Abstract
A total of 104 polypeptides were purified from the low-molecular-mass secretory proteome of Mycobacterium tuberculosis H37Rv using a combination of anion exchange column chromatography and high resolution preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by electroelution. The goal of this study was to identify polypeptides from a low-molecular-mass secretory proteome recognized by human subjects infected with M. tuberculosis and to ascertain the differences in specificity of antigen recognition by the peripheral blood mononuclear cells (PBMCs) and pleural fluid mononuclear cells (PFMCs) of these individuals. The study identified CFP-8 (Rv0496), CFP-11 (Rv2433c), CFP-14.5 (Rv2445c), and CFP-31 (Rv0831c) as novel T-cell antigens apart from previously characterized ESAT-6, TB10.4, CFP10, GroES, MTSP14, MTSP17, CFP21, MPT64, Ag85A, and Ag85B on the basis of recognition by PBMCs of tuberculosis contacts and treated tuberculosis patients. Further, polypeptides prominently recognized by PFMCs of tuberculous pleurisy patients were the same as those recognized by PBMCs of healthy contacts and treated tuberculosis patients. The results of our study indicate the homogeneity of antigenic target recognition by lymphocytes at the site of infection and at the periphery in the human subjects studied and the need to evaluate these antigenic targets as components of future antituberculous vaccines.
Tuberculosis (TB) is one of the major infectious diseases for which no effective vaccine is currently available. Identification of key antigenic targets of the protective human immune response against Mycobacterium tuberculosis is central to the development of an efficacious vaccine against tuberculosis. Secretory proteins of mycobacteria are found to be the major targets of immune recognition during initial stages of infection in various animal models (5, 21, 35) and in humans (12, 20). Subunit vaccines based on culture filtrate proteins (CFPs) of mycobacteria are shown to impart significant levels of protective immunity in different animal models of experimental tuberculosis (1, 6, 23, 34, 39). The secretory proteome of M. tuberculosis consists of numerous actively secreted components, of which many are unique to M. tuberculosis (4, 44, 56, 57). A comparative evaluation of the individual components of the M. tuberculosis secretory proteome in terms of the magnitude of the immune response induced in human subjects is lacking, and this response needs to be evaluated to identify mycobacterial molecules relevant for human immunity to TB. However, identification of such candidate antigens has to rely on hypothetical correlates of protective immunity.
Though not completely understood as yet, cell-mediated immunity is thought to play an important role in resistance to TB. The circulating T-cell response as well as that at the site of infection is considered important. Gamma interferon (IFN-γ) production and a shift in favor of the type 1 over the type 2 immune response are generally regarded as the correlates of protective immunity. The peripheral blood mononuclear cell (PBMC) responses of tuberculin skin test-reactive healthy contacts of TB patients and those of healed TB (memory immune) subjects are suggested as the model of protective immunity against TB (12, 21, 22, 43, 59). According to this hypothesis, antigens recognized by T lymphocytes of this group (i.e., sensitized/infected) but not by those of active TB patients (i.e., diseased) should be considered important for vaccine development.
On the other hand, antigens recognized by pleural fluid mononuclear cells (PFMCs) of patients with tuberculous pleuritis are thought to be useful for development of an anti-TB vaccine (31). Unlike other forms of TB, pleuritis usually resolves without chemotherapy (41) and provides another model to understand immune mechanisms critical for resistance against M. tuberculosis (7). The screening of individual molecules of the secretory proteome of M. tuberculosis in these human models of immunity may lead to identification of vaccine candidates against TB.
During recent years, components of the culture filtrate have been investigated by using narrow-molecular-mass fractions as a guide to identify immunologically active molecules (2). Attempts to screen human immune responses against these narrow-molecular-mass fractions demonstrate that low-molecular-mass proteins are prominently recognized by T lymphocytes (12, 20), while high-molecular-mass proteins are the major targets of B lymphocytes and induce humoral immune responses (26, 27). In view of the potential of low-molecular-mass polypeptides to augment protective cell-mediated immune responses in humans, we purified 104 polypeptides (bands) from the low-molecular-mass (<40 kDa) secretory proteome of M. tuberculosis and evaluated them for recognition by leukocytes from the above-mentioned models of human immunity to TB.
MATERIALS AND METHODS
Growth of M. tuberculosis H37Rv and isolation of culture filtrate proteins. Culture filtrate proteins of M. tuberculosis H37Rv obtained from the National Collection of Type Cultures (London, United Kingdom) were isolated by growing tubercle bacilli in Youman's modified liquid synthetic medium (48) as a stationary pellicle culture. Briefly, bacilli were grown as surface culture in medium for different time periods ranging from 2 weeks to 8 weeks at 37°C. The culture supernatants were filter sterilized (0.22-μm-pore-size membrane filter) and concentrated 100 times by ultrafiltration on an Amicon YM-3 membrane (Millipore, Bedford, MA). The concentrated proteins were desalted by extensive washing in an ultrafiltration chamber with distilled water, with a final exchange in phosphate-buffered saline (PBS; pH 7.2), and were designated RvCFP (culture filtrate proteins of M. tuberculosis H37Rv). The RvCFP were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 16% resolving gels (28), followed by silver staining (32).
Purification of CFPs.
Individual CFPs were purified using a combination of anion exchange column chromatography and high-resolution preparative SDS-PAGE followed by electroelution. Briefly, the RvCFP (200 mg) was separated using DEAE-Sepharose-CL-6B (Sigma, St. Louis, MO) column chromatography as described by Nagai et al. (33) with some modifications. Step gradients of sodium chloride (50 mM to 1 M) in 30 mM Tris-hydrochloride equilibration buffer (pH 8.7) containing 3% (vol/vol) methylcellosolve were used for the elution of proteins. Each fraction was read at 280 nm, and every alternate fraction was analyzed by 16% SDS-PAGE followed by silver staining. The peak fractions of each NaCl gradient having similar protein profiles were pooled. Each chromatography protein pool was subsequently probed with a panel of known monoclonal antibodies (MAb) or polyclonal monospecific antibodies (PAb) to identify previously defined CFPs by enzyme-linked immunosorbent assay (ELISA) and Western immunoblotting (51). The antibodies (protein; H37Rv no.) CS-18 (SOD; Rv3846), α-MPT-53 (MPT-53; Rv2878c), IT-3 (GroES; Rv3418c), IT-4 (16-kDa alpha-crystallin; Rv2031c), IT-20 (14-kDa alpha-crystallin truncated; Rv2031c), IT-10 (20.5-kDa uncharacterized protein), IT-12 (19-kDa lipoprotein; Rv1677), IT-23 (PstS; Rv0934), IT-44 (CFP-32; Rv0577), IT-49 (Ag85 complex; Rv3804c, Rv1886c, and Rv0129c), IT-52 (MPT-51; Rv3803c), IT-59 (33-kDa uncharacterized protein), IT-69 (CFP-20; Rv1932), mc9246 (28-kDa uncharacterized protein), IT-56 (GroEL; Rv0440), CS-44 (GroEL; Rv0440), and IT-57 (KatG/peroxidase complex; Rv1908c) were obtained from the World Health Organization collection (47), while PV-2 (TB 10.4; Rv0288), HYB76-8 (ESAT-6; Rv3875), L24-B4 (MPT-64; Rv1980c), K8483 (CFP-21; Rv1984c), and K8493 (CFP-10; Rv3874) were a kind gift from I. Rosenkrands, Statens Serum Institute, Copenhagen, Denmark. All the MAb and PAb were used according to the instructions provided and have been described previously (47). Proteins of each chromatography pool (5 to 10 mg) were resolved by high-resolution preparative SDS-PAGE (gel dimensions, 16 cm by 14 cm by 1.5 mm) (Hoefer SE 600; Amersham Pharmacia Biotech Inc., San Francisco, CA) overnight under reducing conditions. Longitudinal strips were cut from the right and left sides of the preparative gel and stained with silver nitrate. Stained gel strips were aligned to the edges of unstained gel and used as guide to cut out individual bands from the unstained gel (11). Fine horizontal strips (<1 mm) were excised from the gel and transferred to an electroeluter (Bio-Rad Laboratories, Hercules, CA). Electroelution was carried out at 250 V for 4 h using 25 mM Tris-192 mM glycine electroelution buffer (pH 8.3). The electroeluted proteins were aspirated, and the buffer was exchanged to PBS (pH 7.2) and concentrated on a centricon concentrator (Millipore, Bedford, MA) with a 3-kDa cutoff membrane. The protein concentration was estimated by a Micro bicinchoninic acid (Sigma, St. Louis, MO) method and analyzed by SDS-PAGE and silver staining. The identity of electroeluted proteins was confirmed by reactivity with known MAb/PAb in ELISA, and polypeptides were subsequently stored at −20°C.
