Abstract
Nitric oxide (NO) contributes to mammalian host defense by direct microbicidal activity and as a signaling molecule of innate immune responses. Macrophages produce NO via the inducible NO synthase (iNOS). The genome of Neisseria meningitidis includes two genes, norB (encoding nitric oxide reductase) and cycP (encoding cytochrome c′), both of which detoxify NO in pure cultures of N. meningitidis. We show here that norB, and to a lesser extent cycP, enhance survival of N. meningitidis within primary human macrophages. Furthermore, accumulation of lysosome-associated membrane protein 1 (LAMP-1) is modified in phagosomes containing an isogenic norB mutant of N. meningitidis compared to the wild type. The survival enhancement conferred by norB and cycP is ablated by pretreatment of macrophages with the nitric oxide synthase inhibitor N(G)-monomethyl-l-arginine (L-NMMA). Despite this evidence that NO detoxification confers advantage, we find, using a highly sensitive chemiluminescence technique, that human macrophage-associated [NO] is low even after activation by lipopolysaccharide and interferon alpha. Furthermore, wild-type N. meningitidis further depletes cell-associated NO during phagocytosis by an active mechanism and survives relatively poorly in the presence of L-NMMA, suggesting that the wild-type organism may utilize NO for optimal survival during intracellular life. The natural habitat of N. meningitidis is the human nasopharynx. Using a nasopharyngeal mucosa organ culture system, we show that mutants lacking norB and cycP also survive poorly in nasopharyngeal tissue compared to wild-type N. meningitidis. These findings indicate that the meningococcus requires active NO detoxification systems for optimal survival during experimental nasopharyngeal colonization and processing by human phagocytic cells.
In mammals, nitric oxide (NO) has diverse physiological roles, including involvement in the innate immune response to infection. Reactive oxygen intermediates and reactive nitrogen intermediates each have direct and interdependent antibacterial activities (12), including direct bacterial killing and up- or down-regulation of genes involved in innate immunity and inflammatory responses to microbes (15). Macrophages produce NO via the inducible NO synthase (iNOS) as part of a successful immune response to microbial infection (16, 22). Although NO is produced by human macrophages in small quantities compared to murine macrophages, iNOS mRNA expression, iNOS protein production, NO production, and NO-related antimicrobial activity of human macrophages have each been demonstrated (22, 23).
The human pathogen Neisseria meningitidis, like other gram-negative bacteria, has genes which encode proteins capable of detoxifying NO, including via denitrification. Bacterial denitrification is the process of reduction of nitrate to dinitrogen gas. This is catalyzed by four distinct enzymes: nitrate reductase (nitrate to nitrite), nitrite reductase (nitrite to NO), NO reductase (NO to nitrous oxide), and nitrous oxide reductase (nitrous oxide to dinitrogen). The genome of N. meningitidis includes aniA and norB, which encode, respectively, a nitrite reductase and an NO reductase that are capable of allowing the bacterium to denitrify nitrite to nitrous oxide. Indeed wild-type N. meningitidis is able to utilize NO during microaerophilic respiration and can deplete spiked NO from a closed anaerobic system by reducing it to nitrous oxide, in contrast to an isogenic norB-deficient mutant, which cannot (1). In addition, the genome of N. meningitidis includes cycP, which encodes cytochrome c′, a single heme-containing molecule which, at least in Rhodobacter capsulatus, is able to bind and detoxify NO (6). Although, like NorB, CycP detoxifies NO in pure cultures of N. meningitidis, it is not capable of depleting NO during microaerophilic NO2− respiration by N. meningitidis (1).
To investigate the role of NO detoxification systems in resistance to host defense we measured the survival of norB and cycP mutants during phagocytosis by human monocyte-derived macrophages in vitro. The natural habitat of N. meningitidis is the human nasopharynx. To investigate the role of norB and cycP in successful colonization of this tissue, we also measured the survival of wild-type and mutant strains in an organ culture of human nasopharyngeal mucosa. We show, in both models, that active detoxification of NO by N. meningitidis is important for survival.
MATERIALS AND METHODS
Strains and growth conditions.
Neisseria meningitidis MC58 (serogroup B), whose complete genome sequence has been determined (19), was used as a wild-type strain. Isogenic mutants with interposon disruptions of cycP and norB conferring spectinomycin resistance were described previously (1). For growth on plates, GC agar was supplemented with 1% Vitox. Plates were incubated aerobically in an atmosphere of 5% CO2 at 37°C. Liquid cultures were grown in Mueller-Hinton broth (MHB; Oxoid) in the presence of 10 mM NaHCO3 or 5% CO2 at 37°C, with shaking. All cultures were inoculated with colonies taken from freshly grown aerobic plates. Viable counts were determined by serial dilution of cultures in phosphate-buffered saline (PBS) and plating on warmed GC agar plates. The plates were incubated overnight at 37°C, and the number of CFU per ml was the mean of three determinations. All results were confirmed in at least six independent experiments.
Macrophage culture and infection with N. meningitidis.
Primary human peripheral monocyte-derived macrophages (MDM) were isolated from healthy volunteers, by density centrifugation of heparinized blood using standard techniques (14). Macrophages were cultured for 11 to 13 days in flat-bottom 24-well plates (106 cells/well), in 1 ml RPMI 1640/well (Gibco BRL) supplemented with 2 mM l-glutamine and 10% fetal calf serum (FCS), at 37°C in 95% air, 5% CO2. Where indicated, macrophages were activated with 10 nM lipopolysaccharide from Escherichia coli O55:B5 (Sigma Chemical Co, St. Louis, Mo.), 1000 u/ml alpha interferon (α-IFN) (Roche), or a combination of both.
