Abstract
The development of a subunit protein vaccine for bovine tuberculosis which could be used either in combination with Mycobacterium bovis BCG (to improve the efficacy of that vaccine) or alone would offer significant advantages over currently available strategies. A study was conducted with cattle to determine the protective efficacy of a strategy based on concurrent immunization with an M. bovis culture filtrate (CFP) vaccine and BCG compared to vaccination with either vaccine alone. One group of calves (10 animals per group) was vaccinated subcutaneously with CFP formulated with Emulsigen and combined with a CpG oligodeoxynucleotide (ODN). A second group was vaccinated with both the CFP vaccine and BCG injected at adjacent sites (CFP-BCG). One further group was vaccinated subcutaneously with BCG, while another group served as nonvaccinated control animals. Vaccination with CFP-BCG induced levels of antigen-specific gamma interferon (IFN-γ) and interleukin-2 (IL-2) in whole-blood cultures that were higher than those induced by vaccination with BCG alone. The combination of CFP and BCG did not enhance the production of antibodies to M. bovis CFP compared to vaccination with CFP alone. Vaccination with CFP alone led to delayed antigen-specific IFN-γ and IL-2 responses. Vaccination with CFP-BCG induced a high level of protection against an intratracheal challenge with virulent M. bovis, based on a significant enhancement of six pathological and microbiological parameters of protection compared with the nonvaccinated group. In contrast, vaccination with BCG alone induced a significant enhancement of protection in only one parameter, while CFP alone induced no protection. These results suggest that a combination of a CpG ODN-formulated protein vaccine and BCG offers better protection against bovine tuberculosis than does BCG alone.
Infection with Mycobacterium bovis is a significant human health problem as well as a liability for the cattle industry throughout the world (11), and the control of tuberculosis in both humans and cattle would benefit from the design of improved vaccines or vaccination strategies. The only vaccine that is currently available is M. bovis bacillus Calmette-Guerin (BCG).
Experimental studies using cattle have shown that the vaccination of cattle with BCG under optimal conditions can induce a significant level of protection against the development of tuberculous lesions in animals challenged with virulent M. bovis (6, 7, 30). The protection offered by BCG against bovine tuberculosis is variable and may be influenced by the exposure of animals to environmental mycobacteria, which would interfere with the acquisition of full immunity after vaccination (9). The variable effectiveness of BCG has led to efforts to develop an improved vaccine for bovine tuberculosis. However, BCG offers significant protection under optimal conditions. This leads to the paradigm that novel vaccine strategies could include BCG vaccination in combination with another vaccinating moiety, with the aims of complementing and boosting BCG rather than replacing it entirely.
In contrast to DNA vaccines, which have built-in adjuvant activity in the form of CpG motifs, protein subunits are unlikely to induce cellular immune responses in the absence of an appropriate adjuvant. Therefore, a need exists not only for identifying protective subunits (which also have to be defined for DNA vaccines) but also for selecting optimal adjuvants that strongly favor the development of cellular immune responses. One approach has been the use of the protective protein antigens released from live mycobacteria during in vitro growth. This is based on evidence that live, rather than dead, mycobacteria induce the generation of protective responses (20). Vaccination with culture filtrate proteins (CFP) from Mycobacterium tuberculosis and M. bovis has been shown to induce protection against tuberculosis in mouse models (3, 4, 23). The vaccination of cattle with M. bovis CFP formulated with Emulsigen, an emulsified oil-in-water adjuvant, and combined with a specific CpG oligodeoxynucleotide (ODN) (21) has been shown to induce a significant level of protection against tuberculosis (33). The aims of the present study were to determine if the protection could be enhanced further by increasing the concentration of adjuvant in the vaccine as well as by revaccinating animals twice instead of once. An important additional component of the present study was to determine if a combination of two vaccine strategies, i.e., the live BCG vaccine and the CpG ODN-formulated subunit protein vaccine administered at an adjacent site, could enhance immune responses and confer higher levels of protection against tuberculosis than those induced by either vaccine alone.
MATERIALS AND METHODS
Animals.
Forty Friesian cross female calves (approximately 6 months old) were obtained from tuberculosis-free accredited herds from an area of New Zealand where both farmed and feral animals were free of tuberculosis. The animals were grazed on pasture in a high-security containment unit. Prior to the experiments, the cattle tested negative for reactivity to purified protein derivative from M. bovis (bovine PPD) in a whole-blood gamma interferon (IFN-γ) assay (24). Animal ethics approval was granted for all animal experiments by the local ethics committee.
Bacterial strains.
M. bovis BCG strain Pasteur 1173P2 was used as the vaccine strain, and M. bovis WAg202, originally isolated from a tuberculous possum (Trichosurus vulpecula) in New Zealand, was used as the virulent challenge strain. These strains have been used in previous vaccination-challenge studies with cattle (6, 7, 30). Bacteria were grown to mid-log phase in Tween albumin broth (Dubos broth base; Difco Laboratories, Detroit, Mich.) supplemented with 0.006% (vol/vol) alkalinized oleic acid, 0.5% (wt/vol) albumin fraction V, and 0.25% (wt/vol) glucose. Dilutions were made in Tween albumin broth to obtain the appropriate doses for inoculation. The number of CFU inoculated was determined retrospectively by plating 10-fold dilutions on Middlebrook 7H11 (Difco) agar supplemented with 0.5% (wt/vol) albumin, 0.2% (wt/vol) glucose, and 1% (wt/vol) sodium pyruvate.
