Abstract
Cholera toxin (CT) is an AB5 toxin responsible for the profuse secretory diarrhea resulting from Vibrio cholerae infection. CT consists of a pentameric, receptor-binding B subunit (CTB) and a monomeric A subunit (CTA) that has latent enzymatic activity. In addition to its enterotoxicity, CT has potent mucosal adjuvant activity and can also function as a carrier molecule with many potential applications in cell biology. In earlier studies, the toxic CTA1 domain was replaced by several other antigenic protein domains to produce holotoxin-like chimeras for use as potential mucosal vaccines. In the present study we utilized the twin arginine translocation (tat) system to produce fluorescent CT chimeras, as well as fluorescent chimeras of Escherichia coli heat-labile toxins LTI and LTIIb. Fusion proteins containing either green fluorescent protein (GFP) or monomeric red fluorescent protein (mRFP) and the A2 domain of CT, LTI, or LTIIb were transported to the periplasm of E. coli by the tat system, and the corresponding B polypeptides of CT, LTI, and LTIIb were transported to the periplasm by the sec system. The fluorescent fusion proteins were shown to assemble spontaneously and efficiently with the corresponding B polypeptides in the periplasm to form chimeric holotoxin-like molecules, and these chimeras bound to and entered cultured cells in a manner similar to native CT, LTI, or LTIIb. The GFP and mRFP derivatives of CT, LT, and LTIIb developed here are useful tools for studies on the cell biology of trafficking of the CT/LT family of bacterial enterotoxins. In addition, these constructs provide proof in principle for the development of novel chimeric CT-like or LT-like vaccine candidates containing CTA2 fusion proteins that cannot be delivered to the periplasm of E. coli by use of the sec secretion pathway.
The AB5-type enterotoxins produced by Vibrio cholerae and Escherichia coli have generated interest as potent mucosal adjuvants and immunomodulators, as well as molecular tools to study endocytosis and trafficking from the cell surface to the endoplasmic reticulum (ER) (14, 25). Cholera toxin (CT) and E. coli type I heat-labile enterotoxin (LTI) are approximately 80% identical at the amino acid level and consist of an enzymatically active A subunit and a pentameric, receptor-binding B subunit. The B subunit of CT (CTB) binds to ganglioside GM1 and triggers uptake of the toxin into epithelial cells by endocytosis. Subsequent reduction of the proteolytically nicked A subunit (CTA) releases the active CTA1 fragment within the endoplasmic reticulum, and CTA1 is then translocated to the host cytoplasm to catalyze ADP-ribosylation of Gsα (25). The E. coli type II enterotoxins, LTIIa and LTIIb, are structurally and functionally homologous to CT and LTI; however, they are more divergent at the amino acid sequence level, and their pentameric B subunits bind preferentially to different ganglioside receptors.
Our laboratory and others have constructed stable holotoxin-like molecules with another protein of interest replacing the CTA1 domain of CT (10, 17, 27, 39). These CT-like chimeras demonstrate a number of potential advantages for use as vaccines and molecular tools to study toxin trafficking. These include the absence of the toxic CTA1 domain, the noncovalent association of a fusion protein (consisting of a protein antigen or marker and the CTA2 domain) to a fully functional wild-type receptor-binding B subunit, and maintenance of the ER-targeting KDEL motif of CTA2. Previous studies from our laboratory reported an immunogenic CT chimera containing the serine-rich Entamoeba histolytica protein (39) and a CT chimera containing the MrpH pilus tip antigen from Proteus mirabilis, which protected mice against P. mirabilis urinary tract infection following intranasal immunization (27). Additional chimeras derived from CT, LTI, or LTIIb were characterized by other investigators (9, 12, 30, 35). Assembly of holotoxin-like chimeras in E. coli requires that both the CTA2 fusion protein and the B subunit are transported to the periplasm, where CTA2 of the fusion protein interacts with the nascent B pentamer to promote the assembly process (11, 17). Previous attempts to produce a green fluorescent protein (GFP)-CT chimera proved inefficient when the GFP-CTA2 fusion was exported to the periplasm through the general secretory (sec) system, and the resulting chimera exhibited little or no fluorescence. Further, our efforts to produce several other novel CT fusions as candidate vaccines were unsuccessful when we attempted to deliver the CTA2 fusions to the periplasm of E. coli via the sec pathway (J. K. Tinker, J. Erbe, and R. K. Holmes, unpublished data). In the present study, we developed an alternative method, based on the use of the E. coli twin arginine translocation (tat) system, to export large folded CTA2 fusion proteins to the periplasm of E. coli for incorporation into CT, LTI, and LTIIb chimeras.
The tat secretion system is a sec-independent pathway for translocating folded proteins across the periplasmic membrane of gram-negative bacteria. Tat substrates contain an amino-terminal signal sequence with a characteristic twin arginine motif and generally bind redox cofactors in the cytoplasm (44). The efficient export of active GFP to the E. coli periplasm by fusion to the tat-dependent TorA signal sequence has been previously reported (36, 38, 41). These studies suggest that GFP is properly folded within the cytoplasm prior to its export through the Tat translocase, since GFP is incapable of folding into active form in the periplasm of E. coli (41). Several genes have been identified as components of the tat export pathway in E. coli, and four are thought to be integral membrane proteins (37). The function of these four genes (tatA, tatB, tatC, and tatE) has not been fully elucidated; however, TatA is believed to be the translocation channel and TatC is believed to be the receptor to which preproteins bind (2, 16). In addition, export through the tat pathway can be saturated, and overexpression of these four genes leads to enhanced tat pathway capacity in vivo (37, 45). To direct proteins through the tat pathway, we constructed gene fusions encoding either GFP or monomeric red fluorescent protein (mRFP), as well as the amino-terminal signal sequence of the E. coli tat-dependent trimethylamine N-oxide reductase (TorA) and the carboxyl-terminal A2 domain of CT, LTI, or LTIIb. These fusions were coexpressed with the B subunit of the corresponding enterotoxin in a strain of E. coli that overexpressed TatA, TatB, TatC, and TatE to allow efficient export of the active fluorescent A2 fusion proteins to the periplasm of E. coli. We demonstrated that these fluorescent fusion proteins can assemble efficiently with their corresponding B polypeptides to form enterotoxin-like chimeras in the periplasm and that the resulting chimeras can enter Vero cells and mouse Y1 adrenal cells in culture in a manner similar to that reported for the native enterotoxins. This technique for the efficient assembly of enterotoxin chimeras in vivo will promote further development of novel chimeras for use as vaccine candidates and as molecular tools for studies of protein trafficking in endocytic pathways of target cells.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli NovaBlue is an endA1 K12 derivative [endA1 hsdR17(rK12− mK12−) supE44 thi-1 recA1 gyrA96 relA1 lac(F′ proA+B+ lacIqZΔM15::Tn10[Tcr])] (Novagen, Madison, WI). This strain was used to express and purify the CT, LTI, and LTIIb chimeras and coexpress Tat proteins. E. coli TE1 is a ΔendA derivative of TX1 [F′::Tn10 proA+B+ lacIq Δ(lacZ)M15, glnV44 Δ(hsdM-mcrB)5 Δ(lac-proAB)thi] (18). This strain was used for the cloning of recombinant plasmids as well as expression of His-GFP-CTA2 fusion proteins. Cultures were maintained on Luria-Bertani (34) agar plates supplemented with the appropriate antibiotics. For protein expression, cultures were grown in Terrific Broth (40) at 25°C, unless stated otherwise. Antibiotics were added at a concentration of 75 μg/ml to select for ampicillin resistance (Apr) and of 25 μg/ml to select for chloramphenicol resistance (Cmr).