Study population.
Four patients with tuberculous pleurisy (all males; mean age [years], 40 ± 4) admitted to Nehru Hospital, PGIMER, Chandigarh, India, participated in this study. All patients were diagnosed on the basis of chest roentgenogram. Furthermore, patients with tuberculous pleuritis demonstrated granulomatous pleuritis on closed pleural biopsy, and smear and cultures of sputum or pleural fluid were positive for acid-fast bacilli. All patients had unilateral exudative pleural effusion with predominance of mononuclear cells and were investigated at the onset of antibiotic treatment. Healthy medical, paramedical, and laboratory subjects (n = 7; 3 male [mean age, 33 ± 5] and 4 female [mean age, 31 ± 4]) who had direct contact with tuberculosis patients were termed tuberculosis contacts and were used as categorical representatives of the immune population if they were Mantoux test positive and culture negative for more than 6 months and had normal chest roentgenograms. Healthy treated cases with healed tuberculosis and no history of relapse were categorized as memory immune individuals (n = 6; 3 male [mean age, 29 ± 1] and 3 female [mean age, 30 ± 1]) and were investigated after at least 1 year of completion of treatment. Active pulmonary TB was excluded from healthy contacts and treated TB patients by sputum smears for acid-fast bacilli. All the healthy individuals were evaluated for tuberculin skin test positivity, where induration responses above 10.0 mm were considered as Mantoux positive. All the study subjects used in the study had received Mycobacterium bovis BCG as childhood vaccination and were human immunodeficiency virus negative. Samples of peripheral blood by venipuncture and/or pleural fluid by thoracentesis were collected from the study subjects with prior consent, and the study was approved by the Institutional Ethics Committee.
Isolation of mononuclear cells and proliferation assay.
Mononuclear cells were isolated from pleural fluid and peripheral blood by Ficoll-Hypaque density centrifugation. PBMCs or PFMCs (2 × 105) were added to wells of 96-well flat-bottom sterile tissue culture plates (Greiner Bio-One, Germany) in 0.2 ml of RPMI 1640 supplemented with 100 IU/ml penicillin, 50 μg/ml streptomycin, 1 mM l-glutamine (all from Sigma, St. Louis, MO), 25 mM HEPES (Fluka, Switzerland), 1 mM sodium pyruvate (SRL, Mumbai, India), 5 × 10−5 M β-mercaptoethanol, and 10% heat-inactivated autologous serum. Individual purified CFPs (2 μg/ml), RvCFP (2 μg/ml), and purified protein derivative (PPD; 2 μg/ml) were used for in vitro stimulation. Phytohemagglutinin (PHA) (Sigma, St. Louis, MO) at a concentration of 1 μg/ml was used as a positive control for cell reactivity and viability. Control wells contained responder cells in RPMI 1640 medium alone for determination of background proliferation. Cultures were incubated for 5 days at 37°C in a humidified atmosphere containing 5% CO2. Cell proliferation was measured by the incorporation of [3H]thymidine (BARC, Mumbai, India) that was added at 0.25 μCi per well for the final 18 to 22 h of culture. The cells were harvested onto glass fiber filters using the Nunc cell harvester (Intermed, Denmark), and the incorporated radioactivity was measured in a LKB Rack Beta liquid scintillation counter (model 1214; LKB-Wallac, Palo Alto, Calif). The proliferative responses were expressed as stimulation indices (SI). The SI was calculated by dividing mean counts per minute in antigen-stimulated wells by mean counts per minute in unstimulated wells.
IFN-γ assay.
Levels of IFN-γ released in culture supernatants of PBMC and PFMC cultures after in vitro stimulation with test antigens at day 5 were estimated using a human IFN-γ ELISA reagent set (Opt EIA set; BD Pharmingen, San Diego, Calif). The assay was performed per the manufacturer's instructions. IFN-γ concentration was expressed in pg/ml, and the detection limit of the assay was 2.35 pg/ml.
Detection of antigen-specific serum IgG antibody levels.
Total immunoglobulin G (IgG) antibody specific to individual CFPs and complex mycobacterial antigens of RvCFP and PPD was estimated in sera of study subjects by ELISA. Individual or complex antigens, suspended at a concentration of 2 μg/ml in 100 μl of coating buffer, were allowed to bind to wells of ELISA plates (Greiner Bio-One, Germany,) for 2 h at 37°C. After three washes with PBS-Tween 20 (0.05%) (PBS-T), the wells were blocked with 3% bovine serum albumin (BSA) in PBS-T overnight at 4°C. Serum samples (100 μl) of healthy subjects were added per well at a 1:100 dilution in PBS-T containing 1% BSA. Antigen antibody binding was allowed to proceed for 2 h at 37°C. The plates were washed four times with PBS-T, and 100 μl of horseradish peroxidase-conjugated goat anti-human IgG (Sigma, St. Louis, MO) diluted 1:1,000 in PBS-T containing 1% BSA was added per well. After 90 min the plates were washed six times with PBS-T. The reaction was developed with o-phenylenediamine and hydrogen peroxide in citrate substrate buffer (pH 5.0). The optical density at 492 nm (OD492) was read after the reaction was stopped with 50 μl of 1 M H2SO4.
Characterization of selected CFPs.
Selected CFPs were characterized by amino acid sequence analysis and liquid chromatography-tandem mass spectrometry (LC-MS/MS). To obtain the N-terminal amino acid sequence of selected proteins, CFPs were resolved by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA) by electroblotting with cyclohexylaminopropane sulfonic acid buffer containing 10% methanol. The membrane was stained with 0.1% Coomassie brilliant blue in 10% acetic acid and destained with a solution of 50% methanol-10% acetic acid. Immobilized proteins were subjected to automated Edman degradation. To determine the sequence of internal peptide fragments, selected CFPs were subjected to LC-MS/MS as described previously (19).
RESULTS
Isolation of culture filtrate proteins of M. tuberculosis H37Rv and purification of individual polypeptides (bands). The 2-, 4-, 6-, and 8-week-old culture filtrates were monitored for the presence of the 65-kDa GroEL homolog (HSP-65) by ELISA using IT-56 and CS-44 MAb reactive against GroEL as a marker of autolysis. No reactivity was observed in the 2- and 4-week-old culture filtrates of M. tuberculosis demonstrating an absence of autolysis, while detectable autolysis in 6-week-old culture filtrate and 8-week-old culture filtrate was observed (data not shown). Therefore, 4-week-old culture filtrate was used for further isolation of individual CFPs (Fig. 1A). The yield of M. tuberculosis H37Rv secretory proteins after 4 weeks of stationary growth was 18 ± 2 mg/liter.
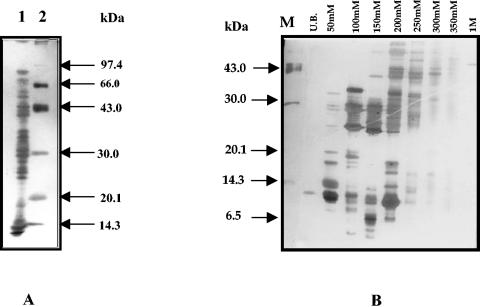
FIG. 1.
SDS-PAGE analysis of M. tuberculosis H37Rv culture filtrate polypeptides (RvCFP; 4 weeks old) and resulting pooled anion exchange chromatography fractions. (A) Lane 1, M. tuberculosis H37Rv culture filtrate polypeptides (RvCFP); lane 2, standard molecular mass markers. (B) Anion exchange chromatography protein pools. From left to right, the lanes are as follows: M, molecular mass markers; UB, pool 1 (fractions 3 to 7); 50 mM, pool 2 (fractions 22 to 26); 100 mM, pool 3 (fractions 40 to 44); 150 mM, pool 4 (fractions 52 to 56); 200 mM, pool 5, (fractions 75 to 79); 250 mM, pool 6 (fractions 104 to 108); 300 mM, pool 7 (fractions 130 to 134); 350 mM, pool 8 (fractions 148 to 152); 1 M, pool 9 (fractions 166 to 170). UB, unbound.
RvCFP was first separated on a DEAE-Sepharose CL-6B column using step gradients of 50 mM, 100 mM, 150 mM, 200 mM, 250 mM, 300 mM, 350 mM, and 1 M sodium chloride in Tris-hydrochloride (pH 8.6) buffer containing 3% methylcellosolve. Alternate column chromatography fractions were resolved by 16% SDS-PAGE, and the fractions were pooled based on similarities in protein profiles as described in Materials and Methods. Each pool consisted of five fractions with the same protein profiles that eluted in the same peak region of the gradient. Each chromatography pool was subsequently analyzed by SDS-PAGE. Nine different pools with markedly different band composition were obtained (Fig. 1B) and used for further purification.
