Abstract
Infection of C57BL/6 (B6) mice with the Lyme disease spirochete Borrelia burgdorferi can result in development of arthritis and carditis. B. burgdorferi induces expression of β2/CD18 integrins, adhesion molecules that mediate the firm adhesion of leukocytes to the endothelium necessary for cellular extravasation during inflammation. The important role of β2/CD18 integrins during extravasation suggests that these molecules play a role in the development of Lyme arthritis and carditis. The dependency of these inflammatory processes on the β2 integrins was investigated in CD18 hypomorph mice, which express low levels of CD18. The results indicate that CD18 deficiency did not abrogate development of Lyme arthritis or carditis. Moreover, it resulted in increased severity of Lyme carditis. B. burgdorferi-infected CD18 hypomorph mice showed an increased macrophage infiltration of the heart, while they produced lower levels of borreliacidal anti-B. burgdorferi antibodies compared to wild-type mice. In accordance with these results, we demonstrate that dendritic cells from CD18 hypomorph mice secrete higher levels of monocyte/macrophage chemoattractant protein 1 (MCP-1/CCL2) in response to B. burgdorferi. Similarly, we show by real-time PCR that B. burgdorferi-infected hearts from CD18 hypomorph mice express increased levels of MCP-1 RNA compared to wild-type mice. Overall, our results indicate that β2 integrin deficiency does not abrogate B. burgdorferi-induced inflammation; rather, it results in increased recruitment of macrophages into the B. burgdorferi-infected heart, likely due to the increased expression of MCP-1 in this tissue. Thus, β2 integrins may play a regulatory role in B. burgdorferi-induced inflammation beyond mediating adhesion of leukocytes to the endothelium.
Lyme disease is a consequence of infection with the two-membrane, diderm (31), spirochete Borrelia burgdorferi (17, 63). B. burgdorferi is transmitted to humans upon feeding of infected Ixodes ticks (40, 59). The spirochete initially establishes infection in the skin and then disseminates to other organs, such as the heart, joints, and nervous system (60). Inflammation is a hallmark of B. burgdorferi infection, which can be manifest as carditis and arthritis. Carditis usually appears several weeks after infection and is estimated to affect 2 to 8% of untreated patients (32). Arthritis develops months after infection, affecting 60% of untreated patients (64). The inflammatory manifestations of Lyme disease can be detrimental to the host; i.e., Lyme carditis can result in serious cardiac conduction abnormalities (44, 61), and joint inflammation may set the stage for development of chronic antibiotic treatment-resistant Lyme arthritis (23, 30, 64). Understanding the mechanism for the development of inflammation in Lyme disease is, therefore, an important aim.
Although the effects of Lyme carditis can be severe, limited information on this disease can be gathered from human patients (32). In contrast, the mouse model of Lyme disease provides an excellent tool to study Lyme carditis. C57BL/6 (B6) mice inoculated with live B. burgdorferi organisms develop Lyme arthritis and carditis 2 to 4 weeks postinfection (3, 68). While Lyme arthritis is characterized by tendonitis and synovitis, with infiltration of neutrophils and other leukocytes (6, 7), the macrophage is the main infiltrating cell found in Lyme carditis (52). Neutrophils and macrophages reach the B. burgdorferi-infected tissues after undergoing a process of extravasation.
Extravasation of immune cells into tissues usually depends on the β2 integrin group of adhesion molecules (33). β2 integrins mediate adhesion of leukocytes to the endothelium, a step required for extravasation (33). All members of the β2 integrin family are heterodimers made up of the common β chain, CD18, which pairs with CD11a, CD11b, CD11c, or CD11d, respectively, forming the corresponding integrins LFA-1, Mac-1/CR3, p150,95, and CD11d/CD18 (33). Therefore, CD18 deficiency results in decreased expression of all β2 integrins (27) and in humans is manifested as the disease leukocyte adhesion deficiency (LAD). Symptoms of LAD include impaired wound healing, severe gingivitis, and recurring necrotic soft tissue infections that can be moderate to life-threatening, depending on the level of expression of functional β2 integrins (34). A mouse model of the moderate form of LAD was developed by Wilson et al. (69). The CD18 hypomorph mice have reduced levels of β2 integrins, with 2 to 16% of normal expression levels of CD18 on leukocytes (69).
We investigated the influence of β2 integrins on the development of the inflammatory infiltrates characteristic of murine Lyme arthritis and carditis. It has been shown that B. burgdorferi induces the expression of β2 integrins on neutrophils (19, 48) and that infiltrating cells in human Lyme arthritis joints express high levels of CD11a/CD18 (LFA-1) (1). These data suggest that β2 integrins play an important role in the inflammatory processes associated with B. burgdorferi infection. CD18 hypomorph mice express low levels of β2 integrins on the surface of immune cells and show decreased cell migration in a thioglycolate-induced peritonitis model (69). Therefore, we tested whether CD18 hypomorph mice on the B6 background develop Lyme arthritis comparable to wild-type (WT) B6 mice. Although we expected to find diminished inflammation in both the joint and the heart of B. burgdorferi-infected CD18 hypomorph mice, we observed that these mice develop arthritis similar to that of infected WT mice. Furthermore, CD18 hypomorph mice developed carditis of aggravated severity, marked by an increased infiltration of macrophages that correlated with increased expression of monocyte/macrophage chemoattractant protein 1 (MCP-1) RNA in the heart of these mice. Our results underscore that β2 integrins play an important role in the control of MCP-1 expression and, therefore, in control of the inflammatory response.
MATERIALS AND METHODS
Bacteria.
Low-passage (passages 2 to 4) cultures of the infectious B. burgdorferi N40 clone D10E9A1-E (kind gift of Jenifer Coburn) (20, 21) were used in all experiments. Spirochetes were grown in complete BSK-H medium (Sigma Chemical Co., St. Louis, Mo.) at 33°C until mid-log phase (5 × 107 cells/ml). A lot of BSK-H medium known to provide high rates of infection was used to grow B. burgdorferi for all experiments, as it has been reported that different lots of BSK-H media influence the pathogenicity of in vitro grown B. burgdorferi (67). B. burgdorferi cell numbers were determined by dark field microscopy.
Mice.
Male CD18 hypomorph mice on the B6 background (B6.129S7-Itgb2tm1Bay/J) were bred at the Tufts University Division of Laboratory Animal Medicine from breeding pairs obtained from Jackson Laboratories (Bar Harbor, Maine) (kind gift of Joan Mecsas). The Institutional Animal Care and Use Committee of Tufts University approved all procedures. Age-matched wild-type B6 mice were purchased (Jackson Laboratories, Bar Harbor, Maine).
Four-week-old male CD18 hypomorph and wild-type mice were infected intradermally in the skin of the tibular area of both hind limbs with a total dose of 104 B. burgdorferi N40 cells per mouse. After infection, mice were followed for development of arthritis by measurement of ankle width with a caliper (Mahr Federal, Providence, RI), and serum was collected once a week through tail bleeding. At 2 to 3 weeks postinfection, at the peak of Lyme disease (based on Lyme arthritis edema measurements) (3, 68), mice were sacrificed by CO2 asphyxiation, and the heart and ankle tissues were harvested. In some experiments, the heart was cut longitudinally, and one half was used for histology (see “Histopathology” below), while the other one was used for DNA extraction (see “B. burgdorferi burden” below). In addition, some hearts were used for immunohistochemistry and/or RNA extraction. All mouse experiments were repeated at least three times.
Histopathology.
