Abstract
Immunodepletion studies of P-4-vaccinated mice indicate that CD4+ and not CD8+ T cells are critical for protection against Leishmania pifanoi (Leishmania mexicana complex). Although a moderate CD8+ T-cell response is elicited by vaccination, CD4+ T cells are the dominant responding population in vitro and at the cutaneous site of infection. These protective T cells produce gamma interferon (IFN-γ), macrophage migration inhibitory factor (MIF), and tumor necrosis factor/lymphotoxin (TNF/LT), each of which significantly contributed to intracellular parasite destruction in vitro. These results indicate that a singular CD4+ T-cell response (IFN-γ, MIF, and/or LT/TNF) can provide protection against New World cutaneous leishmaniasis.
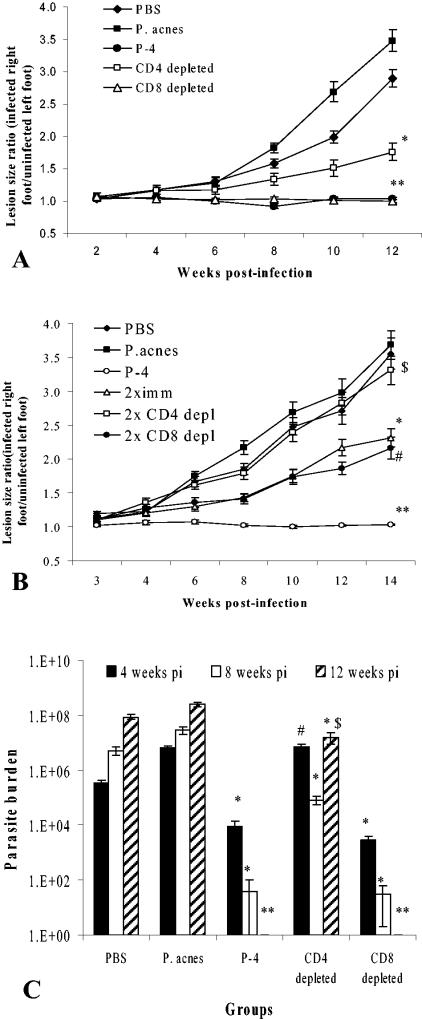
To evaluate the contributions of CD4+ and CD8+ T cells to the protection induced by P-4 vaccination, mice were vaccinated using Propionibacterium acnes (“Corynebacterium parvum”) as an adjuvant (2, 15), and specific T-cell subsets were depleted as described previously (2). Mice were then infected with 106 Leishmania pifanoi promastigotes. While progressive disease in terms of lesion size and parasite burden (Fig. 1) developed for control mice (receiving either phosphate-buffered saline or the adjuvant, P. acnes, alone), vaccinated mice showed no lesion development. Notably, no lesion development could be detected in CD8+-depleted P-4-vaccinated mice. Similar to the P-4-vaccinated group, mice depleted of CD8+ T cells also did not have detectable parasites at 12 weeks postinfection.
FIG. 1.
