Abstract
Studying lung adenocarcinoma (LUAD) early carcinogenesis is challenging, primarily due to the lack of LUAD precursors specimens. We amassed multi-omics data from 213 LUAD and LUAD precursors to identify molecular features underlying LUAD precancer evolution. We observed progressively increasing mutations, chromosomal aberrations, whole genome doubling and genomic instability from precancer to invasive LUAD, indicating aggravating chromosomal instability (CIN). Telomere shortening, a crucial genomic alteration linked to CIN, emerged at precancer stage. Moreover, later-stage lesions demonstrated increasing cancer stemness and decreasing alveolar identity, suggesting epithelial de-differentiation during early LUAD carcinogenesis. The innate immune cells progressively diminished from precancer to invasive LUAD, concomitant with a gradual recruitment of adaptive immune cells (except CD8+ and gamma-delta T cells that decreased in later stages) and upregulation of numerous immune checkpoints, suggesting LUAD precancer evolution is associated with a shift from innate to adaptive immune response and immune evasion mediated by various mechanisms.
Keywords: lung adenocarcinoma, pre-cancer, cancer evolution, immune evasion, chromosomal instability, stemness, alveolar differentiation
INTRODUCTION
Lung cancer remains the leading cause of cancer-related mortality globally, in large part due to frequent diagnosis at late-stage with markedly reduced chances for cure. Early detection through low-dose CT-guided lung cancer screening has demonstrated a significant reduction in lung cancer mortality1. Meanwhile, widespread adoption of chest CT scans for screening or management of other medical conditions has resulted in a significant surge in the detection of indeterminate pulmonary nodules (IPNs)1. While many IPNs are benign, a subset are precursor lesions that may progress to invasive lung adenocarcinoma (LUAD), the most common subtype of lung cancer2. These LUAD precursors include atypical adenomatous hyperplasia (AAH), which may progress into preinvasive adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA)3, 4, 5, 6 and finally to fully invasive LUAD (ADC). Whereas many IPNs can be resected with minimal morbidity7, such invasive intervention may be medically unnecessary if the lesion was destined to remain benign8. In addition, up to 25% of patients may harbor multiple IPNs9, 10, which makes surgical resection more challenging. While chemoprevention to halt or slow the progression of these LUAD precursors to invasive LUAD is appealing in principle, clinical trials to date have been disappointing11, 12, 13, 14, 15, 16, 17, 18, 19. This may be due to a multitude of factors including a lack of biomarkers for risk prediction and lack of effective therapies for early intervention due to our limited understanding of early lung tumorigenesis.
Understanding the molecular mechanisms of the early stages of lung tumorigenesis is essential to discovering new targets for precise diagnosis, prevention, and therapy. However, studying early carcinogenesis of LUAD is challenging primarily due to the scarcity of adequate clinical specimens of precursor lesions, as surgery is not the standard of care. Over the past decade, we and other groups have made extensive efforts to collect and characterize resected LUAD precursors to depict the molecular evolution and associated immune response during early carcinogenesis of LUAD. A series of studies from our group have revealed that LUAD precursors present a simpler molecular landscape20, 21, 22, 23, and more active immunity than invasive LUAD22, 23, 24. However, previous studies have been limited by the small sample size. In addition, the transcriptomic features of these LUAD precursors of different histologic stages have not been systematically investigated.
In this study, we sought to capture the evolutionary processes of early LUAD carcinogenesis by performing multi-regional whole exome sequencing (WES), whole genome sequencing (WGS) and RNA-sequencing (RNA-seq) on a large cohort of resected LUAD and LUAD precursors of various histologic stages, with the intent to improve our understanding of the molecular and immune alterations associated with the initiation and progression of LUAD precursors.
RESULTS
Aggravating chromosomal instability is associated with accumulation of genetic alterations during the neoplastic evolution from precancer to invasive lung adenocarcinoma.
To investigate the genomic alterations during early LUAD carcinogenesis, we analyzed multi-regional whole exome sequencing (WES) data from 472 samples consisting of 213 LUAD and LUAD precursor lesions of various stages (50 AAH, 46 AIS, 70 MIA, and 47 ADC) that presented radiologically as ground glass opacities (GGO) predominant pulmonary nodules (Supplementary Data 1). The median exome sequencing coverage was ~300x. We also performed whole genome sequencing (WGS) in a subset of 26 lesions and their matched normal lung tissue with sufficient DNA at a median coverage of ~45x. There was no significant difference in age, smoking status, or sex between different histologic groups (Supplementary Data 2).
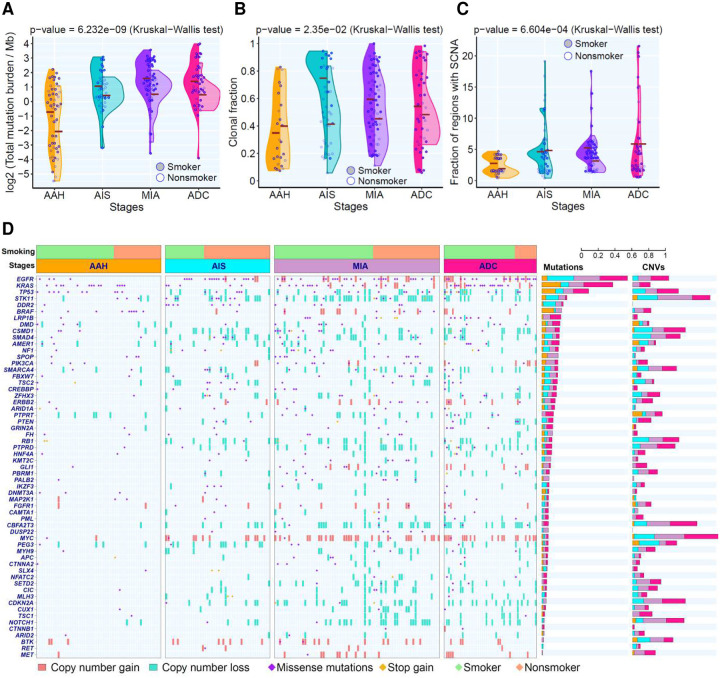
After systematic filtering of single nucleotide variants (SNVs) to remove potential artifacts in formalin-fixed, paraffin-embedded samples, a total of 39,811 mutations from WES were subjected to subsequent analysis. In line with our previous study20, we observed higher total mutational burden (TMB) in later-stage lesions (Fig.1A). TMB was higher in smokers compared to non-smokers across all stages (Fig.1A). Subclonal analysis demonstrated that the proportion of clonal mutations was lower in AAH lesions compared to AIS/MIA/ADC. LUAD precursors from smokers exhibited a higher proportion of clonal mutations than non-smokers in all disease stages (Fig.1B). Furthermore, phylogenetic analysis of LUAD precursors with multi-regional WES data revealed a higher proportion of truncal mutations in later-stage lesions (Fig.S1). Taken together, these results suggest that the neoplastic transformation of LUAD precancers predominantly occurs as a clonal sweep model20.
Figure 1. Progressive genomic evolution from AAH to ADC.
