Abstract
Amebic erythrophagocytosis is characteristic of invasive amebiasis, and mutants deficient in erythrocyte ingestion are avirulent. We sought to understand the molecular mechanisms underlying erythrocyte phagocytosis by Entamoeba histolytica. Following adherence to amebae, erythrocytes became round and crenulated, and phosphatidylserine (PS) was exposed on their outer membrane leaflets. These changes were similar to the effects of calcium treatment on erythrocytes, which we utilized to separate ameba-induced exposure of erythrocyte PS from the process of phagocytosis. The adherence and phagocytosis of calcium-treated erythrocytes were less inhibited by galactose than were those of healthy erythrocytes, suggesting the existence of an amebic coreceptor specific for PS. To test whether PS was recognized by amebae, calcium-treated cells were incubated with annexin V prior to adherence to or ingestion by E. histolytica. Annexin V blocked both adherence (50% ± 12% inhibition; P < 0.05) and phagocytosis (65% ± 10%; P < 0.05), providing evidence that at least one galactose-independent coreceptor was involved in the adherence and ingestion of red blood cells. The coreceptor was inhibited by phospho-l-serine and to a lesser extent by phospho-d-serine but not by phospho-l-threonine, which is consistent with the coreceptor functioning in the adherence and ingestion of erythrocytes via recognition of PS. We expanded our investigations to the highly related but noninvasive parasite Entamoeba dispar and demonstrated that it was deficient in red-blood-cell adherence, induction of PS exposure, and phagocytosis. These findings establish phosphatidylserine involvement in erythrophagocytosis by amebae and suggest the existence of a PS receptor on the surfaces of both E. histolytica and E. dispar.
Entamoeba histolytica is the causative agent of amebiasis. This disease is most prevalent in the poorest areas of the developing world, causing the second-highest rates of morbidity and mortality due to protozoan parasites (57). Clinical manifestations of amebiasis include asymptomatic colonization, colitis, and liver or brain abscesses (24). In patients with invasive amebiasis, E. histolytica causes vast tissue damage (11, 23) that is believed to be a direct result of its ability to induce host cell apoptosis (26, 27, 37, 47) and necrosis (3). The anti-inflammatory nature of cell death via apoptosis may explain the paucity of inflammatory changes with invasive amebiasis (28, 50).
Invasive infection by E. histolytica is thought to involve a stepwise process of adherence and cytolysis followed by phagocytosis. Adherence via the Gal/GalNAc lectin is required for the latter steps to occur (26, 32, 38, 39). Cytolysis occurs in a contact-dependent manner and has been associated with increased intracellular calcium (40), activation of caspase 3 (27), and exposure of phosphatidylserine (PS) on the outer leaflet of the host cell membrane (26). These changes are consistent with the induction of apoptosis, but there is also evidence of necrosis (3) occurring simultaneously during infection.
Although specific mechanisms of cytolysis and phagocytosis remain largely unknown, strains (49), clones (31), and mutants (44, 53) deficient in either of these processes have reduced virulence, supporting a role for these processes in in vivo virulence. Studies of Entamoeba histolytica have suggested the involvement of negatively charged lipids in phagocytosis. Liposomes comprised of phosphatidylserine and dicetylphosphate but not positively charged phosphatidylcholine caused increased actin polymerization in trophozoites (1, 2). The addition of PS to the outer membrane leaflet of viable Jurkat leukemia T cells increased the uptake of these cells (26). Clearance of apoptotic host cells in metazoans involves the recognition of PS on the surfaces of apoptotic host cells via scavenger receptors that recognize the strong negative charge (13, 36, 43, 45) or a stereospecific PS receptor whose identity remains controversial (4, 12, 17, 19). Entamoeba histolytica has been shown to preferentially ingest apoptotic host cells, a finding similar to what has been seen for metazoan systems (26). However, attempts to uncover genes homologous to metazoan receptors have failed, and we are now attempting to understand the specificity of this recognition and the relative contributions of specific lipids.
Erythrophagocytosis is a characteristic feature of E. histolytica infection of humans but is rarely seen during colonization by the nonpathogenic Entamoeba dispar (22).
In these experiments, we studied the physiological target cell, the human erythrocyte, to further understand the basis for the recognition of host cells and the role that phagocytosis has in pathogenesis. The results of this study indicate that in addition to the role of the Gal/GalNAc lectin in adherence and ingestion, there is at least one additional coreceptor involved that recognizes PS. In contrast to the macrophage PS receptor, the amebic receptor was relatively nonstereospecific. In addition, the erythrophagocytosis defect in E. dispar was demonstrated to be multifactorial, encompassing adherence, cytolysis, and phagocytosis.
MATERIALS AND METHODS
Erythrocyte isolation, storage, and calcium treatment.
Human blood, type B Rh+, was collected, heparinized, and sedimented by centrifugation (1,000 × g; 4°C; 10 min) through Ficoll-Paque PLUS (Amersham Biosciences, Piscataway, N.J.) to separate erythrocytes from other blood constituents. Erythrocytes were pelleted, washed twice in HEPES buffer (10 mM HEPES, pH 7.2, 140 mM NaCl, and 0.1% bovine serum albumin with or without 2.5 mM CaCl2), resuspended at a concentration of 1 × 107 cells per ml in HEPES buffer, and stored for up to 48 h (7, 8). Calcium-treated erythrocytes were prepared by incubation in HEPES buffer supplemented with 2.5 mM CaCl2 at 37°C for 48 h.
Culturing of amebae.
Entamoeba histolytica trophozoites (HM1:IMSS) were grown axenically in TYI-S-33 (Trypticase-yeast extract iron serum) medium supplemented with 100 U of penicillin/ml and 100 μg of streptomycin sulfate/ml at 37°C (14). Entamoeba dispar trophozoites (SAW760) were grown xenically in TYI-S-33 medium supplemented with 100 μg of erythromycin/ml. Trophozoites were harvested during log-phase growth by incubation on ice for 10 min, centrifugation at 150 × g and 4°C for 5 min, and resuspension in either HEPES buffer or medium 199 (Gibco BRL, Grand Island, N.Y.) supplemented with 5.7 mM cysteine, 25 mM HEPES, and 0.5% bovine serum albumin at pH 6.8 (M199S) (41). E. histolytica HM1:IMSS was cultured in the presence of bacteria by the addition of the bacteria from a xenic SAW760 culture.
Annexin V staining.
