Abstract
Staphylococcus aureus is a pathogen often found in pneumonia and sepsis. In the context of the resistance of this organism to conventional antibiotics, an understanding of the regulation of natural endogenous antimicrobial molecules is of paramount importance. Previous studies have shown that both human and mouse airways express a variety of these molecules, including defensins, cathelicidins, and the four-disulfide core protein secretory leukocyte protease inhibitor. We demonstrate here by culturing mouse tracheal epithelial cells at an air-liquid interface that, despite the production of Defb1, Defb14, and Defr1 in this system, these cells are unable to clear S. aureus when exposed to this respiratory pathogen. Using an adenovirus (Ad)-mediated gene transfer strategy, we show that overexpression of elafin, an anti-elastase/antimicrobial molecule (also a member of the four-disulfide core protein family), dramatically improves the clearance of S. aureus. In addition, we also demonstrate that this overexpression is efficient in vivo and that intratracheal instillation of Ad-elafin significantly reduced the lung bacterial load and demonstrates concomitant anti-inflammatory activity by reducing neutrophil numbers and markers of lung inflammation, such as bronchoalveolar lavage levels of tumor necrosis factor and myeloperoxidase. These findings show that an increased antimicrobial activity phenotype is provided by the elafin molecule and have implications for its use in S. aureus-associated local and systemic infections.
Staphylococcus aureus infections are closely associated with pneumonia and sepsis, particularly in nosocomial infections (12, 13). This organism can often cause primary pneumonias, in young adults and patients with cystic fibrosis (5, 8, 9). Although pulmonary infections with S. aureus could until recently be controlled with aggressive antibiotic therapy, the increasing association of S. aureus with antimicrobial resistance has become a major concern for clinicians.
In that context, endogenous antimicrobial molecules (AMMs) are gathering interest as alternative microbicidal agents since they are thought to be less likely to induce bacterial resistance than conventional antibiotics (10). This class of molecules includes defensins, cathelicidins, and the four- disulfide core proteins secretory leukocyte protease inhibitor and elafin (29-31). In parallel with human airways, respiratory tract epithelia of mice (a useful species in which to model lung infections) have been shown to express β-defensin molecules, including Defb1 (18), Defb2 (19), Defb3 (2), Defb4 (14), Def b6 (38), Defr1 (21), and the cathelicidin CRAMP (24).
Although little is known about the in vivo activity of these molecules against S. aureus, we have shown that chemically synthesized β-defensin Defb1and Defr1 murine peptides have antimicrobial activity in vitro against this microorganism (18, 21). However, deletion of Defb1 by gene targeting did not lead to increased airway retention of this pathogen after exposure in vivo (20, 22). This suggests that the peptide is either redundant or not crucial in innate immunity against S. aureus in the airways. To investigate innate defenses against S. aureus at the mucosal surface of the airways and to facilitate screening of other potentially important AMMs, we used primary cultures of differentiated mouse tracheal epithelia (at an air-liquid interface [ALI]) as previously described (4). We demonstrate here that Defb1, Defr1, and Defb14 (the orthologue of human β-defensin 3, DEFB103), which display potent activity against S. aureus in vitro (17), are expressed in these epithelial cell cultures. In addition, we show that despite expressing a repertoire of peptides capable of killing S. aureus in vitro, primary ALI cultured cells are unable to clear this respiratory pathogen. In contrast, we show there that adenovirus (Ad)-mediated overexpression of human elafin/skin-derived antileucoprotease/trappin-2 (16, 31, 34, 37), a 9.9-kDa neutrophil elastase inhibitor with antimicrobial activity against S. aureus and Pseudomonas aeruginosa (when added as a purified molecule, leads to killing of S. aureus both in vitro and in vivo in a murine acute lung infection model. This demonstrates that elafin overexpression may have potential therapeutic benefit against S. aureus infections.
MATERIALS AND METHODS
Recombinant replication-deficient Ad constructs.
Two E1-partially E3-deleted type 5 recombinant replication-deficient adenoviruses were used. The Ad-LacZ construct was a gift from J. Gauldie and F. Graham (1), whereas the Ad-elafin construct was generated as described before (28).
Bacteria.
S. aureus C1705 (a clinical strain [9, 18]) was grown initially as colonies on Colombia agar (Unipath, Basingstoke, United Kingdom) and then in 10 ml of tryptone soy broth (Unipath) overnight at 37°C in an orbital shaker (Gallenkamp; Fisher Scientific, Loughborough, United Kingdom) at 200 rpm at room temperature. The resulting suspension was centrifuged at 3,000 rpm for 15 min at room temperature. The supernatant was discarded, and the pellet resuspended in 10 ml of phosphate buffer (8 mM K2HPO4, 2 mM KH2PO4). Suspensions were adjusted with phosphate buffer to an A590 of 1.50, yielding an estimated bacterial concentration of 109 CFU/ml, in accordance with predetermined growth curves.
Serial dilutions were performed with phosphate buffer to obtain the desired concentration of viable bacteria (see below).
Mouse epithelial cell culture.
Female C57BL/6 or BALB/c murine primary tracheal epithelial cells were generated and grown on semipermeable membranes at an ALI according to our previously published method (4). Briefly, tracheae were excised and severed at the proximal limit of the thyroid cartilage and at the junction of the main bronchi. The thyroid gland and other adherent tissues were removed before cutting tracheae longitudinally. Dissociated cells from two tracheae (ca. 4 × 105 cells) were seeded onto one semipermeable support membrane, precoated with 0.5 mg of collagen/ml (24-well plate inserts, 0.4-μm pore size; Corning Costar) in 0.2 ml of culture medium (1:1 Dulbecco modified Eagle medium-Ham’s F-12 medium containing 100 U of penicillin and 100 μg of streptomycin/ml). Cells were incubated at 37°C in 6% CO2 for 3 days. On day 4, medium bathing the apical cell surface was removed, along with any cell debris, and the medium on the outside of the insert replaced with 0.6 ml of Ultroser G (USG) medium. Cells were verified as confluent, with tight junctions, by monitoring transepithelial resistance, as described previously (4). Only cultures with high (>12-kΩ/cm2) resistances were used. In addition, light microscopy confirmed the presence of cells with epithelial morphology and demonstrated the appearance of beating cilia. In selected cases, lipopolysaccharide (Escherichia coli O26:B6 [Sigma]) was added to the surface of the cells at 80 μg/insert and incubated for 2 h at 37°C with 6% CO2. After lipopolysaccharide (LPS) incubation, the cells were harvested and RNA was extracted.