Plasmid construction.
To construct the cholera toxin chimera vector, pJKT21, the nucleotide sequence encoding the TorA leader sequence plus nine amino acids of mature TorA was amplified from the plasmid pETTorA (kindly donated by Tim Yahr, The University of Iowa) using primers TorAHF (GAGCGGATAAAGCTTCCCCTCTAG) and TorASR (GAATTCGCATGCTCTTTCGAGATG). The resulting product was then cloned into the HindIII/SphI large fragment of pARDLR19. pARLDR19 is an arabinose-inducible subclone of pLDR19 (18), containing a multiple cloning site (MCS) 5′ of the coding region for the CTA2-C199S domain and upstream from the CTB gene with an amino-terminal LTIIb leader. The resulting plasmid, pJKT21, includes the amplified TorA leader and the coding sequence for nine amino acids of full-length TorA connected by an MCS to the coding sequence for the CTA2-C199S fragment. This construct will allow in-frame cloning of amplified gene products into the SphI/ClaI sites of the MCS. Both the CTA2 fusion protein and CTB on pJKT21 are produced under the control of the same Salmonella enterica serovar Typhimurium pBAD promoter, and replication is controlled by the p15A origin. Translation of the CTA2 fusion is controlled by a T7 gene 10 ribosome binding site upstream from the TorA leader, and translation of CTB is controlled by the native Shine-Dalgarno sequence within CTA2 located upstream from the LTIIb B leader in pJKT21. A second plasmid, pJKT53, was later constructed that expresses CTB from the T7 gene 10 Shine-Dalgarno sequence and appears to decrease production of CTB relative to the CTA2 fusion protein. The GFPuv and GFPmut3 genes (Clontech Laboratories, Palo Alto, CA) were PCR amplified and cloned into the SphI/ClaI sites of pJKT21. The resulting plasmid pJKT35 expresses the TorA-GFPmut3-CT chimera, and pJKT20 expresses the TorA-GFPuv-CT chimera. To construct pJKT44 expressing the TorA-mRFP-CT chimera, a PCR product of plasmid pmRFP (kindly donated by Bruce Banfield, University of Colorado) was amplified and cloned into the SphI/ClaI of pJKT21. pmRFP expresses mRFP from the reef coral Heteractis crispa (3). To construct the plasmid pJKT28, which expresses wild-type CT with CTA transported through the Tat secretion system, wild-type CTA1 was PCR amplified from pARCT5 (42) and cloned into the SphI/ClaI of pJKT21. The plasmid, pJKT68, contains the gene for full-length E. coli heat-labile toxin (LTI) B subunit, eltB, expressed from its native signal sequence and ribosome binding site, as well as the A2 domain of eltA beginning at amino acid 180 (26). Similar to pJKT21, this plasmid expresses the TorA leader sequence and the eltA fragment connected by an MCS which will allow in-frame cloning of amplified gene products into the SphI/XhoI site. The plasmid pJKT66, for production of E. coli type II heat-labile toxin (LTIIb) chimeras, was constructed in a similar manner as described for pJKT21 and pJKT68. PCR products were cloned, in frame, into the SphI/ClaI sites of the MCS, and the protein fusion junction with the LTIIbA subunit begins at amino acid 178. Expression of chimeras from pJKT68 and pJKT66, like pJKT21, is controlled by the pBAD promoter and induced by the addition of l-arabinose to the medium. The gene encoding mRFP was cloned into pJKT68 to produce the mRFP-LT chimera (pJKT81). The gene encoding GFP was cloned into pJKT68 to produce the GFP-LT chimera (pJKT79). The gene encoding mRFP was cloned into pJKT66 to produce the RFP-LTIIb chimera (pJKT71). Finally, the gene encoding GFP was cloned into pJKT66 to produce the GFP-LTIIb chimera (pJKT75). The plasmids pTatABC and pTatABCE, for the overexpression of Tat proteins, were generously donated by Timothy Yahr, and their construction has previously been described (45). Plasmids used and constructed in this study are listed in Table 1.
TABLE 1.