(A) Violin plot of mutational burden across histologic stage. Each point represents the mutational burden in each lesion from smokers (solid) or non-smokers (hollow). Cross bars represent the mean. Kruskal–Wallis H test was used to compare mutational burden across all stages. (B) Violin plot showing the proportion of clonal mutations in each lesion. Each point represents the clonal fraction in each lesion from smokers (solid violin) or non-smokers (hollow violin). Cross bars represent the mean clonal fraction. The difference across stages was assessed by Kruskal–Wallis H test. Only lesions with a minimum of 10 SNVs were included for subclonal deconvolution analysis. (C) Violin plot of the proportion of chromosomal regions with copy number alterations in each lesion. Each point represents the fraction of chromosomal regions with copy number gain (total copy number > 2.5) or loss (total copy number < 1.5) over the exome capture region across all chromosomes in smokers (solid violin) or non-smokers (hollow violin). Cross bars represent the mean CNV burden. The difference across stages was assessed by Kruskal–Wallis H test. Only regions with a minimum of 50 reads were included to determine copy number alterations. (D) The landscape of cancer gene mutations and copy number aberrations in lesions. Cancer gene mutations were defined as nonsynonymous mutations in known cancer genes identical to those hotspots previously reported and stop-gain variants in tumor suppressor genes. Cancer genes located in chromosomal segments with copy number gains (red) or losses (green) are shown. A threshold of focal copy number ≥3 or ≤1 was used to determine chromosomal gains or losses, respectively.
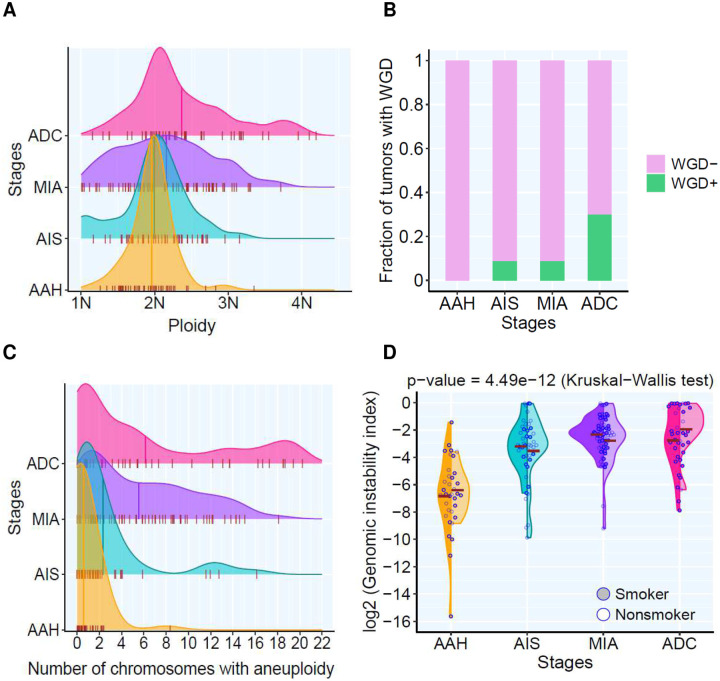
Next, we analyzed somatic copy number alterations (SCNA). SCNA events were observed in AAH frequent lesions, which became more prevalent in AIS, MIA and ADC (Fig.1C, Fig.S2). The frequent chromosome arm aneuploidy (CAA) events reported in invasive LUAD25, including 1q, 5p and 8q gains and 3p, 8p, 9p, 9q, and 13q losses became prevalent after AIS. Moreover, polyploidy started to emerge at the AIS stage and further expanded in MIA and ADC lesions (Fig.2A). Whole genome doubling (WGD) was not detected in AAH lesions, but it was detected in 9% of AIS lesions, 9% of MIA and 30% of ADC lesions (Fig.2B). In addition, we observed a progressively increased number of chromosomes exhibiting aneuploidy (Fig.2C) and a progressive increase in the weighted genomic instability index (wGII, defined as the fraction of genome altered)26 (Fig. 2D) in later-stage lesions. Importantly, wGII was positively correlated with ploidy, SCNA burden and frequency of aneuploidy (Fig.S3 A–C) Taken together, these findings suggest accumulated chromosomal instability along with neoplastic progression during early carcinogenesis of LUAD, particularly after malignant transformation post AIS stage.
Figure 2. Propagated genomic instability in lesions of different histological stages.
(A) Density plot showing the distribution of the ploidy among different histological stages. X-axis shows the density of ploidy numbers. The short whiskers show the estimated ploidy value in each lesion. The vertical cross lines show mean ploidy value of all lesions in AAH, AIS, MIA, and ADC, respectively. (B) The prevalence of whole genome doubling (WGD) among different histological stages. Each bar represents the proportion of lesions with WGD (green) and without WGD (pink) in each stage. (C) Density plot showing the prevalence of the aneuploidy among different histological stages. X-axis shows the number of chromosomes. The short whiskers show the number of chromosomes detected with mosaic aneuploidy in each lesion. The vertical cross lines show mean number of chromosomes carrying aneuploidies from all lesions in AAH, AIS, MIA, and ADC, respectively. (D) Weighted genomic instability index (wGII) amongst different histological stages. Each point represents genomic ability index in each lesion, and the brown cross bars represent the mean genomic instability index of all lesions of each histologic stage in smokers (solid) and non-smokers (hollow), respectively. Kruskal-Wallis H test was used to compare genomic instability indexes across stages.
In addition to SCNA, chromosomal instability can also manifest as structural variants that may have profound impacts on tumor biology27, 28. Leveraging WGS data from LUAD precursors of different stages to investigate the timing of structural variants during early LUAD carcinogenesis, we detected structural variants in lesions of all stages (Fig.S4), suggesting these may be early genomic events.
The top frequently mutated cancer genes in this cohort of LUAD and precursors included EGFR, KRAS, TP53, STK11, and LRP1B, most of which emerged at the precancer AAH stage. In addition, copy number losses in tumor suppressor genes (TSG) such as CDKN2A, TP53, NOTCH1, PTPRD, STK11, and copy number gain in oncogenes such as MYC, EGFR, and MET were detected in precancers of all stages but with higher incidence in later-stages (Fig.1D). These observations indicate that worsening chromosomal instability may have led to copy number gain or loss of critical cancer genes, which subsequently may have contributed to the initiation and progression of LUAD precancers.
Telomere shortening may represent a pivotal early genomic event underlying chromosomal instability during early carcinogenesis of lung adenocarcinoma.
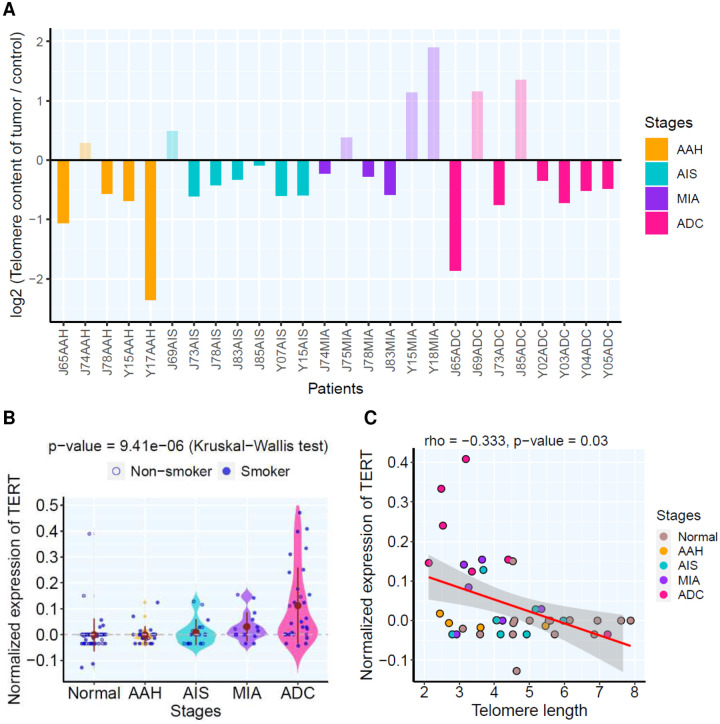
Telomere length shortening has been reported to be causative of chromosomal insstability29 30. In the LUAD precursors for which WGS data was available, telomere shortening (compared to matched normal lung tissues) was a common and early genomic event, which was observed in 19 out of 26 lesions with WGS data available (Fig.3A), including 4 of 5 AAH lesions. In parallel, the expression of telomerase (TERT) gradually increased in later-stage lesions (Fig.3B) and negatively correlated with telomere length (Fig.3C). These data indicate that chromosomal instability, an important cancer hallmark, may be an early genomic event during LUAD carcinogenesis that emerges at precancer stage, while telomere shortening is a potential genomic alteration underlying chromosomal instability.
