Abstract
The Actinobacillus actinomycetemcomitans afeABCD iron transport system, the expression of which is controlled by iron and Fur, was identified in three different isolates. The protein products of this locus are related to bacterial ABC transporters involved in metal transport. Transformation of the Escherichia coli 1017 iron acquisition mutant with a plasmid harboring afeABCD promoted cell growth under iron-chelated conditions. However, insertion disruption of each of the afeABCD coding regions abolished this growth-relieving effect. The replacement of the parental afeA allele with the derivative afeA::EZ::TN<KAN-2> drastically reduced the ability of A. actinomycetemcomitans cells to grow under iron-chelated conditions.
Actinobacillus actinomycetemcomitans has been associated with adult periodontitis (31) and localized juvenile periodontitis, recently renamed localized aggressive periodontitis (LAP) (3, 4). A. actinomycetemcomitans acquires iron from compounds found in the human host, such as hemoglobin and hemin (20), or synthetic chelators (17, 30). The strains tested neither used human lactoferrin or transferrin (20) nor produced siderophores (30). Thus, this pathogen seems to acquire iron through a periplasmic binding protein-dependent transport (PBT) system that functions independently of the TonB-ExbBD energy transducing system and requires neither an outer membrane receptor nor a specific ligand. The Serratia marcescens SfuABC PBT system was the first described (1, 2), and similar systems have been found in other bacteria, including the A. actinomycetemcomitans afuA-Aa system described previously (29). Another type of PBT system is that encoded by the Yersinia pestis yfe locus that contains the yfeABCD and yfeE coding regions (7). Although functionally and organizationally related, YfeABCD is an independent system that shows no significant similarity with the SfuABC-related systems. Here, we report the characterization of an A. actinomycetemcomitans iron acquisition system related to the YfeABCD ABC transporter, which was named AfeABCD to distinguish it from the Y. pestis system and to be consistent with the naming of the AfuABC system already described (29).
Cloning and analysis of the afeABCD iron acquisition system.
A 3,473-bp segment containing four open reading frames (ORFs), the products of which are highly similar to the Y. pestis YfeA, -B, -C, and -D proteins, respectively, was identified by computer analysis of the genome of A. actinomycetemcomitans HK1651, a serotype b strain of the JP2 clone that expresses high leukotoxin activity (19). PCR and reverse transcription (RT)-PCR analyses of total DNA and RNA with the primers listed in Table 2 showed that the serotype f CU1000 and serotype a DF2200N strains, both of which are not JP2-like isolates (22), also contain and express transcriptionally these four ORFs when cultured in AAGM broth (15) containing 100 μM 2,2′-dipyridyl (DIP) (iron-chelated condition). We focused our work on the two latter strains because they are rough, aggregate, and adhere tenaciously to solid surfaces, properties that must be expressed to reproduce the human LAP symptoms in a rat experimental model (27).
TABLE 2.
Oligonucleotide primers used in this work
| Oligonucleotide | Sequence |
|---|---|
| Primers used for RT-PCR | |
| 2546 | 5′-CCAACAATATTCCGGTCG-3′ |
| 2534 | 5′-ACCGGAAATTGCCAGTCC-3′ |
| 1687 | 5′-TGGGGTTAATTACACCGC-3′ |
| 2535 | 5′-ACTACGGCAATAAGGACC-3′ |
| 1689 | 5′-ATCCATTACCAAACTCCG-3′ |
| 2536 | 5′-ATAGAACAAACCAAGCCC-3′ |
| Primers used to clone fur in pACYC184 | |
| 2660 | 5′-CATGCCATGGGCAATTTTCGGTTGTGG-3′ |
| 2661 | 5′-CATGCCATGGTATAGGCGAAAAGTGCGG-3′ |
| Primers used to clone fur in pET100/D-TOPO | |
| 2461 | 5′-CACCATGTCTGAAGAAAA-3′ |
| 2462 | 5′-TATAGGCGAAAAGTGCGG-3′ |
| Primers used to clone afeABCD operon | |
| 2459 | 5′-TTATTTCCCGATGGTCCG-3′ |
| 2460 | 5′-TTTCACTTGGCAACAGGC-3′ |
| Primers used to clone afe promoter region | |
| 2519 | 5′-GCTCTAGAGCCTGCCATTCGTATGTTGG-3′ |
| 2520 | 5′-CCCAAGCTTGGGACTGCTTAAGCTTAACGC-3′ |
| Primers used for 5′ RACEa | |
| 1686 | 5′-GACCGGAATATTGTTGGC-3′ |
| 2518 | 5′-TTCAACGCAAATAGAGGC-3′ |
| 2533 | 5′-TCAAACCAACGCTCAAGG-3′ |
RACE, rapid amplification of cDNA ends.
