Abstract
Work is ongoing to advance seizure forecasting, but the performance metrics used to evaluate model effectiveness can sometimes lead to misleading outcomes. For example, some metrics improve when tested on patients with a particular range of seizure frequencies (SF). This study illustrates the connection between SF and metrics. Additionally, we compared benchmarks for testing performance: a moving average (MA) or the commonly used permutation benchmark. Three data sets were used for the evaluations: (1) Self-reported seizure diaries of 3,994 Seizure Tracker patients; (2) Automatically detected (and sometimes manually reported or edited) generalized tonic-clonic seizures from 2,350 Empatica Embrace 2 and Mate App seizure diary users, and (3) Simulated datasets with varying SFs. Metrics of calibration and discrimination were computed for each dataset, comparing MA and permutation performance across SF values. Most metrics were found to depend on SF. The MA model outperformed or matched the permutation model in all cases. The findings highlight SF’s role in seizure forecasting accuracy and the MA model’s suitability as a benchmark. This underscores the need for considering patient SF in forecasting studies and suggests the MA model may provide a better standard for evaluating future seizure forecasting models.
Keywords: epilepsy, seizure, bioinformatics, statistics
Introduction
Many studies attempt to forecast seizures.1–9 However, patients’ seizure frequency (SF) is usually ignored when reporting the model performance. It has been observed across studies that patients have vastly different SFs.10 It is possible that model performance metrics calculated over a cohort might be influenced by SF and, thus, confound the evaluation of model performance.
Benchmark model selection is another important consideration. Often model performances are compared against a benchmark using random permutations (shuffling) of the predicted seizure labels (details below)1,11,12 Using permutation testing to assess a forecasting model is a very low bar to overcome, and probably does not have any clinical significance. Conversely, a moving average model (“what happened before is likely to happen again’) may be a better litmus test for a successful forecasting tool12.
We hypothesized that (1) there is a SF dependence that affects the performance of some forecasting metrics, and (2) using a moving average model is a better benchmark model compared to permutation testing. This study aims to explore these two hypotheses with simulation data and with two sets of real-world data.
Materials and Methods
Datasets and data preprocessing
Simulated dataset
We produced a structured simulation of 9 seizure diaries with 9 different seizure frequencies respectively. Each diary was a 10000-days-long binary array where 0 indicates there is no seizures and 1 indicates there is at least 1 seizure in that day. The monthly SF is determined by the number of seizure days in a month ranging from 1 seizure day to 9 seizure days per month. Of note, most patients from both clinical datasets had SF values within 1–9/month. All the seizure days occurred consecutively at the beginning of each month (Appendix). This organization of when seizures occurred was arbitrary – the key was that each diary had a prespecified number of seizures per month.
Clinical datasets
Two clinical datasets were evaluated, both approved by BIDMC IRB with Exempt status. We received access to the e-diary data through a data use agreement with Seizure Tracker LLC, facilitated by the International Seizure Diary Consortium. Seizure Tracker10 provided de-identified self-reported diaries. We selected patients based on the recording period and the length of diary (Appendix).
Another dataset was recorded by Empatica’s FDA-cleared Embrace 2, a wearable device for generalized tonic-clonic seizure (GTCS) detection.9 The device has a companion diary app “Mate” which patients sometimes use to manually enter seizures or to delete events that were false alarms. De-identified wearable-derived seizure diaries were provided by Empatica for the purposes of this statistical analysis. We selected patients based on recording duration and on the reliability of their e-diary interactions (Appendix).
Metrics of interest
We focused on 4 commonly used metrics for forecasting: two for calibration (Brier Score and calibration curve) and two for discrimination (area under curve of receiver-operating characteristics (AUCROC), and area under curve of precision-recall curve (AUCPR)).1,2,4–8,11,13
To summarize results across diaries, we categorized each diary into SF bins ranging from 1 seizure day/month to 9 seizure days/month with a 1-seizure day/month bin size and reported the average within each bin. For results on extremely high and low SF, please see Appendix. The number of diaries in each bin was normalized by the total number of diaries for visualization purposes.