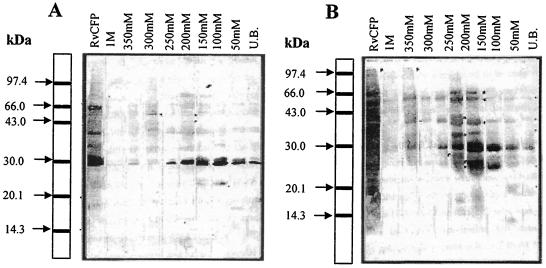
Before separation by preparative SDS-PAGE, protein pools were analyzed for reactivity with TB patients and healthy human sera, as well as with a panel of MAb to facilitate further purification and characterization. The polypeptides in the region of 25 kDa to 30 kDa and of higher molecular mass from all the pools were prominently recognized by antibodies present in the sera of both healthy individuals (Fig. 2A) and TB patients (Fig. 2B). Moreover, polypeptides from the secretory proteome below 40 kDa have been previously demonstrated to contain molecules strongly recognized by CD4+ and CD8+ T lymphocytes of human subjects (12, 58). Therefore, efforts were focused on exploring the mycobacterial secretory proteome below 40 kDa. To identify previously defined proteins of mycobacteria, chromatography protein pools were subjected to reactivity with a panel of known MAb/PAb by ELISA and Western immunoblotting (data not shown). All CFPs were efficiently resolved into different pools obtained by DEAE Sepharose CL-6B column chromatography as evident from the reactivities with different antibodies.
FIG. 2.
Western immunoblot analysis of RvCFP and column chromatography protein pools using healthy tuberculin skin test-positive tuberculosis patient contact sera (A) and moderately advanced pulmonary tuberculosis patient sera (B). Polypeptides of each chromatography pool were separated by 16% SDS-PAGE, transferred on to nitrocellulose membrane (47), and probed with pooled sera of either moderately advanced active pulmonary TB patients (n = 10) or healthy Mantoux-positive tuberculosis contacts (n = 10). A representative blot is shown. UB, unbound.
Further, the panel of known MAb/PAb reactive against low-molecular-mass CFPs allowed the mapping and identification of previously known mycobacterial proteins from the low-molecular-mass (<40-kDa) secretory proteome of M. tuberculosis H37Rv. The proteins identified were TB 10.4 (5 kDa), ESAT-6 (6 kDa), CFP10 (10 kDa), GroES homolog/HSP-10 (10 to 12 kDa), alpha-crystallin homolog (14-kDa truncated isoform), alpha-crystallin/Acr protein (Hspx; 16 kDa), MPT 53 (15 kDa), 19-kDa lipoprotein, CFP-20 (20 kDa), 20.5 kDa (uncharacterized protein), CFP 21 (21 kDa), SOD (23 kDa), MPT64 (24 kDa), MPT-51 (26 to 27 kDa), 28 kDa (uncharacterized protein), Ag85B (29 to 30 kDa), Ag85A (30 to 31 kDa), Ag85C (31 to 32 kDa), 32 kDa (CFP-32), 33 kDa (uncharacterized protein), and PstS (38 kDa).
Individual polypeptide bands of different molecular masses were subsequently purified from different chromatography protein pools using high-resolution preparative SDS-PAGE and electroelution. Polyacrylamide concentrations of 16%, 14%, and 12% were chosen for optimal resolution of molecules between 3 to 15, 15 to 25, and 25 to 40 kDa, respectively. The electroeluted polypeptides were analyzed by SDS-PAGE, and impure fractions were eliminated (data not shown). The preliminary characterization of purified polypeptides (bands) in terms of apparent reactivity with available known MAb was performed (Table 1). In all, 104 polypeptides (SDS-PAGE-separated bands) were purified and were evaluated in the study of human immune responses.
TABLE 1.
Partial characterization of individual purified polypeptides (bands) eluted from different column chromatography pools
| Protein pool and eluted protein no. | Apparent mol mass (kDa)a | Ab reactedb | Degree of reactivityc | Protein identified |
|---|---|---|---|---|
| Pool one | ||||
| 1 | 22.5 | K8483 | ++ | |
| 2 | 21 | K8483 | +++++ | CFP-21 |
| 3 | 20 | K8483 | ++ | |
| Pool two | ||||
| 4 | 3.5 | Nil | − | |
| 5 | 4 | Nil | − | |
| 6 | 4.5 | Nil | − | |
| 7 | 5 | Nil | − | |
| 8 | 6 | Nil | − | |
| 9 | 8 | Nil | − | |
| 10 | 10 | IT-3 | ++ | |
| 11 | 12 | IT-3 | ++ | |
| 12 | 14.5 | α-MPT 53 | +++++ | 15-kDa |
| 13 | 16 | Nil | − | |
| 14 | 16.5 | Nil | − | |
| 15 | 18 | Nil | − | |
| 16 | 19 | IT-12 | +++++ | |
| 17 | 21 | Nil | − | |
| 18 | 23.5 | Nil | − | |
| 19 | 25 | Nil | − | |
| 20 | 26 | Nil | − | |
| 21 | 28 | Nil | − | |
| 22 | 35 | Nil | − | |
| 23 | 37 | Nil | − | |
| Pool three | ||||
| 24 | 18.5 | IT-10/IT-12 | +++ | |
| 25 | 19 | Nil | − | |
| 26 | 20 | Nil | − | |
| 27 | 20.5 | IT-10 | ++++ | 20.5-kDa |
| 28 | 24 | L24B4 | +++++ | MPT64 |
| 29 | 28 | Nil | − | |
| 30 | 29 | Nil | − | |
| 31 | 29.5 | IT-49 | +++++ | Ag85B? |
| 32 | 30.5 | IT-49 | +++++ | Ag85A? |
| 33 | 31 | Nil | − | |
| 34 | 34 | Nil | − | |
| Pool four | ||||
| 35 | 4 | PV-2 | ++ | |
| 36 | 4.5 | PV-2 | +++++ | |
| 37 | 5.5 | PV-2 & | ++ | HYB 76-8 |
| 38 | 6 | HYB 76-8 | +++++ | ESAT-6 |
| 39 | 7 | HYB 76-8 | ++ | |
| 40 | 8 | Nil | − | |
| 41 | 9 | K8493 | ++ | |
| 42 | 9.5 | K8493 | +++ | |
| 43 | 10 | K8493 | +++++ | CFP-10 |
| 44 | 11 | IT-3 | ++ | |
| 45 | 12.5 | IT-3 | ++ | |
| 46 | 13.5 | Nil | − | |
| 47 | 15 | α-MPT-53 | ++ | |
| 48 | 15.5 | Nil | − | |
| 49 | 16 | Nil | − | |
| 50 | 16.5 | Nil | − | |
| 51 | 17 | Nil | − | |
| 52 | 17.5 | Nil | − | |
| 53 | 18 | Nil | − | |
| 54 | 18.5 | Nil | − | |
| 55 | 19.5 | Nil | − | |
| 56 | 20 | Nil | − | |
| 57 | 23 | CS-28 | +++++ | |
| 58 | 25 | Nil | − | |
| 59 | 26 | IT-52 | ++++ | MPT-51 |
| 60 | 27 | Nil | − | |
| 61 | 28.5 | Nil | − | |
| 62 | 33 | Nil | − | |
| 63 | 36 | Nil | − | |
| Pool five | ||||
| 64 | 5 | Nil | − | |
| 65 | 5.5 | Nil | − | |
| 66 | 6 | Nil | − | |
| 67 | 7 | Nil | − | |
| 68 | 8 | IT-3 | ++ | |
| 69 | 9 | IT-3 | +++ | |
| 70 | 10 | IT-3 | +++++ | HSP-10 |
| 71 | 11 | IT-3 | +++ | |
| 72 | 12 | IT-3 | +++ | |
| 73 | 13 | α-MPT-53 | + | |
| 74 | 13.5 | α-MPT-53 | + | |
| 75 | 14 | α-MPT-53 | ++ | |
| 76 | 14.5 | α-MPT-53 | ++ | |
| 77 | 16 | Nil | − | |
| 78 | 17 | Nil | − | |
| 79 | 18 | Nil | − | |
| 80 | 19 | IT-69 | ++++ | |
| 81 | 20 | Nil | − | |
| 82 | 21 | Nil | − | |
| 83 | 21.5 | Nil | − | |
| 84 | 22 | Nil | − | |
| 85 | 22.5 | Nil | − | |
| 86 | 23 | Nil | − | |
| 87 | 23.5 | Nil | − | |
| 88 | 25 | Nil | − | |
| 89 | 26.5 | Nil | − | |
| 90 | 27 | Nil | − | |
| 91 | 28 | Nil | − | |
| 92 | 29 | Nil | − | |
| 93 | 30 | Nil | − | |
| 94 | 32 | IT-44 | +++++ | |
| 95 | 34 | IT-44 | +++ | |
| 96 | 35 | IT-44 | ++ | |
| 97 | 36 | Nil | − | |
| 98 | 38 | Nil | − | |
| 99 | 39 | Nil | − | |
| 100 | 42 | Nil | − | |
| 101 | 43 | Nil | − | |
| Pool eightd | ||||
| 102 | 16 | IT-20 | +++++ | α-crystallin |
| 103 | 26 | mc-9246 | ++++ | 28-kDa |
| 104 | 33 | IT-59 | ++++ | 33-kDa |
Molecular mass was determined by SDS-PAGE analysis.
