Abstract
Helicobacter hepaticus, which induces chronic hepatitis and typhlocolitis in susceptible mouse strains, produces a cytolethal distending toxin (CDT) consisting of CdtA, CdtB, and CdtC. A cdtB-deficient H. hepaticus isogenic mutant (HhcdtBm7) was generated and characterized for colonization parameters in four intestinal regions (jejunum, ileum, cecum, and colon) of outbred Swiss Webster (SW) mice. Inactivation of the cdtB gene abolished the ability of HhcdtBm7 to colonize female mice at both 8 and 16 weeks postinfection (wpi), whereas HhcdtBm7 colonized all of four intestinal regions of three of five males at 8 wpi and then was eliminated by 16 wpi. Wild-type (WT) H. hepaticus was detected in the corresponding intestinal regions of both male and female mice at 8 and 16 wpi; however, colonization levels of WT H. hepaticus in the cecum and colon of male mice were approximately 1,000-fold higher than in females (P < 0.0079) at 16 wpi. Infection with WT H. hepaticus, but not HhcdtBm7, at 8 wpi was associated with significantly increased mRNA level of ileal and cecal gamma interferon (IFN-γ) in females (P < 0.016 and 0.031 between WT H. hepaticus-infected and sham-dosed females, respectively). In contrast, the mRNA levels of IFN-γ were significantly higher in the colon (P < 0.0079) and trended to be higher in the cecum (P < 0.15) in the HhcdtBm7-colonized male mice versus the sham-dosed controls at 8 wpi. In addition, mRNA levels of ileal IFN-γ were significantly higher in the control females than males at 8 wpi (P < 0.016). There were significantly higher Th1-associated immunoglobulin G2a (IgG2a), Th2-associated IgG1 and mucosal IgA (P < 0.002, 0.002, 0.002, respectively) responses in the mice infected with WT H. hepaticus when compared to HhcdtBm7 at 16 wpi. Colonic interleukin-10 (IL-10) expressions at 16 wpi were significantly lower in both female and male mice colonized by WT H. hepaticus or in males transiently colonized through 8 wpi by HhcdtBm7 versus control mice (P < 0.0159). These lines of evidence indicate that (i) H. hepaticus CDT plays a crucial role in the persistent colonization of H. hepaticus in SW mice; (ii) SW female mice are more resistant to H. hepaticus colonization than male mice; (iii) there was persistent colonization of WT H. hepaticus in cecum, colon, and jejunum but only transient colonization of H. hepaticus in the ileum of female mice; (iv) H. hepaticus colonization was associated with down-regulation of colonic IL-10 production.
Multiple pathogenic gram-negative bacteria produce cytolethal distending toxins (CDTs) (reviewed in references 31 and 40). These CDTs are generally tripartite holotoxins, of which subunit B contains an active domain with DNaseI-like activity, whereas subunits A and C appear to function as accessory proteins for the delivery of subunit B into target cells (25). However, a recent study has reported that the Salmonella enterica serovar Typhi CdtB alone has CDT activity, given that there are no homologs of cdtA and cdtC identified within its genome (18). Upon entry into the cytosol, CdtB is translocated into the nucleus, where it causes limited damage to host cell DNA and thereby triggers the DNA damage repair mechanism (26). CDT causes cell cycle arrest and subsequent cellular distension and eventual cell death in cultured mammalian cells. Current in vivo pathogenesis data on the role of bacterial CDT have been inconsistent. The CDT activity in Haemophilus ducreyi and Campylobacter jejuni was reported to be dispensable for colonization and has minimal contribution to pathology in rabbits (37) and scid mice (32), respectively. However, we recently demonstrated that CDT-deficient C. jejuni persistently colonized NF-κB-deficient mice, while its colonization persisted through 2 months postinfection (p.i.) but was eliminated by 4 months p.i. from wild-type (WT) mice (14). In addition, inactivation of cdtB in H. hepaticus significantly attenuated the severity of typhlocolitis in interleukin-10−/− (IL-10−/−) mice, although this mutation appeared to have no effect on the colonization of the mutant (44). Thus, the type of bacterial pathogen and the genetic background of experimental hosts influence colonization and clinical manifestations of infection with CDT-deficient bacterial mutants.
Helicobacter hepaticus was originally isolated from the livers, ceca, and colons of aged A/JCr mice that were controls for a long-term chemical carcinogenesis study (12, 41). It has been documented that the mouse cecum and colon are the primary sites for H. hepaticus colonization (13). In contrast, the colonization status of H. hepaticus in the ileum and small intestine of mice has not been characterized. C. jejuni, which shares 953 protein orthologs with H. hepaticus (50.8% of the predicted open reading frames) (38), colonizes proximal and distal small intestine, cecum, and large intestine in chicks and mice (3, 10, 28). A recent study reported that C. jejuni colonization in the jejunum of humans was associated with immunoproliferative disease of the small intestine (27). Thus, characterization of H. hepaticus colonization in the small intestine of mice will increase our understanding of H. hepaticus pathogenesis.
Natural and experimental infection studies have established that H. hepaticus in susceptible strains of mice causes chronic active hepatitis, typhlocolitis, and hepatocellular carcinoma (15, 19, 20, 41, 42). H. hepaticus-induced liver lesions are more severe in infected male A/JCr mice (13, 33); however, the gender factors contributing to this difference are not understood. In addition, infection by H. hepaticus in immune dysregulated mice induces intestinal pathology that mimics some features of inflammatory bowel disease (IBD) in humans (4, 6, 7, 24). Severity of H. hepaticus-induced typhlocolitis is associated with elevated production of proinflammatory cytokines (e.g., IL-12, tumor necrosis factor α [TNF-α], gamma interferon [IFN-γ]) (24, 29, 42).
Humans are infected by multiple enterohepatic helicobacters that have pathogenic potential (reviewed in reference 11). Human enterohepatic helicobacters H. canis, H. cinaedi, and H. pullorum also contain CDT (5, 39, 43). The immune responses of outbred mice to pathogenic infection should mimic human immune responses and thus are useful models for understanding host-pathogen interactions and identifying potential bacterial virulence factors, particularly those influencing colonization. In this study, we investigated the role of H. hepaticus CDT in intestinal colonization using outbred Swiss Webster (SW) mice. In addition, the colonization status of H. hepaticus in the small intestine was determined; selected cytokine profiles were characterized and compared between WT and CDT-deficient H. hepaticus in both female and male SW mice.
MATERIALS AND METHODS
Bacterial strains, growth media, and conditions.
Escherichia coli strain Top10 was used as a recipient for cloning, mutagenesis, and plasmid propagation and was cultured in Luria-Bertani (LB) broth or agar supplemented with antibiotics ampicillin (50 μg/ml) and chloramphenicol (25 μg/ml) when appropriate. Wild-type (WT) H. hepaticus strain 3B1 (12) was cultured on blood agar (Remel, Lexignton, Kans.) for 2 to 3 days under microaerobic conditions (10% H2, 10% CO2, 80% N2). Chloramphenicol-resistant H. hepaticus mutants were selected on tryptic soy agar supplemented with 5% sheep blood and 25 μg/ml of chloramphenicol (all from Sigma, St. Louis, MO).
