Abstract
Recent studies have shown that activation of the signaling lymphocytic activation molecule (SLAM) family of receptors plays an important role in several aspects of immune regulation. However, translation of this knowledge into a useful clinical application has not been undertaken. One important area where SLAM-mediated immune regulation may have keen importance is in the field of vaccinology. Because SLAM signaling plays such a critical role in the innate and adaptive immunity, we endeavored to develop a strategy to improve the efficacy of vaccines by incorporation of proteins known to be important in SLAM-mediated signaling. In this study, we hypothesized that coexpression of the SLAM adapter EWS-FLI1–activated transcript 2 (EAT-2) along with a pathogen-derived Ag would facilitate induction of beneficial innate immune responses, resulting in improved induction of Ag-specific adaptive immune responses. To test this hypothesis, we used rAd5 vector-based vaccines expressing murine EAT-2, or the HIV-1–derived Ag Gag. Compared with appropriate controls, rAd5 vectors expressing EAT-2 facilitated bystander activation of NK, NKT, B, and T cells early after their administration into animals. EAT-2 overexpression also augments the expression of APC (macrophages and dendritic cells) surface markers. Indeed, this multitiered activation of the innate immune system by vaccine-mediated EAT-2 expression enhanced the induction of Ag-specific cellular immune responses. Because both mice and humans express highly conserved EAT-2 adapters, our results suggest that human vaccination strategies that specifically facilitate SLAM signaling may improve vaccine potency when targeting HIV Ags specifically, as well as numerous other vaccine targets in general.
Development of an effective vaccine to prevent infections by HIV-1 is an important goal. Most recently, a human clinical trial demonstrated that a prophylactic vaccine to HIV may indeed be possible (1). However, the results of that trial combined with recent results derived from the Merck-sponsored STEP trial suggest that a more potent vaccine capable of inducing greater levels of Ag-specific adaptive immune responses may demonstrate greater efficacy to prevent HIV infection (2, 3). Incorporation of adjuvants into vaccine formulations can improve the induction of Ag-specific adaptive immune responses (4-6). Proactive induction and/or harnessing of beneficial innate immune responses may be the mechanism underlying the effectiveness of certain adjuvants to contribute significantly to the ability of vaccines to generate adaptive immune responses (7, 8). We wished to evaluate the potential of improving vaccination efficacy by presenting a target Ag while simultaneously activating novel arms of the mammalian immune system. Whereas in previous studies we and others have explored the potential of modifying TLR-dependent innate immune responses to facilitate improved efficacy of virus-based vaccines (9, 10), in this study we set out to determine if targeted manipulation of the signaling lymphocytic activation molecule (SLAM) family of receptors pathway could also facilitate improved induction of Ag-specific, adaptive immune responses by vaccine platforms.
The SLAM family of receptors currently comprises six distinct members, respectively named SLAM (CD150), 2B4 (CD244), Ly9, CD84, NTB-A (NK, T, and B cell Ag; Ly108 in the mouse), and CRACC (CD2-like receptor activating cytotoxic cells) (11). These receptors are expressed mainly in cells of the hematopoietic lineages, inclusive of innate and adaptive immune cells (12, 13). All SLAM members except 2B4 (which interacts with CD48) are self-ligands and can be triggered by homophilic interactions through their respective extracellular domains to initiate intracellular signaling via recruitment of specific adapters (13-16). The SLAM-associated protein (SAP) family of adapters includes three members named SAP, EWS-LLI1–activated transcript 2 (EAT-2), and EAT-2–related transducer (ERT; ERT is however a nonfunctional pseudogene in humans). These adapters associate with phosphorylated tyrosine-based motifs (immuno-receptor tyrosine based switch motifs) in the cytoplasmic domains of SLAM receptors with high affinity and specificity. Once bound, these adapters either augment or inhibit SLAM-induced intracellular signaling in a variety of immune cells (11). EAT-2 and ERT transduce SLAM-initiated signals via phosphorylation of tyrosine residues directly located in their short C-terminal tails (17). In contrast, the SAP adapter regulates SLAM signaling by recruiting the protein tyrosine kinase FynT.
Various reports indicate a possible role for the binding of SLAM (CD150) receptor in the immunological synapse, whereby SLAM receptor activation acts as a costimulatory molecule facilitating the activation of dendritic cells (DCs) and macrophages. For example, in CD40L-activated human DCs, Ab-mediated ligation of SLAM receptor augmented the secretion of proinflammatory cytokines such as IL-12 and IL-8, but not IL-10 (18). Furthermore, the SLAM receptor was also found to play a role in the production of IL-6 and IL-12 by mouse peritoneal macrophages (19). In addition, macrophages derived from SLAM-deficient mice show a marked reduction in secretion of IL-12, TNF, and NO (19). Because EAT-2 is the only known SLAM-associated adapter protein expressed in DCs and macrophages, it has been proposed that EAT-2 facilitates SLAM-dependent proinflammatory cytokine expression in these cell types (16).
Relative to vaccine augmentation strategies, we note that the TLR adapter MyD88 also facilitates cytokine gene expression during TLR-dependent signaling and that overexpression of MyD88 from DNA-based vaccines facilitated the induction of Ag-specific adaptive immune responses by the vaccine platform (20, 21). Based on these facts, we hypothesized that expression of EAT-2 from a vaccine platform could also augment innate immune responses, potentially resulting in improved APC functions in vivo and consequently improvement in the induction of adaptive immune response generated against a coexpressed target Ag. In this study, we confirmed that an adenovirus (Ad)-based vaccine expressing the SLAM adapter molecule EAT-2 facilitates the induction of several arms of the innate immune system and that these inductions positively correlate with an improved ability of the vaccine to induce stronger cellular immune responses to a coexpressed Ag. Our results highlight for the first time, to our knowledge, the use of a SLAM immune regulatory pathway component to improve vaccination efficacy.
Materials and Methods
Vector construction
The Ad-GFP virus was purified as previously described (22). The open reading frame of EAT-2 gene (GenBank Accession no. NM_012009; http://www.ncbi.nlm.nih.gov/nuccore/148747581) was excised using primers flanked by XhoI and XbaI restriction endonucleases (NEB, Ipswich, MA) from a plasmid (Open Biosystem, Huntsville, AL) and subcloned into the pShuttle vector, which contains a CMV expression cassette. The resulting pShuttle-CMV-EAT2 shuttle plasmid was linearized with PmeI restriction enzyme and homologously recombined with the pAdEasyI Ad5 vector genome as previously described (23) yielding pAd-EAT2. HEK293 cells were transfected with PacI linearized plasmid, and viable virus was obtained and amplified after several rounds of expanding infection. Ad-EAT2 virus was purified using a CsCl2 gradient as previously described (24). The titer obtained was ~2.3 × 1012 viral particles (vp)/ml. Direct sequencing and restriction enzyme mapping were carried out to confirm the integrity of the EAT-2 sequence. To construct Ad-HIV/Gag, the HXB2 Gag gene (GenBank Accession no. K03455; http://www.ncbi.nlm.nih.gov/nuccore/1906382) was blunt-end subcloned into the EcoRV site of pShuttle-CMV. Restriction digests and sequencing were used to confirm the sequence integrity and correct orientation of the resulting shuttle (pShuttle-CMV Gag). Recombination and viral propagation was completed as described above. EAT-2 and Gag expression was verified using Western blotting (data not shown).
Animal procedures
All animal procedures were approved by the Michigan State University (East Lansing, MI) Institutional Animal Care and Use Committee. Adult male C57BL/6 and BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Intravenous injection of animals (8–10 wk old) consisted of injection (via the retroorbital sinus) of 200 μl of a PBS solution (pH 7.4), containing 7.5 × 1010 total virus particles of either Ad-GFP or Ad-EAT2 as previously described (25). Plasma and tissue samples were obtained and processed at the indicated times postinjection as previously described (25). Intramuscular injections were completed by injection of the indicated virus particles in a total volume of 20 μl into the tibialis anterior of the right hind limb.
Cytokine and chemokine analysis
A mouse 7-plex multiplex-based assay was used to determine the indicated cytokine/chemokine plasma concentrations per the manufacturer’s instructions (Bio-Rad, Hercules, CA) via Luminex 100 technology (Luminex, Austin, TX) essentially as previously described (25).
ELISPOT analysis
Splenocytes were harvested from individual mice, and RBCs were lysed using ACK lysis buffer (Invitrogen, Carlsbad, CA). Ninety-six–well Multiscreen high protein binding Immobilon-P membrane plates (Millipore, Billerica, MA) were pretreated with ethanol, coated with mouse anti–IFN-γ or IL-2 capture Abs, incubated overnight, and blocked prior to the addition of 5 × 105 splenocytes/well. Ex vivo stimulation included the incubation of splenocytes in 100 μl medium alone (unstimulated) or medium containing 4 μg/ml Gag-specific peptides (Gag-AMQMLKETI constructed by Genscript, Piscataway, NJ) or peptide no. QBI 304796 (obtained from QBI [Gaithersburg, MD] through the Vaccine Research and Development Branch, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health) for 24 h in a 37°C, 5% CO2 incubator. 15mer Gag-specific peptides were obtained from the National Institutes of Health AIDS Reagent and Reference Program (Rockville, MD) (Cat no. 8117, Lot no. 9). Staining of plates was completed per the manufacturer’s protocol. Spots were counted and photographed by an automated ELISPOT reader system (Cellular Technology, Cleveland, OH). Ready-set Go IFN-γ and IL-2 mouse ELISPOT kits were purchased from eBioscience (San Diego, CA).