After being harvested, ankle tissues were decalcified and fixed overnight in Decalcifier I solution (Surgipath, Richmond, IL) and then kept in formalin until further processing. Hearts were cut in half through bisection across the atria and ventricles, and one half was fixed in phosphate-buffered 4% formalin. After fixation, either tissue was embedded in paraffin, sectioned and hematoxylin-eosin stained (HE). HE-stained sections were histopathologically scored for cellular infiltration in a blind fashion on a scale from 0 to 3 (54): 0, no inflammation; 1, mild inflammation, with less than two small foci of infiltration; 2, moderate inflammation, with 2 or more foci of infiltration; 3, severe inflammation, with focal and diffuse infiltration covering a large area, with the difference that we observed no necrosis of the myocardium.
Immunohistochemistry.
After being harvested, the heart was embedded in 22-oxyacalcitriol, snap-frozen in a dry ice-ethanol bath, and kept at −80°C until further processing. Frozen sections (6 μm thick) were cut from 22-oxyacalcitriol-embedded heart tissues representative of average carditis severity for each group, fixed in acetone for 10 min, air dried, and rehydrated in Tris-buffered saline (TBS). Sections were blocked in 20% swine serum (DAKO, Carpinteria, CA) and then with anti-CD16/CD32 (Pharmingen, San Diego, CA) in TBS-1% swine serum. To eliminate endogenous biotin activity, sections were treated with a biotin blocking system (DAKO, Carpinteria, CA), according to the manufacturer's instructions. After this, sections were stained using the DAKO LSAB+ system (Carpinteria, CA) with the anti-mouse F4/80 biotin antibody (Serotec, Raleigh, NC) or recombinant immunoglobulin G2b (IgG2b)-biotin isotype (E-bioscience), diluted to 0.5 μg/ml in TBS-1% swine serum.
B. burgdorferi burden.
Collected heart tissue was minced with a razor blade and kept frozen at −20°C until processing. DNA was extracted by a modification of the method of Morrison et al. (47). The tissue (half a heart) was incubated for 4 h at 37°C in 200 μl of 1 mg/ml collagenase A (Roche, Indianapolis, IN) in Dulbecco's phosphate-buffered saline without Ca2+/Mg2+ (Cellgro, Herndon, VA). The same volume of lysis solution (100 mM Tris-Hcl, pH 8, 5 mM EDTA, 0.2% sodium dodecyl sulfate, 200 mM NaCl) containing 1 mg/ml proteinase K (Invitrogen, Carlsbad, Calif.) was then added, and the tissue was further incubated overnight at 55°C. The resulting solution was extracted twice with buffer-saturated phenol (Invitrogen, Carlsbad, Calif.) and once with phenol-chloroform-isoamylalcohol (25:24:1; Invitrogen). The DNA was precipitated with 95% ethanol in the presence of 0.3 M Na acetate, pH 5.5, and resuspended in PCR-quality water.
The B. burgdorferi chromosomal RecA and the mouse nidogen genes were amplified by real-time PCR, according to the method described by Morrison et al. (47), with the following modifications: the real-time amplification was performed in a 50-μl volume containing QIAGEN SYBR green mix and 0.3 μM concentrations of the 5′ and 3′ RecA or nidogen primers and 300 ng of sample with an Applied Biosystem 5700 sequence detection system. The amplification was started by an incubation at 95°C for 15 min, followed by 45 cycles of 94°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. Data were analyzed with the GeneAmp5700SDS software and compared to standard curves of B. burgdorferi or mouse genomic DNA to calculate a RecA/nidogen ratio, representative of the number of B. burgdorferi genomes per mouse genome in the samples.
MCP-1 real-time reverse transcription-PCR.
Half of the heart was frozen in liquid nitrogen immediately after sacrifice of the mouse. The tissue was ground with a dry ice-cooled mortar and pestle, and RNA was extracted from the resulting ground tissue with Trizol (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. The RNA was treated with DNase from a DNA-free kit (Ambion, Austin, TX) and reverse transcribed to cDNA, using the Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA). The resulting cDNA was diluted 1:200 or 1:10 for 18S or MCP-1 amplification, respectively. A total of 20 μl of the diluted cDNA was used as a template in the real-time reaction mixture that contained 1× iTaq Sybr Green Supermix with ROX (Bio-Rad, Hercules, CA) and 0.3 μM concentrations of each primer. For the 18S amplification, primers were CGGCTACCACATCCAAGGAA and GCTGGAATTACCGCGGCT (2). For MCP-1 amplification, the primers used were TTAACGCCCCACTCACCTGCTG and GCTTCTTTGGGACACCTGCTGC (2, 71). The amplification, adapted from Akpek et al. (2), was performed on an Applied Biosystem 5700 sequence detection system. The reaction was initiated by incubation at 95°C for 2 min, followed by 40 cycles of 94°C for 15 seconds and 60°C for 1 min. Data were analyzed with the GeneAmp5700SDS software, and the factor of increase in expression relative to the uninfected WT sample was calculated and normalized to the expression of the housekeeping gene, 18S, for each sample.
Borreliacidal assay.
The ability of infected mouse serum to kill live B. burgdorferi in the presence of complement was determined by borreliacidal assay, as previously described (9). Briefly, B. burgdorferi was incubated with the serum samples in the presence of exogenous complement. After a 24-h incubation, B. burgdorferi cells were counted under a dark field microscope, and a percent viability was calculated in relation to complement-only samples. All samples were run in duplicates and scored in a double-blind fashion.
B. burgdorferi, OspC, or C6-specific Ig enzyme-linked immunosorbent assay (ELISA).
Flat-bottom Nunc-Immuno MaxiSorp plates (Rochester, NY) were coated with B. burgdorferi antigen, prepared by sonication as previously described (29), at 10 μg/ml, and with recombinant OspC (kind gift of Nikhat Parveen) at 4 μg/ml in coating buffer (Na2HPO4 0.1 M pH 9) overnight at 4°C or were purchased precoated with C6 (Immunetics, Boston, MA). Coated plates were blocked with blocking buffer (phosphate-buffered saline-0.05% Tween-2% bovine serum albumin; Sigma, St. Louis, MO) for 45 min at 37°C, with gentle shaking. Serum samples, diluted 1:400, were applied onto wells in duplicate and incubated at 37°C for 45 min. After washes, plates were incubated with alkaline phosphatase-conjugated anti-mouse IgG or specific isotype antibody (Southern Biotech, Birmingham, Ala.) for 1 h at 37°C. Plates were developed with a substrate solution prepared with 1 pNPP (Pierce, Rockford, IL) tablet dissolved in 10 ml of 0.2% diethanolamine substrate buffer (Pierce, Rockford, IL). Optical densities were read at a 405-nm wavelength in a SpectraMax Plus spectrophotometer (Molecular Devices, Sunnyvale, CA).
DC challenged with B. burgdorferi and MCP-1 ELISA.
Dendritic cells (DC) from CD18 hypomorph and wild-type mice were generated via granulocyte-macrophage colony-stimulating factor stimulation of mouse bone marrow, as previously described (42). After 10 days of growth in granulocyte-macrophage colony-stimulating factor-containing RPMI medium-10% fetal bovine serum, nonadherent DC were harvested, washed, and plated at 150,000 cells/500 μl/well in RPMI 1640-10% fetal bovine serum supplemented with L-glutamine in 24-well tissue culture plates. After overnight rest, DC were challenged with various B. burgdorferi cell/DC ratios for 24 h, and supernatants were collected and tested for the presence of the chemokine MCP-1 by mouse MCP-1 ELISA (R & D Systems), according to the manufacturer's instructions.
Statistical analysis.
Statistical analysis to compare experimental groups was performed through a Student's two-tailed, unpaired equal variance t test. A P value of ≤0.05 was considered statistically significant. In addition, statistical significance for the borreliacidal assay results, anti-OspC antibody level, and MCP-1 secretion by bone marrow-derived dendritic cells by a Student's t test was also confirmed through use of the nonparametric Mann-Whitney U test.
RESULTS
CD18 hypomorph mice develop Lyme arthritis.
