Abstract
The human immune response to a new recombinant plague vaccine, comprising recombinant F1 (rF1) and rV antigens, has been assessed during a phase 1 safety and immunogenicity trial in healthy volunteers. All the subjects produced specific immunoglobulin G (IgG) in serum after the priming dose, which peaked in value after the booster dose (day 21), with the exception of one individual in the lowest dose level group, who responded to rF1 only. Three subjects, found to have an anti-rV titer at screening, were excluded from the overall analysis. Human antibody functionality has been assessed by quantification of antibody competing for binding to rV in vitro and also by the transfer of protective immunity in human serum into the naïve mouse. Human and macaque IgG competed for binding to rV in vitro with a mouse monoclonal antibody, previously shown to protect mice against challenge with plague, suggesting that this protective B-cell epitope on rV is conserved between these three species. Total IgG to rV in individuals and the titer of IgG competing for binding to rV correlated significantly at days 21 (r = 0.72; P < 0.001) and 28 (r = 0.82; P < 0.001). Passive transfer of protective immunity into mice also correlated significantly with total IgG titer to rF1 plus rV at days 21 (r2 = 98.6%; P < 0.001) and 28 (r2 = 76.8%; P < 0.03). However, no significant vaccination-related change in activation of peripheral blood mononuclear cells was detected at any time. Potential serological immune correlates of protection have been investigated, but no trends specific to vaccination could be detected in cellular markers.
Plague is a potentially fatal infection in humans caused by the bacterium Yersinia pestis. The existing vaccine comprises a suspension of heat-killed whole bacteria. Killed whole-cell vaccines have been in use in humans since 1946, and there is evidence that these formulations provided some protection against the flea-acquired bubonic plague which is endemic in parts of the world (5). However, there is evidence in mice, guinea pigs, and nonhuman primates that such a formulation provides little protection against the most severe pneumonic form of the disease caused by exposure to wild-type F1+ Y. pestis and no protection in mice challenged with an F1− Y. pestis strain (1, 2, 22, 28, 32).
Although Y. pestis produces a variety of potentially protective antigens (including F1 antigen, V antigen, and other Yersinia outer proteins and lipopolysaccharide), most workers consider that antibody against the F1 antigen is the key protective response induced by killed whole-cell vaccines, and no response to V antigen has been detected in mice immunized with these formulations. The killed whole-cell vaccine formulations have a high degree of heterogeneity with variable endotoxin content as well as a high incidence of transient local and systemic side effects, and they require frequent boosting to maintain immunity (3, 15, 19, 24, 25).
The killed whole-cell vaccines are known to be reactogenic in humans (18, 26), with malaise, headaches, local erythema, and induration or mild lymphadenopathy reported in approximately 10% of vaccinees. Allergic reactions induced by immunization with killed whole-cell vaccines, evidenced mainly as urticaria, occur infrequently (22). A live attenuated vaccine (EV76) has also been used in humans. However, in a study in the former USSR, a febrile response was reported in 20% of vaccinees, accompanied by headache, weakness, and malaise. Erythema surrounding the site of vaccination which could reach dimensions of 15 cm2 was frequently reported. Some severe systemic reactions required hospitalization. Numerous unsuccessful attempts were made to reduce the incidence of side effects by administering the vaccine by different routes, including scarification, inhalation, and even intraocularly (18).
Although the killed or attenuated vaccines described above have several shortcomings, they do indicate that protection against both the bubonic and pneumonic forms of plague is achievable. The protective efficacy of F1 antigen alone has been recognized for many years (17), and the immunization of human volunteers with F1 purified from Y. pestis was reported to produce protective immune responses, as assessed by passive transfer into mice (20). The forward development of a subunit vaccine is supported by two observations. First, the major antibody response to Y. pestis (in sera from either vaccinated or convalescent individuals) is known to be directed against the F1 antigen (27). Second, the V antigen has attracted attention as a subunit vaccine component (14) and the superior efficacy of the EV76 vaccine over killed whole-cell vaccines may be explained by the induction of an immune response to V as well as to F1 antigen by the live vaccine only (28).
The side effects of the killed whole-cell vaccines and live attenuated vaccines can be avoided by using F1 and V in a subunit vaccine, thus harnessing the combined protective benefits (28) of the F1 and V subunit antigens. Such vaccines comprising two recombinant proteins, designated rF1 and rV, which are administered as an injectable formulation adsorbed to alhydrogel, are in research (7) and development (31). Experimental evidence indicates that the combination of rF1 plus rV protects immunized animals against pneumonic plague (29). In general, Y. pestis is not endemic in the normal population, and hence it is not possible to carry out phase II/III efficacy clinical trials normally required for licensure. In addition, it is neither practical nor ethical to trial this vaccine for efficacy directly in humans.
Both the rF1 and rV proteins, administered in alhydrogel, have been demonstrated to be highly immunogenic and protective against virulent plague in a number of animal models: mice (11), guinea pigs (12), and cynomolgus macaques (unpublished data). Further, the combination of rF1 plus rV is additive in the protection conferred on the vaccinee (28). In the mouse, the combined immunoglobulin G1 (IgG1) titer to rF1 plus rV has been shown to correlate with protection against challenge (30). Further, protection against plague in the mouse has been demonstrated by the passive transfer of antiserum specific for rF1 plus rV from immunized BALB/c mice into naïve SCID/beige mice (6).
Previously, passive transfer of vaccinee serum from a number of species into the mouse has been carried out to determine the mouse protection index as a measure of whole-cell vaccine potency (16). This passive transfer assay was a 14-day assay in which the mouse protection index was quantified as % mortality over 14 days/average time to death, with a mouse protection index of less than 10 indicative of acceptable potency. Similarly, antiserum from a clinical trial volunteer can be used to immunize mice passively, prior to organism challenge of the recipient mice. This passive transfer of immunity comprising antibody to rF1 plus rV might be expected to be protective against plague, which is predominantly an extracellular infection in which native F1 and V are produced by Y. pestis as virulence factors. A single passive transfer of antiserum will not provide indefinite protection but will serve to inhibit the toxic effects of F1 and V produced by the invading pathogen. Thus, the in vivo phase of the passive transfer assay after a single administration of antiserum will be time-bounded.
Previously, a murine monoclonal antibody (MAb) specific for rV was shown to protect mice by passive immunization against challenge with Y. pestis (8). This MAb (7.3) recognizes an epitope in a region of rV which is required for protection against infection with Y. pestis (8). If this epitope is found to be conserved in different species, it could be exploited to develop a competitive enzyme-linked immunosorbent assay (ELISA) in which antisera from clinical trial volunteers are tested for the ability to compete with MAb 7.3 for binding to the rV antigen.