For MDM infection with N. meningitidis strains, six colonies were transferred from an overnight plate into the appropriate volume of MHB and incubated until an optical density at 600 nm of 0.25 to 0.3. Bacteria were harvested, and pellets were washed twice with PBS. Pellets were resuspended in PBS and opsonized in 10% human serum for 15 min at 37°C with rotation. Opsonized bacteria were harvested and washed 3 times in PBS. Pellets were resuspended in RPMI 1640 to obtain an inoculum of approximately 3 × 107 CFU in 250 μl of RPMI 1640 and declumped by vortexing for 30 s in the presence of 3-mm-diameter glass beads (Fisher Scientific). Wells containing MDM were infected with 250 μl bacterial suspensions for microscopy and killing studies and incubated at 37°C for 60 min (period of maximal internalization). The supernatant fluid was then aspirated, and wells were washed twice with PBS.
Survival assay.
To assay for intracellular killing of bacteria by MDM the minimum bactericidal concentrations (MBCs) of penicillin and gentamicin required to kill 108 N. meningitidis in RPMI 1640 at 37°C in 30 min were experimentally determined to be 10 μg ml−1 and 70 μg ml−1, respectively, for all three strains and were used throughout the experiments to kill bacteria that had not been internalized. After the period of maximum internalization (1 h), macrophages infected with N. meningitidis were bathed in RPMI 1640 containing the MBC of penicillin and gentamicin for 30 min. After antibiotic treatment, wells were washed twice in PBS, and incubated for a further 120 min at 37°C in fresh RPMI 1640. The number of surviving intracellular bacteria was estimated by measuring viable counts from wells before and after lysis of cells with saponin (1%) using a standard dilution technique. This was conducted immediately prior to antibiotic treatment, and 30, 60, 90, or 120 min after addition of antibiotic. To determine whether nitric oxide synthase (NOS) activity affected the rate of bacterial killing by MDM, macrophages were treated with 3 mM L-NMMA for 48 h prior to infection with bacteria.
Microscopy.
The triple staining technique described before (14) was used. In brief, wells were washed twice with PBS, and cells were fixed with 2% fresh paraformaldehyde in PBS. Internalized bacteria were distinguished from external bacteria using direct immunofluorescence with fluorescein isothiocyanate-conjugated anti-N. meningitidis with counterstaining with the nucleic acid stain 4,6-diamidino-2-phenylindole (DAPI). One hundred macrophages were sampled per condition, and the number of macrophage-bound and internalized bacteria was counted using a Leica DMRB 1000 fluorescence microscope. The % internalized macrophages colocalizing with phagolysosomes was determined as previously described (14) by staining with monoclonal antibody H4A3, 1:20 (Iowa Hybridization Bank), a marker of lysosome-associated membrane protein 1 (LAMP-1), which is specific for late endosomal/lysosomal compartments.
Nitric oxide measurements.
NO produced by MDM was measured by chemiluminescence (Sievers, model 280i, nitric oxide analyzer) assay of nitrite accumulation in the supernatant of cultured cells over 24 h incubation at 37°C. Nitrite was quantified by reduction to NO gas in a mixture of acetic acid and KI. We used vanadium(III) chloride in hydrochloric acid to measure nitrate in macrophage supernatants infected with the wild-type and the norB strain. At high temperature (95°C), vanadium(III) converts nitrate as well as nitrite to NO. NO concentrations were calculated from the integral of the detected signal over time and compared to those of a series of standards freshly prepared on the day of the experiment.
Nasopharyngeal mucosa organ culture.
Human respiratory mucosa samples were isolated from inferior turbinates resected from patients with nonallergic nasal obstruction and then used in an air interface model as previously described (20). Squares of mucosa (3 to 4 mm) were treated with antibiotics in minimal essential medium (MEM) for 4 h before washing overnight in MEM and then supported in petri dishes with the epithelial surface projecting above a bed of nonnutrient agar which was perfused with MEM using a wick, and the whole was incubated in a humidified atmosphere with 5% CO2 at 37°C. Only tissue with active ciliary activity observed by microscopy were used in experiments. Phosphate-buffered saline (100 μl) containing 3.5 × 108 cells/ml N. meningitidis strains was dropped onto the surface of the tissue. Infected and control explants were incubated for over 18 h. After 4 h and 18 h incubation, explants were removed from their wells, washed in two changes of PBS, weighed, and then homogenized in a French Press. Viable counts were made from the homogenate using a dilution technique and expressed as CFU per mg of tissue. In order to measure survival of bacteria that penetrated mucosa, the explants were immersed in 0.25% sodium taurocholate for 30 s before washing and processing as above. This treatment kills extramucosal N. meningitidis and, therefore, viable bacteria recovered after sodium taurocholate treatment are assumed to be protected by penetration into the mucosa (20).
Statistical analysis.
Differences between means of at least six experiments were evaluated for statistical significance using the paired Student's t test for data with normal distribution. Nonparametric data were analyzed using the Wilcoxon signed rank test or Mann-Whitney in at least eight separate experiments. All analysis was performed using the SPSS Statistical Software for Windows, version 11.0 (SPSS Inc.).
RESULTS
norB and cycP enhance survival of N. meningitidis after phagocytosis by human macrophages.