Figure 3. The telomere length and TERT expression in each lesion.
(A) Each bar represents the relative telomere length (RTL) in each lesion based on WGS profiling. (B) TERT expression amongst different histological stages. Each blue dot represents normalized expression of TERT in each pulmonary nodule and the solid brown dots represent the mean expression of all lesions of each histologic stage. Kruskal-Wallis H test was used for comparing normalized TERT expression between all stages. (C) The correlation of absolute telomere length by WGS profiling and normalized TERT expression between lesions (only lesions profiled by both WGS and RNAseq) assessed by two-tailed Spearman’s correlation analysis. Each dot represents each lesion from Normal (brown), AAH (orange), AIS (cyan), MIA (purple) and ADC (rose), respectively.
Evolution from pre-cancer to invasive LUAD is associated with increased transcriptomic intratumor heterogeneity and epithelial dedifferentiation.
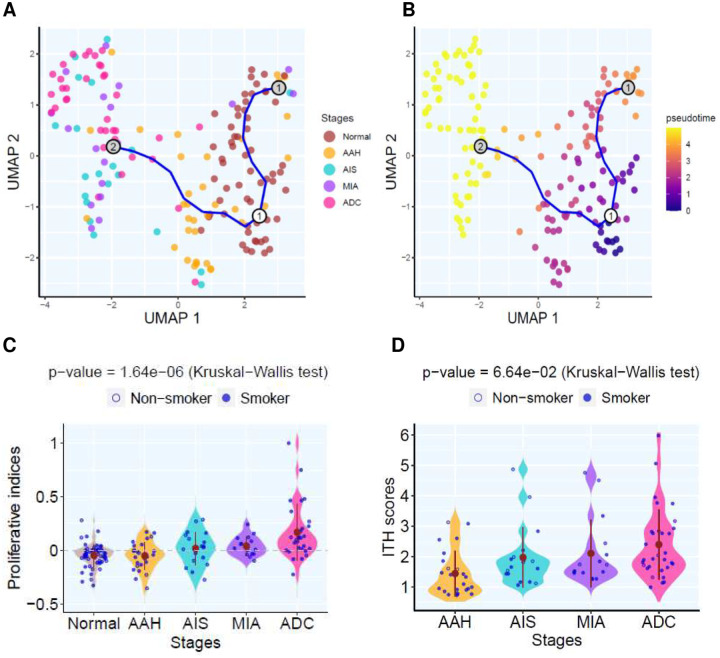
To understand early LUAD carcinogenesis at the transcriptomic level, we performed RNA sequencing (RNA-Seq) on a subset of 168 LUAD and LUAD precursors with adequate tissue available. Principal component analysis (PCA) and hierarchical clustering displayed distinct clusters between normal lung, AAH and AIS/MIA/ADC (Fig.S5A–B) highlighting the transcriptomic divergence at the transition of malignant transformation. Pseudo-time analysis further revealed the evolutionary trajectory from normal lung tissue to AAH, then AIS/MIA/ADC (Fig.4A–B). In addition, the proliferative index based on a pan-cancer proliferative gene signature31 (Supplementary Data 3) was significantly higher in later-stage than early stage lesions (Fig.4C) indicating increasing proliferation rate along with neoplastic progression. Furthermore, network entropy analysis32 to infer transcriptomic intra-tumoral heterogeneity (ITH) uncovered a higher level of transcriptomic ITH in later stage lesions than their early stage counterparts (Fig.4D and Fig.S6), in line with the higher degree of heterogeneity in methylation in later stage LUAD precursors21, 33.
Figure 4. Transcriptomic trajectory and intra-tumoral heterogeneity (ITH) of lesions across distinct pathological stages.
(A) Pseudotime trajectory was estimated using selected genes with high variance and expression in specimens of different pathological stages. Point colors represent histological stage. (B) Trajectory plot colored by estimated pseudo time. (C) The proliferative scores amongst different histological stages. Each blue point represents the proliferative indices in each pulmonary nodule and the solid brown points represent the mean proliferative indices of each histologic stage. Kruskal-Wallis H test was used to compare proliferative indices between all stages. (D) Transcriptomic ITH scores among different histological stages. Each blue point represents ITH in each pulmonary nodule and the solid brown points represent the mean ITH of each histologic stage. Kruskal-Wallis H test was used to compare ITH scores between all stages.
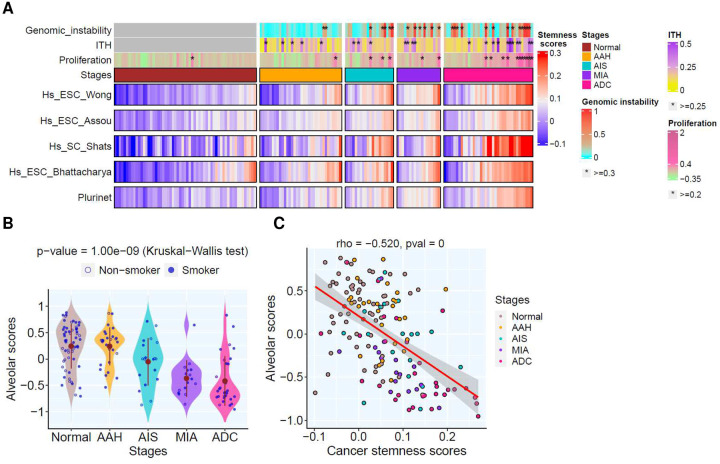
One hallmark of cell plasticity in cancers is dedifferentiation, a process whereby tumor cells lose their specialized properties and revert to less differentiated phenotypes reminiscent of early embryonic development or regenerative processes34. To understand the dedifferentiation process during early LUAD carcinogenesis, we estimated cancer stem cell (CSC) scores35 (Supplementary Data 3), which revealed significantly higher CSC scores and pluripotency signaling in later stage lesions (Fig.5A and Fig.S7A–B). In parallel, we observed a progressive decrease in the alveolar scores36 (Fig.5B, Supplementary Data 3). Importantly, the alveolar scores were negatively associated with the CSC scores (Fig.5C). Further analysis revealed that the expression of pluripotency transcription factors such as FOXM1, OCT4, SOX9, TWIST1, gradually increased, while KLF4 gradually decreased in later-stage lesions (Fig.S8A–E). These results indicate the dedifferentiation of epithelial cells may be regulated by the core pluripotency stem cell transcriptional factors during early LUAD carcinogenesis.
Figure 5. Cancer stemness signatures in lesions of different stages and associated genomic features.