The cognate CU1000 genome fragment was PCR amplified with Pfu DNA polymerase, total DNA, and the primers listed in Table 2; cloned into pCR-BluntII-TOPO; and sequenced. Computer analysis of the nucleotide sequence of the amplicon cloned in pMU375 and pMU376 (Table 1), two independent clones with identical sequences, showed the presence of four ORFs (Fig. 1A). The nucleotide sequence of this CU1000 genomic region has 99% identity with the HK1651 equivalent region, with none of the nucleotide differences affecting the length of the four predicted HK1651 and CU1000 ORFs. RT-PCR analysis of CU1000 RNA isolated from cells cultured under iron-chelated conditions and the appropriate primers (Fig. 1A and Table 2) showed that afeABCD is a polycistronic locus. The cognate amplicons were obtained when either HK1651 or DF2200N RNA was used as a template in the RT-PCR assays.
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Strains | ||
| A. actinomycetemcomitans | ||
| HK1651 | Clinical isolate, genome project | ATCCb |
| CU1000 | Clinical isolate, rough phenotype | 13 |
| CU1060 | Smooth derivative of CU1000 | 13 |
| DF2200N | Clinical isolate, rough phenotype | D. Fine |
| MB1237 | Artificially inducible competent derivative of DF2200N; Cmr | D. Figurski |
| MB1237-MU1 | MB1237 with afeA::EZ::TN<KAN-2>; Cms Kanr | This work |
| E. coli | ||
| DH5α | Used for recombinant DNA methods | Gibco-BRL |
| BN4020 | fur::Tn5 derivative of BN402 | 5 |
| BN4020-T7 | BN4020 harboring the λDE3 phage with T7 RNA polymerase under lacUV5 control; used to overexpress and purify CU1000 Fur protein | This work |
| RRJC1 | BN4020 with fhuF-λplacMu53; used to clone and test expression of fur genes | 28 |
| 1017 | ent::Tn5 derivative of HB101; Kmr; enterobactin production deficient | 11 |
| MB 247 | Host strain containing cloning vector pMB78; Ampr | D. Figurski |
| Plasmids | ||
| pCR-Blunt II-TOPO | Used to clone amplicons; Kmr Zeor | Invitrogen |
| pACYC184 | ColE1-derived cloning vector; Cmr Tetr | 10 |
| pKK232-8 | Promoter cloning vector; Ampr | Amersham |
| pET100/D-TOPO | Directional TOPO expression vector; Ampr | Invitrogen |
| pMB78 | USS-containing cloning vector; Ampr; suicide vector used for allelic exchange | D. Figurski |
| pMU375 | CU1000 afeABCD amplicon cloned in pCR-Blunt II-TOPO | This work |
| pMU376 | CU1000 afeABCD amplicon cloned in pCR-Blunt II-TOPO | This work |
| pMU402 | CU1000 afeABCD cloned in pACYC184 | This work |
| pMU404 | CU1000 afe promoter cloned in pKK232-8; Ampr Cmr | This work |
| pMU428 | CU1000 fur gene cloned in pET100/D-TOPO; Ampr | This work |
| pMU433 | CU1000 fur gene cloned in pACYC184; cloned as NcoI fragment; Cms Tetr | This work |
| pMU461 | pMU402 with EZ::TN<KAN-2> insertion in afeA; Tetr Kmr | This work |
| pMU509 | pMU402 with EZ::TN<KAN-2> insertion in afeB; Tetr Kmr | This work |
| pMU510 | pMU402 with EZ::TN<KAN-2> insertion in afeC; Tetr Kmr | This work |
| pMU511 | pMU402 with EZ::TN<KAN-2> insertion in afeD; Tetr Kmr | This work |
| pMU467 | Insert of pMU461 cloned in pMB78, a pUC18 derivative that does not replicate in A. actinomycetemcomitans; Ampr Kmr | This work |
| pMU500 | EcoRI-NcoI deletion of pACYC184; Cms Tetr | This work |
Amp; ampicillin; Cm, chloramphenicol; Kan, kanamycin; Tet, tetracycline; Zeo, zeocin; r, resistance/resistant; s, sensitive.
