Abstract
In the virulent state (Bvg+), Bordetella bronchiseptica expresses adhesins and toxins that mediate adherence to the upper airway epithelium, an essential early step in pathogenesis. In this study, we used a rabbit tracheal epithelial cell binding assay to test how specific host or pathogen factors contribute to ciliary binding. The host antimicrobial agent surfactant protein A (SP-A) effectively reduced ciliary binding by Bvg+ B. bronchiseptica. To evaluate the relative contributions of bacterial adhesins and toxins to ciliary binding, we used mutant strains of B. bronchiseptica in the binding assay. When compared to Bvg+ or Bvg− phase-locked B. bronchiseptica strains, single-knockout strains lacking one of the known adhesins (filamentous hemagglutinin, pertactin, or fimbriae) displayed an intermediate ciliary binding capacity throughout the coincubation. A B. bronchiseptica strain deficient in adenylate cyclase-hemolysin toxin also displayed an intermediate level of adherence between Bvg+ and Bvg− strains and had the lowest ciliary affinity of any of the Bvg+ phase strains tested. A B. bronchiseptica strain that was missing dermonecrotic toxin also displayed intermediate binding; however, this strain displayed ciliary binding significantly higher than most of the adhesin knockouts tested. Taken together, these findings suggest that virulent-state B. bronchiseptica expresses multiple adhesins with overlapping contributions to ciliary adhesion and that host production of SP-A can provide innate immunity by blocking bacterial adherence to the ciliated epithelium.
Members of the genus Bordetella are gram-negative coccobacilli that can cause respiratory disease in humans and other mammals and include B. pertussis, B. parapertussis, and B. bronchiseptica. B. pertussis is a strict human pathogen and is the causative agent of pertussis (whooping cough), a disease that remains endemic to adult populations despite extensive vaccination programs initiated in the 1940s (3). Subspecies of B. parapertussis cause a milder pertussis-like disease in humans (20) but have also been associated with nonhuman disease (36). B. bronchiseptica infects a wide range of four-legged mammals and results predominantly in asymptomatic infections, although cases of symptomatic disease such as atrophic rhinitis in swine, kennel cough in dogs, and snuffles in rabbits have been well documented (12). Despite their varied host range, recent sequencing of B. pertussis, B. parapertussis, and B. bronchiseptica has shown a high conservation of the pathogenic machinery possibly derived from a single B. bronchiseptica-like ancestor (35). The three strains express highly similar virulent protein profiles under the control of the Bordetella virulence gene activator/sensor two-component signal transduction system (BvgAS) (51). Activation of the virulent (Bvg+) phase is required for successful colonization of the respiratory tract (4, 28, 32).
The conducting airway is protected by an epithelium that provides a physical barrier between respired air and the underlying tissue of the upper respiratory tract. The epithelium generates a “mucociliary escalator” to clear particulate materials, including pathogenic bacteria, from the airway and keep them from reaching the lower lungs (40). The innate immune function of the conducting airway is further complemented by secretion of several antimicrobial molecules that include collectins such as surfactant proteins A and D (SP-A, SP-D) (6). During successful colonization of the mammalian respiratory tract, Bordetella spp. overcome or evade these and other early innate immune defenses, in part by directly binding to the cilia of the respiratory epithelium (33, 45).
Following access of the bacteria to the upper airway, Bordetella can mediate and maintain ciliary adherence via redundant and/or sequential interactions between bacterial attachment molecules and the host cell cilia. Such factors implicated in host-cell attachment and subsequent colonization include filamentous hemagglutinin (FHA), pertactin, fimbriae, and adenylate cyclase-hemolysin (CyaA) toxin (27). These molecules have been studied both in vitro and in vivo to assess their contribution as adhesins and their ability to contribute to colonization of the upper respiratory tract. FHA from B. pertussis has been shown to contribute to human ciliated cell binding (48, 49) and aciliated cell binding (31, 37, 50). FHA from B. bronchiseptica has been shown to mediate binding to L2 cells (43) and to be necessary, but not sufficient, for the colonization of rat trachea (5). Pertactin can contribute to the binding of B. pertussis to CHO (25) or HEp-2 (50) cell lines and has a crystal structure that shows a variety of candidate protein binding sites (10). Fimbriae can contribute to B. pertussis binding to monocytes (18, 19) and to HEp-2 cells (50). CyaA protein can directly bind to erythrocytes (13), and loss of cyaA from B. pertussis can reduce attachment to human ciliated cells (49). A B. bronchiseptica strain that does not express dermonecrotic toxin (DNT) has been shown to differentially colonize swine airways (2). DNT has been suggested to alter ciliary binding in B. avium (47) and may contribute to B. bronchiseptica pathogenesis through host cell adhesion.
Despite evidence for each of the proteins above to contribute to adhesion, many of the studies discussed above have focused on binding to aciliated cells or ciliated cells following a lengthy exposure time (e.g., 30 min to 3 h); it remains unclear if any of these proteins directly contribute to ciliary binding in an effort to overcome mucociliary defense. In this study, we used primary cultured rabbit tracheal epithelial cells (RTEC) as a model for ciliated respiratory epithelium from a natural host of B. bronchiseptica and measured the ability of B. bronchiseptica strains deficient in individual adhesins and/or toxins to directly bind to cilia on a time scale (<5 min) consistent with overcoming mucociliary defense (14). We show that loss of known individual adhesins (FHA, pertactin, or fimbriae) or toxins (CyaA, DNT) reduced ciliary attachment compared to Bvg+ strains but remained significantly higher than Bvg− strains. We also measured the ability of the collectin SP-A to inhibit binding by the phase-locked Bvg+ strain of B. bronchiseptica. These studies represent the first comparative examination of adherence by single-adhesin or -toxin knockout strains of B. bronchiseptica to the ciliary binding of natural host cells and identify a role for a host protein that can disrupt this event.
MATERIALS AND METHODS
Materials.
Dulbecco's modified Eagle medium, fetal bovine serum, Hanks' balanced salt solution (HBSS), and antibiotic-antimycotic (penicillin, streptomycin, and amphotericin B) were obtained from Invitrogen (Carlsbad, CA). Bordet Gengou blood agar plates were obtained from Becton Dickinson and Company (Franklin Lakes, NJ). B. bronchiseptica strains were gifts from Peggy A. Cotter (University of California, Santa Barbara), Seema Mattoo (University of California, Los Angeles), and Jeffery F. Miller (University of California, Los Angeles). Human surfactant protein A was a gift from Jeanne M. Snyder and Rebecca E. Oberley (University of Iowa).
Bacterial strains and growth conditions.