The assays outlined above have been applied to blood samples obtained from volunteers during a phase I clinical trial of the rF1 plus rV vaccine (designated rYP002), which was conducted in healthy adult males primarily to determine the safety of the vaccine. This phase I trial was conducted with fully ethical review of the protocol, and samples were collected with the fully informed consent of the volunteers. The rYP002 vaccine was administered in a double-blind, ascending-dose design, such that groups of six individuals received the vaccine at dose levels of 5 μg rF1 + 5 μg rV, 10 μg rF1 + 10 μg rV, 20 μg rF1 + 20 μg rV, or 40 μg rF1 + 40 μg rV. Attached to each dose group were two individuals who were administered placebo. The vaccine or placebo was administered to individuals in a two-dose intramuscular regimen, with the priming dose on day 1 and the booster dose on day 21. The immunogenicity of the vaccine in humans has been investigated to provide evidence of the development of protective immunity to plague.
MATERIALS AND METHODS
rYP002 vaccine.
The rF1 and rV antigens were produced in Escherichia coli from the expression systems previously described (11), under Good Manufacturing Practice conditions. The vaccine was formulated by adsorption to 20% (vol/vol) alhydrogel (Brenntag Biosector, Frederikssund, Denmark) per ml phosphate-buffered saline (PBS) at the required concentrations of each protein such that concentrations in the range 10 μg rF1 + 10 μg rV per ml up to 80 μg rF1 + 80 μg rV per ml were achieved in a final concentration of 0.26% (wt/vol) alhydrogel to achieve a molar ratio for rF1 to rV of 2:1, as previously described (30). The formulated vaccine was designated rYP002.
Phase 1 clinical trial design.
The rYP002 vaccine was administered to 24 healthy adult males in a double-blind, ascending-dose design, such that groups of six individuals received the vaccine at dose levels of 5 μg,10 μg, 20 μg, and 40 μg of each subunit in a dose volume of 0.5 ml. Attached to each dose group were two individuals who were administered placebo (alhydrogel in PBS), so that there were 32 subjects in the trial in total. The trial was conducted in Europe under protocol R24972 during 2001.
The vaccine or placebo was administered to individuals in a two-dose intramuscular regimen, with the priming dose on day 1 and the booster dose on day 21. For any one individual, the dose level given for priming and boosting was identical. Blood samples (10 ml) were obtained throughout the schedule as follows: days −1, 8, 15, 21, 28, 35, 70, and 91. Serum was separated from these samples, by centrifugation at 1500g for 10 min, and was stored frozen (−20°C) prior to assay for specific IgG and IgG subclass, titer of competing antibody by competitive ELISA and the determination of protective efficacy by passive transfer into mice. Whole blood samples (5 ml) were collected at intervals into sodium-heparin tubes (Vacutainer CPT, no. 362753, Becton Dickinson United Kingdom Ltd.) and transported at ambient temperature (18 to 25°C) for analysis, within 24 h of collection, of cellular responses by flow cytometry.
Titration of IgG to rF1 plus rV.
Human serum samples were assayed for total IgG titer specific for rF1 and rV by a Good Laboratory Practice-validated ELISA methodology modified from that previously described (29, 30) using peroxidase-conjugated antihuman antibodies (The Binding Site, Birmingham, United Kingdom) at 1:2,000 dilution in phosphate-buffered saline (PBS). The concentration of IgG to rF1 and rV in test samples was determined from a calibration curve constructed using MultiCalc (Wallac, Milton Keynes, United Kingdom).
The curve-fitting algorithm used was a five-parameter weighted logistical model. The lower limit of quantification was set at 97.7 U whole serum equivalents (0.977 U in assay). The IgG content of test samples was determined as an absolute value in units/ml serum. Mean values per dose level group have been determined with the calculation of standard errors of the mean. Each batch of test samples was analyzed with a set of calibration standards and quality control samples, and acceptability depended on at least four of the six quality control samples being within ±20% (±25% at the low quality control of their respective nominal values). A constant 1% (vol/vol) serum content (either nonhuman primate or human) was maintained in all calibration standards, serum controls, and test samples.
Isotyping of serum.
Serum isotyping was carried out by minor modification of a previously described procedure (29) which had been qualified as for the IgG ELISA. The distribution of IgG specific for F1 and V across the subclasses IgG1, IgG2, IgG3, and IgG4 was determined and is reported in U/ml.
Mean values per dose level group have been determined with the calculation of standard errors of the mean. The lower limit of quantification was defined as the lowest calibration standard with acceptable (<20%) intra-assay (n = 6) imprecision and inaccuracy. The lower limits of quantification for antibodies to rF1 were: IgG1, 279.9U; IgG2, 312.5 U; and IgG3, 134.9 U; and for antibodies to rV it was: IgG1, 31.3 U; IgG2, 156.3 U; IgG3, 78.1 U; and IgG4, 0.6 U).
Although 24 subjects were vaccinated in the trial, the data reported are for serial samples from 20 individuals, from screening to day 91. The remaining four individuals were excluded from the analysis for the following reasons: no samples were received at any time point from one individual in the 20 μg rYP002 group; serum samples from the remaining three individuals were assayed, but these data have been omitted from the overall data analysis due to the detection of an IgG titer (one individual in the 20-μg group) or IgG4 titer (one individual in the 20-μg group and one individual in the 40-μg group) to rV at screening.
Reference serum.
The reference serum used in subsequent assays was prepared by pooling polyclonal sera collected from hyperimmune cynomolgus macaques immunized with rF1 plus rV under Good Laboratory Practice conditions (study reference MOD 051) to generate a consistent batch (unpublished data) with estimated mean IgG titer to rF1 and rV of 3361 (standard error of the mean 464) and 3433 (standard error of the mean 714) U/ml, respectively. This polyclonal macaque serum was used as the reference serum in the competitive ELISA and passive transfer assays described below.
Competitive ELISA.
Individual serum samples collected in the period from days 21 to 70 were assayed by competitive ELISA in which the human serum competed with MAb 7.3 for binding to rV antigen coated to a microtiter plate. Briefly, rV antigen was coated to 96-well microtiter plates (Dynex) at 5 μg/ml in 0.05 ml PBS (16 to 18 h, 4°C). After washing in PBS with 0.02% Tween 20, plates were blocked with 0.2 ml 5% skimmed milk powder in PBS (37°C, 1 h). After further washing, 0.05 ml MAb 7.3 (1:32,000 in 1% skimmed milk powder in PBS) was added to each well (equivalent to 80 ng/well), and the plates were incubated at 4°C for 16 h. Normal mouse serum, also diluted to 1:32,000, was added to negative control wells (0.05 ml per well). Plates were then washed prior to adding the test serum at a dilution of 1:10 in 1% skimmed milk powder in PBS to starting wells and subsequently double diluting in 1% skimmed milk powder in PBS down the plate. Designated wells on the plate received the macaque reference serum and negative control sera, respectively. Test and control samples were assayed in duplicate. Plates were incubated (1 h, 37°C) prior to washing and addition of HRP-goat anti-mouse IgG (Serotec; 1:2,000 in PBS) followed by incubation (37°C, 1 h).