Plasmids used in this study
| Plasmid | Genotype and relevant featuresa
|
Reference or source | ||||
|---|---|---|---|---|---|---|
| Leader | Gene | Leader | Gene | Features | ||
| pARCT5 | LTIIbB | ctxA | LTIIbB | ctxB | p15a replicon, pBAD* promoter | 42 |
| pARLDR19 | LTIIbB | MCS-ctxA | LTIIbB | ctxB | p15a replicon, pBAD* promoter | This paper |
| pJKT20 | torA | gfp(UV)-ctxA | LTIIbB | ctxB | p15a replicon, pBAD* promoter | This paper |
| pJKT21 | torA | MCS-ctxA | LTIIbB | ctxB | p15a replicon, pBAD* promoter | This paper |
| pJKT28 | torA | ctxA | LTIIbB | ctxB | p15a replicon, pBAD* promoter | This paper |
| pJKT35 | torA | gfp(mut3)-ctxA | LTIIbB | ctxB | p15a replicon, pBAD* promoter | This paper |
| pJKT44 | torA | rfp-ctxA | LTIIbB | ctxB | p15a replicon, pBAD* promoter | This paper |
| pJKT53 | torA | MCS-ctxA | LTIIbB | ctxB | T7gene10 Shine-Dalgarno for ctxB | This paper |
| pJKT66 | torA | MCS-LTIIbA | Native | LTIIbB | p15a replicon, pBAD* promoter | This paper |
| pJKT68 | torA | MCS-eltA | Native | eltB | p15a replicon, pBAD* promoter | This paper |
| pJKT71 | torA | rfp-LTIIbA | Native | LTIIbB | p15a replicon, pBAD* promoter | This paper |
| pJKT75 | torA | gfp(UV)-LTIIbA | Native | LTIIbB | p15a replicon, pBAD* promoter | This paper |
| pJKT79 | torA | gfp(UV)-eltA | Native | eltB | p15a replicon, pBAD* promoter | This paper |
| pJKT81 | torA | rfp-eltA | Native | eltB | p15a replicon, pBAD* promoter | This paper |
| pTatABCE | Native | tatA, tatB, tatC, tatD | MB 1 replicon, pBAD promoter | 45 | ||
Asterisk indicates S. enterica serovar Typhimurium pBAD promoter.
Chimera and subunit purification.
For the production of CT, LTI, and LTIIb chimeras, small-scale cultures (25 ml) were grown in Terrific Broth to an A600 of approximately 1.0, before induction with 0.2% l-arabinose. The cultures were then incubated for an additional 12 to 15 h before they were collected by centrifugation. To prepare periplasmic extracts, cells were resuspended at 25 times the concentration of the original culture volume in 20 mM Tris-buffered saline, pH 7.5, with 1 mg/ml polymyxin B and shaken at 25°C for 15 min; the insoluble debris was removed by centrifugation at 10,000 rpm for 15 min. The CT and LTI chimeric holotoxins were then further purified from the periplasmic extracts over 0.2-ml bed volumes of d-galactose-agarose resin (Pierce, Rockford, IL). Columns were washed with 3 to 5 column volumes of 50 mM Tris-HCl, pH 8.0, and bound holotoxin was eluted with four volumes (0.5 ml) of 50 mM Tris-HCl, pH 8.0, plus 1 M d-galactose. The resulting eluates were analyzed either directly by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting or pooled, dialyzed in Tris-buffered saline-1% glycerol-5 mM phenylmethylsulfonyl fluoride, and stored at 4°C for further characterization by GM1 enzyme-linked immunosorbent assays (ELISAs) as described in the following section. Periplasmic extracts containing LTIIb chimeras were used for fluorescence microscopy without additional purification.
Assays for chimera assembly.
CT and LTI chimera samples purified from d-galactose resin were mixed with a one-half volume of 4× Laemmli sample buffer and boiled for 3 min in the presence of 3% 2-mercaptoethanol or 75 mM dithiothreitol before being loaded onto an SDS-12% polyacrylamide gel for analysis of assembly (34). For Western blotting of CT chimeras, proteins were transferred to nitrocellulose by semidry electroblotting as described by the manufacturer (Bio-Rad, Hercules, CA) and were detected with either a rabbit anti-CTA polyclonal antibody that detected fusion proteins containing the CTA2 domain or a rabbit anti-CTB polyclonal antibody (42). Antibodies against TatA, TatB, and TatC for analysis of Tat expression by Western blotting were donated by Timothy Yahr (University of Iowa). Binding of horseradish peroxidase-conjugated goat anti-rabbit secondary antibody was then detected by chemiluminescence as described by the manufacturer (Dupont/NEN, Wellesley, MA). The antigenic composition of the CT chimeras was demonstrated by GM1-ELISAs using CTA- and CTB-specific antibodies, as described previously (15). The composition of the mRFP-containing CT, LTI, and LTIIb chimeras was also established by GM1 ELISAs (for mRFP-CT and mRFP-LTI) or GD1a ELISAs (for mRFP-LTIIb) using appropriate B subunit-specific and mRFP-specific rabbit primary antibodies and secondary horseradish peroxidase-conjugated goat anti-rabbit secondary antibodies. Each chimera bound to the ganglioside that serves as the specific receptor for its B subunit, and each bound chimera was shown to react with antibodies specific for its B subunit as well as its A2 fusion protein (data not shown). We were unable to acquire a primary anti-GFP antibody that reacted well with GFP under the nondenaturing conditions used for ELISAs, but the fluorescence of the chimeras indicated that their GFP and mRFP domains adopted functional conformations that were most likely similar to those of native GFP and mRFP.
Quantitation of fluorescence and fluorescence microscopy.
Purified fluorescent chimera preparations in phosphate-buffered saline were analyzed for fluorescence in a VersaFluor fluorometer (Bio-Rad, Hercules, CA). GFP-CT was excited at a wavelength of 390 (± 22) nm, and emission was analyzed at a wavelength of 510 (± 10) nm. mRFP-CT was excited at a wavelength of 510 (± 10) nm and emission was analyzed at 590 (± 10) nm using the appropriate filters. Arbitrary units of fluorescence (AFU) for fluorescein isothiocyanate (FITC)-CTB and GFP-CT were compared by setting the fluorescence of the highest concentration of FITC-CTB (50 μg/ml) (Sigma, St. Louis, MO) to 5,000 AFU. The fluorescence of the highest concentration of mRFP-CT (50 μg/ml) was set to 500 AFU because mRFP-CT was substantially less fluorescent than FITC-CTB, and a control that fluoresced at the same wavelength as mRFT-CT was not available. Reported AFUs represent two independent analyses. For fluorescence microscopy, monkey Vero cells or mouse Y1 adrenal tumor cells were grown in 2 ml of Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum for 48 h to subconfluence on uncoated coverslips at 37°C and 5% CO2. The cells were then washed in Dulbecco's modified Eagle medium without serum and incubated in 1 μM ganglioside GM1 for 30 min at 37°C to increase the density of receptors for CT or LTI. Cells were washed again and incubated in 40 μl of 10 μg/ml FITC-CTB or 10 to 100 μg/ml CT and LTI chimeras (as indicated) in phosphate-buffered saline at 4°C for 15 min to allow binding to the plasma membrane. To visualize the LTIIb fluorescent chimeras on Vero cells, 40 μl of periplasmic extract was directly incubated with each coverslip. Subsequently, some cultures were shifted to 37°C for various times to allow toxin internalization to occur. At the times indicated, the cells were immediately washed again, and the coverslips containing the cells were fixed in 3.7% formaldehyde for 1 h at room temperature. The mRFP-CT chimera was consistently brighter and quenched less rapidly than the GFP-CT chimera in these studies. Coverslips were viewed either unmounted or mounted with Vectashield H-1000 (Vector Laboratories, Burlingame, CA) and were visualized with a Zeiss Axioplan 2 microscope with a 40× lens objective and either an FITC filter (excitation at 490/494 nm; emission at 520/525 nm) or a rhodamine filter (excitation at 550 nm; emission at 573 nm).