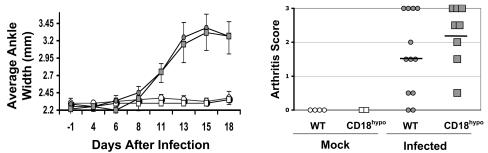
Lyme arthritis develops as a consequence of B. burgdorferi infection in the joint, manifested through edema and/or cellular infiltration. It has been shown that the susceptibility of mice to these two manifestations of Lyme arthritis is controlled by distinct chromosomal loci (51). No significant difference in the course or severity of arthritis between WT and CD18 hypomorph mice following B. burgdorferi infection was observed, as assessed by the measurement of joint edema (Fig. 1, left panel). Three weeks postinfection, we performed a histopathological assessment of cellular infiltration of ankle tissue. As shown in Fig. 1 (right panel), we observed no significant difference in arthritis severity, as measured by cellular infiltration of the ankle tissue. To confirm that CD18 hypomorph mice maintained low-level expression of β2 integrins, we performed CD18, CD11a, CD11b, and CD11c fluorescence-activated cell sorter staining of spleen and lymph node cells from mice at 2 weeks postinfection, when obvious signs of Lyme disease had already developed. Upon B. burgdorferi infection, the level of expression of β2 integrins was slightly up-regulated in WT mice, while it remained low in CD18 hypomorph mice upon B. burgdorferi infection (data not shown). One interpretation of these results is that low expression of β2 integrins is sufficient to allow full development of Lyme arthritis. Alternatively, other adhesion molecules, such as the β1 integrin very late antigen 4 (VLA-4) that mediates CD18-independent adhesion in some cases may contribute to extravasation in CD18 hypomorph mice.
FIG. 1.
Murine Lyme arthritis is unaffected by CD18 deficiency. (Left) The graph depicts the variation in average ankle width (a measure of arthritis edema) over the course of the experiment in uninfected WT (open circles; n = 2 mice) or CD18 hypomorph (open squares; n = 2 mice) and infected WT (filled circles; n = 8 mice) and CD18 hypomorph (filled squares; n = 7 mice) mice, with error bars corresponding to standard deviations of the means. Both ankles were measured for each mouse. Results correspond to one experiment, which is representative of five independent experiments. No significant differences in day of onset or ankle width were observed between the two experimental groups. (Right) Lyme arthritis pathology in WT and CD18 hypomorph mice at 3 weeks postinfection, determined by histopathology score (see Materials and Methods). Each symbol in the graph represents the histopathology score of one individual ankle, with two ankles per mouse included in the analysis, and the average of each experimental group is depicted as a horizontal line. Data correspond to one experiment, representative of three independent experiments. No significant differences were observed between groups (the average ± standard deviation was 1.6 ± 1.2 for WT and 2.25 ± 0.88 for CD18 hypomorph mice; P = 0.22, not significant by an unpaired t test). CD18hypo, CD18 hypomorph mice.
CD18 hypomorph mice develop increased Lyme carditis.
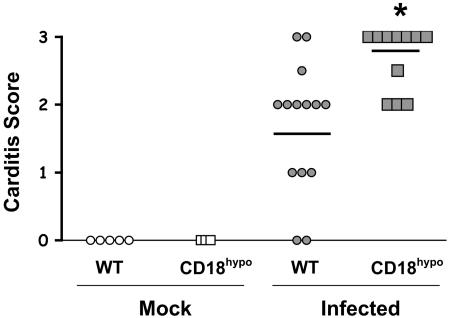
Lyme carditis is another manifestation of B. burgdorferi infection. To assess carditis, heart tissues were harvested 3 weeks postinfection and scored for cellular infiltration. We observed increased carditis severity in CD18 hypomorph mice, compared to WT mice (Fig. 2). Similar results were obtained when carditis was analyzed at 2 weeks postinfection (data not shown). Increased numbers of nucleated cells and larger foci of infiltration were observed in the base of the heart of the CD18 hypomorph mice (Fig. 3). This increase in infiltration of heart tissue suggests that β2 integrins play an important role in homeostasis of B. burgdorferi-induced inflammation.
FIG. 2.
Increased Lyme carditis severity in CD18 hypomorph mice. Lyme carditis pathology in WT (circles) and CD18 hypomorph (CD18hypo; squares) mice at 3 weeks postinfection, determined by histopathology score (see Materials and Methods). Open and filled symbols correspond to mock-infected and B. burgdorferi-infected mice, respectively. Each symbol represents an individual mouse, with the average shown by the horizontal line. The average ± standard deviation was 1.7 ± 0.95 for infected WT (n = 14) and 2.7 ± 0.46 for infected CD18 hypomorph mice (n = 11). Hearts from uninfected WT (n = 5) and CD18 hypomorph mice (n = 3) did not display symptoms of inflammation. The asterisk indicates that the difference is significant (P = 0.004). Data include the pooled results of three independent experiments.
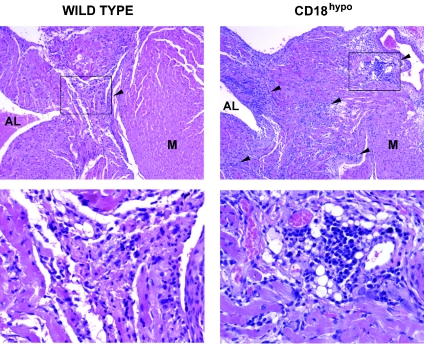
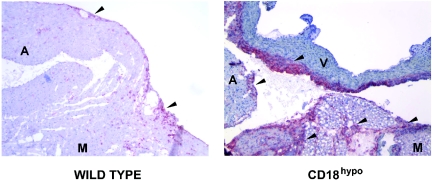
FIG. 3.
HE staining of heart sections from WT and CD18 hypomorph CD18hypo mice, 3 weeks after B. burgdorferi infection. The top left panel shows the base of the heart of an infected wild-type mouse, with a carditis severity score of 2, while the top right panel shows a comparable area from a CD18 hypomorph mouse, with a severity score of 3, both at a magnification of ×100. The scores selected represent the median score for each experimental group. The arrows point to some of the areas of infiltration. Note that the areas of infiltration in the CD18 hypomorph mouse are more abundant, dense, and extensive compared to WT mice. AL, atrium lumen; M, myocardial tissue. The bottom panels correspond to the insets of the picture directly above, blown up to a magnification of ×400. Sparse mononuclear cell infiltration is observed in WT (bottom left panel), compared to the heavy mononuclear cell infiltrate in the CD18 hypomorph (bottom right panel). Pictures were taken with SpotAdvanced software.
Serum of B. burgdorferi-infected CD18 hypomorph mice has reduced borreliacidal activity.