Plates were washed prior to addition of azinobis(3-ethylbenzthiozolinesulfonic acid) (ABTS) substrate (Sigma) with subsequent reading of optical density at 414 nm (OD414). The OD414 determined for each test and the reference serum was adjusted by subtraction of the OD414 determined for the appropriate negative control sera or normal mouse serum. The data were calculated from a titration curve for loss of binding of the mouse antibody, with increased concentration of human serum. From this titration curve, the percentage inhibition of binding of Mab7.3 to rV antigen in the presence of the test and reference serum (where each were at a 1:10 dilution) was determined, and these values were compared to achieve a ratio of percentage inhibition due to the test serum compared with the percentage inhibition due to the reference serum (test:reference). A sample was scored positive if the test:reference ratio was ≥1.0.
Estimation of the half-life of macaque antiserum in the mouse.
Since the passive transfer assay is based on the transfer of a bolus of exogenous macaque antiserum into the mouse, it was necessary to estimate the half-life (t1/2), in order to time-bound the assay. The reference serum was transferred by intraperitoneal injection into 5 naïve BALB/c mice and the concentration of macaque IgG in mouse blood was monitored by daily tail vein sampling.
Establishment of the passive transfer assay.
Equal aliquots of individual serum samples, collected at day 35 from volunteers in all the vaccine dose groups, were pooled by dose group prior to dilution in PBS. The pooled samples were used to passively immunize groups of five BALB/c female mice (Charles River United Kingdom) intraperitoneally (i.e., five recipient mice per donor serum dilution). The pooled serum samples were used undiluted or diluted in the range 75 to 6.3% prior to injection in a constant 0.5-ml volume. Additional groups of 5 mice received the reference serum, either undiluted or in the same dilution range as the test serum. Additional control groups of mice received human serum from the placebo group, either undiluted or diluted in the same range as the test sera.
All serum-treated mice were challenged by the subcutaneous route with 10 CFU equivalent to 10 median lethal doses of Y. pestis GB strain (23) at 2.5 h after passive immunization, together with a group of untreated mice. The survival of mice postchallenge was monitored for up to 10 days, with strict observation of humane endpoints, so that when animals developed the following associated signs which indicated they would not recover, they were culled: ruffled fur, hunched posture, isolation from cagemates, anorexia, and unresponsiveness to external stimuli. All mice surviving at 10 days were humanely culled.
Determination of transferable protective immunity.
Individual serum samples from volunteers collected at prestudy and on days 8, 15, 21, 28, 35, and 70 were passively transferred (intraperitoneally) into groups of five female BALB/c mice at a range of serum dilutions expected to confer approximately 80%, 50% (vol/vol), and 15% protection, as determined from the passive transfer of pooled sera described above. For each test serum, aliquots of 0.5 ml either undiluted or at dilutions in the range (75 to 25% in PBS) were used to passively immunize recipient mice intraperitoneally in groups of five, and the protection conferred was compared with that afforded by the reference serum, which was diluted across the same range. Subsequently, all passively immunized mice were challenged 2.5 h later with 10 median lethal doses of Y. pestis GB strain subcutaneously. The percent protection conferred by each test serum was compared with the percent protection conferred by the reference serum, when each was used at 50% (vol/vol) to passively immunize naïve mice, to achieve a potency ratio of test serum:reference serum. A sample was scored positive in this assay if the ratio of test:reference was ≥1.0
Flow cytometric analysis.
Blood samples were collected into sodium-heparin 8-ml cell separation tubes (Vacutainer CPT, no. 362753, Becton Dickinson United Kingdom Ltd.) and maintained at ambient temperature (18 to 25°C) for up to 24 h prior to analysis. Peripheral blood mononuclear cells (PBMC) were initially separated by centrifugation (3,300 rpm, 25 min) and then washed by transfer into 15 ml tubes with centrifugation (1,800 rpm, 5 min). The cell pellets were washed in staining buffer (phosphate buffered saline, 0.1% sodium azide and 3% human AB serum (Sigma Aldrich, United Kingdom), resuspended in 5 ml staining buffer and incubated for 25 min at room temperature to reduce nonspecific binding of antibody to the cell surface.
Cell surface marker staining was performed by adding the cell suspension (0.1 ml) to flow cytometry tubes, containing combinations of the following fluorochrome-labeled antibodies: CD4-fluorescein isothiocyanate (FITC), CD19-phycoerythrin (PE), CD3-energy-coupled dye (ECD), CD8-phycoerythrin cychrome 5 (PC5), CD8-PE, CD69-PC5, CD25-PE, CD45RO-ECD, HLA-DR-PC5, and CD8-FITC (Beckman Coulter United Kingdom). Samples were vortexed gently and refrigerated (4°C) for 20 min. Isoton II containing 2% paraformaldehyde was added to the samples, which were refrigerated until flow cytometric acquisition within 5 days of sample preparation. 50,000 events were collected in the lymphocyte region for each sample using a Coulter EPICS XL flow cytometer (Beckman Coulter). The positive population was identified using isotype controls.
Statistical analysis.
Data were analyzed with the statistical software package (MINITAB release 13.1) to achieve analysis of variance and of regression with Pearson's coefficient. Student's t test with n − 1 degrees of freedom was used to determine the significance of the difference between paired groups of equal size. The area under the curve was determined by the trapezoid approximation method as described (http://www.duncanwil.co.uk/areacurv.html).
RESULTS
Titration of IgG to rF1 plus rV in serum.
At screening, all individuals were below the lower limit of quantification except for one subject in the 20-μg dose group who was found to have an anti-rV titer prior to immunization, speculated to be due to a previous exposure to Yersinia enterocolitica. The immune response data for this individual has been omitted from the overall analysis. Sera from all individuals who received the placebo were below the lower limit of quantification for assay of IgG to rV and rF1, at every time point tested.
The immunized subjects produced specific antibodies against both subunits (rV and rF1) of the recombinant plague vaccine (rYP002). For each time point tested there was large variation of quantifiable antibody responses between individuals within each dose level group. Therefore, group mean ± standard error of the mean values and ranges have been derived and are presented in Tables 1 and 2. Antibodies to rV were produced within two weeks of the first dose at day 1, with greater levels observed after the booster dose at day 21 (Table 1). One individual in the 5-μg rYP002 group failed to respond with a titer to rV. Across the dose range studied, at least 50% of the maximum mean anti-rV titer was retained for up to 3 months following the first dose. Antibodies to rF1 were also generated within two weeks of the first dose at day 1, with greater levels observed after the booster dose at day 21 (Table 2). Across the dose range studied, at least 50% of the maximum mean anti-rF1 titer was retained for up to 3 months following the first dose, although lack of sample numbers made this difficult to assess for the 20-μg group (Table 2).
TABLE 1.