Erythrocyte surface exposure of phosphatidylserine was quantitated with annexin V-fluorescein isothiocyanate (FITC) binding and flow cytometry. The cells were stained with annexin V per the manufacturer's directions (PharMingen, San Diego, CA) and analyzed using a FACScan cytometer and CellQuest 3.3 software (Becton Dickinson, Franklin Lakes, N.J.). Cytolysis was measured by centrifuging amebae and PHK-26-stained erythrocytes (1:5 ratio) together and incubating them on ice for 20 min to allow rosetting. Samples were either warmed to room temperature or maintained on ice for 25 min. The cell mixture was centrifuged (200 × g for 5 min at 4°C), washed once, and resuspended in annexin V binding buffer supplemented with 110 mM galactose. Samples were resuspended and incubated with annexin V-FITC per the manufacturer's directions and analyzed using a FACScan cytometer and CellQuest 3.3 software.
Adherence assay.
Entamoeba histolytica adherence was assayed with a rosette formation assay (39). Amebae and either healthy or calcium-treated erythrocytes were mixed in 500 μl of M199s medium, centrifuged (200 × g, 4°C, 5 min), and incubated for 30 min on ice. Following incubation, the supernatant was aspirated off, the pellet was suspended with a Pasteur pipette, and 10 μl was placed on a hemacytometer. Adherent amebae were defined as amebae with three or more bound erythrocytes.
Fluorescent labeling and annexin V preincubation.
Prior to calcium treatment, erythrocytes were fluorescently labeled by incubation at 37°C for 20 to 25 min in phosphate-buffered saline containing 47 μM 5- (and 6)-carboxytetramethylrhodamine succinimidyl ester (TAMRA) (Molecular Probes, Eugene, OR). TAMRA is a succinimidyl ester which forms an amide bond between the dye and proteins. Unbound dye was quenched by incubation with an excess of fetal bovine serum at 37°C for 20 min, and the cells were washed twice more with M199s medium before use. Erythrocytes used for flow cytometry were stained with 2 μM PHK-26 (Sigma, St Louis, MO) for 5 min at 25°C. Excess dye was quenched by a 10-min incubation with serum followed by three washes in complete medium. Where indicated, erythrocytes were washed once in annexin V binding buffer (10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2, pH 7.4) and resuspended at 105 cells per 100 μl of annexin V binding buffer with or without 7 μM annexin V for 15 min at 37°C. The cells were then washed twice in annexin V binding buffer before they were added to the amebae (10).
Phagocytosis assays.
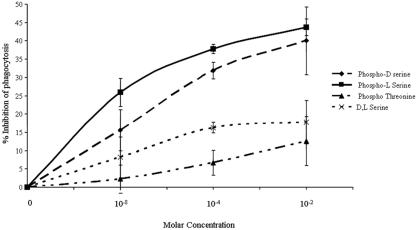
Phagocytosis was assayed by microscopy. Phagocytosis-positive amebae were defined as amebae containing one or more ingested erythrocytes. TAMRA-labeled erythrocytes were mixed with amebic trophozoites in either M199s medium or HEPES buffer. The mixture was centrifuged (200 × g, 4°C, 5 min) and incubated at 37°C for 20 min. Following incubation, ameba host cell rosettes were disrupted by resuspension in 110 mM d-galactose (Sigma Chemicals, St. Louis, MO) in ice-cold water. An additional water wash was used to lyse any extracellular erythrocytes. Cells were then fixed with 3% paraformaldehyde and analyzed by microscopy. Both the numbers of positive amebae and the numbers of intact engulfed erythrocytes were counted. The soluble phosphoester derivatives phospho-l-serine, phospho-d-serine, phospho-l-threonine, d-serine, and l-serine (all from Sigma, St. Louis, MO) were tested for inhibition of phagocytosis by 30-min incubations (amounts ranging from 10−8 to 10−2 M) with amebae prior to the addition of erythrocytes. These results were expressed as a phagocytic index, which was the percentage of amebic trophozoites that had engulfed erythrocytes multiplied by the average number of erythrocytes ingested per ameba (19).
Statistical analysis.
All data were represented as means ± standard deviations (SD), with the statistical significances determined using an unpaired Student t test.
RESULTS
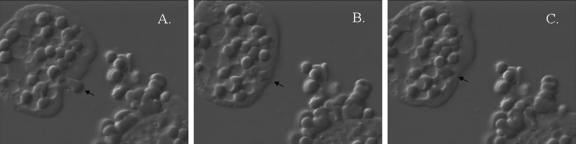
Entamoeba histolytica modifies the surfaces of erythrocytes prior to ingesting them as intact cells. Previous work has shown that contact between E. histolytica and Jurkat leukemia T cells led to the activation of host cell caspase 3, DNA fragmentation, phosphatidylserine exposure, and the ingestion of the host cells as intact particles (26, 27, 37). The phagocytosis of erythrocytes is pathognomonic of amebiasis. It is therefore important to understand whether the ingestion of erythrocytes, which lack mitochondrial and nuclear apoptotic pathways, involves mechanisms similar to those utilized for nucleated cells. Videomicroscopy was used to examine the process of erythrophagocytosis. Initially, amebae adhered to erythrocytes at the leading edges of the trophozoites. Within 40 s, the amebae caused physical distortions on the surface of one erythrocyte (arrow), including membrane blebbing (Fig. 1A). The altered erythrocyte was then ingested as an intact particle (Fig. 1B and C). Changes in the appearance of the red blood cells after their interaction with amebae suggested dynamic modifications of the erythrocyte surface prior to phagocytosis.
FIG. 1.
Erythrophagocytosis by E. histolytica. An intact erythrocyte (arrow) was followed from adherence through ingestion with 40 seconds of elapsed time between each panel. (A) Entamoeba histolytica adherent to an erythrocyte with membrane distortion. (B) Erythrocyte ingested intact. (C) Erythrocyte redistributed within the ameba. A video is available online at http://www.healthsystem.virginia.edu/internet/petri-mann/movies/ehistrbc.mov.