(A) The top bars show genomic instability scores calculated based on WES allelic copy number data, ITH scores estimated using transcriptomic network entropy; and proliferative scores inferred by gene signature. The stars (*) indicate lesions with genomic features greater than the cut-off values. The heatmap panel shows cancer stemness signatures (derived from different resources) grouped in different stages including AAH (typical adenomatous hyperplasia), AIS (adenocarcinoma in situ), MIA (minimally invasive adenocarcinoma), and ADC (invasive adenocarcinoma). (Source data is provided as a source data file). (B) The alveolar scores among different histological stages. Each blue dot represents alveolar score in each pulmonary nodule and the solid brown dots represent the mean alveolar scores within each histologic stage. Kruskal-Wallis H test was used to compare alveolar scores between all stages. (C) Correlation of cancer stemness signatures and alveolar scores amongst different histological stages.
Neoplastic progression of LUAD precursors is associated with transition from innate to adaptive immune response and immune evasion.
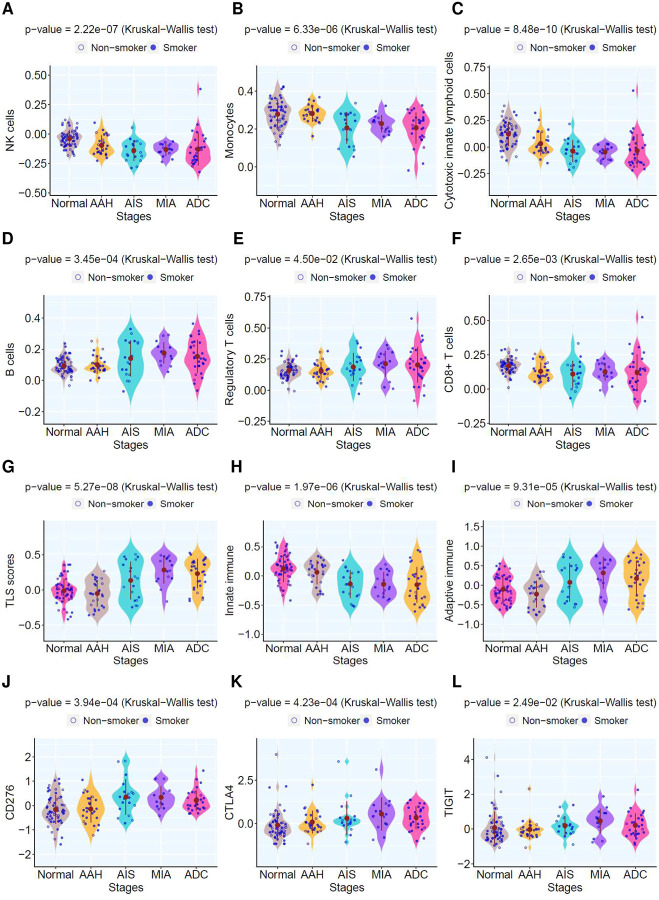
The initiation and development of LUAD precancers is influenced by the intricate interplay between evolving neoplastic cells and host factors, particularly anti-tumor immunity37. We next leveraged the gene expression data to delve into the immune features of LUAD precursors at various stages. Transcriptomic deconvolution demonstrated reduced infiltration of innate immune cells including NK cells, neutrophils, monocytes, eosinophils, and mast cells in later stage lesions (Fig.6A–B, Fig.S9A–D). Conversely, there was an increase in activated myeloid dendritic cells (mDCs) that are known to play important roles in antigen presentation and T cell priming38, as well as various adaptive immune cells, such as B cells, plasma cells, regulatory T cells (Treg), with notable exception of CD8+ T cells and gamma-delta (γδ) T cells with reduced infiltration in later-stage lesions (Fig.6D–F. Fig.S9E–H). The cytotoxic score also decreased in later stages (Fig. 6C).
Figure 6. Immune cell infiltration and immune gene expression in lesions of different histological stages.
Representative innate immune cell infiltration (A–C) and adaptive immune cell infiltration (D–F) based on deconvolution using Consensus amongst different histological stages. Each blue dot represents averaged enrichment score inferred in each pulmonary lesion and the solid brown dots represent the mean enrichment score of all lesions in each histologic stage. Kruskal–Wallis H test was used to compare the enrichment score across stages. The GSVA enrichment score of genes associated with tertiary lymphoid structures (TLS) (G), innate immunity (H) and adaptive immunity (I), respectively. (J–L) Normalized expression of representative immune checkpoint genes across stage.
We further applied GSVA to determine the expression of essential immune genes in LUAD precursors of different stages. Intriguingly, the tertiary lymphoid structure (TLS) score was higher in later-stage lesions (Fig.6G). Correspondingly, the densities of lymphoid follicles, lymphoid aggregates, and TLS, based on pathological assessment, were also higher in later-stage lesions (Fig.S10, S11), aligning with previous pathomics analysis39. This suggests an organized host anti-tumor immune response amid neoplastic progression of LUAD precursors. Consistent with deconvolution analysis, GSVA revealed a decrease in the expression of innate immunity markers (Fig.6H) and an increase in adaptive immunity in later-stage lesions (Fig.6I). Lastly, numerous immune checkpoints were upregulated in later-stage lesions (Fig.6J–L).
Taken together, these findings imply a transition from innate to adaptive immune response during the neoplastic progression from precancer to frankly invasive LUAD. However, neoplastic cells eventually evade host anti-tumor immunity through multiple mechanisms, including an increase in negative immune regulators such as Tregs and immune checkpoints, as well as downregulation of immune effectors such as cytotoxic lymphocytes, leading to progression into invasive LUAD.
DISCUSSION
Despite major advances in its treatment, lung cancer remains the leading cause of cancer-related death. There is an urgent need for effective strategies to prevent the development of this deadly malignancy. While risk avoidance, such as smoking cessation, represents a key strategy for reducing lung cancer risk, up to 20% of lung cancer patients are non-smokers40, 41. Moreover, among smokers with lung cancer, the majority have quit smoking well before their diagnosis, further highlighting the critical need for alternative active approaches for lung cancer interception42,43.
Although it has long been known that LUAD precursors often present as GGO-predominant lung nodules, interception of LUAD has been hindered by our rudimentary understanding of the underlying molecular events and associated tumor microenvironment changes fueling malignant transformation and neoplastic evolution. Recent studies have characterized the molecular and immune features of early-stage LUAD and its precursors20,21,24,44, 45, 46. However, the evolutionary trajectory and intricate crosstalk with host immunity during the initiation and progression of LUAD precursors remain understudied. Leveraging a large cohort of resected LUAD precursors through international collaborations, this study aimed to identify the sequential molecular changes driving LUAD precancer initiation and progression, along with the associated immune response and evasion.
We found that critical driver alterations, including canonical EGFR and KRAS mutations, were detected across the spectrum of lung cancer precursors. This observation underscores the potential for interception of precancerous lesions of LUAD by targeting these early events.
However, a substantial proportion of LUAD precursors lacked targetable driver mutations, presenting a challenge for interception using targeted therapy agents. Alternatively, an immune-based strategy may be more widely applicable, as immune evasion is a universal phenomenon in cancers. Immune prevention has shown success in cancers associated with infectious agents such as hepatitis B47 and human papillomavirus48. However, applying lung cancer immune prevention faces challenges due to our limited understanding of the evolving interplay between premalignant/malignant cells and the host’s anti-tumor immunity during the formation and progression of pre-cancerous lesions.