Anti-V serum IgG concentrationsa
| Day | Serum IgG concn (U/ml)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 μg rYP002
|
10 μg rYP002
|
20 μg rYP002
|
40 μg rYP002
|
|||||||||
| n | Mean (SEM) | range | n | Mean (SEM) | Range | n | Mean (SEM) | Range | N | Mean (SEM) | Range | |
| −1 | 6 | * | 6 | * | 3 | * | 5 | * | ||||
| 8 | 6 | # | <98-136 | 6 | # | <98-910 | 3 | * | 5 | # | <98-4,140 | |
| 15 | 6 | 7,546 (5,007) | <98-32,040 | 6 | 18,248 (7,171) | <98-46,320 | 3 | 1,053 (629) | 335-2,304 | 5 | 35,617 (17,794) | <98-90,190 |
| 21 | 6 | 6,431 (4,380) | <98-28,020 | 6 | 16,584 (6,644) | 372-40,930 | 3 | 1,242 (7605) | 415-2769 | 5 | 27,218 (14,515) | 119-70,790 |
| 28 | 6 | 6,657 (4,103) | <98-26,780 | 6 | 18,590 (6,056) | 703-36,970 | 3 | 2,936 (792) | 1,521-4,256 | 5 | 24,494 (12,020) | 942-58,010 |
| 35 | 6 | 5,975 (3,306) | <98-21,950 | 6 | 20,742 (7,095) | 973-44,390 | 3 | 5,334 (2,101) | 1,488-8,713 | 5 | 26,426 (12,658) | 1,203-64,310 |
| 70 | 6 | 4,991 (2,712) | <98-18,110 | 6 | 15,751 (6,028) | 430-36,760 | 3 | 5,008 (2,900) | 646-10,490 | 5 | 17,762 (8,692) | 820-45,490 |
| 91 | 6 | 4,499 (2,411) | <98-16,140 | 6 | 13,511 (5,797) | 303-37,880 | 2 | 3,137 (2,669) | 475-5,798 | 4 | 15,013 (6,337) | 1,071-33,660 |
Rounded values are shown. n, number of subjects on which determination of the mean and range was based. Negative samples were included as zero in calculation of the mean. The lower limit of quantification (LLQ) was <98 U/ml. *, No range calculable; all samples were below the LLQ. #, No mean calculable; one or two samples positive only.
TABLE 2.
Anti-F1 serum IgG concentrationsa
| Day | Serum IgG concn (U/ml)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 μg rYP002
|
10 μg rYP002
|
20 μg rYP002
|
40 μg rYP002
|
|||||||||
| n | Mean (SEM) | Range | n | Mean (SEM) | Range | n | Mean (SEM) | Range | n | Mean (SEM) | Range | |
| −1 | 6 | * | 6 | * | 3 | * | 5 | * | ||||
| 8 | 6 | 135 (65) | <98-377 | 6 | 198 (151) | <98-944 | 3 | * | 5 | 271 (102) | <98-572 | |
| 15 | 6 | 1,584 (1,127) | 140-7,114 | 6 | 1,432 (539) | 234-3,733 | 3 | # | <98-5,336 | 5 | 2,066 (737) | 358-4,632 |
| 21 | 6 | 1,027 (664) | 116-4,247 | 6 | 1,980 (504) | 127-2,907 | 3 | 3,922 (3,634) | 263-10,980 | 5 | 3,031 (940) | 373-6,205 |
| 28 | 6 | 2,051 (881) | 224-6,161 | 6 | 5,550 (1,499) | 990-11,580 | 3 | 6,590 (4,400) | 3,743-19,970 | 5 | 9,229 (1,268) | 4,510-12,160 |
| 35 | 6 | 2,137 (1,062) | 311-7,335 | 6 | 5,961 (1,554) | 1,649-12,130 | 3 | 12,076 (7,847) | 4,028-27,750 | 5 | 10,298 (1,598) | 4,272-13,860 |
| 70 | 6 | 1,766 (649) | 446-4,714 | 6 | 3,905 (939) | 526-6,504 | 3 | 6,341 (3,170) | 2,627-12,640 | 5 | 7,040 (1,209) | 4,110-11,270 |
| 91 | 6 | 1,534 (519) | 334-3,875 | 6 | 3,409 (871) | 199-5,434 | 2 | 2,246 (53) | 2,193-2,299 | 4 | 7,691 (2,637) | 4,128-15,520 |
See Table 1, footnote a.
Relationship between dose level of vaccine and serum IgG response.
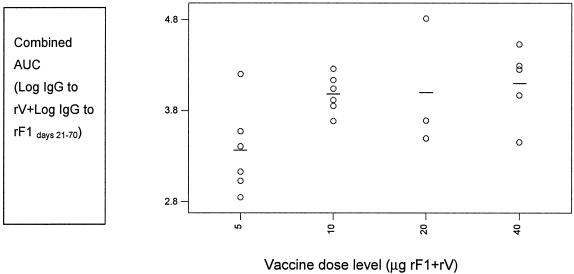
To study a possible dose-response effect, an area-under-the curve (AUC) estimate of IgG serum response to rF1 and to rV was calculated for each subject, during the period days 21 to 70, for each of the vaccine dose groups. The variation in response to both antigens between individuals within each dose group has been studied by plotting the AUC value for combined IgG to rF1 plus rV in the period days 21 to 70 (Fig. 1). A one-way ANOVA was conducted to establish the effect of rYP002 dose level on the serum IgG response over time. The effect of vaccine dose on the combined IgG response to rF1 and rV was statistically significant (f = 3.26, P < 0.05); however the influence of dose on the IgG response to F1 (f = 4.22, P = 0.02) was greater than that on the IgG response to rV (f = 0.93, P = 0.45). The ANOVA indicated that the mean IgG values for rF1 alone, or for rF1 plus rV in combination, were significantly lower for the 5-μg dose group than the means for the other three dose groups. There was no significant effect of vaccine dose on the IgG response to rV.
FIG. 1.
Interdependence of dose level and IgG response to rF1 and rV in individuals across groups. Combined AUC for individuals versus vaccine dose level.
Relationship between serum IgG response and time.
To study the time trends in specific IgG response for each subject, regression analysis of the serum IgG values with time (days 28 to 91) has been carried out. A one-way ANOVA of the resulting slopes as a function of vaccine dose level showed no significant treatment effect for either rF1 (f = 1.27, P = 0.32) or rV (f = 0.92, P = 0.45). The average rate of decline in IgG titer was estimated at 2% per week for rF1 and rV.
Isotyping of serum.
For all subclasses assayed, titers generally developed after the second dose of vaccine and peaked at approximately days 28 to 35. The dominant response to rV was IgG1 followed by IgG2, although the 20-μg dose group showed a strong IgG3 response. By comparison, low levels of IgG4 were generally detected to rV, although one individual in each of the 20-μg and 40-μg groups had a prevaccination IgG4 titer (assumed nonspecific) and developed very high levels of IgG4. The data from these individuals have been omitted from the overall serological analysis. No IgG4 to rF1 was detected at any dose level. The dominant subclass to rF1 was IgG1, closely followed by IgG2 and IgG3.
To study a possible dose-response effect, the IgG1 values for each subject in the period days 21 to 91 were combined to form an AUC for IgG1 to rF1 and rV, allowing comparison between groups. A one-way ANOVA of these AUC values as a function of vaccine dose level showed no significant treatment effect of either rF1 (f = 1.82, P = 0.19) or rV (f = 0.87, P = 0.48).
Competitive ELISA.