B. bronchiseptica strains used in this study are described in Table 1. Wild-type strain RB50 was isolated from a naturally infected rabbit (4). B. bronchiseptica strains RB53, RB54, RB57, RB58, RB63, RBX9, and DF8 were derived from RB50; these derivations are detailed in other studies (1, 4, 5, 16, 29). To create the pertactin-deficient mutant strain SP5, an in-frame deletion in the gene encoding pertactin, prn, extending from codon 227 to codon 756, was constructed, leaving the promoter region, transcriptional start site, and signal sequence of prn intact (Seema Mattoo, personal communication). To create the DNT-deficient strain 50Δdnt, an in-frame, deletion in the gene encoding dermonecrotic toxin, dnt, extending from codon 17 to codon 1461 was constructed, leaving the purported promoter and transcriptional site of dnt intact (Omsland, A., P. Haynes, L. Bennett, and S. Boitano, submitted for publication). All strains were maintained on Bordet Gengou blood agar plates and, prior to use in binding assays, were grown to log phase under constant shaking at 37°C in Stainer Scholte broth (63 mM l-glutamic acid, 2 mM l-proline, 43 mM NaCl, 3.7 mM KH2PO4, 2.7 mM KCl, 1 mM MgCl2, 0.14 mM CaCl2 · 2H2O, 29 mM Tris-HCl, pH 7.6) supplemented with Stainer Scholte supplements (33 mM l-cysteine, 3.6 mM FeSO4 · 7H2O, 3.2 mM nicotinic acid, 48.8 mM glutathione, and 227.1 mM ascorbic acid) (46).
TABLE 1.
Bordetella bronchiseptica strains used in this studya
| Strain | Genotype | Comments | Reference or source |
|---|---|---|---|
| RB50 | WTb | Wild-type isolate from rabbit nares; can switch between Bvg+ and Bvg− dependent on environmental conditions | 4 |
| RB53 | bvgS-C3 | RB50 derivative; a single base pair change in bvgS that results in a phase-locked Bvg+ strain independent of growth conditions | 4 |
| RB54 | ΔbvgS | RB50 derivative; a 1.4-kb deletion in bvgS that results in a phase-locked Bvg− strain independent of growth conditions | 4 |
| RB57 | ΔbvgS, ΔflaA | RB54 derivative; Bvg− phase locked that additionally does not express flagella | 1 |
| DF8 | ΔbvgS, ΔflaA, fhaBr, fhaCr | RB57 derivative; Bvg− phase locked, lacking flagella that ectopically expresses FHA | 5 |
| RBX9 | ΔfhaB | RB50 derivative; does not express FHA | 5 |
| SP5 | Δprn | RB50 derivative; does not express pertactin | See Materials and Methods |
| RB63 | Δfim | RB50 derivative; does not express fimbrial adhesin | 29 |
| RB58 | ΔcyaA | RB50 derivative; does not express CyaA toxin | 16 |
| 50Δdnt | Δdnt | RB50 derivative; does not express DNT | This study |
All strains used were cultured in Bvg+ phase conditions.
WT, wild type.
Eukaryotic cell culture.
RTEC cultures were prepared according to previously described methods (8). Briefly, New Zealand White rabbits were injected in the marginal ear vein with sodium pentobarbital, tracheas were removed, and tracheal epithelial lining was teased away using forceps, sectioned into explants with a scalpel, and seeded onto 15-mm glass coverslips coated with rat-tail collagen. Cultures were incubated at 37°C in a 5% CO2 environment in Dulbecco's modified Eagle medium supplemented with 0.2% NaHCO3, 10% fetal bovine serum, and 1% antibiotic-antimycotic. RTEC were from 6- to 11-day-old mice for all experiments. Prior to use, cultures were washed thoroughly with HBSS; 1.3 mM CaCl2, 5.0 mM KCl, 0.3 mM KH2PO4, 0.5 mM MgCl2, 0.4 mM MgSO4, 137.9 mM NaCl, 0.3 mM Na2PO4, 1% glucose) additionally buffered with 25 mM HEPES, pH 7.4.
Bordetella binding assay.
Binding studies were adapted from previously described methods (14). To control for the binding effects of conditioned growth medium that could be different between bacterial strains, B. bronchiseptica strains from log phase growth, described above, were collected by centrifugation and resuspended into HBSS to an optical density at 600 nm of between 0.20 and 0.34. RTEC were mounted onto an open chamber on an Olympus IX70 microscope stage and adjusted to monitor actively beating ciliated cells. A 60× oil immersion objective with differential interference contrast optics and an additional 1.5× magnification zoom lens allowed for the visualization of both bacteria and cilia (14). RTEC cultures were washed thoroughly with HBSS prior to experimentation. At time zero, HBSS was replaced with 1 ml of the appropriate B. bronchiseptica strain using a syringe and a 16-gauge needle to limit aggregation prior to host cell interaction. Cultures were subsequently washed at 2 and 3 min from the start of coincubation with 1 ml HBSS to remove nonadherent bacteria. Throughout the binding assay, individual images were captured via a Photometrics Cascade charge-coupled device camera (Roper Scientific, Tucson, AZ) onto a personal computer using Image Master 3.0 software (Photon Technology, Inc., NJ) at 1 frame/3 s for 5 min. Captured tagged image file images were analyzed frame by frame using QuickTime software. Bacteria were scored as “attached” if they remained in contact with a ciliated cell for at least eight consecutive frames (24 s). Although bacterial aggregation was rare in all strains except RB58, ciliary attachments that included more than one bacterium were scored as a single attachment. Experiments that tested the inhibition of binding by SP-A were similar to those described above; however, both bacterial suspension and RTEC cultures were preincubated for 30 min in HBSS supplemented with 10 μg/ml native human SP-A or bovine serum albumin (BSA) prior to videomicroscopy. When SP-A effects were tested on only bacterial suspension or host culture, a 30-s preincubation was used in the untested suspension or culture to maintain SP-A concentration during coincubation experiments. The 30-s preincubation did not affect ciliary binding by itself (data not shown). To ensure the reproducibility of experiments, RTEC tissue cultures derived from individual rabbits were tested for binding with up to five B. bronchiseptica strains. All bacterial strains were tested for binding on RTEC tissue cultures derived from at least two and up to four different rabbits.
SEM.
RTEC were coincubated with 250 μl of appropriate B. bronchiseptica strain suspended in HBSS for 5 min and then rinsed with 1 ml HBSS to remove nonadhering bacteria. Cultures were fixed in 3% glutaraldehyde in 100 mM cacodylate buffer (CB; pH 7.2). Fixed samples were washed with 144 mM CB, incubated in 150 mM CB containing 1% tannic acid for 1 h, and washed again. Samples were postfixed in 100 mM CB containing 1% osmium tetroxide (pH 7.2), washed, and dehydrated in a series of graded ethanol washes. The samples were then washed with 100% hexamethyldisilazane and allowed to dry overnight. Samples were mounted, sputter coated with 20-nm gold particles, and imaged with an FEI-Phillips XL30 scanning electron microscope at 10 kV. Scanning electron microscopy (SEM) sample fixation and imaging were performed by the Arizona Health Science Center Core Imaging Facility, Arizona Research Labs, Division of Biotechnology. SEM images were pseudocolored to highlight bacteria by using Photoshop (Adobe Systems Software, San Jose, CA).