Host immunity continuously evolves during cancer development. Our study revealed a dynamic immune response marked by a transition from innate to adaptive immunity with neoplastic progression. During early LUAD carcinogenesis, the broad, non-specific, and rapidly acting innate response serve as the first line of defense. As cancer evolves, anti-tumor immunity gradually transitions to a more specific and potent adaptive immune response both in quantity (higher level of various adaptive immune-cell infiltration) and quality (more organized TLS) in later-stage lesions. Such a transition has also been observed in precancer evolution in oral squamous cell carcinoma49 and colorectal cancers50. This transition marks the host’s attempt to sustain anti-cancer immune surveillance. However, cancer cells eventually evade immune attacks through mechanisms that include increasing negative regulators (e.g., Tregs and immune checkpoints) and decreasing effectors (e.g., CD8+ T cells)
Our findings support a potential role for immune interception of LUAD precursors to prevent lung cancer development. In keeping with this hypothesis, our group has launched two investigator-initiated immune interception trials: Can-Prevent-Lung (NCT04789681, testing reprogramming primarily of innate immunity through canakinumab --anti-IL1β monoclonal antibody treatment) and IMPRINT-Lung (reprogramming adaptive immunity by the anti-PD1 agent pembrolizumab). The planned interim analysis of the Can-Prevent-Lung trial demonstrated that canakinumab has a good safety profile and promising activity in treating persistent high-risk lung nodules51. These promising early successes mark a crucial step toward immune interception for lung cancer prevention. The results in the current study suggest that while both innate and adaptive immunity exhibit potential for immune interception, targeting innate immunity may be more efficient at earlier stages, whereas targeting adaptive immunity may have advantages in later-stage lesions. One major challenge is to distinguish early versus late-stage lesions without surgical resection and pathological assessment. Advanced technologies, including liquid biopsies and radiomics approaches52, may have the potential to characterize the stage and molecular subtype of lesions for precise immune interception.
Neoplastic progression involves the outgrowth of tumor subclones with reduced immunogenicity, allowing escape from immunosurveillance. In the current study, we observed de-differentiation of epithelial cells during precancerous progression, increased cancer cell stemness, and diminished alveolar epithelial cell identity in later-stage lesions. This phenomenon is consistent with observations in genetically engineered mouse models of LUAD53 and other cancers including glioblastoma54, intestinal tumors54, melanoma55, and breast cancer56. In principle, immunity has evolved to protect stem cells, which are essential for normal development and tissue homeostasis57. Emerging evidence highlights the pivotal role of stemness in immune editing and the evolution of cancer57. In the context of LUAD development, increasing stemness may act as a mechanism driving immune evasion, facilitating the transition of LUAD precursors into invasive tumors. Whereas the clinical translation of cancer stem-cell biology is still in its infancy, targeting pre-cancer stem cells may represent a potential cancer interception strategy57.
The scarcity of resected LUAD precursor specimens has impeded our understanding of early carcinogenesis of LUAD. This limitation has become a bottleneck hindering the trials aimed at preventing progression to invasive LUAD. Our multi-omics study on a large cohort of resected LUAD unveiled a transition from innate to adaptive immune response during the early neoplastic evolution. These findings have provided biologic support for our ongoing immunoprevention trials targeting innate immunity (Can-Prevent-Lung) and adaptive immunity (IMPRINT-Lung) for lung cancer interception. Future studies are warranted to delve into cell-cell interactions, key cytokines, chemokines, and their gradients at distinct stages of early LUAD carcinogenesis and provide novel insights for the development of novel and effective precision interception strategies.
Limitations of the study
One important caveat of the current study, common to most other similar studies, is that all the analyses were based on resected specimens, which only provide single molecular snapshots of the evolutionary process of LUAD. While a linear model of evolution from AAH to AIS, MIA, then to ADC was assumed, whether all AAH lesions progress to AIS, MIA, or ADC, and whether every ADC follows the hypothetical linear evolutionary trajectory are unknown. Understanding how the genomic landscape evolves over time with neoplastic progression and its association with patient outcomes requires longitudinal biopsies throughout the disease course, which is impractical in clinical practice. Future studies using animal models or leveraging longitudinal biopsy specimens from interception trials, such as IMPRINT-Lung (NCT03634241) and Can-Prevent-Lung (NCT04789681), may present good opportunities offer to investigate the temporal changes in molecular features during the neoplastic progression of LUAD.
METHODS
Patient cohort
A total of 473 resected tumor specimens and 111 matched adjacent normal lung tissue samples were obtained from 111 patients presenting with GGO-predominant lesions by LDCT-guided screening or incidental findings, who underwent surgery at New York University, Nagasaki Hospital (Japan) and Zhejiang Cancer Hospital (China) from 2014 to 2019. None of these patients received preoperative chemotherapy or radiotherapy (Supplementary Data 1). 472 specimens were subjected to multi-regional whole exome sequencing. Whole RNA sequencing analysis included a subset of 168 samples and whole genome sequencing included 42 of those specimens, respectively. Available demographic information included patient age at date of specimen collection, age at diagnosis, gender, stated race and ethnicity, smoking status and tumor histology based on two independent pathologists’ review. Written informed consent was obtained from all patients. The analysis was performed using de-identified data under the Institutional Review Boards (IRB) at MD Anderson Cancer Center, New York University, Zhejiang Cancer Hospital and Nagasaki University Graduate School of Biomedical Sciences.
Next-Generation Sequencing
Manual macro-dissection on the H&E slides of FFPE specimens was performed to ensure a minimum of 40% diseased (atypical or malignant) cells in each multi-region sample based on the region of interest (ROI) diagnosed by the pathologists. Samples with lower disease content were excluded from further analyses. Adjacent normal lung tissue (≥2 cm from tumor margin, morphologically negative for malignant cells) from the same patients was used as germ line control. DNA and RNA were extracted using Ionic® purification system, respectively (Purigen Biosystems). The resulting genomic DNA was processed using Twist NGS Library Preparation and Capture Kits (#104175) with the Human Twist Comprehensive Exome Panel and subjected to whole-exome sequencing (WES) on the S4 flow cell of NovaSeq 6000 system (Illumina) running NovaSeq Control Software v1.7.5 at 150nt paired-end with dual 10 index reads. The whole-genome sequencing (WGS) run at 150nt paired-end was performed on NovaSeq6000 sequencer (Illumina) by Novogene. The RNA library was prepared with TruSeq® Stranded Total RNA Library Prep Gold (#20020599) and subjected to one lane of S4 flow cell on NovaSeq 6000 running NovaSeq Control Software v1.7.5 at 101nt paired end with dual 8 index reads. The demultiplex of both runs was performed using bcl2fastq v2.20.0.
Single-Nucleotide Variants (SNVs) detection from WES and WGS
Sequencing reads were quality controlled and trimmed by fastp (v0.23.0)58, then mapped to the human reference sequence GRCh38 (hg38) using the Burrows-Wheeler Aligner (BWA)-MEM algorithm (v0.7.17). Duplicate reads were marked using Picard (v1.67) followed by realignment around known indels and base quality recalibration was performed using GATK 3.7. Somatic mutation calls were performed using Mutect (v1.1.7), Varscan2 (v2.4.2), Strelka2 (v2.9.2), Lancet (v1.1.0), SomaticSniper (v0.7.4), allowing at least 0.02 variant allele frequency and coverage of ≥ 20 in tumor and up to maximum of 0.01 allele frequency and coverage of ≥ 10 in normal samples. First, we manually curated a trustworthy list of mutations by combining WUST cancer mutations and TCGA_LUAD mutation profiles, to which SNVs matched are preserved from further filtering. Then those variants detected by at least two above callers were selected, and suspicious artifacts due to sequencing errors in FFPE samples were marked by MicroSEC59 and SOBDetector60. Finally, only single-nucleotide variants (SNVs) 1) detected by at least two callers and 2) not marked as suspicious artifacts and 3) excluded from dbSNP146 and 4) tumor allele frequency >=0.04 and LOD >=10 or included in cosmic database containing census genes were selected. And then the resulting list of somatic SNVs were annotated by multiple databases using Ensembl Variant Effect Predictor (VEP).
