Abstract
Objectives:
To examine the associations of comorbidity and chemotherapy with breast cancer- and non-breast cancer-related death.
Materials and methods:
Included were women with invasive locoregional breast cancer diagnosed in 2004 from seven population-based cancer registries. Data were abstracted from medical records and verified with treating physicians when there were inconsistencies and missing information on cancer treatment. Comorbidity severity was quantified using the Adult Comorbidity Evaluation 27. Treatment guideline concordance was determined by comparing treatment received with the National Comprehensive Cancer Network guidelines. Kaplan–Meier method and multivariable Cox proportional hazards regressions were employed for statistical analyses.
Results:
Of 5852 patients, 76% were under 70 years old and 69% received guideline concordant adjuvant chemotherapy. Comorbidity was more prevalent in women age 70 and older (79% vs. 51%; p < 0.001). After adjusting for tumor characteristics and treatment, severe comorbidity burden was associated with significantly higher cancer-related mortality in older patients (Hazard Ratio [HR] = 2.38, 95% CI 1.08–5.24), but not in younger patients (HR = 1.78, 95% CI 0.87–3.64). Among patients receiving guideline adjuvant chemotherapy, cancer-related mortality was significantly higher in older patients (HR = 2.35, 95% CI 1.52–3.62), and those with severe comorbidity (HR = 3.79, 95% CI 1.72–8.33).
Conclusions:
Findings suggest that, compared to women with no comorbidity, patients with breast cancer age 70 and older with severe comorbidity are at increased risk of dying from breast cancer, even after adjustment for adjuvant chemotherapy and other tumor and treatment differences. This information adds to risk–benefit discussions and emphasizes the need for further study of the role for adjuvant chemotherapy in these patient groups.
Keywords: Breast cancer, Comorbidity, Adjuvant chemotherapy, Survival, Age, Risk–benefit
1. Introduction
Providing appropriate treatment to older patients with breast cancer with comorbidities is a challenge due to lack of high quality evidence from clinical trials. Most patients with breast cancer, however, are age 50 years or older at diagnosis and have at least one comorbidity [1]. With aging of the general population, even more women with comorbidities will be diagnosed and treated for breast cancer.
Studies show a direct relationship between comorbidity and both breast cancer-related and competing-cause mortality, but an inverse association between comorbidity and adjuvant chemotherapy use, such that it is difficult to determine how much of the higher cancer mortality rate in women with comorbidity is due to lack of appropriate adjuvant treatment [2–4]. Admittedly, higher cancer mortality may be due to either differential treatment quality, which is directly correlated with outcome; or it may be due to the direct effects of comorbidities or their treatment on disease biology. Hypertension, cardiovascular disease, and diabetes, for instance, have differential effects on breast cancer survival, disease course, and treatment [2,5,6]. In general, however, more severe comorbidity is associated with under-treatment, a phenomenon that, with adjuvant chemotherapy for breast cancer, may partly be due to withholding chemotherapy because of concern about undue toxicity [7,8]. The impact of comorbidity on the risk of cancer and non-cancer-related death among patients with breast cancer receiving adjuvant chemotherapy, however, has not been well studied.
In this study, we aimed to examine the relationship of comorbidity severity to five-year breast cancer-specific and non-breast cancer mortality in women receiving adjuvant treatment for breast cancer. Data were analyzed from a large population-based pattern of care (PoC) study in which registry data were enhanced with data abstracted from charts, allowing for the inclusion of key modifying factors, including guideline-concordant treatment, age, and others, in the analysis.
2. Methods
2.1. Data Sources
Breast cancer cases diagnosed in 2004 were randomly selected across strata of race/ethnicity in seven population-based state cancer registries (California, Georgia, Kentucky, Louisiana, North Carolina, Minnesota, and Wisconsin) for the Centers for Disease Control and Prevention’s National Program of Cancer Registries (CDC-NPCR) Breast and Prostate Cancer Data Quality and Patterns of Care Study (POC-BP) study [9,10]. Information on initial course of treatment and comorbidities was re-abstracted from medical records at hospitals, pathology laboratories, free-standing radiation facilities, and ambulatory surgery centers to supplement data that these registries routinely collected. Treating physicians were contacted to obtain or verify required information, especially regarding adjuvant chemotherapy, when it was missing or incomplete in hospital medical records. Date of last contact, vital status, and cause of death were obtained from states’ death certificate files and linkages with the National Death Index. All patients were followed through Dec 31, 2009, except those who died prior to this date.
The Institutional Review Board’s approval was obtained from each participating institution.
2.2. Eligibility Criteria and Case Selection
Women age 20 years or older who were residents in the catchment areas and had surgery for microscopically confirmed first primary invasive, nonmetastatic breast cancer (International Classification of Diseases for Oncology, third edition, site codes C50.0–C50.9) in 2004 with no subsequent primary within four months were included. Excluded were cases with previous diagnoses of reportable cancers, Paget’s disease, mesothelioma, Kaposi’s sarcoma, or lymphoma. Cases from Veteran’s Administration hospitals and those identified solely from death certificates or autopsies were also excluded.
The initial sample included 9142 cases. Exclusion criteria eliminated 3290 cases: more than one primary (n = 39); in-situ cancer (n = 1515); unknown American Joint Committee on Cancer (AJCC) stage (n = 256), distant stage (n = 400); unknown tumor size or lymph nodes status (n = 53); unknown hormone receptor status (n = 363); unknown comorbidity status (n = 111); no surgery or unknown surgery type (n = 80); unknown primary treatment status (n = 88); unknown guideline chemotherapy status (n = 142); unknown guideline hormone therapy (n = 124); cases where treatment received was in excess of guidelines (n = 91); loss to follow-up (n = 5); unknown cause of death (n = 23). The final 5852 cases were included in this data analysis.