Cloning of selected cytokines and GAPDH cDNA.
The primers used for cloning IL-10, IL-6, TNF-α, and IFN-γ cDNA are listed in Table 1, whereas the primers for the GAPDH gene were purchased from Applied Biosystems (Foster City, CA). cDNA was synthesized from the RNA template (2 μg) with a reverse primer for each of IL-10, IL-6, TNF-α, IFN-γ, and GAPDH genes and Superscript RNaseH− reverse transcriptase according to the supplier's protocol for first-strand cDNA synthesis (Invitrogen, Carlsbad, CA). Amplicons were cloned into a TOPO vector following the supplier's instruction (Invitrogen), and plasmid DNA was prepared using Aquick Mini kit (QIAGEN Inc., Valencia, CA). The identity of recombinant plasmids was confirmed by DNA sequencing in the ABI 310 sequencer (Applied Biosystems). Concentrations of the plasmid DNA were determined using a spectrophotometer GeneQuan Pro (Amersham Biosciences, Piscataway, NJ).
TABLE 1.
The origin and sequences of the respective primersa
| Primer | Sequences (5′ to 3′) | Orientationb | Sourcec |
|---|---|---|---|
| ZMG38 | (578)TTCACGATATATGCGATAG | Sense | AF163667 |
| ZMG41 | (1253)GACGCGATGCCTCTGTAGCA | Antisense | AF163667 |
| PEP | (207)GACGAATGCTTAGGCTGAT | Antisense | AF163667 |
| CdtCF | (1761)TAAGAAGCCTTGCAACAG | Sense | AF163667 |
| RTF1 | (577)TTCACGATATATGCGATAG | Sense | AF163667 |
| RTR1 | (2018)TTGCACCGCAGAACTTGTTGT | Antisense | AF163667 |
| TM1 | (2263)AGATTGCTTTTGCGCACAAACTCA | Antisense | AF163667 |
| TM2 | (2326)ACTCGATAATTTTGCGAT | Antisense | AF163667 |
| RTF2 | (1)ATGAAAGAGACTTTATTGCTTCA | Sense | AE017148 |
| RTR2 | (290)AGCCTGTGCATACCCTCATA | Antisense | AE017148 |
| mIL-6 | (32)ATGAAGTTCCTCTCTGCAAGAGACT | Sense | X54542 |
| (667)CTAGGTTTGCCGAGTAGATCTCA | Antisense | ||
| mIL-10 | (77)TGCCTGGCTCAGCACTGCTATGCT | Sense | NM_010548 |
| (605)TTCATTTTGATCATCATGTATGCT | Antisense | ||
| IFN-γ | (110)ATGAACGCTACACACTGCATCT | Sense | NM_008337 |
| (572)AGCGACTCCTTTTCCGCTTCCTGA | Antisense | ||
| TNF-α | (157)ATGAGCACAGAAAGCATGATC | Sense | NM_013693 |
| (864)TCACAGAGCAATGACTCCAA | Antisense |
All the primers were designed in this study. The numbers in parentheses denote the start nucleotide of each of the primers in the published nucleotide sequences.
The orientation of the primers to the coding strand of each of the genes.
The accession numbers of the respective genes in the sequence databases of the National Center for Biotechnology Information.
Real-time quantification of H. hepaticus, HH1450, and selected cytokines.
Chromosomal DNA from cultured bacteria was prepared using a High Pure PCR Template kit according to the manufacturer's protocol (Roche Applied Science, Indianapolis, IN). Total DNA and RNA from jejunum, ileum, cecum, and colon were isolated using Trizol reagents following the supplier's procedure (Invitrogen). The numbers of H. hepaticus in each of the intestinal segments were determined by real-time quantitative PCR (Q-PCR) in the Prism Sequence Detection System 7700 (Applied Biosystems) as described elsewhere (17).
For cytokine mRNA quantification, 5 μg of total RNA from samples were converted into cDNA using a High Capacity cDNA Archive kit following the supplier's recommendation (Applied Biosystems). The cDNA levels for IL-6, IL-10, TNF-α, and IFN-γ mRNA were measured by Q-PCR using commercial primers and probes for each of the aforementioned murine cytokines. Briefly, a 25-μl mixture contained 5 μl of cDNA (in duplicate), 1.25 μl of a commercial 20× primer-probe solution (Applied Biosystems), 12.5 μl of 2× master mix (Applied Biosystems), and 6.25 μl of double-distilled H2O. Concentrations of 10, 100, 1000, 104, 105, and 106 copies of recombinant plasmids containing the respective cytokine cDNA inserts were used to generate standard curves. The copy number of each cytokine mRNA among the samples was normalized as copies of each cytokine transcript per 106 copies of GAPDH transcripts.
Transcript levels of H. hepaticus HH1450, which is located immediately downstream of the H. hepaticus cdt operon (38), were measured as described above for murine cytokine quantification with some modifications. Q-PCR was carried out using 2× SyBr Green I PCR master mix (Applied Biosystems) and 500 nM of each of primers RTF2 and RTR2 (Table 1). In addition, the plasmid pVBY9 containing HH1450 was used to generate a standard curve of 10 to 107 copies (45).
Determination of the transcriptional terminator of the cdt operon by reverse transcription-PCR (RT-PCR).
Total RNA from H. hepaticus was isolated using RNAeasy minikit, including a step of DNase treatment according to the supplier's instructions (QIAGEN Inc.). cDNA was synthesized from the RNA template (4 μg) with the respective reverse primer (either RTR1, TM1, or TM2) and Superscript RNaseH- reverse transcriptase according to the supplier's protocol for first-strand cDNA synthesis (Invitrogen). Subsequently the reaction mixture was incubated at 70°C for 10 min, 5 μl of which was then subjected to conventional PCR.
PCR.
The origins of the individual PCR primers used in this study are presented in Table 1. A 50-μl volume of PCR contained the following: 10 to 50 ng of DNA template, 1 × commercial buffer (Roche Applied Science), 100 μg/ml bovine serum albumin (BSA), 500 nM each of forward and reverse primers, and 2.5 units of High Fidelity DNA polymerase (Roche Applied Science). A thermocycling program of 35 cycles in a Thermocycler Genius (Technie Incorporated, Princeton, NJ) was denaturation at 94°C for 1 min, followed by annealing at 50°C to 60°C (based on the respective primers) for 30 s and extension at 72°C for 1 min.
Primer extension.