Evaluation of the contribution of CD4 and CD8 T cells to protection induced by vaccination with the P-4 antigen. (A) BALB/c mice (10/group) were immunized three times at biweekly intervals with purified P-4 antigen along with P. acnes as an adjuvant. Six weeks after the last immunization, depletion of CD4+ and CD8+ T cells was performed as described previously (2). FACS analyses of uninfected mice indicated that 98% of CD4 T cells and 96% of CD8 T cells had been depleted. Mice were then infected with 106 late log L. pifanoi promastigotes. Disease progression was monitored by measurements of lesion development, which is expressed as a ratio of the size of the infected right foot to that of the uninfected left foot. Data shown are average values ± standard errors and are representative of two independent experiments. (Using a Student t test: *, P < 0.02 in comparison to P-4 vaccinated; **, P < 0.002 for P-4 vaccinated and CD8-depleted P-4 vaccinated in comparison to adjuvant control.) (B) T-cell depletion and parasite challenge were performed as indicated for Fig. 1A. However, mice were immunized either twice (2ximm) or three times (P-4) with P-4 antigen together with P. acnes. Groups of mice that were vaccinated twice with P-4 were depleted of either CD4+ or CD8+ T cells; depleted mice are denoted as 2x CD4depl and 2x CD8depl, respectively. Lesion sizes were measured at different time points after infection, as indicated. Ten mice were used per group. Data shown are averaged values ± standard errors for each group. (**, P < 0.002, and *, P < 0.02, compared to adjuvant control; $, P = 0.22, comparison of 2× P-4-vaccinated/ CD4-depleted mice to adjuvant control mice; P = 0.28, 2× P-4-vaccinated CD8-depleted mice compared to P-4-vaccinated mice). (C) Parasite burden analyses of lesions from the site of infection of 3×- P-4-vaccinated and nonvaccinated (adjuvant control) BALB/c mice (from experiment shown in A). The numbers of lesion parasites were determined by limiting-dilution assay at the indicated time points after infection. Data shown are means for six mice per group. Student t test: *, P < 0.02; **, P < 0.001; #, P > 0.05 (when compared to adjuvant control); $, P < 0.001, comparison of P-4-vaccinated and CD4-depleted P-4 vaccinated mice. Note: P = 0.3 when CD8-depleted P-4 vaccinated mice are compared to P-4-vaccinated mice (all three time points).
In contrast, the depletion of CD4+ T cells alone completely reversed protection at 4 weeks postinfection, since parasite burdens in depleted and nonvaccinated mice were identical (Fig. 1C). However, at 8 and 12 weeks postinfection, CD4-depleted P-4-vaccinated mice had parasite burdens somewhat lower than those of control mice but significantly higher than those of P-4-vaccinated mice. Fluorescence-activated cell sorting (FACS) analyses of CD4-depleted P-4-vaccinated mice indicated that CD4+ T cells were reconstituted by 5 to 6 weeks postinfection (data not shown). These results are consistent with earlier studies that demonstrated that transient depletion of CD4+ T cells delayed (by 5 to 7 weeks) but did not prevent the anamnestic response in immune mice (4). The residual protection at 8 to 12 weeks postinfection is likely due to a reconstitution of P-4-specific CD4+ T cells.
These data clearly indicate that CD4+ T cells significantly, if not singularly, are responsible for protection induced by P-4 vaccination. However, it was unclear whether the apparent lack of a CD8+ T-cell response contributing to protection might be due to the ability of an overwhelming CD4+ T-cell response to compensate for a CD8 deficiency. Therefore, to further examine the contribution of CD8+ T cells to protection, mice were vaccinated two times with P-4 together with P. acnes (which results in partial protection), and the effects of CD4+ or CD8+ T-cell depletion were examined. Again, as shown in Fig. 1B, protection was found to depend upon CD4+ and not CD8+ T cells. Consequently, it appeared that CD8+ T cells neither contributed to nor were required for protection against L. pifanoi infection in the P-4-vaccinated BALB/c mice.
To evaluate the effector mechanisms of the CD4+ T cells (Fig. 2), an initial assessment of the cytokine responses of the vaccinated and adjuvant control groups of mice was performed using RNase protection at 4 weeks postinfection, using mRNA isolated from the draining lymph node cells. As shown in Fig. 2, the mice protectively immunized with P-4 expressed 9.6-, 11.5-, and 9.9-fold higher levels of tumor necrosis factor alpha (TNF-α), gamma interferon (IFN-γ), and macrophage migration inhibitory factor (MIF) mRNAs, respectively, than the P. acnes-immunized control mice. In addition, slightly elevated levels of TNF-β (2.5-fold) were found.
FIG. 2.