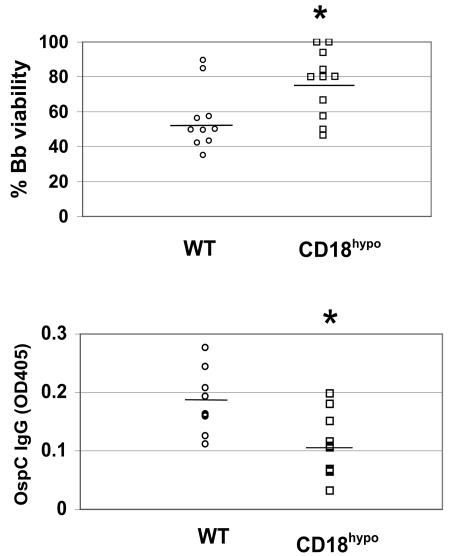
To study how β2 integrins exert their immunomodulatory effect, we looked at anti-B. burgdorferi antibody responses in CD18 hypomorph mice. It has been reported that lack of anti-B. burgdorferi antibody in B-cell-deficient mice results in increased carditis severity (46), similar to what we have observed in CD18 hypomorph mice. We hypothesized that CD18 hypomorph mice have an intrinsic defect in producing anti-B. burgdorferi antibodies that are important for control of B. burgdorferi infection by fixing complement, resulting in B. burgdorferi lysis (37). However, when we compared the total anti-B. burgdorferi sonicate antibody response in CD18 hypomorph and WT mice over the course of the infection, we found no significant qualitative or quantitative differences, as determined by the isotype profile and titer of anti-B. burgdorferi antibodies, respectively (data not shown). In contrast, when we determined the ability of CD18 hypomorph or WT day 21 postinfection immune serum plus exogenous complement to kill B. burgdorferi in a borreliacidal assay (9), we observed that a higher percentage of B. burgdorferi cells survived in the presence of serum from B. burgdorferi-infected CD18 hypomorph mice than from WT mice (Fig. 4, top). Because there are differences in the lipoproteins expressed by in vitro grown and host-adapted B. burgdorferi (15), it was important to determine whether the reduced borreliacidal activity observed against in vitro grown B. burgdorferi correlated with a reduced antibody response against host-adapted B. burgdorferi proteins. OspC is abundantly expressed in host-adapted B. burgdorferi (22), and anti-OspC antibodies have been shown to be, in many cases, the most important borreliacidal antibody (36). Therefore, we looked at the antibody response to recombinant OspC. We indeed found significantly reduced levels of anti-OspC antibodies in the sera of B. burgdorferi-infected CD18 hypomorph mice compared to levels in WT (Fig. 4, bottom). As a control, we determined the levels of antibodies against the C6 peptide, an invariant epitope in the B. burgdorferi protein VlsE that is useful for diagnosis of B. burgdorferi infection (5), in the sera of the two groups of mice and found no significant differences (data not shown). These data indicate that the antibody-mediated borreliacidal activity in CD18 hypomorph mice is reduced, with a defect in the production of anti-OspC antibodies.
FIG. 4.
Reduced borreliacidal activity in the serum of CD18 hypomorph mice. (Top) The percentages of viable B. burgdorferi cells after incubation in serum from infected WT (circles; n = 10) and CD18 hypomorph mice (squares; n = 11) at 3 weeks postinfection are shown. Each symbol represents the serum from one infected mouse, with the average of each experimental group depicted as a horizontal line (the average was 55.99% for WT and 76.31% for CD18 hypomorph mice sera). Samples from four independent experiments were included in this analysis. The asterisk indicates that the difference is statistically significant (P = 0.02). The difference was also statistically significant when the nonparametric Mann-Whitney U test was used. (Bottom) Anti-OspC IgG level in B. burgdorferi-infected WT (circles) and CD18 hypomorph (squares) sera at 3 weeks postinfection. Each symbol corresponds to serum from one mouse. The samples were combined from four independent experiments, as in the top panel. Average optical density (OD) values for B. burgdorferi-infected WT (n = 9) and CD18 hypomorph (n = 10) sera were 0.183 and 0.109, respectively, and are depicted as horizontal lines. The asterisk indicates that the difference is statistically significant (P = 0.008). The difference was also statistically significant when the nonparametric Mann-Whitney U test was used. No anti-OspC IgG was detected in sera from uninfected mice. Bb, B. burgdorferi; CD18hypo, CD18 hypomorph.
CD18 hypomorph mice control B. burgdorferi burden.
The reduced borreliacidal activity of the sera from B. burgdorferi-infected CD18 hypomorph mice could result in increased B. burgdorferi burden and increased carditis. To investigate this, we harvested heart tissue 3 weeks postinfection for DNA extraction. The B. burgdorferi burden in these samples was determined by real-time PCR. We found no significant difference in the B. burgdorferi burden in the hearts of mice from CD18 hypomorph and WT mice (the median was 5.57 B. burgdorferi genomes/105 mouse genomes for WT and 5.8 B. burgdorferi genomes/105 mouse genomes for CD18 hypomorph mice; n = 8 per group from three independent experiments; P = 0.4, not significant). Similar results were obtained with ear tissue samples (data not shown). These data indicate that CD18 hypomorph mice control the amount of B. burgdorferi burden to a level similar to that of WT mice; thus, the difference in carditis severity is not related to increased B. burgdorferi burden in the heart. The maintenance of similar B. burgdorferi burden in the presence of reduced antibody-mediated borreliacidal activity appeared paradoxical; however, other borreliacidal mechanisms, like increased macrophage infiltration, may compensate for this defect.
Increased cardiac macrophage infiltration in B. burgdorferi-infected CD18 hypomorph mice.
The two most important mechanisms for B. burgdorferi elimination appear to be borreliacidal antibodies and macrophage killing (62). We hypothesized that the reduced borreliacidal antibody activity in CD18 hypomorph mice might be compensated by an increase in the recruitment of macrophages to the heart. It has been reported that the most abundant inflammatory cells found in the Lyme carditis lesion are cells of the monocyte/macrophage lineage, characterized by expression of the Mac-1 and F4/80 markers (52). To test whether the increased carditis in the CD18 hypomorph mice was accompanied by increased macrophage infiltration, we stained frozen sections of infected WT and CD18 hypomorph mice with the macrophage marker F4/80 or its corresponding isotype control. The β2 integrin Mac-1 was not used as a marker in this case because its expression is reduced in CD18 hypomorph mice. Heart sections from infected CD18 hypomorph mice showed massive macrophage infiltration compared to mild infiltration in those of WT mice (Fig. 5). These results suggest that, similar to Lyme carditis in WT mice, the macrophage is the main infiltrating cell in the Lyme carditis lesions of CD18 hypomorph mice.
FIG. 5.
Increased macrophage infiltration in hearts of B. burgdorferi-infected CD18 hypomorph mice. Two sections of the base of the heart of wild-type (left panel) and CD18 hypomorph mice (right panel) are shown, corresponding to the median carditis severity for each group. The sections are stained with the macrophage marker F4/80 in red/fuchsia, and counterstained with hematoxylin. Isotype control stainings on serial sections demonstrated minimal background, confirming the specificity of the F4/80 staining. Both pictures were taken at a magnification of ×100. Pericardially localized macrophage infiltration foci are observed in B. burgdorferi-infected hearts in both groups of mice (marked with arrowheads). However, CD18 hypomorph mice show denser and more numerous areas of macrophage infiltration. The more intense blue staining observed in the major blood vessel on the right panel corresponds to normal endothelial cell staining. Pictures were taken with SpotAdvanced software. A, atrium wall; M, myocardium; V, major blood vessel; CD18hypo, CD18 hypomorph.
Dendritic cells from CD18 hypomorph mice show enhanced secretion of MCP-1.
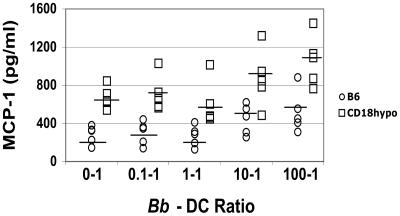
The recruitment of macrophages into the infected heart has been associated with expression of the monocyte/macrophage chemoattractant MCP-1 (52). Dendritic cells are key initiators of the immune reaction to infection via cytokine and chemokine production (18). Thus, we postulated that DCs resident in heart tissue secrete MCP-1, which attracts macrophages. As a measure of how CD18 hypomorph and WT mice respond to the presence of the infectious B. burgdorferi, we looked at the chemokine response of bone marrow-derived DCs to B. burgdorferi. We found that both WT and CD18 hypomorph DCs responded to B. burgdorferi with an increase in MCP-1 secretion, but CD18 hypomorph DCs secrete about twice as much of the chemokine MCP-1 than WT DCs (Fig. 6), reaching the MCP-1 concentration of 1 ng/ml shown to induce maximal migration of monocytes in an in vitro chemotaxis assay (4). In addition, baseline levels of MCP-1 secretion were higher in the CD18 hypomorph mice than in WT mice (Fig. 6). These data suggest that increased levels of MCP-1 expression might be found in the hearts of B. burgdorferi-infected CD18 hypomorph mice.