The primer PEP was labeled at 37°C for 15 min in the mixture (5 μl) containing 1 μl of 5× forward buffer (Invitrogen), 20 ng of PEP, 10 μCi of [γ-33P]ATP (NEN Life Science Products, Boston, MA), 2 units of T4 polynucleotide kinase. The labeled primer was mixed with 5 μl of total H. hepaticus RNA (5 μg) and incubated at 70°C for 10 min; in the control reaction, 1 μl of DNase-free RNase (Roche Applied Science) was added. Primer extension was performed at 37°C for 1 h in a 20-μl volume containing 10 μl of the primer-RNA mixture, 4 μl of 5× first-strand buffer (Invitrogen), 2 μl of 0.1 mM dithiothreitol, 2 μl of 1 mM deoxynucleoside triphosphate solution (dATP, dCTP, dGTP, and dTTP), and 200 units of Superscript RNaseH−reverse transcriptase. Five microliters of sequencing loading dye was added to the reaction mixture. Two microliters of the resulting samples along with the PEP-primed sequencing reaction for plasmid pVBY9 were analyzed on a 5% polyacrylamide sequencing gel.
Minitransposon mutagenesis.
Minitransposon containing chloramphenicol acetyltranferase gene (cat) was constructed using a kit according to the supplier's procedure (Epicenter Technologies, Madison, WI). Briefly, the cat cassette was excised with HincII as previously described (16) and ligated into a HincII site of construction vector pMOD-2. The cat-containing recombinants resistant to ampicillin (Apr) and chloramphenicol (Cmr) were selected and then were subjected to further characterization by DNA sequencing. The resultant Cmr-containing pMOD (namely pMODCm-4) was used to generate the Cmr-minitransposon (referred to as TnCm4) by PCR with primers PF and PR (Epicenter Technologies). The cdtB in plasmid pVBY9 was inactivated using TnCm4 and the in vitro system according to the supplier's recommendation (Epicenter Technologies). Transformants with Apr and Cmr were selected, and the cdtB-inactivated plasmids were screened by PCR with primers ZMG38 and ZMG41 (Table 1), followed by DNA sequencing. The resultant plasmid pVBYMut7 was used for generating isogenic H. hepaticus mutants.
Construction of isogenic mutants.
WT H. hepaticus cells from frozen stock were cultured on blood agar for 2 to 3 days and then washed twice with ice-cold buffer containing 15% (vol/vol) glycerol and 7% (wt/vol) sucrose followed by resuspension in the same buffer. A 50-μl aliquot of cells was mixed with 2 μg of plasmid pVBYMut7 DNA, followed by electroporation (2.5 kV, 12.5 kV/cm) in an Escherichia coli pulser (Bio-Rad, Hercules, CA). Cells were incubated at 37°C for 2 days on blood agar and scraped in Brucella broth containing 25% glycerol, followed by application onto blood agar plates containing 25 μg/ml of chloramphenicol. Isogenic mutants of H. hepaticus isolated after 5 to 10 days' growth under microaerobic conditions were characterized by PCR and DNA sequencing. Isogenic H. hepaticus mutant A7 (HhcdtBm7), which represented the mutation in the cdtB gene, was selected for further characterization in vivo. The CDT activity for WT H. hepaticus and HhcdtBm7 was measured in vitro on HeLa cells as previously described (5).
Experimental design for in vivo infection.
SW mice free of known murine viruses, pathogenic bacteria including Helicobacter spp., and parasites were obtained from Taconic Farms (Germantown, NY). The mice were maintained in an Association for Accreditation and Assessment of Laboratory Animal Care International-accredited facility in static microisolater cages. In a pilot study, female Helicobacter spp.-free SW mice (five per group, 4 to 6 weeks old) were infected with WT H. hepaticus or its CDT-deficient mutant HhcdtBm7 for 6 weeks. Subsequently, 30 male and 30 female SW mice (4 to 6 weeks old) were divided into six groups of 10 mice (either male or female) and were dosed with WT H. hepaticus or HhcdtBm7 or sham-dosed with Brucella broth as a control, respectively. For oral gavage, bacteria were cultured on blood agar, suspended in Brucella broth, and adjusted to 108 organisms/ml as estimated by spectrophotometry at an optical density of 660 nm (OD660). Mice received 0.2 ml of fresh inocula by gastric gavage every other day for three doses.
Five male and five female mice from each group were necropsied at 8 and 16 wpi, respectively. Immediately after euthanasia, contents in the intestine were removed by rinsing with sterile saline. Standardized 1-cm segments of jejunum, ileum, cecum, and colon were collected for culture and RNA/DNA isolation. Tissues for RNA/DNA isolation were frozen in liquid nitrogen immediately after sampling and stored at −70°C prior to use. Representative tissue sections were fixed in 10% buffered formalin for histology.
Histopathology evaluation.
The entire length of the small intestine from the pylorus of the stomach to the ileoceco-colic (ICC) junction was measured and evaluated under a dissection microscope for gross abnormalities. The small intestine of each mouse was divided into three equal segments, Swiss-rolled and embedded in paraffin, sectioned at 5-um thickness, and stained by hematoxylin and eosin (HE) stain for histologic evaluation by a veterinary pathologist (PRN) blinded to experimental groups. The ICC junction and colon were similarly prepared and examined.
Serology and mucosal immunoglobulin A (IgA) responses to H. hepaticus antigens.
Sera was collected from mice at 8 and 16 wpi with WT H. hepaticus or HhcdtBm7 and evaluated by enzyme-linked immunosorbent assay (ELISA) for Th1-associated IgG2a and Th2-associated IgG1 antibody responses. Outer membrane antigens of the type strain of H. hepaticus (ATCC 51449) were prepared and used in the ELISA using standard methods as previously described (42). Feces were collected at 16 wpi and extracted in protease inhibitor (Sigma) for evaluation of IgA responses to H. hepaticus antigens as previously described (42).
Statistical analyses.
Data on the levels of H. hepaticus and cytokine mRNA in the tissues were analyzed using a Mann-Whitney nonparametric t test. Serology and mucosal IgA results were compared using regression, analysis of variance, and the Student's t test. Values of P < 0.05 are considered significant.
RESULTS
Determination of the cdt operon in H. hepaticus.