Benchmark model: Moving average model vs. permutation testing
Moving average model (MA) is a simple causal forecasting model12. It predicts the probability of having seizure events by calculating the rate of seizure-present intervals (here, 24-hour intervals) in the diary history using a lookback window. In this study, we used a 90-day window, during which most SFs would be empirically expected to be steady.8,10 Since MA is intuitive and requires minimal computation (could even be computed manually by a patient/caregiver), we consider MA a candidate benchmark model.
Permutation testing is a widely used benchmark in forecasting tasks.1,11 It permutes the model forecasts and calculates the metrics of interest. This process is then repeated (e.g., 1,000 times). The average metric across all permutations is typically reported.
Improvement over chance (IOC) is another way to quantify a model performance, as shown in Eq. (1).
| (1) |
It can be shown that IOC for a perfectly accurate forecasting model (“truth”) is maximal (Appendix). Therefore, we consider the average result of permutations of truth, denoted permuted truth, as another candidate benchmark test, because it would provide the largest possible IOC for a given SF.
Data Availability
Private data from Seizure Tracker and Empatica were made available upon request from these companies. These data are not public and may be requested by interested investigators subject to project approval. Source code is freely available here. https://github.com/GoldenholzLab/Metric_comparison_and_benchmark.git
Results
After preprocessing, there were 3,994 patients from Seizure Tracker with diary durations of 91–5,337 (median 525) days, and 2,350 patients from Empatica with diary durations of 90–1,551 (median 280) days.
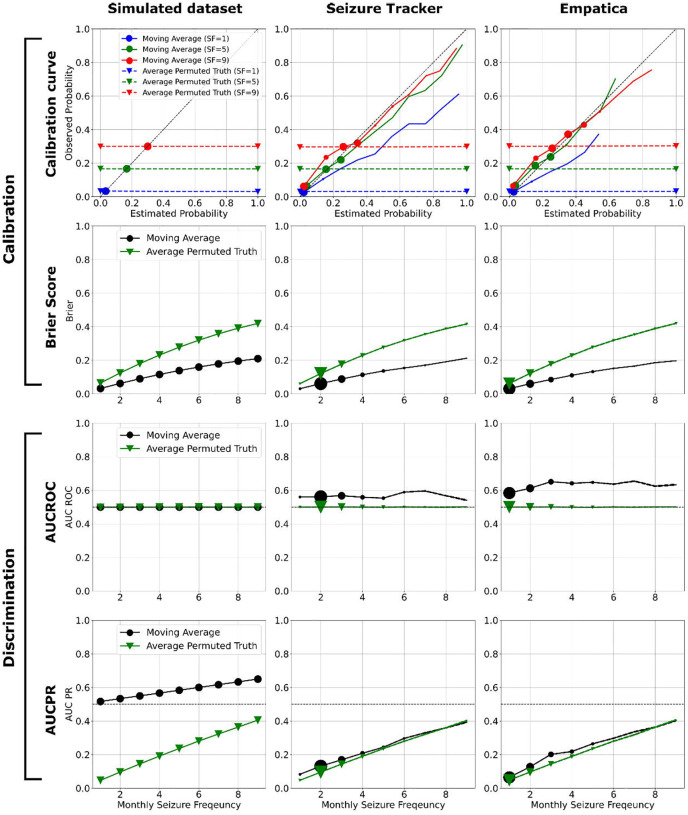
Figure 1 shows the results of comparing the MA and permuted truth, across the four metrics for each of the three datasets. Seizure Tracker and Empatica have more diaries with low SF, as expected10,11. In all twelve comparisons, the MA outperforms the permuted truth, showing MA is a harder baseline to beat. In nine of the comparisons the results depend on SF, usually improving with higher SF except in the case of the Brier Score, where lower (better) Brier score occurs with lower SF. The calibration curves of MA show slight overestimates in probability for low SF but improve as SF increases. All AUCROC values fluctuate around 0.5–0.6 across SF.
Figure 1.