In vitro cell culture
Bone marrow cells were extracted from the femurs and tibiae of male 6- to 8-wk-old BALB/c mice, and red cells were removed using ACK lysis buffer (Invitrogen). Bone marrow cells were cultured in DMEM supplemented with 10% FBS and 30% supernatant derived from confluent L929 cell cultures. At day 7, immature macrophages were collected and plated into 12-well plates for 24 h. This procedure yields a pure population of M-CSF–dependent, adherent macrophages. Murine RAW264.7 macrophages (ATCC TIB71) were maintained in DMEM supplemented with 10% FBS and penicillin–streptomycin following standard procedures. Suspensions of 5 × 105 cells were seeded into each well of 12-well plates. Then, the cells were incubated with 1 ml culture medium alone or with medium containing Ad-EAT2 or Ad-GFP at the multiplicities of infection indicated for 72 h.
Cell staining and flow cytometry
To identify immune cells transduced by Ad-mediated gene transduction, splenocytes derived from Ad-GFP–injected mice were stained with the following Abs: allophycocyanin-Cy7-CD3, Alexa Fluor 700-CD8a, PE-Cy7-NK1.1, and allophycocyanin-CD11c. Splenocytes were then subjected to flow cytometry, and the percentages of Ad-GFP–transduced cells (FITC+) were calculated using FlowJo software. For innate immune cell activation studies, splenocytes were stained with various combinations of the following Abs: PE-CD69 (3 μg/ml), FITC-CD8a, allophycocyanin-CD3, allophycocyanin-Cy7-CD3, Alexa Fluor 700-CD8a, PerCp-Cy5.5-CD19, PE-Cy7-NK1.1, PE–Cy7–TNF-α, allophycocyanin–IFN-γ (4 μg/ml) (all obtained from BD Biosciences, San Diego, CA), and PerCp–Cy5.5–IL-2 (4 μg/ml) (BioLegend, San Diego, CA). For bone marrow-derived macrophage (BMDM) and RAW264.7 cell studies, the following Abs were used: allophycocyanin-CD80, V450-CD86 (BD Biosciences), Alexa Fluor 700–MHC class II, and FITC-CD40 (eBioscience). Cells were incubated on ice with the appropriate Abs for 30 min, washed, and sorted using an LSR-II flow cytometer and analyzed using FlowJo software. For intracellular cytokines staining, cells were surface stained, fixed with 2% formaldehyde (Polysciences, Warrington, PA), permeabilized with 0.2% saponin (Sigma-Aldrich, St. Louis, MO), and stained for intracellular cytokines. We included the violet fluorescent reactive dye (ViViD; Invitrogen) as a viability marker to exclude dead cells from the analysis (26). Blood was isolated by retroorbital bleeds, and PBMCs were isolated using Lympholyte-Mammal (Cedarlane, Burlington, NC). Tetramer staining of PBMCs was completed using a PE-conjugated MHC class I tetramer folded with the AMQMLKETI peptide generated at the National Institutes of Health Tetramer Core Facility (Atlanta, GA). CD8+ T cells were depleted from pooled splenocyte preparations using MACS beads and LS columns per the manufacturer’s protocol (Miltenyi Biotec, Bergisch Gladbach, Germany). %CD8− SFC = SFC CD8-dep/SFC CD8+. Depletion was verified using FACS analysis using allophycocyanin-CD8a and Pacific blue-CD4 Abs (BD Biosciences). FACS analyses revealed >96% depletion of CD8+ T cells (Supplemental Fig. 4).
In vivo CTL assay
BALB/c or C57BL/6 mice were covaccinated with equivalent doses of Ad-HIV/Gag with either Ad-GFP or Ad-EAT2 (totaling 1 × 107 vp for BALB/c and 1 × 109 vp for C57BL/6 mice). At 14 d, syngeneic splenocytes were isolated and either pulsed with an irrelevant peptide specific to the Plasmodium falciparum circumsporozoite Ag (NYDNAGTNL) or with the HIV-Gag immunodominant AMQMLKETI peptide or QBI no. 304796 for 1 h at 37°C. Irrelevant peptide-pulsed cells were subsequently stained with 1 μM CFSE (CFSELow) while Gag-peptides–pulsed cells were stained with 10 μM CFSE (CFSEHigh). Naive and immunized mice were injected with equivalent amount of both CFSELow and CFSEHigh stained cells via the retroorbital sinus. After 5 h, mice were terminally sacrificed, and splenocytes were recovered and sorted on an LSR-II flow cytometer. FlowJo software was used to determine percentages of CFSE-stained cells. %Specific killing = 1 − ([%CFSEHigh/%CFSELow]immunized/[%CFSEHigh/%CFSELow]nonimmunized).
Detection of EAT-2 gene expression in vivo
To determine relative levels of EAT-2 and spleen-derived EAT-2 RNA transcript, spleen tissues were snap frozen in liquid nitrogen, and RNA was harvested from 100 mg frozen tissue using TRIzol reagent (Invitrogen) per the manufacturer’s protocol. After RNA isolation, reverse transcription was performed on 180 ng total RNA using Superscript II (Invitrogen) reverse transcriptase and random hexamers (Applied Biosystems, Foster City, CA) per the manufacturer’s protocol. Reverse transcription reactions were diluted to a total volume of 60 μl, and 2 μl was used as the template in the subsequent PCR reactions. Primers were designed using Primer Bank Web-based software (http://pga.mgh.harvard.edu/primerbank/). The following primers were used: forward primer (5′-CTGGGACTGATCTCAGGGTG-3′) and reverse primer (5′-GAAGGGAACGGGAGAATGGG-3′). Quantitative PCR was carried out on an ABI 7900HT Fast Real-Time PCR System using SYBR Green PCR Mastermix (Applied Biosystems) in a 15-μl reaction. PCRs were subjected to the following procedure: 95°C for 10 min followed by 40 cycles of 95°C for 15 s followed by 60°C for 1 min. The comparative Ct method was used to determine relative gene expression using GAPDH to standardize expression levels across all samples. Relative expression changes were calculated based on comparing experimental levels of a respective spleen transcript with those quantified in spleen samples derived from mock-injected animals.
Statistical analysis
Statistically significant differences in toxicities associated with innate immune responses were determined using one-way ANOVA with a Student–Newman–Keuls post hoc test (p < 0.05). For ELISPOT analysis, a two-way ANOVA was used followed by Bonferroni post hoc test (p < 0.05). For flow cytometry, a one-way ANOVA with a Student–Newman–Keuls post hoc test was used. For in vivo CTL assay, a one-way ANOVA with a Student–Newman–Keuls post hoc test was used. Data of all graphs in this article are presented as mean ± SD. GraphPad Prism software was used for statistical analysis.
Results
EAT-2–expressing Ad vectors enhance Ad vector-induced innate immune responses in vivo
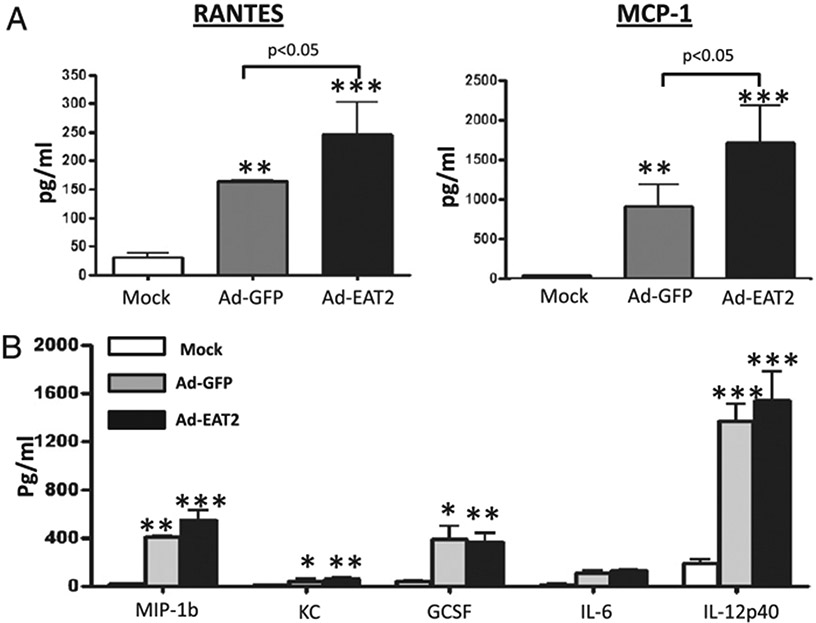
We constructed an E1 and E3 deleted ([E1−])Ad vector specifically designed to express the murine homologue of the SLAM adapter protein EAT-2. These EAT-2–expressing Ad vectors were fully viable, grew to high titers, and were purified and quantified exactly as done for conventional [E1−]Ad vaccines (25). To analyze the maximal impact that Ad-EAT2 expression might have upon innate immune responses, we administered Ad-EAT2 into C57BL/6 mice and measured the levels of cytokines and chemokines in the plasma 6 h postinjection (hpi), a time point we have previously demonstrated to be when maximal induction of proinflammatory cytokines and chemokines occurs after Ad vector administration (9, 27, 28). Administration of the Ad-EAT2 vector into C57BL/6 mice resulted in induction of significantly higher plasma levels of RANTES and MCP-1 at 6 hpi as directly compared with those of mice injected identically and treated with an Ad-GFP control vector (Fig. 1A). The levels of IL-6, IL-12p40, MIP-1β, KC, and G-CSF were also significantly induced by Ad-EAT2 vectors; however, these levels were not statistically different between Ad-EAT2 and Ad-GFP injected mice (Fig. 1).
