Abstract
Leprosy type 1 reactions (T1R) are due to increased cell-mediated immunity and result in localized tissue damage. The anti-inflammatory drug prednisolone is used for treatment, but there is little good in vivo data on the molecular actions of prednisolone. We investigated the effect of prednisolone treatment on tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-10, and transforming growth factor β1 (TGF-β1) mRNA and protein expression in blood and skin biopsies from 30 patients with T1R in India. After 1 month of prednisolone treatment the sizes of the skin granulomas were reduced, as were the grades of cells positive for TNF-α and IL-10 in skin lesions. Increased production of TGF-β1 was seen in skin lesions after 6 months of prednisolone treatment. Expression of mRNA for TNF-α, IL-1β, and TGF-β1 was reduced, whereas no change in IL-10 mRNA expression was detected during treatment. The circulating cytokine profiles were similar in patients with and without T1R, and prednisolone treatment had no detectable effects on cytokine expression in the blood. The data emphasize the compartmentalization of pathology in T1R and the importance of the immune response in the skin. Clinical improvement and cytokine expression were compared. Surprisingly, patients with improved skin and nerve function and patients with nonimproved skin and nerve function had similar cytokine profiles, suggesting that clinical improvement is not directly mediated by the cytokines studied here. This in vivo well-controlled study of the immunosuppressive effects of prednisolone showed that the drug does not switch off cytokine responses effectively.
Leprosy is a chronic infectious disease of the skin and nerves caused by the intracellular pathogen Mycobacterium leprae. It is characterized by a spectrum of clinical forms depending on the host's immune response to M. leprae. Patients with tuberculoid leprosy (TT) have strong cell-mediated immunity (CMI) with elimination of the bacilli, whereas patients with lepromatous leprosy (LL) exhibit defective CMI to M. leprae. Between these two polar forms of disease are the unstable borderline forms, including borderline tuberculoid (BT), borderline, and borderline lepromatous (BL). Leprosy may be complicated by inflammatory reactions. Type 1 (reversal) reactions (T1R), common in borderline forms of leprosy, are due to delayed-type hypersensitivity and an increase in CMI to M. leprae antigens. Skin lesions and affected nerves become more inflamed during the reaction. The neuritis may result in irreversible peripheral nerve damage if it is not adequately treated. Six months of treatment with the glucocorticoid (GC) prednisolone reduces skin inflammation and improves nerve function in about 50 to 80% of the patients (7, 24, 40, 41, 43, 45).
The immune response to M. leprae in TT patients is characterized by expression of protein and mRNA for Th1 cytokines, such as gamma interferon (IFN-γ) and interleukin-2 (IL-2) in skin lesions (1, 25, 47, 49). These cytokines promote CMI and activation of macrophages. In contrast, mRNA for the Th2 cytokines IL-4 and IL-5 is present in skin lesions of LL patients (49). The inflammatory cytokine tumor necrosis factor alpha (TNF-α) is crucial to antimycobacterial immunity and plays an important role in granuloma formation during mycobacterial infection (6, 11, 16, 19, 39). However, excess TNF-α can cause tissue damage, and previous work in our laboratory has shown that high levels of protein and mRNA for TNF-α are present in skin and nerve during tissue-damaging T1R (17). Other groups have demonstrated the presence of protein and mRNA for TNF-α in skin lesions, peripheral blood mononuclear cells (PBMC), and plasma during T1R (30, 32, 36, 38, 42, 50). Hence, down-regulation of TNF-α could be crucial for reducing inflammation during reactions. Expression of tumor necrosis factor β1 (TGF-β1), an important regulator of inflammation and wound healing, is decreased in skin in T1R (18), and thus up-regulation of TGF-β1 could be beneficial for resolution of the reaction.
GCs are used for suppression of immunity and inflammation in chronic inflammatory diseases. The anti-inflammatory actions of GCs are partially due to their capacity to inhibit activation of transcription factors, such as NF-κB, which regulates many genes, including those encoding TNF-α, IL-1β, IL-2, and inducible nitric oxide synthase (iNOS), which are involved in inflammatory responses. Studies have shown that treatment with GCs down-regulates TNF-α mRNA and protein in the blood of patients with inflammatory diseases, such as acute respiratory distress syndrome (29) and multiple sclerosis (14). A down-regulation effect of GCs on many inflammatory cytokines has been observed in vitro, but the findings for the effect of GCs on TGF-β1 and IL-10 expression are conflicting. In leprosy skin lesions, down-regulation effects of prednisolone treatment on IFN-γ, IL-6, IL-10, IL-12, IL-13, and iNOS production (2, 23) and TNF-α, IFN-γ, IL-6, and IL-12 mRNA (31) have been demonstrated. Moreover, a study in Nepal showed that TNF-α and IFN-γ responses, but not IL-10 responses, to M. leprae antigens are reduced in some patients with T1R while they are receiving a high dose of prednisolone (27).
We investigated the effect of prednisolone treatment on the inflammatory cytokines TNF-α and IL-1β and the regulatory cytokines IL-10 and TGF-β1 in the skin and blood of patients with T1R. Our aim was to compare events at the local site of infection (i.e., the skin lesion) and the systemic effects of the drug on cytokine expression. Borderline patients without a reaction and healthy subjects were included as controls.
We hypothesized that prednisolone treatment would result in down-regulation of TNF-α and IL-1β and up-regulation of TGF-β1 and IL-10 in the skin and that treatment would have a similar effect on cytokine expression in the blood. We also hypothesized that a poor clinical outcome of skin and nerve function is associated with high expression of TNF-α and IL-1β and low expression of TGF-β1 and IL-10. Blood samples and skin biopsies were taken from leprosy patients with and without T1R before and during prednisolone treatment. Healthy subjects were included as controls. We studied the effects of treatment on cytokine gene expression, as well as protein production, in blood and skin lesions and on the potential of PBMC to produce cytokines in response to M. leprae.
MATERIALS AND METHODS
Patients.
All patients recruited into this study were attending the Blue Peter Research Centre, Hyderabad, India. The patients were graded clinically and histologically on the leprosy spectrum according to the Ridley-Jopling classification (37). T1R was defined as the appearance of erythema and edema in either existing or new skin lesions within the previous 2 weeks and was confirmed histologically. Patients with T1R were treated with a standard reducing course of steroids that initially consisted of 30 mg oral prednisolone daily, and the dose was reduced by 5 mg each month for 6 months. Patients also received World Health Organization-recommended leprosy multidrug treatment (MDT). Permission was obtained for this study from the local ethics committee of the Blue Peter Research Centre, Hyderabad, India, and the London School of Hygiene and Tropical Medicine ethics committee. Informed consent was obtained in writing.
Clinical improvement.
The severity of T1R was measured by using a numerical severity scale that assessed skin signs, nerve pain and tenderness (NPT), sensory testing (ST), and voluntary muscle testing (VMT). The clinical severity score (CSS) used in this study is a modified version of a protocol developed as part of the ILEP Nerve Function Impairment and Reaction Research Programme and described elsewhere (28). Clinical improvement was defined as any reduction in the CSS for skin signs, NPT, ST, or VMT. Patients were divided into patients who improved (partially or completely) and patients who did not improve for each category after 1 week and 1 month of prednisolone treatment.
Preparation of PBMC and cell culture.
