Abstract
Immunostimulatory CpG oligodeoxynucleotides (ODN) improve host resistance to listeriae. CpG ODN trigger immune cells to produce gamma interferon and “prime” host cells to secrete nitric oxide in response to bacterial exposure. CpG treatment does not protect inducible nitric oxide synthase 2 knockout mice, indicating that NO is critical to CpG-mediated protection against listeriae.
CpG motifs present in bacterial DNA trigger lymphocytes, dendritic cells, and macrophages to produce a variety of immunoprotective cytokines, chemokines, and antibodies (Abs) (1, 9, 14, 16, 21). Synthetic oligodeoxynucleotides (ODN) containing CpG motifs mimic the ability of bacterial DNA to protect the host from infection (5, 8, 11, 12, 15, 18, 23). For example, CpG ODN significantly reduce host susceptibility to Listeria monocytogenes. This immunoprotective effect peaks several days after CpG ODN administration and persists for several weeks (5, 10, 12, 15).
CpG-activated macrophages produce various immunoprotective factors, including large amounts of nitric oxide (NO) (6, 7). Macrophage-derived NO contributes to the clearance of listeria infection (2, 3). Mice lacking inducible nitric oxide synthase 2 (NOS2) are highly susceptible to listeriae (17), consistent with NO playing an important role in host control of this pathogen.
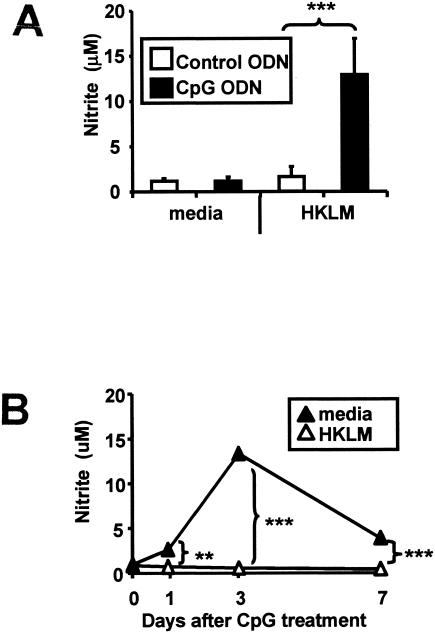
To examine whether NO contributes to CpG-mediated protection against listeriae, 6- to 8-week-old C57BL/6 mice (NCI, Frederick, MD) were treated intraperitoneally (i.p.) with 50 μg of CpG (GCTAGACGTTAGCGT) or control (GCTAGAGCTTAGGCT) ODN. Peritoneal cells were isolated 3 days later by lavage and cultured in vitro with 2 × 105 CFU/ml of heat-killed listeriae (HKL). Nitrite levels in culture supernatants were quantified using the Griess reagent (Sigma, St. Louis, MO) and comparison to a standard curve generated using NaNO2.
As seen in Fig. 1A, CpG ODN had no effect on basal levels of NO production. However, cells from CpG-treated mice produced significantly more NO when cultured with HKL than cells from mice treated with control ODN (P < 0.001). This increased response developed within 1 day of ODN administration, peaked on day 3, and persisted through day 7 (Fig. 1B) (P < 0.01), mirroring the kinetics of CpG-induced protection against listeriae.
FIG. 1.
CpG ODN “prime” host cells to produce NO when exposed to heat-killed L. monocytogenes (HKLM). C57BL/6 mice were injected i.p. with 50 μg of control or CpG ODN. Peritoneal cells isolated 3 (A) or 0 to 7 (B) days later were cultured with 2 × 105 CFU/ml of HKL for 48 h. NO production was measured using the Griess reagent. Data represent the means ± standard deviations (SD) of values for more than seven independently studied mice in two independent experiments. **, P < 0.01; ***, P < 0.001 (two-tailed Student's t test).
