Abstract
Tuberculin shock due to inoculation of Mycobacterium tuberculosis antigens in patients with tuberculosis is a serious syndrome originally described over 100 years ago by Robert Koch. Here, we present experimental evidence that a single M. tuberculosis recombinant protein, CFP-10, triggers this syndrome. Intradermal inoculation of CFP-10 elicits in M. tuberculosis-infected mice high levels of serum tumor necrosis factor alpha and causes tuberculin shock in infected guinea pigs characterized by hypothermia and death within 6 to 48 h after the antigen inoculation. Autopsies of these animals revealed intense polycythemia and hemorrhagic patches in the lung parenchyma, a pathological observation consistent with tuberculin shock. These results point to the possible occurrence of tuberculin shock in sensitive individuals inoculated with highly purified M. tuberculosis recombinant proteins as vaccine candidates or skin test reagents.
Tuberculosis represents a major cause of mortality worldwide, and 1.5 million deaths a year result from this disease. It has been estimated that one-third of the world's population harbor a clinically latent infection of Mycobacterium tuberculosis and that 5 to 10% of these individuals will develop active disease as a result of reactivation of latent infection in their lifetimes. The attenuated strain of Mycobacterium bovis, bacillus Calmette-Guérin (BCG), is currently the only available vaccine against tuberculosis (5, 7). However, the efficacy of BCG in the control of tuberculosis has shown considerable variation in different clinical trials and geographically distinct populations. Identification of individuals infected with tuberculous bacilli, particularly in epidemiological studies, has been traditionally done using the tuberculin skin test. This test is based on the delayed-type hypersensitivity (DTH) reaction elicited by M. tuberculosis antigens (tuberculin). Intradermal injection of tuberculin results in a DTH response in most infected individuals, which peaks at 48 to 72 h after injection (1, 2, 15). The tuberculin test was originally introduced in the 1890s by Robert Koch, who used a boiled crude preparation of M. tuberculosis cultures as the antigen (old tuberculin). However, due to several side effects in many individuals, including shock (tuberculin shock), the test became widely accepted only in the early 1940s, when Florence Seibert introduced and standardized a purified protein derivative of tuberculin (PPD) to replace the old tuberculin. Despite the fact that this preparation is inappropriately referred to as a purified protein (PPD is a preparation consisting of a mixture of several low-molecular-weight proteins of M. tuberculosis), tuberculin shock has never been reported with this antigenic preparation. Although never investigated, it is assumed that the components of the old tuberculin responsible for elicitation of the shock syndrome were either removed from the original preparation or are present in PPD only as trace elements.
Over the past 10 years major efforts have been dedicated to the development of highly purified M. tuberculosis recombinant proteins to be used either as a subunit vaccine or as antigens for a more specific diagnostic skin test (22). Interestingly, the genes encoding some of these antigens (e.g., ESAT-6 and CFP-10) are located in the RD1 genetic region of the M. tuberculosis genome, a region that is deleted from all BCG strains (3, 18). Therefore, in principle, these antigens are good candidates for diagnostic tools because, by definition, vaccination with BCG does not generate cross-reactions to them. Moreover, it has recently been demonstrated that RD1 is apparently responsible for M. tuberculosis virulence and that both CFP-10 and ESAT-6 are required for lysis of alveolar epithelial cells, a virulence phenotype associated with M. tuberculosis but not BCG (12, 14). In other studies, CFP-10 was shown to bind to the J774 mouse macrophage cell line in vitro and to stimulate the production of high levels of tumor necrosis factor alpha (TNF-α) (27).