Venous blood was collected into 10 U/ml heparin, and PBMC were separated on a Ficoll gradient. Aliquots of PBMC in RNAlater (Ambion, Huntingdon, United Kingdom) and aliquots of plasma were stored at −70°C. Production of cytokines by PBMC in response to M. leprae was measured by culturing PBMC in growth medium (RPMI, 5% autologous plasma, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) in wells at 37°C in 5% CO2. M. leprae soluble antigen (MLSA) and tuberculin purified protein derivative (PPD) (Statens Serum Institute, Copenhagen, Denmark) were added to the cultures at a concentration of 10 μg/ml, and concanavalin A (ConA) (Sigma, Pool, United Kingdom) was added to the cultures at a concentration of 5 μg/ml. MLSA was kindly supplied by Patrick Brennan of Colorado State University. After 20 h of incubation culture supernatants were collected and frozen at −70°C for batch testing.
Cytokine measurement by ELISA.
Plasma and supernatants were tested for cytokines using a sandwich enzyme-linked immunosorbent assay (ELISA). Capture and biotinylated detection antibodies directed against TNF-α and IL-10 were purchased from BD Pharmingen (San Diego, Calif.). TGF-β1 was captured using a chicken anti-TGF-β1 antibody (R&D Systems, Abingdon, United Kingdom) and was detected with a mouse anti-TGF-β1,2,3 antibody (R&D Systems), followed by biotinylated anti-mouse immunoglobulin G antibody (Sigma). Standards were prepared by serial dilution of recombinant human TNF-α (rhTNF-α), rhIL-10 (BD Pharmingen), and rhTGF-β1 (R&D Systems). Detection was performed with streptavidin peroxidase (Sigma) conjugated with o-phenylenediamine enzyme substrate (Sigma). The assays were sensitive over concentration ranges of 30 to 2,000 pg/ml for TNF-α and IL-10 and 300 to 5,000 pg/ml for TGF-β1. TGF-β1 is expressed constitutively and secreted in an inactive form that must be cleaved and activated before it can bind to its receptor. Total TGF-β1 (i.e., active and inactive forms) was measured by acid activation of samples. For acid activation, 10 μl of 1 M HCl was added to a 100-μl sample or standard. After mixing and incubation for 10 min, 12 μl of 1 M NaOH was added to neutralize the preparation, and samples were added to an ELISA plate within 10 to 15 min (34).
RNA isolation and reverse transcription.
Isolation of RNA from skin biopsies and PBMC stored in RNAlater was performed using an RNeasy fibrous kit and an RNeasy mini kit (QIAGEN, Crawley, West Sussex, United Kingdom), respectively. Digestion of DNA with DNase I (QIAGEN) was included for all RNA preparations. The RNA yield was determined with a RiboGreen RNA quantitation kit (Molecular Probes, Eugene, OR), and the RNA integrity was checked by agarose gel electrophoresis. cDNA was synthesized from RNA (200 ng/reaction mixture) using an Omniscript reverse transcriptase kit (QIAGEN).
Real-time PCR.
Cytokine gene expression was quantified using real-time PCR assays. Primers were designed across intron-exon boundaries in mRNA sequences (obtained from the Nation Center for Biotechnology Information database) and were synthesized by Sigma-Genosys (United Kingdom). The nucleotide sequences of the forward and reverse primers, respectively, used in this study were as follows: for TNF-α, 5′-TGC TTG TTC CTC AGC CTC TT-3′ and 5′-GGT TTG CTA CAA CAT GG CTA C-3′; for TGF-β1, 5′-GGAGCT GTA CCA GAA ATA CAG-3′ and 5′-TCC ACT TGC AGT GTG TTA TG-3′; for IL-1β, 5′-ATT GCT CAA GTG TCT GAA GC-3′ and 5′-GTA GTG GTG GTC GGA GAT T-3′; for IL-10, 5′-TGA GAA CCA AGA CCC AGA CA-3′ and 5′-TCA TGG CTT TGT AGA TGC CT-3′; and for the hypoxanthine guanine phosphoribosyltransferase 1 (HPRT1) standard, 5′-GCT GGA TTA CAT CAA AGC ACT G-3′ and 5′-TGT TTC ACT CAA TAG TGC TGT GG-3′. Real-time PCRs were performed with a LightCycler (Roche, Idaho Technologies) using QuantiTect SYBR Green PCR master mixture (QIAGEN). The annealing temperature was 58°C for TNF-α, 55°C for TGF-β1 and IL-1β, and 51°C for IL-10. HPRT1 was detected with a probe (QuantiTect gene expression assay) and a QuantiTect probe PCR kit (QIAGEN) used according to the manufacturer's instructions. To quantify gene expression (copies/μl), the unknown samples were compared with a standard curve. To prepare standards for quantification, each target sequence was amplified and gel purified. The stocks were serially diluted from 106 to 10 copies/μl in 2 mg/liter herring sperm DNA (Sigma). The results were expressed as the ratio of the number of cytokine mRNA copies to the number of HPRT mRNA copies.
Immunoperoxidase staining.
Cryostat sections (6 μm) of skin biopsies were fixed in acetone and immunostained with antibodies against TNF-α (MON5006; 1/5; Caltag Medsystems, Silverstone, Towcester, United Kingdom), IL-10 (SC-7888; 1:400; Santa Cruz Biotechnology, Santa Cruz, CA), TGF-β1 (SC-146; 1:10; Santa Cruz Biotechnology), and the macrophage marker CD68 (clone EBM11; M0718; 1:200; Dako Ltd., Ely, United Kingdom). MON5006 and EBM11 were detected with rabbit anti-mouse antisera (Z0259; Dako) at a dilution of 1:50, followed by mouse peroxidase anti-peroxidase antibody (P0850; Dako) at dilution 1:100. SC-7888 was detected with goat anti-rabbit antisera (Z0421; Dako) at a dilution of 1:100, followed by rabbit peroxidase anti-peroxidase antibody (Z0113; Dako) at a dilution of 1:100. SC-146 was detected with biotinylated swine anti-rabbit antisera (P0399; Dako) at a dilution of 1:200, followed by streptavidin-biotin complex-horseradish peroxidase (Dako). Positive staining was visualized using 3,3-diaminobenzidine (Sigma, Pool, United Kingdom)-H2O2. Sections were counterstained with hematoxylin. The controls for the specificity of staining included using normal serum, omitting the primary antibody, and using similar isotype antibodies.
The cell and cytokine staining was assessed by grading the sections with the following scale: 0, negative; 1, a few scattered positive cells; 2, 10 to 30% of the cells were positively stained; 3, 30 to 50% of the cells were positively stained; 4, 50 to 80% of the cells were positively stained; and 5, 80 to 100% of the cells were positively stained. Cellular infiltration was assessed with the following scale: 1, no cellular infiltrate; 2, groups of cells; 3, moderate cellular infiltration; and 3, extensive cellular infiltration. We have used this scale in previous work (18, 20, 23). Slides were evaluated by two independent observers.
Double immunofluorescence staining.
Immunofluorescence staining was performed by serial incubation of cryosections of skin biopsies with mouse anti-human TNF-α (MON5006; 1/5; Caltag Medsystems) or rabbit anti-human IL-10 (SC-7888; 1:400; Santa Cruz Biotechnology), followed by incubation with an isotype-specific fluorochrome (Alexa Fluor 647)-conjugated antibody (rabbit anti-mouse or goat anti-rabbit; Molecular Probes). Sections were washed and incubated with fluorochrome (fluorescein isothiocyanate)-conjugated mouse anti-CD68 antibody (clone KP1; 1:1; Dako). Mounting and counterstaining were performed with Vectashield mounting medium containing propidium iodide (Vector Laboratories Ltd., Peterborough, United Kingdom).
Confocal laser microscopy.
Immunofluorescent labeled sections were examined with an inverted Zeiss LSM510 laser scanning confocal microscope fitted with krypton and argon lasers. Fluorescein isothiocyanate fluorescence was detected with 488-nm excitation and a 505- to 550-nm band-pass emission filter. Propidium iodide fluorescence was detected with 543-nm excitation and a 585-nm long-pass emission filter. Alexa Fluor 647 was detected with 633-nm excitation and a 650-nm long-pass emission filter. The images were superimposed for colocalization analysis. Immunofluorescence staining with negative control antibodies was recorded using exactly the same settings that were used for the specific staining to check for nonspecificity, as well as positive controls to eliminate potential crossover between channels.
