Abstract
We constructed infectious but replication-deficient Semliki Forest virus (SFV) particles carrying recombinant RNA encoding Brucella abortus Cu,Zn superoxide dismutase (SOD). The recombinant SFV particles (SFV-SOD particles) were then evaluated for their ability to induce a T-cell immune response and to protect BALB/c mice against a challenge with B. abortus 2308. Intraperitoneal injection of mice with recombinant SFV-SOD particles did not lead to the induction of SOD-specific antibodies, at least until week 6 after immunization (the end of the experiment). In vitro stimulation of splenocytes from the vaccinated mice with either recombinant Cu,Zn SOD (rSOD) or crude Brucella protein resulted in a T-cell proliferative response and the induction of gamma interferon secretion but not interleukin-4. In addition, the splenocytes exhibited significant levels of cytotoxic T-lymphocyte activity against Brucella-infected cells. The SFV-SOD particles, but not the control virus particles, induced a significant level of protection in BALB/c mice against challenge with B. abortus virulent strain 2308. These findings indicated that an SFV-based vector carrying the SOD gene has potential for use as a vaccine to induce resistance against B. abortus infections.
Brucella abortus is gram-negative, facultative intracellular bacterial pathogen that causes brucellosis in humans and cattle. In the infected host B. abortus multiplies within the phagosomes of reticuloendothelial cells, avoiding the killing effect of the macrophage cells by inhibiting phagosome-lysosome fusion (22). Like resistance to other facultative intracellular bacterial pathogens, resistance to B. abortus depends on acquired cell-mediated immunity (CMI) (51). In this respect, the development of the Th1 subset of CD4+ lymphocytes that secrete gamma interferon (IFN-γ), a crucial cytokine that up-regulates the macrophage anti-Brucella activity, and the development of CD8+ T lymphocytes that are able to lyse Brucella-infected cells (33, 34) are the two main components of the protective response. Live, attenuated vaccines, such as B. abortus strains S19 and RB51, that can stimulate a strong CMI response are usually very effective against brucellosis and have been used to control brucellosis in domestic animals. However, these vaccine preparations are far from ideal, since they have disadvantages; e.g., they are considered virulent or unsafe for human use, and they induce abortion in pregnant cattle (43). In the search for methods that can provide immunity against brucellosis, DNA-based vaccines have been shown to be effective for the delivery of antigenic proteins (3, 22) and to generate a strong cellular response (28). In previous reports, we showed that a DNA vaccine encoding the Cu,Zn superoxide dismutase (SOD) protein of B. abortus is able to induce cytotoxic T-lymphocyte (CTL) activity, high levels of IFN-γ production, and a significant level of protection against challenge with B. abortus virulent strain 2308 in BALB/c mice (30, 37). However, repeated doses and high concentrations of the plasmid containing the Cu,Zn SOD Brucella gene are needed to generate a strong response, probably because of the low in vivo transfection efficiency for this type of plasmid vector (15, 27, 50). Therefore, our recent efforts have been focused on improving the efficiency of the delivery of the SOD gene-based vaccine.
In this connection, self-replicating alphavirus-based expression systems have emerged as potent tools for delivering genetic vaccines, mainly because of their ability to infect cells, resulting in transfection efficiencies that are greater than those of conventional DNA vaccines (24, 39, 46, 51). Such vaccines are based on self-replicating genomes of single-stranded RNA viruses which can induce high levels of transient expression of the cloned genes in transfected cells (14). One such expression system based on Semliki Forest virus (SFV) has been found to be an excellent delivery vector for a wide range of eukaryotic cells and for inducing specific immune responses (8, 10, 23, 25, 29, 38). In the SFV vector system, the structural protein genes are replaced by a foreign gene that is expressed in vivo in a manner similar to viral structural protein genes (24). Thus, the RNA replicon contains the replicase gene and the subgenomic promoter, followed by the heterologous gene and the 5′ and 3′ sequences of the genome that are required for replication. Two approaches to immunize animals with the SFV vector can be used. In the first strategy, the naked RNA replicon that is produced through in vitro transcription can be employed directly for immunization (11). However, the main disadvantage of this approach is the short intracellular half-life of RNA because of its degradation by ubiquitous RNases (4, 49). In the second method, the RNA replicons are packaged into infectious vector particles by cotransfection of cultured cells (38). The advantage of employing this strategy is that SFV particles are generated in vitro from self-sufficient RNAs. The structural proteins required for the packaging of viral particles expressing the foreign protein are supplied in trans by a helper system consisting of two independent RNAs (42). The first RNA includes the capsid protein gene (pSFV-helper C), and a second vector encodes spike protein (pSFV-helper S). The viral replicase encoded by the recombinant vector amplifies all RNA species; however, only the replicon containing the replicase gene and the heterologous gene is packaged into viral particles (23). In this way, suicide particles undergo one round of infection without giving rise to production of new virus progeny because they contain a defective genome that is able to replicate but cannot result in a productive infection (42). After infection by viral particles, RNA replication allows expression of the foreign gene in infected cells, resulting in the induction of strong humoral and cell-mediated immunity in several disease models (6, 7, 12, 13, 32, 52). Moreover, SFV infection by itself induces production of IFN-γ, and infected cells undergo apoptotic death within a few days, which may be important in the molecular mechanisms involving cross-priming of professional antigen-presenting cells and generation of a CTL response (1, 2, 9, 18, 45, 52). Therefore, the objective of the present study was to evaluate the immune response and protective capacity elicited by immunization with SFV particles carrying the Brucella Cu,Zn SOD gene (SFV-SOD particles).