RT-PCR.
Reverse transcription-PCR (RT-PCR) was carried out as previously described (4). Briefly, total RNA was isolated from cells or tissues collected by using RNAzol B as described by the manufacturer (Biogenesis). cDNA synthesis was achieved by using a first strand cDNA synthesis kit (Roche), and the resultant cDNA was used as a template in PCRs with the following primers (forward and reverse): Defb1, 5′-CCAGCTGCCCATCTAATACC-3′ and 5′-AATCCATCGCTCGTCCTTTA-3′; Defb14, 5′-TCTTGTTCTTGGTGCCTGCT-3′ and 5′-TTCTTCTTTCGGCAGCATTT-3′; and Defr1, 5′-ATTTCTCCTGGTGCTGCTGT-3′ and ′-GGTTTGCAGGATCTTTGTC-3′.
The following conditions were used for PCR: denaturation at 94°C for 3 min, followed by 45 cycles of 94°C for 30 s; annealing at temperatures of 54°C for Defb1, 54°C for Defb14, and 60°C for Defr1 for 30 s, with extension at 72°C for 1 min. The amplified products were analyzed on 2% agarose gels by electrophoresis. To confirm RNA amplification of defensin genes, the primers were situated in exons 1 and 2. For elafin RT-PCR, controls were included without reverse transcriptase, and RNA was DNase treated prior to cDNA synthesis according to established methods. Template cDNA was amplified with the primers 5′-CTGTAGATTTTATCAGACTGAAGAG-3′ and 5′-GTCAAGGGCATATCCAACAACAAA-3′ for hypoxanthine phosphoribosyl transferase (Hprt) as a positive control. Southern blotting was performed with Hybond-N membranes (Amersham), and hybridization was carried out with internal radiolabeled oligonucleotide probes (Defb1, 5′-CTGTTGTAAGAGCTGA-3′; Defb14, 5′-GGACGCATTCCTACCAAAAA-3′; and Defr1, 5′CTTATGCCTAAGGCCAATGC3′) for 16 h at 48°C. Filters were washed twice in 0.6 M NaCl-0.06 M trisodium citrate (standard saline citrate) plus 0.5% sodium dodecyl sulfate for 30 min before being exposed to photographic film.
Adenovirus infection of epithelial cells.
In order to increase the efficiency of infection of mouse tracheal epithelial cells (estimated number = 400,000 per insert), Ad infections (multiplicity of infection [MOI] of 100) were carried out in the presence of CaPO4 precipitates (final concentration 5.8 mM Ca2+ and 0.86 mM PO4) generated by adding CaCl2 (4 mM final concentration) to minimal essential Eagle medium (MEEM), a medium with a high enough PO4 concentration to allow for the generation of these precipitates (6). Alternatively, Ad infections were carried out in USG medium. Cells were incubated with these infection media in a total volume of 250 μl for 45 min at 37°C. Cells were washed twice with phosphate-buffered saline (PBS) and then incubated for 18 to 20 h at 37°C in the absence of medium bathing the apical surface.
To assess the Ad infection efficiency, Ad-LacZ-infected epithelial cell layers were fixed for 10 min with a solution comprising 0.2% glutaraldehyde, 0.8% formaldehyde, and 2 mM MgCl2 (in PBS). Fixative was discarded, and 200 μl of staining solution [5 mM K4Fe(CN)6, 5 mM K3 Fe3 (CN)6, 2 mM MgCl2, 0.05% Triton X-100, 0.5 mg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)/ml (in PBS)] was added for 5 h at 37°C. Cells were washed with PBS to remove residual stain, air dried, and photographed.
Ad-elafin quantification and antibacterial activity in vitro.
Mouse tracheal epithelial cells were infected with Ad-elafin (and relevant controls) in MEEM containing CaPO4 precipitates (see above) and in the absence of antibiotics. After 18 to 20 h at 37°C (see above), 20 nl of either phosphate buffer or a C1705 S. aureus suspension (107 CFU/ml) was added to the cells with the use of a special Hamilton precision syringe (Hamilton Company, Reno, NV), followed by incubation for 3 h at 37°C. Cell inserts were washed with both 105 μl of phosphate buffer and 105 μl of phosphate buffer-0.5% Triton X-100. These two washes were then pooled and centrifuged at 8,000 rpm for 10 min. Supernatants were used for elafin quantitation by enzyme-linked immunosorbent assay (ELISA) (27), whereas bacterial cell pellets were suspended in PBS and used for bacterial counts on Colombia agar. Agar plates were incubated overnight at 37°C prior to colony counting. Values represent the mean ± the standard error of three experiments, each performed in triplicate. (Significant differences [P < 0.05] compared to Ad-lacZ-infected cells are indicated in Table 1.)
TABLE 1.