Statistical analysis.
Differences between times and between groups of patients were determined using the Wilcoxon signed-rank test and the Mann-Whitney test, respectively. P values of <0.05 were considered significant.
RESULTS
Thirty patients with T1R, 12 nonreactional patients, and eight healthy controls were recruited between March 2002 and September 2003. The characteristics of the patients with T1R were as follows: 13 BT T1R patients and 17 BL T1R patients; 11 females and 19 males; and 16 to 65 years old. At time of recruitment, 10 patients with T1R were already on MDT, 14 patients with T1R were about to start MDT, and 5 patients with T1R had previously taken and completed MDT. The characteristics of the nonreactional patients were as follows: six BT patients and six BL patients; all male; and 15 to 42 years old. All nonreactional patients were starting MDT at time of recruitment and had not taken MDT within 3 months prior to recruitment. The healthy controls were endemic laboratory staff (two females and six males who were 23 to 41 years old). Blood samples were collected from 16 patients with T1R before prednisolone treatment was started and after 1 and 6 months of prednisolone treatment. Skin biopsies were collected from 29 patients with T1R before prednisolone treatment was started and then during week 1 and months 1 and 6 of prednisolone treatment. For nonreactional patients a skin biopsy and blood sample were collected before MDT was started and after 1 month of MDT. A biopsy and a blood sample were not taken at all times for all patients. For healthy controls one blood sample was taken.
Cytokine mRNA expression in PBMC.
All patients had detectable levels of mRNA for all the cytokines studied. Table 1 shows the median levels of cytokine gene expression in each group at the different times during treatment. The levels of TGF-β1 mRNA in PBMC were significantly higher in healthy controls than in patients with T1R (P < 0.01) and nonreactional patients (P < 0.05). In contrast, the different groups of patients and controls expressed similar levels of mRNA for TNF-α, IL-1β, and IL-10. One month of prednisolone treatment significantly (P < 0.001) increased the expression of IL-10 mRNA in 11 of 14 patients with T1R, and after 6 months of treatment the levels had returned to levels similar to those before treatment. Prednisolone treatment had no significant effect on TNF-α, IL-1β, and TGF-β1 mRNA expression. Nonreactional patients expressed similar levels of TNF-α, IL-1β, IL-10, and TGF-β1 mRNA before treatment and after 1 month of treatment with MDT.
TABLE 1.
Median cytokine plasma levels and gene expression levels in relation to the housekeeping gene in PBMC of patients with T1R, nonreactional patients, and healthy controls
| Cytokine | Median level ina:
|
|||||
|---|---|---|---|---|---|---|
| Patients with T1R
|
Nonreactional patients
|
Healthy controls (n = 7) | ||||
| Before prednisolone (n = 14) | 1 month (n = 14) | 6 months (n = 8) | Before MDT (n = 11) | 1 month (n = 7) | ||
| Plasma levels | ||||||
| TNF-α | 0 (0, 0) | 0 (0, 42) | 0 (0, 91) | 0 (0, 192) | 0 (0, 154) | 64 (0, 149) |
| IL-10 | 0 (0, 76) | 0 (0, 81) | 33 (0, 78) | 0 (0, 123) | 0 (0, 127) | 0 (0, 37) |
| TGF-β1(active) | 327 (0, 596) | 331 (0, 640) | 314 (0, 703) | 325 (0, 603) | 319 (0, 481) | 309 (0, 407) |
| TGF-β1(total) | 7,251 (2,329, 18,548) | 3,134 (1,825, 9,299)b | 3,719 (18,471, 6,299) | 11,142 (4,124, 26,978) | 7,392 (3,461, 18,496) | 4,071 (3,125, 5,072)c |
| mRNA levels | ||||||
| TNF-α | 0.6 (0.2, 2.1) | 0.7 (0.3, 1.8) | 0.6 (0.2, 6.3) | 0.4 (0.2, 5.1) | 0.5 (0.3, 0.5) | 0.4 (0.3, 1.1) |
| IL-1β | 1.2 (0.1, 8.5) | 1.4 (0.2, 5.8) | 0.7 (0.1, 6.7) | 0.8 (0.2, 7.6) | 0.7 (0.1, 1.0) | 0.3 (0.1, 1.8) |
| IL-10 | 12.9 (3.4, 49.3) | 46.1 (6.7, 143.4)d | 14.5 (4.1, 37.5) | 17.9 (2.7, 45.3) | 13.1 (4.8, 26.9) | 8.6 (4.3, 44.4) |
| TGF-β1 | 2.4 (1.9, 5.8) | 2.7 (1.5, 5.7) | 3.9 (1.7, 11.3) | 3.1 (1.2, 4.9) | 2.9 (2.3, 4.6) | 7.3 (3.1, 11.8)e |
The cytokine plasma levels are expressed in picograms per milliliter, and the gene expression levels are expressed as the ratio of the number of cytokine mRNA copies to the number of HPRT mRNA copies. The values in parentheses are 5th and 95th percentiles.
Significantly (P < 0.01) lower than the level before prednisolone treatment.
Significantly (P < 0.05) lower than the level for nonreactional patients before MDT.
Significantly (P < 0.001) higher than the level before prednisolone treatment.
Significantly (P < 0.01) higher than the level for T1R and nonreactional patients before treatment.
Cytokine plasma levels.
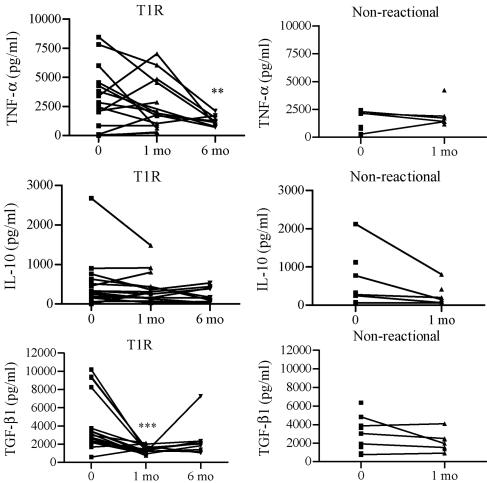
Cytokine plasma levels for patients with T1R, nonreactional patients, and healthy controls were determined using ELISA. Surprisingly, none of the patients with T1R and only two of the nonreactional patients had detectable plasma levels (>30 pg/ml) of TNF-α, whereas TNF-α was detected in six of the seven healthy controls. IL-10 was detected in plasma of two patients with T1R, one nonreactional patient, and one healthy control. The healthy controls produced lower levels of total TGF-β1 than the patients produced, but the difference was significant (P < 0.05) only when nonreactional patients and healthy controls were compared. The median cytokine plasma levels are listed in Table 1. One-third of all the subjects had no detectable levels (<300 pg/ml) of active TGF-β1 in their plasma, and patients and controls produced similar levels of active TGF-β1. The TNF-α plasma levels remained below or just above the positive cutoff in 11 of 14 patients with T1R during the 6 months of treatment with prednisolone. Similarly, the levels of IL-10 and active TGF-β1 did not change in reactional patients during prednisolone treatment. In contrast, the plasma levels of total TGF-β1 were significantly (P < 0.01) reduced after 1 month of treatment. In a few patients a small increase in total TGF-β1 was detected at 6 months, and hence no significant difference in the total TGF-β1 production was detected between the baseline and 6 months. Nonreactional patients had similar plasma levels of these cytokines before MDT and after 1 month of MDT.