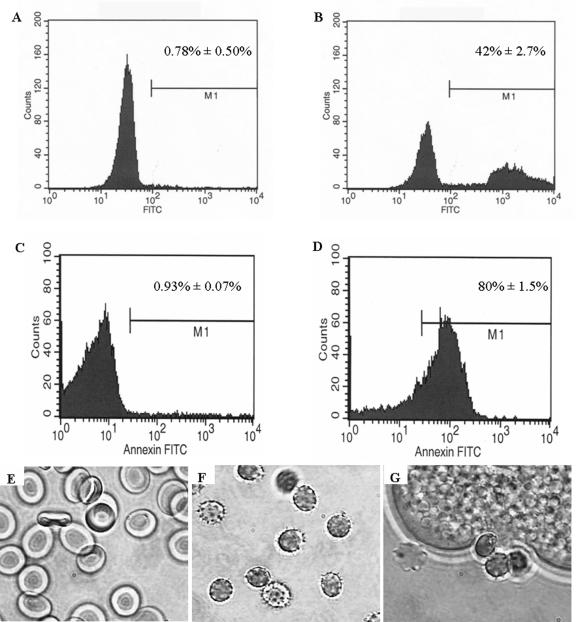
Phosphatidylserine has been demonstrated to be an important trigger for the phagocytosis of apoptotic cells and senescent red blood cells by macrophages (19, 30, 46). To test for PS exposure prior to amebic phagocytosis, we utilized annexin V-FITC staining and flow cytometry. Erythrocytes were incubated in the presence or absence of E. histolytica for 25 min at 25°C and then annexin V-FITC stained before being analyzed by flow cytometry. Less than 1% (0.78% ± 0.50%) of healthy erythrocytes were annexin V-FITC positive prior to the addition of amebae (Fig. 2A), but following the incubation with amebae, 42% (42% ± 2.7%) became annexin V positive (P < 0.001; n = 3) (Fig. 2B). We conclude from these experiments that upon interaction with the amebae, the erythrocytes went through physical changes, which included the exposure of PS on their surfaces prior to their engulfment as intact cells.
FIG. 2.
Erythrocytes exposed to Entamoeba histolytica redistribute phosphatidylserine to the outer leaflet of the membrane and undergo morphological changes. (A to D) PS exposure was measured using annexin V-FITC staining of erythrocytes. Representative histograms of healthy erythrocytes and erythrocytes incubated with E. histolytica trophozoites are shown in panels A and B, respectively. Similarly, annexin V-FITC staining was performed on erythrocytes exposed to treatment with 2.5 mM calcium. Representative histograms of healthy erythrocytes in HEPES buffer for 48 h and erythrocytes in HEPES buffer exposed to 2.5 mM CaCl2 are shown in panels C and D, respectively. (E to G) Representative micrographs of healthy erythrocytes (panel E), crenulated erythrocytes following 48 h calcium treatment (panel F), and erythrocytes following 20 min exposure to E. histolytica (panel G).
Entamoeba histolytica preferentially adheres to and ingests erythrocytes that have exposed PS.
The finding of exposure of PS on the surface of ameba-altered erythrocytes paralleled work that demonstrated caspase-dependent PS exposure on erythrocytes following treatment with calcium (7). Annexin V-FITC staining was performed on erythrocytes which were incubated at 37°C for 48 h in 5% CO2 in HEPES buffer with or without 2.5 mM CaCl2. Less than 1% (0.93% ± 0. 07%) of the healthy erythrocyte population was annexin positive (Fig. 2C); following calcium treatment, however, over 80% (80% ± 1.5%) of the cells were annexin positive (Fig. 2D). In addition to PS exposure, calcium treatment caused physical changes to the erythrocyte surfaces which resembled those coordinated by amebae. Healthy erythrocytes (Fig. 2E) were seen as typical concave cells, but both calcium addition (Fig. 2F) and incubation with amebae (Fig. 2G) led to a round shape and a crenulated appearance. Distortion of erythrocytes was seen only following contact with amebae; neither conditioned medium nor coincubation in the presence of galactose lead to this change. The morphological similarities between erythrocytes that interacted with amebae and erythrocytes treated with calcium indicated that E. histolytica causes PS exposure on the surface of the red blood cell. We concluded that calcium-treated erythrocytes resembled those subjected to the processes of Gal/GalNAc lectin-dependent adherence and cytolysis. These similarities provided a means to examine the role of coreceptors in phagocytosis without the complications of adherence and cytolysis.
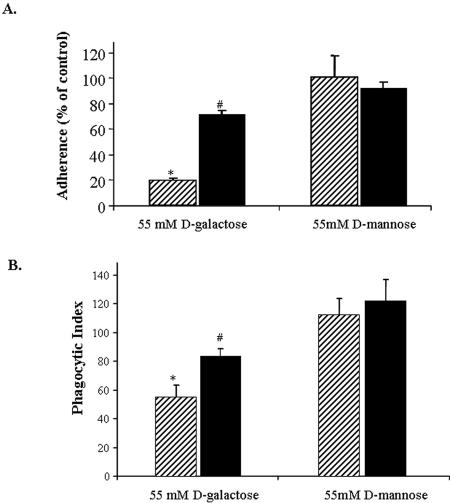
In light of the role PS plays in phagocytosis by metazoans (18, 19), we assayed whether calcium-induced PS exposure contributed to E. histolytica adherence and phagocytosis. This was tested by incubation of erythrocytes (healthy or calcium-treated) with amebae (in a 10:1 erythrocyte-to-ameba ratio) in a rosette-forming assay (Fig. 3A). Adherence to healthy erythrocytes was inhibited by 81% following the addition of 55 mM d-galactose. In contrast, adherence to calcium-treated cells was inhibited by 55 mM d-galactose by only 22%. Similarly, the phagocytic index was higher for calcium-treated erythrocytes than for healthy erythrocytes in 55 mM d-galactose (phagocytic indices of 83 ± 5.0 and 59 ± 7.8 for calcium-treated and healthy erythrocytes, respectively) (Fig. 3B). The adherence of amebae to calcium-treated erythrocytes was not affected by the presence of either mannose, for which there is a known amebic lectin (5), or N-acetylglucosamine, which is known to mediate adherence to apoptotic cells (15) (unpublished data). We concluded that the recognition and ingestion of calcium-treated erythrocytes is mediated by receptors in addition to the galactose-dependent adherence lectin of E. histolytica.
FIG. 3.
Entamoeba histolytica cells adhere to and ingest calcium-treated erythrocytes in a relatively galactose-independent manner. (A) Adherence of E. histolytica to healthy (hatched bars) and calcium-treated (solid bars) erythrocytes. Adherent cells were defined as trophozoites with at least three adherent erythrocytes (means ± SD; n = 6; * indicates a P value of <0.001 compared to healthy cells plus d-mannose; # indicates a P value of <0.01 compared to healthy cells plus d-galactose). (B) Phagocytosis of healthy (hatched bars) or calcium-treated (solid bars) erythrocytes by amebae (reported according to the phagocytic index, i.e., the average number of erythrocytes ingested multiplied by the percentage of amebae positive for ingestion). Following calcium treatment (2.5 mM), erythrocytes were stained with TAMRA and then spun onto amebae and incubated at 37°C for 15 min at a 10:1 erythrocyte-to-ameba ratio. Unengulfed erythrocytes were lysed in water, and amebae containing ingested erythrocytes were counted by microscopy. Data are reported as means ± SD. P values were determined by a two-tailed t test for healthy and calcium-treated cells (* indicates a P value of <0.001 compared to healthy erythrocytes with d-mannose; # indicates a P value of <0.01 compared to healthy erythrocytes with d-galactose; n = 6).