Inflammatory parameters in BALF of C57BL/6 mice sequentially receiving Ad vectors and S. aureusa
| Treatment | No. of mice | Content (range)
|
||
|---|---|---|---|---|
| BALF protein (μg/ml) | BALF MPO (arbitrary units) | BALF TNF (pg/ml) | ||
| PBS-PBS | 3 | 314.7 (232.7-320.4) | 0.031 (0.008-0.26) | 15.0 (3.75-48.75) |
| Ad-LacZ-S. aureus | 6 | 743.2 (579.3-851.6) | 0.094 (0.049-0.299) | 2,283.3* (975.0-3,225.0) |
| Ad-elafin-S. aureus | 8 | 567.0* (433.7-689.3) | 0.059 (0.027-0.165) | 577.5*† (221.9-1,188.1) |
Female C57BL/6 mice received sequential intratracheal treatments of Ad-LacZ or Ad-elafin and then S. aureus as described in Materials and Methods. At 24 h after bacterial administration, BALF was obtained and centrifuged; the cell pellet was used for differential cell analysis, whereas supernatants were used for the determination of total protein and TNF levels and MPO activity (given as median values). *, significantly different from the PBS-PBS group; †, significantly different from the Ad-LacZ-S. aureus group (Mann-Whitney, P < 0.05).
In vivo studies.
Female C57BL/6 mice between 6 and 8 weeks old (Harlan Olac, Bicester, United Kingdom) were treated intratracheally (as described in reference 33) with 40 μl of Ad-LacZ (adenovirus control) or Ad-elafin (3 × 107 PFU in both cases). After 5 days, 4 × 107 CFU of S. aureus (in 40 μl) were administered via the same route. This bacterial dose was shown in previous pilot experiments to produce a substantial inflammatory response in the lung. At 24 h after the bacterial administration, mice were euthanized and bronchoalveolar lavage (BAL) performed (two serial instillations of 0.4 ml of PBS). BAL fluid (BALF) was centrifuged 10 min at 4,000 × g; supernatants were stored at −20°C until further use, whereas each cell pellet was resuspended in 100 μl of PBS. Cell counting (with a hemocytometer) and cytospins (300 rpm for 3 min at room temperature) were then performed. After BALF was recovered, the lungs were harvested for either RNA preparation (storage in RNA later [Sigma]), histology, or bacterial counts. For the latter, half lungs were homogenized in 2 ml of PBS by using a tissue homogenizer (OMNI μH International; Camlab), and homogenates were further diluted 10-fold with PBS. A total of 100 μl of the diluent was plated on Colombia agar plates, which were incubated at 37°C for 24 h.
ELISAs.
ELISA analyses to detect murine tumor necrosis factor alpha (TNF-α) were performed by using the R&D Duoset kit (Abingdon, Oxon, United Kingdom) in accordance with the manufacturer's instructions.
Human elafin was measured by ELISA as described previously (27).
Protein quantification.
Protein was measured by using the bicinchoninic acid method (Pierce) with purified albumin (Pierce) as the standard.
MPO measurement.
A total of 40 μl of BAL fluid was incubated with 60 μl of TMB (3,3′,5,5′-tetramethylbenzidine substrate; 0.1 mg/ml in 0.1 M sodium acetate-citrate buffer [pH 4.9]) containing 0.015% H2O2 (final concentration). After 5 min, the reaction was stopped with concentrated H2SO4, and the absorbance was read at 450 nm (reference filter 560 nm) in a microtiter plate reader (MRX II microtiter plate; Dynex Technologies).
Statistical analysis.
Statistical analyses used either Student t tests or Mann-Whitney tests.
RESULTS
Murine defensin expression in murine primary epithelial cells.
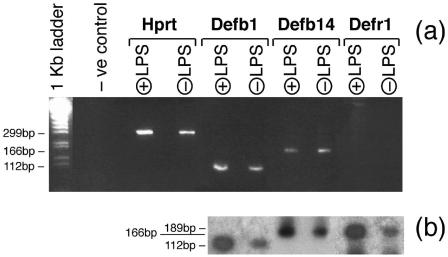
RNA was prepared from C57BL/6 tracheal inserts as detailed in Materials and Methods, and RT-PCR was performed with primers for Hprt (house keeping gene), as well as for the three murine β-defensin genes relevant to antimicrobial activity against S. aureus. Figure 1 demonstrates that murine C57BL/6 tracheal cells express Defb1, the mouse orthologue of human DEFB1, and Defb14, the mouse orthologue of DEFB103. We also show for the first time that Defr1 is expressed in murine epithelial cells. There is no evidence for significant upregulation of these genes after LPS exposure.
FIG. 1.
Expression of defensin genes in mouse primary tracheal epithelial cells. RNA was prepared from female C57BL/6 tracheal epithelial cells (unstimulated or stimulated with LPS for 30 min), and RT-PCR was performed as detailed in Materials and Methods with primers for three murine β-defensin genes (Defb1, Defb14, and Defr1) and for the housekeeping gene Hprt. In the negative control lane (−ve control), RNA was not included in the RT-PCR mixture. (a) Ethidium bromide analysis; (b) Southern blot analysis.
Innate antimicrobial activity of murine epithelial cells against S. aureus.
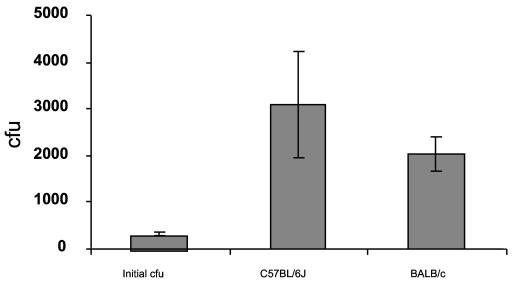
We were interested in examining whether any difference in bacterial killing was evident between BALB/c and C57BL/6 mice since they have a polymorphism in one of their β-defensin genes. BALB/c has Defb8, a classic six cysteine β-defensin, whereas C57BL/6 has a polymorphic allele with three nucleotide and amino acid changes that results in a five-cysteine β-defensin termed Defr1. This molecule has a novel structure, which when made synthetically has potent antibacterial activity (3). Despite the expression of β-defensins in C57BL/6 tracheal cells, neither these cells nor cells derived from BALB/c mice were able to inhibit the growth of an approximately 200 CFU S. aureus inoculum (20 nl of a 107-CFU/ml S. aureus suspension) (Fig. 2). This was considered already as a very small inoculum (identical to that used by Smith et al. [36]), and no attempt was made to further reduce that dose. Indeed, at 3 h postinoculation, an average of 3,077 and 2,050 CFU were recovered from C57BL/6 and BALB/c cells, respectively, compared to 329 in the original inoculum. This clearly demonstrates that, far from being bacteriostatic or bactericidal, the tracheal epithelium was able to promote S. aureus growth under these conditions.