FIG. 6.
MCP-1 chemokine production of CD18 hypomorph (CD18hypo) versus wild-type DCs. MCP-1 secretion, determined by ELISA, by bone marrow-derived DCs from wild-type (open circles) and CD18 hypomorph (open squares) mice at increasing ratios of B. burgdorferi to DCs. Each symbol corresponds to dendritic cells originating from the bone marrow of one mouse, with the average of each experimental group depicted as a horizontal line. Data from three independent experiments were combined. The difference between values for WT and CD18 hypomorph mice was statistically significant at all tested ratios (P < 0.05). The differences were also statistically significant when the nonparametric Mann-Whitney U test was used. Average MCP-1 values for B. burgdorferi cell:DC ratios of 0:1, 0.1:1, 1:1, 10:1, and 100:1 were 281.9, 299.4, 267.4, 442.4, and 521 for WT mice, respectively, and 669.2, 710, 600.4, 874, and 1060.4 for CD18 hypomorph mice, respectively. Bb, B. burgdorferi.
Expression of MCP-1 in hearts of B. burgdorferi-infected CD18 hypomorph mice.
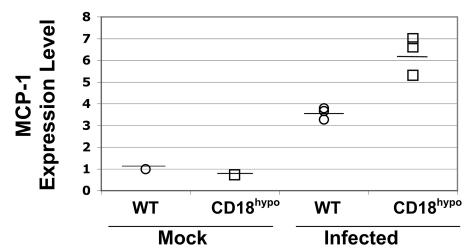
To test the hypothesis that the increased macrophage infiltration in the heart of CD18 hypomorph mice is due to increased secretion of MCP-1 in response to B. burgdorferi, we harvested hearts of uninfected or infected WT and CD18 hypomorph mice at 2 weeks postinfection, when inflammation is still developing and cells are being recruited into the heart. The levels of transcription of the MCP-1 gene and the control gene, 18S, were determined by real-time PCR. In uninfected mice, we found that MCP-1 was expressed at similar levels in WT and CD18 hypomorphs (Fig. 7), which contrasts with the increased MCP-1 secretion by CD18 hypomorph DCs in the absence of B. burgdorferi challenge (Fig. 6) but correlates with the absence of cellular infiltration in uninfected CD18 hypomorph mice (Fig. 2). Upon B. burgdorferi infection, MCP-1 expression was up-regulated threefold in WT and sixfold in CD18 hypomorph mice (Fig. 7). The twofold increase in the level of expression of MCP-1 transcripts in CD18 hypomorph compared to WT mice correlates well with the twofold increase in secretion of MCP-1 by DCs and provides a possible explanation for the increased macrophage infiltration observed in CD18 hypomorph mice.
FIG. 7.
Increased MCP-1 expression in hearts from B. burgdorferi-infected CD18 hypomorph mice. The expression level of MCP-1, relative to the uninfected WT mouse sample, was measured by reverse transcriptase real-time PCR and normalized to 18S expression in each sample. The heart of CD18 hypomorph mice expresses levels of MCP-1 similar to that of reference uninfected WT mice. Upon B. burgdorferi infection, MCP-1 was induced in WT mice (average ± standard deviation, 3.5 ± 0.27) to a lesser extent than in CD18 hypomorph mice (average ± standard deviation, 6.3 ± 0.87). The average of each experimental group is depicted as a horizontal line. The asterisk indicates that the difference is significant (P = 0.006).
DISCUSSION
Lyme arthritis and carditis are a consequence of migration of immune cells into B. burgdorferi-infected joint and heart tissues (6, 7). Because leukocyte migration is considered to depend on β2 integrins (33), we addressed the role of β2 integrins in the development of Lyme arthritis and carditis. We have found that CD18 deficiency results in increased secretion of MCP-1 by CD18 hypomorph DCs and an increase in MCP-1 RNA expression in the B. burgdorferi-infected heart. Our data suggest that this deregulation results in increased macrophage infiltration in the heart and a consequent increase in the severity of carditis.
Contrary to our expectations, development of Lyme arthritis and carditis was not impaired in CD18 hypomorph mice. Leukocytes from CD18 hypomorph mice express low levels (2 to 16%) of β2 integrins (69), and we have confirmed that they do not up-regulate these molecules to WT level upon B. burgdorferi challenge. This indicates that either a low level of expression of CD18 is sufficient for cell extravasation into the B. burgdorferi-infected heart and ankle tissues or that additional adhesion molecules contribute to this extravasation. In this regard, the CD18 null mouse model may prove useful in distinguishing between these two possibilities. However, it is interesting that CD18-independent extravasation of monocytes and neutrophils has been observed in other systems. For example, Streptococcus pneumoniae induces CD18-independent extravasation into the lungs but not in the abdominal wall, suggesting that this effect is tissue-specific (24). Cardiac endothelium is another tissue in which CD18-independent migration has been observed and alternative molecules, such as the VLA-4/VCAM-1 pair, have been proposed to be involved in this migration (14). Furthermore, B. burgdorferi lipoproteins can induce expression of VCAM-1 on endothelial cells (25) and may, therefore, be setting the right conditions for CD18-independent extravasation of cells into tissues. We have observed a slight, but not statistically significant, up-regulation of the β1 chain present in VLA adhesion molecules in leukocytes from B. burgdorferi-infected CD18 hypomorph mice (data not shown), which may contribute to cellular extravasation.
Nonetheless, the observed increase in carditis severity was puzzling, and we tried to address its nature and its cause. We have found that the increased severity of carditis was accompanied by increased macrophage infiltration in the heart. The mainly monocyte/macrophage infiltrate characteristic of Lyme carditis is believed to be determined by production of the monocyte/macrophage chemoattractant MCP-1 in the heart (52). In fact, an increase in MCP-1 production has previously been observed to correlate with cardiac disease, as human patients with acute myocarditis show a twofold increase in MCP-1 serum levels, compared to healthy controls (28). In addition, there is direct evidence that cardiac expression of MCP-1 results in development of myocarditis, since mice transgenic for the MCP-1 gene under control of the α cardiac myosin heavy chain promoter develop myocarditis, with macrophage infiltration (38). MCP-1 is a potent chemoattractant for monocytes, but the MCP-1 receptor CCR2 is also expressed on activated and memory T cells, basophils, DCs, and NK cells (12, 49, 53). Therefore, increased recruitment of these cells may also contribute to increased Lyme carditis severity in CD18 hypomorph mice.
MCP-1 is reportedly secreted by a variety of cell types, including DCs, macrophages, fibroblasts, vascular endothelial cells, and vascular smooth muscle cells (50, 66). Although any of these cell types may be responsible for MCP-1 production in the heart of CD18 hypomorph mice upon sensing B. burgdorferi infection, DCs are professional innate immune cells that can sense the site of infection, move toward it, produce very high amounts of MCP-1, and amplify the response through recruitment of more macrophages and DCs. Although no direct staining of DCs was performed in the heart of CD18 hypomorph mice, a population of heart-resident DCs has been reported to be present in rat hearts (58). Our results indicate that CD18 hypomorph bone marrow-derived DCs secrete twofold more MCP-1 upon B. burgdorferi infection, compared to WT, favoring the notion that DCs may be responsible for increased MCP-1 production in the heart. An increase in MCP-1 secretion by CD18 hypomorph DCs versus WT DCs was also observed in the absence of B. burgdorferi stimulation. The significance of this baseline difference in MCP-1 production is unclear, especially since we have not observed increased infiltration in the hearts and no increase in MCP-1 mRNA expression in the hearts of uninfected CD18 hypomorph mice.