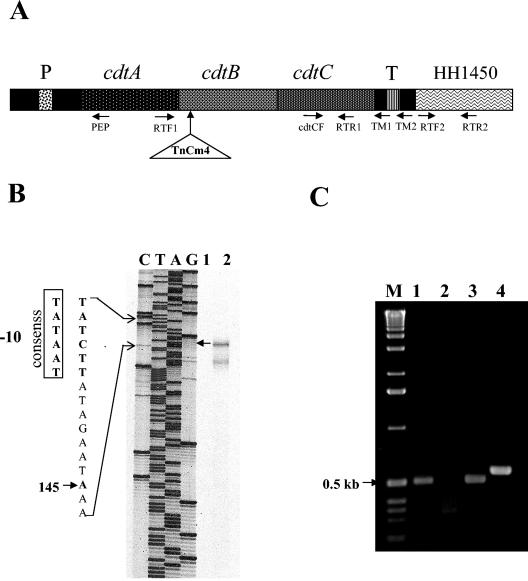
To investigate transcriptional regulation of the cdt genes and create isogenic H. hepaticus mutants, the transcriptional start and termination sites of H. hepaticus cdt operon were characterized by primer extension and RT-PCR, respectively (Fig. 1). The 5′ end of a cDNA product generated by primer extension corresponded to A145 upstream of the start codon of cdtA; the smaller labeled products on the gel probably represent the partially degraded cdt transcripts. In addition, an oligonucleotide sequence similar to the −10 promoter box conserved among bacterial promoters preceded this transcriptional start site (Fig. 1B). These results indicate that nucleotide A145 is the transcriptional start site of the H. hepaticus cdt operon. For determination of the transcriptional termination site, cDNA of total RNA from H. hepaticus was synthesized using primers TM1 (complementary to nucleotides 17 to 38 downstream of the cdtC) and TM2 (complementary to nucleotides 91 to 108 downstream of the cdtC). The respective cDNA templates were then amplified using the primer pair TM1/cdtCF (producing a 500-bp fragment) and TM2/cdtCF (producing a 570-bp fragment). Amplicons of the correct sizes were produced from the TM1-primed cDNA but not from the TM2-primed cDNA (Fig. 1C), indicating that the transcription of the cdt operon was terminated in the region covering nucleotides 38 to 99 downstream of the cdtC stop codon. Successful amplification using RT-PCR with primers RTF1 and RTR1 (complementary to the sequences cdtA and cdtC, respectively) indicates cotranscription of cdtA, cdtB, and cdtC (data not shown). The origins of these PCR products were confirmed by DNA sequencing. These results confirm that the cdt operon in H. hepaticus contains three structural genes cdtA, cdtB, and cdtC previously described by Young et al. (45).
FIG. 1.
Characterization of the transcriptional start and termination sites of the H. hepaticus cdt operon. (A) The gene organization of the cdt operon: the primers for primer extension (PEP) and for RT-PCR as well as the insertional site of TnCm4 within the cdtB are denoted. (B) Primer extension demonstrating the transcriptional start site (A145 upstream of the start codon of the cdtA) is indicated by an arrow. Lanes C, T, A, G: dideoxy-terminated sequence using plasmid pVBY9 DNA as template. This recombinant plasmid containing the cdt operon and the portion of the upstream and downstream regions (45). Lane 1, RNA plus RNase prior to cDNA synthesis; lane 2, RNA minus RNase. (C) RT-PCR-based detection of the transcriptional termination of the cdt opron. Lanes: 1, cDNA with cdtCF/TM1; 2, cDNA with cdtCF/TM2; 3, pVBY9 with cdtCF/TM1; 4, pVBY9 with cdtCF/TM2. A 1-kb ladder (Invitrogen) is located on the left (M).
Colonization of WT H. hepaticus and HhcdtBm7 in outbred SW mice.
To investigate the role of H. hepaticus CDT in colonization, the transposon-mediated mutation in the cdtB gene was created and the H. hepaticus mutant was administered to outbred SW mice of both genders. HhcdtBm7 contained the TnCm4-disrupted mutation within cdtB, which was confirmed by PCR, genomic DNA sequencing, and lack of CDT in vitro activity (data not shown). To investigate whether the TnCm4 insertion within the cdtB gave rise to polar effect on the downstream gene of the cdt operon, transcript copies of the HH1450 that locates immediately downstream of the H. hepaticus cdt operon were quantified by RT-Q-PCR with primers RTF2 and RTR2 (Fig. 1). The levels of the HH1450 transcripts per ng of total RNA in three independent RNA preparations were 1,243 ± 91 copies for HhcdtBm7 and 1,386 ± 176 copies for WT H. hepaticus, indicating that there was no significant change in the HH1450 transcription between WT H. hepaticus and HhcdtBm7. Hence, this cdtB-disrupted mutation had no polar effect on its downstream gene.
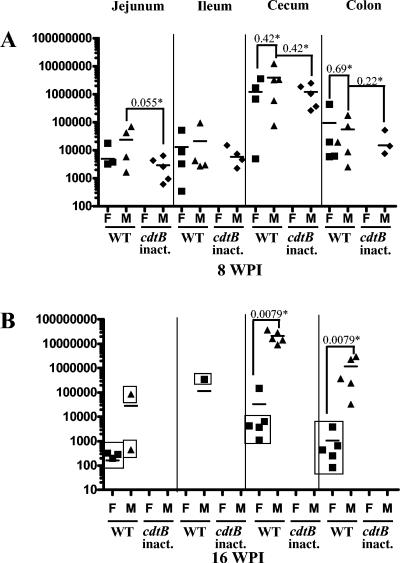
In the pilot study, five female SW mice dosed with HhcdtBm7 were negative by PCR for H. hepaticus in fecal DNA at 2 or 4 wpi and cecal DNA at 6 wpi, whereas the mice dosed with WT H. hepaticus were PCR positive (data not shown). In the longitudinal study, WT H. hepaticus at 8 wpi was variably detected by Q-PCR in jejunum (four of five), ileum (four of five), cecum (five of five), and colon (four of five) of the male SW mice (Fig. 2A). Colonization with WT H. hepaticus persisted in the cecum and colon through 16 wpi in all dosed male mice, but at this later time point, WT H. hepaticus was not detected in one of three or two of three male jejunal or ileal samples, respectively (Fig. 2B). At 8 wpi, WT H. hepaticus was detected by Q-PCR in all dosed female mice with positive results from jejunum (three of five), ileum (four of five), cecum (four of five), and colon (four of five). For each intestinal site that was positive for H. hepaticus at 8 wpi, there were no significant differences in colonization levels between male and female mice (P > 0.22). At 16 wpi in female mice, WT H. hepaticus persisted in the cecum and colon but was detected proximal to the cecum only in the jejunum of three of five female mice. Colonization of WT H. hepaticus in the cecum and colon of male mice significantly increased between 8 and 16 wpi (P < 0.015, cecum; P < 0.03, colon), whereas colonization in the cecum and colon of female mice trended lower through 16 wpi (P < 0.3, cecum; P < 0.15, colon). Consistent with previous reports describing the cecum and colon as the natural niche for H. hepaticus (13), cecal samples contained the highest level of H. hepaticus colonization. Interestingly, at 16 wpi, the levels of H. hepaticus colonization in the cecum and colon from male mice were approximately 1,000-fold higher than those from female mice (P < 0.0079).
FIG. 2.
Q-PCR-based detection of H. hepaticus in four segments of the mouse intestinal tract. Symbols: F, female mice; M, male mice; WT, WT H. hepaticus dosed; cdtB inact., • HhcdtBm7-dosed cdtB inactivated); *, P values for the compared groups. The levels of H. hepaticus in the respective samples are expressed as its genomic copies per μg of mouse DNA. Ileal and jejunal tissues from two male mice of all the groups at 16 wpi were lost during processing. Therefore, the 16-wpi data on the colonization levels and cytokine profiles in the ileum and jejunum of the male mice were based on three mice of each group. The samples negative for H. hepaticus by culture are indicated in boxes.
Male mice at 8 wpi were colonized with HhcdtBm7 in the jejunum, ileum, and cecum at comparable levels to those for WT H. hepaticus (P > 0.05 for all comparisons), whereas HhcdtBm7 was less efficient in colonizing the colon (three out of five) compared to WT H. hepaticus (five out of five). HhcdtBm7 was not detected in the jejunum, ileum, cecum, or colon from any female mice dosed 8 weeks earlier, and the isogenic mutant could not be detected by Q-PCR or culture in either male or female mice at 16 wpi.