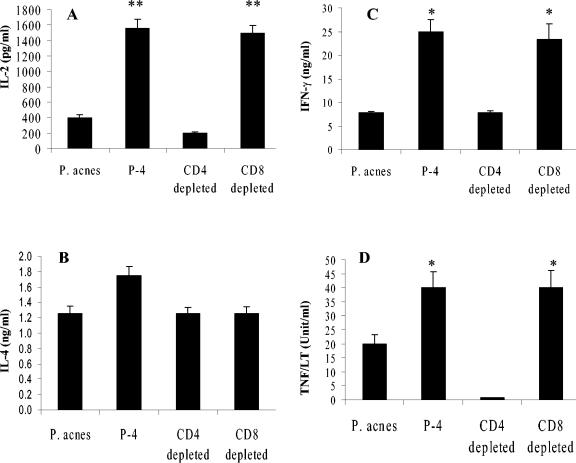
mRNA levels of cytokines in draining lymph nodes. Lymph node cells from control (P. acnes-immunized) and P-4-immunized mice were isolated after 4 weeks of infection and cultured for 72 h in the presence of P-4 antigen. mRNA expression in the cells for different cytokines of interest were determined by RNase protection assay. Intensity for each band (representing a cytokine) was measured by a densitometer. Data were normalized to the level of glyceraldehyde-3-phosphate dehydrogenase expressed in each sample. Each value represents the density of the cytokine relative to corresponding glyceraldehyde-3-phosphate dehydrogenase density, considered to be 100. These results are representative of three experiments.
Given the known role of TNF-α and IFN-γ in protection against leishmaniasis, the comparative levels of these cytokines produced in the vaccinated and control mice were determined; in addition, the levels of interleukin 2 (IL-2) and IL-4 produced were evaluated. After 4 weeks of infection, isolated splenic T cells (P-4 vaccinated, P-4 vaccinated and immunodepleted, and control) were restimulated with P-4 antigen, and cytokine levels were determined (Fig. 3). T cells from P-4-vaccinated mice produced significantly higher levels of IFN-γ and IL-2 in response to antigen (Fig. 3A and C). Both groups (Fig. 3B) produced comparable levels of IL-4. Interestingly, the depletion of CD4+ T cells but not CD8+ T cells resulted in the reduction of IL-2 and IFN-γ levels, suggesting that CD4+ T cells were primarily responsible for the enhanced levels of these cytokines in the protectively vaccinated mice. Further, lymphocytes from the P-4-vaccinated mice (and CD8-depleted P-4-vaccinated mice) secreted higher levels of lymphotoxin (LT)/TNF than those found for control groups of mice (Fig. 3D). In particular, vaccinated mice depleted of CD4+ T cells made negligible levels of LT/TNF, suggesting that these cells are critical for the induction and/or production of these cytokines. These results are consistent with RNase protection assay results, which indicated an enhanced mixture of IFN-γ, LTβ, TNF-β, and TNF-α produced in response to infection in the protectively vaccinated mice (Fig. 2).
FIG. 3.
Cytokine levels produced in response to infection in vaccinated and control mice. The IL-2 (A), IL-4 (B), IFN-γ (C), and TNF-α/LT (D) levels in spleen cell culture supernatants of control and P-4-vaccinated mice. Splenic T lymphocytes were isolated from different groups of mice after 4 weeks of infection and cultured with P-4 antigen as described previously (15). IL-2, IL-4, and IFN-γ levels in the supernatants were measured by enzyme-linked immunosorbent assay. Data shown are means of different cytokine levels in the supernatants from P-4 antigen-containing cultures in three duplicate wells. TNF/LT levels in the spleen culture supernatants were evaluated using a cytotoxicity assay employing MTT and the L929 cell line. The TNF/LT concentration required to achieve 50% cytotoxicity was considered 1 unit. These results are representative of three independent determinations. Student t test: *, P ≤ 0.01; **, P ≤ 0.001 (compared to adjuvant controls).
Overall, these results suggest that CD4+ T cells are primarily responsible for the enhanced levels of IFN-γ and TNF-α/LT produced in response to infection in the P-4-vaccinated mice. Interestingly, CD4+ T-cell depletion had no effect on the levels of IL-4 produced, although a reversal of protection was observed. Consequently, a reduction of the strong Th1-like response (rather than an increased TC2/Th2-like response) appears to be responsible for the lack of protection observed in the CD4-depleted P-4-vaccinated BALB/c mice.