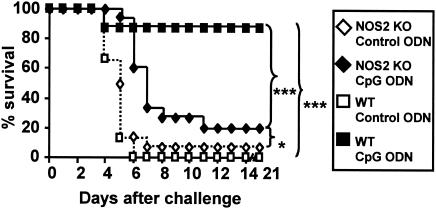
Mice treated with CpG (but not control) ODN survived challenged with 10 50% lethal doses (LD50s) of listeriae (Fig. 2) (P < 0.001) (5, 12, 15). The role of NO was further defined by comparing the rates of survival of NOS2 knockout (KO) and wild-type (WT) mice (Jackson Laboratory, Bar Harbor, ME). NOS2 KO mice were more susceptible to listeria infection than WT mice (1 LD50 = 103 CFU in NOS2 mice but 104 CFU in WT mice). Moreover, CpG ODN treatment failed to protect NOS2 KO mice from listeria challenge (Fig. 2) (P < 0.001).
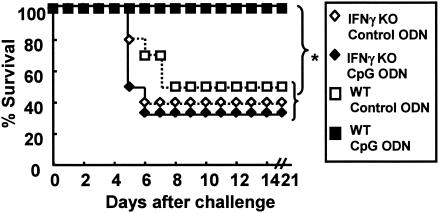
FIG. 2.
CpG ODN promote survival following listeria challenge. C57BL/6 or NOS2 KO mice were injected i.p. with 50 μg of control or CpG ODN (n = 10 to 15/group). Three days later, mice were challenged with 10 LD50 of L. monocytogenes. *, P < 0.05; ***, P < 0.001 (Wilcoxon rank sum test).
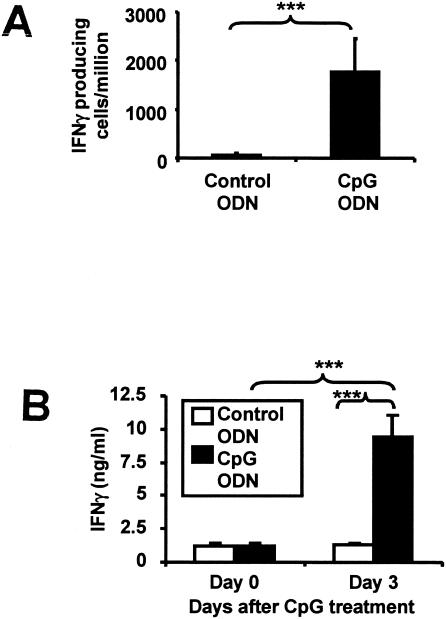
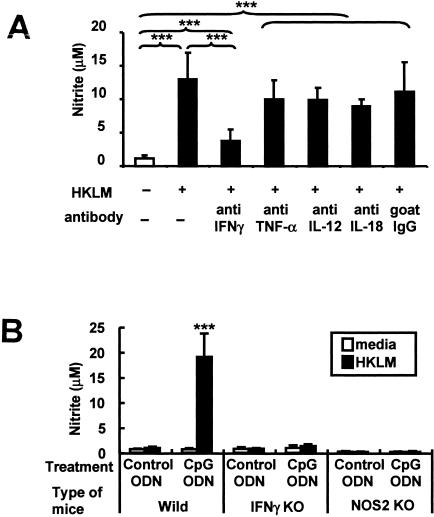
CpG treatment also activates host cells to secrete gamma interferon (IFN-γ) and increases serum IFN-γ levels, as determined by enzyme-linked immunosorbent assay and enzyme-linked immunospot assay (P < 0.001) (Fig. 3; methods used to obtain the results in this figure are described in reference 13). The importance of IFN-γ to NO production was examined by culturing peritoneal cells from CpG-treated mice with HKL plus 0.5 ng/ml of neutralizing Abs against IFN-γ, tumor necrosis factor alpha, interleukin-12, or interleukin-18 (R & D Systems, Minneapolis, MN). As seen in Fig. 4A, only anti-IFN-γ Abs significantly reduced NO production (P < 0.001).
FIG. 3.
CpG ODN treatment induces IFN-γ production. C57BL/6 mice were injected i.p. with 50 μg of CpG ODN. (A) IFN-γ-secreting peritoneal cells were quantified on day 3 by enzyme-linked immunospot assay. (B) Serum IFN-γ levels were determined on day 0 and day 3 by enzyme-linked immunosorbent assay. Data represent the means ± SD of values for five to seven independently studied mice in three independent experiments. ***, P < 0.001 (two-tailed Student's t test).