RESULTS
Development of an in vivo assembly system using Tat secretion in E. coli.
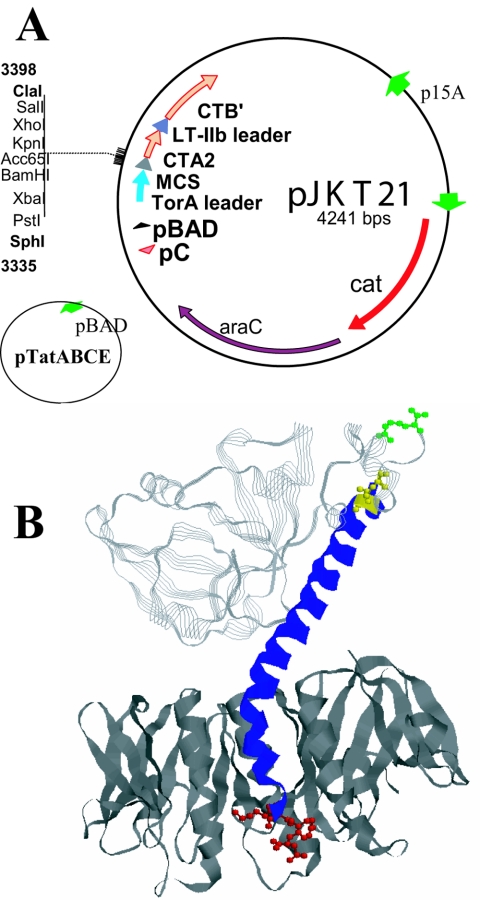
One obstacle to the efficient production of CT chimeras has been the inability to transport some CTA2 fusion proteins to the E. coli periplasm in a soluble and assembly-proficient state. For this study, we investigated the E. coli tat secretion system for transport of such fusion proteins. Figure 1A shows the plasmid pJKT21 into which the coding regions for selected antigens were cloned, in frame, to the coding regions for the E. coli TorA tat-dependent leader peptide at the amino terminus and the CTA2 peptide at the carboxyl terminus. The CTB subunit was coexpressed from the same arabinose-inducible promoter and secreted to the periplasm by use of the sec-dependent E. coli LTIIb B leader peptide. The Tat proteins were expressed simultaneously from pTatABCE (45) in an effort to promote more efficient transport of the fusion proteins into the periplasm. Figure 1B shows a ribbon diagram of the crystal structure of CT with the disulfide bond between amino acids 187 and 199 of CTA, the proteolytic cleavage site at Arg 192 of CTA, and the ER-targeting KDEL sequence corresponding to amino acids 237 to 240 at the carboxyl terminus of CTA; the structures are shown in ball and stick format and are highlighted in yellow, green, and red, respectively. In fusions of foreign proteins to CTA2, the fusion joint is located at amino acid 195 of CTA, and residues of CTA from 1 to 194 are therefore not present in the CT-like chimeras. In addition, Cys 199 of CTA was replaced in the fusion protein by Ser to eliminate the possibility of anomalous disulfide bond formation between the foreign protein and CTA2. However, the ER-targeting KDEL sequence remains at the carboxyl terminus of the fusion proteins. The arabinose-inducible expression of TatA from pTatABCE was confirmed by Western blotting (data not shown). Coding regions for a number of different proteins were cloned into pJKT21 to investigate what fusions could be effectively transported via the tat secretion system. Wild-type CTA was found to be a good substrate for tat-dependent translocation, as were a number of different fluorescent fusion proteins.
FIG. 1.
(A) Plasmid pJKT21 was constructed to facilitate the transport of fusion proteins containing CTA2 to the periplasm of E. coli through the tat secretion system for assembly with CTB into CT-like chimeras. Plasmid pTatABCE was used for overexpression of the Tat components (45). (B) CT structure. The CTA2 domain that is incorporated with fusion proteins in CT-like chimeras corresponds to amino acids 195 to 240 (blue). The nick site in wild-type CTA is Arg 192 (green). The disulfide bond in wild-type CTA is formed between Cys 187 and Cys 199 (yellow); however, the chimeras lack Cys 187 and have a Cys 199-Ser substitution. The KDEL ER targeting signal is located at the carboxyl terminus of CTA from residues 237 to 240 (red).
Production of fluorescent CT chimeras using the tat secretion system in E. coli.