MATERIALS AND METHODS
Animals.
Seven- to eight-week-old female BALB/c mice (obtained from Instituto de Salud Publica, Santiago, Chile) were acclimated and randomly distributed into experimental groups. The mice were kept in conventional animal facilities and received water and food ad libitum.
Bacterial strains and cell line.
The virulent B. abortus strain 2308, the attenuated strain RB51 (41), and RB51-SOD, a strain that overexpresses SOD, were obtained from our culture collection; strains RB51 (41) and RB51-SOD (48) were originally obtained from the Virginia-Maryland Regional College of Veterinary Medicine (Virginia Polytechnic Institute and State University. Blacksburg). The bacterial cells were grown under aerobic conditions in tryptose soy broth (Difco Laboratories, Detroit, Mich.) for 72 h at 37°C. For inoculation, the bacterial suspensions were adjusted spectrophotometrically to an optical density at 600 nm corresponding to 1 × 104 CFU of B. abortus strain 2308. All experiments with live brucellae were performed in biosafety level 2 facilities. Escherichia coli strain DH5α (Life Technologies, Gaithersburg, Md.) was used for producing the necessary plasmid constructs. The E. coli bacteria were routinely grown at 37°C in Luria-Bertani broth or in agar supplemented, when required, with 100 μg of ampicillin per ml. The murine macrophage cell line J774.A1 (H-2d; ATCC TIB 67) was purchased from the American Type Culture Collection (Manassas, Va.). The cells were cultured in complete tissue culture medium (c-RPMI) consisting of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (GIBCO BRL), 2 mM l-glutamine, 100 μg of streptomycin/ml, and 100 IU of penicillin/ml. The COS-7 line (CRL 1651; American Type Culture Collection, Manassas, Va.) was grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% heat-inactivated fetal bovine serum.
Plasmid preparation.
The vectors pSFV4.2, pSFV helper-S (pSFVhS), and pSFV helper-CS219A (pSFVhC) were provided by Peter Liljeström (Karolinska Institute, Stockholm, Sweden). Recombinant plasmid pBAII-3, containing the gene for B. abortus Cu,Zn SOD (sodC) along with its promoter, was initially obtained from a pUC9 genomic library of B. abortus strain 2308 and cloned into pBluescript SK(−) (Stratagene, La Jolla, CA); the resulting plasmid is referred to below as pBSSOD (35). A 514-bp fragment containing only the sodC gene was PCR amplified from pBSSOD by using a primer pair designed based on the nucleotide sequence. (The forward primer was 5′-GGCCTTACGGATCCTCTGGAA-3′, and the reverse primer was 5′-TTATTCGCTCGAGCCGCAGGC-3′.) A restriction site was engineered into each primer by point mutation. The amplified gene fragment was digested with an appropriate restriction enzyme (BamHI/XhoI) and cloned into pSFV4.2 downstream of the viral replicase gene and under the control of the SP6 RNA polymerase promoter (24). The resulting plasmid was designated pSFV-SOD. The recombinant plasmid was electroporated into E. coli DH5α, and a single recombinant colony was selected. Isolation of plasmid DNA was performed using a Wizard Plus SV Minipreps kit (Promega, Madison, Wis.) according to the manufacturer's instructions. The pSFV-SOD plasmid construct was verified by restriction digestion and by sequencing of the complete insert at the Universidad de Concepción sequencing facility.
In vitro transcription and packaging of SFV particles.
Plasmids pSFV-SOD, pSFVhS, and pSFVhC were linearized by SpeI restriction enzyme digestion for 1 h at 37°C. In vitro transcription was performed using 45 U of SP6 RNA polymerase (Promega) per reaction mixture (50 μl). The DNA template was removed by digestion with RQ1 RNase-free DNase (Promega). The pSFV transcripts were capped during transcription using 5 mM Cap analogue (Promega). The RNA replicon was obtained by phenol-chloroform extraction, divided into aliquots, and stored at −80°C. Packaging of the recombinant RNA encoding the SOD protein into SFV particles was done using the two-helper RNA method (42). Briefly, COS-7 cells grown to 80% confluence in DMEM were cotransfected with the recombinant RNA replicon and two helper RNAs. After 24 h of incubation at 37°C with 5% CO2, the medium containing recombinant virus stocks was harvested, and viral particles were purified by ultracentrifugation through a sucrose gradient. The resulting particles were designated SFV-SOD. The control particles were made using plasmid pSFV3 containing the E. coli β-galactosidase sequence purchased from GIBCO BRL (pSFV-lacZ). The viral stock titer was maintained as described by Salminen et al. (40). Briefly, COS-7 cells grown on coverslips were infected with serial dilutions of the virus that was generated, and expression of the SOD protein was visualized by indirect immunofluorescence with a mouse anti-Brucella SOD monoclonal antibody prepared in our laboratory or mouse anti-E. coli β-galactosidase monoclonal antibody (Promega).
Analysis of Cu,Zn SOD protein expression from SFV particles.