TNF-α is a cytokine that has dual antagonistic activities in tuberculosis that are not yet entirely understood; this cytokine is essential for resistance to the disease, but paradoxically it is involved in the tissue damage that occurs during infection. Thus, mice genetically engineered to lack a TNF-α receptor succumb soon after challenge with a few M. tuberculosis organisms (11), and humans with rheumatoid arthritis under treatment with TNF-α inhibitors are highly susceptible to developing tuberculosis (19). However, this cytokine can be a disease promoter during infection, as illustrated by postinfection immunotherapy experiments which show that production of TNF-α in the lungs of infected mice leads to exacerbation of pathology and aggravation of the disease (20). In addition, TNF-α is known to be an important mediator of the Koch's phenomenon (24).
Therefore, it is possible that antigens that, like CFP-10, stimulate TNF-α may trigger a tuberculin-like shock in infected persons if these antigens are used as skin test preparations or as vaccines. To assess this possible outcome, CFP-10 was tested as a skin test reagent in both guinea pigs and mice infected with virulent M. tuberculosis. We show here that this antigen stimulates high levels of systemic production of TNF-α in mice and causes severe side effects in guinea pigs, including hypothermia, hemorrhagic patches in the animals' lungs, and death within 6 to 48 h after the skin test.
MATERIALS AND METHODS
Antigens.
Histidine-tagged derivatives of CFP-10 (9) were expressed and purified as previously described (26), and the Mtb 39 and Mtb 72F M. tuberculosis antigens (10, 25) were kindly provided by Corixa Corp., Seattle, WA. Recombinant CFP-10 was subjected to further purification by fast protein liquid chromatography using a 391Q DEAE anion-exchange minicolumn (Amersham Biosciences). Figure 1 shows that these purification procedures yielded a highly purified CFP-10 preparation, as indicated by Coomassie blue staining of a sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis gel performed with various concentrations of the recombinant antigen. In addition, the recombinant antigens were assayed for the presence of endotoxin using the Limulus amebocyte assay (BioWhittaker, Walkersville, MD) and were shown to contain <0.15 endotoxin unit/mg of protein.
FIG. 1.
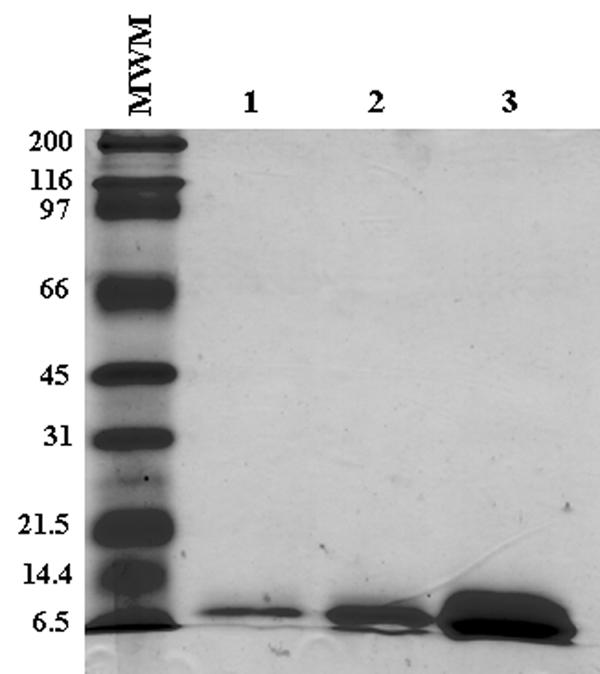
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of purified of recombinant CFP-10 antigen. Different concentrations of the antigen preparation were subjected to electrophoresis (12% polyacrylamide gel), followed by Coomassie blue staining. Lanes 1, 2, and 3 were loaded with 0.4, 2, and 10 μg of purified CFP-10 per lane, respectively. Lane MWM contained molecular weight protein standards. The numbers on the left indicate the molecular weights of the standards.
Animals.
Female C57BL/6 (H-2β), BALB/c (H-2Ad), and C3H/HeJ (H-2k) mice that were 6 to 8 weeks old were obtained from Charles River Laboratories (Portage, MI). Hartley guinea pigs weighing approximately 400 g were obtained from Harlan-Sprague-Dawley Laboratories, North Wilmington, MA. All animals were maintained in specific-pathogen-free conditions. The mice and guinea pigs were infected intravenously (i.v.) with 2 × 105 and 2 × 104 CFU of M. tuberculosis H37Rv, respectively.
