Abstract
Background
Thiamine diphosphate (ThDP) is the active form of thiamine, and it serves as a cofactor for several enzymes, both cytosolic and mitochondrial. Isolated mitochondria have been shown to take up thiamine yet thiamine diphosphokinase is cytosolic and not present in mitochondria. Previous reports indicate that ThDP can also be taken up by rat mitochondria, but the kinetic constants associated with such uptake seemed not to be physiologically relevant.
Results
Here we examine ThDP uptake by mitochondria from several human cell types, including cells from patients with thiamine-responsive megaloblastic anemia (TRMA) that lack a functional thiamine transporter of the plasma membrane. Although mitochondria from normal lymphoblasts took up thiamine in the low micromolar range, surprisingly mitochondria from TRMA lymphoblasts lacked this uptake component. ThDP was taken up efficiently by mitochondria isolated from either normal or TRMA lymphoblasts. Uptake was saturable and biphasic with a high affinity component characterized by a Km of 0.4 to 0.6 μM. Mitochondria from other cell types possessed a similar high affinity uptake component with variation seen in uptake capacity as revealed by differences in Vmax values.
Conclusions
The results suggest a shared thiamine transporter for mitochondria and the plasma membrane. Additionally, a high affinity component of ThDP uptake by mitochondria was identified with the apparent affinity constant less than the estimates of the cytosolic concentration of free ThDP. This finding indicates that the high affinity uptake is physiologically significant and may represent the main mechanism for supplying phosphorylated thiamine for mitochondrial enzymes.
Background
Thiamine is a water-soluble, B-complex vitamin that cannot be synthesized by mammals, and thus thiamine can be obtained only from dietary intake. This can lead to severe consequences in humans when thiamine is limiting; thiamine deficiency may result in beriberi and the Wernike-Korsakoff syndrome [1,2]. Being positively charged and present in relatively low plasma concentrations, thiamine movement across cellular membranes requires transporters. Upon being taken up by a cell, thiamine is rapidly diphosphorylated by thiamine diphosphokinase to give thiamine diphosphate (ThDP) [3]. Thus, thiamine represents only a few percent of the total cellular thiamine/thiamine phosphate derivatives. ThDP serves as a cofactor for several enzymes that are found both in the cytosol (transketolase) and mitochondria (α-ketoglutarate dehydrogenase complex being the most studied example). The intracellular concentration of ThDP has been estimated at 30 μM, with only about 7 percent being free cytosolic and the remainder being enzyme-bound with much of this within mitochondria [4,5]. Previous findings indicate a complex, cell-type dependent regulation of compartmentalization and intracellular pools of thiamine and its phosphorylated derivatives in response to fluctuating extracellular thiamine levels [4-6]. Hence, thiamine transport, including that by mitochondria, is of interest.
Thiamine entry into mammalian cells occurs by a saturable, high affinity transporter that is deficient in humans with thiamine-responsive megaloblastic anemia (TRMA) [7-12]. Thiamine uptake by mitochondria has been demonstrated [13], yet thiamine diphosphokinase is cytosolic and mitochondria cannot convert thiamine to ThDP [14-16]. Barile and coworkers demonstrated saturable uptake of ThDP by rat liver mitochondria characterized by a Km of around 20 μM. Although the estimated concentration, in mice and human cells, of intracellular ThDP is about 30 μM, much of this is found in a low turnover pool representing enzyme-bound ThDP [4,17]. The estimated concentration of free cytosolic ThDP available for intracellular transport is about 10% of the total concentration and thus 2 to 3 μM [4,17,18]. Hence the physiological significance of the mitochondrial ThDP uptake just decribed is uncertain. Within mitochondria, ThDP can be converted to thiamine monophosphate [19]. Thiamine or ThDP entry into mitochondria from TRMA cells has not been studied. Interestingly, ThDP-utilizing enzymes in mitochondria are much less affected (as revealed by loss of activity) upon progressive depletion of thiamine available to TRMA cells than are ThDP-utilizing enzymes of the cytosol [6]. For these reasons, we have examined the uptake of thiamine and especially ThDP by mitochondria from several human cell types, including cells from TRMA patients.