2.3. Comorbidity
Comorbidity burden was measured using the Adult Comorbidity Evaluation-27 (ACE-27) index, which is specific for patients with cancer and has a dose–response relationship to survival [11,12]. This index includes 26 comorbid conditions with three levels of decompensation/severity (i.e., mild, moderate, and severe decompensation). The 26 comorbid conditions were grouped into twelve body organ systems: cardiovascular disease (myocardial infarction, coronary artery disease, congestive heart failure, arrhythmias, hypertension, venous disease, and peripheral arterial disease), respiratory disease, gastrointestinal diseases (hepatic, stomach/intestine, pancreas), renal disease, diabetes mellitus, nervous system (stroke or cerebrovascular accident, dementia, paralysis, neuromuscular disorders), psychiatric, rheumatological, acquired immunodeficiency syndrome (AIDS), cancer (solid tumor, leukemia, lymphoma) excluding the index cancer (i.e., breast cancer), substance abuse (alcohol abuse, illicit drugs), and morbid obesity.
Abstractors were trained with a validated internet-based program to obtain information on comorbidity severity by reviewing medical records. Levels of severity were determined according to diagnosis, medical history, and clinical and laboratory tests [13]. Comorbidities present at or prior to the cancer diagnosis were included; complications caused by cancer or cancer treatment were excluded. Each patient was assigned an overall comorbidity score (0-none, 1-low, 2-moderate, or 3-severe) based on the comorbidity with the highest rank single ailment, except in the situation where two or more moderate decompensations occurred in different organ systems, in which case, the overall comorbidity score was designated severe. A zero comorbidity score was defined as having no comorbidity or no comorbidity mentioned in medical records.
2.4. Cancer Treatment
All patients included in this analysis had a surgical intervention: lumpectomy or mastectomy. Local therapy included three groups: mastectomy, lumpectomy with radiation, and lumpectomy without radiation. Guideline adjuvant chemotherapy was defined based on the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology, version 1, 2003, (https://www.nccn.org/) which applied to the breast cancers diagnosed in 2004. If a patient received chemotherapy, regardless of agent/regimens or dosages, they were included as ‘received chemotherapy’. Adjuvant chemotherapy was categorized into three groups: did not receive chemotherapy because it was not recommended by the guidelines (not indicated by guideline), received chemotherapy recommended by guidelines (received guideline), and did not receive chemotherapy recommended by the guidelines (under treated). Endocrine therapy was grouped into three categories, parallel to those defined for receipt of adjuvant chemotherapy.
2.5. Explanatory Variables
We treated patients 70 years and older the same as younger patients when determining whether adjuvant chemotherapy was recommended by the NCCN guidelines (https://www.nccn.org/) in the univariate analysis, though guidelines acknowledge that data is sparse for those over 70 years old and treatment recommendations should be individualized based on comorbidity burden. Due to the small number of cases, especially in the group of severe comorbidity, sociodemographic variables were not included in the analysis except for age and race/ethnicity (i.e., non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, American Indian/Alaska Native, and Hispanic). Clinical variables included tumor characteristics (i.e., regional lymph node status, tumor size, tumor grade, and hormone receptor status) and treatment type (i.e., surgery, radiation therapy, chemotherapy, and endocrine therapy).
Hormone receptor status was defined as positive [estrogen receptor (ER) + and/or progesterone receptor (PR)+], negative (ER and PR negative), or unknown (no information on ER and PR status). Human epidermal growth factor receptor 2 (HER2) status was defined as positive [3+ by immunohistochemistry (IHC) or amplified by fluorescent in-situ hybridization (FISH)], negative (0 or 1+ by IHC or 2+ by IHC and not amplified by FISH), or unknown.
Cause-specific death (cancer death and non-cancer death) was defined based on SEER cause-specific death classification variable (ICD-10 codes) for sequence 00 and sequence 01 http://seer.cancer.gov/causespecific/. When we calculated five-year cause-specific death rates and hazard ratios, only the specific causes of death were treated as events and death from other causes are treated as censored observation.
2.6. Statistical Analysis
The association of comorbidity level with other explanatory variables were analyzed by X2 test. The Kaplan–Meier (KM) method was employed to calculate cumulative death rates from cancer and non-cancer causes by comorbidity severity overall and stratifying by age. The Cox proportional hazard multivariable regression model was used to examine the association of comorbidity severity with risks of cancer death and non-cancer death, adjusted for age, race/ethnicity, tumor characteristics, and treatment. All estimates were weighted to reflect the population from which the sample was drawn. Data analysis was performed using SAS-callable SUDAAN v 11.0.1 software.
3. Results
Characteristics of the sample (n = 5852), overall and by comorbidity severity, are described in Table 1. The majority of the study population was under age 70 (76%), non-Hispanic white (58%), and had no or mild comorbidity (86%). Breast cancers were most commonly 1.0–2.9 cm (60%), lymph node negative (pN0, 68%), and hormone receptor positive (75%). Chemotherapy use was guideline-concordant in 4009 cases (69%) and endocrine therapy in 4720 (81%) of cases.
Table 1.