Cytokine evaluation.
The levels of culture supernatant and serum TNF-α were analyzed by a sandwich enzyme-linked immunosorbent assay (ELISA), using antibody pairs and procedures available from PharMingen as described previously (26). Supernatants were harvested from mononuclear spleen cell cultures (2 × 106 cells/ml in 96-well flat-bottom plates) stimulated with 0.4, 2, and 10 μg/ml of antigens. To determine serum levels of TNF-α, blood was obtained from the retroorbital capillary bed of mice previously (2 h) inoculated intradermally in the base of tail with recombinant antigens.
Guinea pig skin test and temperature measurement.
Guinea pigs were anesthetized, shaved, and injected intradermally with 0.1 ml of the antigenic solution (Mantoux technique). Reactions were read at 24 h, and the results were expressed as the transverse diameter (in millimeters) of the induration reaction. Rectal temperatures were taken using a digital thermometer (BD Biosciences) for each guinea pig before skin testing and 2 h after skin testing.
Histology.
Guinea pig lung tissue sections were fixed in 10% formalin and embedded in paraffin. Sections (5 μm) were stained with hematoxylin and eosin (H&E).
RESULTS
Systemic production of TNF-α in mice infected with M. tuberculosis.
Unpublished data from our laboratory indicated that CFP-10 did not induce CD4+ T-cell responses in mice of the H-2b or H-2d haplotypes even when the antigen was formulated with complete Freund's adjuvant. Therefore, to perform the current studies, an initial selection of a suitable mouse strain was carried out using the SYFPEITHI major histocompatibility complex class II binding prediction algorithm for H-2 haplotypes (http://syfpeithi.bmi-heidelberg.com/scripts/MHCServer.dll/home.htm). This evaluation indicated that CFP-10 contains several epitope binding motifs for H-2k (scores, ≥25) and no binding motifs for H-2b or H-2d. This prediction was confirmed in experimental studies performed with C3H/HeJ mice (H-2k, immunized subcutaneously with 10 μg of CFP-10 formulated in incomplete Freund's adjuvant) (not shown).
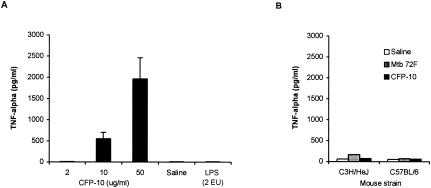
To test the possibility that CFP-10 induced systemic production of TNF-α in infected animals, C3H/HeJ and C57BL/6 mice (H-2b) were infected i.v. with 105 CFU of M. tuberculosis H37Rv, and at day 14 postinfection (a time of intense multiplication of Mycobacterium in the animals' lungs), they were inoculated intradermally (at the base of the tail) with various concentrations of CFP-10. C3H/HeJ mice were ideal for these studies because in addition to being CFP-10 responders, they harbor a mutation in the TLR-4 gene and are hyporesponsive to lipopolysaccharide (LPS) (21). Therefore, the possible effects of LPS contamination (<0.15 EU/mg of protein) in the recombinant antigen preparations are mitigated. Serum levels of TNF-α were determined at 2 h after administration of CFP-10. This time was chosen because TNF-α has a short serum lifetime (4). Figure 2A shows the results and indicates that high concentrations of the cytokine were produced and detected in the sera of mice inoculated with either 50 or 10 μg of the recombinant protein. In contrast, mice inoculated with 2 μg of CFP-10, with 2 EU of LPS (>200 times its content in 50 μg of CFP-10), or with saline alone produced undetectable levels of TNF-α. In addition (Fig. 2B), no TNF-α was detected in M. tuberculosis-infected C57BL/6 mice similarly inoculated with CFP-10 or in sera of M. tuberculosis-infected C3H/HeJ mice (not shown) skin tested with 50 μg of the mycobacterial fused polyprotein recombinant antigen Mtb 72F. This fused molecule is a promising