FIG. 2.
Endogenous antimicrobial activity of murine C57BL/6- and BALB/c-derived tracheal cells. A 200-CFU inoculum of S. aureus (20 nl of either phosphate buffer or of a C1705 S. aureus suspension [107 CFU/ml]) was applied to inserts of either C57BL/6- or BALB/c-derived tracheal epithelial cells, as described in Materials and Methods. Inserts were washed with 105 μl of phosphate buffer and 105 μl of phosphate buffer-0.5% Triton X-100. Washes were pooled, followed by centrifugation at 8,000 rpm for 10 min, and the pellets were used for bacterial counts on nutrient agar plates at 37°C. Values represent means ± the standard errors of three experiments, each performed in triplicate (three inserts).
With this observation in mind, we examined whether recombinant adenovirus (Ad)-mediated overexpression of human elafin, an antimicrobial molecule with no ortholog in mice, was able to limit S. aureus growth in vitro and in vivo.
Optimization of infection of murine C57BL/6 epithelial cells with Ad vectors.
We first tested the Ad expression system by using the E. coli β-galactosidase reporter gene (Ad-LacZ). Because others have reported poor adenoviral gene transfer to airway epithelia due to low apical expression of the coxsackie/adenovirus receptor (26), we used a method incorporating the use of CaPO4 precipitates in the infection protocol in the present study, as previously investigated (6).
Indeed, we show here (Fig. 3) that generation of CaPO4 precipitates in MEEM (a medium particularly well suited for the generation of the latter due to its high concentration of PO4 ions) containing Ad-LacZ significantly increased (two- to threefold) the efficiency of infection of murine C57BL/6 tracheal cells compared to Ad-LacZ infection in the USG medium usually used for these cells (Fig. 3, compare panels D and C). This amended protocol, with MEEM as the medium of choice, was therefore used for all subsequent experiments. Neither CaPO4- nor Ad-mediated cytotoxicity was detected by trypan blue exclusion (not shown).
FIG. 3.
Optimization of infection of murine C57BL/6 tracheal cells with Ad-LacZ. Female C57BL/6 mouse tracheal epithelial cells cultured on inserts (see above) were infected with Ad-LacZ at an MOI of 100, in the presence or absence of CaPO4 precipitates (final concentration of 5.8 mM Ca2+ and 0.86 mM PO4) in MEEM or USG medium. Cells were incubated with this infection medium in a total volume of 250 μl for 45 min at 37°C. Cells were then washed twice with PBS and incubated for 18 to 20 h at 37°C in the absence of media bathing the apical surface. (A) No Ad, USG medium; (B) no Ad, MEEM plus CaPO4; (C) Ad-LacZ, USG medium; (D) Ad-LacZ, MEEM plus CaPO4.
In vitro antibacterial activity of Ad-elafin against S. aureus.
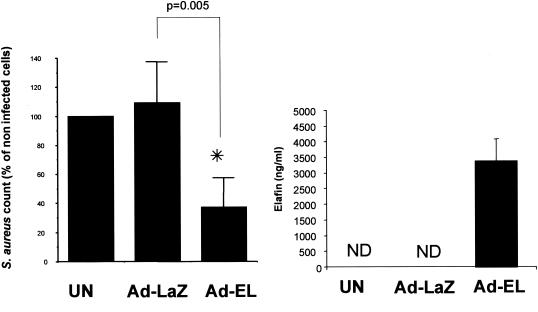
Having established the conditions for optimal Ad infection of murine C57BL/6 tracheal cells, we infected these cells with Ad-elafin and used Ad-LacZ as an Ad control. Figure 4 shows that, in accordance with all our previous studies (28, 30, 32), murine cells do not produce an elafin-related protein since our ELISA did not detect a cross-reacting species from untreated cells or cells treated with Ad-LacZ. In contrast, Ad-elafin infection induced the expression and secretion of very high levels of human elafin (right panel). This level correlated with an efficient reduction of S. aureus numbers (left panel, P = 0.005).
FIG. 4.
Ad-mediated elafin production and antibacterial activity in vitro. Female C57BL/6 mouse tracheal epithelial cells were either uninfected or infected with Ad-LacZ control or Ad-elafin (MOI = 100) in MEEM containing CaPO4 precipitates. After 18 to 20 h at 37°C (see Fig. 3 legend), a 200-CFU inoculum of S. aureus was added (as for Fig. 2). Bacterial counts were performed as described in the same legend, and elafin quantification was performed by ELISA (21). Values represent mean ± the standard error of three experiments, each performed in triplicate (three inserts). ✽, Significant difference (Mann-Whitney, P = 0.005); ND, not detected.
In vivo antibacterial activity of Ad-elafin against S. aureus.
When studying antimicrobial activity against P. aeruginosa (33), we have shown that Ad-LacZ was a useful Ad vector control which, by itself, did not induce a detectable increase in lung inflammation in comparison to PBS treatment. This lack of overt inflammation caused by Ad-LacZ is most probably due to the very low dose of Ad used in our study (3 × 107 PFU). In the present study we compared two experimental groups, Ad-LacZ (3 × 107 PFU) plus S. aureus and Ad-elafin (3 × 107 PFU) plus S. aureus, and one control group, PBS-PBS; the latter served as a control for potential background S. aureus commensals and contamination in the laboratory.