Results
Uptake of thiamine by cells and mitochondria
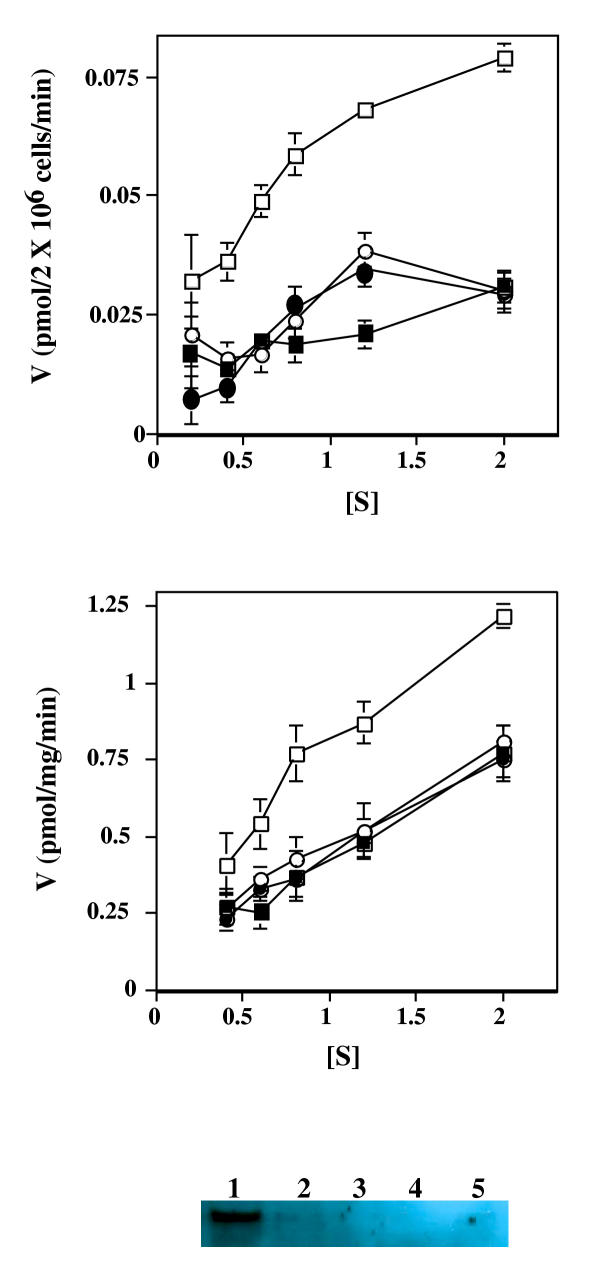
Although our interests primarily were in mitochondrial uptake of thiamine and its derivatives, we first examined cellular uptake of thiamine by the lymphoblast cell lines and found thiamine uptake properties typical of other mammalian cells. Figure 1a indicates thiamine uptake by normal lymphoblasts and lymphoblasts derived from a TRMA patient. The high affinity transport of thiamine by normal lymphoblasts is abolished in the presence of a 100 fold excess of unlabeled thiamine. Under such conditions, some uptake continues from a low affinity (Km in the mM range) transport mechanism [3] and/or from diffusion [20] that characterizes thiamine uptake in all mammalian cells examined to date. Using an expanded range of thiamine concentrations from that shown in fig. 1 in multiple experiments resulted in a Km of 1.0 ± 0.9 μM for the high affinity transport by normal lymphoblasts. As expected, lymphoblasts derived from the TRMA patient showed no high affinity thiamine transport as revealed by thiamine uptake being the same in the absence and presence of excess unlabelled thiamine.
Figure 1.
Uptake of radioactive thiamine by normal and TRMA lymphoblasts and mitochondria isolated from the lymphoblasts. A. Late log phase lymphoblasts from normal (squares) or TRMA individuals (circles) were incubated for 30 minutes with various concentrations of radioactive thiamine. Incubations were carried out in the absence (unfilled symbols) or presence (filled symbols) of a 100 fold excess of non-radioactive thiamine (at each concentration). Cell-associated counts per minute were determined, and the velocity (V) (pmol thiamine per 2 × 106 cells per min.) is plotted versus the concentration (in micromolar) of radioactive thiamine.). Error bars represent SEM for two independent experiments. B. Mitochondria were isolated from lymphoblasts derived from normal (squares) or TRMA individuals (circles) were incubated for 15 minutes with various concentrations of radioactive thiamine. Incubations were carried out in the absence (unfilled symbols) or presence (filled symbols) of a 100 fold excess of non-radioactive thiamine (at each concentration). Mitochondrial-associated counts per minute were determined, and the velocity (V) (pmol thiamine per mg mitochondrial protein per min.) is plotted versus the concentration (in micromolar) of thiamine.). Error bars represent ± SEM for two independent experiments. C. Western anaylsis indicating the presence of the thiamine transporter in plasma membrane fractions and in mitochondrial fractions. Equivalent volumes of subcellular fractions were electrophoretically separated, blotted to a filter, and probed using antisera specific for the human thiamine transporter that is mutated in TRMA individuals. Lane 1, plasma membrane fraction; 2, initial mitochondrial fraction; 3 and 4, successive washes of the mitochondrial fraction; 5, final mitochondrial fraction. 75 micrograms of protein were loaded into each lane with the exception of the lanes containing the washes (3 and 4) which were not quantitated. A faint but reproducible (using different preparations) band was found in the final mitochondrial fraction.