Statistics.
Graphical data are presented as the number of bound bacteria ± standard error of the mean. Significance between attachments by two different bacterial strains at each time point was determined with Student's t test using Prism (GraphPad Software Inc., San Diego, CA). A value of P < 0.05 was used for the determination of significant difference between samples.
RESULTS
Ciliary binding by B. bronchiseptica Bvg phase-locked mutants.
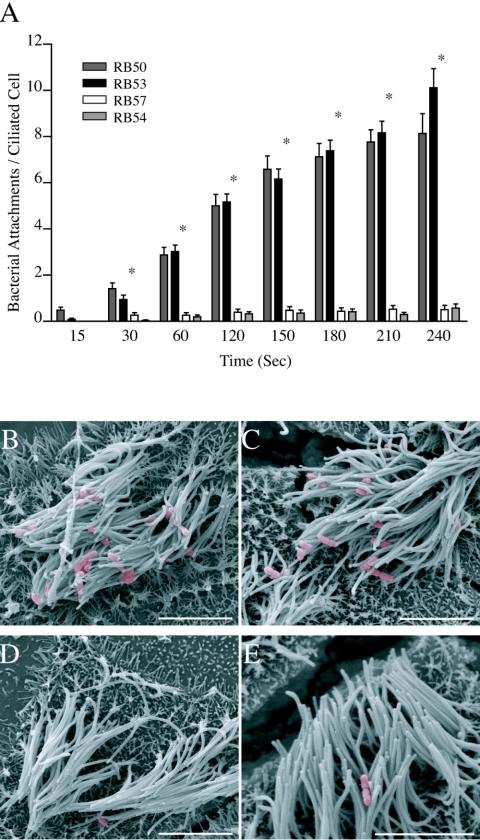
We have previously shown that a wild-type strain of B. bronchiseptica (RB50) grown under Bvg+ conditions preferentially adhered to the cilia of RTEC cultures within seconds of coincubation, whereas a nonmotile, phase-locked Bvg− mutant derivative of RB50 (RB57) showed limited binding over a 5-min coincubation (14). Since RB50 can modulate between Bvg+ and Bvg− phases in response to environmental conditions and because RB57 cells lack the flagella normally associated with the Bvg− phase, we repeated our binding studies with phase-locked Bvg+ (RB53) and phase-locked Bvg− (RB54) strains (Fig. 1A). In the ciliary binding assay, RB53 displayed binding characteristics identical to RB50. Within 30 s, RB53 cells were attached to ciliated cells (0.94 ± 0.19 bacteria/host ciliated cell) and binding steadily increased over time (10.12 ± 0.83 at 240 s). RB54 displayed a binding pattern similar to RB57, with less than a single bacterium attached per ciliated cell on average at any time point over the 240-s experiment. Both RB50 and RB53 bound to ciliated cells at significantly higher numbers than either of the Bvg− strains within 30 s of coincubation and throughout the experiment. To further demonstrate ciliary-specific binding and differences between Bvg+ and Bvg− binding, SEM images were taken after 5 min of coincubation (Fig. 1B through E). SEM images of RB53 and RB50 both displayed similar affinity for the upper portion of ciliated RTEC, with no bacteria associated with exposed aciliated membrane (Fig. 1B and C). In contrast, the Bvg− strains RB54 and RB57 displayed minimal adherence to either ciliated or aciliated areas of the RTEC culture (Fig. 1D and E). Throughout the following experiments, RB50 and RB54 binding data from Fig. 1 are replotted as references for “full binding” and “minimal binding” efficiency.
FIG. 1.
B. bronchiseptica binding to RTEC. (A) Quantification of Bvg+ (wild-type RB50 [dark gray bars] and phase-locked RB53 [black bars]) and Bvg− (phase-locked RB57 [white bars] and phase-locked RB54 [light gray bars]) adherence to ciliated RTEC is shown over time. Scanning electron micrographs of RB50 (B), RB53 (C), RB57 (D), and RB54 (E) binding to RTEC after a 5-min coincubation is also shown. Adherence by Bvg+ strains RB50 and adherence by RB53 were similar throughout the duration of the experiment and were significantly higher than either Bvg− strain within 30 s of coincubation (*). As seen in the SEM image, bacteria adhered preferentially to the upper portion of the cilia, with Bvg+ adhering with greater affinity than Bvg− B. bronchiseptica. Adherence is shown ± standard error with a significance of P < 0.05 (*) for n ≥ 23 for time points at 15 s to 210 s and n ≥ 12 for the 240-time point. Scale bars represent 5 μm.
Ciliary binding by B. bronchiseptica in the presence of SP-A.
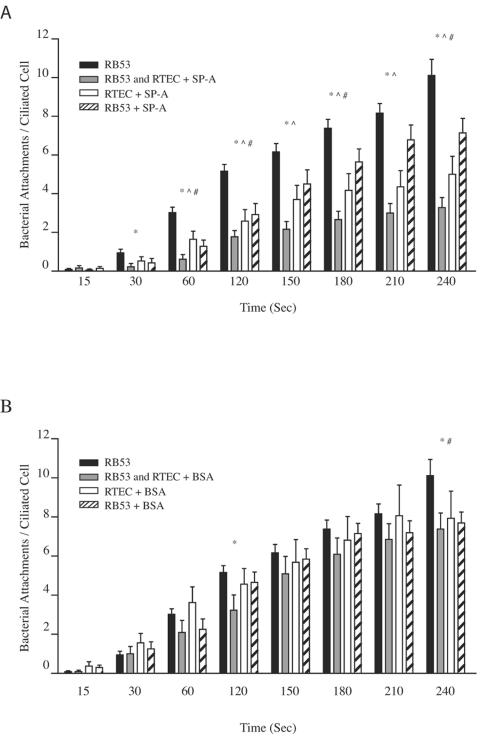
Cells from the conducting airway epithelium produce a variety of antimicrobial agents to prevent bacterial colonization. One of these agents, SP-A, has been shown to directly interact with pathogenic bacteria, including strains of B. pertussis, to aid in bacterial clearance in the airway by opsonization and phagocytosis (30, 44). To test whether SP-A could contribute to innate immunity by reducing ciliary binding by B. bronchiseptica, we preincubated suspensions of RB53 and RTEC cultures for 30 min with native human SP-A protein (5 μg/ml and 10 μg/ml) and repeated the binding assay. Incubation with 5 μg/ml SP-A did not significantly alter RB53 binding to RTEC cilia (data not shown); however, incubation with 10 μg/ml SP-A significantly reduced RB53 binding within 30 s of coincubation, which continued throughout the duration of the experiment (Fig. 2A). In an attempt to determine if SP-A inhibition was bacterial or ciliary specific, the binding assay was repeated after SP-A preincubation with only RTEC cultures or RB53 suspensions. Preincubation of RTEC alone with SP-A significantly reduced the binding capacity of RB53 within 60 s of coincubation and throughout the following time points. Preincubation of RB53 alone with SP-A reduced overall binding capacity, reaching significance at four of the eight time points tested. As a control for nonspecific protein interference, we repeated the experiments with 10 μg/ml BSA in place of SP-A (Fig. 2B). Preincubation of RTEC alone with BSA did not significantly reduce RB53 binding at any of the time points tested. Although preincubation with BSA of bacterial suspensions alone or a dual preincubation of RB53 and RTEC cultures reduced RB53 adherence, this reduction did not establish significance through consecutive analysis points. Additionally, RB53 adherence following dual preincubation with BSA was significantly higher than adherence following dual preincubation with SP-A at 60 s and again at 150 s where binding differences remained significant throughout the duration of analyses, indicative of a specific block of bacterial adherence by SP-A.