Cytokine responses to M. leprae antigen.
The median cytokine levels in culture supernatants of PBMC from patients before they started treatment and from healthy controls are shown in Table 2. Patients with T1R showed significantly (P < 0.05) higher levels of spontaneous production of TNF-α than nonreactional patients showed, whereas ConA- and PPD-induced production of TNF-α was similar in patients and controls. One-half of the patients with T1R showed a higher TNF-α response to MLSA than the nonreactional patients and healthy controls showed, but the difference was not significant. Patients with T1R, nonreactional patients, and healthy controls also produced similar levels of IL-10 in response to ConA, PPD, and MLSA. The levels of spontaneous production of total TGF-β1 were high in all culture supernatants, and stimulation with ConA, PPD, or MLSA did not influence the ability of PBMC to produce total TGF-β1. Moreover, patients and controls produced similar levels of total TGF-β1.
TABLE 2.
Levels of in vitro cytokine production in culture supernatants of PBMC from patients with T1R before prednisolone treatment was started, in nonreactional patients before MDT was started, and in healthy controls
| Prepn | Median cytokine level (pg/ml)a
|
||
|---|---|---|---|
| Patients with T1R (n = 16) | Nonreactional patients (n = 9) | Healthy controls (n = 7) | |
| TNF-α | |||
| Unstimulated | 90 (0, 678)b | 16 (0, 211) | 37 (0, 230) |
| MLSA | 2,634 (0, 7,974) | 912 (249, 2,392) | 1,283 (168, 2,614) |
| PPD | 2,181 (0, 6,437) | 1,284 (214, 3,737) | 1,243 (164, 1,971) |
| ConA | 1,515 (0, 3,937) | 695 (0, 1,996) | 1,257 (52, 4,893) |
| IL-10 | |||
| Unstimulated | 0 (0, 77) | 0 (0, 484) | 0 (0, 64) |
| MLSA | 314 (0, 1,347) | 275 (67, 1,724) | 405 (135, 471) |
| PPD | 225 (0, 1,019) | 311 (112, 2,872) | 259 (114, 641) |
| ConA | 113 (0, 892) | 101 (0, 1,295) | 147 (34, 185) |
| TGF-β1 (total) | |||
| Unstimulated | 2,692 (1,550, 11,926) | 2,418 (1,021, 8,304) | 1,792 (1,132, 11,884) |
| MLSA | 3,048 (1,409, 9,609) | 2,476 (807, 5,672) | 1,517 (1,168, 8,251) |
| PPD | 2,842 (1,503, 9,180) | 2,714 (784, 5,501) | 1,600 (1,086, 7,349) |
| ConA | 2,831 (1,771, 12,125) | 2,928 (875, 2,928) | 1,578 (1,121, 5,785) |
The values in parentheses are 5th and 95th percentiles.
Significantly (P < 0.05) higher than the value for nonreactional patients.
After 1 month of treatment, when all patients with T1R were on a high dose of prednisolone (average dose, 30 mg), PPD- and MLSA-induced TNF-α production was reduced in 8 of the 16 patients with T1R, whereas 4 patients showed an increase and the remainder showed no change in TNF-α production. However, after 6 months of prednisolone treatment, spontaneous (P < 0.01), PPD-induced (P < 0.05), and MLSA-induced (P < 0.01) TNF-α production was significantly lower than production before treatment. Cytokine responses to MLSA during treatment are shown in Fig. 1. Overall, no significant change in TNF-α production was detected in ConA-stimulated cultures during prednisolone treatment. Similar levels of spontaneous and ConA-, PPD-, or MLSA-induced IL-10 were detected in PBMC of patients with T1R before and during prednisolone treatment. In contrast, 1 month of prednisolone treatment significantly (P < 0.01) reduced the levels of total TGF-β1 in culture supernatants of both stimulated and unstimulated PBMC. For most patients the levels of total TGF-β1 stayed low after 6 months of prednisolone treatment, although there was not a significant difference between the baseline and 6 months. Nonreactional patients showed similar levels of spontaneous and ConA-, PPD-, and MLSA-induced TNF-α, IL-10, and total TGF-β1 production before MDT and after 1 month of MDT (Fig. 1). The levels of active TGF-β1 were undetectable or very low in all culture supernatants at all times (data not shown).
FIG. 1.
Levels of TNF-α, IL-10, and total TGF-β1 responses to MLSA in PBMC from patients with T1R and nonreactional patients before and during treatment. PBMC were cultured for 20 h in the presence of MLSA. Cytokine levels in culture supernatants were detected with the ELISA. Each line represents a patient. Statistical differences compared with zero time are indicated as follows: two asterisks, P < 0.01; and three asterisks, P < 0.001.
Cytokine production in skin lesions.
The results of the immunostaining experiment are summarized in terms of semiquantitative grades in Table 3. All skin biopsies collected before treatment had cellular infiltration, and a large proportion of the cells stained positive for the macrophage marker CD68. Most patients with T1R had large granulomas and, overall, significantly (P < 0.05) more cellular infiltration than nonreactional patients. Cellular infiltration was reduced significantly (P < 0.001) after 1 month of prednisolone treatment. However, some patients with T1R continued having moderate to extensive cellular infiltration even after 6 months of treatment. One patient had grade 3 granuloma formation and four patients had grade 2 granuloma formation at 6 months.
TABLE 3.
Scoring of cellular infiltration and cytokine expression in biopsies from skin lesions of patients with and without T1R
| Patients | Wk | No. of patients | Median intensity scorea
|
||||
|---|---|---|---|---|---|---|---|
| Cellular infiltration | TNF-α | IL-10 | TGF-β1 | CD68 | |||
| T1R | 0 | 29 | 2 | 3 | 3 | 1 | 4 |
| 1 | 14 | 2 | 4 | 3 | 1 | 4 | |
| 4 | 29 | 1 | 2 | 2 | 1 | 3 | |
| 26 | 23 | 1 | 2 | 1 | 1 | 3 | |
| BT-BLb | 0 | 10 | 1.5 | 1.5 | 1 | 2 | 3 |
| 4 | 7 | 2 | 2 | 1 | 2 | 3 | |
Scoring of staining was as follows: 0, negative; 1, a few scattered positive cells; 2, 10 to 30% of the cells positively stained; 3, 30 to 50% of the cells positively stained; 4, 50 to 80% of the cells positively stained; and 5, 80 to 100% of the cells positively stained. Cellular infiltration was assessed with the following scale: 1, no cellular infiltrate; 2, groups of cells; 3, moderate cellular infiltration; and 3, extensive cellular infiltration.
BT-BL, borderline patients without reactions.