Culture was performed to confirm viability of H. hepaticus in jejunum, ileum, cecum, and colon from SW mice infected with WT H. hepaticus or HhcdtBm7. The results for the samples from the mice at 8 wpi supported those by Q-PCR. At 16 wpi, the cecum (five out of five) and colon (five out of five) of WT H. hepaticus-dosed male mice as well as the cecum (one out of five) of WT H. hepaticus-dosed female mice, which contained the relatively high levels of H. hepaticus by Q-PCR, were H. hepaticus positive by culture. In contrast, 15 samples (Fig. 2B), 13 of which contained relatively lower levels of H. hepaticus by Q-PCR, were negative for H. hepaticus by culture. These data indicate that Q-PCR is more sensitive for determining the status of H. hepaticus colonization in the murine intestine than culture.
Histopathology.
Gross and histopathological evaluation of the intestines of all mice did not reveal significant lesions.
Serum IgG and mucosal IgA responses to H. hepaticus antigens.
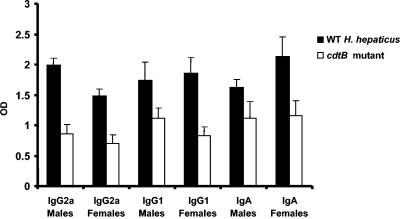
Mice dosed with WT H. hepaticus or its cdtB-deficient mutant HhcdtBm7 had seroconverted to H. hepaticus antigens by 8 wpi (P < 0.0001) (data not shown). At 16 wpi, male mice infected with WT H. hepaticus had developed a progressively higher Th1-associated IgG2a response compared to female mice (P < 0.009) (Fig. 3). The IgG2a responses of both males and females infected with WT H. hepaticus were significantly higher than mice of either sex dosed with the HhcdtBm7 mutant (P < 0.002). Male and female mice colonized with WT H. hepaticus had similar Th2-associated IgG1 responses at 16 wpi (P < 0.40) and, like the IgG2a responses, were significantly higher than mice dosed with the HhcdtBm7 mutant (P < 0.002). Levels of mucosal IgA specific for H. hepaticus were significant only for mice infected with WT H. hepaticus (P < 0.0006; control data not shown). IgA responses of mice dosed with HhcdtBm7 were equivalent to background levels produced by uninfected control mice (P = 0.26). There was a trend for female mice infected with WT H. hepaticus to produce more IgA than male mice (P = 0.09).
FIG. 3.
IgG1, IgG2a, IgA antibody responses to the H. hepaticus antigens. The sera for measuring IgG1 and IgG2a were collected from the mice orally dosed with either WT H. hepaticus or HhcdtBm7 (cdtB inactivated) for 16 wpi. The IgA antibody response to H. hepaticus was measured in fecal extracts from mice orally dosed with either WT H. hepaticus or HhcdtBm7 (cdtB inactivated) by 16 wpi. *, P values for the compared groups. OD, optical density.
Cytokine production in intestinal tissues.
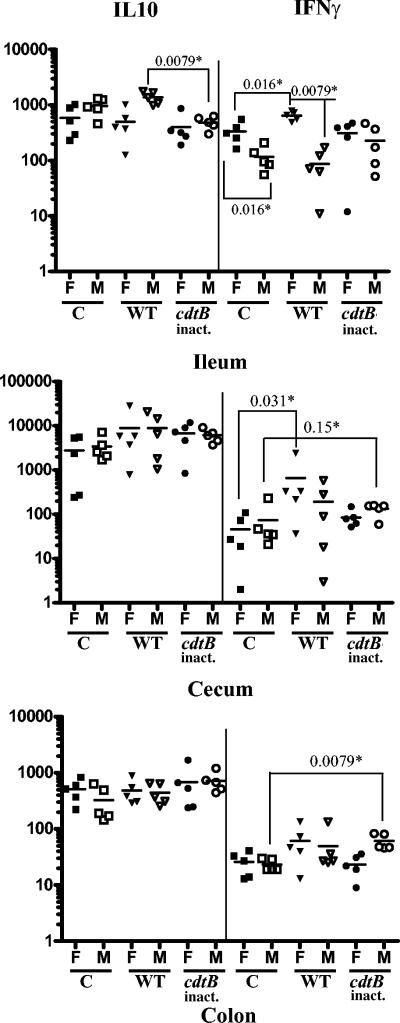
Given that H. hepaticus CDT plays an important role in colonization of SW mice and females were more resistant to H. hepaticus colonization than males, mRNA for three proinflammatory cytokines (IL-6, TNF-α, IFN-γ) and one anti-inflammatory Th2-type cytokine (IL-10) were analyzed using Q-PCR in samples from jejunum, ileum, cecum, and colon. At 8 wpi, infection with WT H. hepaticus significantly increased the level of ileal and cecal IFN-γ transcripts in the female but not in male mice (Fig. 4, P < 0.0079). In contrast, male mice dosed with WT H. hepaticus expressed more ileal IL-10 transcripts than female mice (Fig. 4, P < 0.0079). Both female and male mice dosed with HhcdtBm7 did not express altered levels of ileal IL-10 and IFN-γ; however, the level of IFN-γ mRNA was significantly higher in the colon (Fig. 5, P < 0.0079) and trended to be higher in the cecum without statistical significance (Fig. 4, P < 0.15) from the male mice infected with HhcdtBm7 compared with uninfected male mice. There were no significant differences in expression of IL-6 or TNF-α mRNA in the jejunum, ileum, cecum, and colon among all comparable groups (data not shown).
FIG. 4.
mRNA levels of IL-10 and IFN-γ in the respective intestinal segments. The mice were orally dosed with either Brucella broth, WT H. hepaticus, or HhcdtBm7 (cdtB inactivated) and necropsied at 8 wpi. Symbols: *, P values for the compared groups; C, the controls; WT, WT H. hepaticus-dosed; cdtB inact., HhcdtBm7 (the cdtB inactivated); F, female mice; M, male mice. The levels of the IL-10 and IFN-γ transcripts were expressed as their copy numbers per 106 of the GAPDH transcripts in the same samples.
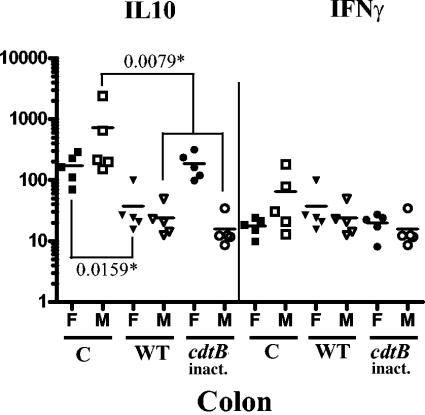
FIG. 5.
mRNA levels of IL-10 and IFN-γ in the colon at 16 wpi. Symbols: *, P values for the compared groups; C, sham dosed; WT, WT H. hepaticus-dosed; cdtB inact., HhcdtBm7 dosed (the cdtB inactivated); F, female mice; M, male mice. The levels of the IL-10 and IFN-γ transcripts were expressed as their copy numbers per 106 of the GAPDH transcripts in the same samples.