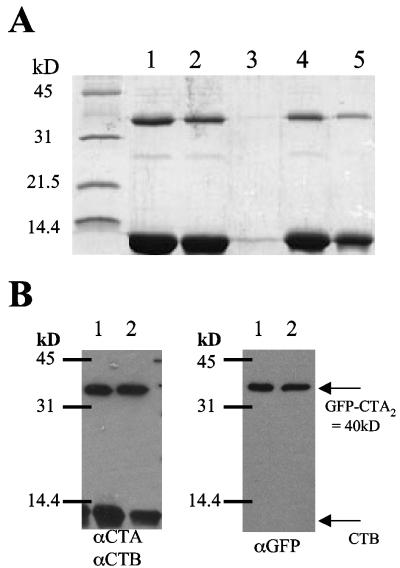
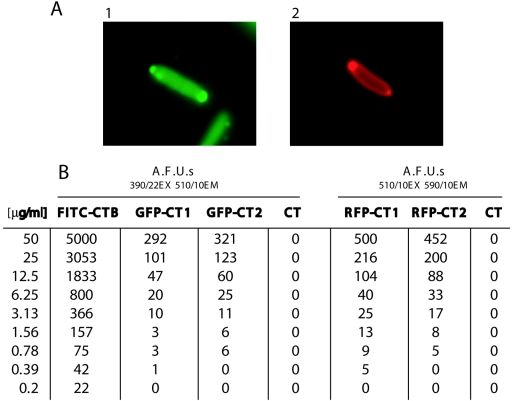
Production of the fluorescent CT chimeras encoded by derivatives of pJKT21 was found to be efficient. Figure 2 shows SDS-PAGE and Western blot analyses of the purified GFPmut3-CTA2/CTB (GFP-CT) chimera. In addition, Fig. 3 shows the analysis of a monomeric RFP-CTA2/CTB (mRFP-CT) chimera. Proteins approximately 6 to 10 kDa smaller than the CTA2 fusions that copurified with the GFP-CT and mRFP-CT chimeras, but did not react strongly with α-CTA, may represent degradation products of the full-length GFP-CTA2 and mRFP-CTA2 fusion proteins. To determine if these chimeras were expressed in active form in the periplasm of E. coli, fluorescence microscopy was performed on the expressing E. coli NovaBlue cultures containing pJKT21 and pTatABCE after overnight induction with 0.2% l-arabinose (Fig. 4A). Quantitation of fluorescence from the GFP-CT, mRFP-CT, and FITC-CTB is shown in Fig. 4B. FITC-CTB was found to be at least 10-fold more fluorescent than the CT holotoxin chimeras. This result was not unexpected since the fluorophore-to-protein ratio of FITC-CTB has been determined to be 4.3 (21), and the maximum ratio is 1 for the GFP-CT and mRFP-CT chimeras.
FIG. 2.
Purification and analysis of the GFPmut3-CTA2/CTB (GFP-CT) chimera. (A) SDS-PAGE. Lane 1, d-galactose eluate 1 from initial preparation; lane 2, d-galactose eluate 2 from initial preparation; lane 3, d-galactose flowthrough; lane 4, d-galactose eluate 1; lane 5, d-galactose eluate 2 from repurification after incubation at 4°C for greater than 1 month. (B) Anti-CTA (α-CTA)/α-CTB and α-GFP Western blots of eluates 1 and 2 described in panel A.
FIG. 3.
The mRFP-CTA2/CTB (mRFP) chimera after in vivo assembly and purification on d-galactose-agarose. (A) SDS-PAGE. (B) α-CTA Western blot.
FIG. 4.
Analysis of CT chimera fluorescence. (A) Expression of the GFP-CT and RFP-CT chimeras in E. coli after an overnight incubation with 0.2% l-arabinose: E. coli NovaBlue plus pTatABCE plus pJKT35 with FITC filter (frame 1) and NovaBlue plus pTatABCE plus pJKT36 with rhodamine filter (frame 2). Both fluorescent proteins exhibit polar localization, and diffuse fluorescence of the periplasm is also evident from the mRFP-CT chimera. (B) Fluorescence spectroscopy of the purified GFP-CT and mRFP-CT chimeras. The numbers 1 and 2 in the designations indicate different purified preparations of the same chimera.
Binding and transport of fluorescent CT chimeras into Y1 and Vero cells.
To determine if the fluorescent CT chimeras were active for binding and transport into tissue culture cells, the proteins were incubated with mouse Y1 adrenal cells or green monkey Vero kidney cells. FITC-conjugated CTB was used as a control. The endocytic pathway of these cells is active at 37°C but does not function at 4°C (29). Incubation of either the GFP-CT or the mRFP-CT on Y1 cells resulted in endocytosis of the fluorescent chimera at 37°C, but only surface labeling was observed at 4°C (Fig. 5). Figure 6A and B show Vero cells after incubation with FITC-CTB or GFP-CT at 4°C or 37°C for 1 h. Transport into the cell was confirmed by a perinuclear concentration of fluorescence. Internalization of the mRFP-CT construct is shown in Fig. 6C. Initial perinuclear concentration and a significant amount of mRFP-CT surface labeling are still visible after 30 min at 37°C, but they are less visible after 1 h at 37°C (data not shown). Internal concentration of the fluorescent marker in Vero cells at 37o is easily distinguished from the surface localization seen at 4°C, indicating that the chimeras are effectively imported into the cell. Incubation of Vero cells with a 10-fold excess of free CTB before the addition of the fluorescent chimera resulted in complete blocking of binding at 4°C and transport at 37°C of GFP-CT and mRFP-CT (data not shown). Although the cells were preincubated with ganglioside GM1 to increase the number of chimera receptors, similar results were obtained when the cells were not preincubated with ganglioside GM1 (data not shown).
FIG. 5.
Binding and internalization of the GFP-CT and mRFP-CT chimeras by Y1 cells. (A) GFP-CT (100 μg/ml) at 4°C (frame 1) and at 37°C (frame 2) for 1 h. (B) mRFP-CT (100 μg/ml) at 4°C (frame 1) and at 37°C (frame 2) for 1 h.
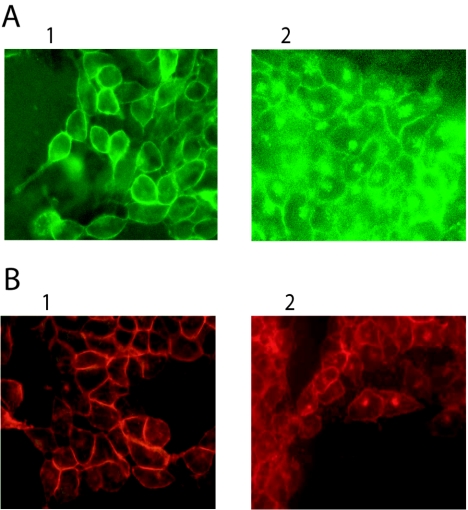
FIG. 6.
Binding and internalization of the GFP-CT and mRFP-CT chimeras by Vero cells. (A) FITC-CTB (10 μg/ml) at 4°C (frame 1) and at 37°C (frame 2) for 1 h. (B) GFP-CT (50 μg/ml) at 4°C (frame 1) and at 37°C (frame 2) for 1 h. (C) RFP-CT (50 μg/ml) at 4°C (frame 1) and at 37°C (frame 2) for 1 h.
Purification and transport of the LTI and LTIIb fluorescent chimeras.