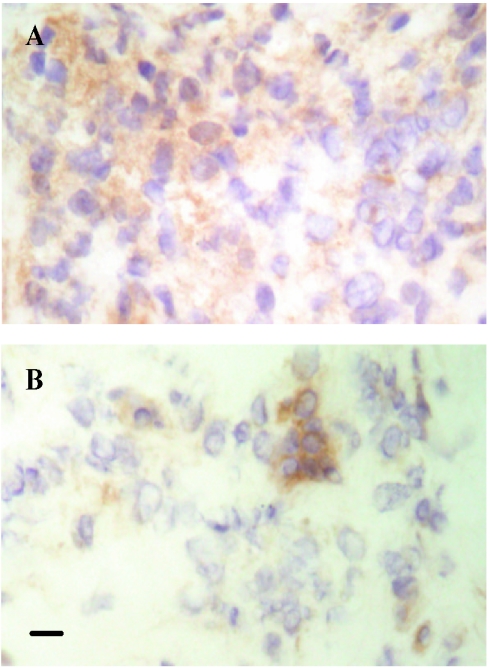
Before treatment the grades for cells staining positive for TNF-α were significantly (P < 0.0001) higher in patients with T1R than in nonreactional patients. Figure 2 shows representative microphotographs of sections of skin in T1R stained for TNF-α. Similarly, skin biopsies from patients with T1R showed significantly (P < 0.0001) more cells positive for IL-10 than skin biopsies from nonreactional patients showed. In contrast, there were significantly (P < 0.05) fewer TGF-β1-positive cells in patients with T1R than in nonreactional patients. After 1 and 6 months of prednisolone treatment the median grades for cells positive for TNF-α and IL-10 were significantly (P < 0.001) reduced. However, not all patients with T1R showed a decrease in TNF-α and IL-10 production. At 6 months two patients still had grade 4 (>50%) for cells staining positive for TNF-α, and one patient had grade 4 for cells staining positive for IL-10. No significant difference was found between the median scores for TNF-α or IL-10 for day 0 and week 1 biopsies during prednisolone treatment. A small but significant increase (P < 0.01) in the grades for cells positive for TGF-β1 was detected after 6 months of prednisolone treatment, although the median grade for TGF-β1 (1) did not change during prednisolone treatment. No change in the grade for TGF-β1-positive staining was detected in biopsies collected from patients with T1R at earlier times during treatment. In nonreactional patients, 1 month of MDT had no discernible effect on cellular infiltration and TNF-α, IL-10, and TGF-β1 production as skin biopsies from nonreactional patients had similar grades before MDT and after 1 month of MDT.
FIG. 2.
Representative microphotographs of tissue sections stained for TNF-α: cryosections of biopsies taken from a patient with T1R before prednisolone treatment was started (A) and after 1 month of prednisolone treatment (B). Bar = 10 μm. Harris hematoxylin counterstaining was used. Original magnification, ×400.
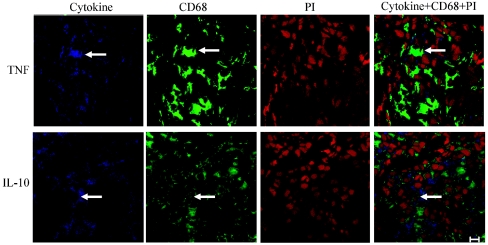
The cytokines studied are predominately produced by cells of the monocyte lineage. To investigate if macrophages are the primary source of TNF-α and IL-10 production in skin lesions in T1R, skin biopsies from four patients were double stained using fluorescence for CD68 and TNF-α or IL-10. A large proportion of cells in the granulomas stained positive for TNF-α, and a large proportion of cells stained positive for CD68 (Fig. 3). When images of the same section were superimposed, most of the CD68-positive cells were shown to also be positive for TNF-α. However, some cells only stained positive for TNF-α, and some cells were negative for both TNF-α and CD68. Similarly, most of the IL-10 staining colocalized with CD68 staining (Fig. 3).
FIG. 3.
Fluorescent double staining for cytokines and CD68 in skin biopsies from patients with T1R before treatment. Three color fluorescence confocal images were obtained for TNF-α and IL-10 (blue) (first panel of each row), CD68 (green) (second panel of each row), and cell nuclei (propidium iodide [PI]) (red) (third panel of each row). The three images were superimposed (fourth panel of each row). The arrows indicate a double-positive cell. Bar = 10 μm. Original magnification, ×630.
Cytokine mRNA expression in skin lesions.
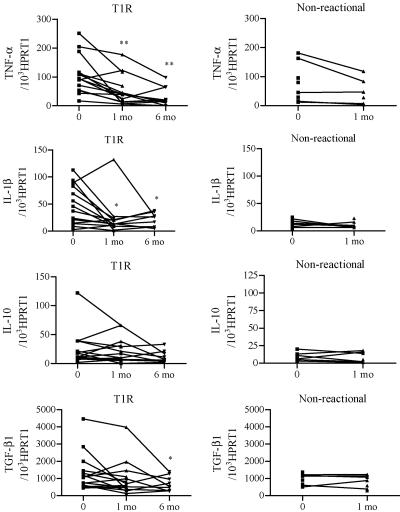
The levels of mRNA for IL-1β were significantly higher in biopsies from patients with T1R than in biopsies from nonreactional patients, whereas similar levels of TNF-α, IL-10, and TGF-β1 were detected in patients with and without T1R (Fig. 4). In patients with T1R, a significant reduction in the mRNA levels for TNF-α (P < 0.01) and IL-1β (P < 0.02) was seen at 1 and 6 months during prednisolone treatment. Likewise, a significant (P < 0.05) decrease in mRNA for TGF-β1 was detected in the month 6 biopsies from some patients with T1R. IL-10 mRNA was expressed at similar levels at all times. The levels of TNF-α, IL-1β, IL-10, and TGF-β1 mRNA were similar in skin biopsies from nonreactional patients before MDT and after 1 month of MDT (Fig. 4).
FIG. 4.
Cytokine mRNA levels in sequential skin biopsies from patients with T1R and nonreactional patients before and during treatment. Levels of cytokine mRNA for TNF-α, IL-1β, TGF-β1, IL-10, and HPRT1 in skin biopsies were quantified using real-time PCR assays. The results are expressed as the ratio of cytokine mRNA to HPRT1 mRNA. Each dot represents a patient. Significant differences compared with zero time are indicated as follows: one asterisk, P < 0.05; and two asterisks, P < 0.01.
Comparison of cytokine expression and clinical outcome.
Clinical improvement of patients with T1R was evaluated by using a clinical severity scale and compared to expression of protein and mRNA for TNF-α, IL-1β, IL-10, and TGF-β1 in skin and blood. After 1 week of prednisolone treatment, 4 of the 13 patients with T1R who had a skin biopsy taken at this time had improved skin signs. The cytokine production in patients who improved at time zero was compared to the cytokine production at 7 days. This showed that patients with improved skin signs had no change in cytokine production after 1 week of treatment. One of two patients with impaired NPT, one of nine patients with impaired ST, and three of nine patients with impaired VMT showed improvement in the corresponding categories after 1 week of treatment. No change in cytokine expression was seen in the few patients with improvement in NPT, ST, or VMT after 1 week of treatment (data not shown).
By definition, all patients with T1R had skin signs (erythema and/or edema) of T1R. After 1 month of prednisolone treatment patients showed complete (n = 14), partial (n = 9), or no (n = 6) improvement of skin signs. Patients who had improved skin signs and patients who did not have improved skin signs had similar skin and blood cytokine profiles (data not shown), indicating that poor clinical skin improvement after 1 month of treatment is not associated with high or low levels of TNF-α, IL-1β, IL-10, or TGF-β1 in the skin or blood. Four of five patients with impaired NPT, 6 of 24 patients with impaired ST, and 9 of 21 patients with impaired VMT showed improvement in the corresponding categories after 1 month of treatment. Patients with improved and nonimproved NPT and VMT had similar skin and blood cytokine profiles (data not shown). A significant (P < 0.01) increase in TGF-β1 mRNA expression was detected in PBMC of patients with improved ST (n = 4) at 1 month, whereas no change in TGF-β1 mRNA expression was detected in patients with nonimproved ST (n = 11) (data not shown). The difference in TGF-β1 mRNA levels between patients with improved ST and patients with nonimproved ST at 1 month was significant (P < 0.01), suggesting that TGF-β1 has a protective role. However, the total and active plasma levels of TGF-β1 were similar in the two groups of patients. Thus, there is no apparent correlation between nerve function impairment and expression of the cytokines studied in leprosy T1R.