Twelve scenarios comparing the performance of MA vs. permuted truth. The calibration curve, brier score, AUCROC, and AUCPR are shown in rows. The results of simulated, Seizure Tracker, and Empatica datasets are shown in columns. In the calibration curves (first row), the monthly seizure frequencies 1, 5, and 9 are shown in blue, green, and red. The results of MA and permuted truth are indicated by solid line and dash line. The marker size indicates the normalized number of diaries within each estimated probability bin. Since the MA outcomes for the simulated dataset are constant, there is only one estimated probability in the calibration curve, resulting in a single marker instead of a solid line. The brier score, AUCROC, and AUCPR of MA and permuted truth are indicated by black and green solid lines respectively in the second, third, and fourth rows. The marker size indicates the normalized number of diaries within each SF bin. Note that in all twelve comparisons, MA performs as well or better than permuted truth. Additionally, within this range of SF, all metrics except AUCROC vary monotonically with seizure frequency. Higher SF values were explored in simulation (Appendix).
Discussion
There are two main findings in our study. First, MA appears to be a better benchmark compared to permutation testing. Second, three of the metrics, the calibration curve, Brier score and AUCPR, show a dependence on SF while AUCROC appears to be relatively SF-independent.
Hence, it is necessary to report individual patient SF with these metrics when comparing model performances across different studies. Bins of seizure frequencies can be used if narrowly defined (as done here). When comparing models evaluated on datasets with different SF ranges, we suggest imputing metric performance for a common SF range and including SF independent metrics, such as AUCROC (Appendix). Critically, some SF values may not be very important to forecast (e.g., daily risk in patients who have a seizure per 2-days, or daily risk in patients with yearly seizures, etc.).
When comparing the performance of MA and permuted truth, we found MA always performs the same or better than permuted truth. Additionally, MA is preferable as a benchmark because it (1) is causal (i.e. does not require knowledge of the future), (2) is easily computed (“back of the envelope calculation”), and (3) is interpretable (“the previous seizure rate will recur”). Conversely, permutation is noncausal (knowledge of the future is required) and requires more computational resources. Anecdotally, our investigations have found MA to be surprisingly accurate in multiple seizure forecasting contexts and therefore a more challenging benchmark to overcome for a candidate model.
There were some differences between the simulation and the clinical datasets. Many of these differences reflect the simplistic assumptions used for the simulation, as well as methodological choices made for our study (Appendix).
The emphasis of this paper is identifying mathematical guideposts for testing algorithms. In contrast, the value of seizure forecasting tools is beyond the scope of this study; patient attitudes, beliefs, desires, and behaviors all need to be accounted for prior to deploying a forecasting tool.
In summary, this study provides insight into the importance of including patients’ seizure frequency in seizure forecasting tasks and demonstrates that MA represents a valuable benchmark with minimal computational complexity.
Supplementary Material
Acknowledgements:
Thanks to the International Seizure Diary Consortium for facilitating data sharing. DG and CC are supported by NINDS K23NS124656. BZ is supported by T32 HL007901-25. Dr. Westover was supported by grants from the NIH (R01NS102190, R01NS102574, R01NS107291, RF1AG064312, RF1NS120947, R01AG073410, R01HL161253, R01NS126282, R01AG073598), and NSF (2014431).
Potential conflicts of interest:
Dr. Goldenholz is an unpaid advisor for Epilepsy AI and Eysz. He has been a paid advisor for Magic Leap. He has been provided speaker fees from AAN, AES and ACNS. He also previously has been a paid consultant for Neuro Event Labs, IDR, LivaNova and Health Advances.Dr. Picard reports personal fees and other from Empatica, Inc., other from Stern Strategy, personal fees from Apple, personal fees from Samsung, personal fees from Harman, personal fees from D.E. Shaw, personal fees from ESME Learning, personal fees from Amazon, personal fees from Partners Healthcare, personal fees from Handelsblatt Media Group, grants from National Institute of Health, grants from Abdul Latif Jameel Clinic for Machine Learning in Health, grants from Samsung, grants from ChildMind Institute, personal fees from Amicus Rx, personal fees from KBTG, grants from NEC, outside the submitted work; In addition, Dr. Picard has a patent Washable Wearable Biosensor US Patent 8,140,143 with royalties paid to Affectiva, Empatica, Media Lab member companies, a patent Methods and Apparatus for Monitoring Patients and Delivering Therapeutic Stimuli. US Patent 8,655,441 with royalties paid to Affectiva, Empatica, Media Lab member companies, a patent Biosensor with Pressure Compensation. US Patent 8,311,605 issued, a patent Method for Biosensor Usage with Pressure Compensation. US Patent 8,396,530 issued, and a patent Biosensor with Electrodes and Pressure Compensation. US Patent 8,965,479 issued and Shareholder in Smart Eye, AB, (who acquired Affectiva and iMotions which works with wearable sensors, which can be broadly applied to many uses in healthcare).