FIGURE 1.
Systemic administration of EAT-2–expressing Ad vector induces cytokine and chemokines responses. C57BL/6 mice (n = 4) were either mock injected or intravenously injected with 7.5 × 1010 vp of either Ad-GFP or Ad-EAT2 vectors. Plasma was harvested at 6 h after virus injection. Cytokine induction was evaluated using a multiplexed bead array-based quantitative system. The bars represent mean ± SD. Statistical analysis was completed using one-way ANOVA with a Student–Newman–Keuls post hoc test; *p < 0.05 was deemed a statistically significant difference; **p < 0.01; ***p < 0.001 (statistically different from mock-injected animals).
Administration of EAT-2–expressing Ad induces activation of innate immune cells
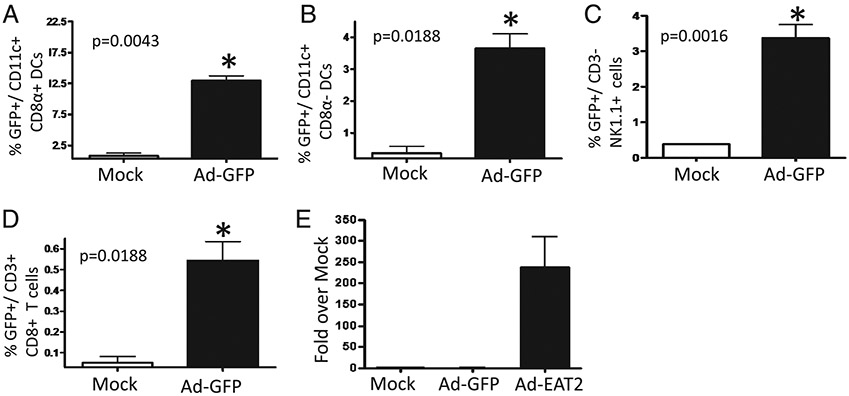
To determine what innate immune cell types may be transduced by the Ad vectors expressing EAT-2 (and potentially be responsible for the enhanced cytokine responses noted in Fig. 1), we analyzed Ad vector transduction of several important classes of innate immune cells found in the spleen by flow cytometry. Using an Ad vector expressing a tracking Ag (GFP), we confirmed that Ad vectors can transduce DCs, NK cells, as well as low levels of T cells upon administration into mice (Fig. 2A-D). Notably, we found that the highest subset of DCs that was transduced by Ad-GFP vectors was CD11c+CD8α+, a distinct subset of DCs shown to play a critical role in shaping adaptive immune responses after vaccination (29) (Fig. 2A) Additionally, we confirmed that after identical administrations of the Ad-EAT2 vector, EAT-2 gene expression was occurring in spleen cells as well (Fig. 2E).
FIGURE 2.
Transduction efficiency of innate immune cells by Ad vectors expressing transgenes. C57BL/6 mice (n = 3) were either mock injected or intravenously injected with 7.5 × 1010 vp Ad-GFP. Splenocytes were harvested 6 hpi, and GFP-expressing cells were detected and identified by gating on FITC+ cells using an LSR-II flow cytometer. A, Ad-GFP transduced CD11c+CD8α+ DCs. B, Ad-GFP–transduced CD11c+CD8α− DCs. C, Ad-GFP–transduced CD3−NK1.1+ cells. D, Ad-GFP–transduced CD3+CD8+ T cells. E, Quantitative RT-PCR for EAT-2 transcript derived from the spleens of mock, Ad-GFP, or Ad-EAT2 injected C57BL/6 mice. The bars represent mean ± SD. Statistical analysis was completed using Student t test; p < 0.05 was deemed a statistically significant difference. *p < 0.05 (statistically different from mock-injected animals).
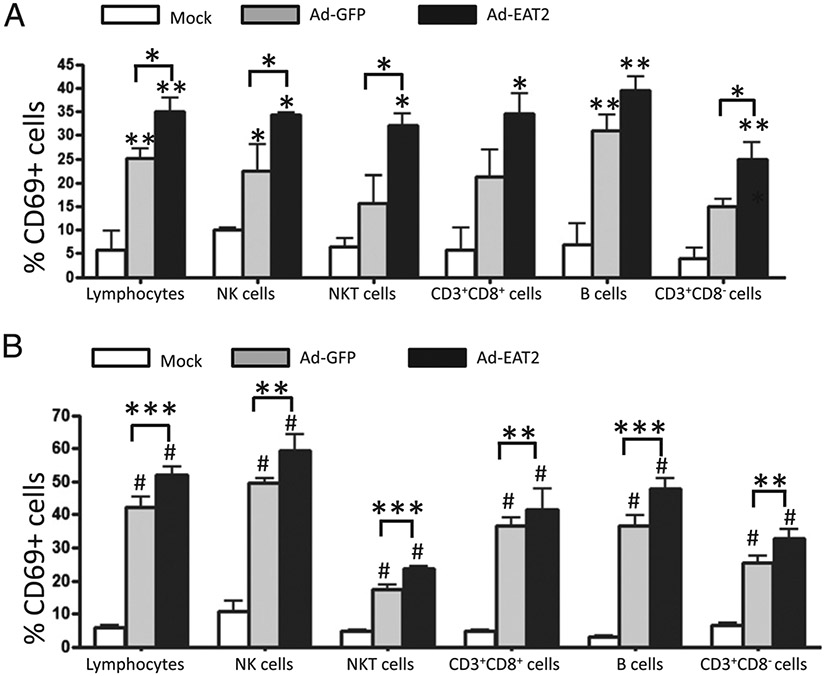
It is widely appreciated that Ads enhance innate immune cell effector functions (30, 31). To evaluate additionally the phenotype of immune cells after exposure or transduction by the Ad vector expressing EAT-2, we analyzed the expression of the lymphocyte activation marker CD69 as well as IFN-γ production in various immune cells shortly after administration of Ad-EAT2 into C57BL/6 mice. Our results confirmed that Ads, in general, significantly induce a rapid activation of NK and NKT cells in both PBMCs and spleens, as confirmed by the presence of increased percentages of CD69-expressing NK and NKT cells in Ad-GFP–treated mice (Fig. 3A, 3B). Specifically, injection of Ad-EAT2 induced significantly higher numbers of CD69-expressing NK and NKT cells, both in PBMCs and splenocytes at 6 hpi, compared with that for the Ad-GFP control vector (Fig. 3A, 3B). At 48 hpi, CD69 expression on NK cells remained significantly higher (p < 0.05) only in PBMCs derived from Ad-EAT2–injected mice compared with that of Ad-GFP–injected mice (Supplemental Fig. 1A). All other differences previously noted at 6 hpi had dissipated by 48 hpi (Supplemental Fig. 1C), suggesting that expression of EAT-2 by Ad vaccines transiently improves the induction of these responses.
FIGURE 3.
Ad-EAT2–mediated activation of innate and adaptive immune cells in vivo. C57BL/6 mice (n = 4) were either mock injected or intravenously injected with 7.5 × 1010 vp of either Ad-GFP or Ad-EAT2. CD69 expression by PBMC- (A) and splenocyte- (B) derived NK, NKT, CD3+CD8+, CD3+CD8−, and B cells was evaluated 6 h after virus injection. PBMCs and splenocytes were harvested, stained, and sorted on an LSR-II flow cytometer. The bars represent mean ± SD. Statistical analysis was completed using one-way ANOVA with a Student–Newman–Keuls post hoc test; p < 0.05 was deemed a statistically significant difference. *p < 0.05; **p < 0.01; ***p < 0.001; ****#p < 0.001 (statistically different from mock injected animals).