To determine if there were differences in cytokine levels depending on whether a patient was already on or had just started MDT at time of recruitment, cytokine mRNA and protein expression data for skin biopsies and blood from these two groups of patients, as well as patients who had previously completed MDT, were compared. No significant difference in cytokine expression was detected between these groups of patients. Moreover, similar patterns of cytokine mRNA and protein expression were detected in patients with BT T1R and in patients with BL T1R. These similarities justify grouping all the patients with T1R together.
DISCUSSION
Although the focal point of the immune response to M. leprae is tissue granulomas of the skin and nerves, the immune response in the blood should reflect what happens at the local site of infection. Surprisingly, circulating cytokine profiles were similar in patients whether or not they had T1R, and prednisolone treatment had no effect or a different effect on cytokine expression in the blood compared to the skin. Protein and mRNA for TNF-α were reduced in the skin after 1 month of prednisolone treatment, whereas no effect of prednisolone treatment on circulating levels of TNF-α mRNA and protein was detected in blood. In a similar way but with a longer time scale than that for the skin, 6 months of prednisolone treatment had an inhibitory effect on the potential of PBMC to produce TNF-α in response to MLSA and PPD. However, it is surprising that we did not see inhibition of the TNF-α response during the first month of treatment when all the patients with T1R were on a high dose of prednisolone. A study in Nepal of 96 patients with T1R showed that TNF-α responses to M. leprae antigens were reduced in some patients with T1R while they were receiving a high dose of prednisolone after 2 weeks of treatment (27). As observed for TNF-α, the effects of prednisolone treatment on IL-1β were different in the skin and the blood. The reason that we did not see a reduction in TNF-α and IL-1β mRNA expression in PBMC during prednisolone treatment may have been that these cytokines are not up-regulated in the blood of leprosy patients with or without T1R. The healthy endemic controls in this study had levels of TNF-α and IL-1β mRNA in PBMC similar those in patients and higher plasma levels of TNF-α protein. In accordance with our findings, others have shown that prednisolone treatment has an inhibitory effect on TNF-α mRNA expression in skin lesions (31) and no effect on TNF-α plasma levels (10, 42). The present study confirmed that the focal point of the immune response during T1R is localized to the skin (and nerves) and suggested that the circulating cytokine profile may not reflect the immune response at the local site of infection. Moreover, the data suggested that using circulating levels of TNF-α diagnostically to confirm reactions or to monitor the response to treatment is not useful.
The high levels of IL-10 in skin lesions of patients with T1R were reduced after 1 month of prednisolone treatment, whereas treatment did not seem to have an effect on IL-10 mRNA expression in skin lesions. The levels of IL-10 mRNA in PBMC increased during the first month of treatment, although prednisolone treatment did not affect the IL-10 plasma levels or the potential of PBMC to produce IL-10 in response to MLSA. Similar conflicting data for the effect of GCs on IL-10 have been obtained for other diseases and experimental models (8, 12, 14, 44). Consistent with our findings, Atkinson et al. (2) showed that prednisolone treatment reduced production of IL-10 in skin lesions in patients with T1R, and Moraes et al. (31) found that IL-10 mRNA expression in skin lesions in patients with T1R is insensitive to prednisolone treatment. Manandhar et al. (27) showed that the IL-10 response to M. leprae antigens in whole-blood assays for patients with T1R is not influenced by prednisolone therapy. Moreover, a clinical study of multiple sclerosis demonstrated that GC treatment resulted in increased circulating levels of IL-10 mRNA and protein (14). It is interesting that IL-10 is up-regulated in reactional skin, although it has important immunoregulatory effects, such as inhibition of expression of TNF-α (21) and Th1 cytokines (48). The presence of both pro- and anti-inflammatory cytokines in the skin lesions highlights the complexity of the regulatory pathways in the granulomas during T1R. Moreover, the differences in cytokine protein and gene expression highlight the importance of studying cytokine protein production alongside mRNA expression.
The suppressive effects of TGF-β include inhibition of IFN-γ, TNF-α, and iNOS production (9, 46) and up-regulation of IL-10 production (26, 35). Hence, the small increase in active TGF-β1 detected in skin lesions during prednisolone treatment may play a role in resolving the T1R and preventing immune-mediated pathology. On the other hand, previous studies (18) have shown that production of active TGF-β1 is low in TT lesions compared to LL lesions, indicating that TGF-β1 may be important in maintaining the balance between control and clearance of M. leprae during infection. A similar role for TGF-β1 in protective immunity versus pathology in malaria has been suggested by Omer et al. (33). There are no published in vivo data on the effect of GC treatment on TGF-β1 in humans, and the results of in vitro studies are conflicting (3, 4, 13), although a xenotransplant model of giant cell arthritis in mice suggested that TGF-β1 mRNA and protein expression is steroid resistant (5). When a patient is given an anti-inflammatory drug, such as prednisolone, the stimulus for the body to make its own anti-inflammatory proteins may be reduced as well. Hence, it is perhaps not so surprising that the levels of IL-10 and TGF-β1 did not increase when the leprosy patients' reactions were ameliorated with prednisolone therapy.
This is one of the largest in vivo studies of cytokine expression in both skin and blood of patients with T1R and nonreactional controls. Most of the patients with T1R in this study were on MDT which included the mild anti-inflammatory drug clofazimine. Previous studies on the effect of prednisolone treatment on cytokine expression in patients with T1R have not looked at the effect of MDT. In the present study, results for cytokine expression in skin biopsies and blood samples collected before MDT was started and after 1 month of MDT from nonreactional patients were used as controls. Our data suggest that 1 month of MDT has no apparent effect on TNF-α, IL-1β, IL-10, and TGF-β1 expression and that it is prednisolone treatment rather than MDT that down-regulates TNF-α, IL-1β, and IL-10 in the skin lesions of patients with T1R.
The data obtained in this study indicate that improvement of skin signs is not directly mediated by the production of TNF-α, IL-1β, IL-10, and TGF-β1, and instead, it seems that prednisolone causes a rapid reduction in edema, followed by a slower effect on expression of these cytokines in the skin. After 6 months of prednisolone treatment the production of TNF-α and IL-10 was still high in some patients. Previous work has shown that production of IFN-γ, IL-6, IL-10, IL-12, and iNOS in skin lesions in patients with T1R is reduced in most patients after 1 month of prednisolone treatment (2, 23). Many of these patients had improved skin signs after 1 week, but the improvement was not associated with reduced expression of any of these cytokines (2). Hence, the reduction in inflammation in skin lesions does not seem to be directly mediated by the effect of prednisolone on cytokines. The fast effect of prednisolone on inflammation might be because GCs diminish the formation of prostaglandins and leukotrienes (15), which are produced by activated mast cells and macrophages and act as vasodilators and bronchoconstrictors. Moreover, some of the effects of prednisolone on inflammation may be due to nonspecific nongenomic mechanisms. These activities result in changes in intracellular processes, such as calcium and sodium transport across the membrane, which are essential for immediate and sustained activation of immune cells (22). Although most patients in this study showed a reduction in erythema and edema of the skin after 1 month of treatment, few patients showed any improvement in nerve function impairment at this time. Only 45 to 50% of patients had reduced CSS for ST and VMT after 6 months of treatment. Thus, the 6-month reducing course of prednisolone was less effective for improving nerve function impairment than for reducing inflammation in skin lesions. Previous studies in Hyderabad (24) and other places (7, 40, 41, 45) showed similar or worse clinical outcomes of nerve function impairment after prednisolone treatment.
Prednisolone is widely used for treatment of leprosy T1R, but clinical improvement varies, and a better understanding of the immunology of T1R is required to improve treatment for these patients. The immune response to M. leprae is cytokine mediated, whereas the involvement of cytokines in reactions is less understood. Leprosy research has focused on cytokines in reactions, but the data obtained in the present study suggest that factors or modulators other than cytokines may be more important, and we do not know what makes the clinical signs resolve in early stages. This study showed that prednisolone does not switch off cytokine responses effectively and highlighted the importance of understanding how prednisolone works. Only by understanding the early resolution can we develop better drugs that reduce inflammation more rapidly.