The question remains whether CD18 hypomorph mice have a T-cell defect that contributes to the development of increased severity of Lyme carditis. SCID mice, which do not have T or B cells, do not resolve Lyme carditis, which is occasionally of increased severity (8, 55), and increased Lyme carditis severity has also been observed in RAG-1−/− mice, which lack T and B cells (46). Interestingly, delayed resolution of Lyme carditis was observed in TcRαβ−/− mice that could be reversed by transfer of Th1 T cells (10). Therefore, a defect in T-cell responses may affect the resolution or, maybe, the severity of Lyme carditis. It is known that CD18−/− mice have a defect in T-cell receptor-mediated responses, with reduced or absent proliferative response to alloantigens or staphylococcal enterotoxin (56). A less pronounced T-cell defect may, therefore, also exist in CD18 hypomorph mice, but this has not been experimentally tested. In this regard, it is also interesting that MCP-1 can skew the T-cell response toward a Th2 phenotype, which may compromise the resolution of Lyme carditis. In any case, it is also important to bear in mind that the clearest role of T cells in Lyme carditis is in the resolution phase, while our study was limited to the acute phase of Lyme carditis.
In contrast to the increase in MCP-1, serum from B. burgdorferi-infected CD18 hypomorph mice has reduced borreliacidal activity and anti-OspC antibody responses compared to that of WT mice. These specific defects occur in the absence of a general defect in anti-B. burgdorferi antibody production. It is unclear why production of antibodies against some B. burgdorferi targets is reduced in CD18 hypomorph mice. A possibility is that T-cell help may be more important for production of antibodies against particular B. burgdorferi proteins, such as OspC, than others, such as VlsE C6. The immunogenicity of each protein may be a factor influencing this difference, since peptide C6 in the highly conserved IR6 segment of VlsE VlsE is highly immunodominant (5, 41) and may induce antibodies more readily than OspC. It is known that B. burgdorferi is capable of inducing specific isotype-switched antibody responses in CD40L-deficient mice, despite the inability of these mice to mediate T-cell-dependent responses (26). However, the production of antibodies against certain targets was impaired in CD40L−/− mice (26), similar to what we found in CD18 hypomorph mice. Furthermore, in a more artificial system, in which B. burgdorferi-pulsed antigen-presenting cells were injected into mice, T-cell help for production of anti-OspC antibodies was provided by αβ and γδ T cells (45).
While we have observed increased carditis severity in CD18 hypomorph mice, arthritis severity was not significantly increased. One important difference between Lyme arthritis and carditis is the predominant infiltrating cell: the monocyte/macrophage in carditis (52) and the neutrophil in arthritis (6, 7). MCP-1 is a CC chemokine with specific monocyte/macrophage chemoattractant activity but no effect on neutrophils (70). Therefore, the alteration in MCP-1 secretion in CD18 hypomorph mice would not, in theory, affect arthritis severity, and in actuality that is what we observed.
How the reduced level of β2 integrins in CD18 hypomorph mice results in increased level of MCP-1 secretion by DCs is an important question. MCP-1 is a chemoattractant that has effects on both the initial adhesion and subsequent migration of cells into tissues (35). More specifically, MCP-1 reportedly induces expression of several β2 integrins on human monocytes, including the alpha chains CD11b and CD11c of Mac-1/CR3 and p150,95 (35). We postulate that the increased expression of MCP-1 in CD18 hypomorph mice reflects an unsuccessful compensatory mechanism by which the cell tries to up-regulate cellular expression of β2 integrins in an autocrine manner. Support for this hypothesis comes from the observation by a number of groups that nitric oxide (NO) inhibits MCP-1 production, while treatment with an inhibitor of the NO synthase greatly enhances MCP-1 mRNA expression (13, 65). CD18 engagement has been shown to induce NO production (39). Therefore, reduced levels of CD18 in CD18 hypomorph mice may reduce production of NO, resulting in increased MCP-1 production. Future work will be aimed to determine the accuracy of this model.
The importance of chemokines in cell recruitment during Lyme disease has recently been demonstrated, to the extent that these chemoattractants may be indispensable for development of Lyme arthritis and carditis. For example, B. burgdorferi-infected mice deficient in the receptor for the neutrophil chemoattractant CXCL1 (KC) fail to recruit neutrophils to the joint and do not develop arthritis (16). This study was based on the observation that the KC and MCP-1 chemokines are expressed at higher levels in joints of high pathology C3H mice compared to low pathology B6 mice (16). Even though these two strains harbor similar numbers of spirochetes, the severity of lesions is higher in C3H mice (43). In contrast, no effect on arthritis severity was found in MCP-1 receptor knockout (CCR2−/−) mice (16). In light of our results, it would be interesting to study the development of carditis in CCR2−/− mice. Our prediction is that carditis development would be reduced or abrogated in these mice.
Recent evidence has pointed out that Toll-like receptor signaling, although essential for control of B. burgdorferi burden, is not required for development of B. burgdorferi-induced inflammation. This has been interpreted as a “dichotomy between host defense and inflammatory Lyme arthritis” (11). The data from the laboratory of Brown et al. (16), discussed above, imply that chemokines play a crucial role in controlling the severity of infiltration in Lyme disease, providing a possible mechanism that resolves the above dichotomy. In this regard, the fact that MyD88-independent secretion of MCP-1 has been observed in a Listeria monocytogenes infection model (57) is extremely interesting. Determining whether MyD88−/− macrophages can produce chemokines in response to B. burgdorferi may further clarify why inflammation still develops in these mice. Our study supports the idea that chemokines determine the severity of inflammation. Furthermore, it highlights the importance of β2 integrins, not only in the initiation of the inflammatory response through adhesion of immune cells to the endothelium but also in the control of this inflammatory response through regulation of chemokine production.
Acknowledgments
This work was funded by NIHRO1 grant AR45386, GRASP Center NIH/NIDDK grant P30 DK34928, and the Eshe Fund.
We are grateful to Jenifer Coburn for challenging discussions, technical advice, and the gift of B. burgdorferi N40, to Nikhat Parveen for the kind gift of the recombinant OspC, to Joan Mecsas and J. M. Balada-Llasat for the initial CD18 hypomorph breeding pair, and to J. Whittum-Hudson for MCP-1 real-time PCR technical advice. We thank members of the laboratory, J. Coburn and L. Hu, for critical reading of the manuscript and G. Calderon and M. Betancur for expert technical help.