Treatment with either Ad-EAT2 or Ad-GFP induced significantly elevated numbers of IFN-γ+ NK cells at both 6 and 48 hpi (p < 0.001 and p < 0.05, respectively). However, no significant differences were observed between the control and experimental viruses, suggesting that overexpression of EAT-2 cannot induce additional inductions of IFN-γ relative to the ability of the Ad vector itself (Supplemental Fig. 2). Finally, at 48 hpi, the number of NKT cells expressing CD69 was also significantly increased (p < 0.05) in PBMCs derived only from Ad-EAT2–injected mice (Supplemental Fig. 2A). CD69 expression on splenic NKT cells isolated from both Ad-EAT2– and Ad-GFP–injected mice were also both significantly (p < 0.05) increased over mock-injected mice (Supplemental Fig. 1C).
Administration of EAT-2–expressing Ads induces greater activation of adaptive immune cells
Because the SLAM family of receptors are expressed in various innate and adaptive immune cells (12) and the activation of T cells and/or B cells can be initiated or accentuated by innate immune system activation (7), we sought to analyze adaptive immune cell responses shortly after administration of Ad-EAT2. Our results confirmed that Ad vector administration in and of itself induces a rapid activation of CD3+CD8+ T cells, CD3+CD8− T cells, and B cells, Ad-dependent responses that can all be further accentuated with expression of EAT-2. For example, injection of Ad-EAT2 resulted in significantly higher numbers of splenic CD69-expressing CD3+CD8+ T cells (p < 0.01), CD3+CD8− T cells (p < 0.01), and B cells (p < 0.001) at 6 hpi (Fig. 3B) compared with the numbers of these cells being induced by the control Ad vector. At 48 hpi, the percentage of splenic CD69-expressing CD3+CD8+ T cells, CD3+CD8− T cells, and B cells derived from Ad-EAT2– and Ad-GFP–injected mice remained significantly higher (p < 0.001) over mock-injected mice; however, no statistical differences were observed between the control and experimental Ad viruses at this time point (Supplemental Fig. 1D). We also evaluated the percentage of CD69-expressing cells in PBMCs from the same animals. At 6 hpi, we observed a significantly (p < 0.05) higher percentage of CD69-expressing total lymphocytes (as defined previously) in Ad-EAT2–injected mice compared with that of Ad-GFP–injected mice. When analyzing specific lymphocyte subsets, we observed a significant increase in the percentage of CD69-expressing CD3+CD8− T cells isolated from Ad-EAT2–injected mice compared with the percentage of identical cells isolated from Ad-GFP–injected mice (p < 0.05) (Fig. 3A). Higher activation of CD3+CD8− T cells and B cells was observed in PBMCs derived from Ad-EAT2–injected mice compared with that of the mock-infected mice; however, no statistically significant differences in activation levels were observed between the control and experimental Ads in these cell types (Fig. 3A). By 48 hpi in PBMCs, any statistically significant differences between control and experimental Ad vector-injected animals had resolved (Supplemental Fig. 1B).
Ad vector expressing EAT-2 enhances T cell responses to a coadministered Ag
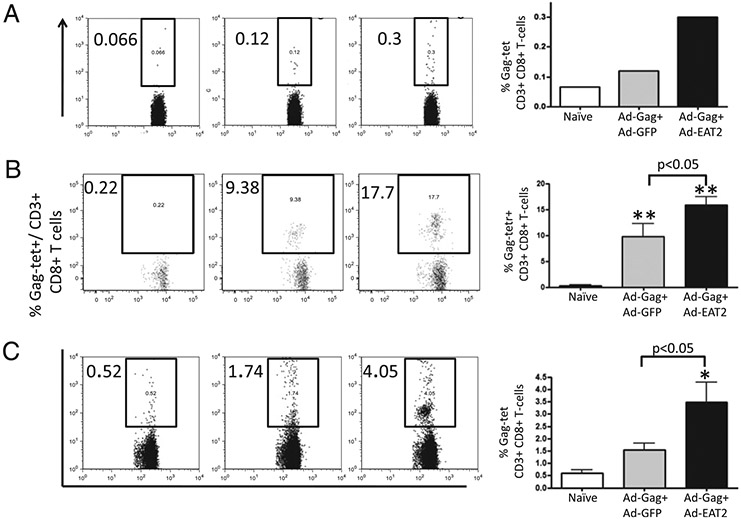
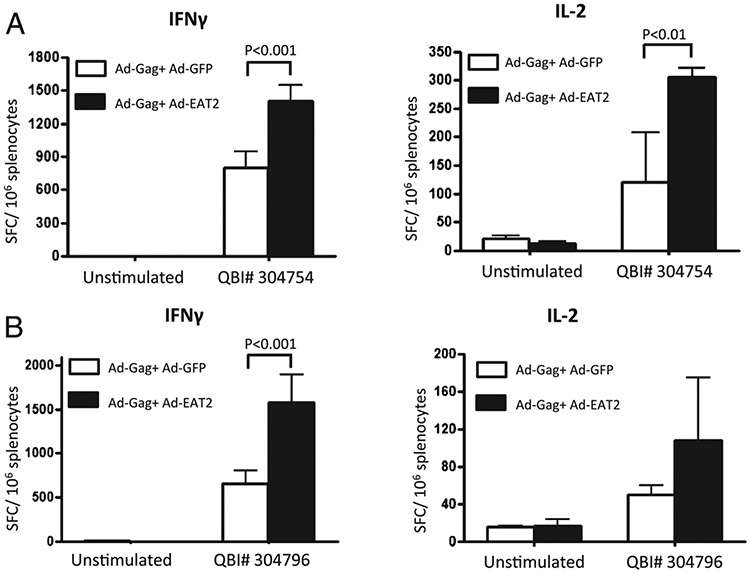
Simultaneous administration of Ags with adjuvants can stimulate the innate immune system to improve significantly the adaptive immune responses to the antigenic target (32, 33). To investigate whether the enhanced innate immune responses promoted by Ad-mediated expression of EAT-2 could influence the adaptive immune responses to a coadministered Ag, we immunized BALB/c or C57BL/6 mice with both an Ad-based vector expressing the HIV-1 clade B Gag protein (HXB2) and the Ad-EAT2 vector or a control vector (Ad-GFP). We performed initial dose-curve studies to identify the lowest dose of Ad-HIV/Gag that generated detectable Gag-specific cellular immune responses in the two distinct strains of mice. As a result, we identified an Ad-HIV/Gag dose of 5 × 106 vp/mouse for BALB/c mice and 5 × 108 vp for C57BL/6 mice as the most relevant experimental doses for these studies (data not shown). Six days after BALB/c mice were i.m. injected with 5 × 106 Ad-HIV/Gag mixed with equivalent amounts of either the control Ad-GFP vector or Ad-EAT2, we were able to detect heightened Gag-specific, tetramer-positive CD8+ T cells in PBMCs derived from mice coimmunized with Ad-HIV/Gag plus Ad-EAT2 compared with that of Ad-HIV/Gag plus Ad-GFP coimmunized mice (Fig. 4A). At 14 dpi, PBMCs (p < 0.05) and splenocytes (p < 0.05) derived from mice coimmunized with Ad-HIV/Gag plus Ad-EAT2 contained higher numbers of Gag-specific tetramer-positive CD8+ T cells compared with that of the respective cell populations isolated from control mice (Fig. 4B, 4C). After ex vivo stimulation with the immunodominant Gag peptides AMQMLKETI (QBI no. 304754 for BALB/c mice) or QBI no. 304796 (for C57BL/6 mice), splenocytes derived from mice coimmunized with Ad-HIV/Gag and Ad-EAT2 contained significantly (p < 0.001) increased numbers of Gag-specific, IFN-γ–secreting cells (Fig. 5A, 5B, respectively). We also observed significantly increased numbers of IL-2–secreting, HIV-Gag peptide specific (QBI no. 304754) splenocytes derived from BALB/c mice coimmunized with Ad-HIV/Gag plus Ad-EAT2 compared with that of Ad-HIV/Gag plus Ad-GFP coimmunized mice (p < 0.01) (Fig. 5A).
FIGURE 4.
HIV-Gag–specific cellular immune responses elicited by Ad-HIV/Gag and Ad-EAT2 coimmunization. BALB/c mice were coimmunized i.m. in the tibialis anterior with equivalent viral particles of Ad-HIV/Gag mixed with either Ad-GFP or Ad-EAT2 (total of 1 × 107 vp mixed prior to injection). At 6 days postinjection (dpi), PBMCs (A) were collected from the immunized mice and stained with a PE-conjugated H2-Kd-AMQMLKETI tetramer complex. At 14 dpi, mice were sacrificed and PBMCs (B) or splenocytes (C) were harvested and stained with a PE-conjugated H2-Kd-AMQMLKETI tetramer complex together with APC-conjugated anti-CD3 and FITC-conjugated anti-CD8 Abs. The bars represent mean ± SD for six mice per group (pool of two for PBMCs) for virus injected and three mice for naive animals. Statistical analysis was completed using one-way ANOVA with a Student–Newman–Keuls post hoc test; p < 0.05 was deemed a statistically significant difference. *p < 0.05; **p < 0.01 (statistically different from mock-injected animals).