FIG. 2.
Ciliary attachment by Bvg+ B. bronchiseptica in the presence of SP-A. The number of phase-locked Bvg+ (RB53) that bound to ciliated RTEC following preincubation of both RB53 and RTEC cultures (gray bars), RTEC cultures alone (white bars), or RB53 alone (striped bars), all with final concentrations of 10 μg/ml of SP-A (A) or BSA (B), are compared to RB53 binding from Fig. 1 (black bars). Preincubation of both RB53 and RTEC cultures with SP-A significantly reduced RB53 binding within 30 s (*) and remained significantly lower throughout the experiment. Preincubation of RTEC alone with SP-A significantly reduced binding within 60 s (^), continuing throughout the experiment, whereas preincubation of RB53 alone significantly reduced binding at the 60-, 120-, 180-, and 240-s analysis points (#). In contrast, preincubation with BSA to assay a nonspecific protein block resulted in binding patterns most comparable to those for BSA-free binding (Fig. 2B). *, #, and ^ denote statistically significant differences at P < 0.05 compared to RB53 data redrawn from Fig. 1, and n ≥ 14 for all time points.
Ciliary binding by B. bronchiseptica with ectopic FHA expression.
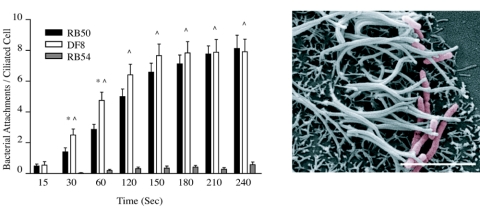
To determine if FHA alone could restore the ability of a Bvg− strain to adhere to cilia, we performed the RTEC binding assay with DF8, a Bvg− flaA mutant strain that ectopically expresses FHA (5). In this strain, the fhaB and fhaC promoters were replaced with a comparably strong flagellin promoter, resulting in the expression of the two genes necessary for FHA production in the Bvg− phase. In the RTEC binding assay, DF8 adhered to cilia at numbers significantly higher than the Bvg− strain RB54 within 30 s and at all other time points tested. Compared to the wild-type strain RB50, DF8 displayed high binding at early time points (30 and 60 s) and bound to ciliated RTEC in similar numbers throughout the duration of the experiment (Fig. 3A). Unlike any of the other strains tested, DF8 displayed a low but significant capacity to bind to aciliated cells and aciliated cell membranes in the RTEC cultures (data not shown). SEM images confirmed high numbers of bacteria associated with cilia and ciliated cells (Fig. 3B). In these images, DF8 exhibited a rod-shaped morphology more typical of the Bvg− phase as opposed to the coccus morphology more typically associated with B. bronchiseptica in the Bvg+ phase. From these studies, we conclude that FHA is sufficient for initial airway epithelial cell attachment in the ciliary binding assay.
FIG. 3.
Ciliary attachment by a Bvg−, FHA-expressing B. bronchiseptica strain. Quantification of DF8 (white bars), a Bvg− strain that expresses FHA, compared to RB50 (black bars) and RB54 (gray bars) is paired with a representative SEM graph of binding following 5-min coincubation. DF8 bound cilia at a rate similar to that of RB50. Binding was significantly higher than RB50 at 30 and 60 s of coincubation. DF8 binding was significantly higher than RB54 from 30 s on. Data for RB50 and RB54 are redrawn from Fig. 1 for comparison. * denotes significantly different binding between DF8 and RB50, and ^ denotes significantly different binding between DF8 and RB54 (P < 0.05), with n = 24 for DF8 at all time points. Error bars in graphs are means ± standard errors. Scale bar in micrograph represents 5 μm.
Ciliary binding by B. bronchiseptica strains lacking single adhesins.
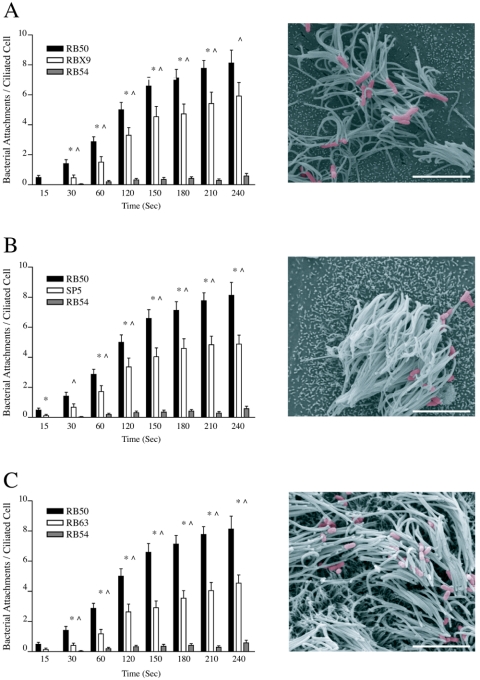
To evaluate if any one of the previously identified adhesins—FHA, pertactin, or fimbriae—contributed to the ciliary binding differences observed when wild-type B. bronchiseptica cells were grown in Bvg+ conditions compared to the Bvg− phase-locked binding, we repeated the RTEC ciliary binding assay with single-adhesin knockout strains (Fig. 4). We first evaluated the role of FHA with the Bvg+ fha mutant B. bronchiseptica strain RBX9 (5). RBX9 displayed an intermediate binding pattern, compared to RB50 and RB54, that was significantly different from both within 30 s and that continued throughout the experiment (Fig. 4A). SEM images verified ciliary attachment of RBX9 to RTEC. In binding assays with SP5, a prn mutant strain deficient in pertactin, a similar intermediate binding pattern was observed. SP5 first displayed significantly different binding from RB50 at 15 s and from RB54 at 30 s (Fig. 4B). By 60 s, SP5 binding significance from RB50 and RB54 continued throughout the experiment. SEM images showed ciliary attachment by SP5 to be slightly lower in numbers but with a pattern similar to that observed with RB50. RB63, a fim mutant strain deficient in fimbriae (29), also displayed an intermediate adherence compared to RB50 and RB54 (Fig. 4C). RB63 binding was significantly different from RB50 and RB54 within 30 s of coincubation. As above, significance was maintained to completion of the experiment. SEM images taken after a 5-min coincubation of RB63 with RTEC were similar to those seen for the other single-adhesin knockouts described above. Compared among each other, there were no significant differences in attachment to cilia between the three single-adhesin knockout strains at any of the time points tested. Our data show that FHA expression in the Bvg− phase is sufficient to induce ciliary binding. Additionally, loss of FHA, pertactin, or fimbriae in the Bvg+ phase reduces ciliary adherence, but loss of any of these single adhesins does not reduce B. bronchiseptica binding to the level observed with Bvg− phase-locked B. bronchiseptica during the 5-min analysis developed to test early pathogen-ciliary interactions.