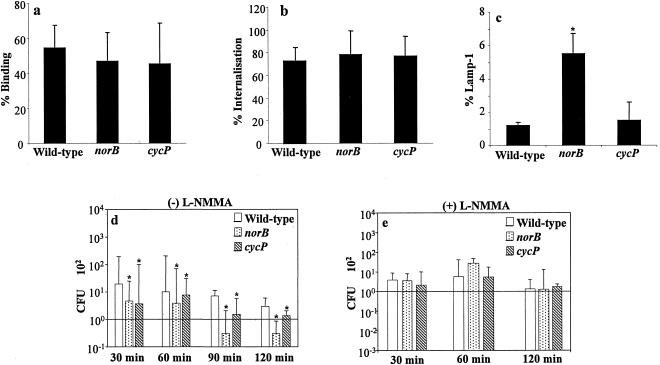
To determine whether the nitric oxide detoxification genes of N. meningitidis enhance intracellular survival during phagocytosis by human macrophages, we investigated the interaction between MDM and the wild-type N. meningitidis strain and norB or cycP mutants. By immunofluorescence microscopy of macrophages, binding (Fig. 1a) and internalization (Fig. 1b) after 1 h incubation of the three strains were indistinguishable. However, the bacterial viability assay revealed that subsequent survival of intracellular cycP and norB strains was impaired compared to the survival of intracellular wild-type N. meningitidis (Fig. 1d) over the period of 120 min after addition of medium containing antibiotics. This was particularly notable for the norB strain, for which intracellular survival was 10-fold lower than that for the wild type. The hypothesis that the survival advantage of wild-type is due to relative resistance to intracellular NO-related molecules is supported by recovery of bacteria from MDM pretreated with the NOS inhibitor L-NMMA (Fig. 1d and 1e). In these cells, the relatively increased survival of the wild-type compared to the norB and cycP mutants was ablated. The raw data used in Fig. 1d and details of the statistical tests used are shown in Table 1.
FIG. 1.
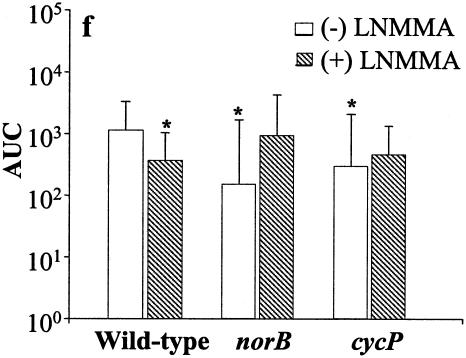
Interaction of N. meningitidis strains, each inoculated at a multiplicity of infection of 30 with human macrophages. (a) % of macrophages to which bacteria are bound externally. (b) % of bacteria internalized by macrophages after 1 h of coinfection. (c) % of bacteria internalized by macrophages that colocalize with LAMP-1. (d) Survival of internalized N. meningitidis. Numbers of CFU of bacteria at 30, 60, 90, and 120 min after antibiotic treatment to kill extracellular bacteria are shown. (e) As for d but macrophage cultures were incubated with L-NMMA prior to the experiment. Analysis for statistical significance was obtained by comparing the AUC of non-L-NMMA-treated wild-type samples with the other conditions used here. (f) Analysis of the areas under the curve from data described in d and e. The data points obtained for each individual experiment were used to obtain a curve, and the area under the curve of each experiment was calculated. The bars in f represent the median of the areas under the curve of all experiments in d and e. *, P < 0.05 versus L-NMMA. Data sets a, b, and c shown as the mean ± standard error of the mean, whereas data sets d, e, and f are expressed as the median. Significance is shown with asterisks (P < 0.05), paired Student's t test (a, b, and c), or Wilcoxon signed rank test (d and e), where the error bars represent the 25th and 75th percentiles.
TABLE 1.
Survival of internalized N. meningitidis after antibiotic treatment in cultures incubated without L-NMMAa
| Parameter | No. of CFU after incubation for:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 min
|
60 min
|
90 min
|
120 min
|
|||||||||
| Wild type | norB | cycP | Wild type | norB | cycP | Wild type | norB | cycP | Wild type | norB | cycP | |
| 16.50 | 8.40 | 11.50 | 53.90 | 17.10 | 920.00 | 500.00 | 3.70 | 1.10 | 1.50 | |||
| 22.80 | 2.70 | 6.85 | 13.20 | 5.00 | 11420.00 | 9415.00 | 11170.00 | 30.35 | 7.70 | 1.20 | ||
| 12.60 | 6.00 | 0 | 34.00 | 50.00 | 8.55 | 7.70 | 5.50 | |||||
| 17.10 | 0.00 | 1.15 | 0 | 1.30 | 7.00 | 0 | 0 | 0 | 0 | 0.05 | ||
| 8.70 | 4.00 | 0.10 | 0.20 | 0.90 | 240.00 | 5.00 | 0 | 0.60 | ||||
| 26.70 | 198.40 | 250.00 | 120.00 | 695.00 | 260.00 | 600.00 | ||||||
| 5.20 | 3.90 | 2.75 | 86.00 | 27.80 | 775.00 | 145.00 | 1.50 | 0.70 | ||||
| 6.35 | 9.20 | 1.65 | 264.00 | 180.00 | 10.60 | 1245.00 | 65.00 | 245.00 | 2.50 | 0.10 | 1.30 | |
| 2.70 | 0.60 | 1.65 | 3.40 | 1.30 | 3.00 | 201.00 | 5.00 | 0.30 | 0.05 | 0.00 | ||
| 1.20 | 0.05 | 1.40 | 5.10 | 0.20 | 0.60 | 2.30 | 1.40 | 1.90 | ||||
| 2.85 | 0.70 | 0.25 | 8.60 | 2.90 | 16.50 | 2.90 | 0.30 | |||||
| 118.33 | 1.10 | 28.30 | 3.35 | 5.85 | 8.33 | |||||||
| 167.80 | 133.33 | 120.00 | 262.23 | 98.87 | 146.40 | |||||||
| 1.00 | 18.50 | 1.67 | 3.50 | 26.75 | 79.82 | 0.42 | 0.17 | 0.09 | ||||
| 0.13 | 0 | 0.27 | 0.77 | 0.13 | 0 | |||||||
| 12.25 | 0.05 | 1.20 | 6.80 | 1.05 | 2.85 | 3.40 | 0 | 0.05 | ||||
| 19.35 | 0.45 | 0.10 | 9.35 | 0.95 | 6.80 | 5.50 | 0.85 | |||||
| 162.70 | 92.75 | 93.65 | 15.00 | 30.40 | 13.00 | 10.20 | 0.50 | 11.35 | ||||