ATCC, American Type Culture Collection.
FIG. 1.
Computer analysis of the A. actinomycetemcomitans CU1000 genomic region containing the afeABCD locus. (A) The four large horizontal arrows indicate the location and direction of transcription of each predicted ORF. The short vertical arrows identify the locations of the insertions of EZ::TN<KAN-2> obtained by in vitro transposition mutagenesis (Epicentre). The short black horizontal arrows identify the locations of the primers used to test the expression of each ORF by RT-PCR. (B) Transcriptional and translational elements controlling the expression of afeABCD. The right and left black horizontal arrows identify the two first and two last codons of afeA and the ORF located upstream of it, respectively. The afe transcription initiation site and the direction of transcription are indicated by the large and bold characters and the arrow, respectively. The predicted matching −10 and −35 promoter regions are also shown. The grey rectangle identifies a predicted Fur box. RBS, ribosome binding site.
The product of ORF1 is related to the Haemophilus influenzae Rd KW20 HI0362 protein (14), which was annotated as the YfeA iron periplasmic binding protein. AfeA contains domains found in bacterial periplasmic metal-binding proteins including the Y. pestis YfeA protein (8), with which AfeA shows 66.5% identity. The production of AfeA is supported by our previous work (16) showing that A. actinomycetemcomitans Y4 cells produce a heme- and iron-regulated periplasmic protein that is immunologically related to H. influenzae YfeA. The latter protein was originally described as a 31-kDa iron-regulated periplasmic protein (18) that most likely represents the H. influenzae Rd KW20 HI0362 protein (14). ORF2 codes for a protein with the highest similarity to H. influenzae Rd KW20 HI0361 (14), which was annotated as the YfeB ATP-binding iron transport protein. AfeB is a predicted cytoplasmic protein with 65.6% identity to Y. pestis YfeB, and it contains Walker A and B ATP-binding motifs found in ABC metal transport proteins (21). The products of ORF3 and ORF4 have the highest similarity to the Pasteurella multocida Pm70 YfeC and YfeD proteins, respectively (24), and contain motifs found in the permease components of ABC-type metal transporters. Accordingly, the AfeC and AfeD proteins are putative integral cytoplasmic membrane proteins with transmembrane helices that contain the EAA motif, which is conserved among the cytoplasmic membrane permeases of ABC transporters (26). In addition to sharing conserved domains, AfeC and AfeD proteins have 67.3% and 57.9% identity with the cognate Y. pestis permeases.
Functional role of the CU1000 AfeABCD transport system in a heterologous bacterial host.