Ab, antibody.
Reactivity (OD492) for MAb/PAb. −, OD of <0.10 (nil); +, OD of 0.10 to 0.20; ++, OD of 0.20 to 0.30; +++, OD of 0.30 to 0.40; ++++, OD of 0.40 to 0.50; +++++, OD of >0.50.
The low-molecular-mass polypeptides resolved in pools six, seven, and eight were apparently identical.
Evaluation of purified polypeptides for recognition by PBMCs of immune human subjects.
PBMCs obtained from immune donors consisting of healthy Mantoux-positive TB contacts and memory immune treated TB individuals were subjected to in vitro stimulation with purified polypeptides. The lymphocyte activating potential of individual purified polypeptides was evaluated on the basis of DNA synthesis using a [3H]thymidine uptake assay, while T-lymphocyte and B-lymphocyte activating abilities were evaluated on the basis of estimates of IFN-γ levels and serum antibody levels, respectively.
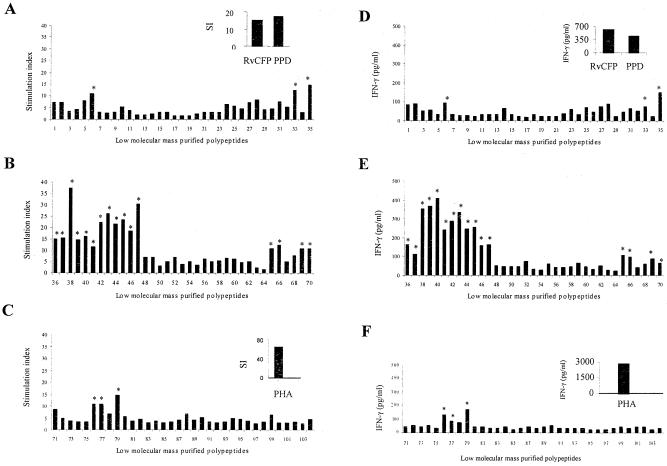
The T- and B-cell responses of two immune populations, i.e., healthy TB contacts and treated TB patients, were found to be directed toward the same set of polypeptides. The screening of purified polypeptides demonstrated predominant recognition of multiple low-molecular-mass polypeptides below 15 kDa by T cells, while antibody reactivity was found to be directed toward polypeptides above 15 kDa, most notably those clustered in the region of 30 kDa. On the basis of the lymphoproliferation assay results, it is clear that several yet uncharacterized key antigen targets exist in the culture filtrate. Out of 104 polypeptides screened for recognition of PBMC, 84 polypeptides were found to induce significant lymphoproliferative responses with T cells from PPD-positive healthy individuals (Fig. 3A to C), using a median SI of 3.0 as the positive cutoff value. From this same study population, the levels of IFN-γ in the culture supernatants in response to stimulation with individual polypeptides were quantified and expressed as median responses (pg/ml) (Fig. 3D to F). Although IFN-γ released in the culture supernatants did not correlate with cellular proliferation in response to every polypeptide, the polypeptides below 15 kDa were found to induce prominent lymphoproliferative as well as IFN-γ responses. Maximum lymphocyte proliferation was observed in response to polypeptide number 38 (median SI, 37.25), while the highest levels of IFN-γ in culture supernatants was observed in response to polypeptide number 40 (median IFN-γ, 406.0 pg/ml). The top 10 polypeptides, which induced prominent T-cell responses in terms of SI and IFN-γ released in culture supernatants, were numbers 36, 38, 39, 40, 41, 42, 43, 44, 47, and 79. These polypeptides were prominently recognized not only by T lymphocytes of healthy tuberculosis contacts but also by the T cells of memory immune subjects, indicating marked homogeneity in the recognition of low-molecular-mass polypeptides by these two immune populations.
FIG. 3.
(A to C) Peripheral blood mononuclear cell proliferation responses (expressed as median SI) of M. tuberculosis H37Rv culture filtrate-purified polypeptides (n = 104) in healthy immune subjects (Mantoux-positive tuberculosis contacts and treated tuberculosis subjects; n = 13). The median counts per minute of culture without antigen was 1,040, and the median induration response of tuberculin skin tests was 25 mm. Median lymphoproliferation responses of RvCFP, PPD, and PHA are shown in the inset. (D to F) IFN-γ responses (expressed as median pg/ml) of PBMCs of healthy immune subjects induced by M. tuberculosis H37Rv culture filtrate-purified polypeptides. The median IFN-γ response of culture without antigen was 14 pg/ml. Median IFN-γ responses of RvCFP, PPD, and PHA are shown in the inset. An asterisk indicates the polypeptides that induced a predominant T-cell response (median SI of ≥10 and median IFN-γ of ≥50.0 pg/ml). All these polypeptides had molecular masses of ≤15 kDa, except polypeptides 33, 77, and 79.
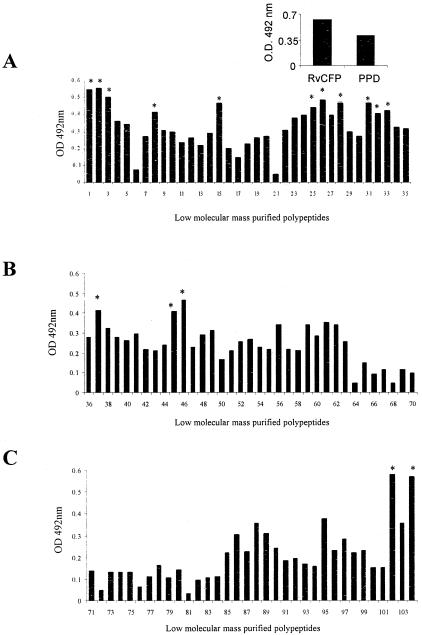
Antigen-specific B-cell responses were evaluated by estimating serum IgG antibody levels against individual antigens and expressed as the median OD492. Maximum antibody levels in the sera of the study subjects were observed for polypeptide number 102 (median OD, 0.580), while 15 other polypeptides exhibited high antibody (IgG) levels (cutoff value of 0.402) (Fig. 4A to C). The positive cutoff was set by using the mean OD of the TB-negative healthy subjects plus 2 standard deviations using PPD as antigen (data not shown). Interestingly, polypeptides 1, 2, 28, 33, 37, 45, and 46 induced both prominent B- and T-cell responses, as evident from marked serum IgG levels, lymphocyte proliferation, and IFN-γ induction.
FIG. 4.
(A to C) Serum antibody (IgG) responses (expressed as median absorbance at 492 nm) of M. tuberculosis H37Rv culture filtrate-purified polypeptides (n = 104) in healthy immune subjects (Mantoux-positive tuberculosis contacts and treated TB subjects; n = 13). The median IgG response of wells without antigen was 0.149, and the median induration response of tuberculin skin tests was 25 mm. Median IgG responses of RvCFP and PPD are shown in the inset. An asterisk indicates the polypeptides that induced a significant B-cell response (median OD of ≥0.402). All these polypeptides had molecular masses of >15 kDa except polypeptides 8, 37, 45, and 46.