Preparation of vaccines.
M. bovis CFP was prepared as follows. M. bovis AN5, the strain used for skin testing in New Zealand, was grown as a pellicle in modified Reed's medium for 6 to 8 weeks. The cultures were centrifuged (3,500 × g for 20 min at 5°C), and the supernatants were sterilely filtered twice through a 0.2-μm filter and dialyzed against distilled water using a 6-kDa-cutoff filter (Amicon YM3 membrane; Amicon, Inc., Beverly, Mass.). The CFP was stored in a freeze-dried form until ready for use. The protein content of the culture filtrate preparation was 70%, as determined by the use of bicinchoninic acid (Pierce, Rockford, Ill.). The CFP was not characterized and likely contained predominantly secreted proteins in addition to some cytoplasmic proteins.
Vaccines were prepared by mixing M. bovis CFP with Emulsigen (MVP Laboratories, Inc., Ralston, Nebr.) and combining it with the CpG oligodeoxynucleotide ODN 2007 (21). Emulsigen was added to a final concentration of 50% (vol/vol). Each vaccine dose contained 0.4 mg of CFP and 0.25 mg of ODN 2007.
Vaccination.
The calves were divided into four groups by use of a randomized stratified sampling system such that all groups contained animals with similar distributions of IFN-γ responses to PPD prepared from Mycobacterium avium (avian PPD) prior to commencement of the trial. Ten calves were vaccinated subcutaneously in the neck with the CFP vaccine and revaccinated with the same vaccine 3 and 6 weeks later. A second group of 10 calves was vaccinated subcutaneously in the neck with the CFP vaccine and injected subcutaneously in the neck with 106 CFU of BCG at a site 2 to 3 cm from the CFP injection site. The animals were revaccinated with the CFP vaccine at weeks 3 and 6. A third group was vaccinated subcutaneously in the neck with 106 CFU of BCG, and a fourth group of 10 calves served as nonvaccinated controls. Heparinized blood samples and sera were collected from the calves at regular intervals to measure cellular and humoral immune responses.
M. bovis challenge and necropsy procedure.
The calves were challenged intratracheally 21 weeks after vaccination with 5 × 103 CFU of virulent M. bovis as previously described (6). All cattle were killed by use of a captive bolt and severance of the carotid artery and then necropsied 15 to 16 weeks after the challenge to assess the protection against tuberculosis. Samples from four thoracic lymph nodes (left and right bronchial and anterior and posterior mediastinal) and the left and right retropharyngeal lymph nodes were collected from all of the animals and processed for bacterial culture and histology. Additional samples were collected from any tuberculous lesions observed in the lungs or other lymph nodes. Lung lesion severity scores were determined with the following scale: 0, no lesions; 1, 1 to 9 lesions; 2, 10 to 29 lesions; 3, 30 to 99 lesions; 4, 100 to 199 lesions; 5, ≥200 lesions. A total lymph node lesion score for each animal was determined by use of the following scale for individual nodes: 0, no lesions; 1, 1 to 19 small lesions (1- to 4-mm diameter); 2, ≥20 small lesions; 3, medium-sized lesions (5- to 9-mm diameter); 4, large lesions (≥10-mm diameter). For bacterial culture, tissue samples were homogenized in a Tenbroeck grinder (Wheaton, Millville, N.J.), decontaminated in 0.75% cetylpyridium chloride for 1 h, centrifuged at 3,500 × g for 20 min, and processed for the isolation of mycobacteria as described previously (6). The numbers of colonies were counted, and the results are expressed as log10 CFU/g of tissue. When no bacteria were isolated, a count of 0.699 log10 CFU/g was recorded. For bacterial isolation from lungs, only samples from lung lesions were subjected to culture, as our previous studies have shown that very few M. bovis cells are isolated from nonlesioned lung samples.
Antibody ELISA.
The M. bovis AN5 culture filtrate was diluted to 3 μg/ml in carbonate buffer (pH 9.6); 100 μl per well was added to 96-well enzyme-linked immunosorbent assay (ELISA) plates (Maxisorp; Nunc, Roskilde, Denmark) and the plates were incubated overnight at 4°C. The antibody ELISA was carried out as described previously (34). Sera were stored at −20°C until tested. Results were expressed as “absorbance indexes,” calculated by expressing the values found for the test sera as fractions of the binding of a strong positive reference serum (7) multiplied by 100. Immunoglobulin G1 (IgG1) and IgG2 responses to M. bovis antigens were measured by using isotype-specific antibodies.
IFN-γ and interleukin-2 (IL-2) assays.
Heparinized blood (1.5 ml) was dispensed into 3 wells of a 24-well plate within 4 h of blood collection, and 100 μl of purified protein derivative prepared from M. bovis (bovine PPD), PPD prepared from Mycobacterium avium (avian PPD) (final concentration of 24 μg/ml; CSL Ltd., Parkville, Victoria, Australia), or phosphate-buffered saline (PBS) was added. The whole-blood cultures were incubated at 37°C for 24 h, and the IFN-γ levels in the plasma supernatants were measured with a sandwich ELISA kit (CSL Ltd.) (24). To show the kinetics of the IFN-γ response, we expressed the IFN-γ levels in ng/ml, using a standard curve prepared for recombinant bovine IFN-γ (29).