To construct fluorescent LTI chimeras, the coding sequence for the TorA leader was cloned into a vector expressing the E. coli LTA2 and LTB gene products (pJKT68) (Fig. 7A). Genes encoding GFP and mRFP were then cloned in frame with the coding sequence for LTA2, and the resulting plasmid transformed into a strain overexpressing TatABCE. After expression in E. coli, the periplasmic extract was purified over d-galactose-agarose, as described for the purification of the CT chimeras. SDS-PAGE analysis of the resulting mRFP-LT chimera is shown in Fig. 7B. Incubation of Vero cells with this chimera resulted in a pattern of surface labeling at 4°C and internalization at 37°C (Fig. 7C) similar to that observed with mRFP-CT. To produce LTIIb chimeras, genes encoding GFP and mRFP were cloned into pJKT66 (Fig. 8A). Expression of the LTIIb fluorescent chimeras in the periplasm of E. coli was confirmed by spectroscopy of the periplasmic extract (data not shown). Periplasmic extracts were analyzed for binding and internalization of the LTIIb chimera. Studies using the mRFP-LTIIb and GFP-LTIIb periplasmic extracts demonstrated that the LTIIb chimeras were able to bind to Vero cells at 4°C and internalize efficiently at 37°C (Fig. 8B).
FIG. 7.
Development of LTI chimeras. (A) Plasmid pJKT68 constructed for transport of LTI chimeras to the periplasm of E. coli through the tat secretion system. Asterisk, coding region for amino acids 162 to 240 of LTI. (B) SDS-PAGE of the mRFP-LTI chimera after purification on d-galactose agarose. Lane 1, flowthrough; lane 2, eluate 1; and lane 3, eluate 2. (C) Binding and internalization of the mRFP-LTI chimera (50 μg/ml) on Vero cells at 4°C (frame 1) or 37°C (frame 2) for 1 h.
FIG. 8.
Development of LTIIb chimeras. (A) Plasmid pJKT66 constructed for the expression and transport of LTIIb chimeras to the periplasm of E. coli through the tat secretion system. Asterisk, amino acids 158 to 243. (B) Binding and internalization of the GFP-LTIIb periplasmic extract on Vero cells at 4°C (frame 1) and 37°C (frame 2) for 1 h or of the RFP-LTIIb chimera at 4°C (frame 3) and 37°C (frame 4) for 1 h.
DISCUSSION
In 1992, Jobling and Holmes reported the construction of holotoxin-like chimeras containing fusion proteins to the CTA2 domain and determined that the A2 domain was both necessary and sufficient to enable a stable association of the fusion proteins with pentameric CTB. These chimeric molecules were functional, immunoreactive, and able to bind to ganglioside GM1 (17). Subsequently, our group and other investigators have continued to develop CT chimeras, as well as E. coli heat-labile toxin (LTI and LTIIb) chimeras as promising mucosal immunogens (9, 10, 12, 19, 20, 22, 27, 31, 35, 39, 43, 46). Bacterial enterotoxins have recently generated interest for a number of other uses as well, including vectors to transport novel therapeutics to intracellular targets and agents to combat autoimmune disease (5, 8, 14, 33). The potential for these chimeric toxins to bind to specific cell types and internalize by a retrograde pathway to the ER also makes them powerful molecular tools to study endocytosis and protein trafficking in eukaryotic cells (6). The markers currently used for cellular trafficking are often direct conjugates of CTB or LTB. The conjugation of fluorescent markers to the B pentamer may alter the receptor binding and transport of these molecules. The fluorescent chimeras developed in this study utilize the wild-type B subunit, and assembly of the chimera is mediated by the association of the wild-type A2 domain of the fusion protein with the corresponding B subunit.
In 2001 Hatic et al. reported the successful production of a GFP-CT chimera by the purification of individual subunits and in vitro reassembly (12). We have also assembled GFP-CT chimeras, as well as a toxin-coregulated pilus A (TcpA)-CT chimera in vitro (data not shown). However, the fluorescence of the GFP fusion protein was largely destroyed during the reassembly process. The results of these experiments demonstrated that in vitro assembly may be an alternative for the construction of some chimeras that are difficult or impossible to produce in the E. coli periplasm using the traditional sec pathway for export of the fusion protein. However, for large-scale production of vaccines, this method would likely be much more time-consuming and expensive than an efficient in vivo assembly process. In previous studies, it was impossible to assemble an active GFP-CT chimera in the periplasm of E. coli for use as a molecular tool to study toxin trafficking (36, 41). To overcome these complications, we adapted the tat translocation system to export CTA2 fusion proteins to the periplasmic space. Active GFP as well as mRFP fusions were effectively transported to the periplasm and properly folded into fluorescent holotoxin-like CT, LTI, and LTIIb chimeras using this system. In addition, these vectors have potential value for the expression and purification of novel chimeric vaccine candidates. We have successfully made a toxin-coregulated pilus F subunit (TcpF)-CT chimera in vivo after transport of the TcpF-CTA2 fusion protein through the tat system (data not shown).
The intracellular trafficking of cholera toxin into Vero cells has been reported previously (1, 4, 28). The time-dependent intracellular transport of CT into Vero cells was determined by utilizing a labeled Cy3 CTB subunit and a Cy5-labeled anti-CTA antibody (28). After 5 min at 37°C, CT was largely associated with the plasma membrane. Incubation for 15 to 20 min at 37°C initiated internalization, and CT was found in a characteristic perinuclear Golgi staining pattern. By 90 min the CTA subunit was localized in the ER. Based upon our observations of the transport of fluorescent chimeras in Vero cells, we recognized a staining pattern that was very similar to patterns that were previously reported (4, 28). Similar to CT, E. coli heat-labile toxin LTI also binds to ganglioside GM1 and moves retrogradely to the ER (13, 23, 24, 32). LTIIb binds to ganglioside GD1a and has been determined to move retrogradely to the ER in Vero cells, although not in human T84 intestinal epithelial cells, and this difference was correlated with the distribution of the receptor gangliosides between lipid rafts and detergent-soluble membrane fractions (7).
In conclusion, we have expressed and purified active, fluorescent enterotoxin chimeras in the periplasm of E. coli by transport of the fusion protein subunit through the tat translocation pathway and transport of the B subunit through the sec translocation pathway. We have demonstrated that the resulting holotoxin-like chimeras are stable, fluorescent, and able to bind to Y1 and Vero cells in tissue culture at 4°C and to internalize into these cells at 37°C. The studies presented here should significantly expand the range of enterotoxin-like chimeras that can be constructed and investigated as novel vaccine candidates, immunomodulators, or molecular tools for studies of cell biology.