COS-7 cells grown on coverslips to 80% confluence were infected with SFV-SOD particles or SFV-LacZ particles at a ratio of 5 infectious units/cell. Cells were cooled on ice and washed twice with ice-cold phosphate-buffered saline (PBS). Recombinant Semliki Forest virus diluted in DMEM was incubated with the cells for 2 h at 37°C to allow virus entry, followed by transfer to complete DMEM (supplemented with 0.5% fetal calf serum). For fluorescence microscopy, cells were cultivated for 24 h. After the medium was removed, the coverslips with the cells were gently rinsed with PBS and fixed for 30 min at room temperature with 0.4% freshly prepared paraformaldehyde in 250 mM HEPES, pH 7.4, 0.1 mM CaCl2, and 0.1 mM MgCl2 buffer. The cells were permeabilized for 30 min with PBS-0.05% Triton X-100 at room temperature and washed four times. Mouse anti-SOD monoclonal antibody was added for 2 h at 37°C (dilution, 1:200). After three washes with PBS-1% bovine serum albumin, the cells were incubated for 30 min with a 1:100 dilution of goat anti-mouse antiserum conjugated with fluorescein isothiocyanate in PBS-1% bovine serum albumin. The cells were washed extensively and embedded in Moviol solution (BBL, Cockeysville, MD). Microscopy and photography of the cells were performed with an AxioPlan2 Zeiss fluorescence microscope.
Immunization.
Mice were injected by the intraperitoneal route with 106 infectious units of recombinant SFV-SOD particles in 200 μl of PBS at zero time and week 3. As negative control, groups of mice were immunized with 106 SFV-LacZ particles or PBS alone. In protection experiments, a positive control group of mice was vaccinated intraperitoneally with 2 × 108 CFU of B. abortus strain RB51 in 0.2 ml of PBS.
Splenocyte cultures and lymphocyte proliferation.
Three weeks after the last immunization, five mice from each group were sacrificed, and their spleens were removed under aseptic conditions. Single-cell suspensions were prepared from the spleens according to a standard procedure (37), and erythrocytes were eliminated with ACK lysis solution (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA [pH 7.3]). Splenocytes from all five mice of each group were first pooled and then cultured at 37°C with 5% CO2 in a 96-well flat-bottom plate at a concentration of 4 × 105 viable cells/well in the presence of no additives (unstimulated control) or one of the following different stimulants: 0.4 μg of crude B. abortus RB51 proteins (CBPs), an extract obtained from bacteria subjected to treatment with a hypertonic salt solution and sonication as described previously (36); 0.04 μg of purified rSOD, obtained by a chromatography affinity procedure (35); or 0.25 μg of concanavalin A (ConA). Splenocytes were cultured in c-RPMI for 3 days and pulsed for 8 h with 0.35 μCi of thymidine (50 Ci/mmol; Amersham, London, United Kingdom) per well. After this, the radioactivity incorporated into the DNA was measured with a liquid scintillation counter. Cell proliferation was expressed as mean counts per minute obtained from triplicate cultures prepared from a cell pool of each experimental or control group composed of five mice.
Cytokine ELISA.
For detection of cytokines, supernatants of the pooled spleen cell cultures described above were collected after 48 h of antigen stimulation and tested for the presence of cytokines by an antigen capture enzyme-linked immunosorbent assay (ELISA) using OptEIA set mouse IFN-γ and interleukin 4 (IL-4) (BD Biosciences, San Diego, CA). All assays were performed in duplicate. The concentrations of IFN-γ and IL-4 in the culture supernatants were calculated by using a liner regression equation obtained from the absorbance values of the standards.
Cytotoxicity assay.
The cytotoxicity assay was carried out as described by He et al. (16). Briefly, stimulator cells were prepared by infecting J774.A1 macrophages at confluent growth with live B. abortus strain RB51-SOD at a ratio of 1:100 for 5 h. Extracellular bacteria were rinsed away with c-RPMI containing 50 μg of gentamicin per ml. Macrophages were scraped off with a sterile rubber policeman and centrifuged at 200 × g for 5 min. The pulsed macrophages were suspended in 5 ml of c-RPMI with 35 μg of mitomycin C per ml in a 37°C water bath for 45 min and then washed by centrifugation with RPMI supplemented with 5% heat-inactivated fetal bovine serum.
The immunized mice were killed by cervical dislocation 4 weeks after viral inoculation. The spleens were removed, and the erythrocytes were eliminated with the ACK lysis solution. Spleen cells from all five mice of each group were pooled and used for the effector cell preparation. Adherent cells were removed by incubation on a plastic dish, and then the enriched T-cell populations were distributed in 24-well cell culture plates (Corning, Corning, N.Y.) at a concentration of 4 × 106 viable cells/well. Stimulator cells were also added to the wells at a concentration of 0.4 × 106 cells/well. Then the mixture of enriched T cells and stimulator cells was incubated at 37°C with 5% CO2. After 5 days of incubation, the live effector cells were obtained by removing the dead cells with Histopaque-1083. Effector cells and target cells (B. abortus RB51-infected J774.A1 macrophages which were not treated with mitomycin C) were mixed at different ratios and incubated for 16 h at 37°C; 200 μl of a 0.036% neutral red solution in PBS was added to stain unlysed target cells. After 30 min, the cells were washed and then lysed with 0.22 ml of a 0.05 M acetic acid-0.05% sodium dodecyl sulfate solution. The amount of dye released was measured by obtaining readings for optical density at 570 nm. As a control for nonlysis and maximal uptake of the neutral red stain, target cells were cultured alone without effector cells. The percentage of specific lysis was established by using the following formula for specific lysis: percentage of specific lysis = (optical density of control − optical density of experimental group)/optical density of control × 100.
Protection experiments.
The protection experiments were performed as described previously (35). Briefly, 6 weeks after vaccination, six mice from each group were challenged by intraperitoneal injection of 104 CFU of B. abortus 2308. Two weeks later, the infected mice were sacrificed, their spleens were homogenized, and dilutions were plated to determine the number of Brucella CFU per spleen. Log10 units of protection were obtained by subtracting the mean log10 CFU for the experimental group from the mean log10 CFU for the corresponding control group.