Distribution of breast cancer by age, race/ethnicity, tumor characteristics, treatment and comorbidity.
| Characteristics | Total | Comorbidity severity |
p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
None |
Mild |
Moderate |
Severe |
|||||||
| Count | % | Count | % | Count | % | Count | % | Count | % | ||
| 5852 | 100.0 | 2499 | 42.7 | 2508 | 42.9 | 619 | 10.6 | 226 | 3.9 | ||
| Age at diagnosis (yrs) | <0.001 | ||||||||||
| < 70 | 4459 | 76.2 | 2210 | 88.4 | 1708 | 68.1 | 428 | 69.1 | 113 | 50.0 | |
| 70+ | 1393 | 23.8 | 289 | 11.6 | 800 | 31.9 | 191 | 30.9 | 113 | 50.0 | |
| Race/ethnicity | <0.001 | ||||||||||
| Non-hispanic Whites | 3400 | 58.1 | 1507 | 60.3 | 1430 | 57.0 | 337 | 54.4 | 126 | 55.8 | |
| Non-hispanic Blacks | 1602 | 27.4 | 534 | 21.4 | 762 | 30.4 | 221 | 35.7 | 85 | 37.6 | |
| AI/AN | 53 | 0.9 | 24 | 1.0 | 18 | 0.7 | 9 | 1.5 | 2 | 0.9 | |
| API | 306 | 5.2 | 166 | 6.6 | 122 | 4.9 | 14 | 2.3 | 4 | 1.8 | |
| Hispanic | 491 | 8.4 | 268 | 10.7 | 176 | 7.0 | 38 | 6.1 | 9 | 4.0 | |
| Tumor size (cm) | |||||||||||
| < 1.0 | 994 | 17.0 | 402 | 16.1 | 463 | 18.5 | 95 | 15.4 | 34 | 15.0 | 0.004 |
| 1.0–2.9 | 3494 | 59.7 | 1503 | 60.1 | 1510 | 60.2 | 354 | 57.2 | 127 | 56.2 | |
| ≥ 3.0 | 1364 | 23.3 | 594 | 23.8 | 535 | 21.3 | 170 | 27.5 | 65 | 28.8 | |
| Regional lymph node | 0.020 | ||||||||||
| pN0 | 3969 | 67.8 | 1691 | 67.7 | 1742 | 69.5 | 389 | 62.8 | 147 | 65.0 | |
| PN1(0.2–2.0 mm) | 1127 | 19.3 | 489 | 19.6 | 468 | 18.7 | 127 | 20.5 | 43 | 19.0 | |
| pN (> 2.0 mm) | 756 | 12.9 | 319 | 12.8 | 298 | 11.9 | 103 | 16.6 | 36 | 15.9 | |
| Grade | 0.025 | ||||||||||
| Well differentiated | 1023 | 17.5 | 418 | 16.7 | 457 | 18.2 | 111 | 17.9 | 37 | 16.4 | |
| Moderate differentiated | 2286 | 39.1 | 963 | 38.5 | 1007 | 40.2 | 238 | 38.5 | 78 | 34.5 | |
| Poorly/undifferentiated | 2280 | 39.0 | 1010 | 40.4 | 915 | 36.5 | 253 | 40.9 | 102 | 45.1 | |
| Unknown | 263 | 4.5 | 108 | 4.3 | 129 | 5.1 | 17 | 2.8 | 9 | 4.0 | |
| Hormone receptora | 0.170 | ||||||||||
| ER+ and/or PR+ | 4409 | 75.3 | 1861 | 74.5 | 1905 | 76.0 | 481 | 77.7 | 162 | 71.7 | |
| ER− and PR− | 1443 | 24.7 | 638 | 25.5 | 603 | 24.0 | 138 | 22.3 | 64 | 28.3 | |
| HER2 statusb | 0.106 | ||||||||||
| Positive | 927 | 15.8 | 423 | 16.9 | 381 | 15.2 | 104 | 16.8 | 19 | 8.4 | |
| Negative | 3907 | 66.8 | 1645 | 65.8 | 1693 | 67.5 | 410 | 66.2 | 159 | 70.4 | |
| Equivocal | 243 | 4.2 | 105 | 4.2 | 99 | 4.0 | 26 | 4.2 | 13 | 5.8 | |
| Unknown | 775 | 13.2 | 326 | 13.1 | 335 | 13.4 | 79 | 12.8 | 35 | 15.5 | |
| Local therapy | <0.001 | ||||||||||
| Mastectomy | 2708 | 46.3 | 1151 | 46.0 | 1152 | 45.9 | 296 | 47.8 | 109 | 48.2 | |
| Lumpectomy + radiation | 2690 | 46.0 | 1179 | 47.2 | 1165 | 46.5 | 264 | 42.7 | 82 | 36.3 | |
| Lumpectomy, no radiation | 454 | 7.8 | 169 | 6.8 | 191 | 7.6 | 59 | 9.5 | 35 | 15.5 | |
| Chemotherapy | <0.001 | ||||||||||
| Not indicated by guideline | 1103 | 18.8 | 427 | 17.1 | 518 | 20.7 | 116 | 18.7 | 42 | 18.6 | |
| Received by guideline | 2906 | 49.7 | 1430 | 57.2 | 1122 | 44.7 | 287 | 46.4 | 67 | 29.7 | |
| Under treated | 1843 | 31.5 | 642 | 25.7 | 868 | 34.6 | 216 | 34.9 | 117 | 51.8 | |
| Endocrine therapy | 0.217 | ||||||||||
| Not indicated by guideline | 1443 | 24.7 | 638 | 25.5 | 603 | 24.0 | 138 | 22.3 | 64 | 28.3 | |
| Received by guideline | 3277 | 56.0 | 1395 | 55.8 | 1401 | 55.9 | 367 | 59.3 | 114 | 50.4 | |
| Under treated | 1132 | 19.3 | 466 | 18.7 | 504 | 20.1 | 114 | 18.4 | 48 | 21.2 | |
AI/AN = American Indian/Alaska Native.