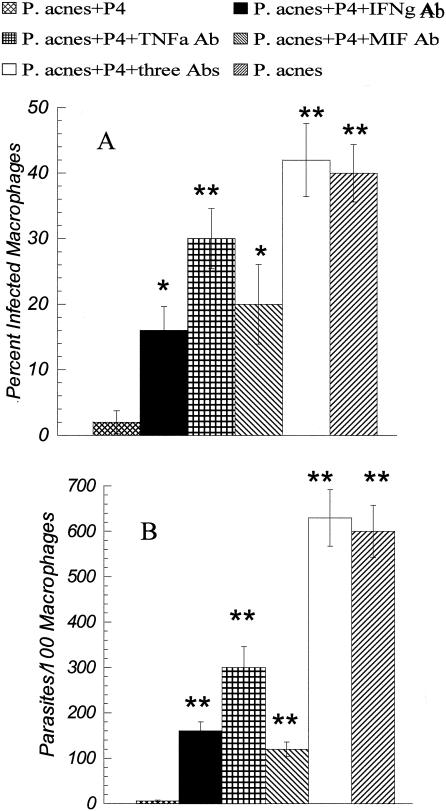
To determine the potential contribution of TNF-α/LT, IFN-γ, and MIF to the control of infection, the effect of specific antibody inhibition was examined in an in vitro macrophage-parasite cytolytic assay. As shown in Fig. 4, the number of parasites detected per 100 macrophages cultured with T cells from P-4-immunized mice was 100-fold less than that of those cultured with T cells from the P. acnes-immunized control mice. Addition of antibodies against either TNF-α/LT, IFN-γ, or MIF significantly increased parasite survival. Addition of the three antibodies simultaneously completely abrogated parasite killing.
FIG. 4.
Cytokine regulation of macrophage-mediated L. pifanoi killing. Shown are the results of macrophage-Leishmania killing assays. Isolated T cells from either P. acnes-vaccinated or P-4 plus P. acnes-vaccinated BALB/c mice were incubated together with L. pifanoi-infected peritoneal exudate macrophages. Antibodies (anti-IFN-γ, anti-TNF-α/LT, and/or anti-MIF) were added to the cultures, as indicated, to a final concentration of 10 μg/ml for each antibody. After 72 h, macrophages were washed, dried, fixed with methanol, and stained with 4′,6′-diamidino-2-phenylindole. The number of parasites per 100 macrophages and the percentage of infected macrophages were determined microscopically. Results are the averaged values of three independent experiments. Student t test: **, P ≤ 0.01; *, P ≤ 0.05 (compared to T cells from P-4-vaccinated mice); in A, P = 0.5, and in B, P = 0.1, when adjuvant control is compared with combination of anti-IFN-γ, anti-TNF-α/LT, and anti-MIF.
Interestingly, TNF/LT appeared to mediate the largest effect on macrophage-mediated parasite killing, since the greatest inhibition was found when anti-TNF antibody was present. TNF-α/LT and MIF have been shown to contribute to the control of cutaneous leishmanial infection caused by Leishmania major (5, 8, 9, 14, 17, 18). LT/TNF-α has been shown to be required for MIF-mediated killing of Leishmania major (5). A role for TNF/LT in the control of infection caused by Leishmania mexicana complex parasites is less clear (6, 13, 16), and both disease exacerbation and resolution have been reported. The role of MIF in New World leishmaniasis has not been previously examined. However, given the role of this cytokine in the host inflammatory response and Th1 T-cell induction (1, 3, 7), these results suggest that further investigation of the contribution of this cytokine to the healing response to New World leishmaniasis is warranted. Together these data indicate that the cytokines TNF-α/LT, IFN-γ, and MIF potentially contribute to the control of L. pifanoi (L. mexicana complex) infection in P-4-vaccinated mice.