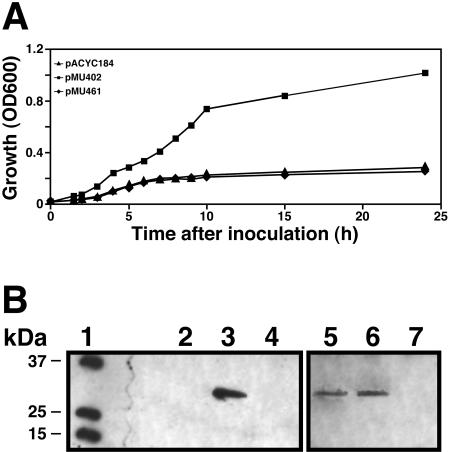
Transformation of the Escherichia coli 1017 enterobactin-deficient strain with pMU402, a pACYC184 derivative harboring the CU1000 afeABCD locus (Table 1), significantly increased the growth of this strain in nutrient broth (NB) containing 25 μM DIP (Fig. 2A). In contrast, the empty cloning vector pACYC184 and pMU461, a pMU402 derivative with an EZ::TN<KAN-2> insertion in afeA, only very poorly enhanced cell growth under iron-chelated conditions (Fig. 2A). A similar defect was observed with E. coli 1017 cells harboring either pMU509, pMU510, or pMU511 (Table 1), in which EZ::TN<KAN-2> disrupted afeB, afeC, and afeD, respectively (data not shown). Immunoblot analysis showed that a 32-kDa protein related to the H. influenzae YfeA protein could be detected in E. coli 1017 cells harboring an intact afeA ORF but not in cells in which the transposon disrupted this coding region (Fig. 2B, lanes 3 and 4, respectively). Supplementation of chelated NB with 100 μM FeCl3 rescued the growth of E. coli 1017 cells harboring pACYC184, pMU402, or pMU461. In contrast, no rescue effect was observed when chelated NB was supplemented with 100 μM ZnCl2 or MnCl2 (data not shown).
FIG. 2.
Growth and expression of AfeA in E. coli 1017 cells harboring different plasmid constructs. (A) The growth of E. coli 1017 cells harboring either the cloning vector pACYC184, pMU404 (afeABCD cloned in pACYC184), or pMU461 (pMU402 with afeA::EZ::TN<KAN-2>) in NB containing 25 μM DIP at 37°C in a rotary shaker was monitored for 24 h. OD600, optical density at 600 nm. (B) Immunoblot analysis of total lysates prepared from E. coli 1017 harboring pACYC184 (lane 2), pMU402 (lane 3), or pMU461 (lane 4); the A. actinomycetemcomitans parental strains DF2200N (lane 5) and MB1237 (lane 6); and the MB1237-MU1 insertion derivative (lane 7). The blotted proteins were probed with anti-H. influenzae YfeA (FimA-like) serum prepared as previously described (16). Lane 1, molecular weight markers.
Phenotype of an A. actinomycetemcomitans isogenic afeA insertion derivative.
Transformation of A. actinomycetemcomitans MB1237, a derivative of DF2200N that harbors the plasmid pMB7 encoding isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible competence functions (M. K. Bhattacharjee, B. A. Perez, S. C. Kachlany, and D. H. Figurski, unpublished data), with pMU467 (Table 1) resulted in the isolation of Kmr transformants. The presence of a 4.2-kbp HindIII-NcoI fragment in MB1237-MU1 but its absence in MB1237 chromosomal DNA (Fig. 3A, lanes 3 and 4) was detected with the aph probe. The same fragment was detected when the MB1237-MU1 DNA was hybridized with the afeA probe (Fig. 3B, lane 4). In contrast, a 1.2-kbp shorter HindIII-NcoI fragment was detected in the DNA of the MB1237 parental strain (Fig. 3B, lane 3), a difference that is consistent with the generation of the afeA::EZ::TN<KAN-2> derivative by allelic exchange. RT-PCR analysis of RNAs isolated from MB1237 and MB1237-MU1 showed that the ORFs located downstream of the transposon insertion site were transcribed as in the parental strain (data not shown), indicating that EZ::TN<KAN-2> did not cause transcriptional polar effects.
FIG. 3.
Analysis of the A. actinomycetemcomitans parental strains and an isogenic insertion derivative. (A and B) Southern blot analysis of total DNA isolated from A. actinomycetemcomitans MB1237 and its insertion derivative MB1237-MU1. HindIII-digested λ DNA (lane 1) and total DNAs from MB1237 (lane 3) and MB1237-MU1 (lane 4) digested with HindIII and NcoI were blotted onto nitrocellulose and probed with radiolabeled aph (A) or afeA (B). Both blots were also probed with λ DNA. Amplicons of aph (A, lane 2) and afeA (B, lane 2) were used as positive controls. (C and D) Growth of the parental strains DF2200N and MB1237 and the insertion derivative MB1237-MU1 on AAGM agar (C) and AAGM agar supplemented with 175 μM DIP (D). Bacterial growth was observed after 48 h of incubation in 5% CO2 at 37°C.