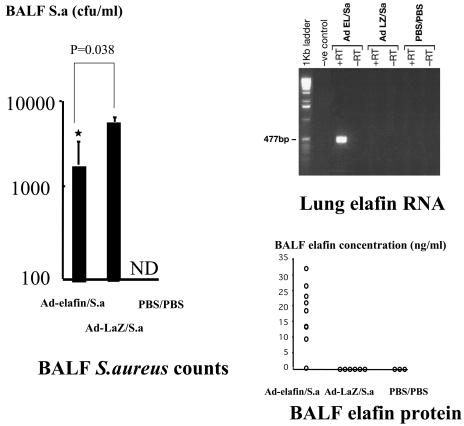
As expected and in keeping with our previous studies (and present in vitro data), no elafin mRNA was detected by RT-PCR in mice receiving Ad-LacZ or PBS, demonstrating that the band detected in the Ad-elafin-S. aureus group (Fig. 5, upper right) was specific to the human elafin transgene. This elafin expression was associated with a reduction in the number of recovered S. aureus in the BALF of Ad-elafin-treated mice, compared to mice given Ad-LacZ (Fig. 5, left panel; P = 0.038). As expected, no bacteria were recovered in the mice treated twice with PBS.
FIG. 5.
Ad-elafin expression and antibacterial activity in vivo. Female C57BL/6 mice were sequentially instilled intratracheally with either Ad-LacZ or Ad-elafin and then with S. aureus (S.a) (see Table 1). At 24 h after bacterial administration, BALF was obtained and centrifuged; the cell pellet was used for differential cell analysis, whereas supernatants were used for bacterial counts (Mann-Whitney analysis) and elafin quantification. In parallel, RNA was isolated from lungs and elafin RT-PCR analysis was performed as described in Materials and Methods. ND, not detected.
Modulation of in vivo inflammatory responses by Ad-elafin gene transfer.
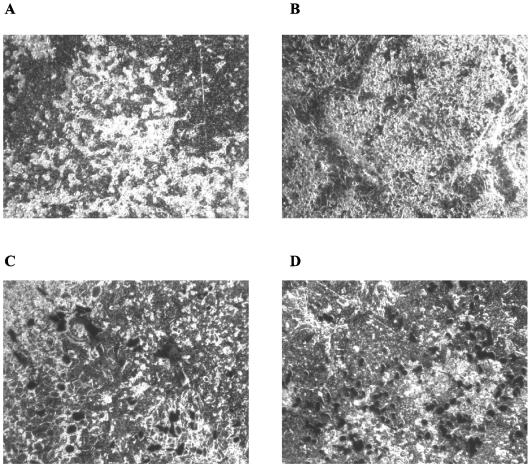
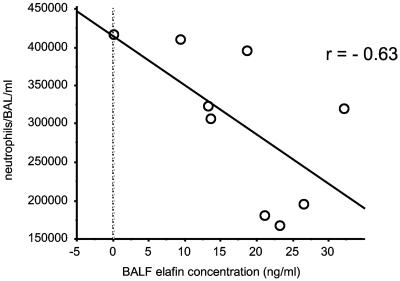
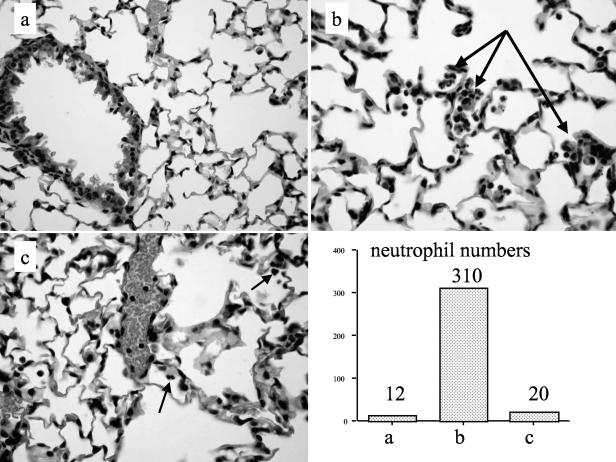
When inflammatory cell profiles in BALF were studied, compared to the PBS/PBS group, unsurprisingly, both the Ad-LacZ plus S. aureus and the Ad-elafin plus S. aureus groups showed increased numbers of total inflammatory cells, particularly neutrophils (P = 0.02 and 0.013, respectively). However, no statistically significant differences in cellular profiles were observed between the Ad-LaZ plus S. aureus and Ad-elafin plus S. aureus groups (data not shown). Interestingly, when the Ad-elafin plus S. aureus group was considered in isolation, there was a strong negative correlation between BAL elafin levels and neutrophil numbers (Fig. 6, r = −0.63, P = 0.069), suggesting that elafin may be associated in this model with a reduction in neutrophil numbers. Also, MPO levels, used as a marker of neutrophil activation, were lower in the Ad-elafin plus S. aureus group, although the difference did not reach statistical significance. A similar reduction (statistically significant) in the proinflammatory mediator TNF-α was noted in the Ad-elafin-S. aureus group, compared to the Ad-LacZ-S. aureus group (Table 1). When total protein levels were measured as an index of blood and/or alveolar barrier disruption, levels were lower in the PBS-PBS group compared to the Ad-groups, but within these there was a trend toward reduction of protein levels in the Ad-elafin-S. aureus group. Mirroring these data, blinded histological analysis showed neutrophilic inflammatory foci in the Ad-LacZ-treated animals, which were reduced in the Ad-elafin-treated mice. Quantification of neutrophils in whole lung sections is also given (representative sections are shown in Fig. 7).
FIG. 6.
Correlation between neutrophil count and elafin concentration in BALF. BALF from mice given Ad-elafin and S. aureus (see Table 1) were analyzed for elafin levels (by ELISA) and neutrophil count (by using cytospins).