IL-2 in the plasma supernatants from whole-blood cultures was assayed by a previously described bioassay (29) based on the uptake of [3H]thymidine by concanavalin A-stimulated lymphoblasts. The results are expressed as stimulation indexes, defined as mean counts per minute (cpm) for bovine PPD-containing plasma supernatants/mean cpm for the PBS-treated plasma supernatant. The addition of a monoclonal antibody against bovine IL-2 to concanavalin A-stimulated lymphoblasts immediately before the addition of the plasma supernatants has been shown to block the IL-2 bioactivity, and this confirmed the specificity of the bioassay.
Measurement of IFN-γ and IL-4 mRNA expression.
IFN-γ and IL-4 mRNA levels in peripheral blood mononuclear cells (PBMCs) were measured by real-time PCR. Heparinized blood was collected from all calves 10 weeks after the initial vaccination. PBMCs were obtained by the centrifugation of blood on Lymphoprep 1.077 (Axis-Shield, Oslo, Norway) and were cultured at a density of 2 × 106 cells/ml in RPMI 1640 medium (Gibco) supplemented with 5% heat-inactivated fetal calf serum (Gibco), 2 mM l-glutamine, 1 mM nonessential amino acids (Sigma), 50 μM 2-mercaptoethanol, 2.9 mM sodium bicarbonate, 1 mM sodium pyruvate, 100 U/ml penicillin (Sigma), and 100 μg/ml streptomycin (Gibco). PBMCs (10 ml) were dispensed into two 25-cm2 flasks, and 720 μl of PBS or bovine PPD (300 μg/ml; CSL) was added to each flask. The cultures were incubated in a humidified incubator at 37°C for 20 h. Total RNAs were prepared from PBMC cultures by the use of TRIzol (Invitrogen, Carlsbad, Calif.), and cDNAs were synthesized by the use of oligo(dT)-primed RNA (3 μg) and 200 U of Superscript II reverse transcriptase (Invitrogen). Cytokine gene expression was measured with a GeneAmp 5700 sequence detection system and sequence detector software (Applied Biosystems, Foster City, Calif.) as described previously (32). A comparative method was used to calculate cytokine mRNA expression, and the bovine PPD-specific expression of IFN-γ and IL-4 mRNAs in PBMCs was expressed in relative quantitative values as described previously (32).
Tuberculin skin test.
A comparative cervical tuberculin skin test was administered 17 weeks after vaccination and 14 weeks after challenge. The animals were inoculated intradermally with a 0.1-ml volume containing 0.05 mg avian PPD or 0.1 mg bovine PPD (AgriQuality, Upper Hutt, New Zealand) in the right side of the neck. The skin fold thickness was measured with calipers prior to injection and 72 h after injection for both bovine and avian PPD. A positive response in the comparative skin test was recorded when the response for bovine PPD was ≥4 mm larger than the response for avian PPD.
Statistical analysis.
Analyses of IFN-γ and IL-2 responses, cytokine mRNA expression, and bacterial counts were undertaken by analysis of variance on log10-transformed data. Analyses of tuberculin skin test responses, antibody responses, and lesion scores used analysis of variance on the raw data. The proportion of animals with lesions was analyzed by using Fisher's exact test with pairwise comparisons.
RESULTS
T-cell responses after vaccination and challenge.
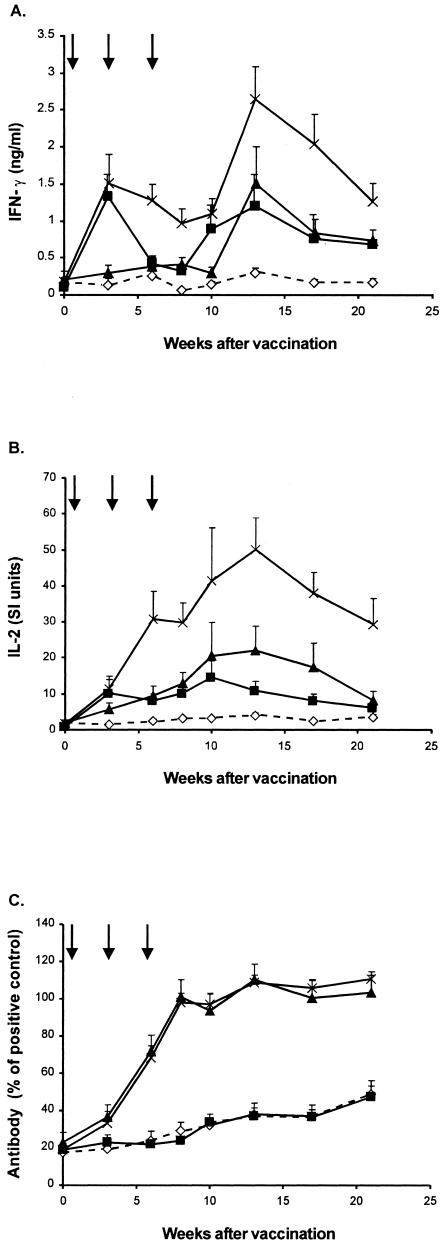
T-cell responses in the animals were measured by the release of IFN-γ and IL-2 from whole blood stimulated with avian and bovine PPD and by bovine PPD-specific cytokine mRNA expression in PBMCs. Immediately prior to vaccination, the mean IFN-γ responses (± standard errors of the means [SEM]) to avian PPD and bovine PPD for all animals were 0.22 ± 0.07 and 0.16 ± 0.04 ng/ml, respectively. Vaccination with BCG alone induced a significant enhancement in the IFN-γ and IL-2 responses to bovine PPD compared to those observed with the nonvaccinated group (Fig. 1). The mean responses for the BCG-vaccinated group were significantly higher than those for the nonvaccinated group at all sampled time points from 3 to 21 weeks after the initial vaccination for IFN-γ and from 3 to 17 weeks for IL-2 (P < 0.05). The combination of M. bovis CFP and BCG vaccination induced very strong bovine PPD-specific IFN-γ and IL-2 responses (Fig. 1). The mean IFN-γ and IL-2 responses for the CFP-BCG group were significantly higher than those for the nonvaccinated group from 6 to 21 weeks after vaccination. The mean IFN-γ responses were higher for the CFP-BCG group than for the BCG-only group at 6, 8, and 13 to 21 weeks (P < 0.05), while the mean IL-2 responses for the CFP-BCG group were higher than those for the BCG-only group from 6 to 21 weeks (P < 0.05). The CFP vaccine alone induced moderate IFN-γ and IL-2 responses, and the means for this group were significantly higher than those for the nonvaccinated group from 13 to 21 weeks for IFN-γ and from 6 to 21 weeks for IL-2 (P < 0.05). The IFN-γ responses of the CFP group were similar to those of the BCG-only group for the 13- to 21-week period, while the IL-2 responses were significantly stronger than those for the BCG-only group at 13 and 17 weeks (P < 0.05). Two weeks after the challenge with M. bovis, the IFN-γ and IL-2 responses to bovine PPD increased markedly in all groups and remained high until the animals were slaughtered (data not shown). There were no significant differences in the IFN-γ and IL-2 responses between the various groups after the challenge.