multiple-epitope M. tuberculosis recombinant vaccine candidate (25). Finally, no TNF-α could be detected in the sera of noninfected H-2k mice skin tested with 50 μg of either CFP-10 or Mtb 72F (Fig. 2B). These results point to a strong and unique ability of CFP-10 to induce systemic production of TNF-α in M. tuberculosis-infected mice. Coincidentally this activity was detected only in mice of the CFP-10 responder haplotype (H-2k). In addition, the strong induction of TNF-α by CFP-10 in infected mice cannot be attributed to possible contamination of the antigenic preparation with endotoxin because only trace amounts of this product were present in the CFP-10 solution (<0.15 EU/mg of protein) and inoculation of C3H/HeJ mice with 2 EU of LPS (>200 times the amount present in CFP-10) resulted in no stimulation of serum TNF-α production. Moreover, no cytokine was detected in the serum of infected C57BL/6 mice (CFP-10 nonresponder haplotype) or in noninfected C3H/HeJ mice skin tested with the same antigenic preparation that elicited high serum levels of the cytokine in M. tuberculosis-infected C3H/HeJ mice (Fig. 2B).
FIG. 2.
Serum levels of TNF-α in mice inoculated intradermally with M. tuberculosis recombinant antigens. C3H/HeJ mice were infected i.v. with 105 CFU of M. tuberculosis H37Rv, and at day 14 postinfection they were inoculated intradermally (at the base of the tail) with various concentrations of CFP-10, with saline, or with LPS. Sera were obtained from blood drawn 2 h later from the retroorbital plexus and assayed for TNF-α by ELISA (A). (B) Serum TNF-α levels determined in both noninfected C3H/HeJ mice and M. tuberculosis-infected C57BL/6 mice skin tested 2 h previously with saline or with 50 μg of either CFP-10 or Mtb 72F antigen. The bars indicate the results obtained for three animals. The data are from one representative experiment of three experiments in which basically the same results were obtained.
The ability of CFP-10 to stimulate lymphoid cells to produce TNF-α was investigated next. C3H/HeJ mice were infected i.v. with virulent M. tuberculosis, and 2 weeks later the animals were sacrificed and mononuclear spleen cells were obtained and cultured for 72 h in the presence of either medium, CFP-10, Mtb 72F, or PPD. Culture supernatants were harvested and assayed by the ELISA for the presence of TNF-α. Figure 3 shows the results and indicates that CFP-10 stimulated the production of the cytokine, which is consistent with the in vivo systemic induction of TNF-α in infected C3H/HeJ mice. In contrast, Mtb 72F, a protective antigen in both mouse and guinea pig models of tuberculosis (25) that is readily recognized by sensitized cells of these animals, did not induce TNF-α. Not surprisingly, high concentrations of PPD (10 μg/ml) induced TNF-α production. These results support the general idea that different antigens of M. tuberculosis are involved in the immunopathology of tuberculosis. Therefore, because TNF-α is apparently one of the main mediators of tuberculin shock and of the pathology associated with Koch's phenomenon (24), these results point to Mtb 72F as a safe vaccine candidate and to CFP-10 as potential pathology promoter antigen.
FIG. 3.
TNF-α production by spleen cells of M. tuberculosis-infected mice. C3H/HeJ mice were infected i.v. with 105 CFU of M. tuberculosis and sacrificed 2 weeks later. Mononuclear spleen cells were obtained and stimulated for 3 days with various concentrations of CFP-10, Mtb 72F, or PPD. Supernatants were harvested and assayed for the presence of TNF-α by the ELISA.
Tuberculin shock in M. tuberculosis-infected guinea pigs skin tested with CFP-10.