Acknowledgments
We thank all the staff and patients at the Blue Peter Research Centre (Hyderabad, India), particularly Syed Muzaffurullah and Mohammed Ismail, for documenting clinical progress and taking and maintaining skin biopsies. We also thank Martin Holland for technical advice on real-time PCR.
The Blue Peter Research Centre is supported by MRC (London, United Kingdom) through Lepra-India. This work and A.K.A. were supported by a grant from the Hospitals and Homes of St. Giles (United Kingdom).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Arnoldi, J., J. Gerdes, and H.-D. Flad. 1990. Immunohistologic assessment of cytokine production of infiltrating cells in various forms of leprosy. Am. J. Pathol. 137:749-753. [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson, S. E., S. Khanolkar-Young, S. Marlowe, S. Jain, R. G. Reddy, S. Suneetha, and D. N. J. Lockwood. 2004. Detection of IL-13, IL-10 and IL-6 in leprosy skin lesions of patients during prednisolone treatment for type 1 (T1R) reactions. Int. J. Lepr. Other Mycobact. Dis. 72:27-34. [DOI] [PubMed] [Google Scholar]
- 3.Batuman, O. A., A. Ferrero, C. Cupp, S. A. Jimenez, and K. Khalili. 1995. Differential regulation of transforming growth factor beta-1 gene expression by glucocorticoids in human T and glial cells. J. Immunol. 155:4397-4405. [PubMed] [Google Scholar]
- 4.Batuman, O. A., A. P. Ferrero, A. Diaz, and S. A. Jimenez. 1991. Regulation of transforming growth factor-beta 1 gene expression by glucocorticoids in normal human T lymphocytes. J. Clin. Investig. 88:1574-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brack, A., H. L. Rittner, B. R. Younge, C. Kaltschmidt, C. M. Weyand, and J. J. Goronzy. 1997. Glucocorticoid-mediated repression of cytokine gene transcription in human arteritis-SCID chimeras. J. Clin. Investig. 99:2842-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britton, W. J., N. Meadows, D. A. Rathjen, D. R. Roach, and H. Briscoe. 1998. A tumor necrosis factor mimetic peptide activates a murine macrophage cell line to inhibit mycobacterial growth in a nitric oxide-dependent fashion. Infect. Immun. 66:2122-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croft, R. P., P. G. Nicholls, J. H. Richardus, and W. C. S. Smith. 2000. The treatment of acute nerve function impairment in leprosy: results from a prospective cohort study in Bangladesh. Lepr. Rev. 71:154-168. [DOI] [PubMed] [Google Scholar]
- 8.De Antonio, S. R., H. M. Blotta, R. L. Mamoni, P. Louzada, M. B. Bertolo, N. T. Foss, A. C. Moreira, and M. Castro. 2002. Effects of dexamethasone on lymphocyte proliferation and cytokine production in rheumatoid arthritis. J. Rheumatol. 29:46-51. [PubMed] [Google Scholar]
- 9.Espevik, T., I. S. Figari, M. R. Shalaby, G. A. Lackides, G. D. Lewis, H. M. Shepard, and M. A. Palladino, Jr. 1987. Inhibition of cytokine production by cyclosporin A and transforming growth factor beta. J. Exp. Med. 166:571-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faber, W. R., A. M. Iyer, T. T. Fajardo, T. Dekker, L. G. Villahermosa, R. M. Abalos, and P. K. Das. 2004. Serial measurements of serum cytokines, cytokine receptors and neopterin in leprosy patients with reversal reactions. Lepr. Rev. 75:274-281. [PubMed] [Google Scholar]
- 11.Flesch, I. E. A., J. H. Hess, I. P. Oswald, and H. E. Kaufmann. 1994. Growth inhibition of Mycobacterium bovis by IFN-gamma stimulated macrophages: regulation by endogenous tumor necrosis factor-alpha and IL-10. Int. Immunol. 6:693-700. [DOI] [PubMed] [Google Scholar]
- 12.Fushimi, T., H. Okayama, T. Seki, S. Shimura, and K. Shirato. 1997. Dexamethasone suppressed gene expression and production of interleukin-10 by human peripheral blood mononuclear cells and monocytes. Int. Arch. Allergy Immunol. 112:13-18. [DOI] [PubMed] [Google Scholar]
- 13.Galon, J., D. Franchimont, N. Hiroi, G. Frey, A. Boettner, M. Ehrhart-Bornstein, J. J. O'Shea, G. P. Chrousos, and S. R. Bornstein. 2002. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 16:61-71. [DOI] [PubMed] [Google Scholar]
- 14.Gayo, A., L. Mozo, A. Suarez, A. Tunon, C. Lahoz, and C. Gutierrez. 1998. Glucocorticoids increase IL-10 expression in multiple sclerosis patients with acute relapse. J. Neuroimmunol. 85:122-130. [DOI] [PubMed] [Google Scholar]
- 15.Guyton, A. C., and J. E. Hall. 1996. The adrenocortical hormones, p. 957-970. In A. C. Guyton (ed.), Textbook of medical physiology, 9th ed. W. B. Saunders Company, Philadelphia, Pa.
- 16.Hirsch, C. S., T. Yoneda, L. Averill, J. J. Ellner, and Z. Toossi. 1994. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-beta 1. J. Infect. Dis. 170:1229-1237. [DOI] [PubMed] [Google Scholar]
- 17.Khanolkar-Young, S., N. Rayment, P. M. Brickell, D. R. Katz, S. Vinayakumar, M. J. Colston, and D. N. Lockwood. 1995. Tumour necrosis factor-alpha (TNF-alpha) synthesis is associated with the skin and peripheral nerve pathology of leprosy reversal reactions. Clin. Exp. Immunol. 99:196-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanolkar-Young, S., D. Snowdon, and D. N. Lockwood. 1998. Immunocytochemical localization of inducible nitric oxide synthase and transforming growth factor-beta (TGF-beta) in leprosy lesions. Clin. Exp. Immunol. 113:438-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56:731-740. [DOI] [PubMed] [Google Scholar]
- 20.Kirkaldy, A. A., A. C. Mousonda, S. Khanolkar-Young, S. Suneetha, and D. N. J. Lockwood. 2003. Expression of CC and CXC chemokines and chemokine receptors in human leprosy skin lesions. Clin. Exp. Immunol. 134:447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kontoyiannis, D., A. Kotlyarov, E. Carballo, L. Alexopoulou, P. J. Blackshear, M. Gaestel, R. Davis, R. Flavell, and G. Kollias. 2001. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J. 20:3760-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipworth, B. J. 2000. Therapeutic implications of non-genomic glucocrticoid activity. Lancet 356:67-89. [DOI] [PubMed] [Google Scholar]
- 23.Little, D., S. Khanolkar-Young, A. Coulthart, S. Suneetha, and D. N. Lockwood. 2001. Immunohistochemical analysis of cellular infiltrate and gamma interferon, interleukin-12, and inducible nitric oxide synthase expression in leprosy type 1 (reversal) reactions before and during prednisolone treatment. Infect. Immun. 69:3413-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lockwood, D. N., S. Vinayakumar, J. N. Stanley, K. P. McAdam, and M. J. Colston. 1993. Clinical features and outcome of reversal (type 1) reactions in Hyderabad, India. Int. J. Lepr. Other Mycobact. Dis. 61:8-15. [PubMed] [Google Scholar]