FIG. 7.
Ad-elafin treatment results in the reduction of neutrophil clusters after intratracheal administration of S. aureus in mice. The histological appearance (magnification, ×400) of representative 3-μm lung sections (fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin) is shown. (b and c) Ad-elafin-S. aureus treatment reduced the numbers of neutrophil clusters (occasional “isolated” neutrophils were observed, see arrows) (c) compared to Ad-LacZ-S. aureus treatment, where multiple clusters were present (b, arrows). (a) None were seen in PBS-PBS-treated animals. Corresponding neutrophil numbers from each section are shown in the relevant bar graph.
DISCUSSION
S. aureus is a major human pathogen associated with localized and systemic infections. In order to facilitate and model the study of microbial infections in mice, cell culture systems have been developed where epithelial cells form a confluent, polarized, ciliated epithelium when grown at an air-liquid interface (4). Using this model, we have previously demonstrated that murine tracheal epithelial cells display many characteristics similar to those of murine tracheal epithelium in vivo and that these cells retain the ability ex vivo to produce important antimicrobial molecules such as β-defensins, which have been specifically demonstrated in the trachea (4). We have also shown previously that Defb2 is expressed in these cells after LPS induction (4). However, we did not follow the in vivo fate of this gene in relation to S. aureus exposure because, in vitro, the Defb2 peptide is a very poor antimicrobial against S. aureus strain C1705 (M. Rolfe et al., unpublished). Here, we have extended these findings by showing for the first time that two further important molecules with activity against S. aureus (Defr1 and Defb14) are also expressed in this system. Defr1 encodes a defensin related peptide (Defr1) that deviates from the canonical six cysteine defensin motif by having the first cysteine replaced with a tyrosine in C57BL/6 mice (21). Surprisingly, we have shown that the five cysteine Defr1 peptide is active in vitro as an antimicrobial and has salt sensitive activity against S. aureus. Defb14 is the mouse orthologue of human DEFB103, which encodes a human peptide (hBD3), shown to have potent activity against S. aureus, including MRSA (17).
However, despite the ability of these cells to secrete these antibacterial molecules (Fig. 1), we have shown here that they are unable to control S. aureus growth in ALI cultures (Fig. 2).
The mechanism for such resistance of S. aureus may be complex and could be due partly to its capacity to modify phosphatidylglycerol, the major phospholipid of S. aureus, with l-lysine, rendering the membrane more cationic and hence repulsive to cationic antimicrobial molecules such as defensins (25). A more specific mechanism could be the production of staphylokinase, which has been shown recently to inactivate human defensins (15). Although we have not demonstrated here the secretion of murine endogenous antimicrobial peptides (and only showed their transcripts [see Fig. 1]), it is likely that these were also produced as demonstrated for HBD-2 in the same ALI system (35). Since it is therefore likely that S. aureus was able to grow even in the presence of these endogenous peptides, we then tested whether overexpressing human elafin, a molecule for which no ortholog exists in the mouse, would endow murine tracheal cells with defense against S. aureus.
Here, using the Ad-overexpressing system described above, we show that murine tracheal epithelial cells producing human elafin were able to reduce S. aureus numbers significantly (Fig. 4). Although the exact mechanism of action was not elucidated and was beyond the scope of the present study, the elafin molecule has a net positive charge of +7, suggesting that its activity against S. aureus (34) may be directed at bacterial membranes.
We show in Fig. 4 that elafin secretion is associated with significant inhibition of growth of S. aureus, although it is unclear whether it acts only to slow the growth of the organisms, directly kills a proportion of the organisms, or a combination of both, since the CFU counts of S. aureus recovered after incubation with Ad-elafin-infected cells were not lower than those added at the outset (not shown). In addition, elafin may not be acting on its own since synergy between neutrophil defensins and cathelicidins has previously been demonstrated against S. aureus (23). In accordance with this, although a direct comparison between the present study and our previous study (34) describing the incubation of synthetic elafin with S. aureus in solution in the test tube is difficult, it seems that “cell-derived” elafin (the present study) is more efficient than synthetic elafin against S. aureus. Indeed, we show here that 3.5 μg/ml (350 nM) can restrict the growth of 200 bacteria by 40%, whereas 3,330 nM would be the extrapolated concentration of synthetic elafin required to kill the same number of bacteria to the same degree when incubated in solution in the test tube.
Having demonstrated a clear antimicrobial effect of elafin ex vivo, we set out to investigate whether it also had a similar effect in vivo. A total of 3 × 107 PFU of Ad-elafin or Ad-LacZ (a dose demonstrated to induce no obvious inflammatory effects per se [33]) were instilled intratracheally 5 days prior to the bacterial challenge. This protocol allowed for robust expression of elafin in the lungs (Fig. 5). Although this did not result in an apparent increase in the expression of the other β-defensins studied here (data not shown), Ad-elafin administration had a beneficial effect in this acute model. Indeed, mirroring the ex vivo data presented in Fig. 4, Ad-elafin instillation reduced the bacterial load in vivo (Fig. 5). Concomitantly, when the Ad-elafin group was considered on its own, there was a negative correlation between lung elafin levels and neutrophil numbers (Fig. 6). The histological analysis of lungs confirmed this finding and showed a reduction in the number of neutrophilic pneumonia-like foci in the alveoli (Fig. 7). This is reminiscent of our previous study, in which P. aeruginosa was administered intratracheally in a protocol similar to the one used here (33). In that study, as here (Table 1), markers of inflammation such as BALF TNF-α and MPO levels were reduced in the Ad-elafin-plus-bacteria group. It could therefore be hypothesized that, in an infective situation, elafin may act directly as a bacteriostatic or bactericidal agent, thereby reducing the infective load. A consequence of this would be a reduction in the cellular inflammatory response, as exemplified by the reduction in cytokine levels, neutrophil activation, and neutrophil-associated “collateral lung damage,” as shown by a reduction in protein leakage in the alveolar compartment (Table 1). Elafin could also have a further direct anti-inflammatory function; indeed, we have shown recently that in vitro elafin inhibits NF-κB (11), an important proinflammatory transcription factor which has been shown to be activated by S. aureus protein A (7). Of note, the mode of action of elafin may be different in noninfective situations, for example, when LPS is used as an inflammatory stimulus, where elafin's chemotactic functions may become prominent (30, 32).