FIGURE 5.
HIV-Gag–specific cellular immune responses elicited by Ad-HIV/Gag and Ad-EAT2 coimmunization. BALB/c mice (n = 6) (A) or C57Bl6 mice (n = 4) (B) were coimmunized i.m. in the tibialis anterior with equivalent viral particles of Ad-HIV/Gag mixed with either Ad-GFP or Ad-EAT2 (total of 1 × 107 vp for BALB/c mice and total of 1 × 109 vp for C57BL/6 mice mixed prior to injection). At 14 dpi, splenocytes were harvested and stimulated ex vivo with the immunodominant peptides (AMQMLKETI) for BALB/c and QBI no. 304796 (EAMSQVTNSATIMMQ) for C57BL/6. Spot-forming cells (SFCs) were quantified using an ELISPOT reader. Data are presented as mean ± SD. Representative data from two independent experiments are shown. Statistical analysis was completed using two-way ANOVA with a Bonferroni post hoc test; p < 0.05 was deemed a statistically significant difference.
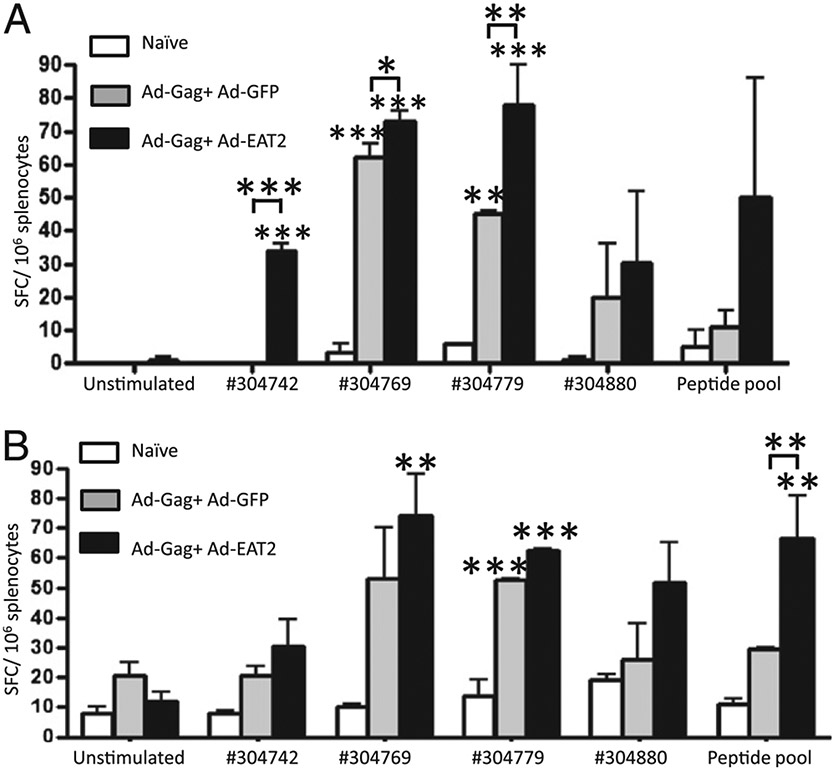
EAT-2 expression during HIV-Gag vaccination also facilitated a broadened induction of HIV-Gag–specific T cell clones, as we observed increased numbers of Gag-specific IFN-γ– and IL-2–cosecreting splenocytes responding to an expanded variety of HIV-Gag–specific peptides present within the full HIV-Gag protein (i.e., QBI no. 304742, 304769, 304779, 304800) and a peptide pool (304790, 403808, and 304826) (Fig. 6A, 6B). In a more global analysis of the extent of HIV-Gag peptide recognition promoted by prior expression of EAT-2, we ex vivo–stimulated splenocyte preparations derived from the immunized mice with HIV-gag–derived peptide pools, each pool containing two to four Gag-specific 15mer peptides spanning the entire HIV-Gag protein sequence. We observed an increased breadth in the immune recognition of HIV-Gag by coexpression of EAT-2, as the number of HIV Gag-specific peptides that triggered cellular responses from splenocytes derived from the Ad-HIV/Gag plus Ad-EAT2 coimmunized mice were significantly increased compared with that of similarly treated splenocytes derived from the Ad-HIV/Gag plus Ad-GFP control mice (Supplemental Fig. 3). CD8 T cell depletion studies suggested that the majority of the T cells responding to the Ags were CD8+ (Supplemental Fig. 4).
FIGURE 6.
Analysis of the breadth of Gag responses. BALB/c mice were coimmunized i.m. with equivalent viral particles of Ad-HIV/Gag mixed with either Ad-GFP or Ad-EAT2 (total dose of 1 × 107 vp mixed prior to injection). At 14 dpi, animals were terminally sacrificed, and splenocytes were harvested and stimulated ex vivo with 15mer HIV-Gag–derived peptides QBI no. 304724 (SLYNTVATLYCVHQR), QBI no. 304753 (GHQAAMQMLKETINE), QBI no. 304754 (AMQMLKETINEEAAE), QBI no. 304769 (PVGEIYKRWIILGLN), QBI no. 304779 (VDRFYKTLRAEQASQ), QBI no. 304800 (GNFRNQRKTVKCFNC), or pool of three peptides (QBI no. 304790, 304808, and 304826), and IFN-γ (A) and IL-2 (B) ELISPOT assays were completed. Bars represent mean ± SD. Statistical analysis was completed using two-way ANOVA with a Bonferroni post hoc test; p < 0.05 was deemed a statistically significant difference. *p < 0.05; **p < 0.01; ***p < 0.001 (statistically different from naive animals).
Assessment of Gag-specific T cell cytokine responses by multiparameter flow cytometry
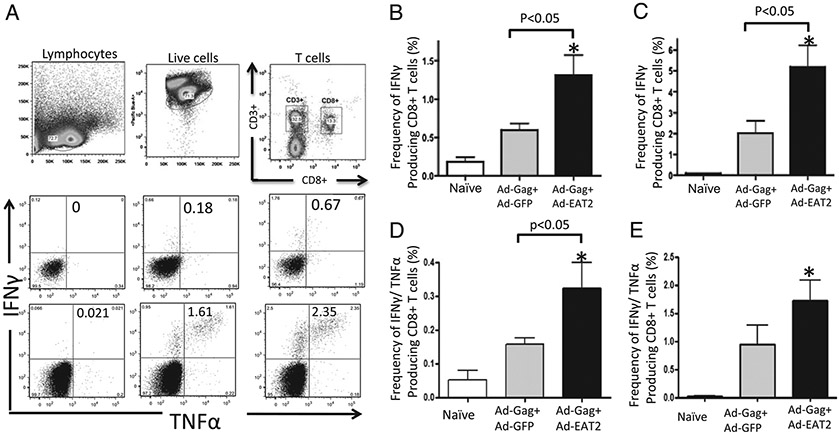
Published reports have shown that the increased presence of Ag-specific T cells that express several cytokines in response to Ags correlate with improved vaccine-induced protective immunity and positively correlate with the induction of long-lived memory responses (34, 35). To enumerate these “polyfunctional” T cells, we evaluated the expression of cytokines in HIV-Gag–specific CD8+ T cells generated after Ad-HIV/Gag and Ad-EAT2 coimmunization. Six-color flow cytometry was used to enumerate the frequency of CD8+ T cells producing IFN-γ, TNF-α, and/or IL-2 after ex vivo stimulation with HIV-Gag–specific peptides. We observed statistically higher numbers of HIV-Gag–specific IFN-γ positive (p < 0.05) (Fig. 7B, 7C) and IFN-γ/TNF-α double-positive (p < 0.05) (Fig. 7D, 7E) CD8+ T cells derived from Ad-HIV/Gag and Ad-EAT2 coimmunized mice compared with that of mice vaccinated with the control vaccines. When evaluating TNF-α or IL-2 single-positive CD8+ T cells, we also observed increased numbers of CD8+ T cells that express TNF-α or IL-2 derived from Ad-HIV/Gag and Ad-EAT2 coimmunized mice compared with that of Ad-HIV/Gag and Ad-GFP coimmunized mice; however, with these numbers of samples, these improved trends did not reach statistically significant levels (data not shown).
FIGURE 7.
Ad-HIV/Gag and Ad-EAT2 coimmunization increases the frequency of HIV-Gag–specific CD8+ T cells. BALB/c (n = 6) or C57BL/6 (n = 4) mice were coimmunized with equivalent viral particles of Ad-HIV/Gag mixed with either Ad-GFP or Ad-EAT2 (1 × 107 total vp for BALB/c and 1 × 109 total vp for C57BL/6 mice). At 14 dpi, the mice were sacrificed, and lymphocytes were isolated from spleens. Multiparameter flow cytometry was used to determine the total frequency of cytokine-producing CD8+ T cells. A, Representative example of the gating strategy used to define the frequency of cytokine-producing CD8+ T cells. Gates were set based on negative control (naive) and placed consistently across samples. B–E, The total frequency of CD8+ T cells derived from BALB/c or C57BL/6 mice expressing IFN-γ (B and C, respectively) or IFN-γ and TNF-α (D and E, respectively) is shown. The bars represent mean ± SD. Statistical analysis was completed using one-way ANOVA with a Student–Newman–Keuls post hoc test; p < 0.05 was deemed a statistically significant difference. *p < 0.05 (statistically different from naive animals).