Acknowledgments
This work was supported in part by an NIH postdoctoral National Research Service Award (T32AI07537) to J.K.T. and an NIH research grant (RO1AI-31940) to R.K.H.
We thank Michael G. Jobling for his advice and insight throughout the project.
Editor: J. D. Clements
REFERENCES
- 1.Bastiaens, P., I. Majoul, P. Verveer, H. Söling, and T. Jovin. 1996. Imaging the intracellular trafficking and state of the AB5 quaternary structure of cholera toxin. EMBO J. 15:4246-4253. [PMC free article] [PubMed] [Google Scholar]
- 2.Behrendt, J., K. Standar, U. Lindenstraub, and T. Bruser. 2004. Topological studies on the twin-translocase component TatC. FEMS Microbiol. Lett. 234:303-308. [DOI] [PubMed] [Google Scholar]
- 3.Campbell, R. E., O. Tour, A. E. Palmer, P. A. Steinbach, G. S. Baird, D. A. Zacharias, and R. Y. Tsien. 2002. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, A., T. Hu, C. Mikoryak, and R. Draper. 2002. Retrograde transport of protein toxins under conditions of COPI dysfunction. Biochim. Biophys. Acta 1589:124-139. [DOI] [PubMed] [Google Scholar]
- 5.De Haan, L., and T. R. Hirst. 2002. Bacterial toxins as versatile delivery vehicles. Curr. Opin. Drug Devel. 5:269-278. [PubMed] [Google Scholar]
- 6.De Haan, L., and T. R. Hirst. 2004. Cholera toxin: a paradigm for multi-functional engagement of cellular mechanisms. Mol. Membr. Biol. 21:77-92. [DOI] [PubMed] [Google Scholar]
- 7.Fujinaga, Y., A. Wolf, C. Rodighiero, H. Wheeler, B. Tsai, L. Allen, M. Jobling, T. Rapoport, R. Holmes, and W. Lencer. 2003. Gangliosides that associate with lipid rafts mediate transport of cholera and related toxins from the plasma membrane to endoplasmic reticulum. Mol. Biol. Cell 14:4783-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George-Chandy, A., K. Eriksson, M. Lebens, I. Nordstrom, E. Schon, and J. Holmgren. 2001. Cholera toxin B subunit as a carrier molecule promotes antigen presentation and increases CD40 and CD86 expression of antigen-presenting cells. Infect. Immun. 69:5716-5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajishengallis, G., E. Harokopakis, S. Hollingshead, M. Russell, and S. Michalek. 1996. Construction and oral immunogenicity of a Salmonella typhimurium strain expressing a streptococcal adhesion linked to the A2/B subunits of cholera toxin. Vaccine 14:1545-1548. [DOI] [PubMed] [Google Scholar]
- 10.Hajishengallis, S. Hollingshead, T. Koga, and M. Russell. 1995. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J. Immunol. 22:4322-4433. [PubMed] [Google Scholar]
- 11.Hardy, S. J. S., J. Holmgren, S. Johansson, J. Sanchez, and T. R. Hirst. 1988. Coordinated assembly of multisubunit proteins: oligomerization of bacterial enterotoxins in vivo and in vitro. Proc. Natl. Acad. Sci. USA 85:7109-7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatic, S. O., J. A. McCann, and W. D. Picking. 2001. In vitro assembly of novel cholera toxin-like complexes. Anal. Biochem. 292:171-177. [DOI] [PubMed] [Google Scholar]
- 13.Hazes, B., and R. Read. 1997. Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry 36:11051-11054. [DOI] [PubMed] [Google Scholar]
- 14.Hirst, T. R. 2001. A toxin with emerging therapeutic potential. Lancet 358(Suppl.):S7. [DOI] [PubMed] [Google Scholar]
- 15.Holmes, R. K., and E. M. Twiddy. 1983. Characterization of monoclonal antibodies that react with unique and cross-reacting determinants of cholera enterotoxin and its subunits. Infect. Immun. 42:914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ize, B., F. Gerard, M. Zhang, A. Chanal, R. Voulhoux, T. Palmer, A. Filloux, and L. Wu. 2002. In vivo dissection of the tat translocation pathway in Escherichia coli. J. Mol. Biol. 317:327-335. [DOI] [PubMed] [Google Scholar]
- 17.Jobling, M. G., and R. K. Holmes. 1992. Fusion proteins containing the A2 domain of cholera toxin assemble with B polypeptides of cholera toxin to form immunoreactive and functional holotoxin-like chimeras. Infect. Immun. 60:4915-4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jobling, M. G., L. M. Palmer, J. L. Erbe, and R. K. Holmes. 1997. Construction and characterization of versatile cloning vectors for efficient delivery of native foreign proteins to the periplasm of Escherichia coli. Plasmid 38:158-173. [DOI] [PubMed] [Google Scholar]
- 19.Kim, B., S. Shin, Y. Yoo, and S. Pyo. 2001. Peroral immunization with Helicobacter pylori adhesion protein genetically linked to cholera toxin A2B subunits. Clin. Sci. 100:291-298. [PubMed] [Google Scholar]
- 20.Kweon, M.-N., M. Yamamoto, F. Watanabe, S. Tamura, F. W. van Ginkel, A. Miyauchi, H. Takagi, Y. Takeda, T. Hamabata, K. Fujihashi, J. R. McGee, and H. Kiyono. 2002. A nontoxic chimeric enterotoxin adjuvant induces protective immunity in both mucosal and systemic compartments with reduced IgE antibodies. J. Infect. Dis. 186:1261-1269. [DOI] [PubMed] [Google Scholar]
- 21.Lauer, S., B. Goldstein, R. L. Nolan, and J. P. Nolan. 2002. Analysis of cholera toxin-ganglioside interactions by flow cytometry. Biochemistry 41:1742-1751. [DOI] [PubMed] [Google Scholar]
- 22.Lee, S., S. Halperin, D. Salloum, A. MacMillan, and A. Morris. 2003. Mucosal immunization with a genetically engineered pertussis toxin S1 fragment-cholera toxin subunit A chimeric protein. Infect. Immun. 71:2272-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lencer, W. I., C. Constable, S. Moe, P. A. Rufo, A. Wolf, M. G. Jobling, S. Ruston, J. L. Madara, R. K. Holmes, and T. R. Hirst. 1997. Proteolytic activation of cholera toxin and Escherichia coli labile toxin by entry into host epithelial cells. Signal transduction by a protease-resistant variant. J. Biol. Chem. 272:15562-15568. [DOI] [PubMed] [Google Scholar]