In conclusion, we demonstrated here that elafin is able to confer an increased antimicrobial phenotype to primary murine tracheal epithelial cells which, despite the endogenous expression of at least three β-defensins (shown at the RNA level) with in vitro relevance to S. aureus killing, were otherwise unable to contain the growth of this pathogen in vitro.
This phenotype was replicated in vivo, in a model of acute inflammation in C57BL/6 mice. We believe that these data have implications both for the development of improved murine models of S. aureus lung infections and for the consideration of elafin as a potential therapeutic agent against S. aureus-associated local and systemic infections.
Acknowledgments
This study was supported by the MRC and the Salvesen Emphysema Research Fund.
We thank C. Doherty (Edinburgh University Medical School) for help with the bacterial strains and M. Rolfe (MRC Human Genetics Unit, Western General Hospital) for aid in the preparation of cell samples. A. Carrothers (MRC Human Genetics Unit) provided help with statistical analysis and Tara Sheldrake provided excellent technical assistance. We also wish to acknowledge A. J. Simpson for reviewing the manuscript.
Editor: J. N. Weiser
REFERENCES
- 1.Addison, C. L., M. Hitt, D. Kunsken, and F. L. Graham. 1997. Comparison of the human versus murine cytomegalovirus immediate-early gene promoters for transgene expression by adenoviral vectors. J. Gen. Virol. 78:1653-1661. [DOI] [PubMed] [Google Scholar]
- 2.Bals, R., X. Wang, R. L. Meegalla, S. Wattler, D. J. Weiner, M. C. Nehls, and J. M. Wilson. 1999. Mouse beta-defensin 3 is an inducible antimicrobial peptide expressed in the epithelia of multiple organs. Infect. Immun. 67:3542-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campopiano, D. J., D. J. Clarke, N. C. Polfer, P. E. Barran, R. J. Langley, J. R. Govan, A. Maxwell, and J. R. Dorin. 2004. Structure-activity relationships in defensin dimers: a novel link between beta-defensin tertiary structure and antimicrobial activity. J. Biol. Chem. 279:48671-48679. [DOI] [PubMed] [Google Scholar]
- 4.Davidson, D. J., F. M. Kilanowski, S. H. Randell, D. N. Sheppard, and J. R. Dorin. 2000. A primary culture model of differentiated murine tracheal epithelium. Am. J. Physiol. Lung Cell Mol. Physiol. 279:L766-L778. [DOI] [PubMed] [Google Scholar]
- 5.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevsky, J. Bell, R. N. Jones, and M. Beach. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin. Infect. Dis. 32:S114-S132. [DOI] [PubMed] [Google Scholar]
- 6.Fasbender, A., J. H. Lee, R. W. Walters, T. O. Moninger, J. Zabner, and M. J. Welsh. 1998. Incorporation of adenovirus in calcium phosphate precipitates enhances gene transfer to airway epithelia in vitro and in vivo. J. Clin. Investig. 102:184-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez, M. I., A. Lee, B. Reddy, A. Muir, G. Soong, A. Pitt, A. Cheung, and A. Prince. 2004. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat. Med. 10:842-848. [DOI] [PubMed] [Google Scholar]
- 8.Govan, J. R., and J. W. Nelson. 1992. Microbiology of lung infection in cystic fibrosis. Br. Med. Bull. 48:912-930. [DOI] [PubMed] [Google Scholar]
- 9.Govan, J. R., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock, R. E. W. 2001. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 1:156-164. [DOI] [PubMed] [Google Scholar]
- 11.Henriksen, P. A., M. Hitt, Z. Xing, J. Wang, C. Haslett, R. A. Riemersma, D. J. Webb, Y. V. Kotelevtsev, and J. M. Sallenave. 2004. Adenoviral gene delivery of elafin and secretory leukocyte protease inhibitor attenuates NF-κB-dependent inflammatory responses of human endothelial cells and macrophages to atherogenic stimuli. J. Immunol. 172:4535-4544. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670-1673. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 14.Jia, H. P., S. A. Wowk, B. C. Schutte, S. K. Lee, A. Vivado, B. F. Tack, C. L. Bevins, and P. B. McCray, Jr. 2000. A novel murine beta-defensin expressed in tongue, esophagus, and trachea. J. Biol. Chem. 275:33314-33320. [DOI] [PubMed] [Google Scholar]
- 15.Jin, T., M. Bokarewa, T. Foster, J. Mitchell, J. Higgins, and A. Tarkowski. 2004. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J. Immunol. 172:1169-1176. [DOI] [PubMed] [Google Scholar]
- 16.Meyer-Hoffert, U., N. Wichmann, L. Schwichtenberg, P. C. White, and O. Wiedow. 2003. Supernatants of Pseudomonas aeruginosa induce the Pseudomonas-specific antibiotic elafin in human keratinocytes. Exp. Dermatol. 12:418-425. [DOI] [PubMed] [Google Scholar]
- 17.Midorikawa, K., K. Ouhara, H. Komatsuzawa, T. Kawai, S. Yamada, T. Fujiwara, K. Yamazaki, K. Sayama, M. A. Taubman, H. Kurihara, K. Hashimoto, and M. Sugai. 2003. Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect. Immun. 71:3730-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison, G. M., D. J. Davidson, F. M. Kilanowski, D. W. Borthwick, K. Crook, A. I. Maxwell, J. R. Govan, and J. R. Dorin. 1998. Mouse beta defensin-1 is a functional homolog of human beta defensin-1. Mamm. Genome 9:453-457. [DOI] [PubMed] [Google Scholar]