Estimation of telomere length
Telomerehunter (v1.1.0)61 was applied to quantify telomere content and composition using 10 telomere variant repeats including TCAGGG, TGAGGG, TTGGGG, TTCGGG, TTTGGG, ATAGGG, CATGGG, CTAGGG, GTAGGG and TAAGGG in matched tumor and normal samples.
Identification of chromosomal instability related events
Tumor purity was inferred using TITAN framework62 and ASCAT63, somatic copy number alterations (SCNAs) were detected using CNVkit64. The allelic copy number profiles and corresponding ploidy of tumor samples were generated applying “FACETS” packages65 using the matched germline data. Whole-Genome Doubling (WGD) was determined (p-value <0.001 for samples with ploidy ≤3) based on random simulation test of WGD. Each sample, s was represented as an aberration profile of major and minor allele copy numbers at chromosome arm resolution. From which Ns, the total number of aberrations (relative to diploid) and Ps, the probabilities of loss/gain for major and minor allele at each chromosome arm were calculated. 10,000 simulations were run for each sample. In each simulation, Ns sequential aberrations, based on Ps, were applied to a diploid profile. A P-value for genome doubling was obtained by counting the percentage of simulations in which the proportion of chromosome arms with a major allele copy number ≥2 was higher than that observed in the sample. The weighted Genome Instability Index (wGII) was calculated to estimate the proportion of the genome with aberrant copy number compared with the median ploidy, weighted on per-chromosome length basis. The mosaic chromosomal aneuploidies were identified using MAD-seq66, based on fitting a mixture model of alternate allele frequencies (AAFs) at heterozygous loci. The subclonal architecture reconstruction was inferred by CliP using a penalized likelihood model67.
Detection of structural variants
Five variant callers were used to identify somatically acquired structural variants from matched tumor and germline whole genome sequencing data: DELLY68, LUMPY69, BRASS (BReakpoint AnalySiS) (https://github.com/cancerit/BRASS/), Manta70 and SVABA71. These were merged into a final call set using SURVIVOR72, a graph-based algorithm to identify overlapping breakpoint junctions across different callers, accepting all structural-variant calls made by two or more of the five algorithms to obtain best trade-off between sensitivity and specificity. “gGnome” package was used to graph the genomic intervals.
Bulk RNAseq processing and gene expression matrix construction
Initially, raw sequencing data underwent quality control and adapter trimming using FASTP (v0.20.0). Subsequently, ribosomal RNAs (rRNAs) were eliminated using SortMeRNA73, followed by mapping to human transcriptome reference (hg38) using STAR aligner. The expected transcript counts were quantified using RSEM (v1.3.3). Then outliner samples were removed via voom (voomWithQualityWeights) and RSEM transcripts were filtered with a minimum of two counts in all samples and variance stabilized transform (VST, DESeq2) was applied. Batch effects were assessed with Principal Component Analysis (PCA) and removed with LIMMA via linear modeling (removeBatchEffect) using DESeq2 (v1.38.3). The normalization of gene expression matrix was performed by subtracting the median of each transcript across all samples, and only transcripts mapped to coding genes (GENCODE -human release 38) were selected for downstream analysis.
Pseudotime analysis of specimens diagnosed with different histological stages
The trajectory paths were inferred using “tradeSeq”74 and “monocle” packages74 on selected genes with high variance and expression across all the samples diagnosed with different pathological stages.
Tumor heterogeneity analysis using transcriptomic profiling
The transcriptome-based ITH was estimated using nJSD, an entropy-based distance metric between two networks of tumor and matched normal samples, with Jensen-Shannon Divergence (JSD)32.
Deconvolution of tumor infiltrating immune cells
The content of tumor infiltrating NK cells, monocytes, cytotoxicity innate lymphoid cells, neutrophils, eosinophils, activated dendritic cells, B cells, regulatory T cells, CD8+ T cells were estimated using Consensus75. The mast cells resting, plasma cells were calculated using Cibersort76. The infiltration of naïve CD4+ T cells, memory CD4+ T cells and activated myeloid dendritic cells were inferred using xCell77 based on bulk RNAseq expression matrix.
Gene Set Variation Analysis (GSVA)
The normalized gene expression matrix was then processed to produce ssGSEA enrichment scores by GSVA, which calculates per sample overexpression level of a particular gene list by comparing the ranks of the genes in that list with those of all other genes78. A list of gene sets that are functionally associated with proliferation, cancer cell stemness, alveolar differentiation, tertiary lymphoid structures (TLS), innate immunity and adaptive immunity were used as gene signatures (Supplementary Data 3). Differential expression at the gene set level was assessed using a multivariate linear model and the empirical Bayes method in LIMMA.
Assessment of lymph nodes aggregates (LA) and tertiary lymphoid structures (TLS) in H&E-stained image
The archived Hematoxylin and Eosin (H&E) stained pathology slides were first scanned at 20X magnification using Aperio AT2 scanner and uploaded to the digital image analysis software HALO-AI-v3.5 (Indica Labs) (https://indicalab.com/halo-ai/). Then the deep learning tissue classification algorithm was applied to annotate some representative ROIs under the pathologist’s supervision, and the entire tissue section was classified into LA/TLS versus lung tissue, finally LA/TLS were manually assessed for tissue classification accuracy and the numbers of lymph nodes aggregates including TLSs on individual slide were quantified.
Statistical analysis
All statistical analyses were performed using R software version 4.1.0. Violin plots were generated using “geom_violin” function in ggplot2 (v.0.9.1) to represent data point density along the Y-axis, and the “stat_summary” function from ggplot2 (v.0.9.1) was used to calculate the mean as the center point. Differences in TMB, fraction of clonal mutations, relative telomere length, genomic instability, proliferation, cancer cell stemness and normalized gene expression, immune cell infiltration, TLS scores between the lesions of different stages were assessed using the Kruskal–Wallis H test. Two-sided Spearman’s correlation coefficient was used to access the association between two variables. Confidence intervals for proportions were computed using a 2-sample z-test without continuity correction. All tests were carried out at the 5% significance level with Benjamini-Hochberg correction for multiple testing.
ACKNOWLEDGEMENTS
This study was supported in part by the MD Anderson Precancer Atlas through the institution’s Strategic Research Initiative Development (STRIDE) program, National Cancer Institute of the National Institute of Health Research Project Grant (R01CA234629-01), the AACR-Johnson & Johnson Lung Cancer Innovation Science Grant (18-90-52-ZHAN), the Specialized Program of Research Excellence (SPORE) of lung cancer, the MD Anderson Physician Scientist Program, the MD Anderson Lung Cancer Moon Shot Program, MD Anderson Lung Cancer Interception Program, MD Anderson Lung Cancer Genomics Program. We thank MD Anderson Cancer Center’s Advanced Technology Genomics Core (ATGC) (CA016672, NIH1S10OD024977-01) for performing WES and RNAseq profiling. We thank MDACC’s Flow Cytometry and Cellular Imaging Core Facility (FCCICF) for providing the resource for H&E image scanning. We also thank MDACC’s Biospecimen Extraction Facility (BEF) and Purigen Biosystems (purigenbio.com) for performing DNA/RNA extraction. We appreciate Rong Yao, Jinzhen Chen, Eric Sisson, Stan Bujnowski for providing excellent technical support for the high-performance cluster (HPC) resource (http://hpcweb.mdanderson.edu/citing.html). We thank Drs. Shawna M. Hubert, Ling-Zhi Hong and Run-Zhe Chen for their coordination of clinical samples, Drs. Jian-Rong Li and Wei Hong from Baylor college of medicine, Chia-Chin Wu, Xiao-Gang Wu from MD Anderson Cancer Center for constructive suggestions. We also thank Mrs. Sophie Rydin, a fearless cancer fighter for her generous support to lung cancer prevention research.