Editor: J. T. Barbieri
REFERENCES
- 1.Akin, E., J. Aversa, and A. C. Steere. 2001. Expression of adhesion molecules in synovia of patients with treatment-resistant lyme arthritis. Infect. Immun. 69:1774-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akpek, E. K., D. A. Jabs, H. C. Gerard, R. A. Prendergast, A. P. Hudson, B. Lee, and J. A. Whittum-Hudson. 2004. Chemokines in autoimmune lacrimal gland disease in MRL/MpJ mice. Invest. Ophthalmol. Vis. Sci. 45:185-190. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong, A. L., S. W. Barthold, D. H. Persing, and D. S. Beck. 1992. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 47:249-258. [DOI] [PubMed] [Google Scholar]
- 4.Audran, R., T. Lesimple, M. Delamaire, C. Picot, J. Van Damme, and L. Toujas. 1996. Adhesion molecule expression and response to chemotactic agents of human monocyte-derived macrophages. Clin. Exp. Immunol. 103:155-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacon, R. M., B. J. Biggerstaff, M. E. Schriefer, R. D. Gilmore, Jr., M. T. Philipp, A. C. Steere, G. P. Wormser, A. R. Marques, and B. J. Johnson. 2003. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J. Infect. Dis. 187:1187-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barthold, S., D. S. Beck, G. M. Hansen, G. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 7.Barthold, S. W., D. H. Persing, A. L. Armstrong, and R. A. Peeples. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am. J. Pathol. 139:263-273. [PMC free article] [PubMed] [Google Scholar]
- 8.Barthold, S. W., C. L. Sidman, and A. L. Smith. 1992. Lyme borreliosis in genetically resistant and susceptible mice with severe combined immunodeficiency. Am. J. Trop. Med. Hyg. 47:605-613. [DOI] [PubMed] [Google Scholar]
- 9.Belperron, A. A., and L. K. Bockenstedt. 2001. Natural antibody affects survival of the spirochete Borrelia burgdorferi within feeding ticks. Infect. Immun. 69:6456-6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bockenstedt, L. K., I. Kang, C. Chang, D. Persing, A. Hayday, and S. W. Barthold. 2001. CD4+ T helper 1 cells facilitate regression of murine Lyme carditis. Infect. Immun. 69:5264-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirschning, J. F. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 173:2003-2010. [DOI] [PubMed] [Google Scholar]
- 12.Bonecchi, R., G. Bianchi, P. P. Bordignon, D. D'Ambrosio, R. Lang, A. Borsatti, S. Sozzani, P. Allavena, P. A. Gray, A. Mantovani, and F. Sinigaglia. 1998. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosco, M. C., M. Puppo, S. Pastorino, Z. Mi, G. Melillo, S. Massazza, A. Rapisarda, and L. Varesio. 2004. Hypoxia selectively inhibits monocyte chemoattractant protein-1 production by macrophages. J. Immunol. 172:1681-1690. [DOI] [PubMed] [Google Scholar]
- 14.Bowden, R. A., Z. M. Ding, E. M. Donnachie, T. K. Petersen, L. H. Michael, C. M. Ballantyne, and A. R. Burns. 2002. Role of α4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circ. Res. 90:562-569. [DOI] [PubMed] [Google Scholar]
- 15.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71:3371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown, C. R., V. A. Blaho, and C. M. Loiacono. 2003. Susceptibility to experimental Lyme arthritis correlates with KC and monocyte chemoattractant protein-1 production in joints and requires neutrophil recruitment via CXCR2. J. Immunol. 171:893-901. [DOI] [PubMed] [Google Scholar]
- 17.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 18.Carbone, F. R., and W. R. Heath. 2003. The role of dendritic cell subsets in immunity to viruses. Curr. Opin. Immunol. 15:416-420. [DOI] [PubMed] [Google Scholar]
- 19.Cinco, M., E. Panfili, G. Presani, and S. Perticarari. 2000. Interaction with Borrelia burgdorferi causes increased expression of the CR3 integrin and increased binding affinity to fibronectin via CR3. J. Mol. Microbiol. Biotechnol. 2:575-579. [PubMed] [Google Scholar]
- 20.Coburn, J., S. W. Barthold, and J. M. Leong. 1994. Diverse Lyme disease spirochetes bind integrin αIIbβ3 on human platelets. Infect. Immun. 62:5559-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coburn, J., J. M. Leong, and J. K. Erban. 1993. Integrin αIIbβ3 mediates binding of the Lyme disease agent Borrelia burgdorferi to human platelets. Proc. Natl. Acad. Sci. USA 90:7059-7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crother, T. R., C. I. Champion, X. Y. Wu, D. R. Blanco, J. N. Miller, and M. A. Lovett. 2003. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect. Immun. 71:3419-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crowley, H., and B. T. Huber. 2003. Host-adapted Borrelia burgdorferi in mice expresses OspA during inflammation. Infect. Immun. 71:4003-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doerschuk, C. M., R. K. Winn, H. O. Coxson, and J. M. Harlan. 1990. CD18-dependent and -independent mechanisms of neutrophil emigration in the pulmonary and systemic microcirculation of rabbits. J. Immunol. 144:2327-2333. [PubMed] [Google Scholar]
- 25.Ebnet, K., K. D. Brown, U. K. Siebenlist, M. M. Simon, and S. Shaw. 1997. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J. Immunol. 158:3285-3292. [PubMed] [Google Scholar]
- 26.Fikrig, E., S. W. Barthold, M. Chen, I. S. Grewal, J. Craft, and R. A. Flavell. 1996. Protective antibodies in murine Lyme disease arise independently of CD40 ligand. J. Immunol. 157:1-3. [PubMed] [Google Scholar]
- 27.Fischer, A., B. Lisowska-Grospierre, D. C. Anderson, and T. A. Springer. 1988. Leukocyte adhesion deficiency: molecular basis and functional consequences. Immunodefic. Rev. 1:39-54. [PubMed] [Google Scholar]
- 28.Fuse, K., M. Kodama, H. Hanawa, Y. Okura, M. Ito, T. Shiono, S. Maruyama, S. Hirono, K. Kato, K. Watanabe, and Y. Aizawa. 2001. Enhanced expression and production of monocyte chemoattractant protein-1 in myocarditis. Clin. Exp. Immunol. 124:346-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glickstein, L., M. Edelstein, and J. Z. Dong. 2001. Gamma interferon is not required for arthritis resistance in the murine Lyme disease model. Infect. Immun. 69:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross, D. M., T. Forsthuber, M. Tary-Lehmann, C. Etling, K. Ito, Z. A. Nagy, J. A. Field, A. C. Steere, and B. T. Huber. 1998. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281:703-706. [DOI] [PubMed] [Google Scholar]
- 31.Gupta, R. S., D. Y. Chan, and L. Siminovitch. 1978. Evidence for functional hemizygosity at the Emtr locus in CHO cells through segregation analysis. Cell 14:1007-1013. [DOI] [PubMed] [Google Scholar]
- 32.Haddad, F. A., and R. B. Nadelman. 2003. Lyme disease and the heart. Front. Biosci. 8:s769-s782. [DOI] [PubMed] [Google Scholar]
- 33.Harris, E. S., T. M. McIntyre, S. M. Prescott, and G. A. Zimmerman. 2000. The leukocyte integrins. J. Biol. Chem. 275:23409-23412. [DOI] [PubMed] [Google Scholar]
- 34.Hogg, N., and P. A. Bates. 2000. Genetic analysis of integrin function in man: LAD-1 and other syndromes. Matrix Biol. 19:211-222. [DOI] [PubMed] [Google Scholar]
- 35.Jiang, Y., D. I. Beller, G. Frendl, and D. T. Graves. 1992. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J. Immunol. 148:2423-2428. [PubMed] [Google Scholar]
- 36.Jobe, D. A., S. D. Lovrich, R. F. Schell, and S. M. Callister. 2003. C-terminal region of outer surface protein C binds borreliacidal antibodies in sera from patients with Lyme disease. Clin. Diagn. Lab. Immunol. 10:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kochi, S. K., R. C. Johnson, and A. P. Dalmasso. 1991. Complement-mediated killing of the Lyme disease spirochete Borrelia burgdorferi. Role of antibody in formation of an effective membrane attack complex. J. Immunol. 146:3964-3970. [PubMed] [Google Scholar]