Mitochondria isolated from normal lymphoblasts also were found to take up thiamine (fig. 1b) in a manner similar to that of cellular uptake, with both a high and low affinity component. Using an expanded range of thiamine concentrations in multiple experiments resulted in a Km of 2.1 ± 0.4 μM for high affinity thiamine uptake by normal lymphoblast mitochondria. Interestingly, mitochondria from TRMA lymphoblasts did not possess a "high affinity" thiamine transport capacity as did mitochondria form normal lymphoblasts. No difference in uptake of thiamine by TRMA mitochondria was found in the presence and absence of excess unlabelled thiamine (fig. 1b).
This finding suggests that cellular and mitochondrial uptake of thiamine may be mediated by the same transporter since TRMA is defined by mutation within the thiamine transporter located on the plasma membrane [7-12]. Using antiserum specific for the human thiamine transporter that is mutated in TRMA individuals, western analysis consistently resulted in a faint but detectable band within the isolated mitochondrial suspension (fig. 1c), even after extensive and multiple washing.
Uptake of ThDP by mitochondria
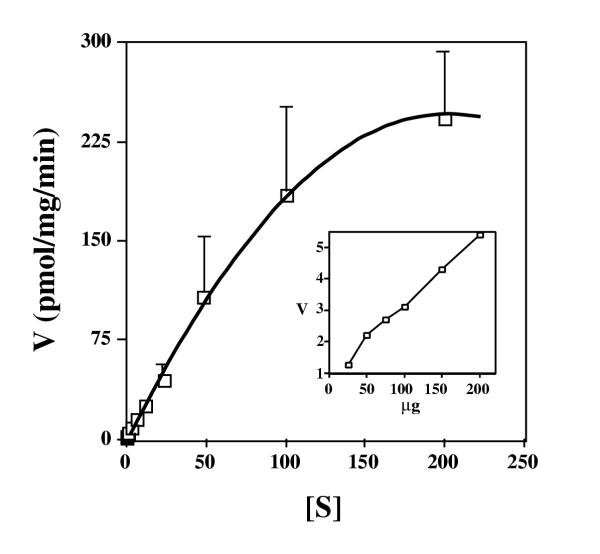
Although mitochondria from lymphoblasts (above) and other mammalian cells [13] were found to take up thiamine, the physiological significance of the uptake is unknown given that thiamine diphosphokinase is cytosolic and mitochondria cannot convert thiamine to ThDP [14,16,21]. We thus were interested in uptake of ThDP by mitochondria, a possibility demonstrated previously with rat liver [14]. Mitochondria from normal human lymphoblasts were able to take up ThDP in a saturable, biphasic manner (fig. 2) with a first saturation in the submicromolar range (described below) and a second at much higher concentrations of ThDP. Time course experiments indicated ThDP uptake was linear for at least 20 minutes (data not shown), and uptake was linear with the amount of mitochondrial protein added (inset of fig. 2).
Figure 2.
The rate of uptake of ThDP by mitochondria isolated from normal lymphoblasts. Mitochondria were isolated from normal lymphoblasts and were incubated for 15 minutes with various concentrations of radioactive ThDP. Mitochondrial-associated counts were determined, and the velocity (V) (pmol ThDP per mg mitochondrial protein per min.) is plotted versus the concentration in micromolar of ThDP ([S]). Error bars represent SEM for four independent experiments. The inset shows uptake (V, pmol ThDP per mg mitochondrial protein per min.) versus varying amounts of resuspended mitochondria (μg of protein) in the presence of 2 M radioactive ThDP.