Despite the fact that tuberculin shock is difficult to demonstrate in mice (13), in the experiments described above most infected C3H/HeJ mice skin tested with CFP-10 showed clear signs of lethargy or prostration that lasted for 1 to 10 h after the test (not shown). In fact, one mouse died ∼4 h after the skin test. Conversely, because guinea pigs have been traditionally employed to study tuberculin shock, this animal species was used to further investigate clinical side effects of inoculation of CFP-10 in M. tuberculosis-infected animals. Guinea pigs have been used for more than 100 years to standardize tuberculin preparations, and death (tuberculin shock) was indeed an important readout originally used by Robert Koch to evaluate the possible immunotherapeutic properties of tuberculin preparations (6, 16).
Hartley guinea pigs weighing approximately 400 g were infected i.v. with 2 × 104 CFU of M. tuberculosis (8), and 6 weeks later groups of animals were skin tested either with 10 μg of CFP-10 or with Mtb 39 (100-μl solution injected intradermally). The clinical outcome of the skin tests was monitored using the following readouts: (i) rectal temperature changes measured 2 h after the skin test; (ii) DTH at the inoculation site, expressed as the diameter of the induration reaction 20 to 24 h after the skin test; and (iii) animal death within 6 to 48 h after the skin test.
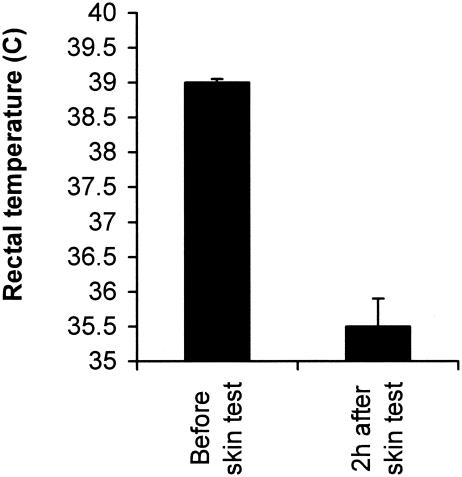
The results showed that in all animals intradermal inoculation of CFP-10 caused a drop in the rectal temperature of approximately ∼4°C (Fig. 4), which is consistent with both TNF-α and tuberculin shocks (13, 17, 23). No rectal temperature change was observed in infected guinea pigs skin tested with either saline, Mtb 39, or PPD (not shown). In addition, ∼60% of the guinea pigs developed DTH to Mtb 39 with an induration reaction of 8 ± 3 mm (not shown). In contrast, none of the animals skin tested with CFP-10 developed DTH; rather, a generalized cutaneous hyperemia and prostration occurred in 100% of the animals approximately 3 to 4 h after the skin test. No reaction (weal or erythematic) was seen at the skin test site immediately after inoculation of the antigen or at later times, which indicates that no immediate-type hypersensitivity was involved in the delayed upsurge of the generalized cutaneous hyperemia (not shown). However, within 6 to 36 h, 50% of the CFP-10-tested animals died (Table 1). The autopsies of these animals revealed intense polycythemia in the lungs associated with hemorrhagic patches dispersed throughout the organ's parenchyma (Fig. 5).
FIG. 4.
Body temperature changes in M. tuberculosis-infected guinea pigs skin tested with CFP-10. The rectal temperature was measured before and 2 h after the skin test using a digital thermometer. The bars indicate the standard errors of the means for temperatures taken from six animals. The data are from one representative experiment of two experiments in which basically the same results were obtained.
TABLE 1.
Death of M. tuberculosis-infected guinea pigs subsequent to skin tests performed with recombinant antigensa
| Antigen | Death rate (no. of deaths/no. tested) |
|---|---|
| CFP-10 | 5/10 |
| Mtb 39 | 0/6 |
Two groups of guinea pigs were infected with M. tuberculosis, and 6 weeks later the animals were skin tested with 10 μg of recombinant antigen (100 μl intradermally). The death of guinea pigs skin tested with CFP-10 occurred within 6 to 36 h after antigen inoculation. All guinea pigs were previously skin tested at 4 weeks postinfection with 2 μg of PPD, and 100% of them responded with DTH reactions ranging from 12 to 18 mm of induration (not shown).