The addition of 175 μM DIP to AAGM agar reduced drastically the growth of MB1237-MU1 while the parental strains DF2200N and MB1237 each grew as a dense lawn similar to that produced by these strains in the absence of DIP (compare the cognate samples in Fig. 3C and D). Overall, the two parental strains grew on plates containing DIP up to 300 μM, while MB1237-MU1 did not grow in AAGM containing 200 μM DIP (data not shown). The strains DF2200N and MB1237 grew around filter disks containing either heme or FeCl3 when plated on AAGM containing 325 μM DIP. These two strains did not show any detectable growth when the filter disks were saturated with human transferrin, human lactoferrin, or hemoglobin. The same response was detected when MB1237-MU1 cells were plated on AAGM agar containing 200 μM DIP and provided with the same iron sources. No effect was observed when the filter disks were saturated with 100 μM ZnCl2 or MnCl2 (data not shown). Immunoblot analysis with anti-H. influenzae YfeA antibodies showed that a 32-kDa protein was present in the cell lysates of the DF2200N and MB1237 (Fig. 2B, lanes 5 and 6) parental strains but not in the cell lysate of the afeA insertion derivative MB1237-MU1 (Fig. 2B, lane 7).
Molecular and functional analyses of the afe promoter region.
The afe transcription initiation site was mapped to the C located 47 nucleotides upstream of the afeA initiation codon (Fig. 1B) using total RNA isolated from cells cultured under iron-chelated conditions, the 5′ rapid amplification of cDNA ends system (Invitrogen), and the primers listed in Table 2. However, the G located at position −48 could not be excluded as the initiation site due to the C tailing of the cDNA. Transformation of E. coli DH5α with pMU404, a derivative of pKK232-8 (Table 1) in which this intergenic region was cloned upstream of the promoterless cat gene in the orientation shown in Fig. 1B, resulted in the isolation of ampicillin- and chloramphenicol-resistant colonies.
Figure 4 shows that the addition of increasing concentrations of CU1000 Fur to a mixture containing a 32P-labeled fragment harboring the afeA promoter region decreased the mobility of the probe (lanes 1 to 5), with no free probe being detected in the presence of 250 ng of Fur (lane 5). Addition of increasing amounts of unlabeled probe to reaction mixtures containing either 100 ng (lanes 6 to 8) or 250 ng (lanes 9 to 11) of Fur resulted in the gradual disappearance of probe-Fur complexes with a concomitant increase in the amount of free probe. The increase in Fur concentration also resulted in the formation of complexes that displayed increasing sizes, an observation consistent with the formation of different Fur-DNA complexes at different Fur-probe ratios (6).
FIG. 4.
Binding of Fur to the afe promoter region determined by DNA gel mobility shift assays. An end-labeled fragment containing the afe promoter (P) region was incubated with either 0 ng (lane 1), 25 ng (lane 2), 50 ng (lane 3), 100 ng (lane 4), or 250 ng (lane 5) of purified Fur. Competition assays were done using 100 ng (lanes 6 to 8) or 250 ng (lanes 9 to 11) of Fur and either 100 ng (lanes 6 and 9), 250 ng (lanes 7 and 10), or 500 ng (lanes 8 and 11) of unlabeled DNA probe. Fur was overexpressed in E. coli BN4020-T7 harboring pMU428 and purified by affinity chromatography. The gel mobility shift assays were done using the conditions described before (25).