Statistical analysis.
The data for the lymphocyte proliferation, detection of cytokine, and protection experiments were analyzed with Student's paired t test. The data for CTL lysis were subjected to an analysis of variance, and the means were compared by using Tukey's honestly significant difference procedure (SAS System for mixed models; SAS Institute Inc., Cary, N.C.).
RESULTS
Expression of the Brucella Cu,Zn SOD protein by SFV-SOD.
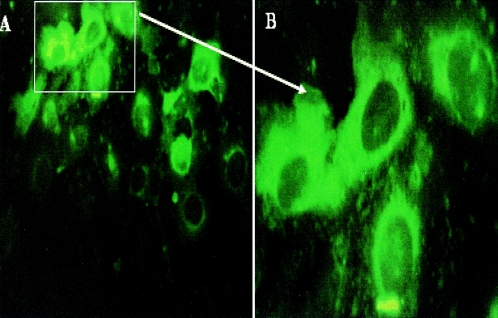
Expression of the Cu,Zn SOD protein was monitored in COS-7 cells infected with the recombinant Semliki Forest virus particles. As shown in Fig. 1, cytoplasm in cells infected with SFV-SOD particles showed a strong reaction with the monoclonal anti-SOD antibody, indicating high levels of expression of the Cu,Zn SOD protein. In contrast, cells infected with SFV-LacZ particles did not show any positive reaction with monoclonal anti-SOD antibody (data not shown).
FIG. 1.
Detection of SOD Brucella protein in COS-7 cells by immunofluorescence. COS-7 cells were infected with SFV-SOD recombinant particles and then cultured for 24 h. Subsequently, the cells were fixed and probed with anti-SOD monoclonal antibody. (A) Original magnification, ×400; (B) Original magnification, ×1,000.
Immune response of mice vaccinated with SFV-SOD.
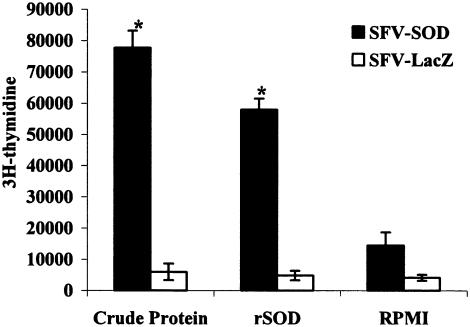
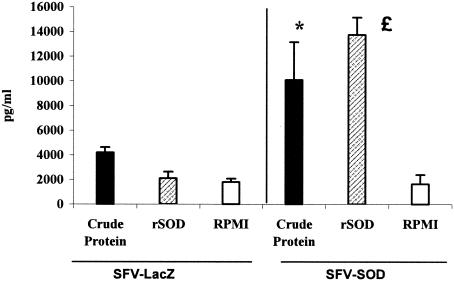
We have reported previously that SOD protein delivery from a DNA-based vaccine is able to induce proliferative capacity in T cells when they are confronted in vitro with SOD antigen (37). In order to evaluate the cell-mediated immunity to Brucella rSOD and CBPs, the proliferative responses and cytokine profiles of spleen cells from mice immunized with SFV-SOD and SFV-LacZ were determined. As shown in Fig. 2, 6 weeks after immunization, splenocytes from mice immunized with SFV-SOD exhibited a significant proliferative response to rSOD and CBPs (P < 0.003 for a comparison with mice immunized with saline alone). Only low levels of spontaneous proliferation occurred in cultures with no antigen stimulation (medium control). The splenocytes from mice in both groups had similar very high levels of proliferative responses to the mitogen ConA throughout the study (data not shown). With respect to the cytokine profile, at 6 weeks after immunization, the supernatants from cultures of spleen cells from SFV-SOD-vaccinated mice stimulated with rSOD or CBPs showed significantly higher levels of IFN-γ (P < 0.0002 and P < 0.01, respectively) than the supernatants from cultures of spleen cells from SFV-LacZ-vaccinated mice (Fig. 3). In addition, no IL-4 was detected in any of the culture supernatants of splenocytes stimulated with specific antigen of B. abortus (data not shown). Splenocytes from all groups of mice exhibited similar levels of IFN-γ and IL-4 production upon stimulation with ConA (data not shown). Specific anti-SOD antibody was not detected by ELISA in sera from mice immunized with SFV-SOD or SFV-LacZ (data not shown).
FIG. 2.
Lymphocyte proliferation assay. BALB/c mice were immunized with recombinant SFV-SOD particles or control recombinant SFV-LacZ particles. The T-cell proliferative response was measured 3 weeks after the last immunization. Splenocytes (4 × 105 cells per well) from five mice of each group were pooled and stimulated in vitro with CBPs (4 μg/ml) and purified rSOD (0.4 μg/ml) as the antigen. The data are representative of data from two separate experiments. Each bar indicates the average number of counts per minute for triplicate cultures of cells; the error bars indicate standard deviations. An asterisk indicates that the P value is <0.003 compared with the value for SFV-LacZ-immunized control mice.
FIG. 3.