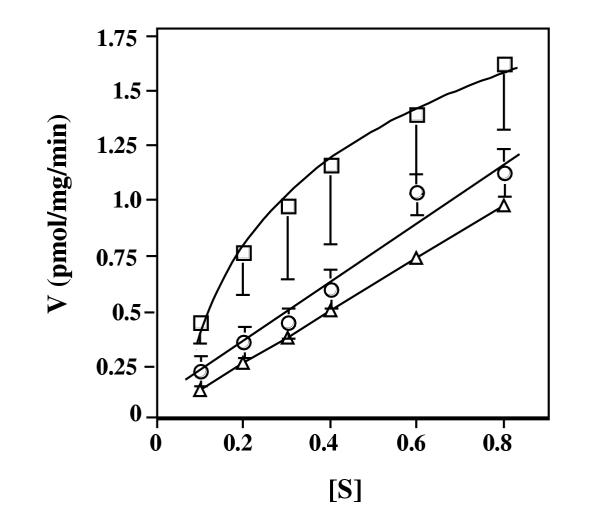
Although the biphasic nature of the uptake is readily seen in various plots of the data, the existence of the high affinity component perhaps is best illustrated in fig. 3 in which the uptake at submicromolar concentrations of ThDP is illustrated (open squares). Uptake of ThDP was repeated in the presence of 30 μM non-radioactive ThDP, a concentration that is 100 to about 40 fold excess over the concentration of radioactive ThDP that was used. Given the Km values for the high and low affinity components (see below), this excess should abolish most of the high affinity uptake but have little to no effect on the low affinity uptake. The uptake due solely to the low affinity component was calculated using the kinetic parameters determined for this component and was plotted as open triangles. As seen in fig. 3, uptake in the presence of 30 μM non-radioactive ThDP (open circles) was essentially identical to that calculated for the low affinity component and abolition of the high affinity uptake indeed was observed.
Figure 3.
The rate of uptake of ThDP at submicromolar concentrations by mitochondria from normal lymphoblasts. Mitochondria were isolated from normal lymphoblasts and were incubated for 15 minutes with various concentrations of radioactive ThDP in the absence (open squares) or presence (open circles) of 30 μM nonradioactive ThDP. Mitochondrial-associated counts were determined, and the velocity (V) (pmol ThDP per mg mitochondrial protein 15 min.) is plotted versus the concentration in micromolar of radioactive ThDP ([S]). The calculated V versus [S] for the low affinity component only, using the kinetic parameters for that component, also is plotted (open triangles). Error bars represent ± SEM for four independent experiments in the absence and three experiments in the presence of non-radioactive ThDP.
Using the data from 4 independent experiments resulted in the determination of a Km of 0.38 μM for the high affinity (table 1) and 115 μM for the low affinity uptake components. The high affinity Km value compares favorably with the estimated 2 to 3 μM intracellular concentration of free ThDP.
Table 1.
Kinetic constants for the high affinity component of ThDP uptake by mitochondria isolated from various cell types.
| cell line | Km | Vmax |
| normal lymphoblasts | 0.38 | 2.0 |
| TRMA lymphoblasts | 0.60 | 1.5 |
| fibroblasts | 0.41 | 10 |
| neuroblastoma | 0.20 | 39 |
| glyB | 0.41 | 0.8 |
Km, micromolar; Vmax, pmol per mg protein per min. Values for normal lymphoblasts are derived from a Lineweaver-Burk plot using the mean velocity values averaged from 4 independent experiments. Two independent experiments were performed for TRMA lymphoblasts, fibroblasts, and neuroblastomas, and one experiment was performed for glyB cells.
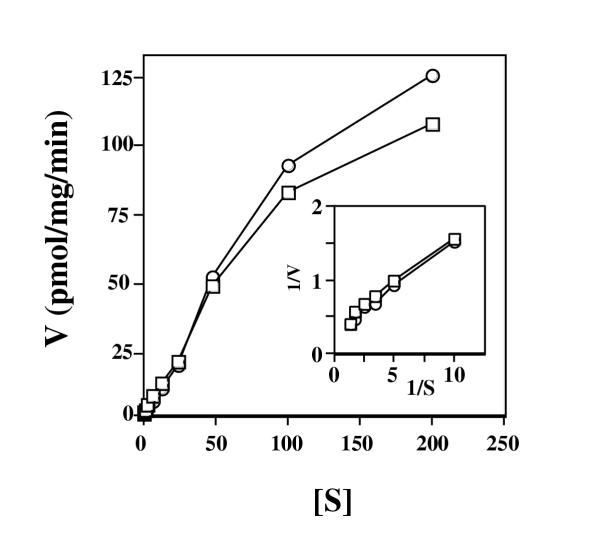
Mitochondria isolated from TRMA lymphoblasts took up ThDP in an essentially identical saturable, biphasic fashion as uptake by normal mitochondria (fig. 4). The inset compares the Lineweaver-Burk plot of the high affinity uptake component for mitochondria of both cell types. A high affinity Km of 0.60 (table 1) was calculated for mitochondrial uptake of ThDP for TRMA lymphoblasts, a value that is essentially the same as that for mitochondria isolated from normal lymphoblasts. The results indicate that although mitochondria from TRMA lymphoblasts cannot take up thiamine with high affinity, they can efficiently import ThDP, the active form of thiamine.
Figure 4.