The iron and Fur regulation of transcription initiation from the afe promoter was tested by measuring chloramphenicol acetyltransferase (CAT) production in E. coli BN4020 (fur) harboring the promoter reporter construct pMU404 (Table 1). Figure 5A shows a representative immunoblot of total lysates prepared from BN4020 cells harboring pMU404 together with either pMU500, a pACYC184 derivative with no fur, or pMU433, a pACYC184 derivative harboring the CU1000 fur gene (Table 1). Densitometry of the signals produced by undiluted samples (samples 1 and 2) showed that BN4020 cells harboring pMU404 and pMU500 produced more CAT (A and C, sample 1) than cells harboring pMU404 and pMU433 (A and C, sample 2) when both cell samples were cultured in the presence of 100 μM FeCl3. In contrast, more CAT was produced in BN4020 cells harboring pMU404 and pMU433 (B and D, samples 2) than in cells containing pMU404 and pMU500 (B and D, sample 1) when cultured in the presence of 100 μM DIP. Analysis of 1:2 and 7:10 dilutions produced results similar to those obtained with the undiluted cell lysates (Fig. 5). The addition of 50 μM heme to the culture medium produced a regulatory effect similar to that observed with the addition of FeCl3 (data not shown).
FIG. 5.
Expression analysis of the afe promoter region. (A and B) Total lysates prepared from approximately the same amount of E. coli BN4020 cells harboring the cat reporter construct pMU404 together with either the empty cloning vector pMU500 (samples 1, 3, and 5) or the plasmid pMU433 that harbors the CU1000 fur gene (samples 2, 4, and 6) were size fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed with anti-CAT serum. Bacterial cells were cultured under either iron-rich (A) or iron-chelated (B) conditions and the same volumes of undiluted lysates (samples 1 and 2) and 7:10 (samples 3 and 4) and 1:2 (samples 5 and 6) dilutions of the lysates were loaded in a 12.5% polyacrylamide gel. The antigen-antibody complexes were detected with horseradish peroxidase-labeled protein A and chemiluminescence on an X-ray film. (C and D) Densitometry of the signals shown in panels A and B, respectively. IDV, integrated density value = Σ (each pixel value − background).
Conclusions.
The AfeABCD system is present and expressed in three different serotype strains of A. actinomycetemcomitans, two of which do not belong to the JP2 clone. The expression of this metal transporter, which is regulated by FeCl3 and heme, in either an isogenic or a nonisogenic background relieves the cell growth inhibition imposed by iron-chelated conditions. It is apparent that, under the conditions used in this work, AfeABCD transports Fe3+ but not other divalent metals, unlike the YfeABCD (7) and SitABCD (9, 23) related systems. Taken together, these observations suggest that AfeABCD could play a role in the pathogenesis of LAP by allowing A. actinomycetemcomitans to prosper under the iron-limiting conditions of the human oral cavity, imposed mainly by the apo-lactoferrin present in saliva (12). While it is evident that AfeABCD is required for iron acquisition under the laboratory conditions we used, it is possible that other systems such as AfuABC, either by itself or in conjunction with AfeABCD, could be involved in iron acquisition during the different stages of LAP. Furthermore, computer analysis of the HK1651 genome showed the presence of at least eight genes or gene clusters coding for putative iron transport functions, including two afuABC-like gene clusters. This redundancy in genes coding for iron acquisition functions most likely underscores the biological importance of this metal and the possibility that these systems are differentially expressed during the colonization and infection of the human host as it has been described for other bacterial pathogens. These possibilities can be tested now that a representative animal model (27) and the genetic methods to obtain isogenic derivatives of rough strains have been developed.
Nucleotide sequence accession number.
The nucleotide sequence of the A. actinomycetemcomitans CU1000 genomic region described in this work was deposited in GenBank under the accession number AY762615.
Acknowledgments
Miami University research funds and Public Health Service grant DE13657-02 supported the research work presented in this report.