Quantitative ELISA analysis of IFN-γ secreted by lymphocytes upon stimulation with different antigens. Splenocytes (4 × 105 cells per well) from five mice of each group were pooled and stimulated in vitro with CBPs (4 μg/ml), rSOD (0.4 μg/ml), or RPMI 1640 (negative control) for 48 h. The data are representative of the data from two separate experiments. Each bar indicates the geometric mean of the response, and the error bars indicate standard deviations. The asterisk and the pound sign indicate statistically significant differences compared to RPMI 1640 (P < 0.01 and P < 0.0002, respectively).
Inoculation with SFV-SOD particles induces a cytotoxic T-lymphocyte response in BALB/c mice.
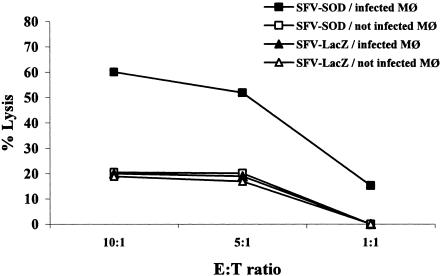
In order to evaluate if recombinant SFV-SOD particles were able to induce antigen-specific CTL, we quantified the ability of T cells from vaccinated or nonvaccinated control mice to specifically lyse target cells expressing endogenous Brucella antigen. To do this, effector T cells derived from mice inoculated with SFV-SOD particles were confronted with J774.A1 macrophages infected with B. abortus strain RB51-SOD or with SFV expressing E. coli β-galactosidase (SFV-LacZ) as a control. As shown in Fig. 4, purified T cells derived from mice inoculated with SFV-SOD specifically lysed strain RB51-SOD-infected J774.A1 macrophages. The lysis level was approximately 63% when the effector/target ratio was 10:1. The effector T cells derived from SFV-LacZ-inoculated mice produced low levels (∼20%) of lysis. When the same effector T cells were confronted with uninfected macrophages, the levels of lysis were approximately 15%. These results indicate that SFV-SOD particles are able to induce significant levels of specific cytotoxic T-cell activity.
FIG. 4.
Specific cytotoxic activity of total T cells. Six weeks after immunization, splenocytes from five mice immunized with SFV-SOD particles or the control SFV-LacZ particles were pooled and stimulated for 5 days with J774.A1 macrophages (Mφ) infected with B. abortus RB51-SOD at a ratio of cells to RB51-SOD of 1:100. These effector cells were incubated with J774.A1 cells alone or with J774.A1 cells infected with RB51-SOD. A neutral red uptake assay was used to measure target lysis. The data are representative of the data from two separate experiments and are means for triplicate estimates; the standard deviations did not exceed 20% of the means. E, effector cells; T, target cells.
SFV-SOD particles induce significant protection against virulent Brucella infection.
Protection experiments were carried out by challenging vaccinated and control mice by intraperitoneal injection of virulent B. abortus 2308, and the level of infection was evaluated by determining the numbers of CFU in their spleens. The results indicate that immunization with SFV-SOD resulted in a significant degree of protection (1.52-log increase in protection) compared to the degree of infection in saline-inoculated controls (P < 0.01). In comparison, vaccination with live B. abortus strain RB51 induced 1.9-log protection. No significant difference in the number of CFU was seen between groups injected with SFV-LacZ and groups injected with PBS (Table 1). These results indicate that the SFV-SOD vaccine induced a significant degree of protection against Brucella infection.
TABLE 1.
Protection of mice against challenge with B. abortus 2308 after immunization with RNA particles coding for Cu,Zn SODa
| Vaccine (dose) | Log10 CFU of B. abortus 2308 in spleen (mean ± SD) | Log10 units of protection |
|---|---|---|
| Saline control | 3.07 ± 0.45 | 0 |
| Live RB51 (2 × 108 CFU) | 1.17 ± 0.35b | 1.9 |
| SFV-SOD | 1.55 ± 0.84c | 1.52 |
| SFV-LacZ | 2.91 ± 0.3 | 0.06 |
Mice were challenged intraperitoneally with 104 CFU of strain 2308 2 weeks prior to sacrifice.
P < 0.005 compared to the control groups.
P < 0.01 compared to the control groups.
DISCUSSION
The Semliki Forest virus-based expression system has been shown to be a potent tool for production of genetic vaccines. The main advantage of this system is its greater transfection efficiency, which results in the delivery of the foreign proteins into a wide range of eukaryotic cells. Packaging of alphavirus replicons that contain exogenous genes into recombinant particles can be achieved very efficiently in culture systems (24). Entry and replication of recombinant SFV particles in cells result in shutting off host protein synthesis and in redirecting the cell's metabolism to express virus-encoded proteins. Previous studies have shown that an SFV vector can be used efficiently to induce cellular and humoral responses in a variety of animal models (14, 25, 39, 51). The recombinant SFV particles can be easily generated by cotransfection with independent RNAs. The virus particles produced are safer to use as vaccines than the DNA immunization procedure since they are not capable of causing a productive infection because of their defective genome (42). In vivo, the antigen expressed in the host cell must be processed and presented in a highly efficient way in order to generate the high levels of CTL activity and antibody response (53). For the induction of a cellular immune response, lymphoid cells must respond either to the cells that primarily contain the foreign protein or to antigen-presenting cells that have taken up and processed material from such cells (17).