FIG. 4.
Ciliary attachment by mutant B. bronchiseptica strains missing single-adhesin proteins. Individual graphs quantifying the adherence of single-adhesin knockout strains of B. bronchiseptica mutant strains are presented as in Fig. 3. (A) RBX9, a fha mutant strain deficient in FHA expression; (B) SP5, a prn mutant strain deficient in pertactin expression; (C) RB63, a fim mutant strain deficient in fimbrial protein expression. All single-adhesin mutant strains displayed an intermediate binding capacity with a ciliary adherence significantly higher than that of RB54 within 30 s that was maintained throughout the experiments. Binding by RBX9 and RB63 was significantly lower than RB50 beginning at 30 s and continuing throughout the experiment. Binding by SP5 was significantly lower than RB50 at the 15-s time point and again at 60 s, where it remained significantly lower throughout the experiment. Markings are as described in the legend to Fig. 3, except n ≥ 22 for single-adhesin knockout strains. Scale bar in micrograph represents 5 μm.
Ciliary binding by B. bronchiseptica strains lacking single toxins.
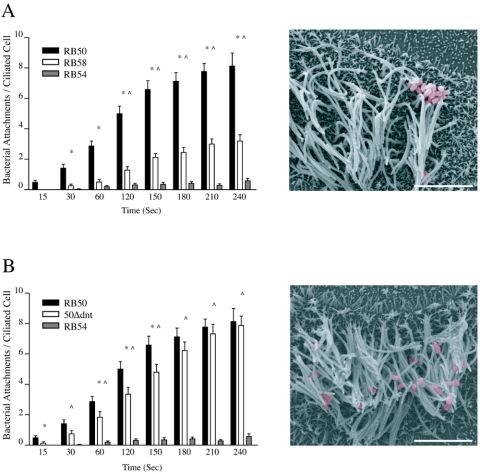
Bordetella expresses a variety of toxins including CyaA and DNT. Because CyaA has been reported to have adhesive properties (16), we evaluated a cyaA mutant B. bronchiseptica strain, RB58, in the RTEC binding assay. Similar to the single-adhesin knockout mutants, RB58 displayed significantly reduced ciliary binding compared to RB50 starting at 30 s and significantly increased ciliary binding compared to RB54 starting at 120 s (Fig. 5A). Compared to RBX9 and SP5, RB58 binding to ciliated RTEC was significantly lower within 60 s and throughout the experiment. RB58 binding was also significantly lower than RB63 at 60 s and 120 s; however, this significance was not maintained throughout the experiment. SEM images confirmed a reduced number of RB58 binding sites on ciliated RTEC in culture (Fig. 5B). We also evaluated the dnt mutant B. bronchiseptica strain, 50Δdnt, which is unable to express DNT. 50Δdnt ciliary adherence was significantly higher than RB54 within 30 s of coincubation and throughout the duration of the experiment. Although 50Δdnt also displayed consistently lower binding than RB50, this difference reached significance at only four of the eight time points tested (Fig. 5C). SEM images showed that 50Δdnt ciliary binding was comparable to RB50 (Fig. 5D). These data highlight the multifunctionality of the CyaA protein and its contribution to ciliary adhesin, a property not shared by all Bordetella toxins, including DNT.
FIG. 5.
Ciliary attachment by mutant B. bronchiseptica strains missing single-toxin proteins. Individual graphs quantifying the adherence of single-toxin knockout strains of B. bronchiseptica are presented as in Fig. 3. (A) RB58, a cyaA mutant strain deficient in CyaA toxin; (B) 50Δdnt, a dnt mutant strain deficient in DNT expression. RB58 displayed significantly higher binding than RB54 within 120 s and significantly lower ciliary binding than RB50 within 30 s of coincubation. 50Δdnt adherence was also significantly higher than that of RB54 within 30 s; however, significance compared to that for RB50 binding was variable. Markings are as described in the legend to Fig. 3, except n ≥ 24 for single-toxin knockout strains.
DISCUSSION
In order to successfully infect the conducting airway, primary colonizing bacteria must overcome the inherent innate immunity of the respiratory tract. This includes evading the mucociliary escalator and an environment of antimicrobial factors. We have developed an in vitro model tissue culture system to assay one of the mechanisms that Bordetella use to overcome the mucociliary escalator—direct binding to cilia on host epithelium (14). We show that an antimicrobial host factor produced in the conducting airway, SP-A, can reduce ciliary binding by B. bronchiseptica. We also evaluated roles for several BvgAS-regulated surface virulence factors to mediate adherence to host cell cilia, including but not limited to the adhesins FHA, pertactin, and fimbriae and the toxin CyaA. Using phase-locked Bvg+ or Bvg− B. bronchiseptica, we confirmed that initial interaction with the ciliated cell via direct binding was dependent on positive BvgAS expression. Additionally, we evaluated the contributions of individual adhesins and toxins in ciliary attachment by comparing ciliary binding by B. bronchiseptica strains deficient in single-adhesin or -toxin expression to ciliary binding by wild-type Bvg+ or Bvg− phase-locked strains. Our results show that loss of any of three identified adhesins (FHA, fimbriae, or pertactin) or a multifunctional toxin (CyaA) from B. bronchiseptica reduces ciliary adherence in comparison to Bvg+ strains but does not reduce ciliary adherence to the level observed by Bvg− strains. The ability of B. bronchiseptica to express several factors that can directly interact with host cell cilia to overcome mucociliary clearance and the evidence that host cells can block this interaction by the secretion of certain factors suggest that this is an essential determining step in bacterial colonization of the conducting airway.