API = Asian or Pacific Islander.
ER = Estrogen receptor.
PR = Progesterone receptor.
HER = Human epidermal growth factor receptor 2.
Borderline ER or PR was included in the ER+ and/or PR+ group.
Her2 positive [3+ by immunohistochemistry (IHC) or amplified by fluorescent in-situ hybridization (FISH)], negative (0, 1+ or 2+ by IHC and not amplified by FISH).
In univariate analyses, the comorbidity score varied significantly by age, race/ethnicity, tumor size, nodal status, and grade, type of local therapy and use of chemotherapy, but not by tumor hormone receptor status or use of endocrine therapy (Table 1). Those age 70 and older (p < 0.001) and non-Hispanic blacks (p < 0.001) were more likely to have more severe comorbidity. Larger tumor size (p = 0.004), greater number of involved lymph nodes (p = 0.020), and poorly differentiated tumor grade (p = 0.025) were associated with more severe comorbidity. Receipt of lumpectomy without radiation (p < 0.001) and lack of guideline-concordant chemotherapy (under treated, p < 0.001) were associated with more severe comorbidity.
Fig. 1 and Table 2 demonstrate cancer-specific and non-cancer mortality by comorbidity level and age. For cancer mortality, the cumulative mortality was consistently higher for those with severe comorbidity compared to the other three comorbidity groups, for which the curves were overlapping. In unadjusted analyses, compared to no comorbidity, the hazard of cancer-specific mortality was 2.41 in those age <70 year (95% confidence interval (CI), 1.27–4.58) and 3.13 in those age 70 and older (95% CI, 1.45–6.77) with severe comorbidity. For non-cancer mortality, the cumulative mortality increased with increasing comorbidity level, overall and by age.
Fig. 1.
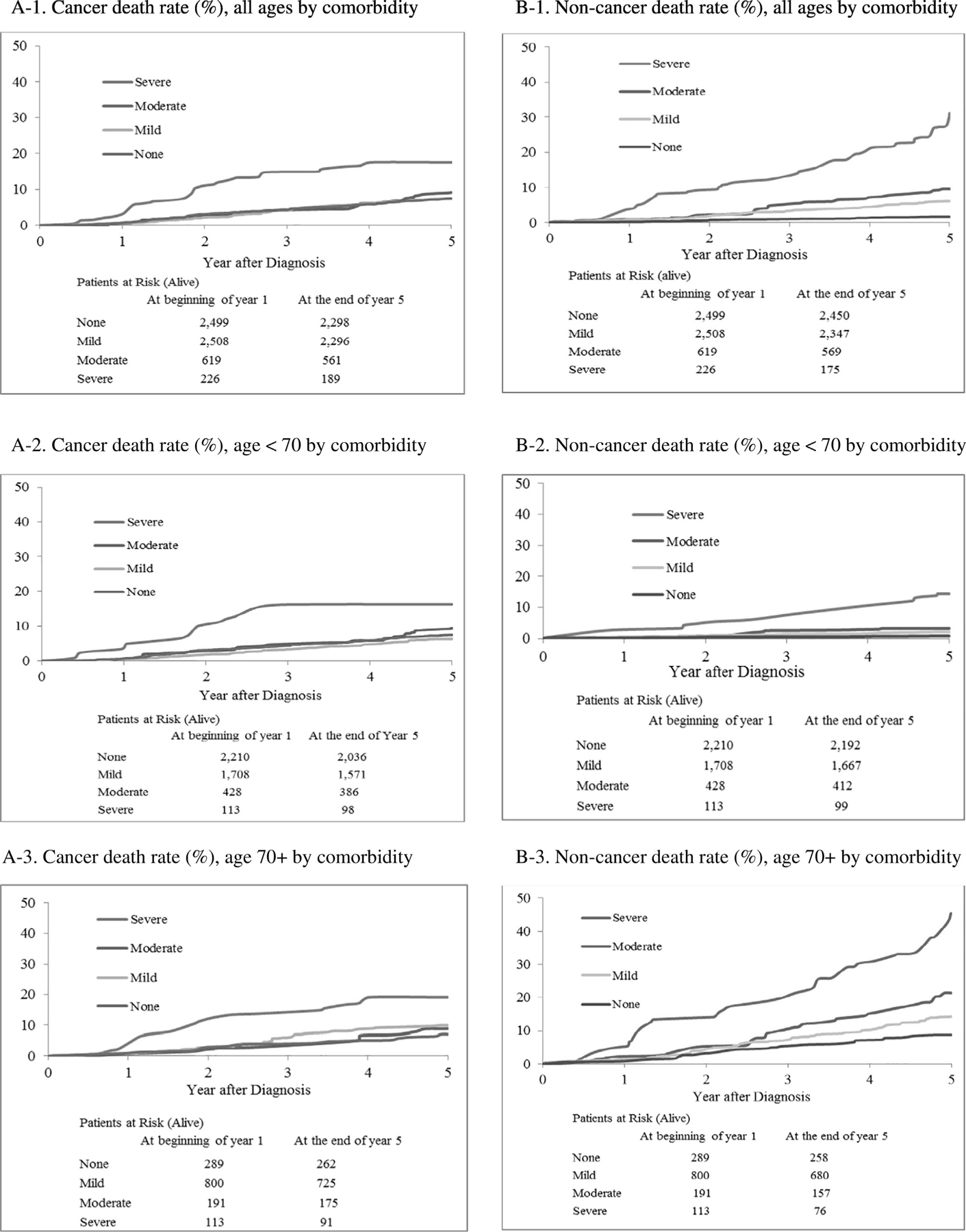
Five-year cumulative cause-specific death rate (%) by age and comorbidity.