FIG. 4.
IFN-γ-dependent NO production following CpG treatment. C57BL/6 and IFN-γ KO mice were treated with 50 μg of CpG ODN. Peritoneal cells were isolated 3 days later and stimulated with HKL. (A) Effect of cytokine-specific neutralizing Abs on NO production. (B) Comparison of cells from WT, IFN-γ KO, and NOS2 KO mice. Data represent the means ± SD of values for six to nine independently studied mice in more than two independent experiments. ***, P < 0.001 versus values for all other groups (Student's t test). TNF-α, tumor necrosis factor alpha; IL-12, interleukin-12; IL-18, interleukin-18.
To confirm the importance of IFN-γ to NO production, CpG ODN were administered to IFN-γ and NOS2 KO mice (Jackson Laboratory). Whereas CpG treatment “primed” immune cells from normal mice to produce NO when exposed to HKL, no such effect was observed on cells from either KO strain (Fig. 4B) (P < 0.001). Moreover, CpG ODN treatment failed to protect IFN-γ KO mice from listeria infection (Fig. 5).
FIG. 5.
CpG ODN fail to protect IFN-γ KO mice from listeria infection. WT and IFN-γ KO mice were injected i.p. with 50 μg of control or CpG ODN (n = 5 to 6/group). Three days later, mice were challenged with 1 LD50 of L. monocytogenes (WT, 104 CFU; IFN-γ KO, 0.5 × 103 CFU). *, P < 0.05 (Wilcoxon rank sum test).
CpG ODN stimulate a complex immunomodulatory cascade involving multiple cell types, cytokines, and chemokines (1, 9, 14, 16, 21). This complexity impeded efforts to identify the contribution of specific factors to CpG-mediated host protection. Current findings establish that IFN-γ-dependent NO production is a critical component of host resistance to listeriae elicited by CpG ODN.
Intracellular bacteria take up residence in host macrophages to avoid immune surveillance/destruction (19, 20, 22). These pathogens are cleared when the macrophages are activated to produce bactericidal factors, such as NO. Evidence that IFN-γ-dependent NO contributes to protection against listeriae is provided by Fig. 2, showing that CpG treatment protects WT but not NOS2 KO mice from infection (Fig. 2) (P < 0.001). Similarly, Datta et al. recently showed that NO contributes to CpG-mediated resistance against leishmania (4). The possibility of a role for IFN-γ in the regulation of NO production is supported by the finding that anti-IFN-γ Abs selectively blocked NO secretion by CpG-stimulated cells (Fig. 4A). In addition, cells from CpG-treated IFN-γ KO mice failed to produce NO in response to HKL (Fig. 4B). These findings confirm and extend previous results indicating that CpG ODN up-regulate the production of IFN-γ, which in turn modulates the host's ability to produce NO in response to infection (4).