| 350.00 | 3.80 | 10.20 | 21.35 | 2.00 | 14.20 | 5.95 | 0.05 | 1.95 | ||||
| 3.70 | 0.04 | 3.37 | 8.91 | 1.11 | 1.67 | 0.63 | 0.46 | 1.39 | ||||
| 343.33 | 722.50 | 452.50 | 1560.00 | 720.83 | 533.33 | |||||||
| 63.80 | 3.85 | 8.00 | 22.55 | 1.40 | 2.45 | |||||||
| 35.55 | 5.20 | 4.50 | 70.70 | 3.85 | 3.85 | |||||||
| Median value | 16.5 | 3.88 | 2.75 | 9.13 | 2.9 | 6.8 | 735 | 49.5 | 245 | 2.9 | 0.46 | 1.3 |
| 25th percentile | 3.28 | 0.35 | 1.2 | 3.9 | 1 | 2.06 | 210.75 | 5 | 0 | 0.63 | 0.05 | 0.07 |
| 75th percentile | 91.07 | 11.53 | 11.5 | 66.5 | 28.58 | 22.15 | 1163.75 | 231.25 | 600 | 5.95 | 1.1 | 1.93 |
Comparison of data by Wilcoxon signed rank test analysis of statistically significant differences gave the following P values (with incubation times in parentheses): for wild type versus norB, P = 0.012 (30 min), 0.022 (60 min), 0.018 (90 min), and 0.01 (120 min); for wild type versus cycP, P = 0.02 (30 min), 0.024 (60 min), 0.028 (90 min), and 0.023 (120 min).
LAMP-1 incorporation by meningococcal phagosomes is influenced by norB.
By immunofluorescence microscopy, approximately 1% of macrophage-internalized wild-type N. meningitidis were observed to colocalize with lysosome-associated protein 1 (LAMP-1) after 1 h of incubation and, by inference, be within terminal phagolysosomes (Fig. 1c). However, colocalization of the norB mutant with LAMP-1 was fivefold greater than observed for the wild-type and cycP strains. These data suggest that the mechanism of impaired survival of norB and cycP mutants may differ and that activity of norB may influence incorporation of glycoproteins into the meningococcal phagosome.
Nitric oxide concentrations in association with human macrophages are low.
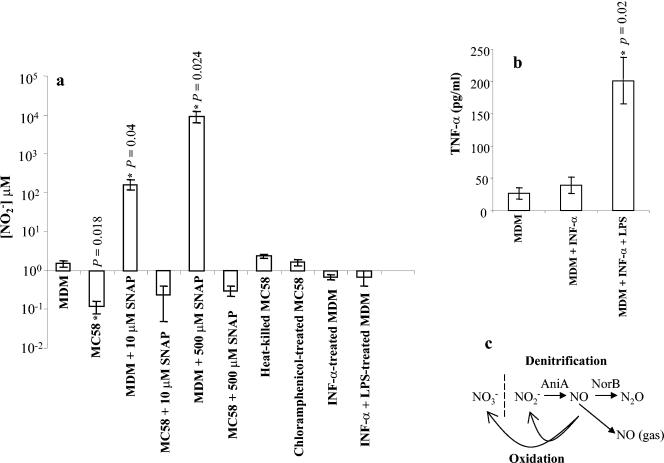
Our observation, that NO detoxification systems enhance intracellular survival of N. meningitidis implies that NO has biologically significant microbicidal activity in human macrophages. However, although experimental infection of MDM and cell lines with intact bacteria, lipopolysaccharide, or killed bacteria (3), including N. meningitidis (4) leads to transcription of the gene encoding iNOS, we found, using highly sensitive chemiluminescence, that resting human MDM produce a low level of NO, which is subsequently oxidized to nitrite (Fig. 2a). Low [NO] was observed even following activation by proinflammatory agonists such as α-IFN and lipopolysaccharide at concentrations capable of eliciting tumor necrosis factor alpha release (Fig. 2a and 2b). These data imply that NO concentration is tightly regulated in activated cells.
FIG. 2.
Variations in nitric oxide concentrations in cultures of human macrophages. (a) Nitric oxide concentrations were measured by chemiluminescense in supernatants from MDM only, MDM incubated with RPMI 1640 containing 10 μM or 500 μM SNAP, and MDM infected with 3 × 107 N. meningitidis (wild type) in normal RPMI 1640 or RPMI 1640 containing 10 μM or 500 μM SNAP. To assess the effect of de novo protein synthesis, samples were also collected from supernatants from macrophages infected with heat-killed or chloramphenicol-treated bacteria. The effect of macrophage activation on nitrite levels was studied in macrophages treated with α-IFN and lipopolysaccharide. (b) Relationship between macrophage activation and tumor necrosis factor alpha (TNF-α) production. Data are shown as the mean ± standard error of the mean. Significance, compared to MDM only, is shown with asterisks to indicate significant differences after Bonferroni correction. (c) A scheme showing the interconversions between different nitrogen oxides.
Consumption of NO by N. meningitidis in macrophage cultures.
Strikingly, during cocultivation of MDM with viable N. meningitidis, the low steady-state [NO2−] decreased even further (Fig. 2a). This effect of nitrite depletion was demonstrated to require viable bacteria capable of protein synthesis by comparison with heat-killed and chloramphenicol-treated N. meningitidis as controls (Fig. 2a). The NO releaser compound S-nitrosopenicillamine (SNAP) caused accumulation of nitrite in culture medium in the absence of N. meningitidis, but in the presence of bacteria there was gross depletion (Fig. 2a). It is notable that SNAP leads to NO production in excess of the sum of [SNAP] and resting macrophage NO production. This suggests some direct or indirect cellular effect that enhances NO production in primary human MDM.