The reason for the lack of a CD8 T-cell contribution to protection was not clear. Previous vaccine studies employing P. acnes as an adjuvant have resulted in the induction of effector CD4+ and CD8+ T cells (2, 10). Further, epitope scanning of the P-4 sequence (11, 12) indicated the existence of 10 Kd epitopes (nonamers) with scores of 18 to 24, suggesting that several H-2d major histocompatibility complex class I epitopes potentially exist within the P-4 protein. Consequently, the T-cell response (CD4 and CD8) of noninfected, P-4-vaccinated mice was further examined (Table 1). When T lymphocytes were restimulated with P-4 protein in vitro using irradiated spleen cells as antigen-presenting cells, both CD4+ and CD8+ cells were observed to proliferate. These levels were significantly above that found in control mice (vaccinated with P. acnes alone). Overall, in the P-4-vaccinated mice, there were 3.5- and 1.4-fold increases in the level of IFN-γ-producing CD4+ and CD8+ cells, respectively, over those in control mice (P. acnes vaccinated). However, among the responding BLAST cell populations, a higher percentage of memory/responding and IFN-γ-producing BLAST CD4+ T cells than CD8+ T cells was observed in response to P-4 antigen (Table 1). Notably, 85% of the IFN-γ-producing BLAST cells were CD4+ T cells. These results indicated that vaccination with P-4 antigen, together with P. acnes as an adjuvant, elicited antigen-specific CD8+ T cells. However, there was a predominant CD4+ T-cell response.
TABLE 1.
FACS analysis of T-cell responses of P-4-vaccinated micea
| Group | % CD4 cells with:
|
% CD8 cells with:
|
% CD4 IFN-γ cellsb with:
|
% CD8, IFN-γ cellsb with:
|
||||
|---|---|---|---|---|---|---|---|---|
| P. acnes | P-4 | P. acnes | P-4 | P. acnes | P-4 | P. acnes | P-4 | |
| In vitro T-cell response to purified P-4 Ag | 40.33 ± 0.71 | 67.43 ± 0.61 | 15.13 ± 0.31 | 21.20 ± 0.66 | 4.14 ± 0.15 | 16.50 ± 0.61* | 5.87 ± 0.15 | 10.07 ± 0.25 |
| In vitro T-cell response to L. pifanoi infected macrophages | 28.20 ± 0.56 | 34.73 ± 0.57 | 8.79 ± 0.52 | 7.01 ± 0.21 | 3.85 ± 0.22 | 11.87 ± 0.15* | 0.81 ± 0.05 | 1.21 ± 0.18 |
| Ear dermis T cells from infected mice stimulated with purified P-4 Ag | 13.00 ± 1.00 | 14.00 ± 0.80 | 12.67 ± 1.53 | 8.33 ± 1.26 | 3.59 ± 0.40 | 25.37 ± 0.67* | 16.20 ± 0.36 | 14.43 ± 0.61 |
Results from FACS analyses of the responding T-cell subpopulations from noninfected P-4-vaccinated or adjuvant control mice. Isolated T lymphocytes were stimulated for 72 h with purified P-4 antigen (Ag) in the presence of irradiated spleen cells as antigen-presenting cells or were cultured in the presence of L. pifanoi amastigote-infected starch-elicited PEC macrophages (1:10, macrophage:parasite ratio). Alternatively, cells from the site of infection were analyzed in P-4 thrice-vaccinated mice at 4 weeks postinfection. FACS analyses for cell surface markers and intercellular IFN were performed as previously described (2). All results are mean values ± standard errors obtained from groups of three mice. *, P ≤ 0.001, P-4 vaccinated mice in comparison to adjuvant (P. acnes) control mice, using a Student t test.
Cells producing IFN-γ.