Dr. Westover is a co-founder, scientific advisor, and consultant to Beacon Biosignals and has a personal equity interest in the company. He also receives royalties for authoring Pocket Neurology from Wolters Kluwer and Atlas of Intensive Care Quantitative EEG by Demos Medical.
Footnotes
Ethical statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Ethics approval statement:
This study was deemed IRB Exempt by the BIDMC IRB.
Data Availability
Private data from Seizure Tracker and Empatica were made available upon request from these companies. These data are not public and may be requested by interested investigators subject to project approval. Source code is freely available here. https://github.com/GoldenholzLab/Metric_comparison_and_benchmark.git
Bibliography
- 1.Leguia MG, Rao VR, Tcheng TK, et al. Learning to generalize seizure forecasts. Epilepsia. 2023;64. [DOI] [PubMed] [Google Scholar]
- 2.Proix T, Truccolo W, Leguia MG, et al. Forecasting seizure risk in adults with focal epilepsy: a development and validation study. Lancet Neurol. Lancet Publishing Group; 2021;20:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinkmann BH, Karoly PJ, Nurse ES, et al. Seizure Diaries and Forecasting With Wearables: Epilepsy Monitoring Outside the Clinic. Front Neurol Frontiers Media S.A.; 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karoly PJ, Cook MJ, Maturana M, et al. Forecasting cycles of seizure likelihood. Epilepsia. Blackwell Publishing Inc.; 2020;61:776–786. [DOI] [PubMed] [Google Scholar]
- 5.Stirling RE, Maturana MI, Karoly PJ, et al. Seizure Forecasting Using a Novel Sub-Scalp Ultra-Long Term EEG Monitoring System. Front Neurol. Frontiers Media S.A.; 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasseri M, Pal Attia T, Joseph B, et al. Ambulatory seizure forecasting with a wrist-worn device using long-short term memory deep learning. Sci Rep. Nature Research; 2021;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onorati F, Regalia G, Caborni C, et al. Prospective Study of a Multimodal Convulsive Seizure Detection Wearable System on Pediatric and Adult Patients in the Epilepsy Monitoring Unit. Front Neurol. Frontiers Media S.A.; 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldenholz DM, Goldenholz SR, Romero J, Moss R, Sun H, Westover B. Development and Validation of Forecasting Next Reported Seizure Using e-Diaries. Ann Neurol. John Wiley and Sons Inc.; 2020;88:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Onorati F, Regalia G, Caborni C, et al. Multicenter clinical assessment of improved wearable multimodal convulsive seizure detectors. Epilepsia. Blackwell Publishing Inc.; 2017;58:1870–1879. [DOI] [PubMed] [Google Scholar]
- 10.Ferastraoaru V, Goldenholz DM, Chiang S, Moss R, Theodore WH, Haut SR. Characteristics of large patient-reported outcomes: Where can one million seizures get us? Epilepsia Open. Wiley-Blackwell Publishing Ltd; 2018;3:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snyder DE, Echauz J, Grimes DB, Litt B. The statistics of a practical seizure warning system. J Neural Eng. 2008;5:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenholz DM, Eccleston C, Moss R, Westover MB. Prospective validation of a seizure diary forecasting falls short. Epilepsia [online serial]. Epilepsia; Epub 2024. Apr 12. Accessed at: https://pubmed.ncbi.nlm.nih.gov/38606580/. Accessed April 15, 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brier GW. Verification of forecasts expressed in terms of probability. Mon Weather Rev. 1950;78:1–3. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Private data from Seizure Tracker and Empatica were made available upon request from these companies. These data are not public and may be requested by interested investigators subject to project approval. Source code is freely available here. https://github.com/GoldenholzLab/Metric_comparison_and_benchmark.git
Private data from Seizure Tracker and Empatica were made available upon request from these companies. These data are not public and may be requested by interested investigators subject to project approval. Source code is freely available here. https://github.com/GoldenholzLab/Metric_comparison_and_benchmark.git