phenotype (cell wall anchoring of Alp2 and ScpB and adherence) was observed upon complementation of the mutant strain, excluding the possibility that the mutant phenotype was due to a polar effect on the downstream gene orfX. However, the inactivation of srtA did not dramatically alter the virulence of NEM316 in a neonatal rat sepsis model, as we only observed a fourfold increase in the LD50 of the mutant strain. Until now, only two LPXTG-containing proteins, ScpB and CspA, have been associated with GBS virulence by promoting resistance to phagocytosis (8, 29). These two enzymes, which are likely substrates for SrtA, are members of the cell envelope-associated proteases and belong to the subtilisin-like serine protease subfamily (29). The role of ScpB in virulence has been demonstrated in a model of reconstitution of C5-deficient mice with human C5a (8) since this enzyme does not cleave murine C5a (7). A recent report indicated that a CspA− mutant of the GBS strain COH1 displayed a 10-fold increase in its LD50 in a neonatal rat model (29). However, it is not known if these enzymes need to be anchored to the bacterial cell wall to fulfill their biological roles during the infectious process. We hypothesize that these enzymes remain enzymatically active and are either retained in the membrane of the SrtA− mutant or released into the culture medium.
We recently reported that the virulence of a d-alanyl-lipoteichoic acid mutant of NEM316 was severely impaired in the same animal model (with a 2-log increase in the LD50) (45). The fact that the virulence of the SrtA− mutant was not so dramatically reduced suggests that the anchoring of SrtA-dependent LPXTG-containing proteins does not constitute a key event for GBS virulence, at least in the neonatal rat intraperitoneal model.
The adherence of GBS to epithelial cells is required for colonization of the rectal and vaginal tracts of the mother and for persistence in the lungs of the neonate. S. agalactiae is part of the normal digestive flora of humans and animals, and both SrtA+ and SrtA− GBS strains can singly colonize the intestines of axenic mice at similarly high levels. However, in a competitive assay with equal numbers of both strains, the SrtA-expressing strain was stably established at a high level throughout a 23-day experiment, whereas the srtA-deficient strain was cleared rapidly and was almost entirely eliminated by the end of the experiment. These results strongly suggest that the cell wall anchoring of SrtA-dependent LPXTG-containing proteins is required for colonization and persistence in the digestive tracts of mice. These results correlate with our in vitro data showing that the SrtA− mutant was about 10-fold less adherent to the human epithelial cell lines Caco-2, from a colorectal adenocarcinoma, and HeLa, from a cervical carcinoma. It is therefore tempting to speculate that SrtA is required for rectal and vaginal GBS carriage in humans.
Surprisingly, after intranasal inoculation the wild-type and SrtA− GBS strains were similarly cleared from the lungs of neonatal rat pups, although in vitro the mutant strain was significantly less adherent to rat and human pulmonary epithelial cell lines. Lung homeostasis is achieved via the phagocytosis of inhaled foreign material by alveolar macrophages, and neonates manifest an increased susceptibility to lung infections which may possibly be due to a deficiency in the function of alveolar macrophages (35). Several studies have demonstrated a deficient phagocytic capacity of alveolar macrophages in neonates compared to that in adults (2, 28), including humans (26), but these results are controversial (14, 35, 52). Our results indicate that the phagocytic capacity of alveolar macrophages from newborn rats enables the rapid clearance of a massive lung infection with GBS. This finding is consistent with the observation that the CR3 receptor, which is known to mediate opsonin-independent GBS phagocytosis (1), is highly expressed by neonatal alveolar macrophages (35). Thus, it is likely that in our model of lung infection the phagocytic killing of bacteria masked the SrtA-dependent colonization of the pulmonary epithelial surfaces by GBS. Whether these results can be extrapolated to human pathogenesis remains to be demonstrated.
Acknowledgments
We thank G. Lindahl for the kind gift of anti-R28/Alp2 antibodies.
This work was supported by the Institut National de la Santé et de la Recherche Médicale, the Pasteur Institute (PTR no. 17 and GPH no. 09), and the University of Paris V.