The rate of uptake of ThDP by mitochondria isolated from normal and TRMA lymphoblasts. Mitochondria were isolated from normal (open squares) or TRMA (open circles) lymphoblasts and were incubated for 15 minutes with various concentrations of radioactive ThDP. Mitochondrial-associated counts were determined, and the velocity (V) (pmol ThDP per mg mitochondrial protein per min.) is plotted versus the concentration in micromolar of ThDP ([S]). The inset shows the Lineweaver-Burk plot (1/V vs. 1/S) of the high affinity component of ThDP uptake for normal (open squares) and TRMA (open circles) derived mitochondria.
Previous work has indicated that different cell types may differentially regulate intracellular pools of thiamine and/or its phosphorylated derivatives [6]. Thus, we examined ThDP uptake by several other cell types. As shown in table 1, high affinity uptake of ThDP by mitochondria from fibroblasts and neuroblastoma cells was essentially the same as that for normal and TRMA lymphoblasts as revealed by the similar Km values. The apparent affinity for ThDP characteristic of the high affinity uptake component was essentially identical for all cell types examined, however variation was seen in transport capacity, as revealed by substantially different values for Vmax (table 1). Mitochondria from all of the cell types examined also possessed the low affinity uptake characterized by Km's similar to that of normal lymphoblasts but with a greater range of values being found (20 to 115 μM).
The final entry in table 1 is for ThDP uptake by mitochondria isolated from glyB cells. GlyB cells are a Chinese hamster ovary cell line that is deficient in the transport of folate into mitochondria. For reasons discussed below, ThDP uptake was examined in these cells. As seen in table 1, high affinity uptake was found with kinetic constants similar to those of the human cell types. The low affinity uptake component was also similar to that by human mitochondria (data not shown).
Discussion + Conclusion
As has been reported for mitochondria of rat liver [13], mitochondria from human lymphoblasts were found herein to take up thiamine in a saturable manner characterized by a Km of 2.1 μM. Upon entry into a cell, thiamine is rapidly diphosphorylated to ThDP, resulting in a low intracellular thiamine concentration [4,17]. The Km determined here is about an order of magnitude greater than the estimated intracellular thiamine concentration [4], raising questions about the efficiency of such uptake.
Surprisingly, mitochondria derived from TRMA lymphoblasts lacked the high affinity uptake of thiamine. TRMA is caused by mutations which destroy the high affinity thiamine transporter of the plasma membrane [7-12]. The similar Km values found for cellular and mitochondrial uptake of thiamine for normal lymphoblasts and the lack of such uptake by TRMA mitochondria and TRMA cells suggests that high affinity thiamine import into mitochondria may be carried out by the same transporter or a variant form, perhaps generated by differential splicing, of that serving on the plasma membrane. Although western analysis using anti-human transporter (that is mutated in TRMA individuals) antiserum supports this interpretation, further experiments need to be carried out to substantiate the suggestion of a shared thiamine transporter between these two membrane systems. Even if true, the physiological significance of thiamine uptake by mitochondria is unknown since mitochondria cannot form ThDP from thiamine [14-16].
We find that mitochondria from a variety of human cell types efficiently take up ThDP. Uptake is biphasic with a high and a low affinity component. The Km values characteristics of the high affinity uptake component (all around 0.4 μM) are comparable to the estimated intracellular concentration of free (non-enzyme bound) cytosolic ThDP of around 3 μM [4]. This suggests that the high affinity uptake system is the physiologically relevant mechanism responsible for ThDP entry into mitochondria. Earlier work with rat liver mitochondria identified a ThDP uptake system with an estimated Km of around 20 μM [14]. This is of the same order of magnitude that we find for the low affinity component in the human cells examined herein. The previous work used a less sensitive procedure of examining ThDP uptake and did not examine uptake below 10 μM. This would explain the lack of identification in the previous work of the high affinity uptake component.
There is a high degree of amino acid similarity among folate transporters and the thiamine transporter of the plasma membrane [7-10]. Recently, it was found that in murine cells there can be a substantial efflux of ThDP mediated by the reduced folate carrier protein [22]. GlyB cells are a Chinese hamster ovary cell line derivative that are deficient in the transport of folates into mitochondria, and the responsible mitochondrial transporter has recently been identified and its gene cloned [23]. We wondered if the mitochondrial folate transporter was responsible for uptake of ThDP into mitochondria, making analogies to the ability of the plasma membrane folate transporter being able to transport ThDP. However, this is not the case as glyB cells that lack the mitochondrial folate transporter were found to take up ThDP with high affinity kinetics similar to that of mitochondria of human cells.