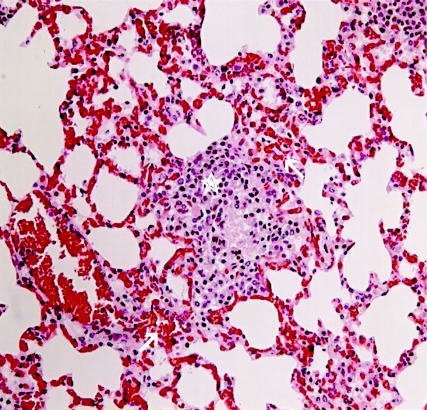
FIG. 5.
H&E-stained lung section of an M. tuberculosis-infected guinea pig skin tested with CFP-10. The lung was harvested during the autopsy of an M. tuberculosis-infected guinea and skin tested with 10 μg of CFP-10. The guinea pig died ∼10 h after the skin test. Lung sections were formalin fixed and H&E stained. Note the intense polycythemia in the lung tissue and hemorrhagic patches (arrows) surrounding a typical tuberculous granuloma (star). Identical pathology was observed in lung sections of six other infected animals that died after intradermal inoculation of CFP-10.
In addition, it is important to point out that noninfected control guinea pigs immunized with CFP-10 formulated with complete Freund's adjuvant and skin tested with the recombinant antigen developed typical DTH but no tuberculin shock (not shown). This observation is in full agreement with the notion that tuberculin shock happens as a consequence of an acute discharge of immunological mediators surrounding the tuberculous lesions present in vital organs, such as lungs. Moreover, it is important to mention that elicitation of tuberculin shock in infected guinea pigs after the skin test with CFP-10 occurred only in animals infected for at least 4 weeks (not shown), a time which coincides with the development of tuberculous lesions in the animal's lungs. Therefore, similar to the tuberculin shock elicited by old tuberculin, these results suggest that inoculation of CFP-10 in either BCG-vaccinated individuals or in persons with latent tuberculosis, in contrast to patients with active disease, should not result in this serious side effect because these persons do not have large tuberculous lesions in their tissues.
DISCUSSION
The results presented in this paper indicate that intradermal inoculation of CFP-10 in M. tuberculosis-infected guinea recapitulates all of the manifestations of a classical tuberculin shock. These findings are particularly relevant to current strategies aiming to use recombinant proteins for the development of either a subunit vaccine for tuberculosis or a more specific skin test reagent for diagnosis of the disease. For approximately 100 years the generalized toxemia or tuberculin shock that results from inoculation of infected animals or humans with Mycobacterium antigens has been considered to have less clinical significance because it occurs only when high concentrations of a crude antigen preparation are used. However, the current study warns that there could be a reoccurrence of this serious complication due to the possible use of highly purified recombinant proteins. These purified recombinant proteins have been tested as vaccine candidates or skin test reagents at doses ranging from 2 to 20 μg. These doses by far exceed the amounts, if any, in the preparations of crude or semipurified native antigens used in the current skin tests for diagnosis of tuberculosis (e.g., the Mantoux test). The conventional Mantoux test is performed with preparations of PPD containing 1 to 2 tuberculin units or approximately 0.2 μg of total Mycobacterium proteins. Tests performed with larger amounts, although rarely fatal in humans, can cause a febrile reaction and/or acute inflammation around old or recent tuberculosis lesions in the lungs and elsewhere in individuals with either latent or active disease. Therefore, vaccination or skin testing highly sensitive individuals with 2 to 20 μg of a recombinant antigen like CFP-10 is likely to result in serious consequences. However, in contrast to this suggestion, intradermal vaccination with BCG, despite exposing the hosts to large amounts of Mycobacterium antigens, is relatively safe from the immunological point of view and only rarely causes minor complications at the inoculation site in hypersensitive individuals. This clinical observation is nonetheless consistent with the premise proposed in the present study because the gene encoding CFP-10 (located in the RD1 genetic region of the M. tuberculosis genome) is deleted from all BCG strains (3, 18). Therefore, because BCG does not produce CFP-10 and possibly other immunologically potent antigens, vaccination with these organisms is less likely to elicit tuberculin shock. Although the data are circumstantial, this is an interesting possibility that deserves further investigation.