In addition to coordinated ciliary beat and mucus production, cells of the respiratory epithelium can produce a variety of antimicrobial factors that directly interact with bacteria or other factors and can recruit a full inflammatory response (7). SP-A is an antimicrobial protein that contains both a collagen-like domain and a calcium dependent (C-type) lectin domain (collectins) and is produced by airway epithelial cells in the conducting airway (6, 22, 42). In knockout murine models, loss of SP-A increases susceptibility to bacterial infection (26). This loss of host defense can be through a multitude of antimicrobial mechanisms of action by SP-A, including agglutination, opsonization, modulation of inflammation, or direct inhibition of bacterial growth (6, 9, 30, 52). Although SP-A had limited effects on wild-type B. pertussis, strains that expressed mutant lipopolysaccharide were susceptible to enhanced aggregation, permeablization, and opsonization, leading to monocyte phagocytosis by SP-A protein (44). Although we did not directly test permeablization or opsonization, SP-A did not cause aggregation of B. bronchiseptica (data not shown) but did reduce Bordetella-ciliary interactions. The protective effect of SP-A in the ciliary binding assay was best observed when SP-A was preincubated with both bacteria and host cultures; however, a consistent reduction in ciliary binding was observed when either bacterial suspensions or host cultures were preincubated with SP-A. Although we cannot rule out a direct lipopolysaccharide binding activity contributing to the block of ciliary attachment, we speculate that it is more likely that the lectin-like domains inherent to SP-A are blocking exposed sugars important in pathogen/host ciliary attachment. Independent of the mechanism, the role for SP-A in directly preventing the ciliary attachment of B. bronchiseptica provides a novel antimicrobial defense in the conducting airway to prevent bacterial colonization.
FHA is a cell surface-associated adhesin that can participate in Bordetella binding to a variety of cell types (31, 37-39, 48-50). Although FHA is highly conserved among Bordetella spp. —B. bronchiseptica FHA and B. pertussis FHA are similar in molecular mass and structure and contain shared epitopes—culture supernatants of B. bronchiseptica contain less FHA than B. pertussis supernatants (21). FHA from B. pertussis is the most extensively studied in Bordetella and has at least three separate binding activities: a glycosaminoglycan binding site (15, 31), an integrin binding arginine-glycine-aspartate (RGD) sequence (38, 39), and a carbohydrate recognition domain (37, 48). In our studies, the expression of FHA in a Bvg− background is sufficient to restore ciliary binding whereas the loss of expression of FHA in a Bvg+ background reduces, but does not eliminate, early ciliary attachment. These data are different than that seen in a previous assay of B. pertussis fha mutant strain binding to rabbit ciliated cells, where loss of FHA resulted in only 5.8% of the wild-type binding (39). However, our studies examine polarized epithelial cells and focus on ciliary binding in a 5-min experiment, whereas the previous study used a 30-min coincubation with isolated ciliated cells. The use of isolated ciliated cells can also limit ciliary-specific attachment by exposing the basolateral membrane to Bordetella and thus may invoke an attachment not normally available to colonizing bacteria. Because mammalian cilia contain a variety of glycosylated proteins (17) and B. pertussis FHA binding to human cilia includes a lactose domain (48), the observed FHA-dependent ciliary binding likely involves the carbohydrate binding domain. We have also shown that ectopic expression of FHA in a Bvg− background (i.e., the DF8 strain) restores ciliary binding and induces aciliated membrane binding by B. bronchiseptica. These data are in agreement with the ability of FHA to contribute to aciliated cell binding in a variety of lung epithelial cell lines (50) and may include contributions from all three of the FHA binding domains.
In our ciliary binding assay, loss of fimbriae or pertactin was equivalent to the loss of FHA in the reduction of ciliary binding by Bvg+ strains and no single adhesin was necessary for this important early step in B. bronchiseptica colonization of the conducting airway. Previous results assaying the role of pertactin in adhesion required extended coincubations and varied depending on the aciliated epithelial cell model used. For example, in studies using a binding assay with a lung laryngeal epithelial cell line (HEp2), prn mutant strains had adherence similar to that of wild-type strains of B. pertussis (41). In cell models using CHO or HeLa cells, prn mutant strains had a decreased binding capacity and host attachment involved an RGD sequence within the pertactin protein (25). Fimbriae are multisubunit extracellular filamentous proteins expressed by most gram-negative pathogenic bacteria that aid in bacterial attachment to host tissues (23). When purified, B. pertussis fimbriae or fimbrial subunits can bind to the sulfated sugars chondroitin sulfate, heparin sulfate, and dextran sulfate (11) and mediate Bordetella binding to human monocytes, (18) which can help to coordinate FHA binding (19). In cellular assays, B. pertussis binding to HEp-2 cells required both fimbriae and FHA; however, fimbriae did not contribute to binding of the bronchial-derived cell line NCI-H292 (11, 50). Contrasting results have been found in B. bronchiseptica studies using a centrifugation binding assay, where fim mutant strains did not show a decrease in aciliated epithelial cell binding, although fimbriae were important to the establishment of colonization in an animal model (29). The reduction of ciliary binding in the fim mutant B. bronchiseptica strain presented in this study suggests that fimbriae contribute to ciliary attachment and may help to explain the lack of colonization in the animal model. Our data are consistent with a redundant contribution by Bordetella adhesins rather than a dominant effect of FHA in ciliary attachment. However, we cannot rule out that the loss of individual adhesins (e.g., pertactin and fimbriae) may indirectly contribute to binding through their interactions with other adhesins (e.g., FHA) and thus a more supportive role in ciliary attachment.
In addition to adhesins, there are several toxins under BvgAS control in B. bronchiseptica that could potentially contribute to colonization through adhesion. We tested two of these toxins, CyaA and DNT, in the RTEC binding assay. CyaA is a multifunctional bacterial surface-associated enzyme; the hemolysin domain of CyaA may mediate cellular attachment and allow for internalization of the calmodulin-activated adenylate cyclase toxin domain into host cells, where it catalyzes the uncontrolled conversion of ATP to cyclic AMP (24). In an in vitro assay with human ciliated cells, loss of CyaA from B. pertussis significantly reduced ciliary binding (49). Our data show that cyaA mutant B. bronchiseptica cells were similarly reduced in ciliary binding. Although these data are consistent with a direct contribution of CyaA to bacterial binding of cilia, previous studies have shown a physical association between CyaA and FHA in B. pertussis in vitro (53). Thus, similar to that seen with adhesins, we cannot rule out an indirect effect of CyaA on FHA or other B. bronchiseptica adhesins that result in the low binding capacity of this mutant. Purified DNT has been reported to cause dermonecrosis when injected intradermally, enhance DNA and protein synthesis in cell cultures, and alter host cell cytoskeleton via small GTPase activity; however, an in vivo phenotype associated with any of these properties has yet to be established (27). B. avium strains have been shown to have reduced attachment to turkey tracheal rings when dnt-deficient strains are compared to wild-type strains in a 3-h incubation assay (47). Although there was decreased ciliary binding by 50Δdnt compared to RB50 at some of the time points tested, our results are inconsistent with a DNT contribution to ciliary adhesion and the role for DNT in Bordetella pathogenesis remains elusive.
All three adhesin mutant strains and the cyaA mutant strain displayed a significantly reduced capacity to bind host cilia in our assay; however, all of these strains displayed significantly high ciliary binding compared to Bvg− strains in the RTEC binding assay. These results suggest that B. bronchiseptica relies on multiple adhesins to maximize adherence to the ciliated respiratory epithelium. The redundancy in ciliary adhesiveness highlights the importance of this process in the establishment of colonization in the host. Additionally, we might consider the potential of sequential binding by diverse adhesins that might initiate cell signaling in the host epithelium. Such a paradigm has been shown between B. pertussis fimbriae and FHA on human monocytes (18). Further studies are required to determine additional adhesin molecules for B. bronchiseptica, their ciliary receptors, and their function in ciliary binding.