DECLARATION OF INTERESTS
J.J.Z. reports research funding from Merck, Johnson and Johnson, Novartis, Summit, Hengenix and consultant fees from BMS, Johnson and Johnson, AstraZeneca, Geneplus, OrigMed, Innovent, Varian, Catalyst outside the submitted work. I.I.W reports Honoraria from Genentech/Roche, Bayer, Bristol-Myers Squibb, Astra Zeneca/Medimmune, Pfizer, HTG Molecular, Asuragen, Merck, GlaxoSmithKline, Guardant Health, Oncocyte, Flame, and MSD; Research support from Genentech, Oncoplex, HTG Molecular, DepArray, Merck, Bristol-Myers Squibb, Medimmune, Adaptive, Adapt immune, EMD Serono, Pfizer, Takeda, Amgen, Karus, Johnson & Johnson, Bayer, Iovance, 4D, Novartis, and Akoya. J.V.H. reports honorariums from AstraZeneca, Boehringer-Ingelheim, Catalyst, Genentech, GlaxoSmithKline, Guardant Health, Foundation medicine, Hengrui Therapeutics, Eli Lilly, Novartis, Spectrum, EMD Serono, Sanofi, Takeda, Mirati Therapeutics, BMS, BrightPath Biotherapeutics, Janssen Global Services, Nexus Health Systems, EMD Serono, Pneuma Respiratory, Kairos Venture Investments, Roche and Leads Biolabs. D.E.G. reports research funding from Astra-Zeneca, BerGenBio, Karyopharm, and Novocure; stock ownership in Gilead; consultant/advisory fees from Abbvie, Astra-Zeneca, Catalyst Pharmaceuticals, Daiichi-Sankyo, Elevation Oncology, Janssen Scientific Affairs, LLC, Jazz Pharmaceuticals, Regeneron Pharmaceuticals, and Sanofi; and serving as co-founder and chief scientific officer of OncoSeer Diagnostics, Inc. S.H. reports consulting fees from Guardant Health and AstraZeneca. S.M.D serves on the Scientific Advisory Boards for Early Diagnostics Inc. and LungLife AI, Inc. and has received research funding from Johnson & Johnson Lung Cancer Initiative and Novartis. The other authors declare no competing interests.
Footnotes
Supplementary Files
DATA AVAILABILITY
The raw sequence data has been deposited at European Genome-phenome Archive (EGA), which is hosted by The European Bioinformatics Institute (EBI) and the Centre for Genomic Regulation (CRG) under the accession code: EGAD50000000395 (RNAseq), EGAD50000000396 (WGS), EGAD50000000397 (WES), EGAD00001004960 (WES). Further information about EGA is available at https://ega-archive.org. All other data may be found within the main manuscript or Supplementary Information or available from the authors upon request.
REFERENCES
- 1.Aberle DR, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365, 395–409 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart BW, Wild C, International Agency for Research on Cancer, World Health Organization. World cancer report 2014. International Agency for Research on Cancer WHO Press; (2014). [Google Scholar]
- 3.Detterbeck FC, Homer RJ. Approach to the ground-glass nodule. Clin Chest Med 32, 799–810 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Kodama K, et al. Treatment strategy for patients with small peripheral lung lesion(s): intermediate-term results of prospective study. Eur J Cardiothorac Surg 34, 1068–1074 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Mun M, Kohno T. Efficacy of thoracoscopic resection for multifocal bronchioloalveolar carcinoma showing pure ground-glass opacities of 20 mm or less in diameter. J Thorac Cardiovasc Surg 134, 877–882 (2007). [DOI] [PubMed] [Google Scholar]
- 6.Ohtsuka T, Watanabe K, Kaji M, Naruke T, Suemasu K. A clinicopathological study of resected pulmonary nodules with focal pure ground-glass opacity. Eur J Cardiothorac Surg 30, 160–163 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Sroufe R, Kong FM. Triaging early-stage lung cancer patients into non-surgical pathways: who, when, and what? Transl Lung Cancer R 4, 438–447 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black WC, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med 371, 1793–1802 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomonaga N, et al. Analysis of Intratumor Heterogeneity of EGFR Mutations in Mixed Type Lung Adenocarcinoma. Clin Lung Cancer 14, 521–526. [DOI] [PubMed] [Google Scholar]
- 10.Nambu A, et al. Focal area of ground-glass opacity and ground-glass opacity predominance on thin-section CT: discrimination between neoplastic and non-neoplastic lesions. Clin Radiol 60, 1006–1017 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med 321, 129–135 (1989). [DOI] [PubMed] [Google Scholar]
- 12.van Zandwijk N, Dalesio O, Pastorino U, de Vries N, van Tinteren H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the EUropean Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. Journal of the National Cancer Institute 92, 977–986 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Peto R, et al. Randomised trial of prophylactic daily aspirin in British male doctors. British medical journal 296, 313–316 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slatore CG, Littman AJ, Au DH, Satia JA, White E. Long-term use of supplemental multivitamins, vitamin C, vitamin E, and folate does not reduce the risk of lung cancer. American journal of respiratory and critical care medicine 177, 524–530 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumberg J, Block G. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study in Finland. Nutrition reviews 52, 242–245 (1994). [DOI] [PubMed] [Google Scholar]
- 16.Cook NR, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. Jama 294, 47–55 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Omenn GS, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. Journal of the National Cancer Institute 88, 1550–1559 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Karp DD, et al. Randomized, double-blind, placebo-controlled, phase III chemoprevention trial of selenium supplementation in patients with resected stage I non-small-cell lung cancer: ECOG 5597. J Clin Oncol 31, 4179–4187 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lippman SM, et al. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. Journal of the National Cancer Institute 93, 605–618 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Hu X, et al. Multi-region exome sequencing reveals genomic evolution from preneoplasia to lung adenocarcinoma. Nat Commun 10, 2978 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X, et al. Evolution of DNA methylome from precancerous lesions to invasive lung adenocarcinomas. Nat Commun 12, 687 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang C, et al. Genomic Landscape and Immune Microenvironment Features of Preinvasive and Early Invasive Lung Adenocarcinoma. J Thorac Oncol 14, 1912–1923 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen K, et al. Multiomics Analysis Reveals Distinct Immunogenomic Features of Lung Cancer with Ground-Glass Opacity. Am J Respir Crit Care Med 204, 1180–1192 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dejima H, et al. Immune evolution from preneoplasia to invasive lung adenocarcinomas and underlying molecular features. Nat Commun 12, 2722 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao B, et al. Genomic landscape and evolution of arm aneuploidy in lung adenocarcinoma. Neoplasia 23, 870–878 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burrell RA, et al. Replication stress links structural and numerical cancer chromosomal instability. Nature 494, 492–496 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Belzen I, Schonhuth A, Kemmeren P, Hehir-Kwa JY. Structural variant detection in cancer genomes: computational challenges and perspectives for precision oncology. NPJ Precis Oncol 5, 15 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nat Rev Mol Cell Biol 11, 220–228 (2010). [DOI] [PubMed] [Google Scholar]