Based on these comparative analyses, immunoreactive polypeptides were grouped into two categories. Specifically, 18 polypeptides inducing predominant T-cell responses (median SI of >10 and median IFN-γ of ≥50.0 pg/ml as arbitrary cutoff values) but no significant B-cell (IgG antibody) response (median OD of <0.402) were designated polypeptides inducing predominant T-cell-mediated immune responses and were referred to as group I. On the other hand, out of 16 polypeptides inducing significant B-cell responses in terms of serum IgG antibody levels (median OD of ≥0.402), 10 were found also to induce substantial levels of T-cell responses (median SI of >5.0 and median IFN-γ of >50.0 pg/ml as arbitrary cutoff values). These polypeptides (group II) were designated polypeptides inducing both antibody and cell-mediated immune responses. The remaining six polypeptides inducing predominant antibody responses alone were not characterized. The immune responses of selected group I and II polypeptides in the healthy immune population are summarized in Table 2.
TABLE 2.
Immunoreactivity and characterization of group I and group II purified polypeptides
| PGa | Protein no. | Group I and group II purified polypeptides
|
||||||
|---|---|---|---|---|---|---|---|---|
| Immunoreactivityb
|
Characterization
|
|||||||
| Median SI | Median IFN-γ (pg/ml) | Median IgG (OD492) | Apparent mol mass (kDa) | Theoretical mol mass (kDa)c | H37Rv annotation no. | Identity | ||
| I | 6 | 10.82 | 94.00 | 0.071 | 4.5 | Unknown | Unknown | Unknown |
| 35 | 14.62 | 147.50 | 0.312 | 4.0 | Unknown | Unknown | ESAT-6 family member?d | |
| 36 | 15.15 | 160.00 | 0.277 | 4.5 | 10.391 | Rv0288 | TB10.4d | |
| 38 | 37.25 | 350.00 | 0.320 | 6.0 | 11.75 | Rv3875 | ESAT-6df | |
| 39 | 14.73 | 367.00 | 0.275 | 7.0 | Unknown | Unknown | ESAT-6 family member?a | |
| 40 | 16.09 | 406.00 | 0.258 | 8.0 | 34.80 | Rv0496 | CFP-8a (conserved hypothetical protein)f | |
| 41 | 11.70 | 241.00 | 0.297 | 9.0 | Unknown | Unknown | ESAT-6 family member?d | |
| 42 | 22.47 | 288.00 | 0.216 | 9.5 | Unknown | Unknown | ESAT-6 family member?d | |
| 43 | 26.21 | 334.00 | 0.212 | 10.0 | 10.79 | Rv3874 | CFP-10d | |
| 44 | 21.51 | 247.00 | 0.238 | 11.0 | Unknown | Unknown | Unknown | |
| 47 | 30.30 | 161.00 | 0.228 | 15.0 | 20.18 | Rv0164 | MTSP17f | |
| 65 | 10.92 | 107.00 | 0.145 | 5.5 | Unknown | Unknown | Unknown | |
| 66 | 12.12 | 98.00 | 0.088 | 6.0 | Unknown | Unknown | Unknown | |
| 69 | 10.67 | 89.00 | 0.116 | 9.0 | Unknown | Unknown | Unknown | |
| 70 | 10.84 | 65.00 | 0.095 | 10.0 | 14.45 | Rv3418C | GroES (hsp-10/MPT-57)df | |
| 76 | 10.84 | 137.00 | 0.061 | 14.5 | 14.47 | Rv2445C | CFP 14.5g (ndkA)f | |
| 77 | 10.85 | 87.00 | 0.109 | 16.0 | 17.21 | Rv1827 | MTSP14f | |
| 79 | 14.42 | 178.00 | 0.104 | 18.0 | 16.292, 17.21 | Rv2185C, Rv1827 | CFP-18g (mixed sample) | |
| II | 1 | 7.00 | 82.00 | 0.545 | 22.5 | 24.35, 22.66 | Rv0632C, Rv1626, Rv0905 | CFP22.5a Mixed sample (Enoyl-CoA-hydratase two-component response regulator)f |
| 2 | 7.18 | 88.00 | 0.555 | 21-22.0 | 18.67 | Rv1984C | CFP-21 (cutinase precursor)d | |
| 25 | 5.52 | 71.00 | 0.442 | 19.0 | Unknown | Unknown | Unknown | |
| 28 | 8.15 | 89.00 | 0.463 | 24.0 | 26.15 | Rv1980C | MPT-64def | |
| 31 | 7.53 | 65.50 | 0.468 | 29.5 | 29.85 | Rv1886C | Ag85B (MPT-59, mycolyl transferase)def | |
| 32 | 5.20 | 50.70 | 0.402 | 30.5 | 31.44 | Rv3804C | Ag85A (MPT-44, mycolyl transferase)def | |
| 33 | 12.17 | 75.00 | 0.418 | 31.0 | 30.18 | Rv0831C | CFP-31g (hypothetical protein)f | |
| 37 | 15.38 | 110.00 | 0.416 | 5.5 | Unknown | Unknown | ESAT-6 family member?d | |
| 45 | 23.65 | 253.00 | 0.408 | 12.5 | 10.00, 11.00, 12.50 | Rv3874, Rv3592, Rv3914 | CFP 12.5g Mixed sample (10 kDa, 11 kDa conserved hypothetical protein, 12.5 kDa thioredoxin/MPT46)f | |
| 46 | 18.32 | 156.00 | 0.464 | 13.5 | 11.00 | Rv2433C | CFP-11g (conserved hypothetical protein)e | |
PG, polypeptide group (I or II).
The immunoreactivity of purified polypeptides was determined in terms of PBMC responses in healthy immune subjects (healthy Mantoux test-positive TB contacts and treated TB subjects; n = 13).
Identified from http://www.ncbi.nih.gov/BLAST/Blast.cgi.
Identity determined by MAb/PAb reactivity.
Identity determined by N-terminal sequencing.
Identity determined by electrospray mass spectrometry. CoA, coenzyme A.
Novel T-cell antigen identified in this study.
Characterization of group I and group II polypeptides selected on the basis of PBMC response.
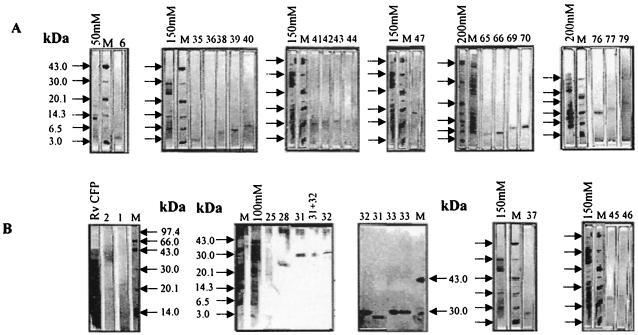
The SDS-PAGE analysis of group I and group II polypeptides is shown in Fig. 5. On the basis of reactivity to known MAb/PAb, the immunoreactive polypeptides 36, 38, 43, 70, 2, 28, 31, and 32 were identified as TB 10.4, ESAT-6, CFP-10, GroES, CFP-21, MPT-64, putative Ag85B, and Ag85 A, respectively. On the basis of N-terminal sequencing, polypeptides 28, 31, 32, and 46 were characterized as MPT-64, Ag85B, Ag85A, and CFP-11 (Rv2433c), respectively. The identity of polypeptides 38, 70, 28, 31, and 32 was confirmed as ESAT-6, GroES, MPT-64, Ag85B, and Ag85A by LC-MS/MS of tryptic digests. The immunoreactive polypeptides that were MAb nonreactive, polypeptides 33, 40, 47, 76 and 77, were identified by LC-MS/MS as Rv0831c, Rv0496, Rv0164, Rv 2445c (ndkA), and Rv1827, respectively. Of these proteins, Rv1827and Rv0164 have been recently described as MTSP14 and MTSP17, respectively, by Lim et al. (30). Others were designated CFP-31, CFP-8, and CFP-14.5 as shown in Table 2. Interestingly, CFP-8, which showed an apparent molecular mass of 8 kDa by SDS-PAGE (Fig. 5), was found to have a theoretical molecular mass of 34.08 kDa on the basis of database searches (http://www.ncbi.nlm.nih.gov/BLAST/BLAST.cgi). Polypeptides 1, 45, and 79 were characterized as mixed samples on the basis of LC-MS/MS (Table 2). Taking the results of molecular characterization into account, this study identified CFP-8 (Rv0408), MTSP17 (Rv0164), ndkA (Rv2445c), MTSP14 (Rv1827), CFP-31 (Rv0831c), and CFP-11 (Rv2433c) as immunodominant human T-cell antigens apart from ESAT-6, TB 10.4, CFP-10, GroES, CFP-21, MPT-64, Ag85B, and Ag85A recognized by PBMCs of the immune population (Table 2). MTSP14 (Rv1827), a mycobacterial protein described as a T-cell antigen in this study and in a previous study by Lim et al. (30), should be considered the same protein as CFP17 identified by Weldingh et al. (55).