Cytotoxic CD8+ T cells from Ad-EAT2 coimmunized mice exhibit improved Gag-specific cytotoxicity in vivo
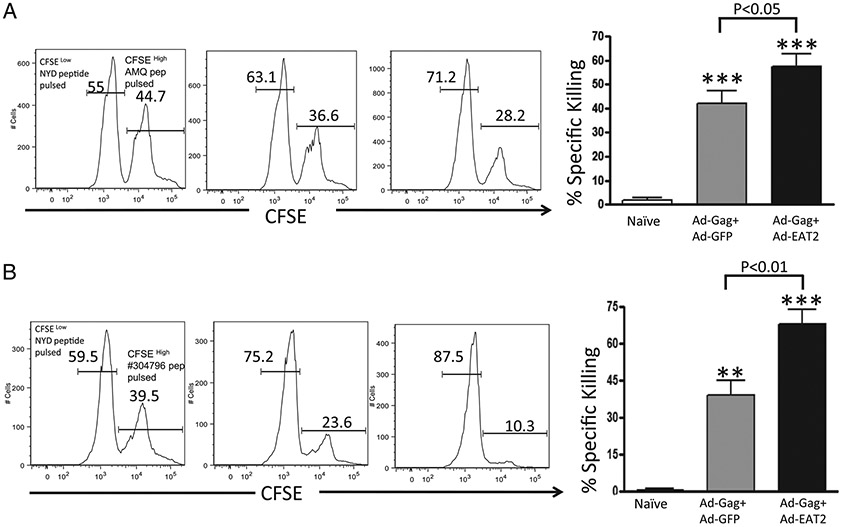
The direct measurement of in vivo functionality of CD8+ T lymphocytes (CTLs) to specifically kill Ag-presenting target cells provides a critical assessment as to their overall functional capacity. To evaluate the in vivo cytolytic activity of the Gag-specific CD8+ T lymphocytes generated after Ad-HIV/Gag and Ad-EAT2 coimmunization, mice were coimmunized with Ad-HIV/Gag plus Ad-EAT2 or Ad-HIV/Gag plus Ad-GFP. Fourteen days after vaccination, the two groups of mice were then injected with CFSE-labeled syngeneic splenocytes pulsed with the Gag-derived peptides AMQMLKETI or QBI no. 304796. The elimination of the peptide-pulsed splenocytes (CFSEhigh) was then examined by a flow cytometry-based CTL assay (36). Our results demonstrated that HIV-Gag–specific CTL activities induced in mice coimmunized with Ad-HIV/Gag and Ad-EAT2 were significantly higher compared with those of control mice (p < 0.05 for BALB/c and p < 0.01 for C57BL/6 mice) (Fig. 8A, 8B).
FIGURE 8.
Increased cytolytic activity of the Gag-specific T cell in vivo in Ad-HIV/Gag and Ad-EAT2 coimmunized mice. BALB/c (n = 6) (A) or C57BL/6 (n = 4) (B) mice were coimmunized with equivalent viral particles of Ad-HIV/Gag mixed with either Ad-GFP or Ad-EAT2 (1 × 107 total vp for BALB/c and 1 × 109 total vp for C57BL/6 mice). At 14 dpi, syngeneic splenocytes were pulsed with either an irrelevant peptide (NYD-pep) and stained with 1 μM CFSE (CFSELow) or with the HIV-Gag–specific peptides (AMQ peptide for BALB/c and QBI no. 304796 for C57BL/6 mice) and labeled with 10 μM CFSE (CFSEHigh). Five hours after adoptive transfer into either naive or immunized mice, splenocytes were harvested and sorted using an LSR-II flow cytometer. Percentage CFSE-positive cells were quantified using FlowJo software. %Specific killing = 1 − ([%CFSEHigh/%CFSELow]immunized/[%CFSEHigh/%CFSELow]nonimmunized). Representative figure of two combined independent experiments is shown for BALB/c mice. **p < 0.01; ***p < 0.001 (statistically different from naive animals).
Ad vector expressing EAT-2 induces greater expression of CD40, CD80, CD86, and MHC class II molecules
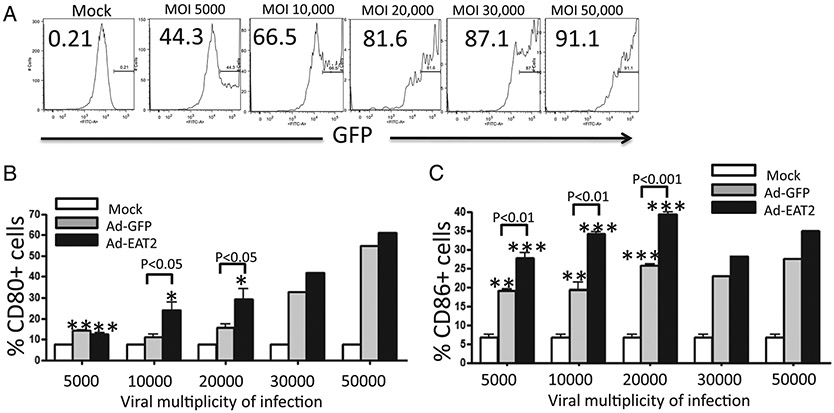
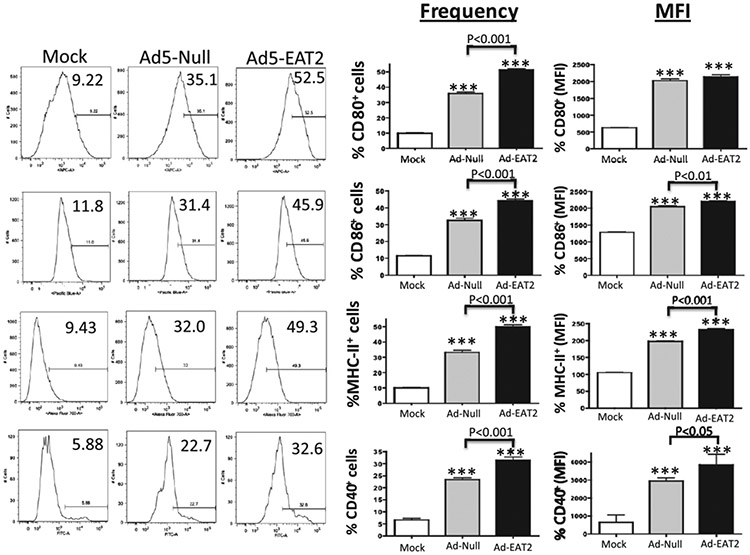
To investigate the mechanisms underlying the enhanced Gag-specific adaptive immune responses generated after Ad-HIV/Gag and Ad-EAT2 coimmunization, we evaluated the expression of markers associated with APC function, in this instance analyzing BMDMs. Isolated BMDMs were initially infected with the Ad-EAT2 or Ad-GFP vectors at escalating multiplicities of infection (5,000–50,000 vp/cell). Cells were then analyzed for the surface expression of CD80 and CD86 costimulatory molecules by flow cytometry. Mock-infected BMDMs expressed low levels of CD80 and CD86, whereas infection with the control Ad virus significantly induced the expression of CD80 and CD86 molecules (Fig. 9). Notably, Ad-EAT2 infection greatly enhanced the expression of CD80 and CD86 compared with that of BMDMs infected with the control Ad (Fig. 9). These effects were not seen if the BMDMs were infected with a UV irradiation-inactivated Ad-EAT2 vector, confirming that EAT-2 gene expression was necessary for the effect (data not shown). RAW264.7 cells were also infected with either the Ad-EAT2 or Ad control vector. Similar to the results we obtained in BMDMs, Ad infection resulted in significant increases in the expression of CD40, CD80, CD86, and MHC class II molecules (Fig. 10). Importantly, Ad-EAT2 infection resulted in significantly higher levels of CD40, CD80, CD86, and MHC class II molecules compared with those of cells infected with the Ad control virus (Fig. 10).
FIGURE 9.
EAT2 overexpression augments CD80 and CD86 expression by BMDMs. In vitro-cultured murine BMDMs (500,000 cells/well) were mock infected or infected with the Ad-EAT2 or Ad-GFP control for 72 h at the multiplicity of infection (MOI) shown. A, Expression of GFP on BMDMs after infection with escalating doses of Ad-GFP at 72 h. B, Expression of CD80 on BMDMs 72 hpi with escalating doses of Ad-EAT2 (black) or Ad-GFP (gray). C, CD86 expression in BMDMs infected with various doses of Ad-EAT2 or Ad-GFP at 72 hpi. Data are representative of three independent experiments with similar results. Samples were plated in triplicate and are expressed as mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001 (statistically different from uninfected cells).