FIG. 1.
Mean levels of IFN-γ (A) and IL-2 (B) released from bovine PPD-stimulated whole-blood cultures and serum antibody responses to M. bovis culture filtrate (C) for animals vaccinated with CFP (▴) (n = 10), BCG (▪) (n = 10), or CFP and BCG (×) (n = 9) and for nonvaccinated animals (⋄) (n = 10). IFN-γ levels are presented as mean concentrations in plasma (ng/ml), IL-2 levels are given as mean stimulation indexes (SI), and antibody levels are given as percentages of a positive control serum. The bars above the data points represent SEM. The arrows indicate vaccination times (0 for BCG and 0, 3, and 6 weeks for CFP).
The cytokine responses in the peripheral blood of animals after vaccination were analyzed further by measuring IFN-γ and IL-4 mRNA expression in PBMCs 10 weeks after the initial vaccination. The mean bovine PPD-specific IFN-γ and IL-4 responses for the BCG and CFP-BCG groups were stronger than the responses for the nonvaccinated group (P < 0.01) (Table 1).
TABLE 1.
IFN-γ and IL-4 mRNA expression in PBMC cultures from cattle
| Vaccine group | Cytokine mRNA expressiona
|
|
|---|---|---|
| IFN-γ | IL-4 | |
| No vaccine | 1.0 ± 0.3 | 0.8 ± 0.3 |
| CFP/Emulsigen/CpG ODN | 1.7 ± 0.6 | 1.3 ± 0.5 |
| CFP/Emulsigen/CpG ODN + BCG | 5.3** ± 2.2 | 3.4** ± 1.3 |
| BCG | 7.9** ± 4.9 | 2.7** ± 0.9 |
Bovine PPD-specific cytokine mRNA expression in PBMCs from cattle (9 or 10 animals per group) 10 weeks after initial vaccination is shown as mean relative quantitation values ± SEM. **, significantly different from the nonvaccinated group (P < 0.01).
Antibody responses after vaccination.
Significant antibody responses to M. bovis CFP were observed for the group vaccinated with CFP and the group vaccinated with CFP-BCG. The mean responses for these groups 3 to 21 weeks after initial vaccination were significantly higher than the means for the BCG-vaccinated and nonvaccinated groups (P < 0.01) (Fig. 1C). The M. bovis-specific IgG1-to-IgG2 ratios in sera from animals vaccinated with CFP and CFP-BCG were also measured and compared. Ten weeks after vaccination, the mean ratios of IgG1 to IgG2 (± SEM) were 1.8 ± 0.3 for the CFP-vaccinated group and 1.4 ± 0.5 for the CFP-BCG-vaccinated group. Following the challenge, the antibody responses for the CFP and CFP-BCG groups remained high, while the mean responses for the BCG-vaccinated and nonvaccinated groups increased to similar levels (data not shown).
Tuberculin skin test responses.
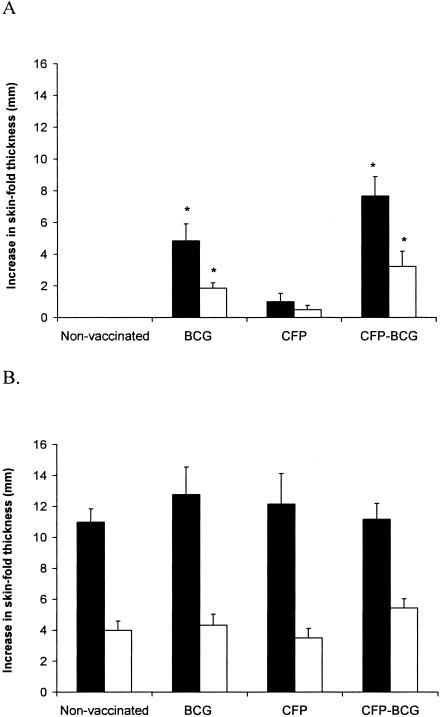
Seventeen weeks after vaccination, all of the calves were subjected to a comparative cervical tuberculin skin test. The mean responses for the different groups are shown in Fig. 2. For both the BCG and CFP-BCG groups, the mean responses to bovine PPD after vaccination were significantly larger than those for the nonvaccinated group. With the comparative cervical skin test, responses to bovine and avian PPD were measured, and positive responses (bovine PPD response − avian PPD response = ≥4 mm) were recorded for 2/10 animals vaccinated with BCG and 6/9 animals vaccinated with CFP-BCG. No significant differences were seen in the comparative cervical skin test responses between the groups 14 weeks after the challenge with M. bovis.