This CD8+ T-cell response, although low, suggested that these cells could, in fact, potentially contribute to protection in the vaccinated mice. However, it was possible that P-4 protein-pulsed macrophages were not representative of antigen presentation during infection. Consequently, the level of P-4 presentation by peritoneal macrophages infected with L. pifanoi was examined. As shown in Table 1, the IFN-γ response of CD4+ T cells to infected macrophages was significantly higher for P-4-immunized mice than for control mice (11.9% versus 3.85%; 3.1-fold increase). In contrast, there was only a small increase in the percentage of IFN-γ-producing CD8+ T cells (0.81% for control versus 1.21% for P-4 vaccinated; 1.5-fold increase). These data suggest that the dominant T-cell response to P-4 antigen presentation to infected macrophages was a CD4+ T-cell response. These results are consistent with total IFN-γ production determined by enzyme-linked immunosorbent assay (Fig. 3) for the P-4-vaccinated mice, which was unchanged in the CD8-depleted mice. Further, the responding lymphocyte cell populations at the cutaneous site of infection were examined at 4 weeks postinfection. The percentage of CD4+ T cells producing IFN-γ was 25.8% in P-4-immunized mice, whereas 3.57% of the CD4+ T cells in control mice were producing IFN-γ; this represents a 7.2-fold increase in CD4+ IFN-γ-producing cells in the P-4-vaccinated mice. However, the levels of IFN-γ-producing CD8+ T cells were comparable for both groups of mice. These results are consistent with immunodepletion results and indicate a lack of enhanced CD8+ T-cell activity and contribution to parasite control at the site of infection of P-4-vaccinated mice. Therefore, it appears that although P-4 antigen-specific CD8+ T cells are elicited during vaccination, CD4+ T cells predominate and CD8+ T cells are not significantly activated/retained at the site of infection above levels found in control mice. Hence, the lack of CD8+ T-cell contribution to protection appears to reflect the overall CD4+/CD8+ proportions of P-4-specific T populations in the vaccinated mice.
In conclusion, in the current study, we have further characterized the immune response elicited by the P-4 antigen in L. pifanoi-infected BALB/c mice in terms of the involvement of CD4+/CD8+ T cells and the cytokine effectors. The protection mediated by P-4 is long lived and independent of CD8+ T cells and appears to involve the production of MIF and TNF/LT, as well as IFN-γ. Although P-4 antigen-specific CD8+ T cells are elicited in the vaccinated mice, a predominant CD4+ T-cell response is found and appears to control infection. This is the first demonstration of which we are aware of complete protection against cutaneous leishmaniasis in vaccinated mice being afforded by CD4 T cells alone. These observations suggest that CD8+ T cells may not always be critical in the maintenance of protection against New World cutaneous leishmaniasis.
Acknowledgments
We thank Cheryl Bergman for help with the TNF/LT analyses.
This work was supported by a grant from the National Institutes of Health, AI-27811.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bacher, M., C. N. Metz, T. Calandra, K. Mayer, J. Chesney, M. Lohoff, D. Gemsa, T. Donnelly, and R. Bucala. 1996. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc. Natl. Acad. Sci. USA 93:7849-7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colmenares, M., P. E. Kima, E. Samoff, L. Soong, and D. McMahon-Pratt. 2003. Perforin and gamma interferon are critical CD8+ T-cell-mediated responses in vaccine-induced immunity against Leishmania amazonensis infection. Infect. Immun. 71:3172-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denkinger, C. M., M. Denkinger, J. J. Kort, C. Metz, and T. G. Forsthuber. 2003. In vivo blockade of macrophage migration inhibitory factor ameliorates acute experimental autoimmune encephalomyelitis by impairing the homing of encephalitogenic T cells to the central nervous system. J. Immunol. 170:1274-1282. [DOI] [PubMed] [Google Scholar]