Editor: V. J. DiRita
REFERENCES
- 1.Antal, J. M., J. V. Cunningham, and K. J. Goodrum. 1992. Opsonin-independent phagocytosis of group B streptococcus: role of complement receptor type three. Infect. Immun. 60:1114-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakker, J. M., E. Broug-Holub, H. Kroes, E. P. van Rees, G. Kraal, and J. F. van Iwaarden. 1998. Functional immaturity of rat alveolar macrophages during postnatal development. Immunology 94:304-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett, T. C., and J. R. Scott. 2002. Differential recognition of surface proteins in Streptococcus pyogenes by two sortase gene homologs. J. Bacteriol. 184:2181-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckmann, C., J. D. Waggoner, T. O. Harris, G. S. Tamura, and C. E. Rubens. 2002. Identification of novel adhesins from group B streptococci by use of phage display reveals that C5a peptidase mediates fibronectin binding. Infect. Immun. 70:2869-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bierne, H., S. K. Mazmanian, M. Trost, M. G. Pucciarelli, G. Liu, P. Dehoux, L. Jansch, F. Garcia-del Portillo, O. Schneewind, and P. Cossart. 2002. Inactivation of the srtA gene in Listeria monocytogenes inhibits anchoring of surface proteins and affects virulence. Mol. Microbiol. 43:869-881. [DOI] [PubMed] [Google Scholar]
- 6.Biswas, I., A. Gruss, S. D. Ehrlich, and E. Maguin. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J. Bacteriol. 175:3628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohnsack, J. F., J. K. Chang, and H. R. Hill. 1993. Restricted ability of group B streptococcal C5a-ase to inactivate C5a prepared from different animal species. Infect. Immun. 61:1421-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bohnsack, J. F., K. Widjaja, S. Ghazizadeh, C. E. Rubens, D. R. Hillyard, C. J. Parker, K. H. Albertine, and H. R. Hill. 1997. A role for C5 and C5a-ase in the acute neutrophil response to group B streptococcal infections. J. Infect. Dis. 175:847-855. [DOI] [PubMed] [Google Scholar]
- 9.Bolken, T. C., C. A. Franke, K. F. Jones, G. O. Zeller, C. H. Jones, E. K. Dutton, and D. E. Hruby. 2001. Inactivation of the srtA gene in Streptococcus gordonii inhibits cell wall anchoring of surface proteins and decreases in vitro and in vivo adhesion. Infect. Immun. 69:75-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celli, J., and P. Trieu-Cuot. 1998. Circularisation of Tn916 is required for expression of the transposon-encoded transfer functions: characterisation of long tetracycline-inducible transcripts reading through the attachment site. Mol. Microbiol. 28:103-118. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, Q., D. Stafslien, S. S. Purushothaman, and P. Cleary. 2002. The group B streptococcal C5a peptidase is both a specific protease and an invasin. Infect. Immun. 70:2408-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chmouryguina, I., A. Suvorov, P. Ferrieri, and P. P. Cleary. 1996. Conservation of the C5a peptidase genes in group A and B streptococci. Infect. Immun. 64:2387-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comfort, D., and R. T. Clubb. 2004. A comparative genome analysis identifies distinct sorting pathways in gram-positive bacteria. Infect. Immun. 72:2710-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conly, M. E., and D. P. Speert. 1991. Human neonatal monocyte-derived macrophages and neutrophils exhibit normal nonopsonic and opsonic receptor-mediated phagocytosis and superoxide anion production. Biol. Neonate 60:361-366. [DOI] [PubMed] [Google Scholar]
- 15.Cossart, P., and R. Jonquieres. 2000. Sortase, a universal target for therapeutic agents against gram-positive bacteria? Proc. Natl. Acad. Sci. USA 97:5013-5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz-Rodz, A. L., and M. S. Gilmore. 1990. High efficiency introduction of plasmid DNA into glycine treated Enterococcus faecalis by electroporation. Mol. Gen. Genet. 224:152-154. [DOI] [PubMed] [Google Scholar]
- 17.Dramsi, S., P. Trieu-Cuot, and H. Bierne. 2005. “Sorting sortases”: a nomenclature proposal for the various sortases of gram-positive bacteria. Res. Microbiol. 156:289-297. [DOI] [PubMed]
- 18.Farley, M. M. 2001. Group B streptococcal disease in nonpregnant adults. Clin. Infect. Dis. 33:556-561. [DOI] [PubMed] [Google Scholar]
- 19.Fischetti, V. A., V. Pancholi, and O. Schneewind. 1990. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol. Microbiol. 4:1603-1605. [DOI] [PubMed] [Google Scholar]