We are grateful to D. Dyer, University of Oklahoma Health Science Center, for making available the nucleotide sequence of the A. actinomycetemcomitans HK 1651 genome (http://www.stdgen.lanl.gov/oralgen/). We also thank D. Figurski (College of Physicians and Surgeons of Columbia University) and D. Fine and S. Kachlany (University of Medicine and Dentistry of New Jersey) for giving us the A. actinomycetemcomitans strains and plasmid constructs used in this work. We thank C. Wood, coordinator of the Miami University Center of Bioinformatics and Functional Genomics, for support and assistance with automated DNA sequencing and nucleotide sequence analysis.
Editor: F. C. Fang
REFERENCES
- 1.Angerer, A., S. Gaisser, and V. Braun. 1990. Nucleotide sequence of sfuA, sfuB, and sfuC genes of Serratia marcescens. J. Bacteriol. 172:572-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angerer, A., B. Klupp, and V. Braun. 1992. Iron transport systems of Serratia marcescens. J. Bacteriol. 174:1378-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo, M. 2002. Localized juvenile periodontitis or localized aggressive periodontitis. J. Mass. Dent. Soc. 51:14-18. [PubMed] [Google Scholar]
- 4.Armitage, G. C. 1999. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 4:1-6. [DOI] [PubMed] [Google Scholar]
- 5.Bagg, A., and J. Neilands. 1985. Mapping of a mutation affecting regulation of iron uptake systems in Escherichia coli K-12. J. Bacteriol. 161:450-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baichoo, N., and J. D. Helmann. 2002. Recognition of DNA by Fur: a reinterpretation of the Fur box consensus sequence. J. Bacteriol. 184:5826-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bearden, S. W., and R. D. Perry. 1999. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol. Microbiol. 32:403-414. [DOI] [PubMed] [Google Scholar]
- 8.Bearden, S. W., T. M. Staggs, and R. D. Perry. 1998. An ABC transport system of Yersinia pestis allows utilization of chelated iron by Escherichia coli SAB11. J. Bacteriol. 180:1135-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer, E., I. Bergevin, D. Malo, P. Gros, and M. F. Cellier. 2002. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect. Immun. 70:6032-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, A. C., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daskaleros, P. A., J. A. Stoebner, and S. M. Payne. 1991. Iron uptake in Plesiomonas shigelloides: cloning of the genes for the heme-iron uptake system. Infect. Immun. 59:2706-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine, D. H., D. Furgang, and F. Beydouin. 2002. Lactoferrin iron levels are reduced in saliva of patients with localized aggressive periodontitis. J. Periodontol. 73:624-630. [DOI] [PubMed] [Google Scholar]
- 13.Fine, D. H., D. Furgang, H. C. Schreiner, P. Goncharoff, J. Charlesworth, G. Ghazwan, P. Fitzgerald-Bocarsly, and D. H. Figurski. 1999. Phenotypic variation in Actinobacillus actinomycetemcomitans during laboratory growth: implications for virulence. Microbiology 145:1335-1347. [DOI] [PubMed] [Google Scholar]
- 14.Fleischmann, R. D., M. D. Adams, O. White, R. A. Clayton, E. F. Kirkness, A. R. Kerlavage, C. J. Bult, J.-F. Tomb, B. A. Dougherty, J. M. Merrick, K. McKenney, G. Sutton, W. FitzHugh, C. Fields, J. D. Gocayne, J. Scott, R. Shirley, L.-I. Liu, A. Glodek, J. M. Kelley, J. F. Weidman, C. A. Phillips, T. Spriggs, E. Hedblom, M. D. Cotton, T. R. Utterback, M. C. Hanna, T. Nguyen, D. M. Saudek, R. C. Brandon, L. D. Fine, J. L. Fritchman, J. L. Fuhrmann, N. S. M. Geoghagen, C. L. Gnehm, L. A. McDonald, K. V. Small, C. M. Fraser, H. O. Smith, and J. C. Venter. 1995. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 269:496-512. [DOI] [PubMed] [Google Scholar]