- 19.Morrison, G. M., D. J. Davidson, and J. R. Dorin. 1999. A novel mouse beta defensin, Defb2, which is upregulated in the airways by lipopolysaccharide. FEBS Lett. 442:112-116. [DOI] [PubMed] [Google Scholar]
- 20.Morrison, G., F. Kilanowski, D. Davidson, and J. Dorin. 2002. Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect. Immun. 70:3053-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison, G. M., M. Rolfe, F. M. Kilanowski, S. H. Cross, and J. R. Dorin. 2002. Identification and characterization of a novel murine beta-defensin-related gene. Mamm. Genome 13:445-451. (Erratum, 13:603.) [DOI] [PubMed] [Google Scholar]
- 22.Moser, C., D. J. Weiner, E. Lysenko, R. Bals, J. N. Weiser, and J. M. Wilson. 2002. β-Defensin 1 contributes to pulmonary innate immunity in mice. Infect. Immun. 70:3068-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagaoka, I., S. Hirota, S. Yomogida, A. Ohwada, and M. Hirata. 2000. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm. Res. 49:73-79. [DOI] [PubMed] [Google Scholar]
- 24.Nizet, V., T. Ohtake, X. Lauth, J. Trowbridge, J. Rudisill, R. A. Dorschner, V. Pestonjamasp, J. Piraino, K. Huttner, and R. L. Gallo. 2001. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature 414:454-457. [DOI] [PubMed] [Google Scholar]
- 25.Peschel, A., and L. V. Collins. 2001. Staphylococcal resistance to antimicrobial peptides of mammalian and bacterial origin. Peptides 22:1651-1659. [DOI] [PubMed] [Google Scholar]
- 26.Pickles, R. J., J. A. Fahrner, J. M. Petrella, R. C. Boucher, and J. M. Bergelson. 2000. Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer. J. Virol. 74:6050-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid, P. T., M. E. Marsden, G. A. Cunningham, C. Haslett, and J. M. Sallenave. 1999. Human neutrophil elastase regulates the expression and secretion of elafin (elastase-specific inhibitor) in type II alveolar epithelial cells. FEBS Lett. 457:33-37. [DOI] [PubMed] [Google Scholar]
- 28.Sallenave, J. M., Z. Xing, A. J. Simpson, F. L. Graham, and J. Gauldie. 1998. Adenovirus-mediated expression of an elastase-specific inhibitor (elafin): a comparison of different promoters. Gene Ther. 5:352-360. [DOI] [PubMed] [Google Scholar]
- 29.Sallenave, J. M. 2000. The role of secretory leukocyte proteinase inhibitor and elafin (elastase-specific inhibitor/skin-derived antileukoprotease) as alarm antiproteinases in inflammatory lung disease. Respir. Res. 1:87-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallenave, J. M., G. A. Cunningham, R. M. James, G. McLachlan, and C. Haslett. 2003. Regulation of pulmonary and systemic bacterial lipopolysaccharide responses in transgenic mice expressing human elafin. Infect. Immun. 71:3766-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schalkwijk, J., O. Wiedow, and S. Hirose. 1999. The trappin gene family: proteins defined by an N-terminal transglutaminase substrate domain and a C-terminal four-disulfide core. Biochem. J. 340:569-577. [PMC free article] [PubMed] [Google Scholar]
- 32.Simpson, A. J., G. A. Cunningham, D. J. Porteous, C. Haslett, and J. M. Sallenave. 2001. Regulation of adenovirus-mediated elafin transgene expression by bacterial lipopolysaccharide. Hum. Gene Ther. 12:1395-1406. [DOI] [PubMed] [Google Scholar]
- 33.Simpson, A. J., W. A. Wallace, M. E. Marsden, J. R. Govan, D. J. Porteous, C. Haslett, and J. M. Sallenave. 2001. Adenoviral augmentation of elafin protects the lung against acute injury mediated by activated neutrophils and bacterial infection. J. Immunol. 167:1778-1786. [DOI] [PubMed] [Google Scholar]
- 34.Simpson, A. J., A. I. Maxwell, J. R. Govan, C. Haslett, and J. M. Sallenave. 1999. Elafin (elastase-specific inhibitor) has anti-microbial activity against gram-positive and gram-negative respiratory pathogens. FEBS Lett. 452:309-313. [DOI] [PubMed] [Google Scholar]
- 35.Singh, P. K., H. P. Jia, K. Wiles, J. Hesselberth, L. Liu, B. A. Conway, E. P. Greenberg, E. V. Valore, M. J. Welsh, T. Ganz, B. F. Tack, and P. B. McCray, Jr. 1998. Production of beta-defensins by human airway epithelia. Proc. Natl. Acad. Sci. USA 95:14961-14966. (Erratum, 96:2569.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, J. J., S. M. Travis, E. P. Greenberg, and M. J. Welsh. 1996. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 85:229-236. [DOI] [PubMed] [Google Scholar]
- 37.Wiedow, O., J. M. Schroder, H. Gregory, J. A. Young, and E. Christophers. 1990. Elafin: an elastase-specific inhibitor of human skin—purification, characterization, and complete amino acid sequence. J. Biol. Chem. 265:14791-14795. (Erratum, 266:3356, 1991.) [PubMed] [Google Scholar]
- 38.Yamaguchi, Y., S. Fukuhara, T. Nagase, T. Tomita, S. S. Hitomi, Kimura, H. Kurihara, and Y. Ouchi. 2001. A novel mouse beta-defensin, mBD-6, predominantly expressed in skeletal muscle. J. Biol. Chem. 276:31510-31514. [DOI] [PubMed] [Google Scholar]