In this study, we showed that injection of recombinant SFV particles containing the Cu,Zn superoxide dismutase gene from the bacterium B. abortus was able to generate a protective immune response in BALB/c mice. It is well documented that the cellular immune response plays a major role in the establishment of a protective response against Brucella (19, 20, 44, 51), and for this reason the design of a preventive vaccine against brucellosis must be based on its capacity to generate a strong CMI response. Our group has previously demonstrated that SOD protein delivered by different means is able to generate high levels of a protective immune response in BALB/c mice against challenge with B. abortus virulent strain 2308. The protective immune response always consisted of IFN-γ-secreting T cells and CD8+ T cells with cytotoxic activity against infected cells of the same haplotype (35, 37). A DNA vaccine carrying the sodC gene induces a strong T-cell cytotoxic activity and protection against B. abortus 2308 challenge (30). However, some unresolved difficulties related to integration of DNA plasmid into the host chromosome causing cell transformation (26, 31), tolerance to DNA vector (28), or generation of autoimmune disease (5, 47) and low efficiency in DNA transfection (15, 50) have limited the progress of DNA vaccines in clinical trials.
Induction of a T-cell immune response by immunization with SFV-SOD particles was evaluated by measuring the splenocyte proliferative response and the cytokine produced after in vitro stimulation of spleen cells with purified recombinant SOD or CBPs. Immunizations with SFV-SOD resulted in a strong Th1-type response with a high T-cell proliferative response, a high level IFN-γ, and no IL-4 detected in T-cell cultures from vaccinated mice. Moreover, the SFV-SOD vaccine also induced T cells with cytotoxic activity, whereas lymphocytes obtained from the immunized mice were able to lyse B. abortus RB51-SOD-infected macrophages. Cytotoxic T cells are probably necessary for elimination of an intracellular pathogen such as Brucella because of the bacterium's ability to proliferate in the host phagocytic cells and avoid the extracellular defense mechanisms (21). We failed to detect SOD-specific antibodies in the serum from mice vaccinated with SFV-SOD. This finding is similar to our previous results obtained with a DNA vaccine, which showed that mice vaccinated with plasmid DNA carrying the SOD gene (pcDNA-SOD) was able to exhibit a cellular immune response and a protective immune response in the absence of detectable levels of SOD-specific antibodies in the serum (30). These findings suggest that CMI, but not antibodies, to SOD is important in protective immunity against brucellosis.
Our protection studies demonstrated that immunization with SFV-SOD resulted in the generation of a high-level protective response whose level is similar to that conferred by immunization with live RB51 vaccine. Further studies are required to delineate the role of different T-cell types in the protection induced by vaccination with SFV-SOD particles and to examine its usefulness for veterinary and human applications.
Acknowledgments
This work was supported by grant 1010851 from Fondo Nacional de Investigación Cientifica y Tecnologica (FONDECYT), Santiago, Chile.
We are grateful to Ramesh Vemulapalli, Purdue University, for comments on the manuscript.
Editor: J. D. Clements
REFERENCES
- 1.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 2.Albert, M. L. S. F Pearce, L. M. Francisco, B. Sauter, P. Roy, and R. L. Silverstein. 1998. Immature dendritic cells phagocytose apoptotic cells via v/5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188:1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Mariri, A., A. Tibor, P. Mertens, X. De Bole, P. Michel, J. Godfroid, K. Walravens, and J.-J. Letesson. 2001. Induction of immune response in BALB/c mice with a DNA vaccine encoding bacterioferritin or P39 of Brucella spp. Infect. Immun. 69:6264-6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson, C., N. Vasconcelos, M. Sievertzon, D. Haddad, S. Liljeqvist, P. Berglund, P. Liljeström, M. Ahlborg, S. Ståhl, and K. Berzins. 2001. Comparative immunization study using RNA and DNA constructs encoding a part of the Plasmodium falciparum antigen Pf332. Scand. J. Immunol. 54:117-124. [DOI] [PubMed] [Google Scholar]
- 5.Beger, E., B. Deocharan, M. Edelman, B. Erblich, Y. Gu, and C. Putterman. 2002. A peptide DNA surrogate accelerates autoimmune manifestations and nephritis in lupus-prone mice. J. Immunol. 168:3617-3626. [DOI] [PubMed] [Google Scholar]
- 6.Bennett, S., F. Carbone, F. Karamalis, J. Miller, and W. Heath. 1997. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J. Exp. Med. 186:65-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berglund, P., M. N. Fleeton, C. Smerdou, and P. Liljestrom. 1999. Immunization with recombinant Semliki Forest virus induces protection against influenza challenge in mice. Vaccine 17:497-507. [DOI] [PubMed] [Google Scholar]
- 8.Berglund, P., M. Quesada-Rolander, P. Putkonen, G. Biberfeld, R. Thorstensson, and P. Liljestrom. 1997. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS Res. Hum. Retrovir. 13:1487-1495. [DOI] [PubMed] [Google Scholar]