At 16 wpi, both female and male mice infected by WT H. hepaticus had significantly down-regulated IL-10 mRNA expression compared to the control mice in the colon (Fig. 5; P < 0.015, female; P < 0.0079, male). In addition, infection with HhcdtBm7 down-regulated the IL-10 mRNA expression only in males (P < 0.0079) but not in females (P < 0.9) that had eliminated colonization prior to 8 wpi. At 16 wpi, there were no significant differences in the levels of IL-10 or IFN-γ transcripts in the ileum and cecum among all the comparable groups (data not shown).
DISCUSSION
Many gram-negative pathogenic bacteria, including Actinobacillus actinomycetemcomitans, Campylobacter spp., E. coli, H. ducreyi, enterohepatic Helicobacter species, Salmonella enterica serovar Typhi, and Shigella species, produce CDT, a heat-labile cytotoxin that causes cell cycle arrest, subsequent cell distension, and eventual cell death in cultured mammalian cells (40). It has been reported that CDT activity was not required for colonization of C. jejuni and H. hepaticus in immunodeficient mice, including scid, NF-κB-deficient, and IL-10−/− mice (14, 32, 44). However, the C. jejuni cdtB mutant was eliminated by 4 months p.i. from C57BL/129 mice, indicating that CDT is necessary for persistent colonization of C. jejuni in immunocompetent mice (14). In this study, we demonstrated that CDT is critical for persistent colonization of H. hepaticus in outbred SW mice. Loss of HhcdtBm7 colonization in female mice by 8 wpi and male mice by 16 wpi was supported by the serological data indicating that mice infected with the CDT-deficient mutant developed significantly lower Th1-associated IgG2a, Th2-associated IgG1 and IgA responses than those mice colonized by WT H. hepaticus. Results from our pilot study indicated that HhcdtBm7 could be eliminated from female mice as early as 2 wpi. Inability of HhcdtBm7 to persistently colonize does not appear to be due to a polar effect of the mutation, because the H. hepaticus cdtA, cdtB, and cdtC genes constitute a single operon and the insertion of minitransposon in the cdtB did not affect the transcription of HH1450 immediately downstream of the cdt operon. Recently, Young et al. (44) reported that CDT-deficient H. hepaticus mutants induced less severe typhlocolotis than WT H. hepaticus, but its colonization levels were not different from WT H. hepaticus in C57BL/6 IL-10−/− mice. The contrasting results of our study may be explained by use of outbred, immunocompetent SW mice compared to IL-10-deficient mice. We hypothesize that successful colonization of CDT-deficient bacterial mutants in immunodeficient mice results from the inefficient immune host responses. The molecular mechanisms underlying the role of CDT in colonization are unclear at present. H. hepaticus CDT may suppress IFN-γ responses to helicobacter infection, perhaps by inhibiting T- and B-cell function as demonstrated for A. actinomycetemcomitans CDT (34-36). This notion is supported by clearance of HhcdtBm7 by 8 wpi in female SW mice, which was associated with a significantly elevated IFN-γ expression in the ileum; in contrast, the elevated ileal IFN-γ expression was not observed in male mice which were colonized with the mutant at least through 8 wpi. In addition, the subsequent elimination of the CDT isogenic mutant from the male mice by 16 wpi may be due to the gradually increased expression of IFN-γ in the cecum and colon. The importance of IFN-γ in protecting hosts from bacterial infection has been demonstrated in other mouse models. Increased IFN-γ expression in Yersinia-specific T cells and activated macrophages in Peyers patches and mesenteric lymph nodules was shown to be essential for clearing Y. enterocolitica infection from C57BL/6 mice, whereas BALB/c mice that did not respond with elevated Yersinia-associated IFN-γ expression were susceptible to Yersinia infection (1, 2).
In H. hepaticus, 953 of the predicted open reading frames (50.8%) have orthologs in C. jejuni (38). C. jejuni colonizes the cecum and large intestine as primary sites but also colonizes proximal distal small intestine in chicks and mice (3, 10, 28). Importantly, a recent study reported that the C. jejuni colonization in the jujenum of humans was associated with immunoproliferative small intestinal disease with characteristic lymphoepithelial lesions caused by infiltration of CD20-positive (a B-cell marker) centrocyte-like lymphocytes (27). Given the fact that H. hepaticus causes typhlitis in A/J mice (13, 42), these studies prompted us to characterize the status of H. hepaticus colonization in the small intestine of mice. WT H. hepaticus persistently colonized the jejunum of both male and female mice but in the ileum was eliminated by 16 wpi from the female mice, whereas HhcdtBm7 colonized the jejunum and ileum of male SW mice only through 8 wpi. H. hepaticus colonization in ileum of female SW mice was associated with the up-regulation of ileal IFN-γ mRNA at 8 wpi. The levels of ileal IFN-γ mRNA between the control and WT H. hepaticus-dosed female mice were not significantly different at 16 wpi when H. hepaticus was eliminated from this niche. These results suggest that the colonization of H. hepaticus in this intestinal niche at an early time postinfection may play an important role in the up-regulation of IFN-γ expression.
Our data indicated that female SW mice are more resistant to both WT H. hepaticus and HhcdtBm7 infection than male mice. Gender effect on the colonization by WT H. hepaticus and HhcdtBm7 in SW mice appears to be associated with different IFN-γ responses in the intestine by 8 wpi between female and male mice. In the ileum and cecum of female mice, but not males, after being infected with WT H. hepaticus the level of IFN-γ was significantly increased. This result is consistent with the recent finding that the IFN-γ transcripts were increased in the cecum of female A/JCr mice compared to males after H. hepaticus infection for 1 month (29). However, elevated cecal IFN-γ in female A/JCr mice persisted through 3 months postinfection, and cecal IL-10 and TNF-α mRNA levels were also increased. These differences may be ascribed to different genetic backgrounds between inbred A/JCr and the outbred SW mice. Although the difference in IFN-γ expression between female and male SW mice may explain the sex-dependent colonization outcomes for WT H. hepaticus and HhcdtBm7 in SW mice, we cannot rule out potential additional host and other intestinal microbial factors that could contribute to the observed sex effect on H. hepaticus colonization.