- 25.Longley, J., A. Haregewoin, T. Yemaneberhan, T. Warndorff van Diepen, J. Nsibami, D. Knowles, K. A. Smith, and T. Godal. 1985. In vivo responses to Mycobacterium leprae: antigen presentation, interleukin-2 production, and immune cell phenotypes in naturally occurring leprosy lesions. Int. J. Lepr. Other Mycobact. Dis. 53:385-394. [PubMed] [Google Scholar]
- 26.Maeda, H., and A. Shiraishi. 1996. TGF-beta contributes to the shift toward Th2-type responses through direct and IL-10-mediated pathways in tumor-bearing mice. J. Immunol. 156:73-78. [PubMed] [Google Scholar]
- 27.Manandhar, R., N. Shrestha, C. R. Butlin, and P. W. Roche. 2002. High levels of inflammatory cytokines are associated with poor clinical response to steroid treatment and recurrent episodes of type 1 reactions in leprosy. Clin. Exp. Immunol. 128:333-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marlowe, S. N. S., R. A. Hawsworth, C. R. Butlin, P. G. Nicholls, and D. N. J. Lockwood. 2004. Clinical outcomes in a randomized controlled study comparing azathioprine and prednisolone versus prednisolone alone in the treatment of severe leprosy type 1 reactions in Nepal. Trans. R. Soc. Trop. Med. Hyg. 98:602-609. [DOI] [PubMed] [Google Scholar]
- 29.Meduri, G. U., E. A. Tolley, G. P. Chrousos, and F. Stentz. 2002. Prolonged methylprednisolone treatment suppresses systemic inflammation in patients with unresolving acute respiratory distress syndrome: evidence for inadequate endogenous glucocorticoid secretion and inflammation-induced immune cell resistance to glucocorticoids. Am. J. Respir. Crit. Care Med. 165:983-991. [DOI] [PubMed] [Google Scholar]
- 30.Moraes, M. O., E. N. Sarno, A. S. Almeida, B. C. Saraiva, J. A. Nery, R. C. Martins, and E. P. Sampaio. 1999. Cytokine mRNA expression in leprosy: a possible role for interferon-gamma and interleukin-12 in reactions (RR and ENL). Scand. J. Immunol. 50:541-549. [DOI] [PubMed] [Google Scholar]
- 31.Moraes, M. O., E. N. Sarno, R. M. Teles, A. S. Almeida, B. C. Saraiva, J. A. Nery, and E. P. Sampaio. 2000. Anti-inflammatory drugs block cytokine mRNA accumulation in the skin and improve the clinical condition of reactional leprosy patients. J. Investig. Dermatol. 115:935-941. [DOI] [PubMed] [Google Scholar]
- 32.Moubasher, A. D., N. A. Kamel, H. Zedan, and D. D. Raheem. 1998. Cytokines in leprosy, I. Serum cytokine profile in leprosy. Int. J. Dermatol. 37:733-740. [DOI] [PubMed] [Google Scholar]
- 33.Omer, F. M., J. A. Kurtzhals, and E. M. Riley. 2000. Maintaining the immunological balance in parasitic infections: a role for TGF-beta? Parasitol. Today 16:18-23. [DOI] [PubMed] [Google Scholar]
- 34.Omer, F. M., and E. M. Riley. 1998. Transforming growth factor beta production is inversely correlated with severity of murine malaria infection. J. Exp. Med. 188:39-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Othieno, C., C. S. Hirsch, B. D. Hamilton, K. Wilkinson, J. J. Ellner, and Z. Toossi. 1999. Interaction of Mycobacterium tuberculosis-induced transforming growth factor beta1 and interleukin-10. Infect. Immun. 67:5730-5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parida, S. K., G. E. Grau, S. A. Zaheer, and R. Mukherjee. 1992. Serum tumor necrosis factor and interleukin 1 in leprosy and during lepra reactions. Clin. Immunol. Immunopathol. 63:23-27. [DOI] [PubMed] [Google Scholar]
- 37.Ridley, D. S., and W. H. Jopling. 1966. Classification of leprosy according to immunity. A five-group system. Int. J. Lepr. Other Mycobact. Dis. 34:255-273. [PubMed] [Google Scholar]
- 38.Sarno, E. N., G. E. Grau, L. M. Vieira, and J. A. Nery. 1991. Serum levels of tumour necrosis factor-alpha and interleukin-1 beta during leprosy reactional states. Clin. Exp. Immunol. 84:103-108. [PMC free article] [PubMed] [Google Scholar]
- 39.Saunders, B. M., and A. M. Cooper. 2000. Restraining mycobacteria: role of granulomas in mycobacterial infections. Immunol. Cell Biol. 78:334-341. [DOI] [PubMed] [Google Scholar]
- 40.Saunderson, P., S. Gebre, K. Desta, P. Byass, and D. N. Lockwood. 2000. The pattern of leprosy-related neuropathy in the AMFES patients in Ethiopia: definitions, incidence, risk factors and outcome. Lepr. Rev. 71:285-308. [DOI] [PubMed] [Google Scholar]
- 41.Schreuder, P. A. 1998. The occurrence of reactions and impairments in leprosy: experience in the leprosy control program of three provinces in northeastern Thailand, 1987-1995 [correction of 1978-1995]. III. Neural and other impairments. Int. J. Lepr. Other Mycobact. Dis. 66:170-181. [PubMed] [Google Scholar]
- 42.Sehgal, V. N., S. N. Bhattacharya, D. Chattopadhaya, and K. Saha. 1993. Tumor necorsis factor: status in reactions in leprosy before and after treatment. Int. J. Dermatol. 32:436-439. [DOI] [PubMed] [Google Scholar]
- 43.Sugumaran, D. S. 1997. Steroid therapy for paralytic deformities in leprosy. Int. J. Lepr. Other Mycobact. Dis. 65:337-344. [PubMed] [Google Scholar]
- 44.Tabardel, Y., J. Duchateau, D. Schmartz, G. Marecaux, M. Shahla, L. Barvais, J. L. Leclerc, and J. L. Vincent. 1996. Corticosteroids increase blood interleukin-10 levels during cardiopulmonary bypass in men. Surgery 119:76-80. [DOI] [PubMed] [Google Scholar]
- 45.van Brakel, W. H., and I. B. Khawas. 1996. Nerve function impairment in leprosy: an epidemiological and clinical study-part 2: results of steroid treatment. Lepr. Rev. 67:104-118. [DOI] [PubMed] [Google Scholar]
- 46.Vodovotz, Y., C. Bogdan, J. Paik, Q. Xie, and C. Nathan. 1993. Mechanism of suppression of macrophage nitric oxide release by transforming growth factor beta. J. Exp. Med. 178:605-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volc-Platzer, B., H. Stemberger, T. Luger, T. Radaszkiewicz, and G. Wiedermann. 1988. Defective intralesional interferon-gamma activity in patients with lepromatous leprosy. Clin. Exp. Immunol. 71:235-240. [PMC free article] [PubMed] [Google Scholar]
- 48.Wang, P., P. Wu, M. I. Siegel, R. W. Egan, and M. Billah. 1995. Interleukin (IL)-10 inhibits nuclear factor kappaB activation in human monocytes. J. Biol. Chem. 270:9558-9563. [DOI] [PubMed] [Google Scholar]
- 49.Yamamura, M., K. Uyemura, R. J. Deans, K. Weinberg, T. H. Rea, B. R. Bloom, and R. L. Modlin. 1991. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science 254:277-279. [DOI] [PubMed] [Google Scholar]
- 50.Yamamura, M., X. H. Wang, J. D. Ohmen, K. Uyemura, T. H. Rea, B. R. Bloom, and R. L. Modlin. 1992. Cytokine patterns of immunologically mediated tissue damage. J. Immunol. 149:1470-1475. [PubMed] [Google Scholar]