In summary, we evaluated the ability of an antimicrobial protein to inhibit the ciliary attachment of Bordetella and the relative importance of several B. bronchiseptica adhesins and toxins in adherence to cilia. We found that SP-A could decrease ciliary binding by the phase-locked Bvg+ strain of B. bronchiseptica, representing a novel activity of SP-A in host defense. Additionally, FHA, fimbriae, pertactin, and CyaA all participated in Bvg+-dependent ciliary adherence; none of these factors were shown to be necessary for pathogen/host binding. Future studies examining the molecular mechanism of SP-A interference in Bordetella binding and identifying additional adhesin molecules on the surfaces of B. bronchiseptica cells, or specific interactions between adhesins and toxins that alter ciliary binding, will provide preventative or therapeutic targets against primary colonizing bacteria.
Acknowledgments
We thank Jeffery F. Miller, Peggy A. Cotter, Seema Mattoo, Jeanne M. Snyder, and Rebecca E. Oberley for their kind gifts of bacterial mutants and SP-A that made this work possible. We thank Peggy McCuskey for her expertise in scanning electron microscopy, Anders Omsland and Seema Mattoo for their critical reading of the manuscript and Robert A. Heinzen for his help in setting up the binding assay.
This work was supported by NIH grants HL64039 and P30ES06694 and an award through The University of Arizona Institute for Collaborative Research (BIO5).
Editor: D. L. Burns
REFERENCES
- 1.Akerley, B. J., P. A. Cotter, and J. F. Miller. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell 80:611-620. [DOI] [PubMed] [Google Scholar]
- 2.Brockmeier, S. L., K. B. Register, T. Magyar, A. J. Lax, G. D. Pullinger, and R. A. Kunkle. 2002. Role of the dermonecrotic toxin of Bordetella bronchiseptica in the pathogenesis of respiratory disease in swine. Infect. Immun. 70:481-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cherry, J. D. 1999. Epidemiological, clinical, and laboratory aspects of pertussis in adults. Clin. Infect. Dis. 28(Suppl. 2):S112-S117. [DOI] [PubMed] [Google Scholar]
- 4.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cotter, P. A., M. H. Yuk, S. Mattoo, B. J. Akerley, J. Boschwitz, D. A. Relman, and J. F. Miller. 1998. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect. Immun. 66:5921-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crouch, E., and J. R. Wright. 2001. Surfactant proteins A and D and pulmonary host defense. Annu. Rev. Physiol. 63:521-554. [DOI] [PubMed] [Google Scholar]
- 7.Diamond, G., D. Legarda, and L. K. Ryan. 2000. The innate immune response of the respiratory epithelium. Immunol. Rev. 173:27-38. [DOI] [PubMed] [Google Scholar]
- 8.Dirksen, E. R., J. A. Felix, and M. J. Sanderson. 1995. Preparation of explant and organ cultures and single cells from airway epithelium. Methods Cell Biol. 47:65-74. [DOI] [PubMed] [Google Scholar]
- 9.Eggleton, P., and K. B. Reid. 1999. Lung surfactant proteins involved in innate immunity. Curr. Opin. Immunol. 11:28-33. [DOI] [PubMed] [Google Scholar]
- 10.Emsley, P., I. G. Charles, N. F. Fairweather, and N. W. Isaacs. 1996. Structure of Bordetella pertussis virulence factor P.69 pertactin. Nature 381:90-92. [DOI] [PubMed] [Google Scholar]
- 11.Geuijen, C. A., R. J. Willems, and F. R. Mooi. 1996. The major fimbrial subunit of Bordetella pertussis binds to sulfated sugars. Infect. Immun. 64:2657-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodnow, R. A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44:722-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray, M. C., W. Ross, K. Kim, and E. L. Hewlett. 1999. Characterization of binding of adenylate cyclase toxin to target cells by flow cytometry. Infect. Immun. 67:4393-4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groathouse, N. A., R. A. Heinzen, and S. Boitano. 2003. Functional BvgAS virulence control system in Bordetella bronchiseptica is necessary for induction of Ca2+ transients in ciliated tracheal epithelial cells. Infect. Immun. 71:7208-7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hannah, J. H., F. D. Menozzi, G. Renauld, C. Locht, and M. J. Brennan. 1994. Sulfated glycoconjugate receptors for the Bordetella pertussis adhesin filamentous hemagglutinin (FHA) and mapping of the heparin-binding domain on FHA. Infect. Immun. 62:5010-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvill, E. T., P. A. Cotter, M. H. Yuk, and J. F. Miller. 1999. Probing the function of Bordetella bronchiseptica adenylate cyclase toxin by manipulating host immunity. Infect. Immun. 67:1493-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hastie, A. T., M. J. Krantz, and F. P. Colizzo. 1990. Identification of surface components of mammalian respiratory tract cilia. Cell Motil. Cytoskelet. 17:317-328. [DOI] [PubMed] [Google Scholar]
- 18.Hazenbos, W. L., C. A. Geuijen, B. M. van den Berg, F. R. Mooi, and R. van Furth. 1995. Bordetella pertussis fimbriae bind to human monocytes via the minor fimbrial subunit FimD. J. Infect. Dis. 171:924-929. [DOI] [PubMed] [Google Scholar]
- 19.Hazenbos, W. L., B. M. van den Berg, C. W. Geuijen, F. R. Mooi, and R. van Furth. 1995. Binding of FimD on Bordetella pertussis to very late antigen-5 on monocytes activates complement receptor type 3 via protein tyrosine kinases. J. Immunol. 155:3972-3978. [PubMed] [Google Scholar]
- 20.Heininger, U., K. Stehr, S. Schmitt-Grohe, C. Lorenz, R. Rost, P. D. Christenson, M. Uberall, and J. D. Cherry. 1994. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatr. Infect. Dis. J. 13:306-309. [DOI] [PubMed] [Google Scholar]
- 21.Jacob-Dubuisson, F., B. Kehoe, E. Willery, N. Reveneau, C. Locht, and D. A. Relman. 2000. Molecular characterization of Bordetella bronchiseptica filamentous haemagglutinin and its secretion machinery. Microbiology 146:1211-1221. [DOI] [PubMed] [Google Scholar]
- 22.Khubchandani, K. R., K. L. Goss, J. F. Engelhardt, and J. M. Snyder. 2001. In situ hybridization of SP-A mRNA in adult human conducting airways. Pediatr. Pathol. Mol. Med. 20:349-366. [PubMed] [Google Scholar]
- 23.Kuehn, M. J. 1997. Establishing communication via gram-negative bacterial pili. Trends Microbiol. 5:130-132. [DOI] [PubMed] [Google Scholar]
- 24.Ladant, D., and A. Ullmann. 1999. Bordetella pertussis adenylate cyclase: a toxin with multiple talents. Trends Microbiol. 7:172-176. [DOI] [PubMed] [Google Scholar]