Also, colonization of H. hepaticus was associated with the down-regulation of IL-10 production in the colon of SW mice by 16 wpi. The role of IL-10 in the development of H. hepaticus-driven intestinal diseases in mice has been well documented. H. hepaticus colonization in IL-10-deficient mice can significantly accelerate severity of typhlocolits, suggesting that IL-10 is required for suppressing progression of intestinal pathology (23, 24, 44). In 129/RAG2−/− mice, H. hepaticus colonization induces severe typhlocolitis and colon tumors (9, 21, 22, 30). Adoptive transfer of CD4+CD45RBloCD25+ T-regulatory cells from wild-type mice to 129/RAG2−/− mice both before and after infection with H. hepaticus inhibits this pathology (8, 9, 22, 30). In contrast, this protective effect was abrogated when Treg cells from IL-10−/− mice were used, indicating that IL-10 plays a pivotal role in this process (9, 22, 30). Hence, the down-regulation of IL-10 production in the murine colon by H. hepaticus colonization may promote H. hepaticus-induced colonic carcinoma in 129/RAG2−/− mice (8, 9). This effect also occurred in the colon of male mice which eliminated HhcdtBm7 by 16 wpi but was not noticed in the colon of female mice which eliminated the mutant by 2 wpi, indicating that the colonization of H. hepaticus and not CDT activity led to down-regulation of colonic IL-10 expression. We speculate that additional virulence factors (e.g., secretory proteins) from H. hepaticus suppress the IL-10-producing cell populations in the colon. Further investigation into the interplay between the H. hepaticus, immune cells, cytokines, and epithelia in this lower bowel niche will provide a valuable model to dissect molecular mechanisms controlling colon carcinoma in humans.
In summary, we demonstrated that H. hepaticus CDT and sex differences have significant impact on colonization by H. hepaticus in outbred SW mice. The elevated IFN-γ production in the murine intestine is associated with loss of initial (in females) and persistent (in males) colonization of the CDT-deficient H. hepaticus mutant as well as the reduction of WT H. hepaticus colonization levels in SW females. H. hepaticus-mediated down-regulation of IL-10 in the colon of SW mice may play a role in H. hepaticus-induced colitis and colon carcinoma observed in other mouse models. These results provide new insights into H. hepaticus colonization in male and female SW mice, including the important roles of CDT and host immune responses.
Acknowledgments
We thank David Schauer and Vincent Young for providing plasmid pVBY9 and Kathleen Cormier, Erinn Stefanich, and Jeff Bajko for histologic processing of tissues.
This study was supported by NIH grants R01 CA67529 (J.G.F.), R01 AI50952 (J.G.F.), P01 CA26731, and P30ES02109.
Editor: J. T. Barbieri
REFERENCES
- 1.Autenrieth, I. B., M. Beer, E. Bohn, S. H. Kaufmann, and J. Heesemann. 1994. Immune responses to Yersinia enterocolitica in susceptible BALB/c and resistant C57BL/6 mice: an essential role for gamma interferon. Infect. Immun. 62:2590-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autenrieth, I. B., V. Kempf, T. Sprinz, S. Preger, and A. Schnell. 1996. Defense mechanisms in Peyer's patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect. Immun. 64:1357-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beery, J. T., M. B. Hugdahl, and M. P. Doyle. 1988. Colonization of gastrointestinal tracts of chicks by Campylobacter jejuni. Appl. Environ. Microbiol. 54:2365-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill, R. J., C. J. Foltz, J. G. Fox, C. A. Dangler, F. Powrie, and D. B. Schauer. 1997. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect. Immun. 65:3126-3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien, C. C., N. S. Taylor, Z. Ge, D. B. Schauer, V. B. Young, and J. G. Fox. 2000. Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J. Med. Microbiol. 49:525-534. [DOI] [PubMed] [Google Scholar]
- 6.Chin, E. Y., C. A. Dangler, J. G. Fox, and D. B. Schauer. 2000. Helicobacter hepaticus infection triggers inflammatory bowel disease in T cell receptor αβ mutant mice. Comp. Med. 50:586-594. [PubMed] [Google Scholar]
- 7.Erdman, S. E., J. G. Fox, C. A. Dangler, D. Feldman, and B. H. Horwitz. 2001. Typhlocolitis in NF-kappa B-deficient mice. J. Immunol. 166:1443-1447. [DOI] [PubMed] [Google Scholar]
- 8.Erdman, S. E., T. Poutahidis, M. Tomczak, A. B. Rogers, K. Cormier, B. Plank, B. H. Horwitz, and J. G. Fox. 2003. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am. J. Pathol. 162:691-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erdman, S. E., V. P. Rao, T. Poutahidis, M. M. Ihrig, Z. Ge, Y. Feng, M. Tomczak, A. B. Rogers, B. H. Horwitz, and J. G. Fox. 2003. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 63:6042-6050. [PubMed] [Google Scholar]
- 10.Fauchere, J. L., M. Veron, A. Lellouch-Tubiana, and A. Pfister. 1985. Experimental infection of gnotobiotic mice with Campylobacter jejuni: colonisation of intestine and spread to lymphoid and reticulo-endothelial organs. J. Med. Microbiol. 20:215-224. [DOI] [PubMed] [Google Scholar]
- 11.Fox, J. G. 2002. The non-H. pylori helicobacters: their expanding role in gastrointestinal and systemic diseases. Gut 50:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox, J. G., F. E. Dewhirst, J. G. Tully, B. J. Paster, L. Yan, Taylor, N. S., M. J. Collins, Jr., P. L. Gorelick, and J. M. Ward. 1994. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J. Clin. Microbiol. 32:1238-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox, J. G., X. Li, L. Yan, R. J. Cahill, R. Hurley, R. Lewis, and J. C. Murphy. 1996. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect. Immun. 64:1548-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox, J. G., A. B. Rogers, M. T. Whary, Z. Ge, N. S. Taylor, S. Xu, B. H. Horwitz, and S. E. Erdman. 2004. Gastroenteritis in NF-κB-deficient mice is produced with wild-type Campylobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect. Immun. 72:1116-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox, J. G., L. Yan, B. Shames, J. Campbell, J. C. Murphy, and X. Li. 1996. Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect. Immun. 64:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge, Z., K. Hiratsuka, and D. E. Taylor. 1995. Nucleotide sequence and mutational analysis indicate that two Helicobacter pylori genes encode a P-type ATPase and a cation-binding protein associated with copper transport. Mol. Microbiol. 15:97-106. [DOI] [PubMed] [Google Scholar]
- 17.Ge, Z., D. A. White, M. T. Whary, and J. G. Fox. 2001. Fluorogenic PCR-based quantitative detection of a murine pathogen, Helicobacter hepaticus. J. Clin. Microbiol. 39:2598-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haghjoo, E., and J. E. Galan. 2004. Salmonella typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. USA 101:4614-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hailey, J. R., J. K. Haseman, J. R. Bucher, A. E. Radovsky, D. E. Malarkey, R. T. Miller, A. Nyska, and R. R. Maronpot. 1998. Impact of Helicobacter hepaticus infection in B6C3F1 mice from twelve National Toxicology Program two-year carcinogenesis studies. Toxicol. Pathol. 26:602-611. [DOI] [PubMed] [Google Scholar]
