Abstract
Practical relevance:
Trichomonosis of the large intestine of the cat was described as a cause of chronic diarrhea over 20 years ago. The trichomonad was identified as Tritrichomonas foetus, with a genotype that is distinct from venereal T foetus of cattle.
Clinical challenges:
Despite multiple means for diagnosis of the infection, including light microscopy, protozoal culture and PCR amplification using species-specific primers, tests with even greater sensitivity are needed. Feline trichomonosis is resistant to all commonly used antiprotozoal drugs. Ronidazole is currently the only drug demonstrated to be effective in eliminating the infection from cats; however, this drug has a narrow safety margin and clinical resistance is increasingly recognized. The more we learn about trichomonosis in cats, the more complicated and controversial the infection has become, ranging from what we should call the organism to whether we should even bother trying to treat it.
Global importance:
Feline trichomonosis is recognized to occur worldwide and is regarded as one of the most common infectious causes of colitis in the domestic cat. The infection is widespread in catteries and shelters; and, while remission of diarrhea may occur over time, persistence of the infection is common.
Evidence base:
This review provides a comprehensive examination of what is currently known about feline trichomonosis and pinpoints areas, based on the authors’ opinion, where further research is needed.
The culprit of infection
Trichomonads are members of the class Parabasalia, order Trichomonadida. These protozoa reside as parasites or commensals of animals, where they live in mucous membrane-lined, anaerobic-to-microaerophilic, non-sterile organ cavities such as the gastrointestinal and reproductive tracts. As is the case for most anaerobic protozoa, trichomonads lack many of their own biosynthetic pathways and rely on the salvage of nutrients from their host for survival. They are spindle to tear-drop shaped, highly motile flagellates, similar in size to Giardia species. Only trophozoites are present in the life cycle (no true cyst stage), division is by binary fission, and transmission occurs directly between hosts via ingestion of trophozoites.
Trophozoites bear characteristic numbers of anteriorly directed flagella. In addition there is a single, posteriorly directed flagellum that arises at the anterior end and courses along the body creating an undulating membrane, which is a characteristic feature. A rigid, rod-shaped organelle, the axostyle, runs through the trophozoite and protrudes from the posterior end (Figure 1).
Figure 1.
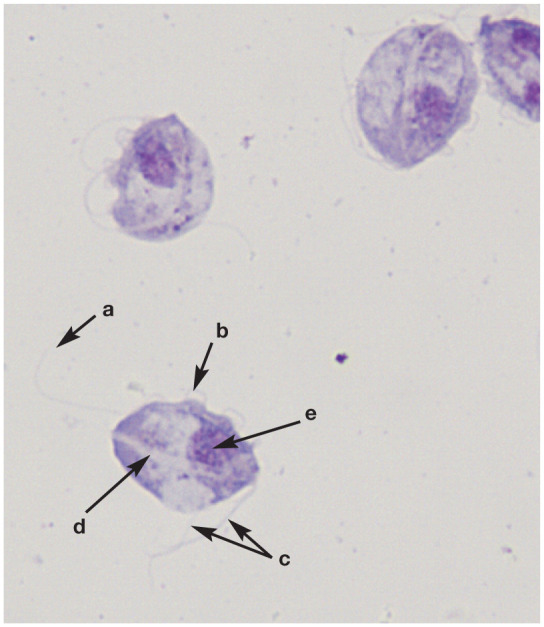
Cytological preparation of cultured feline Tritrichomonas species; note the posterior flagellum (a), undulating membrane (b), anterior flagella (c), axostyle (d) and nucleus (e). Wright-Giemsa, x100 magnification

Table 1.