Serum samples from individuals in each of the dose groups in the period days 21 to 70 of the immunization schedule were also assayed for their ability to compete with a constant concentration of the murine MAb 7.3 for binding to rV on a solid phase. The human serum displaced the mouse monoclonal antibody, to produce a titration curve for loss of binding of the mouse antibody, with increased concentration of human serum. The percentage inhibition of binding of MAb 7.3 to rV in the presence of the test and reference serum (where each were at a 1:10 dilution) was calculated for each individual at each time point and a ratio of test:reference serum derived.
Effect of dose level on the development of competing antibody.
When the development of an antibody titer which competed with MAb 7.3 for binding to rV was monitored by competitive ELISA with time, the majority of individuals in the 10-μg, 20-μg, and 40-μg rYP002 groups had competitive antibody by day 28, whereas the majority of individuals in the 5 μg group (5/6) had competitive antibody by day 70.
Effect of time on development of competing antibody.
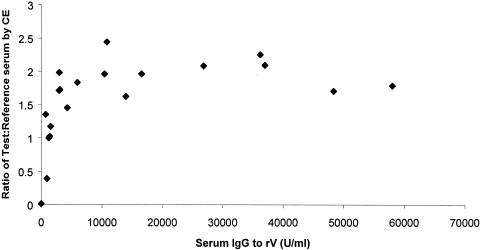
When the total number of individuals across the immunization groups with competitive antibody to rV was monitored using the competitive ELISA assay, the following proportions had developed antibody which competed with MAb 7.3 for binding to rV: 12/20 at day 21, 18/20 at day 28, and 14/20 at day 70. A one-way ANOVA of IgG titer to rV (expressed as an AUC for rV over days 28 to 91) as a function of vaccine dose showed no significant effect of treatment (f = 0.92, P = 0.45). However, Pearson correlation of log10 IgG to rV at days 21, 28, and 70 for all individuals across all rYP002 dose groups, against the ratio of test:reference serum determined by competitive ELISA, showed a significant correlation at days 21 (r = 0.72, P < 0.001) and 28 (r = 0.82, P = <0.001) but not at day 70 (r = 0.43, P = 0.06) when serum IgG titers were declining.
The relationship between serum IgG to rV and the test:reference serum ratio for all individuals across the rYP002 dose groups at day 28 is shown (Fig. 2). The one individual who had a preexisting IgG titer to rV as well as the two individuals with a preexisting IgG4 titer to rV were not included in the regression analysis, but their serum samples were assayed by competitive ELISA. The individual from the 20-μg rYP002 group with preexisting IgG titer was positive by competitive ELISA at days 8, 21, 28, and 70 (data not shown). This was not the case for the other two individuals (one from each of the 20-μg and 40-μg rYP002 groups) who were negative by competitive ELISA at days 8 and 21 but positive at days 28 and 70.
FIG. 2.
Correlation of IgG to rV with test:reference serum ratio determined by competitive ELISA for day 28 serum samples. The ratios are plotted against individual serum IgG titers to rV.
The mean AUC21-70 for IgG to rV for all individuals with a titer of competing antibody by competitive ELISA and the mean AUC for IgG to rV for all individuals without detectable competing antibody, were compared using a Student's t test. There was a significant difference between the means at day 21 (t = 3.08, P = <0.01) but not at days 28 or 70.
Estimation of the half-life of the reference serum in the mouse.
The t1/2 of the reference serum, which comprised a polyclonal macaque IgG specific for rF1 plus rV, in the mouse was estimated to be 8 days for IgG to rV and six days for IgG to rF1, with a decline to undetectable levels by 10 days (data not shown). The duration of the passive transfer assay was therefore limited to 10 days. As for the test sera, the dominant isotype in the reference serum was IgG1.
Establishment of passive transfer assay.
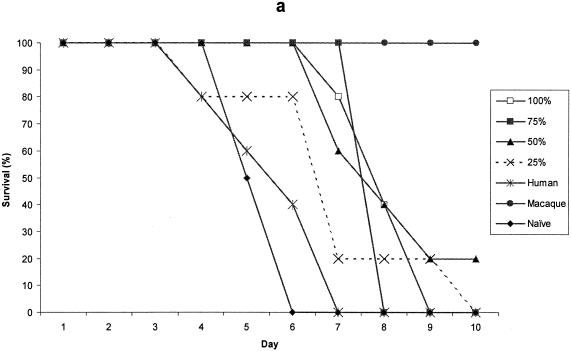
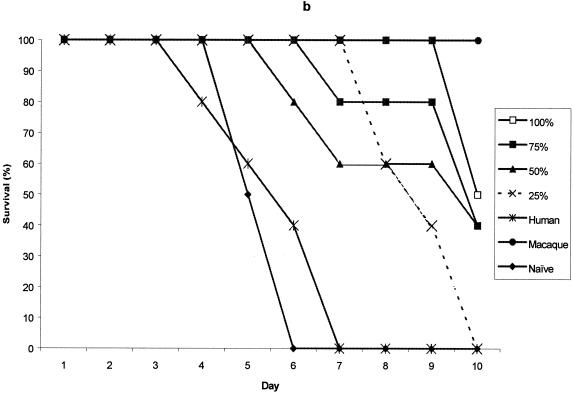
Pooled test sera from each of the vaccine dose groups on day 35, conferred some degree of protective immunity on recipient mice for the first 7 days postchallenge. The protection conferred was dose-related, so that it declined with dilution of the serum. Mice receiving human serum from the placebo groups did not survive and neither did untreated control mice. The assay was terminated at day 10, at which time pooled serum from the 40-μg rYP002 group was outperforming sera from the other dose groups (data not shown).
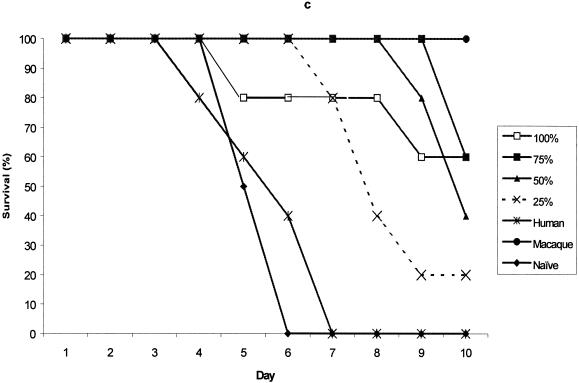
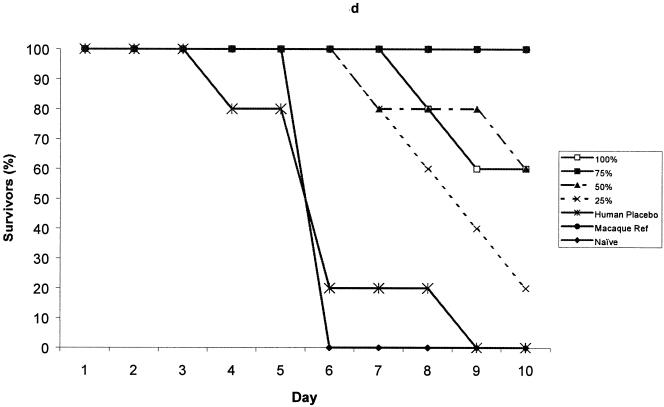
The protection provided by pooled test sera, collected from each of the rYP002 dose groups, was compared with that provided by the reference serum, either undiluted or diluted across the same range (75% to 25% in PBS). The data for day 35 serum samples from the 5 μg rYP002, 10 μg rYP002, 20 μg rYP002, and 40 μg rYP002 groups are shown in Fig. 3. Although sera from all the groups except the 5 μg rYP002 group conferred some partial protection at this time point, only sera from the 40 μg rYP002 group conferred protection equivalent to the reference serum (Fig. 3d).