FIGURE 10.
Increased expression of CD40, CD80, CD86, and MHC class II in Ad-EAT2–infected RAW264.7 cells. RAW264.7 cells (500,000 cells/well) were mock infected or infected with 20,000 vector particles/cell of Ad-EAT2 (black) or Ad-Null (gray). Seventy-two hours later, cells were stained with Abs specific for CD40, CD80, CD86, and MHC class II and analyzed on an LSR-II flow cytometer. Data are representative of four independent experiments with similar results. Samples were plated in triplicate and are expressed as mean ± SD. ***p < 0.001 (statistically different from uninfected cells).
Discussion
The SLAM family of receptors modulate multiple innate and adaptive immune responses through their intracellular signaling adapters SAP, EAT-2, and ERT (12, 15, 16). This report provides evidence supporting an important new strategy to improve the efficacy of vaccines in general, and that of Ad-based vaccines specifically, by expression of SLAM system-derived adapters simultaneous with Ag-specific vaccinations. More specifically, in this study we confirmed these notions by using an Ad vaccine genetically engineered to express the SLAM adapter molecule EAT-2 along with the HIV-derived Ag Gag. The inclusion of the Ad-EAT2 vector in Ad-HIV-gag vaccine mixtures significantly impacted upon the innate immune responses induced by the vaccine mixture, and more importantly specifically improved multiple, Ag-specific adaptive (cellular) immune responses to the target Ag present in the vaccine mixture, in this instance the HIV Gag protein.
There are a number of mechanisms as to how expression of EAT-2 might facilitate induction of Ag-specific immune responses in vivo. NK cells represent a subset of innate immune cells that have been shown to play an important role in bridging innate and adaptive immune responses by influencing DC function (37), providing signals for augmenting Th1 immune responses (38-40), and inducing tumor-specific CTLs (41). In addition, it has been shown recently that NK cell-mediated cytotoxicity of Ag-expressing target cells induces robust Ag-specific adaptive immune responses (42). EAT-2 has been shown to be indispensable in activating NK cell-mediated cytotoxicity by acting as a down-stream adapter protein facilitating signaling from the SLAM family receptor CRACC (in mice) (43, 44) or NTB-A (in humans) (45). Ad-mediated transduction of the EAT-2 gene enhanced NK cell activation (especially prolonged in those NK cells found in the circulation), activations that may facilitate the activation and/or maturation of DCs thereby biasing the HIV-Gag–specific immune profile toward a Th1 response (40). Of interest, we noted that Ads induced significantly elevated levels of IFN-γ in NK cells regardless of EAT-2 overexpression. These results suggest that EAT-2 overexpression enhances immune responses to the co-injected Ag in a mechanism that does not involve induction of higher levels of IFN-γ–producing NK cells. For example, in addition to NK cells, we also observed increased activation of NKT cells after Ad-mediated transduction of the EAT-2 gene. Several reports have shown that enhancing the activation of NKT cells can also positively influence the initial activation of DCs and/or NK cells, thereby increasing DC-dependent adaptive (cellular) immune responses (46-50). Our data suggest that harnessing this potential capability of NKT cells (in this instance via expression of EAT-2) may be an important goal of “next-generation” vaccines.
Previous reports have shown that SLAM-derived signaling can also increase the Ag-presentation capabilities of APCs (18, 19). We note that EAT-2 is the only SLAM adapter molecule currently known to be expressed in APCs (16). These previous observations suggest that Ad-mediated transduction of EAT-2 into DCs may have directly facilitated the induction of Ag-specific adaptive immune responses observed in this study. We confirmed that EAT-2 overexpression within several different types of APC triggers the induction of CD40, CD80, CD86, and MHC class II, all of which are critical costimulatory molecules that augment subsequent APC activation of T cells. Although it is known that Ads can transduce APCs and DCs in vivo, other cell types may also be transduced by the Ad vector expressing EAT-2 and also be affecting our in vivo results. It is conceivable that EAT-2 overexpression also facilitated SLAM-independent functions in Ad-EAT2–transduced immune cells. SAP family adapters can also interact, by way of their SH-2 domain, with other classes of receptors expressed in immune cells including CD22 (expressed in both CD8a DCs and B cells) and FcγRIIB (51, 52). Future studies will be required to delineate which aspects of the augmented EAT-2–dependent immune responses may be mediated by these or other receptors.
Various studies in mouse models and non-human primates suggest that improving the breadth of the Ag-specific cellular immune responses elicited by a vaccine is positively correlated with an improved ability of the vaccine to induce protective immunity, for example after pathogen challenge of vaccinated subjects (53, 54). Our results demonstrate that vaccine mixtures possessing the ability to express EAT-2 along with a target Ag increased the breadth of the cellular immune responses to the Ag, a result that correlated with a significantly improved induction of Ag-specific cytolytic T cell activity in vivo. We confirmed these results in both C57BL/6 and BALB/c mice (two mouse strains that can bias adaptive immune responses to a Th1 or Th2 response, respectively) indicating that the “adjuvant” effect of EAT-2 is not specifically limited by immunogenetic background differences of the host animal, at least in this species. In conclusion, our findings suggest that enhancing SLAM signaling by expressing EAT-2 during Ag vaccination can serve to improve the ability of a vaccine to stimulate the innate immune system and subsequently induce improved, Ag-specific adaptive immune responses.
Supplementary Material
Acknowledgments
We thank Michigan State University laboratory animal support facilities for assistance in the humane care and maintenance of the animals used in this work. We specifically thank Dr. Sungjin Kim for generous advice and suggestions.
This work was supported by the MSU Foundation as well as the Osteopathic Heritage Foundation (to A.A.). Y.A.A. was supported by the King Abdullah bin Abdulaziz Scholarship, Ministry of Higher Education, Kingdom of Saudi Arabia.
Abbreviations used in this article:
- Ad
adenovirus
- BMDM
bone marrow-derived macrophage
- CRACC
CD2-like receptor activating cytotoxic cells
- DC
dendritic cell
- dpi
days postinjection
- EAT-2
EWS-FLI1–activated transcript 2
- ERT
EAT-2–related transducer
- hpi
hours postinjection
- SAP
SLAM-associated protein
- SLAM
signaling lymphocytic activation molecule
- vp
viral particle
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, and Adams E, et al. 2009. Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N. Engl. J. Med 361: 2209–2220. [DOI] [PubMed] [Google Scholar]
- 2.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al. ; Step Study Protocol Team. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372: 1881–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, et al. ; Step Study Protocol Team. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372: 1894–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, Bergen IM, Castillo R, Lambrecht BN, and Tschopp J. 2008. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol 181: 3755–3759. [DOI] [PubMed] [Google Scholar]
- 5.Mbow ML, De Gregorio E, Valiante NM, and Rappuoli R. 2010. New adjuvants for human vaccines. Curr. Opin. Immunol 22: 411–416. [DOI] [PubMed] [Google Scholar]
- 6.Wille-Reece U, Flynn BJ, Loré K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, and Seder RA. 2005. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc. Natl. Acad. Sci. USA 102: 15190–15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki A, and Medzhitov R. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol 5: 987–995. [DOI] [PubMed] [Google Scholar]
- 8.Pulendran B, and Ahmed R. 2006. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124: 849–863. [DOI] [PubMed] [Google Scholar]
- 9.Appledom DM, Aldhamen YA, Depas W, Seregin SS, Liu CJ, Schuldt N, Quach D, Quiroga D, Godbehere S, Zlatkin I, et al. 2010. A new adenovirus based vaccine vector expressing an Eimeria tenella derived TLR agonist improves cellular immune responses to an antigenic target. PLoS ONE 5: e9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartman ZC, Osada T, Glass O, Yang XY, Lei GJ, Lyerly HK, and Clay TM. 2010. Ligand-independent toll-like receptor signals generated by ectopic overexpression of MyD88 generate local and systemic antitumor immunity. Cancer Res. 70: 7209–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veillette A, Dong Z, Pérez-Quintero LA, Zhong MC, and Cruz-Munoz ME. 2009. Importance and mechanism of ‘switch’ function of SAP family adapters. Immunol. Rev 232: 229–239. [DOI] [PubMed] [Google Scholar]
- 12.Veillette A. 2006. Immune regulation by SLAM family receptors and SAP-related adaptors. Nat. Rev. Immunol 6: 56–66. [DOI] [PubMed] [Google Scholar]