- 38.Kolattukudy, P. E., T. Quach, S. Bergese, S. Breckenridge, J. Hensley, R. Altschuld, G. Gordillo, S. Klenotic, C. Orosz, and J. Parker-Thornburg. 1998. Myocarditis induced by targeted expression of the MCP-1 gene in murine cardiac muscle. Am. J. Pathol. 152:101-111. [PMC free article] [PubMed] [Google Scholar]
- 39.Kurose, I., H. Saito, S. Miura, H. Ebinuma, H. Higuchi, N. Watanabe, S. Zeki, T. Nakamura, M. Takaishi, and H. Ishii. 1997. CD18/ICAM-1-dependent oxidative NF-κB activation leading to nitric oxide production in rat Kupffer cells cocultured with syngeneic hepatoma cells. J. Clin. Investig. 99:867-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lane, R. S., J. Piesman, and W. Burgdorfer. 1991. Lyme borreliosis: relation of its causative agent to its tick vectors and hosts in North America and Europe. Annu. Rev. Entomol. 36:587-609. [DOI] [PubMed] [Google Scholar]
- 41.Liang, F. T., A. L. Alvarez, Y. Gu, J. M. Nowling, R. Ramamoorthy, and M. T. Philipp. 1999. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J. Immunol. 163:5566-5573. [PubMed] [Google Scholar]
- 42.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 43.Ma, Y., K. P. Seiler, E. J. Eichwald, J. H. Weis, C. Teuscher, and J. J. Weis. 1998. Distinct characteristics of resistance to Borrelia burgdorferi-induced arthritis in C57BL/6N mice. Infect. Immun. 66:161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marcus, L. C., A. C. Steere, P. H. Duray, A. E. Anderson, and E. B. Mahoney. 1985. Fatal pancarditis in a patient with coexistent Lyme disease and babesiosis. Demonstration of spirochetes in the myocardium. Ann. Intern. Med. 103:374-376. [DOI] [PubMed] [Google Scholar]
- 45.Mbow, M. L., N. Zeidner, N. Panella, R. G. Titus, and J. Piesman. 1997. Borrelia burgdorferi-pulsed dendritic cells induce a protective immune response against tick-transmitted spirochetes. Infect. Immun. 65:3386-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKisic, M. D., W. L. Redmond, and S. W. Barthold. 2000. Cutting edge: T cell-mediated pathology in murine Lyme borreliosis. J. Immunol. 164:6096-6099. [DOI] [PubMed] [Google Scholar]
- 47.Morrison, T. B., Y. Ma, J. H. Weis, and J. J. Weis. 1999. Rapid and sensitive quantification of Borrelia burgdorferi-infected mouse tissues by continuous fluorescent monitoring of PCR. J. Clin. Microbiol. 37:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morrison, T. B., J. H. Weis, and J. J. Weis. 1997. Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J. Immunol. 158:4838-4845. [PubMed] [Google Scholar]
- 49.Rollins, B. J. 1997. Chemokines. Blood 90:909-928. [PubMed] [Google Scholar]
- 50.Rollins, B. J., T. Yoshimura, E. J. Leonard, and J. S. Pober. 1990. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am. J. Pathol. 136:1229-1233. [PMC free article] [PubMed] [Google Scholar]
- 51.Roper, R. J., J. J. Weis, B. A. McCracken, C. B. Green, Y. Ma, K. S. Weber, D. Fairbairn, R. J. Butterfield, M. R. Potter, J. F. Zachary, R. W. Doerge, and C. Teuscher. 2001. Genetic control of susceptibility to experimental Lyme arthritis is polygenic and exhibits consistent linkage to multiple loci on chromosome 5 in four independent mouse crosses. Genes Immun. 2:388-397. [DOI] [PubMed] [Google Scholar]
- 52.Ruderman, E. M., J. S. Kerr, S. R. Telford III, A. Spielman, L. H. Glimcher, and E. M. Gravallese. 1995. Early murine Lyme carditis has a macrophage predominance and is independent of major histocompatibility complex class II-CD4+ T cell interactions. J. Infect. Dis. 171:362-370. [DOI] [PubMed] [Google Scholar]
- 53.Sallusto, F., D. Lenig, C. R. Mackay, and A. Lanzavecchia. 1998. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 187:875-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Satoskar, A. R., J. Elizondo, G. M. Monteforte, L. M. Stamm, H. Bluethmann, P. Katavolos, and S. R. Telford III. 2000. Interleukin-4-deficient BALB/c mice develop an enhanced Th1-like response but control cardiac inflammation following Borrelia burgdorferi infection. FEMS Microbiol. Lett. 183:319-325. [DOI] [PubMed] [Google Scholar]
- 55.Schaible, U. E., S. Gay, C. Museteanu, M. D. Kramer, G. Zimmer, K. Eichmann, U. Museteanu, and M. M. Simon. 1990. Lyme borreliosis in the severe combined immunodeficiency (SCID) mouse manifests predominantly in the joints, heart, and liver. Am. J. Pathol. 137:811-820. [PMC free article] [PubMed] [Google Scholar]
- 56.Scharffetter-Kochanek, K., H. Lu, K. Norman, N. van Nood, F. Munoz, S. Grabbe, M. McArthur, I. Lorenzo, S. Kaplan, K. Ley, C. W. Smith, C. A. Montgomery, S. Rich, and A. L. Beaudet. 1998. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J. Exp. Med. 188:119-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serbina, N. V., W. Kuziel, R. Flavell, S. Akira, B. Rollins, and E. G. Pamer. 2003. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity 19:891-901. [DOI] [PubMed] [Google Scholar]
- 58.Spencer, S. C., and J. W. Fabre. 1990. Characterization of the tissue macrophage and the interstitial dendritic cell as distinct leukocytes normally resident in the connective tissue of rat heart. J. Exp. Med. 171:1841-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spielman, A. 1994. The emergence of Lyme disease and human babesiosis in a changing environment. Ann. N. Y. Acad. Sci. 740:146-156. [DOI] [PubMed] [Google Scholar]
- 60.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 61.Steere, A. C., W. P. Batsford, M. Weinberg, J. Alexander, H. J. Berger, S. Wolfson, and S. E. Malawista. 1980. Lyme carditis: cardiac abnormalities of Lyme disease. Ann. Intern. Med. 93:8-16. [DOI] [PubMed] [Google Scholar]
- 62.Steere, A. C., J. Coburn, and L. Glickstein. 2004. The emergence of Lyme disease. J. Clin. Investig. 113:1093-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Steere, A. C., R. L. Grodzicki, A. N. Kornblatt, J. E. Craft, A. G. Barbour, W. Burgdorfer, G. P. Schmid, E. Johnson, and S. E. Malawista. 1983. The spirochetal etiology of Lyme disease. N. Engl. J. Med. 308:733-740. [DOI] [PubMed] [Google Scholar]
- 64.Steere, A. C., R. T. Schoen, and E. Taylor. 1987. The clinical evolution of Lyme arthritis. Ann. Intern. Med. 107:725-731. [DOI] [PubMed] [Google Scholar]
- 65.Tsao, P. S., B. Wang, R. Buitrago, J. Y. Shyy, and J. P. Cooke. 1997. Nitric oxide regulates monocyte chemotactic protein-1. Circulation 96:934-940. [DOI] [PubMed] [Google Scholar]
- 66.Van Coillie, E., J. Van Damme, and G. Opdenakker. 1999. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 10:61-86. [DOI] [PubMed] [Google Scholar]
- 67.Wang, G., R. Iyer, S. Bittker, D. Cooper, J. Small, G. P. Wormser, and I. Schwartz. 2004. Variations in Barbour-Stoenner-Kelly culture medium modulate infectivity and pathogenicity of Borrelia burgdorferi clinical isolates. Infect. Immun. 72:6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weis, J. J. 2002. Host-pathogen interactions and the pathogenesis of murine Lyme disease. Curr. Opin. Rheumatol. 14:399-403. [DOI] [PubMed] [Google Scholar]
- 69.Wilson, R. W., C. M. Ballantyne, C. W. Smith, C. Montgomery, A. Bradley, W. E. O'Brien, and A. L. Beaudet. 1993. Gene targeting yields a CD18-mutant mouse for study of inflammation. J. Immunol. 151:1571-1578. [PubMed] [Google Scholar]
- 70.Yoshimura, T., and E. J. Leonard. 1992. Human monocyte chemoattractant protein-1: structure and function. Cytokines 4:131-152. [PubMed] [Google Scholar]
- 71.Yoshimura, T., N. Yuhki, S. K. Moore, E. Appella, M. I. Lerman, and E. J. Leonard. 1989. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 244:487-493. [DOI] [PubMed] [Google Scholar]