FIG. 3.
Survival of mice passively immunized with day 35 serum pooled from the 5-, (a), 10-, (b), 20-, (c), and 40-μg (d) dose groups prior to challenge. Values are survival rates postchallenge for up to 10 days. Negative control mice received human placebo serum (human) or were naive.
Determination of transferable protective immunity for individual serum samples.
The effect of time and dose level on the development of transferable protective immunity was studied by repeating the passive transfer experiments with individual (rather than pooled) sera collected from the 20 and 40 μg rYP002 dose groups at days 21, 28, and 70. Serum samples from all individuals receiving the placebo were tested and at each time point were negative in the passive transfer assay. At day 21, serum from only 4/8 vaccinated individuals tested was scored positive by passive transfer with a test:reference ratio ≥1.0; at day 28 3/8 sera from vaccinees scored positive. Passive transfer of individual sera from the 10 μg rYP002 dose group was tested only at day 70, at which time only 2/6 individuals were scored positive. At this time point, 3/5 individuals tested in the 40 μg rYP002 group were also positive by passive transfer bringing the overall total to 5/14 sera tested scoring positive by passive transfer at day 70.
Sera from the three individuals who were excluded from the analysis due to an antibody titer to rV at screening were also tested by passive transfer, in an attempt to determine whether the preexisting titer was protective. When tested at the day 21 and 28 time points, none of these sera were scored positive in the passive transfer assay. By day 70, only one of the three sera was scored positive, indicating that the preexisting IgG4 titer to rV in this individual from the 20 μg rYP002 group did not confer any cross-protection as determined by this assay.
Correlation between total IgG to rF1 plus rV and transferable protective immunity.
For all individuals across the dose groups, the specific log10 values for IgG to rF1 and to rV have been determined and a regression analysis for these values against the ratio of test:reference derived from the passive transfer assay at days 21 to 70 has been conducted. Significant correlations were obtained at day 21 for IgG to rF1 and rV (r2 = 98.6%; P < 0.001). At day 28, IgG to rV only was significantly related to the test:reference ratio (r2 = 76.8%; P < 0.03), whereas at day 70, only the IgG to rF1 was significantly related to the ratio, but the degree of correlation was weak (r = 55.7%, P < 0.01).
The difference between the mean combined AUC21-70 for IgG to rF1 plus rV for all individuals with a positive score by passive transfer and the mean combined AUC for IgG to rF1 plus rV for all individuals without a positive score by passive transfer, was compared by Student's t test but was not significant at any time point.
Cell counts.
Prior to vaccination, leukocyte counts in peripheral blood ranged between 3.99 × 109 and 1.32 × 1010 cells/ml and for most subjects were within the accepted normal range. There was wide variation between individuals and no significant effect of vaccination could be detected.
In contrast, lymphocyte counts which comprised 22.1 to 44.6% of leukocytes, were within the normal range for all subjects prior to immunization, and fluctuated within the normal range until immediately after the second dose was administered, when they decreased. This decrease was attributed to a loss of cells to the draining lymph nodes (9) and also occurred for the placebo group, possibly in response to the injection of alhydrogel as a mild immunostimulant. Normal levels of lymphocytes, as a percentage of leukocytes in the peripheral blood, were restored from day 35.
Flow cytometric analysis.
The response to immunization involves activation at the cellular level. Flow cytometric analysis was carried out to investigate whether immunization resulted in changes in markers of cellular activation or gross changes in cell counts and whether either of these represents an immune correlate. However, large intra- and interindividual variation existed in all treatment groups including placebo, and thus the median was used to describe group tendencies.
Within the lymphocyte population, changes in the number and percentage of CD 19+ B cells, CD3+CD4+ T helper cells and CD3+CD8+ cytotoxic T cells in peripheral blood were monitored at several time-points following immunization in comparison with preimmunization levels. Large natural interindividual variation existed prior to immunization, in all lymphocyte parameters investigated. No significant differences were observed in the number or percentages of B-cell and T-cell populations between placebo and any dose group at any time-point following immunization and in comparison with preimmunization levels.
The expression of the memory and activation markers CD45RO, CD25, and HLA-DR was investigated on CD4+ and CD8+ lymphocytes. High expression of CD45RO was determined to examine memory lymphocyte populations. CD25 and HLA-DR were examined on CD45ROhi lymphocytes and CD45RO negative cells. Again, large variation existed between individuals and no significant differences were detected in the expression of activation or memory markers between placebo and any dose group. No significant differences were found in expression of activation or memory markers between baseline and any time-point postimmunization.
DISCUSSION
This is the first reported clinical trial for safety and immunogenicity of a recombinant subunit vaccine for plague. None of the formerly licensed killed whole-cell plague vaccines have been subjected to a randomized clinically controlled field study in humans (10). Although controlled clinical studies are desirable, the sporadic incidence of plague in endemic areas of the world means that such studies would be difficult to conduct. Thus, an assessment of efficacy for the rYP002 vaccine will depend on the determination of immune correlates of protection which this small study has started to investigate.
The measurement of specific IgG titers to rF1 and rV in groups of immunized individuals revealed that the vaccine was immunogenic, but that there was a wide range of responses in each dose group, as expected for the human immune response. The dominant subclass was IgG1, with some IgG2 and IgG3 to both antigens, while IgG4 was detectable to rV antigen only. This subclass profile in humans is indicative of a Th2 response, which is the anticipated response to this vaccine since this has also been seen in the mouse (11, 30) and nonhuman primates (unpublished data).
The serological data reported are for 20 of the 24 individuals in the trial. While no samples were received from one individual, three further individuals were excluded from the analysis. Serum from one individual had an IgG titer to rV at screening and competed with MAb 7.3 for binding to rV as early as day 8, but conferred no protection by passive transfer at day 21 or later. A prior environmental exposure to Yersinia enterocolitica could have induced an anti-V titer which cross-reacted with the Y. pestis sequence rV. The fact that serum from this individual did not score positive by passive transfer at any time point, despite high IgG titers to rV, suggests that the preexisting anti-V titer conferred no protective benefit. On the other hand, two individuals with an apparent IgG4 titer to rV at screening but who were negative by competitive ELISA at days 8 and 21 appeared to respond to the vaccine at day 28 with positive scores by competitive ELISA; one of these individuals had a positive score by passive transfer at day 28, the other was negative at all time points tested. This sample size (n = 3) is too small to draw any sound conclusions about the effect of preexisting titers to V, possibly due to exposure to another species of Yersinia, on protection against plague.