- 4.Jin, F. S., R. J. Youle, V. G. Johnson, J. Shiloach, R. Fass, D. L. Longo, and S. H. Bridges. 1991. Suppression of the immune response to immunotoxins with anti-CD4 monoclonal antibodies. J. Immunol. 146:1806-1811. [PubMed] [Google Scholar]
- 5.Juttner, S., J. Bernhagen, C. N. Metz, M. Rollinghoff, R. Bucala, and A. Gessner. 1998. Migration inhibitory factor induces killing of Leishmania major by macrophages: dependence on reactive nitrogen intermediates and endogenous TNF-alpha. J. Immunol. 161:2383-2390. [PubMed] [Google Scholar]
- 6.Khaskhely, N. M., M. Maruno, H. Uezato, A. Takamiyagi, S. T. Ramzi, K. M. Al-Kasem, K. Kariya, T. Toda, Y. Hashiguchi, E. A. Gomez Landires, and S. Nonaka. 2002. Low-dose UVB contributes to host resistance against Leishmania amazonensis infection in mice through induction of gamma interferon and tumor necrosis factor alpha cytokines. Clin. Diagn. Lab. Immunol. 9:677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koebernick, H., L. Grode, J. R. David, W. Rohde, M. S. Rolph, H. W. Mittrucker, and S. H. Kaufmann. 2002. Macrophage migration inhibitory factor (MIF) plays a pivotal role in immunity against Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 99:13681-13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liew, F. Y., C. Parkinson, S. Millott, A. Severn, and M. Carrier. 1990. Tumour necrosis factor (TNF alpha) in leishmaniasis. I. TNF alpha mediates host protection against cutaneous leishmaniasis. Immunology 69:570-573. [PMC free article] [PubMed] [Google Scholar]
- 9.Nashleanas, M., S. Kanaly, and P. Scott. 1998. Control of Leishmania major infection in mice lacking TNF receptors. J. Immunol. 160:5506-5513. [PubMed] [Google Scholar]
- 10.Rachamim, N., and C. L. Jaffe. 1993. Pure protein from Leishmania donovani protects mice against both cutaneous and visceral leishmaniasis. J. Immunol. 150:2322-2331. [PubMed] [Google Scholar]
- 11.Rammensee, H., J. Bachmann, N. P. Emmerich, O. A. Bachor, and S. Stevanovic. 1999. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213-219. [DOI] [PubMed] [Google Scholar]
- 12.Rammensee, H. G., K. Falk, and O. Rotzschke. 1993. Peptides naturally presented by MHC class I molecules. Annu. Rev. Immunol. 11:213-244. [DOI] [PubMed] [Google Scholar]
- 13.Satoskar, A., and J. Alexander. 1995. Sex-determined susceptibility and differential IFN-gamma and TNF-alpha mRNA expression in DBA/2 mice infected with Leishmania mexicana. Immunology 84:1-4. [PMC free article] [PubMed] [Google Scholar]
- 14.Satoskar, A. R., M. Bozza, M. Rodriguez Sosa, G. Lin, and J. R. David. 2001. Migration-inhibitory factor gene-deficient mice are susceptible to cutaneous Leishmania major infection. Infect. Immun. 69:906-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soong, L., S. M. Duboise, P. Kima, and D. McMahon-Pratt. 1995. Leishmania pifanoi amastigote antigens protect mice against cutaneous leishmaniasis. Infect. Immun. 63:3559-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soong, L., J. C. Xu, I. S. Grewal, P. Kima, J. Sun, B. J. Longley, Jr., N. H. Ruddle, D. McMahon-Pratt, and R. A. Flavell. 1996. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4:263-273. [DOI] [PubMed] [Google Scholar]
- 17.Wilhelm, P., U. Ritter, S. Labbow, N. Donhauser, M. Rollinghoff, C. Bogdan, and H. Korner. 2001. Rapidly fatal leishmaniasis in resistant C57BL/6 mice lacking TNF. J. Immunol. 166:4012-4019. [DOI] [PubMed] [Google Scholar]
- 18.Xu, D., S. J. McSorley, L. Tetley, S. Chatfield, G. Dougan, W. L. Chan, A. Satoskar, J. R. David, and F. Y. Liew. 1998. Protective effect on Leishmania major infection of migration inhibitory factor, TNF-alpha, and IFN-gamma administered orally via attenuated Salmonella typhimurium. J. Immunol. 160:1285-1289. [PubMed] [Google Scholar]