A similar depletion of nitrite in supernatants from macrophages infected with a norB mutant strain compared to those infected with wild-type N. meningitidis was observed (data not shown). This is not surprising, as the norB strain still possesses an active aniA gene, whose protein product reduces nitrite to NO (Fig. 2c); therefore, any nitrite that could potentially accumulate would be reduced to NO by the AniA protein and then become reoxidized by molecular oxygen, yielding nitrate plus nitrite. Nitrate is a major oxidation product in some cell systems and in human tissues. Measurement of nitrate in macrophage supernatants infected with wild-type and the norB strain revealed a small reduction (compare the uninfected control MDM) of [nitrate] in the presence of MDM infected with wild-type N. meningitidis, whereas the nitrate concentration remained constant in norB mutant-infected MDM (data not shown).
Intracellular viability of N. meningitidis is relatively impaired by inhibition of iNOS.
Active removal of NO from cultures by N. meningitidis might imply that this organism is able to utilize NO for microaerophilic respiration in the cell. Consistent with this, we found that there was a small but significant impairment of survival of wild-type bacteria in MDM pretreated with L-NMMA over the period of the experiment (see Fig. 1d, 1e, and 1f), suggesting that synthesis of NO is a requirement for optimal survival of wild-type N. meningitidis in the intracellular environment. The raw data and area under the curve (AUC) calculations used to formulate Fig. 1d and e are shown in Tables 1 and 2.
TABLE 2.
Survival of internalized N. meningitidis after antibiotic treatment in cultures incubated with L-NMMAa
| Parameter | No. of CFU after incubation for:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 min
|
60 min
|
120 min
|
|||||||
| Wild type | norB | cycP | Wild type | norB | cycP | Wild type | norB | cycP | |
| 20.00 | 43.75 | 82.75 | 29.90 | 42.30 | 20.00 | 9.40 | 13.40 | 8.40 | |
| 7.00 | 4.60 | 10.60 | 1.70 | 3.60 | 5.50 | 4.40 | 12.90 | 2.10 | |
| 9.80 | 0 | 28.60 | |||||||
| 3.80 | 2.50 | 6.40 | 8.30 | 7.80 | 3.00 | 0 | 0 | 0.10 | |
| 3.70 | 9.40 | 9.90 | 0.20 | 24.40 | 5.20 | 1.30 | 0.80 | 1.70 | |
| 53.00 | 34.00 | ||||||||
| 3.30 | 75.00 | 120.40 | 27.80 | 0.05 | 0 | 0.30 | |||
| 8.10 | 4.90 | 0.60 | 260.00 | 178.00 | 106.00 | 2.50 | 1.70 | 2.60 | |
| 2.00 | 0.40 | 0.90 | 5.60 | 5.20 | 0.30 | 0.10 | 0 | ||
| 0.10 | 0.10 | 0.70 | 0.90 | 605 | 2.80 | 0.50 | 0.60 | 1.50 | |
| 2.30 | 0.05 | 0.20 | 4.80 | 9.00 | 3.60 | 2.10 | 1.70 | ||
| Median value | 3.8 | 3.55 | 2.1 | 6.95 | 29.2 | 5.5 | 1.3 | 1.25 | 1.7 |
| 25th percentile | 2.15 | 0.18 | 0.5 | 1.5 | 4.65 | 4.1 | 0.18 | 0.08 | 0.2 |
| 75th percentile | 8.95 | 8.28 | 10.08 | 58.5 | 100.88 | 23.9 | 4 | 13.03 | 2.35 |
Comparison of data gave the following P values (with incubation times in parentheses): for wild type versus norB, P = 0.866 (30 min), 0.779 (60 min), and 0.889 (120 min); for wild type versus cycP, P = 0.767 (30 min), 0.314 (60 min), and 0.553 (120 min); and for wild type versus wild type + L-NMMA, P = 0.263 (30 min), 0.036 (60 min), and 0.5 (120 min).
Mutations in norB and cycP impair survival within nasopharyngeal mucosa.
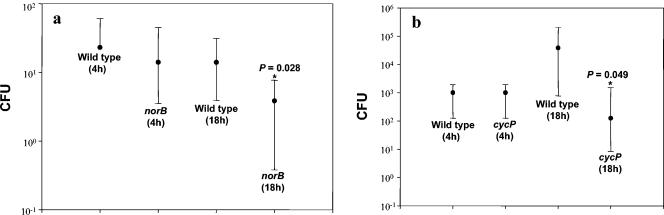
Having shown that the two NO detoxification systems of N. meningitidis protect against macrophage NO during intracellular processing, we determined whether these gene products also have a role in bacterial protection in a relevant model of mucosal colonization. Both norB and cycP exhibited significantly impaired survival over 18 h of coculture with human nasopharyngeal explants. Plots showing median viable counts of bacteria recovered after 4 h and 18 h from eight experiments using explants derived from individual hosts are shown in Fig. 3. Primary data from these experiments are shown in Table 3. After treatment with sodium taurocholate for 30 s to kill extramucosal bacteria, there was significantly greater recovery of wild-type than norB or cycP strains after 18 h, supporting the conclusion that detoxification mechanisms enhance the survival of N. meningitidis within nasopharyngeal mucosa.
FIG. 3.