Table 2.
Unadjusted and adjusteda Hazard Ratio (HR) with 95% Confidence Interval (CI) of cause-specific death by age group and comorbidity.
| Comorbidity severity | Patients at risk | Cancer death HR (95% CI) |
Non-cancer death HR (95% CI) |
||||
|---|---|---|---|---|---|---|---|
| Count | Unadjusted | Adjusteda | Count | Unadjusted | Adjusteda | ||
| Total | 5852 | 508 | 311 | ||||
| None | 2499 | 201 | Reference | Reference | 49 | Reference | Reference |
| Mild | 2508 | 212 | 1.00 (0.78–1.29) | 0.99 (0.76–1.29) | 161 | 3.66 (2.40–5.58) | 1.72 (1.09–2.70) |
| Moderate | 619 | 58 | 1.22 (0.84–1.78) | 1.05 (0.71–1.55) | 50 | 5.78 (3.46–9.67) | 2.61 (1.51–4.49) |
| Severe | 226 | 37 | 2.70 (1.69–4.33) | 1.93 (1.18–3.16) | 51 | 21.1 (12.9–34.6) | 5.14 (2.99–8.83) |
| Model (age < 70)b | |||||||
| Total | 4459 | 368 | 89 | ||||
| None | 2210 | 174 | Reference | Reference | 18 | Reference | Reference |
| Mild | 1708 | 137 | 0.83 (0.62–1.12) | 0.91 (0.68–1.23) | 41 | 2.75 (1.32–5.76) | 2.79 (1.31–5.92) |
| Moderate | 428 | 42 | 1.25 (0.82–1.90) | 1.13 (0.73–1.74) | 16 | 4.34 (1.63–11.6) | 4.32 (1.56–12.0) |
| Severe | 113 | 15 | 2.41 (1.27–4.58) | 1.78 (0.87–3.64) | 14 | 20.6 (8.50–49.7) | 19.0 (7.27–49.4) |
| Model (age 70+) b | |||||||
| Total | 1393 | 140 | 222 | ||||
| None | 289 | 27 | Reference | Reference | 31 | Reference | Reference |
| Mild | 800 | 75 | 1.43 (0.83–2.47) | 1.21 (0.69–2.11) | 120 | 1.67 (1.01–2.80) | 1.57 (0.92–2.68) |
| Moderate | 191 | 16 | 1.21 (0.54–1.71) | 0.93 (0.40–2.18) | 34 | 2.56 (1.39–4.73) | 2.26 (1.20–4.26) |
| Severe | 113 | 22 | 3.13 (1.45–6.77) | 2.38 (1.08–5.24) | 37 | 6.26 (3.49–11.2) | 4.39 (2.36–8.16) |
Adult Comorbidity Evaluation-27 index (ACE-27).
Full model included age, race, tumor characteristics (regional lymph nodes, tumor size, grade, ER/PR receptor, and HER2 status), and treatment (Surgery, chemotherapy and hormonal therapy).
In age <70, and age 70+ full model not included age.
The adjusted hazard ratio of death from cancer and non-cancer causes, by comorbidity severity, controlling for age, race, tumor characteristics, and treatment, are presented in Table 2. In the adjusted analyses, the hazard of cancer-related death was significantly higher among those with the severe comorbidity compared to no comorbidity (HR = 1.93, 95% CI, 1.18–3.16). When stratified by age, however, the adverse effect of severe comorbidity on cancer-related death was significant among patients age 70 and older (HR = 2.38, 95% CI: 1.08–5.24), but not among patients younger than age 70 years (HR = 1.78, 95% CI: 0.87–3.64). For non-cancer-related mortality, in the adjusted model, the risk of death increased in a dose-dependent fashion with increasing comorbidity level: compared to no comorbidity, HR = 1.72 (95% CI: 1.09–2.70) for mild, HR = 2.61 (95% CI: 1.51–4.49) for moderate, and HR = 5.14 (95% CI: 2.99–8.83) for severe comorbidity. Stratification by age saw little change in this pattern.
To further explore the effect of guideline-concordant chemotherapy on mortality by age and comorbidity burden, we stratified by receipt of guideline concordant chemotherapy versus under treated, excluding those in whom guidelines did not recommend chemotherapy be used (Table 3). Those who were undertreated had a higher risk of non-cancer death (HR = 7.42, 95% CI: 5.02–11.0), but not cancer death (HR = 1.01, 95% CI: 0.79–1.27) at five years. Cancer mortality did not vary by age or comorbidity level among patients who were undertreated. Among patients receiving guideline concordant chemotherapy, however, five-year cancer mortality varied by age and comorbidity level. Cancer mortality was higher in patients age 70 and older versus those < 70 years (17.7% vs 9.2%; HR = 2.35, 95% CI: 1.52–3.62) and in those with severe comorbidity versus no comorbidity (21.4% vs 8.4%; HR = 3.79, 95% CI: 1.72–8.33). The risk of non-cancer-related mortality was higher in patients both age 70 and older and in patients with higher comorbidity burden, regardless of receipt of guideline concordant chemotherapy or not.
Table 3.