FIG. 2.
Skin test responses to avian PPD (white bars) and bovine PPD (black bars) 17 weeks after vaccination (A) and 14 weeks after challenge (B). The data are expressed as mean (± SEM) increases in skin thickness (mm) between the time of inoculation and 72 h later. *, significantly higher than the mean of the nonvaccinated group (P < 0.05).
Vaccination with CFP and BCG induces better protection against M. bovis challenge than does vaccination with BCG alone.
Following the challenge with M. bovis, macroscopic tuberculous lesions were found in only the lungs and thoracic lymph nodes of the animals, except for two animals (one each from the BCG and CFP groups) in which an M. bovis culture-positive lesion was found in a mesenteric lymph node. These two animals also had lesions in the lungs or thoracic lymph nodes. The CFP-BCG group exhibited significant reductions in five parameters associated with pathology compared to the nonvaccinated group (Table 2). In contrast, the BCG group had a significant reduction in only one parameter of pathology. The proportion of animals with macroscopic tuberculous lung lesions and the proportion of animals with lymph node lesions in the CFP-BCG group were less than the proportions in the nonvaccinated group (P < 0.05). The mean lymph node severity scores of the CFP-BCG and BCG groups were lower than the score of the nonvaccinated group (P < 0.05), but the mean lung severity score was significantly lower only for the CFP-BCG group (P < 0.05) compared to the nonvaccinated group. The CFP-BCG group was the only group with a significantly smaller mean number of lymph node lesions per animal (P < 0.05). The lung lesions were comprised of a series of small nodular lesions of 2 to 5 mm in diameter with yellow caseous centers. The lymph node lesions ranged in size from 1 to 30 mm in diameter, with yellow calcified centers. The lesions found in the two mesenteric lymph nodes were small lesions of 2 to 3 mm in diameter.
TABLE 2.
Pathological findings following challenge of calves with M. bovisc
| Vaccine group | No. of animals with macroscopic lesions/total no. of animals
|
Mean lung scorea ± SEM | Mean total LN scoreb ± SEM | Mean no. of LNs (± SEM) with macroscopic TB lesions per animal | |
|---|---|---|---|---|---|
| Lung lesions | LN lesions | ||||
| No vaccine | 9/10 | 10/10 | 3.40 ± 0.48 | 7.10 ± 1.04 | 2.50 ± 0.22 |
| CFP/Emulsigen/CpG ODN | 10/10 | 10/10 | 4.30 ± 0.21 | 8.60 ± 1.30 | 3.00 ± 0.37 |
| CFP/Emulsigen/CpG ODN + BCG | 4/9* | 4/9* | 1.56* ± 0.76 | 2.22* ± 1.06 | 1.11* ± 0.46 |
| BCG | 5/10 | 8/10 | 2.00 ± 0.76 | 3.50* ± 1.17 | 1.70 ± 0.40 |
Lung lesion scores: 0, no lesions; 1, 1 to 9 lesions; 2, 10 to 29 lesions; 3, 30 to 99 lesions; 4, 100 to 199 lesions; 5, ≥200 lesions.
Total lymph node (LN) lesion scores for individual animals based on scores for individual nodes: 0, no lesions; 1, 1 to 19 small lesions (1- to 4-mm diameter); 2, ≥20 small lesions; 3, medium-sized lesion (5- to 9-mm diameter); 4, large lesion (≥10-mm diameter).
*, significantly different from nonvaccinated group (P < 0.05).
The mean number of M. bovis cells cultured from the thoracic lymph nodes of the CFP-BCG group was significantly lower than that for the nonvaccinated group (P < 0.05) (Table 3), while there were no differences among the other groups. There were no significant differences among the mean numbers of M. bovis cells isolated from lung lesions for the different groups.
TABLE 3.
Microbiological findings following challenge of calves with M. bovis
| Vaccine group | No. of M. bovis culture-positive animals/total no. of animals | Mean bacterial count (log10 CFU/g of tissue) ± SEMa
|
|
|---|---|---|---|
| Lung lesionsb | Lymph node lesions | ||
| No vaccine | 10/10 | 3.226 ± 0.198 | 2.506 ± 0.225 |
| CFP/Emulsigen/CpG ODN | 10/10 | 3.048 ± 0.142 | 2.602 ± 0.204 |
| CFP/Emulsigen/CpG ODN + BCG | 8/9 | 2.632 ± 2.079 | 1.485* ± 0.160c |
| BCG | 9/10 | 2.885 ± 0.204 | 1.970 ± 0.209 |
When no bacteria were isolated, a count of 0.699 log10 CFU/g was recorded.
Bacterial culture was only undertaken with lung lesions; for the nonvaccinated group, n = 9; for the CFP/Emulsigen/CpG ODN group, n = 10; for the CFP/Emulsigen/CpG ODN + BCG group, n = 4; for the BCG group, n = 5.