Viable yield of N. meningitidis strains from lysates of nasopharyngeal explant mucosa. Viable counts of wild type and norB (a) (n = 8) or wild type and cycP (b) (n = 10) strains recovered from nasopharyngeal explants from human donors, following 4 h and 18 h incubation. Data are shown as the median, where the error bars represent the 25th and 75th percentiles. Significance is shown with asterisks (P < 0.05), Mann-Whitney.
TABLE 3.
Median viable bacterial countsa
| Parameter | No. of CFU after incubation for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 4 h
|
18 h
|
|||||||
| Wild type | norB | Wild type | cycP | Wild type | norB | Wild type | cycP | |
| 40.26 | 14.16 | 16666.67 | 3500.00 | 20.38 | 3.85 | 25000000 | 1578947 | |
| 55.56 | 51.59 | 1800.00 | 4.00 | 69.46 | 10.17 | 121212.1 | 32.26 | |
| 76.36 | 13.92 | 2000.00 | 3.23 | 14.01 | 1.14 | 6060.61 | 571.43 | |
| 5.79 | 4.70 | 1363.64 | 400.00 | 11.32 | 0.76 | 238095.2 | 1818.18 | |
| 0.00 | 42.55 | 178.57 | 125.00 | 3.79 | 6.38 | 108.11 | 10.71 | |
| 0.00 | 0.00 | 85.71 | 344.83 | 0.00 | 0.00 | 2758.62 | 7.69 | |
| 111.11 | 714.29 | 40.00 | 7.69 | 50.00 | 4.76 | |||
| 645.16 | 357.14 | 26.40 | 3.40 | 71428.57 | 218.75 | |||
| Median value | 23.03 | 14.04 | 1004.39 | 351 | 17.2 | 5.12 | 38744.59 | 125.5 |
| 25th percentile | 0 | 3.53 | 127.98 | 34.25 | 4 | 1.05 | 770.74 | 8.45 |
| 75th percentile | 60.73 | 44.81 | 1950 | 635.71 | 29.8 | 26.55 | 208874.5 | 1506.49 |
Comparison of data by Mann-Whitney analysis of statistically significant differences gave the following P values (with incubation times in parentheses): for wild type versus norB, P = 0.937 (4 h) and 0.028 (18 h); for wild type versus cycP, P = 0.328 (4 h) and 0.049 (18 h).
The median yield of wild-type organisms from the lysates of the two series of experiments was different; we have previously shown that there is very wide variation in the recovery of experimentally inoculated Neisseria meningitidis from explants derived from different hosts (20). Despite the large variation between different hosts, variations between replicates for any given host are small, and so pairwise experiments in which explants were inoculated with wild-type versus mutant strains yield statistically valid results. It was not possible to carry out three-way comparisons with tissue from a given donor because of the small amount of tissue available.
DISCUSSION
Infection and colonization of the nasopharyngeal mucosa is a crucial step in the pathogenesis of meningococcal disease. The nasopharyngeal mucosa is rich in NO-producing cells, including macrophages and epithelial cells, and local concentrations of NO have been shown to reach concentrations which are likely to be greatly in excess of that required to produce toxic effects in most bacteria (10).
We previously demonstrated that N. meningitidis has at least two NO detoxification systems (1). CycP and NorB are proteins which protect N. meningitidis from the toxicity of NO donors such as sodium nitroprusside and nitrosothiols. The work presented here shows that NO detoxification contributes to bacterial survival during experimental colonization and immune evasion. Mutation of norB has a more severe effect on survival of N. meningitidis than mutation of cycP. This is consistent with our earlier observations in pure cultures, in which the toxicity of NO was greater in the norB than the cycP mutant (1). Neither binding nor internalization of N. meningitidis by human macrophages was affected by mutation of norB or cycP, but there was at least a 10-fold drop in the number of norB organisms recovered from macrophages when compared with macrophages infected with the wild-type. Furthermore, mutation of norB was associated with a fivefold increase in the proportion of meningococcal phagosomes containing lysosome-associated membrane protein, a glycoprotein which richly populates late endosomal/phagosomal compartments. The survival advantage afforded by norB and cycP was shown to be due to their involvement in NO metabolism by pretreating macrophages with the NOS inhibitor L-NMMA. This ablated the difference in survival between wild-type and mutant strains.
The data reported here are apparently paradoxical. In unstimulated murine peritoneal macrophages, it has been observed that mean concentrations of approximately 40 μmol nitrite accumulate over 20 h incubation (21), whereas we observed a median accumulation of approximately 0.5 μmol in human MDM. Despite this very low measured [NO] there is clear protection afforded by the NO detoxification systems during intracellular processing of the organism by human macrophages. We have previously demonstrated that another bacterial NO detoxification mechanism, the Salmonella flavohemoglobin Hmp, also protects Salmonella enterica serovar Typhimurium from NO-related bactericidal activity of human macrophages (5, 18). There is no homologue of Hmp in Neisseria.
Since the [NO] is low, it is possible that the impact of NO on bacterial survival in human macrophages is primarily due to its action as a signaling molecule rather than directly via toxic damage caused by the free radical. It may also be relevant that the inhibition of iNOS with L-NMMA resulted in decreased intracellular viability of wild-type N. meningitidis. NO can be used as a respiratory substrate (via NorB), and so it is possible that NO production by host tissue provides an alternative respiratory pathway for the bacterium within this microaerophilic environment. This possibility is supported by our previous observation that N. meningitidis utilizes NO in microaerobic cultures in vitro (1) and by the observation reported here that N. meningitidis depletes NO and nitrite in macrophage cultures even in the presence of 500 μM SNAP. We speculate that N. meningitidis utilizes NO within the intracellular environment. It is clear from the results presented here that N. meningitidis is able to deplete not only NO produced by activated macrophages but also the accumulated NO produced by donation of as much as 500 μM SNAP.