Phosphatidylserine-dependent adherence and phagocytosis of calcium-treated erythrocytes by E. histolytica.
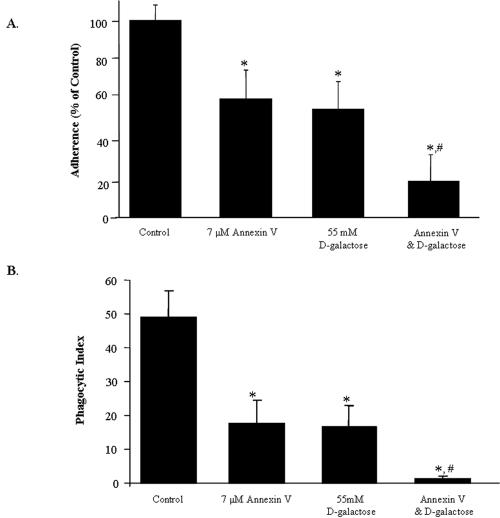
E. histolytica has been shown to recognize negatively charged lipids both as constituents of liposomes (2) and as present on the surface of nucleated cells during phagocytosis (26) in a manner similar to that of metazoans. Annexin V has been demonstrated to bind PS on the surfaces of apoptotic cells and mask it from recognition by mammalian macrophages, thereby inhibiting the clearance of apoptotic cells (10, 56). To determine whether PS was required for the ingestion of erythrocytes by E. histolytica, we incubated erythrocytes in annexin V prior to any interaction with amebae. Adherence of amebae to annexin V-preincubated erythrocytes (healthy or calcium treated) was examined with a rosetting assay (5:1 erythrocyte-to-ameba ratio; Fig. 4A). Annexin V caused no statistical change in the percentage of amebae adherent to healthy erythrocytes (data not shown). However, the preincubation of calcium-treated erythrocytes with annexin V caused a 48% reduction in adherence compared with a control. The effects of both 55 mM galactose and 7 μM annexin V were additive, resulting in an 81% inhibition of adherence compared with a control.
FIG. 4.
Adherence and phagocytosis of calcium-treated erythrocytes are blocked by annexin V and galactose in an additive manner. (A) Human erythrocytes were preincubated for 24 h with 2.5 mM CaCl2 in order to induce apoptotic-like surface changes. These cells were washed and then incubated with 7 μM annexin V. Each of the three samples was statistically significantly different from the control (* indicates a P value of <0.05 compared to calcium-treated erythrocytes without d-galactose or annexin V; n = 10). The addition of annexin V to galactose further reduced adherence by 15% when compared to galactose alone (# indicates a P value of <0.01; n = 10). (B) Following calcium treatment (2.5 mM) cells were stained with TAMRA, quenched, and preincubated with 7 μM annexin V. Cells were spun onto amebae and incubated at 37°C for 15 min at a 5:1 erythrocyte-to-ameba ratio. Uningested erythrocytes were lysed in water, and the cells were counted by microscopy. Data are reported as means ± SD. Differences between control cells (calcium-treated cells without annexin or galactose) and all other treatments were statistically significant (* indicates a P value of <0.0001 compared to the control). Also, preincubation with annexin V and addition of galactose were additive (# indicates a P value of <0.002 compared to calcium-treated erythrocytes with d-galactose; n = 5).
The phagocytosis of calcium-treated erythrocytes following preincubation with 7 μM annexin V was also examined by microscopy (Fig. 4B). A 5:1 ratio of erythrocytes to amebae was used, resulting in lower total phagocytic indices than in other experiments. Pretreatment of healthy erythrocytes or amebae with 7 μM annexin V caused no statistical change in the phagocytic index of ingestion (data not shown). Phagocytosis of calcium-treated erythrocytes was blocked by 63% by preincubation with 7 μM annexin V. Again, the effects of galactose and annexin V preincubation were additive, leading to a 97% inhibition of phagocytosis compared to a control.
Previous work by Fadok et al. showed that soluble phosphoester serine derivatives that mimicked the polar head group of PS blocked PS-dependent uptake of apoptotic cells by macrophages (19). In order to test whether amebae specifically recognized PS, we preincubated trophozoites with phospho-l-serine, phospho-d-serine, phospho-l-threonine, and dl-serine. Both 10 mM phospho-l-serine as well as phospho-d-serine inhibited E. histolytica ingestion of calcium-treated erythrocytes by more than 30% (Fig. 5). These data suggested not only that PS exposed in the outer leaflet of the erythrocytic membrane was recognized by the amebae but also that amebae recognize either stereoisomer phospho-l- or phospho-d-serine.We infer from these data that E. histolytica recognized PS that was exposed on the outer membrane leaflet of erythrocytes, providing additional adherence and eventual ingestion.
FIG. 5.
Phospho-l- and phospho-d-serine both inhibit E. histolytica ingestion of calcium-treated erythrocytes. Phospho-d-serine, phospho-l-serine, phospho-l-threonine, and dl-serine were added to M199S medium and incubated with E. histolytica for 30 min prior the performance of a phagocytosis assay. Erythrocytes were added at a 1:5 ratio (amebae to erythrocytes) and incubated at 37°C for 20 min. Amebae were fixed, counted, and scored for phagocytosis. Data are represented as means ± SD (n = 3).
The nonpathogenic Entamoeba dispar preferentially ingests calcium-treated erythrocytes.