FIG. 5.
SDS-PAGE analysis of group I (A) and group II (B) polypeptides purified by preparative electroelution. M, molecular mass markers. Arrows on the left indicate low-molecular-mass markers (from top to bottom, 43.0, 30.0, 20.1, 14.3, 6.5, and 3.0 kDa, unless otherwise indicated); the number on top identifies the polypeptide from a total 104 purified polypeptides (Table 1).
Evaluation of purified polypeptides for recognition by PFMCs of tuberculosis pleuritis patients.
In order to identify polypeptides prominently recognized by lymphocytes from the site of inflammation, we evaluated pleural fluid mononuclear cell responses of tuberculous pleuritis patients. Another purpose of this recognition study was to detect possible differences in fine specificity for purified low-molecular-mass secretory polypeptides between T lymphocytes of peripheral blood obtained from contacts and recall immunity individuals and T lymphocytes of pleural fluid obtained from tuberculous pleuritis patients. The study identified polypeptides 36, 37, 38, 39, 40, 42, 43, 46, 47, 65, 67, 70, 71, and 81 as low-molecular-mass polypeptides prominently recognized by T lymphocytes of pleural fluid, taking a mean SI of 10.0 and 500 pg/ml of IFN-γ released as arbitrary cutoff values (Fig. 6). All these polypeptides demonstrated molecular masses below 15 kDa except polypeptide 81 and were also prominently recognized by peripheral blood T lymphocytes of contacts and treated TB individuals. With the exception of polypeptides 67, 71, and 81, all polypeptides were the same as those prominently recognized (group I and group II) by T lymphocytes of peripheral blood of the previous patient groups. These results demonstrate considerable homogeneity in antigenic target recognition by T lymphocytes at two sites.
FIG. 6.
(A to C) Pleural fluid mononuclear cell proliferation responses (expressed as mean SI) of M. tuberculosis H37Rv culture filtrate-purified polypeptides (n = 104) in tuberculous pleuritis patients (n = 4). The absence of a bar indicates that lymphoproliferation was not carried out. The standard error of the mean was below 30%. The background value (i.e., proliferation in medium alone) of M. tuberculosis antigens was 2,111 ± 235 cpm (mean ± standard error of the mean). Mean lymphoproliferation responses of RvCFP, PPD, and PHA are shown in the inset (D to F). IFN-γ responses (expressed as mean pg/ml) of PFMCs of tuberculosis pleuritis patients induced by M. tuberculosis H37Rv culture filtrate-purified polypeptides. The background IFN-γ response of culture without antigen was 44 ± 14 pg/ml (mean ± standard error of the mean). Mean IFN-γ responses of RvCFP, PPD, and PHA are shown in the inset. Asterisks indicate the polypeptides that induced predominant T-cell responses (mean SI of ≥10 and mean IFN-γ of ≥500 pg/ml). All these polypeptides had molecular masses of ≤15 kDa except polypeptide 81.
DISCUSSION
The sequencing of the whole genome of M. tuberculosis H37Rv ushered an era of renewed interest in the field of TB prophylaxis and led to the identification of a plethora of putative candidates for vaccine development (17). However, the practical utility of such candidate antigens as vaccine components requires critical in vitro experimental evaluation in various models of immunity. Studies in the field of proteomics and immunology are essential to obtain functional data, as it will be of great help to analyze information obtained by the genomics approach. Moreover, the proteomics approach has been instrumental in identifying new open reading frames that were not predicted by genomics (25). Studies involving native proteins rather than recombinant ones are needed, as the latter may lack similar immunogenic characteristics (42). Therefore, in the absence of an efficient method to predict protective antigens, we evaluated individual polypeptides purified from the immunodominant low-molecular-mass secretory proteome of M. tuberculosis for recognition by lymphocytes of human subjects with protective immunity.
In view of the complex nature of the M. tuberculosis H37Rv low-molecular-mass proteome and existence of multiple CFPs of overlapping molecular masses, we employed a simple and effective method to purify individual polypeptides with increased precision compared to a multielution technique (2). The combination of techniques used in the study enabled us to purify multiple polypeptides with yields sufficient for screening in immunoreactivity experiments. Moreover, the polypeptide preparations were found to be nontoxic in preliminary lymphocyte proliferation studies (data not shown), as charged SDS molecules are removed through the dialysis membrane during electroelution (11, 52). The technique was appropriate for comparative evaluation of individual molecules of complex culture filtrate preparations, reducing the need for laborious methods, such as conventional column chromatographic purification or those relying on two-dimensional separation procedures. However, the precision required for the purification of uncharacterized polypeptides can be increased using a strategy based on preparative isoelectric focusing as the first step, followed by separation according to size in SDS-PAGE, as elegantly described by Covert et al. (19) and others (11, 55). Moreover, this method also has an inherent lacuna considering that M. tuberculosis CFPs have an extremely narrow pI range. As shown in this study, five different polypeptides from the immunodominant 30-kDa region were purified, despite the limited pI heterogeneity. These polypeptides include Ag85A from 50 mM, Ag85B from 100 mM (both react with IT-49), CFP-31 (Rv0831c) from 100 mM, CFP-32 (reacts with IT-44) from 200 mM, and CFP-33 (reacts with IT-59) from the 350 mM NaCl gradient pool. Similarly the separation obtained by combining the techniques used clearly demonstrates the efficient resolution of polypeptides with similar molecular masses.
Presumably, the best candidate vaccine antigens would be those recognized by T lymphocytes of healthy TB contacts or healed TB patients but not recognized by those of active pulmonary TB patients (12, 21, 43, 59). Hence, we started the present study by characterizing purified polypeptides on the basis of the peripheral blood lymphocyte responses of these immune categories using human subjects. In agreement with previous reports that screened human lymphocyte responses using narrow-molecular-mass secreted antigen fractions (12, 20), it was observed that T cells from healthy contacts and treated TB patients recognize multiple targets from the low-molecular-mass secretory proteome with a trend toward stronger recognition of molecules below 15 kDa. The predominant T-cell responses to low-molecular-mass proteins may be due to their small size, which renders them more susceptible to proteolytic degradation, processing, intracellular trafficking, and presentation (8, 46). Low-molecular-mass polypeptides have also been shown to be strongly recognized by T lymphocytes of mouse models of memory immunity (3, 13). The prominent recognition of low-molecular-mass polypeptides (<15 kDa) by T lymphocytes of treated TB patients, a memory immune population, observed in this study is consistent with these findings. Antigen repertoire recognition and the magnitude of blood lymphocyte responses against these antigens were found to be more or less similar in the two immune categories, i.e., healthy contacts and memory immune individuals. Hence, responses of these two categories to the 104 polypeptides screened were not expressed separately. In recognition of the role of antibodies in protective responses against intracellular pathogens (14, 15, 16, 49) and the requirement of balance between Th1 and Th2 responses rather than a biased Th1 or Th2 response (38), we estimated antigen-specific IgG levels in the serum of the immune population. The antibody responses evaluated against purified polypeptides were found to be most notably against the polypeptides in the region of 30 to 40 kDa. These observations can also be justified in light of similar studies using chromatographically separated protein pools and sera from healthy contacts. In the studies most of the polypeptides above 30 kDa showed prominent seroreactivity. It was not surprising to observe greater antibody responses to higher-molecular-mass antigens since a direct relationship between molecular mass and antibody response has been reported earlier (8, 26, 27).
To detect possible differences in the specificity of mycobacterial antigens between T lymphocytes from peripheral blood and from the site of infection, we investigated the pleural fluid lymphocyte responses of tuberculous pleurisy patients. To our surprise, considerable homogeneity in the recognition of multiple low-molecular-mass polypeptides below 15 kDa by T lymphocytes of pleural effusion and peripheral blood of the immune patients studied was observed. It is tempting to speculate that this homogeneity may be due to migration of antigen-specific lymphocytes at the site of infection from the peripheral blood of tuberculous pleurisy patients. However, pleural exudates and peripheral blood-derived lymphocytes of tuberculous pleurisy patients have been previously shown to differ both in phenotype and in fine specificity for mycobacterial antigens (31). The observations made in the study were surprising in the context that immune functions at the site of tuberculous infection are considered to be differentially regulated, and blastogenic responses in pleural mononuclear cells are preserved even in the face of cutaneous tuberculin anergy and inhibition of blood mononuclear cell responses (10). The characterization of antigens recognized by lymphocytes from peripheral blood and pleural fluid revealed the presence of many new targets in the culture filtrate. Rv1827, Rv1626, Rv3875, Rv3418c, Rv1980c, Rv1886c, and Rv3804c, which have been defined as potent T-cell antigens in an earlier study employing proteomics and a mouse model of infection (19), were also identified as dominant human T-cell targets in the present study. Our study confirmed the immunodominance of the ESAT-6 family members for induction of a Th1 response. Considering the proposed role of the ESAT-6 family proteins in virulence and pathogenesis (24, 29, 36, 53, 54), it will be interesting to evaluate targets identified on the basis of immune recognition in this study for their immunoprotective potential in direct vaccine experiments, which will also validate the utility of the patient population used in the study.