Five-year cause-specific death rate and Unadjusted Hazard Ratio (HR) with 95% Confidence Interval (CI) by chemotherapy guideline, age group, and comorbidity among women with an indication for adjuvant chemotherapy.
| Patients at Risk | Cancer death |
Non-cancer death |
|||||
|---|---|---|---|---|---|---|---|
| Count | Five-year death rate | Unadjusted HR (95% CI) | Count | Five-year death rate | Unadjusted HR (95% CI) | ||
| Chemotherapy | |||||||
| Received by guideline | 2906 | 284 | 9.9 | Reference | 57 | 2.1 | Reference |
| Under treated | 1843 | 206 | 11.8 | 1.01 (0.79–1.27) | 203 | 11.7 | 7.42 (5.02–11.0) |
| Chemotherapy received, according to guideline | |||||||
| Age group | |||||||
| Age < 70 | 2679 | 245 | 9.2 | Reference | 43 | 1.7 | Reference |
| Age 70 + | 227 | 39 | 17.7 | 2.35 (1.52–3.62) | 14 | 6.8 | 5.65 (2.60–12.3) |
| Comorbidity | |||||||
| None | 1430 | 120 | 8.4 | Reference | 14 | 1.0 | Reference |
| Mild | 1122 | 120 | 10.8 | 1.21 (0.87–1.69) | 22 | 2.1 | 1.97 (0.76–5.09) |
| Moderate | 287 | 30 | 10.8 | 1.40 (0.86–2.29) | 15 | 5.5 | 4.46 (1.64–12.1) |
| Severe | 67 | 14 | 21.4 | 3.79 (1.72–8.33) | 6 | 10.8 | 16.5 (4.50–60.8) |
| Under treated (Chemotherapy indicated by guideline, but not received) | |||||||
| Age group | |||||||
| Age <70 | 1015 | 114 | 11.5 | Reference | 40 | 4.2 | Reference |
| Age 70 + | 828 | 92 | 12.2 | 1.08 (0.75–1.56) | 163 | 21.0 | 5.88 (3.80–9.10) |
| Comorbidity | |||||||
| None | 642 | 77 | 12.3 | Reference | 26 | 4.3 | Reference |
| Mild | 868 | 85 | 10.4 | 0.80 (0.53–1.21) | 112 | 13.6 | 3.22 (1.88–5.51) |
| Moderate | 216 | 22 | 11.0 | 0.78 (0.40–1.52) | 27 | 13.2 | 4.78 (2.45–9.33) |
| Severe | 117 | 22 | 21.7 | 1.82 (0.99–3.33) | 38 | 37.8 | 13.6 (7.44–24.9) |
Comorbidity severity based on Piccirillo Comorbidity Scores (PCS).
4. Discussion
This large study of patterns of care for breast cancer, with detailed information about comorbidity using the ACE-27 comorbidity score and the availability of five-year mortality data, provided a unique opportunity to explore the associations among comorbidity burdens and mortality outcome by age and receipt of guideline concordant care, including chemotherapy, for breast cancer. As expected, comorbidity burden was directly correlated with older age and non-breast cancer-related mortality. The cumulative risk of breast cancer-related mortality was higher in patients with severe comorbidity, but, after stratifying by age and controlling for demographic factors, tumor characteristics and treatment, the adverse effect of severe comorbidity on breast cancer-related mortality risk was only significant in patients age 70 and older. Furthermore, in the analysis stratified by use of guideline concordant chemotherapy, in unadjusted analyses, it appeared that benefits of adjuvant chemotherapy with regard to breast cancer survival are greater in younger patients and those without high comorbidity burdens.
The adverse effect of comorbidity burden on breast cancer-specific and overall mortality is well documented [2,14–16]. Most of these studies, however, were based on data from outside the United States. We provide data from a large dataset derived from seven cancer registries in the United States that confirms these findings. Adjustment for age and stage, however, does not negate the relationship of comorbidity with survival in women with breast cancer [2,14,17,18]. We confirm these findings in our study and show a dose-dependent association between comorbidity and non-breast cancer related mortality. With regard to breast cancer-related mortality, however, after adjusting for tumor characteristics and treatment, the relationship was only significant among patients age 70 and older with a severe comorbidity burden, implying that younger women and older women with lesser comorbidity burdens obtain benefits from guideline concordant therapies, whereas the benefit of guideline concordant treatment may be attenuated by severe comorbidity in patients age 70 and older.
There are at least four explanations for the relationship between comorbidity and breast cancer-specific survival. One, comorbidity may be associated, directly or indirectly, with the development of later stage, more aggressive, or more treatment-resistant disease [19]. Second, comorbidity may lead to poor organ function, such as cardiac, respiratory, or renal function, making it difficult to receive optimal therapy and leading to increased risk of death from breast cancer due to inability to provide sufficient treatment. Third, the coexisting illness may make the patient more vulnerable to toxicity from chemotherapy, leading to dose reduction or early discontinuation of treatment and, therefore, worse outcome [20]. Fourth, medications for comorbidities may affect tumor virulence or patient side effect risk and make treatment less effective [21]. In our study, it is not possible to determine which of these mechanisms is involved and/or most important, but, as the population ages and there is more interest in adjuvant therapies in a wider range of patients, it will be important to include these considerations both in observational and prospective studies.
This study has many strengths. We employed a large, geographically diverse database, with tumor registry data augmented with chart abstraction, and used the record-based ACE-27 comorbidity measure, although a claims-based version of the ACE-27 has been reported elsewhere [22]. Record re-abstraction enabled capture of detailed information about tumor characteristics, treatment, and severity of comorbid conditions. Whereas most studies of comorbidity were limited to older patients with Medicare, this study sample included patients ranging in age from 20 to 99 years. The database included well-defined variables, pertaining to demographic, patient, tumor and treatment characteristics, facilitating analyses across the full range of comorbidity levels. Use of the ACE-27, a robust, validated, chart-based instrument developed specifically for patients with cancer to study presence and severity of comorbidity, is a tremendous strength of the study [23]. Other studies used less powerful measures of comorbidity [2,7,24,25]. The widely used Charlson Comorbidity index [26,27] can be used with administrative databases, but includes fewer diagnoses, little information about disease severity, and tends to overestimate comorbidity burden. The Cumulative Illness Rating Scale, on the other hand, is perhaps the most comprehensive index, but it requires detail only available when collected prospectively [28].