Editor: J. B. Bliska
REFERENCES
- 1.Ballas, Z. K., W. L. Rasmussen, and A. M. Krieg. 1996. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 157:1840-1845. [PubMed] [Google Scholar]
- 2.Beckerman, K. P., H. W. Rogers, J. A. Corbett, R. D. Schreiber, M. L. McDaniel, and E. R. Unanue. 1993. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J. Immunol. 150:888-895. [PubMed] [Google Scholar]
- 3.Boockvar, K. S., D. L. Granger, R. M. Poston, M. Maybodi, M. K. Washington, J. B. Hibbs, Jr., and R. L. Kurlander. 1994. Nitric oxide produced during murine listeriosis is protective. Infect. Immun. 62:1089-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Datta, N., S. Mukherjee, L. Das, and P. K. Das. 2003. Targeting of immunostimulatory DNA cures experimental visceral leishmaniasis through nitric oxide up-regulation and T cell activation. Eur. J. Immunol. 33:1508-1518. [DOI] [PubMed] [Google Scholar]
- 5.Elkins, K. L., T. R. Rhinehart-Jones, S. Stibitz, J. S. Conover, and D. M. Klinman. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291-2298. [PubMed] [Google Scholar]
- 6.Gao, J. J., E. G. Zuvanich, Q. Xue, D. L. Horn, R. Silverstein, and D. C. Morrison. 1999. Cutting edge: bacterial DNA and LPS act in synergy in inducing nitric oxide production in RAW 264.7 macrophages. J. Immunol. 163:4095-4099. [PubMed] [Google Scholar]
- 7.Ghosh, D. K., M. A. Misukonis, C. Reich, D. S. Pisetsky, and J. B. Weinberg. 2001. Host response to infection: the role of CpG DNA in induction of cyclooxygenase 2 and nitric oxide synthase 2 in murine macrophages. Infect. Immun. 69:7703-7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gramzinski, R. A., D. L. Doolan, M. Sedegah, H. L. Davis, A. M. Krieg, and S. L. Hoffman. 2001. Interleukin-12- and gamma interferon-dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect. Immun. 69:1643-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 10.Ito, S., K. J. Ishii, H. Shirot, and D. M. Klinman. 2004. CpG oligodeoxynucleotides improve the survival of pregnant and fetal mice following Listeria monocytogenes infection. Infect. Immun. 72:3543-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juffermans, N. P., J. C. Leemans, S. Florquin, A. Verbon, A. H. Kolk, P. Speelman, S. J. H. van Deventer, and T. van der Poll. 2002. CpG oligodeoxynucleotides enhance host defense during murine tuberculosis. Infect. Immun. 70:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klinman, D. M., J. Conover, and C. Coban. 1999. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect. Immun. 67:5658-5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klinman, D. M., and T. B. Nutman. 1994. ELIspot assay to detect cytokine-secreting murine and human cells sect. 6.19, p. 1-8. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology, 7th ed. Greene Publishing Associates, Brooklyn, N.Y.
- 14.Klinman, D. M., A.-K. Yi, S. L. Beaucage, J. Conover, and A. M. Krieg. 1996. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12 and interferon γ. Proc. Natl. Acad. Sci. USA 93:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krieg, A. M., L. Love-Homan, A.-K. Yi, and J. T. Harty. 1998. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161:2428-2434. [PubMed] [Google Scholar]
- 16.Krieg, A. M., A.-K. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-549. [DOI] [PubMed] [Google Scholar]
- 17.MacMicking, J. D., C. Nathan, G. Hom, N. Chartrain, D. S. Fletcher, M. Trumbauer, K. Stevens, Q. W. Xie, K. Sokol, N. Hutchinson, et al. 1995. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81:641-650. [DOI] [PubMed] [Google Scholar]
- 18.Pyles, R. B., D. Higgins, C. Chalk, A. Zalar, J. Eiden, C. Brown, G. Van Nest, and L. R. Stanberry. 2002. Use of immunostimulatory sequence-containing oligonucleotides as topical therapy for genital herpes simplex virus type 2 infection. J. Virol. 76:11387-11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raupach, B., and S. H. Kaufmann. 2001. Immune responses to intracellular bacteria. Curr. Opin. Immunol. 13:417-428. [DOI] [PubMed] [Google Scholar]
- 20.Shiloh, M. U., and C. F. Nathan. 2000. Reactive nitrogen intermediates and the pathogenesis of Salmonella and mycobacteria. Curr. Opin. Microbiol. 3:35-42. [DOI] [PubMed] [Google Scholar]
- 21.Takeshita, F., C. A. Leifer, I. Gursel, K. J. Ishii, S. Takeshita, M. Gursel, and D. M. Klinman. 2001. Cutting edge: role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J. Immunol. 167:3555-3558. [DOI] [PubMed] [Google Scholar]
- 22.Vázquez-Boland, J. A., M. Kuhn, P. Berche, T. Chakraborty, G. Domínguez-Bernal, W. Goebel, B. González-Zorn, J. Wehland, and J. Kreft. 2001. Listeria pathogenesis and molecular virulence determinants. Clin. Microbiol. Rev. 14:584-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmermann, S., O. Egeter, S. Hausmann, G. B. Lipford, M. Rocken, H. Wagner, and K. Heeg. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160:3627-3630. [PubMed] [Google Scholar]