- 13.Veillette A, Dong Z, and Latour S. 2007. Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes. Immunity 27: 698–710. [DOI] [PubMed] [Google Scholar]
- 14.Flaig RM, Stark S, and Watzl C. 2004. Cutting edge: NTB-A activates NK cells via homophilic interaction. J. Immunol 172: 6524–6527. [DOI] [PubMed] [Google Scholar]
- 15.Ma CS, Nichols KE, and Tangye SG. 2007. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu. Rev. Immunol 25: 337–379. [DOI] [PubMed] [Google Scholar]
- 16.Calpe S, Wang N, Romero X, Berger SB, Lanyi A, Engel P, and Terhorst C. 2008. The SLAM and SAP gene families control innate and adaptive immune responses. Adv. Immunol 97: 177–250. [DOI] [PubMed] [Google Scholar]
- 17.Veillette A. 2006. NK cell regulation by SLAM family receptors and SAP-related adapters. Immunol. Rev 214: 22–34. [DOI] [PubMed] [Google Scholar]
- 18.Bleharski JR, Niazi KR, Sieling PA, Cheng G, and Modlin RL. 2001. Signaling lymphocytic activation molecule is expressed on CD40 ligand-activated dendritic cells and directly augments production of inflammatory cytokines. J. Immunol 167: 3174–3181. [DOI] [PubMed] [Google Scholar]
- 19.Wang N, Satoskar A, Faubion W, Howie D, Okamoto S, Feske S, Gullo C, Clarke K, Sosa MR, Sharpe AH, and Terhorst C. 2004. The cell surface receptor SLAM controls T cell and macrophage functions. J. Exp. Med 199: 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeshita F, Tanaka T, Matsuda T, Tozuka M, Kobiyama K, Saha S, Matsui K, Ishii KJ, Coban C, Akira S, et al. 2006. Toll-like receptor adaptor molecules enhance DNA-raised adaptive immune responses against influenza and tumors through activation of innate immunity. J. Virol 80: 6218–6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartman ZC, Kiang A, Everett RS, Serra D, Yang XY, Clay TM, and Amalfitano A. 2007. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J. Virol 81: 1796–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appledom DM, McBride A, Seregin S, Scott JM, Schuldt N, Kiang A, Godbehere S, and Amalfitano A. 2008. Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors. Gene Ther. 15: 1606–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, and Vogelstein B. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95: 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ng P, and Graham FL. 2002. Construction of first-generation adenoviral vectors. Methods Mol. Med 69: 389–414. [DOI] [PubMed] [Google Scholar]
- 25.Appledom DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N, and Amalfitano A. 2008. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol 181: 2134–2144. [DOI] [PubMed] [Google Scholar]
- 26.Perfetto SP, Chattopadhyay PK, Lamoreaux L, Nguyen R, Ambrozak D, Koup RA, and Roederer M. 2006. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J. Immunol. Methods 313: 199–208. [DOI] [PubMed] [Google Scholar]
- 27.Seregin SS, Aldhamen YA, Appledom DM, Schuldt NJ, McBride AJ, Bujold M, Godbehere SS, and Amalfitano A. 2009. CR1/2 is an important suppressor of adenovirus-induced innate immune responses and is required for induction of neutralizing antibodies. Gene Ther: 16: 1245–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seregin SS, Appledorn DM, McBride AJ, Schuldt NJ, Aldhamen YA, Voss T, Wei J, Bujold M, Nance W, Godbehere S, and Amalfitano A. 2009. Transient pretreatment with glucocorticoid ablates innate toxicity of systemically delivered adenoviral vectors without reducing efficacy. Mol. Ther: 17: 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindsay RW, Darrah PA, Quinn KM, Wille-Reece U, Mattel LM, Iwasaki A, Kasturi SP, Pulendran B, Gall JG, Spies AG, and Seder RA. 2010. CD8+ T cell responses following replication-defective adenovirus serotype 5 immunization are dependent on CD11c+ dendritic cells but show redundancy in their requirement of TLR and nucleotide-binding oligomerization domain-like receptor signaling. J. Immunol 185: 1513–1521. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Huang X, and Yang Y. 2008. A critical role for type I IFN-dependent NK cell activation in innate immune elimination of adenoviral vectors in vivo. Mol. Ther: 16: 1300–1307. [DOI] [PubMed] [Google Scholar]
- 31.Muruve DA 2004. The innate immune response to adenovirus vectors. Hum. Gene Ther: 15: 1157–1166. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, Wilson JM, Schmieg J, Kronenberg M, Nakayama T, Taniguchi M, Koezuka Y, and Tsuji M. 2002. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J. Exp. Med 195: 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wille-Reece U, Flynn BJ, Loré K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, and Seder RA. 2006. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J. Exp. Med 203: 1249–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darrah PA Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med 13: 843–850. [DOI] [PubMed] [Google Scholar]
- 35.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107: 4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Huang CT, Huang X, and Pardoll DM. 2004. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat. Immunol 5: 508–515. [DOI] [PubMed] [Google Scholar]
- 37.Vitale M, Della Chiesa M, Carlomagno S, Pende D, Aricò M, Moretta L, and Moretta A. 2005. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood 106: 566–571. [DOI] [PubMed] [Google Scholar]
- 38.Mailliard RB, Son YI, Redlinger R, Coates PT, Giermasz A, Morel PA, Storkus WJ, and Kalinski P. 2003. Dendritic cells mediate NK cell help for Th1 and CTL responses: two-signal requirement for the induction of NK cell helper function. J. Immunol 171: 2366–2373. [DOI] [PubMed] [Google Scholar]
- 39.Piccioli D, Sbrana S, Melandri E, and Valiante NM. 2002. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J. Exp. Med 195: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martín-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, and Sallusto F. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol 5: 1260–1265. [DOI] [PubMed] [Google Scholar]
- 41.Kelly JM, Darcy PK, Markby JL, Godfrey DI, Takeda K, Yagita H, and Smyth MJ. 2002. Induction of tumor-specific T cell memory by NK cell-mediated tumor rejection. Nat. Immunol 3: 83–90. [DOI] [PubMed] [Google Scholar]
- 42.Krebs P, Barnes MJ, Lampe K, Whitley K, Bahjat KS, Beutler B, Janssen E, and Hoebe K. 2009. NK-cell-mediated killing of target cells triggers robust antigen-specific T-cell-mediated and humoral responses. Blood 113: 6593–6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cruz-Munoz ME, Dong Z, Shi X, Zhang S, and Veillette A. 2009. Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat. Immunol 10: 297–305. [DOI] [PubMed] [Google Scholar]
- 44.Tassi I, and Colonna M. 2005. The cytotoxicity receptor CRACC (CS-1) recruits EAT-2 and activates the PI3K and phospholipase Cgamma signaling pathways in human NK cells. J. Immunol 175: 7996–8002. [DOI] [PubMed] [Google Scholar]
- 45.Eissmann P, and Watzl C. 2006. Molecular analysis of NTB-A signaling: a role for EAT-2 in NTB-A-mediated activation of human NK cells. J. Immunol 177: 3170–3177. [DOI] [PubMed] [Google Scholar]
- 46.Fujii S, Shimizu K, Smith C, Bonifaz L, and Steinman RM. 2003. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med 198: 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermans IE, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, and Cerundolo V. 2003. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol 171: 5140–5147. [DOI] [PubMed] [Google Scholar]
- 48.Cerundolo V, Silk JD, Masri SH, and Salio M. 2009. Harnessing invariant NKT cells in vaccination strategies. Nat. Rev. Immunol 9: 28–38. [DOI] [PubMed] [Google Scholar]
- 49.Hermans IF, Silk JD, Gileadi U, Masri SH, Shepherd D, Farrand KJ, Salio M, and Cerundolo V. 2007. Dendritic cell function can be modulated through cooperative actions of TLR ligands and invariant NKT cells. J. Immunol 178: 2721–2729. [DOI] [PubMed] [Google Scholar]
- 50.Dondji B, Deak E, Goldsmith-Pestana K, Perez-Jimenez E, Esteban M, Miyake S, Yamamura T, and McMahon-Pratt D. 2008. Intradermal NKT cell activation during DNA priming in heterologous prime-boost vaccination enhances T cell responses and protection against Leishmania. Eur. J. Immunol 38: 706–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ostrakhovitch EA, Wang Y, and Li SS. 2009. SAP binds to CD22 and regulates B cell inhibitory signaling and calcium flux. Cell. Signal 21: 540–550. [DOI] [PubMed] [Google Scholar]
- 52.Li C, Chung B, Tao J, Iosef C, Aoukaty A, Wang Y, Tan R, and Li SS. 2008. The X-linked lymphoproliferative syndrome gene product SAP regulates B cell function through the FcgammaRIIB receptor. Cell. Signal 20: 1960–1967. [DOI] [PubMed] [Google Scholar]
- 53.Liu J, O’Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, Abbink P, Coffey RT, Grandpre LE, Seaman MS, et al. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kong WP, Wu L, Wallstrom TC, Fischer W, Yang ZY, Ko SY, Letvin NL, Haynes BF, Hahn BH, Korber B, and Nabel GJ. 2009. Expanded breadth of the T-cell response to mosaic human immunodeficiency virus type I envelope DNA vaccination. J. Virol 83: 2201–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.