We also recognize limitations deserving mention. This retrospective study is limited by what was recorded in the medical record, which is a poor source of information about physician and patient decision making, referrals, and patient compliance. Limited subgroup sizes precluded exploration of the influence that race/ethnicity and socioeconomic status on risk of death by comorbidity. Abstractors reviewed records from both hospital and physician offices, but not all physician offices. Residual confounding may also be an issue; although women with severe comorbidity may have been less likely to receive chemotherapy than women with no or less severe comorbidity, the main analysis was adjusted for differences in receipt of guideline concordant treatment. Analyses do not include dose-intensity, specific regimen of chemotherapy, or completion of planned chemotherapy, so the extent to which these factors may have played a role is uncertain. Inclusion of comorbidity measures into future randomized clinical trials will facilitate exploration into the effect of comorbidity severity and chemotherapy dose intensity on outcomes. Regarding the ACE-27, we obtained severity of comorbid conditions but possible misclassification may still exist with medical records as the sole source of information. Moreover, data were collected years after breast cancer diagnosis, medical records were either destroyed or not accessible for approximately 20% of sampled cases, and small numbers in some sub-categories may have limited power to detect differences in care. Finally, use of death certificate files and linkage with the National Death Index to identify cause of death, may have led to some misclassification, including classifying other causes of cancer death as breast cancer death.
In conclusion, for patients age 70 and older with severe comorbidity, we found higher hazard of breast cancer-related death, even after adjusting for race/ethnicity, tumor characteristics, and treatment. Furthermore, it appeared that the benefit of guideline concordant chemotherapy was greater in younger persons and those without severe comorbidity. Current NCCN guidelines state that “there are limited data to make chemotherapy recommendations for those > 70 years old” and “treatment should be individualized with consideration of comorbid conditions” [29]. We provide data indicating that, compared to women with no comorbidity, patients with breast cancer age 70 and older with severe comorbidity are at increased risk of dying from breast cancer, even after adjustment for guideline concordant adjuvant chemotherapy and other tumor and treatment differences. These findings support use of guideline-concordant adjuvant chemotherapy in women with early stage breast cancer with comorbidity at mild and moderate levels, but raise questions about whether older women and women with severe comorbidity may derive less benefit from adjuvant chemotherapy, and whether higher thresholds of risk may be needed to justify use of adjuvant chemotherapy in patients with severe comorbidity and patients who are age 70 and older. This information, the fact that data supporting use of adjuvant chemotherapy in older women is limited to those healthy enough to participate in clinical trials [30], and toxicity concerns even in healthy older women, adds to the risk–benefit discussion and emphasizes the need for further study to clarify the role of adjuvant chemotherapy for breast cancer in older women.
Funding/Support:
The Breast and Prostate Cancer Data Quality and Patterns of Care Study was supported by the Centers for Disease Control and Prevention through cooperative agreements with the California Cancer Registry (Public Health Institute) (1-U01-DP000260), Emory University (1-U01-DP000258), Louisiana State University Health Sciences Center (1-U01-DP000253), Minnesota Cancer Surveillance System (Minnesota Department of Health) (1-U01-DP000259), Medical College of Wisconsin (1-U01-DP000261), University of Kentucky (1-U01-DP000251), and Wake Forest University (1-U01-DP000264). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Conflict of Interest/Disclosures
Dr. Kimmick has served on speakers boards and been a consultant for AstraZeneca, Pfizer, and Novartis and has had research funding from AstraZeneca, Pfizer, and Roche. Dr. Anderson has had research funding from AstraZeneca and Roche. None of the other authors have financial interests, activities, relationships, or affiliations that would pose a conflict of interest with the content of this manuscript.