- 20.Freter, R., H. Brickner, J. Fekete, M. M. Vickerman, and K. E. Carey. 1983. Survival and implantation of Escherichia coli in the intestinal tract. Infect. Immun. 39:686-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaillot, O., C. Poyart, P. Berche, and P. Trieu-Cuot. 1997. Molecular characterization and expression analysis of the superoxide dismutase gene from Streptococcus agalactiae. Gene 204:213-218. [DOI] [PubMed] [Google Scholar]
- 22.Garandeau, C., H. Reglier-Poupet, I. Dubail, J. L. Beretti, P. Berche, and A. Charbit. 2002. The sortase SrtA of Listeria monocytogenes is involved in processing of internalin and in virulence. Infect. Immun. 70:1382-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geoffroy, C., J. L. Gaillard, J. E. Alouf, and P. Berche. 1987. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect. Immun. 55:1641-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson, R. L., M. K. Lee, C. Soderland, E. Y. Chi, and C. E. Rubens. 1993. Group B streptococci invade endothelial cells: type III capsular polysaccharide attenuates invasion. Infect. Immun. 61:478-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaser, P., C. Rusniok, F. Chevalier, C. Buchrieser, L. Frangeul, M. Msadek, M. Zouine, E. Couvé, L. Lalioui, C. Poyart, P. Trieu-Cuot, and F. Kunst. 2002. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol. Microbiol. 45:1499-1514. [DOI] [PubMed] [Google Scholar]
- 26.Grigg, J., J. Riedler, C. F. Robertson, W. Boyle, and S. Uren. 1999. Alveolar macrophage immaturity in infants and young children. Eur. Respir. J. 14:1198-1205. [DOI] [PubMed] [Google Scholar]
- 27.Gutekunst, H., B. J. Eikmanns, and D. J. Reinscheid. 2004. The novel fibrinogen-binding protein FbsB promotes Streptococcus agalactiae invasion into epithelial cells. Infect. Immun. 72:3495-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall, S. L., and M. P. Sherman. 1992. Intrapulmonary bacterial clearance of type III group B streptococcus is reduced in preterm compared with term rabbits and occurs independent of antibody. Am. Rev. Respir. Dis. 145:1172-1177. [DOI] [PubMed] [Google Scholar]
- 29.Harris, T. O., D. W. Shelver, J. F. Bohnsack, and C. E. Rubens. 2003. A novel streptococcal surface protease promotes virulence, resistance to opsonophagocytosis, and cleavage of human fibrinogen. J. Clin. Investig. 111:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonsson, I. M., S. K. Mazmanian, O. Schneewind, M. Verdrengh, T. Bremell, and A. Tarkowski. 2002. On the role of Staphylococcus aureus sortase and sortase-catalyzed surface protein anchoring in murine septic arthritis. J. Infect. Dis. 185:1417-1424. [DOI] [PubMed] [Google Scholar]
- 31.Kharat, A., and A. Tomasz. 2003. Inactivation of the srtA gene affects localization of surface proteins and decreases adhesion of Streptococcus pneumoniae to human pharyngeal cells in vitro. Infect. Immun. 71:2758-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lachenauer, C. S., R. Creti, J. L. Michel, and L. C. Madoff. 2000. Mosaicism in the alpha-like protein genes of group B streptococci. Proc. Natl. Acad. Sci. USA 97:9630-9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lancefield, R. C., and R. Hare. 1935. The serological differentiation of pathogenic and non-pathogenic strains of hemolytic streptococci from parturient women. J. Exp. Med. 61:335-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebrun, M., J. Mengaud, H. Ohayon, F. Nato, and P. Cossart. 1996. Internalin must be on the bacterial surface to mediate entry of Listeria monocytogenes into epithelial cells. Mol. Microbiol. 21:579-592. [DOI] [PubMed] [Google Scholar]
- 35.Lee, P. T., P. G. Holt, and A. S. McWilliam. 2000. Role of alveolar macrophages in innate immunity in neonates: evidence for selective lipopolysaccharide binding protein production by rat neonatal alveolar macrophages. Am. J. Respir. Cell. Mol. Biol. 23:652-661. [DOI] [PubMed] [Google Scholar]
- 36.Lee, S. F., and T. L. Boran. 2003. Roles of sortase in surface expression of the major protein adhesin P1, saliva-induced aggregation and adherence, and cariogenicity of Streptococcus mutans. Infect. Immun. 71:676-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mazmanian, S. K., G. Liu, E. R. Jensen, E. Lenoy, and O. Schneewind. 2000. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl. Acad. Sci. USA 97:5510-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazmanian, S. K., H. Ton-That, and O. Schneewind. 2001. Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol. Microbiol. 40:1049-1057. [DOI] [PubMed] [Google Scholar]
- 39.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nizet, V., and C. E. Rubens. 2000. Pathogenic mechanisms and virulence factors of group B streptococci. ASM Press, Washington, D.C.