- 25.Leininger, E., M. Roberts, J. G. Kenimer, I. G. Charles, N. Fairweather, P. Novotny, and M. J. Brennan. 1991. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc. Natl. Acad. Sci. USA 88:345-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeVine, A. M., M. D. Bruno, K. M. Huelsman, G. F. Ross, J. A. Whitsett, and T. R. Korfhagen. 1997. Surfactant protein A-deficient mice are susceptible to group B streptococcal infection. J. Immunol. 158:4336-4340. [PubMed] [Google Scholar]
- 27.Locht, C., R. Antoine, and F. Jacob-Dubuisson. 2001. Bordetella pertussis, molecular pathogenesis under multiple aspects. Curr. Opin. Microbiol. 4:82-89. [DOI] [PubMed] [Google Scholar]
- 28.Martinez de Tejada, G., P. A. Cotter, U. Heininger, A. Camilli, B. J. Akerley, J. J. Mekalanos, and J. F. Miller. 1998. Neither the Bvg− phase nor the vrg6 locus of Bordetella pertussis is required for respiratory infection in mice. Infect. Immun. 66:2762-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattoo, S., J. F. Miller, and P. A. Cotter. 2000. Role of Bordetella bronchiseptica fimbriae in tracheal colonization and development of a humoral immune response. Infect. Immun. 68:2024-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCormack, F. X., and J. A. Whitsett. 2002. The pulmonary collectins, SP-A and SP-D, orchestrate innate immunity in the lung. J. Clin. Investig. 109:707-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menozzi, F. D., R. Mutombo, G. Renauld, C. Gantiez, J. H. Hannah, E. Leininger, M. J. Brennan, and C. Locht. 1994. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect. Immun. 62:769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merkel, T. J., S. Stibitz, J. M. Keith, M. Leef, and R. Shahin. 1998. Contribution of regulation by the bvg locus to respiratory infection of mice by Bordetella pertussis. Infect. Immun. 66:4367-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, J. F., S. A. Johnson, W. J. Black, D. T. Beattie, J. J. Mekalanos, and S. Falkow. 1992. Constitutive sensory transduction mutations in the Bordetella pertussis bvgS gene. J. Bacteriol. 174:970-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reference deleted.
- 35.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35:32-40. [DOI] [PubMed] [Google Scholar]
- 36.Porter, J. F., K. Connor, and W. Donachie. 1994. Isolation and characterization of Bordetella parapertussis-like bacteria from ovine lungs. Microbiology 140:255-261. [DOI] [PubMed] [Google Scholar]
- 37.Prasad, S. M., Y. Yin, E. Rodzinski, E. I. Tuomanen, and H. R. Masure. 1993. Identification of a carbohydrate recognition domain in filamentous hemagglutinin from Bordetella pertussis. Infect. Immun. 61:2780-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Relman, D., E. Tuomanen, S. Falkow, D. T. Golenbock, K. Saukkonen, and S. D. Wright. 1990. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell 61:1375-1382. [DOI] [PubMed] [Google Scholar]
- 39.Relman, D. A., M. Domenighini, E. Tuomanen, R. Rappuoli, and S. Falkow. 1989. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc. Natl. Acad. Sci. USA 86:2637-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynolds, H. Y. 2002. Modulating airway defenses against microbes. Curr. Opin. Pulm. Med. 8:154-165. [DOI] [PubMed] [Google Scholar]
- 41.Roberts, M., N. F. Fairweather, E. Leininger, D. Pickard, E. L. Hewlett, A. Robinson, C. Hayward, G. Dougan, and I. G. Charles. 1991. Construction and characterization of Bordetella pertussis mutants lacking the vir-regulated P.69 outer membrane protein. Mol. Microbiol. 5:1393-1404. [DOI] [PubMed] [Google Scholar]
- 42.Saitoh, H., H. Okayama, S. Shimura, T. Fushimi, T. Masuda, and K. Shirato. 1998. Surfactant protein A2 gene expression by human airway submucosal gland cells. Am. J. Respir. Cell. Mol. Biol. 19:202-209. [DOI] [PubMed] [Google Scholar]
- 43.Sakurai, Y., H. Suzuki, and E. Terada. 1993. Purification and characterisation of haemagglutinin from Bordetella bronchiseptica. J. Med. Microbiol. 39:388-392. [DOI] [PubMed] [Google Scholar]
- 44.Schaeffer, L. M., F. X. McCormack, H. Wu, and A. A. Weiss. 2004. Bordetella pertussis lipopolysaccharide resists the bactericidal effects of pulmonary surfactant protein A. J. Immunol. 173:1959-1965. [DOI] [PubMed] [Google Scholar]
- 45.Soane, M. C., A. Jackson, D. Maskell, A. Allen, P. Keig, A. Dewar, G. Dougan, and R. Wilson. 2000. Interaction of Bordetella pertussis with human respiratory mucosa in vitro. Respir. Med. 94:791-799. [DOI] [PubMed] [Google Scholar]
- 46.Stainer, D. W., and M. J. Scholte. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J. Gen. Microbiol. 63:211-220. [DOI] [PubMed] [Google Scholar]
- 47.Temple, L. M., A. A. Weiss, K. E. Walker, H. J. Barnes, V. L. Christensen, D. M. Miyamoto, C. B. Shelton, and P. E. Orndorff. 1998. Bordetella avium virulence measured in vivo and in vitro. Infect. Immun. 66:5244-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tuomanen, E., H. Towbin, G. Rosenfelder, D. Braun, G. Larson, G. C. Hansson, and R. Hill. 1988. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J. Exp. Med. 168:267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tuomanen, E., and A. Weiss. 1985. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J. Infect. Dis. 152:118-125. [DOI] [PubMed] [Google Scholar]
- 50.van den Berg, B. M., H. Beekhuizen, R. J. L. Willems, F. R. Mooi, and R. van Furth. 1999. Role of Bordetella pertussis virulence factors in adherence to epithelial cell lines derived from the human respiratory tract. Infect. Immun. 67:1056-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss, A. A., and S. Falkow. 1984. Genetic analysis of phase change in Bordetella pertussis. Infect. Immun. 43:263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu, H., A. Kuzmenko, S. Wan, L. Schaffer, A. Weiss, J. H. Fisher, K. S. Kim, and F. X. McCormack. 2003. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J. Clin. Investig. 111:1589-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaretzky, F. R., M. C. Gray, and E. L. Hewlett. 2002. Mechanism of association of adenylate cyclase toxin with the surface of Bordetella pertussis: a role for toxin-filamentous haemagglutinin interaction. Mol. Microbiol. 45:1589-1598. [DOI] [PubMed] [Google Scholar]