The reason for the low concentration of NO in association with human macrophages, even when activated, is unclear. In murine models it has long been established that infection of macrophages with whole bacteria or lipopolysaccharide greatly enhance expression of iNOS and that this helps to control infection (16, 21). In humans, despite dramatic elevation of NO concentrations during infection in vivo, in vitro stimulation of macrophages does not consistently result in significant elevations of iNOS expression, suggesting that the mechanisms controlling iNOS expression in murine macrophages differ from those of humans (3). Given these differences between human and murine macrophages in NO production, and that N. meningitidis is a strictly human pathogen, it was important for us to approach this study with human tissue despite the large variations observed between primary cells and tissues derived form different hosts.
Unlike the Salmonella Hmp protein, NorB is part of a high-flux respiratory pathway in N. meningitidis (1). It is interesting that the NO dioxygenase Hmp of S. enterica serovar Typhimurium only confers a twofold protection from macrophage killing, whereas the denitrifying NorB protein confers on N. meningitidis a 10-fold protection. This observation raises interesting questions and suggests that the mode of NO detoxification may also impact the survival of the organism within the host. Denitrification is associated with respiration under oxygen-depleted conditions and thus nitrite and nitric oxide reductase activities are likely to be more active under the hypoxic conditions that prevail within mucosa or within ischemic tissues of patients with meningococcal sepsis. In well-oxygenated tissues (e.g., interstitial fluid or arterial blood), however, the major sink for NO may be via its direct reaction with oxygen to form nitrate or nitrite rather than nitrous oxide, which is the product of NorB. Previous clinical observations have revealed that nitrite plus nitrate concentration is elevated in arterial blood of patients with meningococcal disease (2).
We found that the role of internalization of wild-type and norB strains by macrophages was equivalent. We colocalized intracellular organisms with a LAMP marker in order to confirm entry of organisms into phagosomes, as we have previously used this marker to study meningococcal processing by human macrophages (14). Unexpectedly, we found that phagosomes of the norB strain were enriched with LAMP. We did not perform a formal study of phagosomal maturation as this was not the purpose of our study, but phagosomal incorporation of LAMP may either be a consequence of the altered rate of death of norB or higher intracellular concentrations of NO during macrophage uptake of norB. In Leishmania phagosomes NO concentration has been shown to regulate phagosomal membrane constituents including LAMP (7), and we are currently investigating the effect of NO consumption by N. meningitidis upon phagosomal maturation more formally.
Colonization of the nasopharynx is a prerequisite step in the pathogenesis of meningococcal disease, and 5 to 10% of the human population carry the organism at any one time (24). However, the nasopharynx produces higher concentrations of NO than other parts of the respiratory tract, the major sources being epithelial cells (via iNOS/eNOS) and macrophages (via iNOS) (11). During experimental infection of the human nasopharynx, N. meningitidis transcytoses the epithelium and can penetrate the basement membrane collagen to reside within subepithelial tissue (13) and has been observed deep within tonsillar tissue (in intracellular locations) of naturally colonized patients who have undergone tonsillectomy (17). Therefore, norB and cycP may contribute to successful colonization of the human nasopharynx by virtue of their protective role against NO. A clinical observation of relevance is that meningococcal infection commonly occurs following upper respiratory tract viral infection (9), and it is possible that N. meningitidis is able to resist the relatively high NO toxicity associated with such infections, allowing it to replicate to sufficiently high numbers to increase the penetration into and survival within the bloodstream.
We used techniques to measure relative intracellular and intramucosal viability of N. meningitidis that warrant discussion. To measure intramacrophage viability we used the gentamicin protection assay. This is widely used, but some investigators have reservations about its accuracy, particularly in relation to potential compartmentalization of the drug within the cell. The technique rests upon the observation that gentamicin penetrates cells poorly; thus, viable bacteria released from a lysed cell culture likely derive from an intracellular location. In fact, gentamicin has been shown to enter cells, albeit poorly, by pinocytosis (8). Gentamicin is inactive at the low pH observed within phagosomes (8), but we have shown here that the norB phagosome may be different from that of wild-type N. meningitidis, suggesting a possible confounder. However, the cycP phagosome was unaltered, and cycP also exhibited reduced intracellular viability by the gentamicin protection assay.
We were careful to show that the MBCs of the wild-type, norB, and cycP strains were identical. To measure survival within mucosa, we immersed explants in sodium taurochlate for 30 s. This rapidly kills extramucosal bacteria, and we have previously validated the technique as a measure of intramucosal viability of N. meningitidis (20). Again, we were careful to show that the sensitivity to sodium taurocholate of the three strains used was equivalent. To demonstrate that there was equivalent initial association of all three strains with the mucosa, we measured the yield of the three strains within lysates (not treated with sodium taurocholate) after 4 h of coincubation and found no difference between them (data not shown), suggesting that the differences observed at 18 h are due to reduced viability of the mutant strains rather than an effect on adherence to the surface of epithelial cells.
In summary, we find that a nitric oxide detoxification system of Neisseria meningitidis protects the organism from intracellular killing by human macrophages despite low levels of NO measured in association with these phagocytes and also allows the bacteria to colonize human nasopharyngeal mucosa more effectively in vitro. NO resistance and consumption may be key events contributing to successful nasopharyngeal colonization, escape into the bloodstream, and ultimately catastrophic bacteremia in humans.
Acknowledgments
This work was supported by a Project Grant from the Wellcome Trust to J.W.B.M. and R.C.R. (award no. 056362/z/98/z).
We thank Linda Goodwin, Margaret Lee, and Anne Cooke for technical assistance.