Mitochondria from three human cell types – lymphoblasts, fibroblasts, and neruoblastoma cells – all possessed a high affinity ThDP uptake component characterized by equivalent apparent affinity for ThDP as revealed by essentially identical Km values. Previous studies indicate the existence of a complex, cell-type dependent regulation of compartmentalization and intracellular pools of thiamine and/or its phosphorylated derivatives in response to fluctuating extracellular thiamine levels [4,6,17]. The cell lines used here were also used in the studies leading to this conclusion. Clearly, differences in mitochondrial transporter affinities do not contribute to the cell-type dependent regulation of ThDP compartmentalization. However, we did find significant differences in ThDP uptake capacity with respect to cell type as revealed by variation in the Vmax values. Neuroblastoma mitochondria possessed the largest uptake capacity, having a Vmax 4 to 30 fold higher than that of the other cell types examined. Interestingly, of the three cell types neuroblastoma cells also are the most resistant to changes in mitochondrial ThDP-utilizing enzyme activity upon progressive depletion of the thiamine made available to the cells [6]. This suggests that cell-dependent variation in ThDP uptake capacity by mitochondria may contribute to the cell-dependent regulation of ThDP compartmentalization. As such regulation was most clearly revealed upon progressively depleting thiamine from cells [6], it will be of interest to examine possible changes in mitochondrial transport capacity in response to thiamine depletion.
Studies on the sensitivity of ThDP-utilizing enzymes to progressive depletion of thiamine that is available to the cell indicate that such enzymes in mitochondria are significantly less sensitive than cytosolic enzymes in TRMA cells [6]. This could be interpreted as efficient import of thiamine/ThDP into mitochondria in TRMA cells even though thiamine inefficiently enters these cells due to the lack of the high affinity thiamine transporter. Although we found a lack of mitochondrial uptake of thiamine in TRMA cells, our finding of an intact, high affinity ThDP transport mechanism for TRMA mitochondria is consistent with and offers an explanation for such an interpretation.
Materials and Methods
Radiochemicals
[3H]thiamine (1 Ci/mmol, radiochemical purity greater than 97%) and [3H]thiamine diphosphate (1.4 Ci/mmol, radiochemical purity greater than 98 %) were purchased from Moravek Biochemical Inc (Brea, CA).
Cell culture
Normal lymphoblasts, TRMA lymphoblasts and fibroblasts cell lines were obtained and have been characterized as described [6]. The human neuroblastoma cells were an SY-SY5Y cell line, a thrice-cloned subline of SK-N-SH [24]. GlyB cells, a Chinese hamster ovary K1 subline, are deficient in the transport of folate into mitochondria [23] and were a kind gift from L. Chasin (Columbia University). All cell types were growth at 37°C in the presence of 10 μM thiamine in RPMI 1640 medium supplemented with 10% heat-inactive fetal calf serum, 2 mM L-glutamine and 1 g/L penicillin/streptomycin, except glyB cells which were grown in MEM medium. Cells were grown and used at late log phase or at 80–90% confluency.
Cellular thiamine transport
Cells were harvested, washed four times with 40 ml of ice-cold transport buffer (145 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM glucose, 10 mM HEPES, pH 7.4), titered, and preincubated for 30 min. at 37°C after resuspending 3 × 107 cells in 1 ml of transport buffer. Various amounts of [3H]thiamine (to give submicromolar and micromolar final concentrations) were added and the reactions were incubated for 30 min. The specific activity of the radioactive thiamine was the same for each concentration used. The cells were collected by rapid filtration onto glass fiber filters (type A/E, Gelman Sciences, Ann Arbor, MI) and washed via filtration with 10 ml of cold transport buffer. After thorough drying (overnight at 60°C), the amount of labeled thiamine taken up by the cells was determined by scintillation counting [25]. For each concentration, the uptake in the presence of a 100-fold excess of unlabelled thiamine was performed to assess the contribution to uptake from a low affinity (Km in the mM range) component [3] and/or from diffusion [20].
Isolation of mitochondria
Mitochondria were isolated from about 3 × 108 cells according to published procedures [26,27]. The final mitochondrial pellet was suspended in suspension buffer (140 mM KCl, 0.3 mM EDTA, 5 mM MgCl2, 10 mM HEPES, pH 7.4) to give a protein concentration of 3–4 mg/ml. Protein concentration was determined using the Bio-Rad DC Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA). The isolation and purity of the mitochondrial preparations were monitored by western analysis using anti-cytochrome C antiserum and by microscopy. Western analysis also was performed on the subcellular fractions using antiserum raised against a human thiamine transporter-specific peptide. The antiserum detects a protein of 55 KD (predicted size of the plasma membrane thiamine transporter) that is not detected in cells from TRMA individuals that possess a premature stop codon within the transporter gene (unpublished results).