- 24.Lencer, W. I., C. Constable, S. Moe, M. G. Jobling, H. M. Webb, S. Ruston, J. L. Madara, T. R. Hirst, and R. K. Holmes. 1995. Targeting of cholera toxin and Escherichia coli heat-labile toxin to polarized epithelia: role of COOH-terminal KDEL. J. Cell Biol. 131:951-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lencer, W. I., T. R. Hirst, and R. K. Holmes. 1999. Membrane traffic and the cellular uptake of cholera toxin. Biochim. Biophys. Acta 1450:177-190. [DOI] [PubMed] [Google Scholar]
- 26.Leong, J., A. C. Vinal, and W. S. Dallas. 1985. Nucleotide sequence comparison between heat-labile toxin B-subunit cistrons from Escherichia coli of human and porcine origin. Infect. Immun. 48:73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, X., J. L. Erbe, C. V. Lockatell, D. E. Johnson, M. G. Jobling, R. K. Holmes, and H. L. Mobley. 2004. Use of a translational fusion of the MrpH fimbrial adhesion binding domain with cholera toxin A2 domain, coexpressed with the cholera toxin B subunit, as an intranasal vaccine to prevent experimental urinary tract infection by Proteus mirabilis. Infect. Immun. 72:7306-7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Majoul, I., P. Bastiaens, and H. Söling. 1996. Transport of an external Lys-Asp-Glu-Leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin in vero cells. J. Cell Biol. 133:777-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamdouh, Z., M. C. Giocondi, R. Laprade, and C. Le Grimellec. 1996. Temperature dependence of endocytosis in renal epithelial cells culture. Biochim. Biophys. Acta 1282:171-173. [DOI] [PubMed] [Google Scholar]
- 30.Martin, M., G. Hajishengallis, D. J. Metzger, S. M. Michalek, T. D. Connell, and M. W. Russell. 2001. Recombinant antigen-enterotoxin A2/B chimeric mucosal immunogens differentially enhance antibody responses and B7-dependent costimulation of CD4+ T cells. Infect. Immun. 69:252-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin, M., D. Metzger, S. Michalek, T. Connell, and M. Russell. 2001. Distinct cytokine regulation by cholera toxin and type II heat-labile toxins involves differential regulation of CD40 ligand on CD4+ T cells. Infect. Immun. 69:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Middlebrook, J., and R. Dorland. 1984. Bacterial toxins: cellular mechanisms of action. Microbiol. Rev. 48:199-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmond, R. J., J. A. Luross, and N. A. Williams. 2002. Immune modulation by the cholera-like enterotoxins. Expert Rev. Mol. Med. 2002:1-16. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 35.Sanchez, J., G. Wallerstrom, M. Fredriksson, J. Angstrom, and J. Holmgren. 2002. Detoxicification of cholera toxin without removal of its immunoadjuvanticity by the addition of (STa-related) peptides to the catalytic subunit: a potential new strategy to generate immunostimulants for vaccination. J. Biol. Chem. 277:33369-33377. [DOI] [PubMed] [Google Scholar]
- 36.Santini, C. A. Bernadac, M. Zhang, A. Chanal, B. Ize, C. Blanco, and L. Wu. 2001. Translocation of jellyfish green fluorescent protein via the Tat system of Escherichia coli and change of its periplasmic localization in response to osmotic up-shock. J. Biol. Chem. 276:8159-8164. [DOI] [PubMed] [Google Scholar]
- 37.Sargent, F., U. Gohlke, E. de Leeuw, N. R. Stanley, T. Palmer, H. R. Saibil, and B. Berks. 2001. Purified components of the Escherichia coli Tat protein transport system form a double-layered ring structure. Eur. J. Biochem. 268:3361-3367. [DOI] [PubMed] [Google Scholar]
- 38.Spence, E., M. Sarcina, N. Ray, S. Geir Moller, C. W. Mullineaux, and C. Robinson. 2003. Membrane-specific targeting of green fluorescent protein by the Tat pathway in the cyanobacterium Synechocystis PCC6803. Mol. Microbiol. 48:1481-1489. [DOI] [PubMed] [Google Scholar]
- 39.Sultan, F., L. Jin, M. G. Jobling, R. K. Holmes and S. Stanley, Jr. 1998. Mucosal immunogenicity of a holotoxin-like molecule containing the serine-rich Entamoeba histolytica protein (SREHP) fused to the A2 domain of cholera toxin. Infect. Immun. 66:462-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tartof, K. D., and C. A. Hobbs. 1987. Improved media for growing plasmid and cosmid clones. Focus 9:12. [Google Scholar]
- 41.Thomas, J. D., R. A. Daniel, J. Errington, and C. Robinson. 2001. Export of active green fluorescent protein to the periplasm by the twin-arginine translocase (Tat) pathway in Escherichia coli. Mol. Microbiol. 39:47-53. [DOI] [PubMed] [Google Scholar]
- 42.Tinker, J. K., J. L. Erbe, W. G. Hol, and R. K. Holmes. 2003. Cholera holotoxin assembly requires a hydrophobic domain at the A-B5 interface: mutational analysis and development of an in vitro assembly system. Infect. Immun. 71:4093-4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toida, N., G. Hajishengallis, H. Wu, and M. Russell. 1997. Oral immunization with the saliva-binding region of Streptococcus mutans AgI/II genetically coupled to the cholera toxin B subunit elicits T-helper cell responses in gut-associated lymphoid tissues. Infect. Immun. 65:909-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiner, J. H., P. T. Bilous, G. M. Shaw, S. P. Lubitz, L. Frost, G. H. Thomas, J. A. Cole, and R. J. Turner. 1998. A novel and ubiquitous system for membrane targeting and secretion of cofactor-containing proteins. Cell 93:93-101. [DOI] [PubMed] [Google Scholar]
- 45.Yahr, T., and W. T. Wickner. 2001. Functional reconstruction of bacterial Tat translocation in vitro. EMBO J. 20:2472-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu, J., and W. Langrudge. 2001. A plant-based multicomponent vaccine protects mice from enteric diseases. Nat. Biotechnol. 19:548-552. [DOI] [PubMed] [Google Scholar]