Of special note, the Ag85 complex proteins, which were previously shown to be recognized by the PBMCs of healthy tuberculosis contacts (50) and by PFMCs of tuberculous pleurisy patients (31) in countries of nonendemicity, were found to be subdominant in this study when T-cell responses of the blood were taken into consideration. Both Ag85A and B were found to induce substantial levels of antibodies in healthy tuberculosis contacts and were evident from immunoblotting experiments with chromatography protein pools using healthy contact sera. The Ag85 complex is a conserved protein complex having the mycolyl transferase activity required for cell wall biosynthesis of all mycobacterial species (9). It has already been reported that environmental mycobacteria have a direct antagonistic influence on the Th1 response and can skew the immune response toward the Th2 direction (40); however, the precise mechanism of this interference is still unknown. High serum antibody levels and subdominant T-cell responses against Ag85 complex antigens in a scenario of endemicity (observed in this study) can be explained on the basis of preexposure and the high environmental burden of saprophytic mycobacterial species diverting Ag85 complex-specific cellular immune response toward a humoral type. Interestingly, Ag85 complex antigens have been reported to be prominently recognized by thoracic lymph node cells of memory immune mice (18) and bronchoalveolar lavage (BAL) cells of active pulmonary TB patients (45). Moreover, the proportion of Ag85-reactive BAL cells was found to be high in the lungs of healthy TB contacts (44) compared to ESAT-6, the antigen that was prominently recognized by blood T lymphocytes (37). Considering this fact, it will be interesting to evaluate polypeptides purified in this study for recognition by BAL cells to find diversity, if any, in antigen recognition at the level of blood and lungs. We are currently evaluating BAL cell responses to purified low-molecular-mass polypeptides in order to decipher the antigen specificity for lymphocytes at the actual site of disease. It will be of great help to come to a logical conclusion regarding molecules that have relevance for human immunity to TB and the development of a new generation of efficacious vaccines.
Acknowledgments
This work was funded by a grant from the Department of Biotechnology and Indian Council of Medical Research of the Government of India, New Delhi, and NIH NIAID grant AI-75320. S.B.S. and R. K. are recipients of a Senior Research Fellowship of the Council of Scientific and Industrial Research, New Delhi, India. M.K. is a recipient of Senior Research Fellowship of University Grants Commission, India.
We thank the Central Instrumentation facility of the PGIMER for help with N-terminal amino acid sequencing of mycobacterial proteins. We thank all the volunteers who donated blood for this study.
Editor: J. L. Flynn
REFERENCES
- 1.Andersen, P. 1994. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect. Immun. 62:2536-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, P., and I. Heron. 1993. Simultaneous electroelution of whole SDS-polyacrylamide gels for the direct cellular analysis of complex protein mixtures. J. Immunol. Methods 161:29-39. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, P., and I. Heron. 1993. Specificity of a protective memory immune response against Mycobacterium tuberculosis. Infect. Immun. 61:844-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen, P., D. Askgaard, L. Ljungqvist, J. Bennedsen, and I. Heron. 1991. Proteins released from Mycobacterium tuberculosis during growth. Infect. Immun. 59:1905-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen, P., D. Askgaard, L. Ljungqvist, M. W. Bentzon, and I. Heron. 1991. T-cell proliferative response to antigens secreted by Mycobacterium tuberculosis. Infect. Immun. 59:1558-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldwin, S. L., C. D'Souza, A. D. Roberts, B. P. Kelly, A. A. Frank, M. A. Lui, J. B. Ulmer, K. Huygen, D. N. McMurray, and I. M. Orme. 1998. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66:2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes, P. F., S. Lu., J. S. Abrams, E. Wang, M. Yamamura, and R. L. Modlin. 1993. Cytokine production at the site of disease in human tuberculosis. Infect. Immun. 81:3482-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassey, E. O. E., P. F. Life, D. Catty, J. S. H. Gaston, and D. S. Kumararatne. 1996. T-cell response to mycobacterial proteins: a comparative study of tuberculous and control immunoblots of Mycobacterium tuberculosis and M. bovis BCG. Tuberc. Lung Dis. 77:146-153. [DOI] [PubMed] [Google Scholar]
- 9.Belisle, J. T., V. D. Vissa, T. Sievert, K. Takayama, P. J. Brennan, and G. S. Besra. 1997. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 276:1420-1422. [DOI] [PubMed] [Google Scholar]
- 10.Berger, H., and E. Mejia. 1973. Tuberculous pleurisy. Chest 63:88-92. [DOI] [PubMed] [Google Scholar]
- 11.Bhaskar, S., S. P. Khanna, and R. Mukherjee. 2000. Isolation, purification and immunological characterization of novel low molecular weight protein antigen CFP6 from culture filtrate of M. tuberculosis. Vaccine 18:2856-2866. [DOI] [PubMed] [Google Scholar]
- 12.Boesen, H., B. N. Jensen, T. Wilcke, and P. Andersen. 1995. Human T-cell responses to secreted antigen fraction of Mycobacterium tuberculosis. Infect. Immun. 63:1491-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandt, L., T. Oettinger, A. Holm, and P. Andersen. 1996. Key epitopes on the ESAT-6 antigen recognized in mice during the recall of protective immunity to Mycobacterium tuberculosis. J. Immunol. 157:3527-3533. [PubMed] [Google Scholar]
- 14.Casadevall, A. 2003. Antibody-mediated immunity against intracellular pathogens: two-dimensional thinking comes full circle. Infect. Immun. 71:4225-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Casadevall, A., and L. Pirofski. 2003. Antibody-mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 24:474-478. [DOI] [PubMed] [Google Scholar]
- 16.Chamber, M. A., D. Gavier-Widen, and R. G. Hewinson. 2004. Antibody bound to the surface antigen MPB 83 of Mycobacterium bovis enhances survival against high dose and low dose challenge. FEMS Immunol. Med. Microbiol. 41:93-100. [DOI] [PubMed] [Google Scholar]
- 17.Cole, S. T., R. Brosch. J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeglr, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 18.Cooper, A. M., J. E. Callahan, M. Keen, J. T. Belisle, and I. M. Orme. 1997. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tuberc. Lung Dis. 75:57-63. [DOI] [PubMed] [Google Scholar]
- 19.Covert, B. A., J. S. Spencer, I. M. Orme, and J. T. Belisle. 2001. The application of proteomics in defining the T-cell antigens of Mycobacterium tuberculosis. Proteomics 1:574-586. [DOI] [PubMed] [Google Scholar]
- 20.Demissie, A., P. Ravn, J. Olobo, T. M. Doherty, T. Eguale, M. Geletu, W. Hailu, P. Andersen, and S. Britton. 1999. T-cell recognition of Mycobacterium tuberculosis culture filtrate fractions in tuberculosis patients and their household contacts. Infect. Immun. 67:5967-5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasløv, K., A. Andersen, S. Nagai, A. Gottschau, T. Sørensen, and P. Andersen. 1995. Guinea pig cellular immune responses to proteins secreted by Mycobacterium tuberculosis. Infect. Immun. 63:804-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Havlir, D. V., R. S. Wallis, W. H. Boom, T. M. Daniel, K. Chervenak, and J. J. Ellner. 1991. Human immune responses to Mycobacterium tuberculosis antigens. Infect. Immun. 59:665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz, M. A., B. W. Lee, B. J. Dillon, and G. Harth. 1995. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 92:1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu, T., S. M. Hingley-Wilson, B. Chen. M. Chen, A. Z. Dai, P. M. Morin, C. B. Markes, J. Padiyar, C. Goulding, M. Gingery, D. Eisenberg, R. G. Russell, S. C. Derrick, F. M. Collins, S. L. Morris, C. H. King and W. R. Jacobs, Jr. 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. USA 100:12420-12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jungblut, P. R., E. C. Muller, J. Mattow, and S. H. E. Kaufmann. 2001. Proteomics reveals open reading frames in Mycobacterium tuberculosis H37Rv not predicted by genomics. Infect. Immun. 69:5905-5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laal, S., K. M. Samanich, M. G. Sonnenberg, J. T. Belisle, J. O'Leary, M. S. Simberkoff, and S. Zolla-Pazner. 1997. Surrogate marker of preclinical tuberculosis in human immunodeficiency virus infection: antibodies to an 88-kDa secretory antigen of Mycobacterium tuberculosis. J. Infect. Dis. 176:133-143. [DOI] [PubMed] [Google Scholar]