Uptake of thiamine and ThDP by mitochondria
Uptake of thiamine and ThDP by mitochondria was determined by a rapid filtration procedure [26,28]. Incubations were performed at 37°C by rapidly mixing 30 μl of mitochondrial suspension (ca. 100 micrograms of protein) with 220 μl of incubation buffer (140 mM KCl, 0.3 mM EDTA, 5 mM MgCl2, 10 mM Mes, pH 6.5) containing labeled thiamine or ThDP at various concentrations. The uptake was stopped at 15 min. by the addition of 2 ml of ice-cold stop buffer (100 mM KCl, 100 mM mannitol, 10 mM potassium phosphate, pH 7.4) and the mitochondria were collected by rapid filtration on 0.45 μM Millipore membrane filters. The filters were immediately washed with 5 ml of stop buffer via filtration, and they were subsequently dried at 60°C overnight. The amount of labeled thiamine or ThDP taken up by the mitochondria was determined by scintillation counting. Background binding was determined by using a 100 fold excess of unlabelled thiamine or ThDP in parallel reactions.
Authors' Contributions
QS carried out most of the experiments and participated in writing the manuscript. CKS conceived of the study, participated in its design and coordination, performed a few of the thiamine uptake by cells experiments, and participated in writing the manuscript.
Abbreviations
thiamine diphosphate (ThDP); thiamine-responsive megaloblastic anemia (TRMA)
Acknowledgments
Acknowledgements
This work was supported by the NIH, AA12014-02.
Contributor Information
Qilin Song, Email: tony.song@vanderbilt.edu.
Charles K Singleton, Email: charles.k.singleton@vanderbilt.edu.
References
- Hazell AS, Todd KG, Butterworth RF. Mechanisms of neuronal cell death in Wernicke's encephalopathy. Metab Brain Disease. 1998;13:97–122. doi: 10.1023/A:1020657129593. [DOI] [PubMed] [Google Scholar]
- Singleton CK, Martin PR. Molecular mechanisms of thiamine utilization. Curr Mol Med. 2001;1:197–207. doi: 10.2174/1566524013363870. [DOI] [PubMed] [Google Scholar]
- Bettendorff L, Wins P. Mechanism of thiamine transport in neuroblastoma cells. J Biol Chem. 1994;269:14379–14385. [PubMed] [Google Scholar]
- Bettendorff L. Thiamine homeostasis in neuroblastoma cells. Neurochem Int. 1995;26:295–302. doi: 10.1016/0197-0186(94)00123-C. [DOI] [PubMed] [Google Scholar]
- Bettendorff L, Wins P, Lesourd M. Subcellular localization and compartmentation of thiamine derivatives in rat brain. Biochim Biophys Acta. 1994;1222:1–6. doi: 10.1016/0167-4889(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Pekovich SR, Martin PR, Poggi V, Singleton CK. Sensitivity to thiamine deficiency in cultured human cells is dependent on cell type and is greatly increased in cells from thiamine-responsive megaloblastic anemia patients. J Nutri Biochem. 1998;9:215–222. doi: 10.1016/S0955-2863(97)00187-3. [DOI] [Google Scholar]
- Diaz G, Banikazemi M, Oishi K, Desnick RJ, Gelb B. Mutations in a new gene encoding a thiamine transporter cause thiamine-responsive megaloblastic anemia syndrome. Nat Genet. 1999;22:309–312. doi: 10.1038/10385. [DOI] [PubMed] [Google Scholar]
- Dutta B, Huang W, Molero M, kekuda R, Leibach F, Devoe L, Ganapathy V, Prasad P. Cloning of the human thiamine transporter, a member of the folate transporer family. J Biol Chem. 1999;45:31925–31929. doi: 10.1074/jbc.274.45.31925. [DOI] [PubMed] [Google Scholar]
- Fleming J, Tartaglini E, Steinkamp M, Schorderet D, Cohen N, Neufeld E. The gene mutated in thiamine-responsive anemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nat Genet. 1999;22:305–308. doi: 10.1038/10379. [DOI] [PubMed] [Google Scholar]
- Labay V, Raz T, Baron D, Mandel H, Williams H, t Barrett, Szargel R, McDonald L, Shalata A, Nosaka K, Gregory S, Cohen N. Mutations in SLC19A2 cause thiamine-responsive megaloblastic anemia associated with diabetes mellitus and deafness. Nat Genet. 1999;22:300–304. doi: 10.1038/10372. [DOI] [PubMed] [Google Scholar]