- 20.Ihrig, M., M. D. Schrenzel, and J. G. Fox. 1999. Differential susceptibility to hepatic inflammation and proliferation in AXB recombinant inbred mice chronically infected with Helicobacter hepaticus. Am. J. Pathol. 155:571-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kullberg, M. C., J. F. Andersen, P. L. Gorelick, P. Caspar, S. Suerbaum, J. G. Fox, A. W. Cheever, D. Jankovic, and A. Sher. 2003. Induction of colitis by a CD4+ T cell clone specific for a bacterial epitope. Proc. Natl. Acad. Sci. USA 100:15830-15835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kullberg, M. C., D. Jankovic, P. L. Gorelick, P. Caspar, J. J. Letterio, A. W. Cheever, and A. Sher. 2002. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J. Exp. Med. 196:505-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kullberg, M. C., A. G. Rothfuchs, D. Jankovic, P. Caspar, T. A. Wynn, P. L. Gorelick, A. W. Cheever, and A. Sher. 2001. Helicobacter hepaticus-induced colitis in interleukin-10-deficient mice: cytokine requirements for the induction and maintenance of intestinal inflammation. Infect. Immun. 69:4232-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kullberg, M. C., J. M. Ward, P. L. Gorelick, P. Caspar, S. Hieny, A. Cheever, D. Jankovic, and A. Sher. 1998. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect. Immun. 66:5157-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lara-Tejero, M., and J. E. Galan. 2001. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect. Immun. 69:4358-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lara-Tejero, M., and J. E. Galan. 2002. Cytolethal distending toxin: limited damage as a strategy to modulate cellular functions. Trends Microbiol. 10:147-152. [DOI] [PubMed] [Google Scholar]
- 27.Lecuit, M., E. Abachin, A. Martin, C. Poyart, P. Pochart, F. Suarez, D. Bengoufa, J. Feuillard, A. Lavergne, J. I. Gordon, P. Berche, L. Guillevin, and O. Lortholary. 2004. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N. Engl. J. Med. 350:239-248. [DOI] [PubMed] [Google Scholar]
- 28.Lee, A., J. L. O'Rourke, P. J. Barrington, and T. J. Trust. 1986. Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni: a mouse cecal model. Infect. Immun. 51:536-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livingston, R. S., M. H. Myles, B. A. Livingston, J. M. Criley, and C. L. Franklin. 2004. Sex influence on chronic intestinal inflammation in Helicobacter hepaticus-infected A/JCr mice. Comp. Med. 54:301-308. [PubMed] [Google Scholar]
- 30.Maloy, K. J., L. Salaun, R. Cahill, G. Dougan, N. J. Saunders, and F. Powrie. 2003. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 197:111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickett, C. L., and C. A. Whitehouse. 1999. The cytolethal distending toxin family. Trends Microbiol. 7:292-297. [DOI] [PubMed] [Google Scholar]
- 32.Purdy, D., C. M. Buswell, A. E. Hodgson, K. McAlpine, I. Henderson, and S. A. Leach. 2000. Characterisation of cytolethal distending toxin (CDT) mutants of Campylobacter jejuni. J. Med. Microbiol. 49:473-479. [DOI] [PubMed] [Google Scholar]
- 33.Rogers, A. B., S. R. Boutin, M. T. Whary, N. Sundina, Z. Ge, K. Cormier, and J. G. Fox. 2004. Progression of chronic hepatitis and preneoplasia in Helicobacter hepaticus-infected A/JCr mice. Toxicol. Pathol. 32:668-677. [DOI] [PubMed] [Google Scholar]
- 34.Shenker, B. J., R. H. Hoffmaster, T. L. McKay, and D. R. Demuth. 2000. Expression of the cytolethal distending toxin (Cdt) operon in Actinobacillus actinomycetemcomitans: evidence that the CdtB protein is responsible for G2 arrest of the cell cycle in human T cells. J. Immunol. 165:2612-2618. [DOI] [PubMed] [Google Scholar]
- 35.Shenker, B. J., R. H. Hoffmaster, A. Zekavat, N. Yamaguchi, E. T. Lally, and D. R. Demuth. 2001. Induction of apoptosis in human T cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J. Immunol. 167:435-441. [DOI] [PubMed] [Google Scholar]
- 36.Shenker, B. J., T. McKay, S. Datar, M. Miller, R. Chowhan, and D. R. Demuth. 1999. Actinobacillus actinomycetemcomitans immunosuppressive protein is a member of the family of cytolethal distending toxins capable of causing a G2 arrest in human T cells. J. Immunol. 162:4773-4780. [PubMed] [Google Scholar]
- 37.Stevens, M. K., J. L. Latimer, S. R. Lumbley, C. K. Ward, L. D. Cope, T. Lagergard, and E. J. Hansen. 1999. Characterization of a Haemophilus ducreyi mutant deficient in expression of cytolethal distending toxin. Infect. Immun. 67:3900-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suerbaum, S., C. Josenhans, T. Sterzenbach, B. Drescher, P. Brandt, M. Bell, M. Droge, B. Fartmann, H. P. Fischer, Z. Ge, A. Horster, R. Holland, K. Klein, J. Konig, L. Macko, G. L. Mendz, G. Nyakatura, D. B. Schauer, Z. Shen, J. Weber, M. Frosch, and J. G. Fox. 2003. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proc. Natl. Acad. Sci. USA 100:7901-7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor, N. S., Z. Ge, Z. Shen, F. E. Dewhirst, and J. G. Fox. 2003. Cytolethal distending toxin: a potential virulence factor for Helicobacter cinaedi. J. Infect. Dis. 188:1892-1897. [DOI] [PubMed] [Google Scholar]
- 40.Thelestam, M., and T. Frisan. 2004. Cytolethal distending toxins. Rev. Physiol. Biochem. Pharmacol. 152:111-133. [DOI] [PubMed] [Google Scholar]
- 41.Ward, J. M., J. G. Fox, M. R. Anver, D. C. Haines, C. V. George, M. J. Collins, Jr., P. L. Gorelick, K. Nagashima, M. A. Gonda, R. V. Gilden, J. G. Tully, R. J. Russel, R. E. Benveniste, B. J. Paster, F. E. Dewhirst, J. C. Donovan, L. M. Anderson, and J. M. Rice. 1994. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J. Natl. Cancer Inst. 86:1222-1227. [DOI] [PubMed] [Google Scholar]
- 42.Whary, M. T., T. J. Morgan, C. A. Dangler, K. J. Gaudes, N. S. Taylor, and J. G. Fox. 1998. Chronic active hepatitis induced by Helicobacter hepaticus in the A/JCr mouse is associated with a Th1 cell-mediated immune response. Infect. Immun. 66:3142-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young, V. B., C. C. Chien, K. A. Knox, N. S. Taylor, D. B. Schauer, and J. G. Fox. 2000. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J. Infect. Dis. 182:620-623. [DOI] [PubMed] [Google Scholar]
- 44.Young, V. B., K. A. Knox, J. Pratt, J. S. Cortez, L. S. Mansfield, A. B. Rogers, J. G. Fox, and D. B. Schauer. 2004. In vitro and in vivo characterization of Helicobacter hepaticus cytolethal distending toxin mutants. Infect. Immun. 72:2521-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young, V. B., K. A. Knox, D. B. Schauer. 2000. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect. Immun. 68:184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]