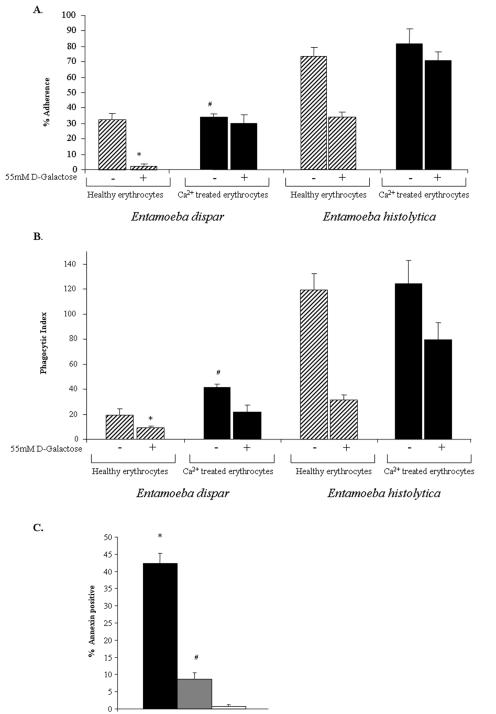
Erythrophagocytosis has been demonstrated to be a characteristic distinguishing between Entamoeba histolytica and the nonpathogenic commensal Entamoeba dispar (22). E. dispar rarely ingests erythrocytes during colonization of the host or during in vitro assays (16). To test whether the inability of E. dispar to ingest cells was due to reduced adherence, a phagocytosis defect, or an inability to cause PS exposure, we examined each process. Entamoeba histolytica HM1:IMSS was cultured with the bacterial flora from E. dispar strain SAW760 to provide a control for these experiments. The contrast between the adherence of E. dispar and that of E. histolytica to erythrocytes was measured using a rosette-forming assay (Fig. 6A). Entamoeba dispar adherence to healthy or calcium-treated erythrocytes was significantly reduced compared to that of E. histolytica. E. dispar adherence to calcium-treated erythrocytes was less hindered by the addition of 55 mM d-galactose than was its adherence to healthy erythrocytes (2.3% ± 1.5% versus 30% ± 5.9%; adherence to healthy cells compared to adherence to calcium-treated cells in 55 mM d-galactose; mean ± SD; P < 0.002; n = 3). To measure phagocytosis, xenic E. dispar was incubated with healthy or calcium-treated erythrocytes (20:1 erythrocyte-to-ameba ratio), for 25 min at 37°C and assayed by microscopy (Fig. 6B). The experiments showed that E. dispar had a low rate of phagocytosis of healthy cells which was nearly completely abolished by the addition of galactose. However, calcium-treated erythrocytes were ingested at a higher rate that was not as dramatically affected by the addition of 55 mM d-galactose (phagocytic indices, 42 ± 2.3 and 22 ± 5.0, respectively). While E. dispar ingested PS-exposing erythrocytes, it was far less phagocytic than E. histolytica. Despite the lack of an effect on adherence, calcium treatment was accompanied by increased phagocytosis.
FIG. 6.
E. dispar displays reduced adherence, PS exposure, and phagocytosis of erythrocytes relative to E. histolytica, along with an even greater defect in cytolysis. (A) Adherence of amebae to healthy (hatched bars) and calcium-treated (black bars) erythrocytes. Adherent cells were defined as trophozoites with at least three adherent erythrocytes (means ± SD; n = 3; * indicates a P value of <0.01 for E. dispar adherence to healthy versus calcium-treated erythrocytes in 55 mM d-galactose; # indicates a P value of <0.001 for E. dispar adherence to calcium-treated erythrocytes compared to that of E. histolytica). (B) Phagocytosis of healthy or calcium-treated erythrocytes by amebae (reported as the phagocytic index described in the legend to Fig. 3; means ± SD; n = 3). Following calcium treatment (2.5 mM), cells were stained with TAMRA, quenched, and then spun onto amebae and incubated at 37°C for 15 min at a 20:1 erythrocyte-to-ameba ratio. Uningested erythrocytes were lysed in water, and the cells were counted by microscopy (* indicates a P value of <0.005 for E. dispar ingestion of healthy erythrocytes with 55 mM d-galactose compared to that of healthy erythrocytes without d-galactose; # indicates a P value of <0.01 for E. dispar ingestion of calcium-treated erythrocytes in 55 mM d-galactose versus E. dispar ingestion of healthy erythrocytes with 55 mM d-galactose). (C) Exposure of PS on the outer leaflet of the membrane of erythrocytes measured by annexin V-FITC staining and flow cytometry. Surface changes caused by E. histolytica (black bars), E. dispar (grey bars), or M199S alone (white bars) are shown (* indicates a P value of <0.001 for healthy erythrocytes incubated with E. histolytica compared to that of healthy erythrocytes in M199S; # indicates a P value of <0.05 for healthy erythrocytes incubated with E. dispar compared to that of healthy erythrocytes in M199S. Data are reported as means ± SD; n = 3).
Given that E. dispar could still ingest PS-exposing erythrocytes, we questioned whether it was defective in causing PS to be exposed to the outer leaflet of erythrocyte membranes. Either E. histolytica or E. dispar was added to PKH-26-labeled erythrocytes at a 1:5 ratio (ameba to erythrocyte) to allow rosette formation. Samples were then either warmed to 25°C or retained on ice as a control. The use of a temperature of 25°C rather than the physiologic temperature of 37°C reduced the levels of erythrophagocytosis. Cells were then washed in 110 mM d-galactose, subjected to annexin V-FITC staining, and analyzed by flow cytometry (Fig. 6C). E. histolytica was more efficient at promoting the exposure of PS on the surface of erythrocytes at 25°C (43%) than E. dispar (8.7%). Both E. histolytica and E. dispar inductions of PS exposure were statistically significantly higher than that of M199S medium alone (0.8%).
DISCUSSION
Erythrophagocytosis is pathognomonic of amebiasis (49), and deficiencies in this process have been associated with reduced virulence (31). In this work we attempted to determine what ligands were recognized on erythrocytes for ingestion by E. histolytica and whether these were also recognized by Entamoeba dispar. Our conclusions from this study are as follows: (i) E. histolytica causes physical changes on the surfaces of erythrocytes prior to ingestion, including the exposure of PS; (ii) PS is specifically recognized by an amebic coreceptor during the ingestion of erythrocytes; and (iii) the commensal parasite E. dispar is relatively deficient in each step of erythrophagocytosis.
Entamoeba histolytica caused apparent surface changes on erythrocytes, including a change in the cell shape from concave to spherical, which were followed by membrane blebbing and PS exposure. These changes are consistent with both the aging of erythrocytes (9) and calcium treatment (7). The physical modifications of the erythrocyte membranes led to changes in their properties of adherence to amebae. Galactose was able to only partially block the recognition and consequent ingestion of calcium-treated erythrocytes; this was consistent with the existence of an additional receptor that recognized a ligand specific to the altered red-blood-cell surface. This theory was corroborated by the reduced galactose inhibitions of adherence and ingestion of calcium-treated erythrocytes by amebae compared to those of healthy erythrocytes. We surmise from these data that surface changes rendered by contact with amebae expose a new ligand on the erythrocytes, which can be recognized by an amebic receptor apart from the Gal/GalNAc lectin.