- Rindi G, Casirola D, Poggi V, Vizia BD, Patrini C, Laforenza U. Thiamine transport by erythrocytes and ghosts in thiamine responsive megaloblastic anaemia. J Inhert Metab Dis. 1992;15:231–242. doi: 10.1007/BF01799637. [DOI] [PubMed] [Google Scholar]
- Rindi G, Patrini C, Laforenza U, Mandel H, Berant M, Viana MB, Poggi V, Zarra AMF. Further studies on erythrocyte thiamine transportand phosphorylation in seven patients with thiamine-responsive megaloblastic anemia. J Inhert Metab Dis. 1994;17:667–677. doi: 10.1007/BF00712009. [DOI] [PubMed] [Google Scholar]
- Barile M, Passarella S, Quagliariello E. Uptake of thiamine by isolated rat liver mitochondria. Biochem Biophys Res Commun. 1986;141:466–473. doi: 10.1016/s0006-291x(86)80196-6. [DOI] [PubMed] [Google Scholar]
- Barile M, Passarella S, Quangliariello E. Thiamine pyrophosphate uptake into isolated rat liver mitochondria. Arch Biochem Biophys. 1990;280:352–357. doi: 10.1016/0003-9861(90)90341-u. [DOI] [PubMed] [Google Scholar]
- Deus B, Blum H. Biochim Biophys Acta. 1970;219:489–492. doi: 10.1016/0005-2736(70)90229-4. [DOI] [PubMed] [Google Scholar]
- Voskoboyev AI, Averin VA. Vopr Med Khim. 1981;27:239–243. [PubMed] [Google Scholar]
- Bettendorff L. The compartmentation of phosphorylated thiamine derivatives in cultured neuroblastoma cells. Biochim Biophys Acta. 1994;1222:7–14. doi: 10.1016/0167-4889(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Tallaksen CME, Bohmer T, Bell H, Karlsen J. Concomitant determination of thiamine and its phosphate esters in human blood and serum by high performance liquid chromatography. J Chromat. 1991;564:127–136. doi: 10.1016/0378-4347(91)80075-N. [DOI] [PubMed] [Google Scholar]
- Barile M, Valenti D, Brizio C, Quagliariello E, Passarella S. Rat liver mitochondria can hydrolze thiamine pyrophosphate to thiamine monophosphate which can cross the mitochondrial membrane in a carrier-mediated process. FEBS Let. 1998;435:6–10. doi: 10.1016/S0014-5793(98)01007-2. [DOI] [PubMed] [Google Scholar]
- Rindi G. Some aspects of thiamine transport in mammals. J Nutri Sci Vitamin. 1992;S12:379–382. doi: 10.3177/jnsv.38.special_379. [DOI] [PubMed] [Google Scholar]
- Deus B. Assay of thiamine pyrophosphokinase. Meth Enzym. 1970;18:221–225. [Google Scholar]
- Zhao R, Gao F, Wang Y, Diaz GA, Gelb BD, Goldman ID. Impact of the reduced folate carrier on the accumulation of active thiamin metabolites in murine leukemia cells. J Biol Chem. 2000;276:1114–1118. doi: 10.1074/jbc.M007919200. [DOI] [PubMed] [Google Scholar]
- Titus S, Moran R. Retrovirally Mediated Complementation of the glyB Phenotype: Cloning of a human gene encoding the carrier for entry of folates into mitochondria. J Biol Chem. 2000;275:36811–36817. doi: 10.1074/jbc.M005163200. [DOI] [PubMed] [Google Scholar]
- Ross RA, Spengler BA, Biedler JL. Coordinate morphological and biochemical interconversion of human neuroblastoma cells. J Natl Cancer Inst. 1983;71:741–747. [PubMed] [Google Scholar]
- Singleton CK. Identification and characterization of the thiamine transporter gene of Saccharomyces cerevisiae. Gene. 1997;199:111–121. doi: 10.1016/S0378-1119(97)00354-5. [DOI] [PubMed] [Google Scholar]
- Horne D, Holloway R, Said H. Uptake of 5-formyltetrahydrofolate in isolated rat liver mitochondria is carrier-mediated. J Nutr. 1992;122:2204–2209. doi: 10.1093/jn/122.11.2204. [DOI] [PubMed] [Google Scholar]
- Pederson P, Greenwalt J, Feynafarje B, Hullihen J, Decker G, Soper J, Bustamante E. Preparation and characterization of mitochondria and submitochondrial particlesof rat liver and liver-derived tissues. Meth Cell Biol. 1978;20:411–481. doi: 10.1016/s0091-679x(08)62030-0. [DOI] [PubMed] [Google Scholar]
- Said H, McAlister-Henn L, Mohammadkhani R, Horne D. Uptake of biotin by isolated rat liver mitochondria. Am J Physiol. 1992;263:G81–G86. doi: 10.1152/ajpgi.1992.263.1.G81. [DOI] [PubMed] [Google Scholar]