Finally, for the first time, in this work we identified and characterized a molecular mediator of the tuberculin shock, a phenomenon described over 100 years ago. The mechanism by which some M. tuberculosis antigens, such as CFP-10, sensitize the host during the infection process to develop a pathological response and other antigens, like Mtb 72F, induce protection, has not been resolved. However, the definition of a molecular mediator of tuberculin shock should help future investigators understand the mechanisms underlining these opposite responses.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health: AI 43528 to A. Campos-Neto and AI 44373 to S. G. Reed.
Editor: J. L. Flynn
REFERENCES
- 1.American Thoracic Society. 1981. The tuberculin skin test. Am. Rev. Respir. Dis. 124:356-363. [Google Scholar]
- 2.American Thoracic Society and Centers for Disease Control. 1991. Diagnostic standards and classification of tuberculosis. Am. Rev. Respir. Dis. 142:725-735. [DOI] [PubMed] [Google Scholar]
- 3.Berthet, F. X., P. B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology 144:3195-3203. [DOI] [PubMed] [Google Scholar]
- 4.Beutler, B. A., I. W. Milsark, and A. Cerami. 1985. Cachectin/tumor necrosis factor: production, distribution, and metabolic fate in vivo. J. Immunol. 135:3972-3977. [PubMed] [Google Scholar]
- 5.Brewer, T. F., and G. A. and Colditz. 1995. Relationship between bacille Calmette-Guerin (BCG) strains and the efficacy of BCG vaccine in the prevention of tuberculosis. Clin. Infect. Dis. 20:126-135. [DOI] [PubMed] [Google Scholar]
- 6.Burke, D. S. 1993. Of postulates and peccadilloes: Robert Koch and vaccine (tuberculin) therapy for tuberculosis. Vaccine 11:795-804. [DOI] [PubMed] [Google Scholar]
- 7.Colditz, G. A., T. F. Brewer, C. S. Berkey, M. E. Wilson, E. Burdick, H. V. Fineberg, and F. Mosteller. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698-702. [PubMed] [Google Scholar]
- 8.Coler, R. N., Y. A. Skeiky, P. J. Ovendale, T. S. Vedvick, L. Gervassi, J. Guderian, S. Jen, S. G. Reed, and A. Campos-Neto. 2000. Cloning of a Mycobacterium tuberculosis gene encoding a purified protein derivative protein that elicits strong tuberculosis-specific delayed-type hypersensitivity. J. Infect. Dis. 182:224-233. [DOI] [PubMed] [Google Scholar]
- 9.Dillon, D. C., M. R. Alderson, C. H. Day, T. Bement, A. Campos-Neto, Y. A. Skeiky, T. Vedvick, R. Badaro, S. G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dillon, D. C., M. R. Alderson, C. H. Day, D. M. Lewinsohn, R. Coler, T. Bement, A. Campos-Neto, Y. A. Skeiky, I. M. Orme, A. Roberts, S. Steen, W. Dalemans, R. Badaro, and S. G. Reed. 1999. Molecular characterization and human T-cell responses to a member of a novel Mycobacterium tuberculosis mtb39 gene family. Infect. Immun. 67:2941-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 12.Guinn, K. M., M. J. Hickey, S. K. Mathur, K. L. Zakel, J. E. Grotzke, D. M. Lewinsohn, S. Smith, and D. R. Sherman. 2004. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 51:359-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han, S. H., and R. S. Weiser. 1967. Systemic tuberculin sensitivity in mice. I. Factors contributing to active tuberculin shock. J. Immunol. 98:1152-1157. [PubMed] [Google Scholar]