- 9.Blackman, M. J., and A. G. Morris. 1984. γ-Interferon production and cytotoxicity of spleen cells from mice infected with Semliki Forest virus. J. Gen. Virol. 65:955-961. [DOI] [PubMed] [Google Scholar]
- 10.Chen, M., K.-F. Hu, B. Rozell, C. Orvell, B. Morein, and P. Liljeström. 2002. Vaccination with recombinant alphavirus or immune-stimulating complex antigen against respiratory syncytial virus. J. Immunol. 169:3208-3216. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, W. F., C. F. Hung, C. Y. Chai, K. F. Hsu, L. He, C. M. Rice, M. Ling, and T. C. Wu. 2001. Enhancement of Sindbis virus self-replicating RNA vaccine potency by linkage of Mycobacterium tuberculosis heat shock protein 70 gene to an antigen gene. J. Immunol. 166:6218-6226. [DOI] [PubMed] [Google Scholar]
- 12.Fleeton, M. N., B. J. Sheahan, E. A. Gould, G. J. Atkins, and P. Liljeström. 1999. Recombinant Semliki Forest virus particles encoding the prME or NS1 proteins of louping ill virus protect mice from lethal challenge. J. Gen. Virol. 80:1189-1198. [DOI] [PubMed] [Google Scholar]
- 13.Fleeton, M. N., P. Liljeström, B. Sheahan, and G. Atkins. 2000. Recombinant Semliki Forest virus particles expressing louping ill virus antigens induce a better protective response than plasmid-based DNA vaccines or an inactivated whole particle vaccine. J. Gen. Virol. 81:749-758. [DOI] [PubMed] [Google Scholar]
- 14.Frolov, I., T. A. Hoffman, B. M. Pragai, S. A. Dryga, H. V. Huang, S. Schlesinger, and C. M. Rice. 1996. Alphavirus-based expression vectors: strategies and applications. Proc. Natl. Acad. Sci. USA 93:11371-11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashida, M., R. Mahato, K. Kawabata, T. Miyao, M. Nishikawa, and Y. Takakura. 1996. Pharmacokinetics and targeted delivery of proteins and genes. J. Control. Release 41:91-97. [Google Scholar]
- 16.He, J., R. Vemulapalli, A. Zeytun, and G. G. Schurig. 2001. Induction of specific cytotoxic lymphocytes in mice vaccinated with Brucella abortus RB51. Infect. Immun. 69:5502-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, A. Y. C., P. Golumbek, M. Ahmadzadeh, E. Jaffee, D. Pardoll, and H. Levitsky. 1994. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science 264:961-965. [DOI] [PubMed] [Google Scholar]
- 18.Huckriede, A., L. Bungener, M. Holtrop, J. de Vries, B. L. Waarts, T. Daemen, and J. Wilschut. 2004. Induction of cytotoxic T lymphocyte activity by immunization with recombinant Semliki Forest virus: indications for cross-priming. Vaccine 22:1104-1113. [DOI] [PubMed] [Google Scholar]
- 19.Jiang, X., and, C. L., Baldwin. 1993. Effects of cytokines on intracellular growth of Brucella abortus. Infect. Immun. 61:124-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, S., and, A. J., Winter. 1992. Survival of virulent and attenuated strain of Brucella abortus in normal and gamma interferon-activated murine peritoneal macrophages. Infect. Immun. 60:3011-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko. J., and G. Splitter. 2003. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin. Microbiol. Rev. 16:65-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurar, E., and G. Splitter. 1997. Nucleic acid vaccination of Brucella abortus ribosomal L7/L12 gene elicits immune response. Vaccine 15:1851-1857. [DOI] [PubMed] [Google Scholar]
- 23.Liljeström, P. 1994. Alphavirus expresion system. Curr. Opin. Biotechnol. 5:495-500. [DOI] [PubMed] [Google Scholar]
- 24.Liljeström, P., and H. Garoff. 1991. A new generation of animal cell expression vectors based on Semliki Forest virus replicon. Bio/Technology 9:1356-1361. [DOI] [PubMed] [Google Scholar]
- 25.Lundstrom, K. 2003. Semliki Forest virus vectors for rapid and high-level expression of integral membrane proteins. Biochim. Biophys. Acta 1610:90-96. [DOI] [PubMed] [Google Scholar]
- 26.Martin, Y., S. E. Parker, R. Hedstrom, J. Norman, P. Hobart, and D. Lew. 1999. Plasmid DNA malaria vaccine: the potential for genomic integration after intramuscular injection. Hum. Gene Ther. 10:759-768. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery, D. L., B. Jeffrey, J. Ulmer, J. Donnelly, and M. A. Liu. 1997. DNA vaccines. Pharmacol. Ther. 74:195-205. [DOI] [PubMed] [Google Scholar]
- 28.Mor, G., G. Yamshchikov, M. Sedegah, M. Takeno, R. Wang, R. A. Houghten, S. Hoffman, and D. M. Klinman. 1996. Induction of neonatal tolerance by plasmid DNA vaccination of mice. J. Clin. Investig. 98:2700-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris-Downes, M., K. Phenix, J. Smyth, B. Sheahan, S. Lileqvist, D. Mooney, P. Liljeström, D. Todd, and G. Atkins. 2001. Semliki Forest virus-based vaccines: persistence, distribution and pathological analysis in two animal systems. Vaccine 19:1978-1988. [DOI] [PubMed] [Google Scholar]
- 30.Muñoz-Montesino, C., E. Andrews, R. Rivers, A. Gonzáles-Smith, G. Moraga-Cid, H. Folch, and A. Oñate. 2004. Intraspleen delivery of a DNA vaccine coding for superoxide dismutase (SOD) of Brucella abortus induces SOD-specific CD4+ and CD8+ T cells. Infect. Immun. 72:2081-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichols, W. W., B. J. Led, S. V. Manam, and P. J. Troilo. 1995. Potential DNA vaccine integration into host cell genome. Ann. N. Y. Acad. Sci. 772:30-39. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson, C., B. Makitalo, P. Berglund, F. Bex, P. Liljeström, G. Sutter, V. Erfle, P. ten Haaft, J. Heeney, G. Biberfeld, and R. Thorstensson. 2001. Enhanced simian immunodeficiency virus-specific immune responses in macaques induced by priming with recombinant Semliki Forest virus and boosting with modified vaccinia virus Ankara. Vaccine 19:3526-3536. [DOI] [PubMed] [Google Scholar]