- 15.Goncharoff, P., J. K. Yip, H. Wang, H. C. Schreiner, J. A. Pai, D. Furgang, R. H. Stevens, D. H. Figurski, and D. H. Fine. 1993. Conjugal transfer of broad-host-range incompatibility group P and Q plasmids from Escherichia coli to Actinobacillus actinomycetemcomitans. Infect. Immun. 61:3544-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graber, K., L. M. Smoot, and L. A. Actis. 1998. Expression of iron binding proteins and hemin binding activity in the dental pathogen Actinobacillus actinomycetemcomitans. FEMS Microbiol. Lett. 163:135-142. [DOI] [PubMed] [Google Scholar]
- 17.Grenier, D., A. Leduc, and D. Myrand. 1997. Interaction between Actinobacillus actinomycetemcomitans lipopolysaccharides and human hemoglobin. FEMS Microbiol. Lett. 151:77-81. [DOI] [PubMed] [Google Scholar]
- 18.Harkness, R. E., P. Chong, and M. Klein. 1992. Identification of two iron-repressed periplasmic proteins in Haemophilus influenzae. J. Bacteriol. 174:2425-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haubek, D., K. Poulsen, J. Westergaard, G. Dahlen, and M. Kilian. 1996. Highly toxic clone of Actinobacillus actinomycetemcomitans in geographically widespread cases of juvenile periodontitis in adolescents of African origin. J. Clin. Microbiol. 34:1576-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashida, H., K. Poulsen, and M. Kilian. 2002. Differences in iron acquisition from human haemoglobin among strains of Actinobacillus actinomycetemcomitans. Microbiology 148:3993-4001. [DOI] [PubMed] [Google Scholar]
- 21.Higgins, C. 1992. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8:67-113. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan, J. B., H. C. Schreiner, D. Furgang, and D. H. Fine. 2002. Population structure and genetic diversity of Actinobacillus actinomycetemcomitans strains isolated from localized juvenile periodontitis patients. J. Clin. Microbiol. 40:1181-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kehres, D. G., A. Janakiraman, J. M. Slauch, and M. E. Maguire. 2002. SitABCD is the alkaline Mn2+ transporter of Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:3159-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.May, B. J., Q. Zhang, L. L. Li, M. L. Paustian, T. S. Whittam, and V. Kapur. 2001. Complete genomic sequence of Pasteurella multocida, Pm70. Proc. Natl. Acad. Sci. USA 98:3460-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochsner, U. A., A. L. Vasil, and M. L. Vasil. 1995. Role of the ferric uptake regulator of Pseudomonas aeruginosa in the regulation of siderophore and exotoxin A expression: purification and activity on iron-regulated promoters. J. Bacteriol. 177:7194-7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saurin, W., W. Koster, and E. Dassa. 1994. Bacterial binding protein-dependent permeases: characterization of distinctive signatures for functionally related integral cytoplasmic membrane proteins. Mol. Microbiol. 12:993-1004. [DOI] [PubMed] [Google Scholar]
- 27.Schreiner, H. C., K. Sinatra, J. B. Kaplan, D. Furgang, S. C. Kachlany, P. J. Planet, B. A. Perez, D. H. Figurski, and D. H. Fine. 2003. Tight-adherence genes of Actinobacillus actinomycetemcomitans are required for virulence in a rat model. Proc. Natl. Acad. Sci. USA 100:7295-7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wertheimer, A., M. E. Tolmasky, L. A. Actis, and J. H. Crosa. 1994. Structural and functional analyses of mutant Fur proteins with impaired regulatory function. J. Bacteriol. 176:5116-5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willemsen, P. T. J., I. Vulto, M. Boxem, and J. De Graaf. 1997. Characterization of a periplasmic protein involved in iron utilization of Actinobacillus actinomycetemcomitans. J. Bacteriol. 179:4949-4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winston, J. L., C.-K. Chen, M. E. Neiders, and D. W. Dyer. 1993. Membrane protein expression by Actinobacillus actinomycetemcomitans in response to iron availability. J. Dent. Res. 72:1366-1373. [DOI] [PubMed] [Google Scholar]
- 31.Zambon, J. J. 1985. Actinobacillus actinomycetemcomitans in human periodontal disease. J. Clin. Periodontol. 12:1-20. [DOI] [PubMed] [Google Scholar]