- 41.Osaki, M., D. Takamatsu, Y. Shimoji, and T. Sekizaki. 2002. Characterization of Streptococcus suis genes encoding proteins homologous to sortase of gram-positive bacteria. J. Bacteriol. 184:971-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pallen, M. J., A. C. Lam, M. Antonio, and K. Dunbar. 2001. An embarrassment of sortases—a richness of substrates? Trends Microbiol. 9:97-102. [DOI] [PubMed] [Google Scholar]
- 43.Paterson, G. K., and T. J. Mitchell. 2004. The biology of gram-positive sortase enzymes. Trends Microbiol. 12:89-95. [DOI] [PubMed] [Google Scholar]
- 44.Poyart, C., E. Pellegrini, O. Gaillot, C. Boumaila, M. Baptista, and P. Trieu-Cuot. 2001. Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae. Infect. Immun. 69:5098-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poyart, C., E. Pellegrini, M. Marceau, M. Baptista, F. Jaubert, M. C. Lamy, and P. Trieu-Cuot. 2003. Attenuated virulence of Streptococcus agalactiae deficient in d-alanyl-lipoteichoic acid is due to an increased susceptibility to defensins and phagocytic cells. Mol. Microbiol. 49:1615-1625. [DOI] [PubMed] [Google Scholar]
- 46.Poyart-Salmeron, C., C. Carlier, P. Trieu-Cuot, A. Courtieu, and P. Courvalin. 1990. Transferable plasmid-mediated antibiotic resistance in Listeria monocytogenes. Lancet 335:1422-1426. [DOI] [PubMed] [Google Scholar]
- 47.Rubens, C. E., S. Smith, M. Hulse, E. Y. Chi, and G. van Belle. 1992. Respiratory epithelial cell invasion by group B streptococci. Infect. Immun. 60:5157-5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 49.Schubert, A., K. Zakikhany, G. Pietrocola, A. Meinke, P. Speziale, B. J. Eikmanns, and D. J. Reinscheid. 2004. The fibrinogen receptor FbsA promotes adherence of Streptococcus agalactiae to human epithelial cells. Infect. Immun. 72:6197-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schubert, A., K. Zakikhany, M. Schreiner, R. Frank, B. Spellerberg, B. J. Eikmanns, and D. J. Reinscheid. 2002. A fibrinogen receptor from group B Streptococcus interacts with fibrinogen by repetitive units with novel ligand binding sites. Mol. Microbiol. 46:557-569. [DOI] [PubMed] [Google Scholar]
- 51.Schuchat, A. 1998. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin. Microbiol. Rev. 11:497-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Speer, C. P., M. Wieland, R. Ulbrich, and M. Gahr. 1986. Phagocytic activities in neonatal monocytes. Eur. J. Pediatr. 145:418-421. [DOI] [PubMed] [Google Scholar]
- 53.Stalhammar-Carlemalm, M., T. Areschoug, C. Larsson, and G. Lindahl. 1999. The R28 protein of Streptococcus pyogenes is related to several group B streptococcal surface proteins, confers protective immunity and promotes binding to human epithelial cells. Mol. Microbiol. 33:208-219. [DOI] [PubMed] [Google Scholar]
- 54.Stalhammar-Carlemalm, M., L. Stenberg, and G. Lindahl. 1993. Protein Rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J. Exp. Med. 177:1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi, S., Y. Aoyagi, E. E. Adderson, Y. Okuwaki, and J. F. Bohnsack. 1999. Capsular sialic acid limits C5a production on type III group B streptococci. Infect. Immun. 67:1866-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamura, G. S., and C. E. Rubens. 1995. Group B streptococci adhere to a variant of fibronectin attached to a solid phase. Mol. Microbiol. 15:581-589. [DOI] [PubMed] [Google Scholar]
- 57.Tettelin, H., V. Masignani, M. J. Cieslewicz, J. A. Eisen, S. Peterson, M. R. Wessels, I. T. Paulsen, K. E. Nelson, I. Margarit, T. D. Read, L. C. Madoff, A. M. Wolf, M. J. Beanan, L. M. Brinkac, S. C. Daugherty, R. T. DeBoy, A. S. Durkin, J. F. Kolonay, R. Madupu, M. R. Lewis, D. Radune, N. B. Fedorova, D. Scanlan, H. Khouri, S. Mulligan, H. A. Carty, R. T. Cline, S. E. Van Aken, J. Gill, M. Scarselli, M. Mora, E. T. Iacobini, C. Brettoni, G. Galli, M. Mariani, F. Vegni, D. Maione, D. Rinaudo, R. Rappuoli, J. L. Telford, D. L. Kasper, G. Grandi, and C. M. Fraser. 2002. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. Proc. Natl. Acad. Sci. USA 99:12391-12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ton-That, H., S. K. Mazmanian, K. F. Faull, and O. Schneewind. 2000. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. Sortase catalyzed in vitro transpeptidation reaction using LPXTG peptide and NH(2)-Gly(3) substrates. J. Biol. Chem. 275:9876-9881. [DOI] [PubMed] [Google Scholar]
- 59.Trieu-Cuot, P., and P. Courvalin. 1983. Nucleotide sequence of the Streptococcus faecalis plasmid gene encoding the 3′5"-aminoglycoside phosphotransferase type III. Gene 23:331-341. [DOI] [PubMed] [Google Scholar]