Editor: F. C. Fang
REFERENCES
- 1.Anjum, M..F., T. M. Stevanin, R. C. Read, and J. W. Moir. 2002. Nitric oxide metabolism in Neisseria meningitidis. J. Bacteriol. 184:2987-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baines, P. B., S. Stanford, D. Bishop-Bailey, J. A. Sills, A. P. J. Thomson, J. A. Mitchell, S. C. Fear, C. A. Hart, and A. J. Petros. 1999. Nitric oxide production in meningococcal disease is directly related to disease severity. Crit. Care Med. 27:1187-1190. [DOI] [PubMed] [Google Scholar]
- 3.Bogdan, C. 2001. Nitric oxide and the immune response. Nat. Imuunol. 2:907-916. [DOI] [PubMed] [Google Scholar]
- 4.Constantin, D., D. Ala'Aldeen, and S. Murphy. 2002. Transcriptional activation of nitric oxide synthase-2, and NO-induced cell death, in mouse cerebrovascular endothelium exposed to Neisseria meningitidis. J. Neurochem. 81:270-276. [DOI] [PubMed] [Google Scholar]
- 5.Crawford, M. J., and D. E. Goldberg. 1998. Role of the Salmonella flavohemoglobin in protection from nitric oxide. J. Biol. Chem. 273:12543-12547. [DOI] [PubMed] [Google Scholar]
- 6.Cross, R., D. Lloyd, R. K. Poole, and J. W. Moir. 2001. Enzymatic removal of nitric oxide catalyzed by cytochrome c′ in Rhodobacter capsulatus. J. Bacteriol. 183:3050-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunninghan, A. C. 2002. Parasitic adaptive mechanisms in infection by leishmania. Exp. Mol. Pathol. 72:132-141. [DOI] [PubMed] [Google Scholar]
- 8.Drevets, D. A., B. P. Canono, P. J. Leenen, and P. A. Campbell. 1994. Gentamicin kills intracellular Listeria monocytogens. Infect. Immun. 62:2222-2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison, L. H., C. W. Armstrong, S. R. Jenkins, M. W. Harmon, G. W. Ajello, G. B. Miller, and C. V. Broone. 1991. A cluster of meningococcal disease on a school bus following epidemic of influenza. Arch. Intern. Med. 151:1005-1009. [PubMed] [Google Scholar]
- 10.Lundberg, J. O., S. T. Farkas, E. Witzberb, J. Rinder, J. Lidholm, A. Anggaard, T. Hokfelt, J. M. Lundberg, and K. Alvin. 1995. Hugh nitric oxide production in human sinuses. Nat. Med. 1:370-373. [DOI] [PubMed] [Google Scholar]
- 11.Lundberg, J. O., and E. Weitzberg. 1999. Nasal nitric oxide in man. Thorax 54:947-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathan, C., M. U. Shiloh. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97:8841-8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Read, R. C., A. Fox, K. Miller, T. Gray, N. Jones, R. Borrows, D. M. Jones, and R. G. Finch. 1995. Experimental infection of human nasal mucosal explants with Neisseria meningitidis. J. Med. Microbiol. 42:353-361. [DOI] [PubMed] [Google Scholar]
- 14.Read, R. C., S. Zimmerli, C. Broaddus, D. A. Sanan, D. S. Stephens, and J. D. Ernst. 1996. The (α2→8)-linked polysialic acid capsule of group B Neisseria meningitidis modifies multiple steps during interaction with human macrophages. Infect. Immun. 64:3210-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnappinger, D., S. Ehrt, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: Insights into the phagosomal environment. J. Exp. Med. 198:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiloh, M. U., J. D. MacMicking, S. Nicholson, J. E. Brause, S. Potter, M. Marino, F. Fang, M. Dinauer, and C. Nathan. 1999. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity 10:29-38. [DOI] [PubMed] [Google Scholar]
- 17.Sims, R. J., M. M. Harrison, E. R. Moxon, and C. M. Tang. 2000. Underestimation of meningococci in tonsollar tissue by nasopharyngeal swabbing. Lancet 356:1653-1654. [DOI] [PubMed] [Google Scholar]
- 18.Stevanin, T. M., R. K. Poole, E. A. G. Demoncheaux, and R. C. Read. 2002. Flavohemoglobin Hmp protects Salmonella enterica serovar Typhimurium from nitric oxide related killing by human macrophages. Infect. Immun. 70:4399-4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. F. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 20.Townsend, R. L. Goodwin, T. M. Stevanin, P. B. Silcocks, A. Parker, M. C. Maiden, and R. C. Read. 2002. Invasion by Neisseria meningitidis varies widely between clones and among nasopharyngeal mucosae derived from adult human hosts. Microbiology 148:1467-1474. [DOI] [PubMed] [Google Scholar]
- 21.Vazquez-Torres, A., J. Jones-Carson, P. Mastroeni, H. Ischiropoulos, and F. C. Fang. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J. Exp. Med. 192:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinberg, J. B., M. A. Misukonis, P. J. Shami, S. N. Mason, D. J. Sauls, W. A. Dittman, E. R. Wood, G. K. Smith, B. Mcdonald, K. E. Bachus, A. F. Haney, and D. L. Granger. 1995. Human mononuclear phagocyte inducible nitric-oxide synthase (iNOS) - analysis of iNOS messenger-RNA, iNOS protein, biopterin, and nitric-oxide production by blood monocytes and peritoneal-macrophages. Blood 86:1184-1195. [PubMed] [Google Scholar]
- 23.Weinberg, J. B. 1999. Human mononuclear phagocyte nitric oxide production and inducible nitric oxide synthase expression, p. 95-150. In F. C. Fang (ed.), Nitric oxide and infection. Academic Press, New York, N.Y.
- 24.Yazdakhah, S. P., and D. A. Caugant. 2004. Neisseria meningitidis: an overview of the carriage state. J. Med. Microbiol. 53:821-832. [DOI] [PubMed] [Google Scholar]