- 33.Oliveira, S. C., and G. A. Splitter. 1996. Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine 14:959-962. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira, S. C., J. S. Harms, E. L. Rech, R. S. Rodarte, A. L. Bocca, A. M. Goes, and G. A. Splitter. 1998. The role of T cell subsets and cytokines in the regulation of an intracellular bacterial infection. Braz. J. Med. Biol. Res. 32:77-84. [DOI] [PubMed] [Google Scholar]
- 35.Oñate, A. A., R. Vemulapalli, E. Andrews, G. G. Schuring, S. Boyle, and H. Folch. 1999. Vaccination with live Escherichia coli expressing Brucella abortus Cu/Zn superoxide dismutase protects mice against virulent B. abortus. Infect. Immun. 67:986-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oñate, A., E. Andrews, A. Beltran, G. Eller, G. Schurig, and H. Folch. 2000. Frequent exposure of mice to crude Brucella abortus proteins down-regulates immune response. J. Vet. Med. 47:677-682. [DOI] [PubMed] [Google Scholar]
- 37.Oñate, A. A., S. Cespedes, A. Cabrera, R. Rivers, A. Gonzalez, C. Munoz, H. Folch, and E. Andrews. 2003. A DNA vaccine encoding Cu,Zn superoxide dismutase of Brucella abortus induces protective immunity in BALB/c mice. Infect. Immun. 71:4857-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polo, J. M., B. A. Belli, D. A. Driver, I. Frolov, S. Sherrill, M. J. Hariharan, K. Townsend, S. Perri, S. J. Mento, D. J. Jolly, S. M. W. Chang, S. Schlesinger, and T. W. Dubensky, Jr. 1999. Stable alphavirus packaging cell lines for Sindbis virus- and Semliki Forest virus-derived vectors. Proc. Natl. Acad. Sci. USA 96:4598-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pushko, P., M. Braya, G. V. Ludwiga, M. Parkera, A. Schmaljohna, A. Sanchez, P. B. Jahrlinga, and J. F. Smitha. 2001. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 19:142-153. [DOI] [PubMed] [Google Scholar]
- 40.Salminen, A., J. M. Wahlberg, M. Lobigs, P. Liljeström, and H. Garoff. 1992. Membrane fusion process of Semliki Forest virus. II. Cleavage-dependent reorganization of the spike protein complex controls virus entry. J. Cell Biol. 116:349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schurig, G. G., R. M. Roop, T. Bagchi, S. Boyle, D. Buhrman, and N. Sriranganathan. 1991. Biological properties of RB51, a stable rough strain of Brucella abortus. Vet. Microbiol. 28:171-188. [DOI] [PubMed] [Google Scholar]
- 42.Smerdou, C., and P. Liljeström. 1999. Two-helper RNA system for production of recombinant Semliki Forest virus particles. J. Virol. 73:1092-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith, L. D., and T. A. Ficht. 1990. Pathogenesis of Brucella. Crit. Rev. Microbiol. 17:209-230. [DOI] [PubMed] [Google Scholar]
- 44.Stevens, M. G., G. W. Pugh, and L. B. Tabatabai. 1992. Effects of gamma interferon and indomethacin in preventing Brucella abortus infection in mice. Infect. Immun. 60:4407-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Storni, T., F. Lechner, I. Erdmann, T. Bachi, A. Jegerlehner, T. Dumrese, T. M. Kundig, C. Ruedl, and M. F. Bachmann. 2002. Critical role for activation of antigen-presenting cells in priming of cytotoxic T cell responses after vaccination with virus-like particles. J. Immunol. 168:2880-2886. [DOI] [PubMed] [Google Scholar]
- 46.Strauss, J. H., and E. G. Strauss. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58:491-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsunoda, I., N. Tolley, L. Whitton, H. Kobayashi, and R. Fujinami. 1999. Exacerbation of viral and autoimmune animal models for multiple sclerosis by bacterial DNA. Brain Pathol. 9:481-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vemulapalli, R., S. Cravero, C. L. Calvert, T. E. Toth, N. Sriranganathan, S. M. Boyle, O. L. Rossetti, and G. G. Schurig. 2000. Characterization of specific immune responses of mice inoculated with recombinant vaccinia virus expressing an 18-kilodalton outer membrane protein of Brucella abortus. Clin. Diagn. Lab. Immunol. 7:114-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vignuzzi, M., S. Gerbaud, S. Van der Werf, and N. Escriou. 2001. Naked RNA immunization with replicons derived from poliovirus and Semliki Forest virus genomes for the generation of a cytotoxic T cell response against the influenza A virus nucleoprotein. J. Gen. Virol. 82:1737-1747. [DOI] [PubMed] [Google Scholar]
- 50.Wiethoff, C., and R. Middaugh. 2003. Barriers to nonviral gene delivery. J. Pharm. Sci. 92:203-217. [DOI] [PubMed] [Google Scholar]
- 51.Zhan, Y., and C. Cheers. 1993. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect. Immun. 61:4899-4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou, X., P. Berglund, G. Rhodes, S. E. Parker, M. Jondal, and P. Liljeström. 1994. Self-replicating Semliki Forest virus RNA as recombinant vaccine. Vaccine 12:1510-1514. [DOI] [PubMed] [Google Scholar]
- 53.Zhou, X., P. Berglund, H. Zhao, P. Liljeström, and M. Jondal. 1995. Generation of cytotoxic and humoral immune responses by nonreplicative recombinant Semliki Forest virus. Proc. Natl. Acad. Sci. USA 92:3009-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]