- 29.O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol 11, 171–181 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aviv A, Anderson JJ, Shay JW. Mutations, Cancer and the Telomere Length Paradox. Trends Cancer 3, 253–258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramaker RC, et al. RNA sequencing-based cell proliferation analysis across 19 cancers identifies a subset of proliferation-informative cancers with a common survival signature. Oncotarget 8, 38668–38681 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park Y, Lim S, Nam JW, Kim S. Measuring intratumor heterogeneity by network entropy using RNA-seq data. Sci Rep 6, 37767 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung H, et al. DNA methylation loss promotes immune evasion of tumours with high mutation and copy number load. Nat Commun 10, 4278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warrier NM, Kelkar N, Johnson CT, Govindarajan T, Prabhu V, Kumar P. Understanding cancer stem cells and plasticity: Towards better therapeutics. Eur J Cell Biol 102, 151321 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Liu Q, Lei J, Zhang X, Wang X. Classification of lung adenocarcinoma based on stemness scores in bulk and single cell transcriptomes. Comput Struct Biotechnol J 20, 1691–1701 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han G, et al. An atlas of epithelial cell states and plasticity in lung adenocarcinoma. Nature, (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egen JG, Ouyang W, Wu LC. Human Anti-tumor Immunity: Insights from Immunotherapy Clinical Trials. Immunity 52, 36–54 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. Myeloid dendritic cells: Development, functions, and role in atherosclerotic inflammation. Immunobiology 220, 833–844 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Chen P, et al. Pathomic Features Reveal Immune and Molecular Evolution from Lung Preneoplasia to Invasive Adenocarcinoma. Mod Pathol, 100326 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang T, et al. Genomic and evolutionary classification of lung cancer in never smokers. Nat Genet 53, 1348–1359 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakelee HA, et al. Lung cancer incidence in never smokers. J Clin Oncol 25, 472–478 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umar A, Loomans-Kropp HA. Immuno-Interception for Patients with High-Risk Cancer. Cancer Prev Res (Phila) 13, 493–496 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Blackburn EH. Cancer interception. Cancer Prev Res (Phila) 4, 787–792 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Chen H, et al. Genomic and immune profiling of pre-invasive lung adenocarcinoma. Nat Commun 10, 5472 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, et al. Deciphering cell lineage specification of human lung adenocarcinoma with single-cell RNA sequencing. Nat Commun 12, 6500 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yanagawa J, et al. Single-Cell Characterization of Pulmonary Nodules Implicates Suppression of Immunosurveillance across Early Stages of Lung Adenocarcinoma. Cancer Res 83, 3305–3319 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Hu C, Xie X, Qi L, Li C, Li S. Immune Checkpoint Inhibitors in HBV-Caused Hepatocellular Carcinoma Therapy. Vaccines (Basel) 11, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang JW, Hung CF, Huh WK, Trimble CL, Roden RB. Immunoprevention of human papillomavirus-associated malignancies. Cancer Prev Res (Phila) 8, 95–104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johnson SD, Levingston C, Young MRI. Premalignant Oral Lesion Cells Elicit Increased Cytokine Production and Activation of T-cells. Anticancer Res 36, 3261–3270 (2016). [PMC free article] [PubMed] [Google Scholar]
- 50.Cui GL. Immune battle at the premalignant stage of colorectal cancer: focus on immune cell compositions, functions and cytokine products. Am J Cancer Res 10, 1308–1320 (2020). [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J. The Interim Analysis of Can-Prevent-Lung Trial: Canakinumab for The Prevention of Lung Cancer. In: IASLC-WCLC 2023) (2023). [Google Scholar]
- 52.Saad MB, et al. Predicting benefit from immune checkpoint inhibitors in patients with non-small-cell lung cancer by CT-based ensemble deep learning: a retrospective study. Lancet Digit Health 5, e404–e420 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dost AFM, et al. Organoids Model Transcriptional Hallmarks of Oncogenic KRAS Activation in Lung Epithelial Progenitor Cells. Cell Stem Cell 27, 663–678 e668 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwitalla S, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 152, 25–38 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Landsberg J, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature 490, 412–+ (2012). [DOI] [PubMed] [Google Scholar]
- 56.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clarke MF. Clinical and Therapeutic Implications of Cancer Stem Cells. N Engl J Med 380, 2237–2245 (2019). [DOI] [PubMed] [Google Scholar]
- 58.Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ikegami M, et al. MicroSEC filters sequence errors for formalin-fixed and paraffin-embedded samples. Commun Biol 4, 1396 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diossy M, et al. Strand Orientation Bias Detector to determine the probability of FFPE sequencing artifacts. Brief Bioinform 22, (2021). [DOI] [PubMed] [Google Scholar]
- 61.Feuerbach L, et al. TelomereHunter - in silico estimation of telomere content and composition from cancer genomes. Bmc Bioinformatics 20, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ha G, et al. TITAN: inference of copy number architectures in clonal cell populations from tumor whole-genome sequence data. Genome Res 24, 1881–1893 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Loo P, et al. Allele-specific copy number analysis of tumors. Proc Natl Acad Sci U S A 107, 16910–16915 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Talevich E, Shain AH, Botton T, Bastian BC. CNVkit: Genome-Wide Copy Number Detection and Visualization from Targeted DNA Sequencing. PLoS Comput Biol 12, e1004873 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen R, Seshan VE. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res 44, e131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kong Y, et al. Detecting, quantifying, and discriminating the mechanism of mosaic chromosomal aneuploidies using MAD-seq. Genome Res 28, 1039–1052 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang YJ, Yu KX, Zhu HT, Wang WY. CliP: A model-based method for subclonal architecture reconstruction using regularized maximum likelihood estimation. Cancer Res 80, (2020). [Google Scholar]
- 68.Rausch T, Zichner T, Schlattl A, Stutz AM, Benes V, Korbel JO. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics 28, i333–i339 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Layer RM, Chiang C, Quinlan AR, Hall IM. LUMPY: a probabilistic framework for structural variant discovery. Genome Biol 15, R84 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen X, et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 32, 1220–1222 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Wala JA, et al. SvABA: genome-wide detection of structural variants and indels by local assembly. Genome Res 28, 581–591 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeffares DC, et al. Transient structural variations have strong effects on quantitative traits and reproductive isolation in fission yeast. Nat Commun 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kopylova E, Noe L, Touzet H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics 28, 3211–3217 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Trapnell C, et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol 32, 381–386 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jimenez-Sanchez A, Cast O, Miller ML. Comprehensive Benchmarking and Integration of Tumor Microenvironment Cell Estimation Methods. Cancer Res 79, 6238–6246 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling Tumor Infiltrating Immune Cells with CIBERSORT. Methods Mol Biol 1711, 243–259 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol 18, 220 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. Bmc Bioinformatics 14, 7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw sequence data has been deposited at European Genome-phenome Archive (EGA), which is hosted by The European Bioinformatics Institute (EBI) and the Centre for Genomic Regulation (CRG) under the accession code: EGAD50000000395 (RNAseq), EGAD50000000396 (WGS), EGAD50000000397 (WES), EGAD00001004960 (WES). Further information about EGA is available at https://ega-archive.org. All other data may be found within the main manuscript or Supplementary Information or available from the authors upon request.