Previous experiments had implicated negatively charged lipids in recognition and phagocytosis by E. histolytica. Bailey et al. demonstrated that liposomes comprised of the negatively charged lipids PS and dicetyl phosphate triggered actin polymerization in E. histolytica trophozoites, whereas phosphatidylcholine, phosphatidylethanolamine, and phosphatidic acid did not (1). Entamoeba histolytica also showed increased ingestion of Jurkat leukemia T cells with added PS but not with added phosphatidylcholine, phosphatidylethanolamine, or phosphatidic acid on their surfaces (26). These experiments showed that PS promoted ingestion of host cells, but there was no direct evidence suggesting the utilization of host cell PS exposure for ingestion by E. histolytica.
Phosphatidylserine involvement in erythrocyte recognition and uptake was tested by the addition of annexin V to bind and mask exposed PS on the erythrocyte surface. Annexin V has been shown to bind to PS on apoptotic cells (51) and interfere with the interactions between apoptotic cells and macrophages (19, 52). Annexin V inhibition of both adherence and phagocytosis and its additive nature with galactose suggest a coordinated role of the Gal/GalNAc lectin and a PS coreceptor in erythrophagocytosis.
Phosphatidylserine exposure has been well characterized on the surfaces of apoptotic cells (18, 19) as a signal for clearance in metazoans. There are many examples of receptors that recognize PS in mammals, including the stereospecific macrophage PS receptor (17) and multiple scavenger receptors, which include CD36 (20, 42, 48). Contrary to evidence pertaining to macrophages, the amebic receptor does not appear to recognize PS in a stereospecific manner; both phospho-l- and phospho-d-serine inhibited amebic phagocytosis, suggesting the amebic receptor functions as a scavenger receptor. There are no homologues of scavenger receptors or the mammalian PS receptor in either E. histolytica genome database (available from The Institute for Genomic Research and the Sanger Centre). In addition, recent evidence suggests that the mammalian PS receptor does not participate in the endocytosis of apoptotic cells (4, 12). Therefore, the nature of the PS receptor that mediates recognition and endocytosis remains obscure for both amebae and mammals. The use of a PS receptor to ingest apoptotic cells by amebae is an interesting example of convergent evolution and illustrates the pressures on a parasite to emulate its host.
Entamoeba dispar was initially distinguished from E. histolytica by biochemical differences (21), but much effort is being made to determine the genetic differences between these two species (54, 55). E. dispar is noninvasive and rarely ingests host cells in vivo. The nature of the differences separating E. dispar and E. histolytica remains unknown, but the genome sequences of the two species are very closely related. One important finding was that the Gal/GalNAc adherence lectin was less prevalent on the surface of E. dispar than on that of E. histolytica (34). Additional information has been published by Pimenta et al. describing smaller vesicle formation by E. dispar than by E. histolytica during the ingestion of bacteria (35). Otherwise, little is known about the capacity for E. dispar to perform phagocytosis.
Given the noninvasive nature of Entamoeba dispar, it was important to address whether deficiencies existed in the processes of adherence, cytolysis, or phagocytosis. E. dispar exhibited reduced adherence to erythrocytes and little ability to cause externalization of PS to the outer leaflet of the membrane of erythrocytes. However, E. dispar was able to ingest PS-exposing erythrocytes, albeit more slowly than E. histolytica. The discrepancy between the ingestion of erythrocytes by E. dispar and that by E. histolytica may be explained by multiple factors, such as reduced Gal/GalNAc lectin expression, cell size, and long-term xenic growth conditions. Regardless of these differences, E. dispar can ingest erythrocytes exposing PS in a fashion similar to E. histolytica.
Future studies will focus on identifying the amebic receptors for PS as well as other receptors for apoptotic cells. A few candidates have already been uncovered through the work of other laboratories. Identification of a receptor would allow us to directly address the contribution of phagocytosis to virulence. We propose a stepwise model for amebic pathogenicity. First, the Gal/GalNAc lectin is used to adhere amebae to terminal galactose residues on host cells (33). Once the membranes are within close proximity, secretion of amebapore (6, 29) and cysteine proteinases (25) can damage the host cells, allowing calcium to flow into the cells and activate host cell apoptotic factors. As a direct result of this damage, PS is exposed on the surfaces of the erythrocytes, where amebic coreceptors can recognize and bind to them. This recognition then stimulates the phagocytic machinery, yielding ingestion of the damaged host cells. We hypothesize that amebic induction of host cell apoptosis leads to the rapid removal of dying tissue prior to the release of toxic cellular content, allowing the amebae to cause chronic infection by limiting inflammation.
Acknowledgments
This work was supported by National Institutes of Health grants AI26649 to W.A.P. and AI053678 to C.D.H.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Bailey, G. B., D. B. Day, C. Nokkaew, and C. C. Harper. 1987. Stimulation by target cell membrane lipid of actin polymerization and phagocytosis by Entamoeba histolytica. Infect. Immun. 55:1848-1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey, G. B., E. D. Nudelman, D. B. Day, C. F. Harper, and J. R. Gilmour. 1990. Specificity of glycosphingolipid recognition by Entamoeba histolytica trophozoites. Infect. Immun. 58:43-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berninghausen, O., and M. Leippe. 1997. Necrosis versus apoptosis as the mechanism of target cell death induced by Entamoeba histolytica. Infect. Immun. 65:3615-3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bose, J., A. D. Gruber, L. Helming, S. Schiebe, I. Wegener, M. Hafner, M. Beales, F. Kontgen, and A. Lengeling. 2004. The phosphatidylserine receptor has essential functions during embryogenesis but not in apoptotic cell removal. J. Biol. 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bracha, R., D. Kobiler, and D. Mirelman. 1982. Attachment and ingestion of bacteria by trophozoites of Entamoeba histolytica. Infect. Immun. 36:396-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bracha, R., Y. Nuchamowitz, M. Leippe, and D. Mirelman. 1999. Antisense inhibition of amoebapore expression in Entamoeba histolytica causes a decrease in amoebic virulence. Mol. Microbiol. 34:463-472. [DOI] [PubMed] [Google Scholar]