*, significantly different from the nonvaccinated group (P < 0.05).
DISCUSSION
The efficacy of BCG vaccination can vary widely in both humans and cattle (9, 13). This has led to renewed efforts to develop novel tuberculosis vaccines that induce a more reliable level of protection. From this perspective, a promising area of research is the development of subunit vaccines, particularly those based upon the culture filtrate proteins (CFP) of the bacillus, which in mice stimulate IFN-γ secretion by CD4+ T cells and can give levels of protection similar to those induced by BCG (1). In addition, these protein vaccines have been shown to induce higher levels of protection against tuberculosis than BCG in a mouse model when the animals were sensitized to environmental mycobacteria prior to vaccination (5).
CFP represents a complex mixture of mycobacterial antigens which have a variety of immunogenic properties. However, studies with guinea pigs have shown that the protection is relatively ephemeral (3). In contrast, immune protection conferred by BCG can be very long lasting (2). This suggests that BCG and CFP induce different facets of the immune response.
We tested the hypothesis that a combination of CpG ODN-formulated CFP and BCG would offer substantial protection against tuberculosis in cattle. Our data showed that the CFP-BCG regimen induced a significant enhancement in six pathological and microbiological parameters of protection. While there was a trend towards protection for the BCG-vaccinated animals, vaccination with BCG alone conferred a significant enhancement of protection in only one parameter. These results suggest that a combination of a subunit protein vaccine with BCG may be a better option for vaccination against tuberculosis than vaccination with BCG alone. We have previously shown that a DNA prime-BCG boost regimen may be better than a single vaccine for the protection of cattle against tuberculosis (25). Vaccination with CFP alone did not confer protection against tuberculosis, suggesting that there is a synergism between the CFP components and the BCG vaccine in terms of inducing a protective immune response. CFP is a complex cocktail of antigens that have a range of immunomodulatory effects. The enhanced IFN-γ response observed for cattle vaccinated with both BCG and CFP concurs with the ability of formulated CFP to potentiate an IFN-γ response. Some secreted antigens of virulent mycobacteria have been shown to exert potent immunosuppressive effects (19). These effects may explain the failure of the CFP preparation to provide protection when used alone. An implication is that CFP constitutes a complex mixture of immunosuppressive and immunopotentiating antigens, inviting studies aimed at identifying the discrete antigens acting as immunoenhancers for the current BCG vaccine.
In the present trial, the IFN-γ and IL-2 responses in CFP-vaccinated cattle were stronger than those seen previously for animals vaccinated with this vaccine (33), yet there was no protection with the current CFP preparation. The differences in immune responses and protection may be related to the concentration of adjuvant used for preparing the vaccine and the number of boosters given. For the present trial, M. bovis CFP was mixed with 50% Emulsigen and the animals were given two boosters, while in the previous trial the adjuvant concentration in the vaccine was 50% for the initial vaccination and reduced to 30% for a single revaccination. The higher concentration of adjuvant used in the vaccine formulation and a vaccination regimen of three injections may have contributed to an immune response which was not associated with protection. In both trials, the CFP vaccine formulation included CpG ODN, which is known to have strong immunostimulatory effects on cells of the innate immune system, including dendritic cells and macrophages (14), and to enhance the generation of a Th1 response to vaccines (35). The addition of the specific CpG ODN used in the CFP vaccine preparation was essential for the induction of protective immunity (33).
In vaccination/challenge trials in cattle, the induction of an IFN-γ response to bovine PPD early after vaccination (2 weeks postvaccination) is generally associated with protection, and no protection was observed in a BCG vaccination trial in which a delay in the induction of an IFN-γ response was observed (8). In the present study, a strong early IFN-γ response was associated with protection, with the strongest cellular immune responses observed with animals vaccinated with CFP and BCG. Our data suggest that CFP on its own leads to an IFN-γ response which is delayed compared to the vigorous early response which occurs following BCG vaccination. This delayed IFN-γ response was associated with a poor level of protection. This raises the question of which particular cell types releasing IFN-γ are recruited by CFP versus BCG in cattle and what relative contributions they give to resistance. Recent data suggested that CFP is a strong inducer of IFN-γ secretion by bovine γδ T cells (26). However, the exact role of this subpopulation of T cells in bovine tuberculosis remains unclear. Recent experiments with cattle showed that the depletion of γδ T cells does not modify the ability of animals to generate a delayed-type hypersensitivity response (17) and does not affect the development of tuberculosis following an intranasal challenge with M. bovis (16).
Vaccination with CFP either alone or in combination with BCG induced a strong antibody response. The antibody responses to M. bovis CFP were similar for these two groups, indicating that BCG did not boost the antibody levels beyond those induced by CFP alone. The ratio of antigen-specific IgG1 to IgG2 was also similar, although it is possible that antibody responses to particular M. bovis antigens may have varied with the two vaccination strategies. This suggests that in bovine tuberculosis, the development of a vigorous antibody response against M. bovis does not preclude the expression of a strong cell-mediated immune response, as long as an appropriate IFN-γ response is recruited. These observations lend credence to the emerging concept that the Th1/Th2 responses in vaccinated humans or experimental animals are not mutually exclusive (12, 15, 18, 22). BCG vaccination of cattle induces both IFN-γ and IL-4 mRNA expression but no antibodies (10, 31, 33), whereas in the present study, vaccination with CFP and BCG induced a strong antibody response in addition to significant IFN-γ and IL-4 responses. Therefore, in cattle, a strong Th2 component of the immune response (characterized by IL-4 and antibody production) does not mask the acquisition of protection against tuberculosis as long as a main immune system correlate of protection, notably IFN-γ production, is induced.