References
- [1].Wu X, Richardson LC, Kahn AR, Fulton JP, Cress RD, Shen T, et al. Survival difference between non-Hispanic black and non-Hispanic white women with localized breast cancer: the impact of guideline-concordant therapy. J Natl Med Assoc 2008;100 (5):490–8. [DOI] [PubMed] [Google Scholar]
- [2].Louwman WJ, Janssen-Heijnen ML, Houterman S, Voogd AC, van der Sangen MJ, Nieuwenhuijzen GA, et al. Less extensive treatment and inferior prognosis for breast cancer patient with comorbidity: a population-based study. Eur J Cancer 2005;41 (5):779–85. [DOI] [PubMed] [Google Scholar]
- [3].Land LH, Dalton SO, Jorgensen TL, Ewertz M. Comorbidity and survival after early breast cancer. A review. Crit Rev Oncol Hematol 2012;81(2):196–205. [DOI] [PubMed] [Google Scholar]
- [4].Berglund A, Wigertz A, Adolfsson J, Ahlgren J, Fornander T, Warnberg F, et al. Impact of comorbidity on management and mortality in women diagnosed with breast cancer. Breast Cancer Res Treat 2012;135(1):281–9. [DOI] [PubMed] [Google Scholar]
- [5].Braithwaite D, Tammemagi CM, Moore DH, Ozanne EM, Hiatt RA, Belkora J, et al. Hypertension is an independent predictor of survival disparity between African-American and white breast cancer patients. Int J Cancer 2009;124(5):1213–9. [DOI] [PubMed] [Google Scholar]
- [6].Kimmick G, Fleming ST, Sabatino SA, Wu XC, Hwang W, Wilson JF, et al. Comorbidity burden and guideline-concordant care for breast cancer. J Am Geriatr Soc 2014;62 (3):482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hawfield A, Lovato J, Covington D, Kimmick G. Retrospective study of the effect of comorbidity on use of adjuvant chemotherapy in older women with breast cancer in a tertiary care setting. Crit Rev Oncol Hematol 2006;59(3):250–5. [DOI] [PubMed] [Google Scholar]
- [8].Lee L, Cheung WY, Atkinson E, Krzyzanowska MK. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J Clin Oncol 2011;29 (1):106–17. [DOI] [PubMed] [Google Scholar]
- [9].German RR, Wike JM, Bauer KR, Fleming ST, Trentham-Dietz A, Namiak M, et al. Quality of cancer registry data: findings from CDC-NPCR’s breast and prostate cancer data quality and patterns of care study. J Registry Manag 2011;38(2):75–86. [PubMed] [Google Scholar]
- [10].Wu XC, Lund MJ, Kimmick GG, Richardson LC, Sabatino SA, Chen VW, et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol 2012;30(2):142–50. [DOI] [PubMed] [Google Scholar]
- [11].Piccirillo JCI, Claybour P, et al. The measurement of comorbidity by cancer registries. J Registry Manag 2003;30:8–14. [Google Scholar]
- [12].Piccirillo JTR, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2006;291:2441–7. [DOI] [PubMed] [Google Scholar]
- [13].Johnson APJ, Creech C, et al. Validation of a comorbidity education program. J Registry Manag 2001;28:125–31. [Google Scholar]
- [14].Houterman S, Janssen-Heijnen ML, Verheij CD, Louwman WJ, Vreugdenhil G, van der Sangen MJ, et al. Comorbidity has negligible impact on treatment and complications but influences survival in breast cancer patients. Br J Cancer 2004;90(12): 2332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sheppard AJ, Chiarelli AM, Marrett LD, Nishri ED, Trudeau ME. Stage at diagnosis and comorbidity influence breast cancer survival in first nations women in Ontario, Canada. Cancer Epidemiol Biomarkers Prev 2011;20(10):2160–7. [DOI] [PubMed] [Google Scholar]
- [16].Land LH, Dalton SO, Jensen MB, Ewertz M. Impact of comorbidity on mortality: a cohort study of 62,591 Danish women diagnosed with early breast cancer, 1990–2008. Breast Cancer Res Treat 2012;131(3):1013–20. [DOI] [PubMed] [Google Scholar]
- [17].Carlsen K, Hoybye MT, Dalton SO, Tjonneland A. Social inequality and incidence of and survival from breast cancer in a population-based study in Denmark, 1994–2003. Eur J Cancer 2008;44(14):1996–2002. [DOI] [PubMed] [Google Scholar]
- [18].Cronin-Fenton DP, Norgaard M, Jacobsen J, Garne JP, Ewertz M, Lash TL, et al. Comorbidity and survival of Danish breast cancer patients from 1995 to 2005. Br J Cancer 2007;96(9):1462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol 2011;29(1):40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Budman DR, Berry DA, Cirrincione CT, Henderson IC, Wood WC, Weiss RB, et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The cancer and leukemia group B. J Natl Cancer Inst 1998;90(16):1205. [DOI] [PubMed] [Google Scholar]
- [21].Henry NL, Stearns V, Flockhart DA, Hayes DF, Riba M. Drug interactions and pharmacogenomics in the treatment of breast cancer and depression. Am J Psychiatry 2008;165(10):1251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fleming ST, Sabatino SA, Kimmick G, Cress R, Wu XC, Trentham-Dietz A, et al. Developing a claim-based version of the ACE-27 comorbidity index: a comparison with medical record review. Med Care 2011;49(8):752–60. [DOI] [PubMed] [Google Scholar]
- [23].Piccirillo JCC, Zequeira R, et al. Inclusion of comorbidity into oncology data registry. J Registry Manag 1999;26:66–70. [Google Scholar]
- [24].Siegelmann-Danieli N, Khandelwal V, Wood GC, Mainali R, Prichard J, Murphy TJ, et al. Breast cancer in elderly women: outcome as affected by age, tumor features, comorbidities, and treatment approach. Clin Breast Cancer 2006;7(1):59–66. [DOI] [PubMed] [Google Scholar]
- [25].Field TS, Bosco JL, Prout MN, Gold HT, Cutrona S, Pawloski PA, et al. Age, comorbidity, and breast cancer severity: impact on receipt of definitive local therapy and rate of recurrence among older women with early-stage breast cancer. J Am Coll Surg 2011;213(6):757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40(5):373. [DOI] [PubMed] [Google Scholar]
- [27].D’Hoore W, Bouckaert A, Telquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol 1996; 49(12):1429. [DOI] [PubMed] [Google Scholar]
- [28].Wedding U, Roehrig B, Klippstein A, Steiner P, Schaeffer T, Pientka L, et al. Comorbidity in patients with cancer: prevalence and severity measured by cumulative illness rating scale. Crit Rev Oncol Hematol 2007;61(3):269–76. [DOI] [PubMed] [Google Scholar]
- [29].Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Invasive breast cancer version 1.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2016;14(3):324–54. [DOI] [PubMed] [Google Scholar]
- [30].Kimmick G Adjuvant chemotherapy for breast cancer in older women: emerging evidence to aid in decision making. Curr Treat Options Oncol 2011;12(3):286–301. [DOI] [PubMed] [Google Scholar]