- 27.Laal, S., K. M. Samanich, M. G. Sonnenberg, S. Zolla-Pazner, J. M. Phadtare, and J. T. Bellisle. 1997. Human humoral responses to antigens of Mycobacterium tuberculosis: immunodominance of high-molecular-mass antigens. Clin. Diagn. Lab. Immunol. 4:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 29.Lewis, K. N., R. Liao, K. M. Guinn, M. J. Hickey, S. Smith, M. A. Behr, and D. R. Sherman. 2003. Deletion of RD1 from Mycobacterium tuberculosis mimics bacilli Calmette-Guerin attenuation. J. Infect. Dis. 187:117-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim, J. H., H. J. Kim, K. S. Lee, E. K. Jo, C. H. Song, S. B. Jung, S. Y. Kim, J. S. Lee, T. H. Paik, and J. K. Park. 2004. Identification of new T-cell stimulating antigens from Mycobacterium tuberculosis culture filtrate. FEMS Microbiol. Lett. 232:51-59. [DOI] [PubMed] [Google Scholar]
- 31.Manca, F., G. Rossi, M. T. Valle, S. Lantero, G. L. Pira, D. Fenoglio, J. D. Bruin, M. Costantini, G. Damiani, B. Balbi, and F. Celada. 1991. Limited clonal heterogeneity of antigen-specific T cells localizing in the pleural space during mycobacterial infection. Infect. Immun. 59:503-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrissey, J. H. 1981. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal. Biochem. 117:307-310. [DOI] [PubMed] [Google Scholar]
- 33.Nagai, S., H. G. Wiker, M. Harboe, and M. Kinomoto. 1991. Isolation and partial characterization of major protein antigens in the culture fluid of Mycobacterium tuberculosis. Infect. Immun. 59:372-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pal, P. G., and M. A. Horwitz. 1992. Immunization with extracellular proteins of Mycobacterium tuberculosis induces cell-mediated immune responses and substantial protective immunity in a guinea pig model of pulmonary tuberculosis. Infect. Immun. 60:4781-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollock, J. M., and P. Andersen. 1997. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovis in cattle. Infect. Immun. 65:2587-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pym, A. S., P. Brodin, R. Brosch, M. Huerre, and S. T. Cole. 2002. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 46:709-717. [DOI] [PubMed] [Google Scholar]
- 37.Ravn, P., A. Demissie, T. Eguale, H. Wondwosson, D. Lein, H. Amaudy, A. S. Mustafa, A. K. Jensen, A. Holm, I. Rosenkrands, F. Oftung, J. Olobo, C. F. von-Reyn, and P. Andersen. 1999. Human T-cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637-645. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes, S. G., and S. P. Graham. 2002. Is “timing” important for cytokine polarization. Trends Immunol. 23:246-249. [DOI] [PubMed] [Google Scholar]
- 39.Roberts, A. D., M. G. Sonnenberg, D. J. Ordway, S. K. Furney, P. J. Brennan, J. T. Belisle, and I. M. Orme. 1995. Characterization of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology 85:502-508. [PMC free article] [PubMed] [Google Scholar]
- 40.Rook, G. A. W., G. M. Bahr, and J. L. Stanford. 1981. The effect of two distinct forms of cell mediated response to mycobacteria on the protective efficacy of BCG. Tubercle 62:63-68. [DOI] [PubMed] [Google Scholar]
- 41.Roper, W. H., and J. J. Waring. 1955. Primary serofibrinous pleural effusion in military personnel. Am. Rev. Tuberc. 71:616-634. [DOI] [PubMed] [Google Scholar]
- 42.Rosenkrands, I., K. Weldingh, P. Ravn, L. Brandt, P. Hojrup, P. B. Rasmussen, A. R. Coates, M. Singh, P. Mascagni, and P. Andersen. 1999. Differential T-cell recognition of native and recombinant Mycobacterium tuberculosis GroES. Infect. Immun. 67:5552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schoel, B., H. Gulle, and S. H. E. Kaufmann. 1991. Heterogeneity of the repertoire of T cells of tuberculosis patients and healthy contacts of Mycobacterium tuberculosis antigens separated by high-resolution techniques. Infect. Immun. 60:1717-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwander, S. K., M. Torres, C. Carranza, C., D. Escobedo, M. Tarry-Lehman, P. Andersen, Z. Toosi, J. L. Ellner, E. A. Rich, and E. Sada. 2000. Pulmonary mononuclear cell responses to antigens of Mycobacterium tuberculosis in healthy tuberculosis contacts of patients with active tuberculosis and healthy controls from the community. J. Immunol. 165:1479-1485. [DOI] [PubMed] [Google Scholar]
- 45.Schwander, S. K., M. Torres, E. Sada, C. Carranza, E. Ramos, M. Tarray-Lehman, R. S. Wallis, J. Sierra, and E. A. Rich. 1998. Enhanced responses to Mycobacterium tuberculosis antigens by human alveolar lymphocytes during active pulmonary tuberculosis. J. Infect. Dis. 178:1434-1445. [DOI] [PubMed] [Google Scholar]
- 46.Skjot, R. L., T. Oettinger, I. Rosenkrands, P. Ravn, I. Brock, S. Jacobsen, and P. Andersen. 2000. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect. Immun. 68:214-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonnenberg, M. G., and J. T. Belisle. 1997. Definition of Mycobacterium tuberculosis culture filtrate proteins by two-dimensional polyacrylamide gel electrophoresis, N-terminal amino acid sequencing, and electrospray mass spectrometry. Infect. Immun. 65:4515-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subrahmanyam, D. 1964. Studies on the polyglycerophosphatides of Mycobacterium tuberculosis. Can. J. Biochem. 42:1195-1201. [DOI] [PubMed] [Google Scholar]
- 49.Teitelbaum, R., A. Glatman-Freedman, B. Chen, J. B. Robbins, E. K. Unanue, A. Casadevall, and B. R. Bloom. 1998. A monoclonal antibody recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. USA 95:15688-15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres, M., T. Herrera, H. Villareal, E. A. Rich, and E. Sada. 1998. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect. Immun. 66:176-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tuszynski, G. P., and L. Warren. 1975. Removal of sodium dodecyl sulphate from proteins. Anal. Biochem. 67:55-58. [DOI] [PubMed] [Google Scholar]
- 53.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6 specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wards, B. J., G. W. de Lisle, D. M. Collins. 2000. An ESAT-6 knockout mutant of Mycobacterium bovis produced by homologous recombination will contribute to the development of a live tuberculosis vaccine. Tuber. Lung Dis. 80:185-189. [DOI] [PubMed] [Google Scholar]
- 55.Weldingh, K., I. Rosenkrands, S. Jacobsen, P. B. Rasmussen, M. J. Elhay, and P. Andersen. 1998. Two-dimensional electrophoresis for analysis of Mycobacterium tuberculosis culture filtrate and purification and characterization of six novel proteins. Infect. Immun. 66:3492-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiker, H. G., M. Harboe, and S. Nagai. 1991. Localization index for distinction between extracellular and intracellular antigens of Mycobacterium tuberculosis. J. Gen. Microbiol. 137:875-884. [DOI] [PubMed] [Google Scholar]
- 57.Wiker, H. G., S. L. Michell, R. G. Hewinson, E. Spierings, S. Nagai, and M. Harboe. 1999. Cloning, expression and significance of MPT-53 for identification of secreted proteins of Mycobacterium tuberculosis. Microb. Pathog. 26:207-219. [DOI] [PubMed] [Google Scholar]
- 58.Wilkinson, K. A., J. T. Belisle, M. Mincek, R. J. Wilkinson, and Z. Toosi. 2000. Enhancement of the human T-cell response to culture filtrate fractions of Mycobacterium tuberculosis by microspheres. J. Immunol. Methods 235:1-9. [DOI] [PubMed] [Google Scholar]
- 59.Wilsher, M. L., C. Hagan, R. Prestidge, A. U. Wellis, and G. Murison. 1999. Human in vitro immune responses to Mycobacterium tuberculosis. Tuber. Lung Dis. 79:371-377. [DOI] [PubMed] [Google Scholar]