Vaccination with both CFP and BCG induced positive tuberculin skin test responses in a proportion of the vaccinated animals. These responses would impede discrimination between animals which received the combination vaccines and animals actively infected with tuberculosis. However, novel differential diagnostic tests using tuberculosis-specific antigens such as ESAT-6 and CFP-10, which are not expressed by BCG, could be used to discriminate between vaccinated cattle and those infected with M. bovis (27). CFP could be produced by mutant strains of M. bovis which are deficient in the expression of ESAT-6 and CFP-10 (28).
In summary, we have described a novel vaccination strategy consisting of a combination of a subunit protein vaccine and the live attenuated M. bovis BCG strain. This approach was shown to impart better protection against bovine tuberculosis than a single injection with BCG.
Acknowledgments
We thank Keith Hamel, Denise Keen, Natalie Parlane, Allison McCarthy, and Gary Yates for expert technical assistance and Lilian Morrison for statistical analyses.
This work was supported financially by the New Zealand Ministry of Agriculture and Forestry (Policy Management), the Foundation for Research, Science and Technology, and DEFRA (Department for Environment, Food, and Rural Affairs) (United Kingdom).
Editor: J. L. Flynn
REFERENCES
- 1.Andersen, P. 1994. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect. Immun. 62:2536-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aronson, N. E., M. Santosham, G. W. Comstock, R. S. Howard, L. H. Moulton, E. R. Rhoades, and L. H. Harrison. 2004. Long-term efficacy of BCG vaccine in American Indians and Alaska natives: a 60-year follow-up study. JAMA 291:2086-2091. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin, S. L., C. D'Souza, A. D. Roberts, B. P. Kelly, A. A. Frank, M. A. Lui, J. B. Ulmer, K. Huygen, D. N. McMurray, and I. M. Orme. 1998. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66:2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosio, C. M., and I. M. Orme. 1998. Effective, nonsensitizing vaccination with culture filtrate proteins against Mycobacterium bovis infections in mice. Infect. Immun. 66:5048-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt, L., J. Feino Cunha, A. Weinreich Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buddle, B. M., G. W. de Lisle, A. Pfeffer, and F. E. Aldwell. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123-1130. [DOI] [PubMed] [Google Scholar]
- 7.Buddle, B. M., D. Keen, A. Thomson, G. Jowett, A. R. McCarthy, J. Heslop, G. W. de Lisle, J. L. Stanford, and F. E. Aldwell. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10-16. [DOI] [PubMed] [Google Scholar]
- 8.Buddle, B. M. 2001. Vaccination of cattle against Mycobacterium bovis. Tuberculosis 81:125-132. [DOI] [PubMed] [Google Scholar]
- 9.Buddle, B. M., B. J. Wards, F. E. Aldwell, D. M. Collins, and G. W. de Lisle. 2002. Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine 20:1126-1133. [DOI] [PubMed] [Google Scholar]
- 10.Buddle, B. M., D. N. Wedlock, N. A. Parlane, L. A. L. Corner, G. W. de Lisle, and M. A. Skinner. 2003. Revaccination of neonatal calves with Mycobacterium bovis BCG reduces the level of protection against bovine tuberculosis induced by a single vaccination. Infect. Immun. 71:6411-6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosivi, O., J. M. Grange, C. J. Daborn, M. C. Raviglione, T. Fujikura, D. Cousins, R. A. Robinson, H. F. Huchzermeyer, I. de Kantor, and F. X. Meslin. 1998. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg. Infect. Dis. 4:59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erb, K. J., C. Trujillo, M. Fugate, and H. Moll. 2002. Infection with the helminth Nippostrongylus brasiliensis does not interfere with efficient elimination of Mycobacterium bovis BCG from the lungs of mice. Clin. Diagn. Lab. Immunol. 9:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fine, P. E. M. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 14.Häcker, G., B. Redecke, and H. Häcker. 2002. Activation of the immune system by bacterial CpG-DNA. Immunology 105:245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung, Y. J., R. LaCourse, L. Ryan, and R. J. North. 2002. Evidence inconsistent with a negative influence of T helper 2 cells on protection afforded by a dominant T helper 1 response against Mycobacterium tuberculosis lung infection in mice. Infect. Immun. 70:6436-6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy, H. E., M. D. Welsh, D. G. Bryson, J. P. Cassidy, F. I. Forster, C. J. Howard, R. A. Collins, and J. M. Pollock. 2002. Modulation of immune responses to Mycobacterium bovis in cattle depleted of WC1(+) gamma delta T cells. Infect. Immun. 70:1488-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy, H. E., M. D. Welsh, J. P. Cassidy, D. G. Bryson, F. Forster, J. McNair, B. Gangadharan, C. J. Howard, and J. M. Pollock. 2003. The role of WC1(+) gamma delta T-cells in the delayed-type hypersensitivity (DTH) skin-test reaction of Mycobacterium bovis-infected cattle. Vet. Immunol. Immunopathol. 93:169-176. [DOI] [PubMed] [Google Scholar]