- 7.Bratosin, D., J. Estaquier, F. Petit, D. Arnoult, B. Quatannens, J. P. Tissier, C. Slomianny, C. Sartiaux, C. Alonso, J. J. Huart, J. Montreuil, and J. C. Ameisen. 2001. Programmed cell death in mature erythrocytes: a model for investigating death effector pathways operating in the absence of mitochondria. Cell Death Differ. 8:1143-1156. [DOI] [PubMed] [Google Scholar]
- 8.Bratosin, D., S. Leszczynski, C. Sartiaux, O. Fontaine, J. Descamps, J. J. Huart, J. Poplineau, F. Goudaliez, D. Aminoff, and J. Montreuil. 2001. Improved storage of erythrocytes by prior leukodepletion: flow cytometric evaluation of stored erythrocytes. Cytometry 46:351-356. [DOI] [PubMed] [Google Scholar]
- 9.Bratosin, D., J. Mazurier, J. P. Tissier, C. Slomianny, J. Estaquier, F. Russo-Marie, J. J. Huart, J. M. Freyssinet, D. Aminoff, J. C. Ameisen, and J. Montreuil. 1997. Molecular mechanisms of erythrophagocytosis. Characterization of the senescent erythrocytes that are phagocytized by macrophages. C. R. Acad. Sci. Ser. III 320:811-818. [DOI] [PubMed] [Google Scholar]
- 10.Callahan, M. K., M. S. Halleck, S. Krahling, A. J. Henderson, P. Williamson, and R. A. Schlegel. 2003. Phosphatidylserine expression and phagocytosis of apoptotic thymocytes during differentiation of monocytic cells. J. Leukoc. Biol. 74:846-856. [DOI] [PubMed] [Google Scholar]
- 11.Chadee, K., and E. Meerovitch. 1985. Entamoeba histolytica: early progressive pathology in the cecum of the gerbil (Meriones unguiculatus). Am. J. Trop. Med. Hyg. 34:283-291. [DOI] [PubMed] [Google Scholar]
- 12.Cikala, M., O. Alexandrova, C. N. David, M. Proschel, B. Stiening, P. Cramer, and A. Bottger. 2004. The phosphatidylserine receptor from Hydra is a nuclear protein with potential Fe(II) dependent oxygenase activity. BMC Cell Biol. 5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devitt, A., O. D. Moffatt, C. Raykundalia, J. D. Capra, D. L. Simmons, and C. D. Gregory. 1998. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 392:505-509. [DOI] [PubMed] [Google Scholar]
- 14.Diamond, L. S., D. R. Harlow, and C. C. Cunnick. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 15.Duvall, E., A. H. Wyllie, and R. G. Morris. 1985. Macrophage recognition of cells undergoing programmed cell death (apoptosis). Immunology 56:351-358. [PMC free article] [PubMed] [Google Scholar]
- 16.Espinosa-Cantellano, M., A. Gonzales-Robles, B. Chavez, G. Castanon, C. Arguello, A. Lazaro-Haller, and A. Martinez-Palomo. 1998. Entamoeba dispar: ultrastructure, surface properties and cytopathic effect. J. Eukaryot. Microbiol. 45:265-272. [DOI] [PubMed] [Google Scholar]
- 17.Fadok, V. A., D. L. Bratton, D. M. Rose, A. Pearson, R. A. Ezekewitz, and P. M. Henson. 2000. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405:85-90. [DOI] [PubMed] [Google Scholar]
- 18.Fadok, V. A., A. de Cathelineau, D. L. Daleke, P. M. Henson, and D. L. Bratton. 2001. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. J. Biol. Chem. 276:1071-1077. [DOI] [PubMed] [Google Scholar]
- 19.Fadok, V. A., D. R. Voelker, P. A. Campbell, J. J. Cohen, D. L. Bratton, and P. M. Henson. 1992. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148:2207-2216. [PubMed] [Google Scholar]
- 20.Fadok, V. A., M. L. Warner, D. L. Bratton, and P. M. Henson. 1998. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (αvβ3). J. Immunol. 161:6250-6257. [PubMed] [Google Scholar]
- 21.Gatti, S., C. Cevini, A. Bruno, M. Ramsan, L. Marchi, and M. Scaglia. 1997. First isolation and characterization in humans of Entamoeba histolytica (laboratory-made) zymodeme XX. Parasitol. Res. 83:716-718. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Ruiz, A., R. Haque, A. Aguirre, G. Castanon, A. Hall, F. Guhl, G. Ruiz-Palacios, M. A. Miles, and D. C. Warhurst. 1994. Value of microscopy in the diagnosis of dysentery associated with invasive Entamoeba histolytica. J. Clin. Pathol. 47:236-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin, J. L. 1972. Human amebic dysentery. Electron microscopy of Entamoeba histolytica contacting, ingesting, and digesting inflammatory cells. Am. J. Trop. Med. Hyg. 21:895-906. [PubMed] [Google Scholar]
- 24.Haque, R., C. D. Huston, M. Hughes, E. Houpt, and W. A. Petri, Jr. 2003. Amebiasis. N. Engl. J. Med. 348:1565-1573. [DOI] [PubMed] [Google Scholar]
- 25.Hellberg, A., R. Nickel, H. Lotter, E. Tannich, and I. Bruchhaus. 2001. Overexpression of cysteine proteinase 2 in Entamoeba histolytica or Entamoeba dispar increases amoeba-induced monolayer destruction in vitro but does not augment amoebic liver abscess formation in gerbils. Cell Microbiol. 3:13-20. [DOI] [PubMed] [Google Scholar]
- 26.Huston, C. D., D. R. Boettner, V. Miller-Sims, and W. A. Petri, Jr. 2003. Apoptotic killing and phagocytosis of host cells by the parasite Entamoeba histolytica. Infect. Immun. 71:964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huston, C. D., E. R. Houpt, B. J. Mann, C. S. Hahn, and W. A. Petri, Jr. 2000. Caspase 3-dependent killing of host cells by the parasite Entamoeba histolytica. Cell Microbiol. 2:617-625. [DOI] [PubMed] [Google Scholar]
- 28.Kretschmer, R., M. L. Collado, M. G. Pacheco, M. C. Salinas, M. Lopez-Osuna, M. Lecuona, E. M. Castro, and J. Arellano. 1985. Inhibition of human monocyte locomotion by products of axenically grown E. histolytica. Parasite Immunol. 7:527-543. [DOI] [PubMed] [Google Scholar]