- 14.Hsu, T., S. M. Hingley-Wilson, B. Chen, M. Chen, A. Z. Dai, P. M. Morin, C. B. Marks, J. Padiyar, C. Goulding, M. Gingery, D. Eisenberg, R. G. Russell, S. C. Derrick, F. M. Collins, S. L. Morris, C. H. King, and W. R. Jacobs, Jr. 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. USA 100:12420-12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huebner, R. E., M. F. Schein, and J. B. Bass, Jr. 1993. The tuberculin skin test. Clin. Infect. Dis. 17:968-975. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann, S. H. 2003. A short history of Robert Koch's fight against tuberculosis: those who do not remember the past are condemned to repeat it. Tuberculosis (Edinburgh) 83:86-90. [DOI] [PubMed] [Google Scholar]
- 17.Kettelhut, I. C., W. Fiers, and A. L. Goldberg. 1987. The toxic effects of tumor necrosis factor in vivo and their prevention by cyclooxygenase inhibitors. Proc. Natl. Acad. Sci. USA 84:4273-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohan, A. K., T. R. Cote, J. A. Block, A. M. Manadan, J. N. Siegel, and M. M. Braun. 2004. Tuberculosis following the use of etanercept, a tumor necrosis factor inhibitor. Clin. Infect. Dis. 39:295-299. [DOI] [PubMed] [Google Scholar]
- 20.Moreira, A. L., L. Tsenova, M. H. Aman, L. G. Bekker, S. Freeman, B. Mangaliso, U. Schroder, J. Jagirdar, W. N. Rom, M. G. Tovey, V. H. Freedman, and G. Kaplan. 2002. Mycobacterial antigens exacerbate disease manifestations in Mycobacterium tuberculosis-infected mice. Infect. Immun. 70:2100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. V. Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 22.Reed, S. G., and A. Campos-Neto. 2003. Vaccines for parasitic and bacterial diseases. Curr. Opin. Immunol. 15:456-460. [DOI] [PubMed] [Google Scholar]
- 23.Rook, G. A., and R. al Attiyah. 1991. Cytokines and the Koch phenomenon. Tubercle 72:13-20. [DOI] [PubMed] [Google Scholar]
- 24.Rook, G. A., and J. L. Stanford. 1996. The Koch phenomenon and the immunopathology of tuberculosis. Curr. Top. Microbiol. Immunol. 215:239-262. [DOI] [PubMed] [Google Scholar]
- 25.Skeiky, Y. A., M. R. Alderson, P. J. Ovendale, J. A. Guderian, L. Brandt, D. C. Dillon, A. Campos-Neto, Y. Lobet, W. Dalemans, I. M. Orme, and S. G. Reed. 2004. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J. Immunol. 172:7618-7628. [DOI] [PubMed] [Google Scholar]
- 26.Skeiky, Y. A., P. J. Ovendale, S. Jen, M. R. Alderson, D. C. Dillon, S. Smith, C. B. Wilson, I. M. Orme, S. G. Reed, and A. Campos-Neto. 2000. T cell expression cloning of a Mycobacterium tuberculosis gene encoding a protective antigen associated with the early control of infection. J. Immunol. 165:7140-7149. [DOI] [PubMed] [Google Scholar]
- 27.Trajkovic, V., G. Singh, B. Singh, S. Singh, and P. Sharma. 2002. Effect of Mycobacterium tuberculosis-specific 10-kilodalton antigen on macrophage release of tumor necrosis factor alpha and nitric oxide. Infect. Immun. 70:6558-6566. [DOI] [PMC free article] [PubMed] [Google Scholar]