- 18.Lagranderie, M., and A. M. Balazuc. 2002. Development of mixed Th1/Th2 type immune response and protection against Mycobacterium tuberculosis after rectal or subcutaneous immunization of newborn and adult mice with Mycobacterium bovis BCG. Scand. J. Immunol. 55:293-303. [DOI] [PubMed] [Google Scholar]
- 19.Natarajan, K., V. K. Latchumanan, B. Singh, S. Singh, and P. Sharma. 2003. Down-regulation of T helper 1 responses to mycobacterial antigens due to maturation of dendritic cells by 10-kDa Mycobacterium tuberculosis secretory antigen. J. Infect. Dis. 187:914-928. [DOI] [PubMed] [Google Scholar]
- 20.Orme, I. M. 2001. Immunology and vaccinology of tuberculosis: can lessons from the mouse be applied to the cow? Tuberculosis 81:109-113. [DOI] [PubMed] [Google Scholar]
- 21.Pontarollo, R. A., R. Rankin, L. A. Babiuk, D. L. Godson, P. J. Griebel, R. Hecker, A. M. Krieg, and S. van Drunen Littel-van den Hurk. 2002. Monocytes are required for optimum in vitro stimulation of bovine peripheral blood mononuclear cells by non-methylated CpG motifs. Vet. Immunol. Immunopathol. 84:43-59. [DOI] [PubMed] [Google Scholar]
- 22.Rhodes, S. G., N. Palmer, S. P. Graham, A. E. Bianco, R. G. Hewinson, and H. M. Vordermeier. 2000. Distinct response kinetics of gamma interferon and interleukin-4 in bovine tuberculosis. Infect. Immun. 68:5393-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts, A. D., M. G. Sonnenberg, D. J. Ordway, S. K. Furney, P. J. Brennan, J. T. Belisle, and I. M. Orme. 1995. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology 85:502-508. [PMC free article] [PubMed] [Google Scholar]
- 24.Rothel, J. S., S. L. Jones, L. A. Corner, J. C. Cox, and P. R. Wood. 1990. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust. Vet. J. 67:134-137. [DOI] [PubMed] [Google Scholar]
- 25.Skinner, M. A., B. M. Buddle, D. N. Wedlock, D. Keen, G. W. deLisle, R. E. Tascon, J. Candido Ferraz, D. B. Lowrie, P. J. Cockle, M. H. Vordermeier, and R. G. Hewinson. 2003. A DNA prime-Mycobacterium bovis BCG boost vaccination strategy for cattle induces protection against bovine tuberculosis. Infect. Immun. 71:4901-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vesosky, B., O. C. Turner, J. Turner, and I. M. Orme. 2004. Gamma interferon production by bovine γδ T cells following stimulation with mycobacterial mycolylarabinogalactan peptidoglycan. Infect. Immun. 72:4612-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wards, B. J., G. W. de Lisle, and D. M. Collins. 2000. An esat6 knockout mutant of Mycobacterium bovis produced by homologous recombination will be useful for development of a live tuberculosis vaccine. Tuber. Lung Dis. 80:1985-1989. [DOI] [PubMed] [Google Scholar]
- 29.Wedlock, D. N., F. E. Aldwell, D. M. Collins, G. W. de Lisle, T. Wilson, and B. M. Buddle. 1999. Immune responses induced in cattle by virulent and attenuated Mycobacterium bovis strains: correlation of delayed type hypersensitivity with ability of strains to grow in bovine macrophages. Infect. Immun. 67:2172-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wedlock, D. N., B. Vesosky, M. A. Skinner, G. W. de Lisle, I. M. Orme, and B. M. Buddle. 2000. Vaccination of cattle with Mycobacterium bovis culture filtrate proteins and interleukin-2 for protection against bovine tuberculosis. Infect. Immun. 68:5809-5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wedlock, D. N., D. L. Keen, F. E. Aldwell, P. Andersen, and B. M. Buddle. 2002. Effect of adjuvants on immune responses of cattle vaccinated with culture filtrate proteins from Mycobacterium tuberculosis. Vet. Immunol. Immunopathol. 86:79-88. [DOI] [PubMed] [Google Scholar]
- 32.Wedlock, D. N., M. A. Skinner, N. A. Parlane, H. M. Vordermeier, R. G. Hewinson, G. W. de Lisle, and B. M. Buddle. 2003. Vaccination with DNA vaccines encoding MPB70 or MPB83 or a MPB70 prime/protein boost does not protect cattle against bovine tuberculosis. Tuberculosis 83:339-349. [DOI] [PubMed] [Google Scholar]
- 33.Wedlock, D. N., M. A. Skinner, H. M. Vordermeier, R. G. Hewinson, R. Hecker, S. van Drunen Littel-van den Hurk, and B. M. Buddle. Vaccination of cattle with Mycobacterium bovis culture filtrate proteins and CpG oligodeoxynucleotides induces protection against bovine tuberculosis. Vet. Immunol. Immunopathol., in press. [DOI] [PubMed]
- 34.Wood, P. R., L. A. Corner, J. S. Rothel, J. L. Ripper, T. Fifis, B. S. McCormick, B. Francis, L. Melville, K. Small, L. De Witte, J. Tolson, T. J. Ryan, G. W. de Lisle, J. C. Cox, and S. L. Jones. 1992. A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet. Microbiol. 31:71-79. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann, S., O. Egeter, S. Hausmann, G. B. Lipford, M. Rocken, H. Wagner, and K. Heeg. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160:3627-3630. [PubMed] [Google Scholar]