Some relationship between serum IgG titer specific for rV and the ability of this antibody to compete with MAb 7.3 for binding to rV has been demonstrated in the period days 21 to 70 for the 20 subjects included in the data analysis. The number of individuals determined to have a titer of competing antibody increased to a maximum of 18/20 at day 28, 7 days after administration of the second dose. Regression analysis of serum IgG titer to rV with the test:reference ratio by competitive ELISA indicated a significant correlation across the vaccine dose groups, at days 21 and 28. However, no significant correlation was found at day 70, when IgG titers in serum were in decline for all the dose groups.
The competitive ELISA assay measured antibody competing with MAb 7.3 for binding to rV only, whereas the passive transfer assay measured the protective capacity of antibody directed to both antigens. Evidence of transferable protective immunity by passive transfer was detected in individuals in the two highest dose groups (20 μg and 40 μg) at days 21 and 28, with individuals in the 10-μg group also becoming positive by day 70, so that a maximum of 5/14 individuals tested were positive in the passive transfer assay at day 70. Nevertheless, a significant correlation was found between total IgG to rF1 and rV and transferable protective immunity by passive transfer at day 21; at day 28, the IgG titer to rV only correlated significantly with the test:reference serum ratio determined in the passive transfer assay. In contrast, the IgG titer to rF1 only, correlated with protective immunity measured by passive transfer at day 70.
In the 6 weeks (days 28 to 70), titers were in gradual decline (estimated at 2% per week); however protection by passive transfer was maximal at day 70. Thus, it is not surprising that statistical correlation between the two indices was lost. It is likely that although circulating antibody titers were in decline from peak values around day 35, residual antibody was still functional and indeed would have been undergoing affinity maturation which would off-set the decline in titer. The macaque reference serum was very protective when passively transferred into the mouse. Both the macaque and human sera were predominantly of the IgG1 isotype and would be expected to have a similar half-life in the mouse (4).
In the competitive ELISA, the human sera were able to displace MAb 7.3 from rV as effectively as the reference serum, suggesting that each source of serum was of similar binding affinity to rV. Thus, the finding that only 5 of 14 human sera tested at day 70 were more protective than the reference serum by passive transfer, would suggest that the human response to this vaccine has not plateaued at the 40-μg maximum dose level used here and that higher dose levels of vaccine should be considered for future clinical trials. However, the application of the competitive ELISA and passive transfer assays has demonstrated that individuals enrolled in the phase I clinical trial have developed antibody competing with MAb 7.3 for binding to rV and transferable protective immunity which correlates at early time points with the serum IgG titer to rF1 and rV.
The immune correlates of protection investigated here rely on measuring the function of specific serological antibody. The assays suffer the disadvantage that the natural decline in functional antibody with time (in the absence of a further booster dose) may render the assays less accurate and predictive at later time points. The data presented above indicate that beyond day 28, the competitive ELISA and passive transfer assays no longer give significant correlation with total IgG to rV and/or rF1. However, it is expected that cellular memory to the vaccine will be maintained for many months. Exposure of an immunized individual to plague, even in the presence of only a low antibody titer, would stimulate memory B and T lymphocytes, triggering rapid antibody production. An assay of cell-mediated immunity would be advantageous in determining the degree of memory recall response which prevails in the individual after serum antibody titers have declined.
To this end, whole blood samples from individuals in the phase I clinical trial were subjected to flow cytometric analysis to determine activation status and recall response of their immune effector cells, principally B- and T-cell repositories of immunological memory. Large variations in cell counts and surface markers were expected and observed between individuals within each dose group. A decrease in the numbers of lymphocytes as a percentage of the PBMC population was observed at 24 h after the booster dose of rYP002 at all dose levels of vaccine and also of placebo. This temporary depletion may be due to migration, activation, and subsequent antibody secretion in the spleen and/or lymph nodes (9).
The natural variation in display of cell surface markers by individuals within each vaccine dose group as well as the placebo group meant that no significant changes could be detected that were related to vaccination. However, neither were any pathophysiological alterations observed in lymphocyte subsets as a result of vaccination. Assays of cell-mediated immunity are under development in which immune effector cells are derived from a vaccinee's whole blood sample and their activation on reexposure to the rF1 and rV antigens in vitro is determined in order to assess the degree of memory an individual has for the vaccine. They would be particularly valuable to apply when antibody titer has declined to low levels, in order to determine the need for a booster dose.
In summary, this is the first report of the human immune response to this recombinant vaccine for plague. The data gained indicate that the vaccine was immunogenic in recipients at all dose levels tested, although data from only three subjects were available at the 20-μg dose level and the lowest dose level (5 μg) was suboptimal. The titer of specific IgG developed by individuals at 21 and 28 days post-initial vaccination and 7 days postboost correlated with the development of a titer of antibody competing for binding to the rV antigen and with transferable protective immunity.
There has been debate as to whether plague is an eradicable disease, given the existence of plague reservoirs in wild rodent populations in certain parts of the world (13). Although bubonic plague is primarily a disease of rodents that is spread by fleas in nature, humans are occasionally infected either by flea bite or by inhalational exposure, usually through a secondary host, for example, a wild rabbit or prairie dog or domestic cat or, rarely, through another infected person (21). The future availability of this vaccine, to provide more comprehensive protection in humans, will bring the goal of disease prevention or eradication closer.
Acknowledgments
We are grateful to Avecia Biotechnology for vaccine manufacture and supply and to Debbie Bell, Debbie Rogers, Dave Rawkins, Mark Brown, Tony Stagg, and Rosa Taylor for expert technical assistance. We are grateful to T. G. Brookes and A. Simpson for clinical advice and to G. E. Westlake for critical review.
Editor: D. L. Burns
REFERENCES
- 1.Anderson, G. W., S. E. C. Leary, E. D. Williamson, R. W. Titball S.L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Recombinant V antigen protects mice against pnuemonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun. 64:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, G. P., D. G. Heath, G. W. Anderson, Jr., S. L. Welkos, and A. M. Friedlander. 1996. Fraction 1 capsular antigen (F1) purification from Yersinia pestis CO92 and an Escherichia coli recombinant strain and efficacy against lethal plague challenge. Infect. Immun. 64:2180-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartelloni, P. J., J. D. Marshall, Jr., and D. C. Cavanaugh. 1973. Clinical and serological responses to plague vaccine U.S.P. Mil. Med. 138:720-722. [PubMed] [Google Scholar]
- 4.Brekke, O. H., and I. Sandlie. 2003. Therapeutic antibodies for human diseases at the dawn of the twenty-first century. Nat. Rev. Drug Discovery 2:52-62. [DOI] [PubMed] [Google Scholar]
- 5.Cavanaugh, D. C., B. L. Elisberg, C. H. Llewellyn, J. D. Marshall, J. H. Rust, J. E. Williams, and K. F. Meyer. 1974. Plague immunization. V. Indirect evidence for the efficacy of plague vaccine. J. Infect. Dis. 129:S37-S40. [DOI] [PubMed] [Google Scholar]
- 6.Green, M., D. Rogers, P.Russell, A. J. Stagg, D. L. Bell, S. M. Eley, R. W. Titball and E. D. Williamson. 1999. The SCID/beige mouse as a model to investigate protection against Yersinia pestis. FEMS Immunol. Med. Microbiol. 23:107-113. [DOI] [PubMed] [Google Scholar]
- 7.Heath, D. G., G. W. Anderson, J. M. Mauro, S. L. Welkos, G. P. Andrews, J. Adamovicz, and A. M. Friedlander. 1998. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 16:1131-1137. [DOI] [PubMed] [Google Scholar]
- 8.Hill, J., S. E. C. Leary, K. F. Griffin, E. D. Williamson, and R. W. Titball. 1997. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect. Immun 65:4476-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janeway, C. A., Jr., P. Travers, M. Walport, and J. D. Capra. 1999. Immunobiology: the immune system in health and disease, 4th ed. Garland, London, England.