- 29.Leippe, M., J. Andra, and H. J. Muller-Eberhard. 1994. Cytolytic and antibacterial activity of synthetic peptides derived from amoebapore, the pore-forming peptide of Entamoeba histolytica. Proc. Natl. Acad. Sci. USA 91:2602-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEvoy, L., P. Williamson, and R. A. Schlegel. 1986. Membrane phospholipid asymmetry as a determinant of erythrocyte recognition by macrophages. Proc. Natl. Acad. Sci. USA 83:3311-3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orozco, E., G. Guarneros, A. Martinez-Palomo, and T. Sanchez. 1983. Entamoeba histolytica. Phagocytosis as a virulence factor. J. Exp. Med. 158:1511-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orozco, E., M. A. Rodriquez, C. F. Murphy, R. A. Salata, W. A. Petri, R. D. Smith, and J. I. Ravdin. 1987. Entamoeba histolytica: cytopathogenicity and lectin activity of avirulent mutants. Exp. Parasitol. 63:157-165. [DOI] [PubMed] [Google Scholar]
- 33.Petri, W. A., Jr., R. D. Smith, P. H. Schlesinger, C. F. Murphy, and J. I. Ravdin. 1987. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J. Clin. Investig. 80:1238-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pillai, D. R., S. Kobayashi, and K. C. Kain. 2001. Entamoeba dispar: molecular characterization of the galactose/N-acetyl-d-galactosamine lectin. Exp. Parasitol. 99:226-234. [DOI] [PubMed] [Google Scholar]
- 35.Pimenta, P. F., L. S. Diamond, and D. Mirelman. 2002. Entamoeba histolytica Schaudinn, 1903 and Entamoeba dispar Brumpt, 1925: differences in their cell surfaces and in the bacteria-containing vacuoles. J. Eukaryot. Microbiol. 49:209-219. [DOI] [PubMed] [Google Scholar]
- 36.Platt, N., H. Suzuki, Y. Kurihara, T. Kodama, and S. Gordon. 1996. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc. Natl. Acad. Sci. USA 93:12456-12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ragland, B. D., L. S. Ashley, D. L. Vaux, and W. A. Petri, Jr. 1994. Entamoeba histolytica: target cells killed by trophozoites undergo DNA fragmentation which is not blocked by Bcl-2. Exp. Parasitol. 79:460-467. [DOI] [PubMed] [Google Scholar]
- 38.Ravdin, J. I., B. Y. Croft, and R. L. Guerrant. 1990. Cytopathogenic mechanisms of Entamoeba histolytica. J. Exp. Med. 152:377-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravdin, J. I., and R. L. Guerrant. 1981. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J. Clin. Investig. 68:1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ravdin, J. I., F. Moreau, J. A. Sullivan, W. A. Petri, Jr., and G. L. Mandell. 1988. Relationship of free intracellular calcium to the cytolytic activity of Entamoeba histolytica. Infect. Immun. 56:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravdin, J. I., P. Stanley, C. F. Murphy, and W. A. Petri, Jr. 1989. Characterization of cell surface carbohydrate receptors for Entamoeba histolytica adherence lectin. Infect. Immun. 57:2179-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren, Y., R. L. Silverstein, J. Allen, and J. Savill. 1995. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J. Exp. Med. 181:1857-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rigotti, A., S. L. Acton, and M. Krieger. 1995. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J. Biol. Chem. 270:16221-16224. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez, M. A., and E. Orozco. 1986. Isolation and characterization of phagocytosis- and virulence-deficient mutants of Entamoeba histolytica. J. Infect. Dis. 154:27-32. [DOI] [PubMed] [Google Scholar]
- 45.Savill, J., J. Smith, C. Sarraf, Y. Ren, F. Abbott, and A. Rees. 1992. Glomerular mesangial cells and inflammatory macrophages ingest neutrophils undergoing apoptosis. Kidney Int. 42:924-936. [DOI] [PubMed] [Google Scholar]
- 46.Schlegel, R. A., M. Callahan, S. Krahling, D. Pradhan, and P. Williamson. 1996. Mechanisms for recognition and phagocytosis of apoptotic lymphocytes by macrophages. Adv. Exp. Med. Biol. 406:21-28. [DOI] [PubMed] [Google Scholar]
- 47.Seydel, K. B., and S. L. Stanley, Jr. 1998. Entamoeba histolytica induces host cell death in amebic liver abscess by a non-Fas-dependent, non-tumor necrosis factor alpha-dependent pathway of apoptosis. Infect. Immun. 66:2980-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tait, J. F., and C. Smith. 1999. Phosphatidylserine receptors: role of CD36 in binding of anionic phospholipid vesicles to monocytic cells. J. Biol. Chem. 274:3048-3054. [DOI] [PubMed] [Google Scholar]
- 49.Trissl, D., A. Martinez-Palomo, M. de la Torre, R. de la Hoz, and E. Perez de Suarez. 1978. Phagocytosis of human erythrocytes by Entamoeba histolytica. Quantitative study. Arch. Investig. Med. (Mexico) 9(Suppl. 1):219-222. (In Spanish with English summary.) [PubMed] [Google Scholar]
- 50.Tsutsumi, V., and A. Martinez-Palomo. 1988. Inflammatory reaction in experimental hepatic amebiasis. An ultrastructural study. Am. J. Pathol. 130:112-119. [PMC free article] [PubMed] [Google Scholar]
- 51.van Engeland, M., L. J. Nieland, F. C. Ramaekers, B. Schutte, and C. P. Reutelingsperger. 1998. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31:1-9. [DOI] [PubMed] [Google Scholar]
- 52.Verhoven, B., R. A. Schlegel, and P. Williamson. 1995. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J. Exp. Med. 182:1597-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vines, R. R., G. Ramakrishnan, J. B. Rogers, L. A. Lockhart, B. J. Mann, and W. A. Petri, Jr. 1998. Regulation of adherence and virulence by the Entamoeba histolytica lectin cytoplasmic domain, which contains a beta2 integrin motif. Mol. Biol. Cell 9:2069-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willhoeft, U., H. Buss, and E. Tannich. 2002. The abundant polyadenylated transcript 2 DNA sequence of the pathogenic protozoan parasite Entamoeba histolytica represents a nonautonomous non-long-terminal-repeat retrotransposon-like element which is absent in the closely related nonpathogenic species Entamoeba dispar. Infect. Immun. 70:6798-6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willhoeft, U., H. Buss, and E. Tannich. 1999. DNA sequences corresponding to the ariel gene family of Entamoeba histolytica are not present in E. dispar. Parasitol. Res. 85:787-789. [DOI] [PubMed] [Google Scholar]
- 56.Williamson, P., S. van den Eijnde, and R. A. Schlegel. 2001. Phosphatidylserine exposure and phagocytosis of apoptotic cells. Methods Cell Biol. 66:339-364. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization. 1997. WHO/PAHO/UNESCO report. A consultation with experts on amoebiasis. Mexico City, Mexico 28-29 January, 1997. Epidemiol. Bull. 18:13-14. [PubMed] [Google Scholar]