- 10.Jefferson, T., V. Demicheli, and M. Pratt. 2001.Vaccines for preventing plague (Cochrane Review). The Cochrane Library, vol. 4. Oxford University, Oxford, England. [DOI] [PMC free article] [PubMed]
- 11.Jones, S. M., F. Day, A. J. Stagg and E.D.Williamson. 2001. Protection conferred by a fully recombinant sub-unit vaccine against Yersinia pestis in male and female mice of four inbred strains. Vaccine. 19:358-366. [DOI] [PubMed] [Google Scholar]
- 12.Jones, S. M., K. F. Griffin, I. Hodgson, and E. D. Williamson. 2003. Protective efficacy of a fully recombinant plague vaccine in the guinea pig. Vaccine. 21:3912-3918. [DOI] [PubMed] [Google Scholar]
- 13.Keeling, M. J., and C. A. Gilligan. 2000. Metapopulation dynamics of bubonic plague. Nature 407:903-906. [DOI] [PubMed] [Google Scholar]
- 14.Leary, S. E. C., K. F. Griffin, H. S. Garmory., E. D. Williamson, and R. W. Titball. 1997. Expression of an F1/V fusion protein in attenuated Salmonella typhimurium and protection of mice against plague. Microb. Pathog. 23:167-179. [DOI] [PubMed] [Google Scholar]
- 15.Marshall, J. D., P. J. Bartelloni, D. C. Cavanaugh, P. J. Kadull, and K. F. Meyer. 1974. Plague immunisation. II. Relation of adverse clinical reactions to multiple immunisations with killed vaccine. J. Infect. Dis. 129:S19-S25. [DOI] [PubMed] [Google Scholar]
- 16.Marshall, J. D., D. C. Cavanaugh, P. J. Bartelloni, and K. F. Meyer. 1974. Plague immunization. III. Serologic response to multiple inoculations of vaccine. J. Infect. Dis. 129:S26-S29. [DOI] [PubMed] [Google Scholar]
- 17.Meyer, K. F. 1953. Recent studies on the immunity response to administration of different plague vaccines. Bull. W.H.O. 9:619-636. [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer, K. F., D. C. Cavanaugh, P. J. Bartelloni, and J. D. Marshall. 1974. Plague immunization. I. Past and present trends. J. Infect. Dis. 129:S13-S18. [DOI] [PubMed] [Google Scholar]
- 19.Meyer, K. F., G.Smith, L. E. Foster, J. D. Marshall, and D. C. Cavanaugh. 1974. Plague immunization. IV. Clinical reactions and serological responses to inoculations of Haffkine and freeze-dried plague vaccine. J. Infect. Dis. 129:S30-S36. [DOI] [PubMed] [Google Scholar]
- 20.Meyer, K. F., J. A. Hightower, F. R. McCrumb. 1974. Plague immunization. VI. Vaccination with the fraction1 antigen of Yersinia pestis. J. Infect. Dis. 129:S41-45. [DOI] [PubMed] [Google Scholar]
- 21.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis-etiologic agent of plague. Clin. Microbiol. Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reisman, R. E. 1970. Allergic reactions due to plague vaccine. J. Allergy 46:49-56. [DOI] [PubMed] [Google Scholar]
- 23.Russell, P., S. M. Eley, S. E. Hibbs, R. J. Manchee, A. J. Stagg, and R.W. Titball. 1995. A comparison of plague USP and EV76 Vaccine induced protection against Yersinia pestis in a murine model. Vaccine 13:1551-1556. [DOI] [PubMed] [Google Scholar]
- 24.Speck, R. S., and H. Wolochow. 1957. Studies on the experimental epidemiology of respiratory infections. VIII. Experimental pneumonic plague in Macacus rhesus. J. Infect. Dis. 100:58-69. [DOI] [PubMed] [Google Scholar]
- 25.von Metz, E., D. M. Eisler, and G. A. Hottle. 1971. Immunogenicity of plague vaccines in mice and guinea pigs. Appl. Microbiol. 22:84-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams, J. E., P. L. Altieri, S. Berman, J. P. Lowenthaland, and D. C. Cavanaugh. 1980. Potency of killed plague vaccines prepared from avirulent Yersinia pestis. Bull. W.H.O. 58:753-756. [PMC free article] [PubMed] [Google Scholar]
- 27.Williams, J. E., L. Arntzen, G. L. Tyndal, and M. Isaacson. 1986. Application of enzyme immunoassays for the confirmation of clinically suspect plague in Nambia, 1982. Bull World Health Organ 64:745-752. [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson, E. D., S. M. Eley, K. F. Griffin, M. Green, P. Russell, S. E. C. Leary, P. C. F. Oyston, T. Easterbrook, K. M. Reddin, A. Robinson, andR. W. Titball. 1995. A new improved sub-unit vaccine for plague: the basis of protection. FEMS Immunol. Med. Microbiol. 12:223-230. [DOI] [PubMed] [Google Scholar]
- 29.Williamson, E. D., S. M. Eley, A. J. Stagg, M. Green, P. Russell, and R. W. Titball. 1997. A sub-unit vaccine elicits IgG in serum, spleen cell cultures and bronchial washings and protects immunised animals against pneumonic plague. Vaccine 15:1079-1084. [DOI] [PubMed] [Google Scholar]
- 30.Williamson, E. D., P. M. Vesey, K. J. Gillhespy, S. M. Eley, M. Green, and R. W. Titball. 1999. An IgG1 titer to the F1 and V antigens correlates with protection against plague in the mouse model. Clin. Exp. Immunol. 116:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson, E. D. 2001. Plague vaccine research and development J. Appl. Microbiol. 91:606-608. [DOI] [PubMed] [Google Scholar]
- 32.Williamson, E. D., S. M. Eley, A. J. Stagg, M. Green, P. Russell, and R. W. Titball. 2001. A single dose sub-unit vaccine protects against pneumonic plague. Vaccine 19:566-571. [DOI] [PubMed] [Google Scholar]