Advances in recognition, diagnosis, treatment and understanding of disease pathogenesis of feline trichomonosis
| Year | Discovery | Reference(s) |
|---|---|---|
| 1996 | • Trichomonads described in feces of three Pixie-bob kittens with Colitis | 8,9 |
| 1999 | • Feline trichomonosis is refractory to treatment using common antiprotozoal drugs | 12 |
| 2001 | • Feline trichomonads molecularly identified as Tritrichomonas foetus | 1,14 |
| • Experimental infection of cats with feline T foetus results in diarrhea | 10 | |
| 2002 | • Culture and PCR methods optimized for diagnosis of feline T foetus infection | 15,16 |
| 2004 | • Prevalence of T foetus in US purebred cats reported to be 31% | 17 |
| 2005 | • Histological lesions described associated with feline T foetus infection | 11 |
| 2006 | • Ronidazole reported as effective for treatment of feline T foetus infection | 18 |
| 2007/2008 | • Cross-transmission studies between cats and cattle demonstrate biological and pathogenic differences between feline and bovine T foetus isolates | 19,20 |
| 2010 | • Feline T foetus and bovine T foetus demonstrated to be genetically distinct | 21–24 |
| 2013 | In vitro studies of feline T foetus identify cytopathic effects on intestinal epithelium | 25 |
| • Proposal to rename the feline trichomonad Tritrichomonas blagburni to distinguish it from the ‘cattle genotype’ of T foetus | 26 |
Epidemiology and transmission
Tritrichomonas species have now been described in cats residing in many countries, with prevalence of infection ranging from 2–59%.17,27–47 Trichomonosis is presumed to be transmitted from cat to cat via the fecal–oral route. Trichomonads do not form true cysts and therefore do not persist for more than a few hours under clean, dry and aerobic conditions. 15 Under laboratory conditions, trichomonads can survive for several days in moist feces 15 and up to 24 h in contaminated water or urine.48,49 Garden slugs fed canned cat food spiked with feline Tritrichomonas species can subsequently pass trichomonads in their feces for several days. While slugs are an improbable italic for normal dissemination of infection, these findings support the idea that transport of trichomonads from one time and location to another is possible through the intestinal tract of a second species. 50
T foetus is sexually transmitted in cattle; however, there is little evidence for venereal transmission of T foetus in cats. Studies conducted on the reproductive organs of purebred cats, where a high prevalence of intestinal T foetus infection was identified, found no light microscopic, immunohistochemical or molecular evidence of colonization by T foetus. 51 Infection of the feline uterus with T foetus was reported in one cat, although it was unclear whether T foetus was a primary or opportunistic pathogen. 52 One of the authors (JLG) has also observed and molecularly identified T foetus in purulent exudate from the uterus of a cat with pyometra, but this event is suspected to be rare. From these data, it is unlikely that reproductive tract infection by feline T foetus has a significant role in transmission of the disease in cats or is a frequent cause of reproductive tract pathology in breeding catteries.
Relationship between Tritichomonas species from different hosts
The question regarding the relationship between feline isolates of T foetus, bovine isolates of T foetus and porcine Tritrichomonas suis has been a italic of considerable debate. As discussed on page 263, several avenues have been pursued in an attempt to explore this.
Clinical presentation
Feline Tritrichomonas species infection is characterized by a waxing and waning large bowel diarrhea.12,72 The duration of diarrhea in the published literature ranges from 1 day to 8 years, and 59% of owners report that their cat has had diarrhea ‘since adoption’. 72 Frequency of bowel movements ranges from once to eight times a day; 72 the feces often contains mucus (59% of cats) and/or fresh blood (46% of cats), and there may be accompanying straining (43% of cats). 72 Diarrhea is typically semi-formed to ‘cow pie’ in consistency and malodorous. In most cases infected cats maintain good health, and a normal appetite and body condition, which presumably reflects confinement of the infection to the colon. However, some kittens will develop fecal incontinence, and overt swelling and inflammation of the anal region from fecal scalding.
Cats with diarrhea and concurrent trichomonosis are generally young (median age, 1 year),30,31,72,73 but have ranged in age from 1 month to 16 years.13,72 Older infected cats may be more likely to be clinically healthy or may have a long history of diarrhea dating back to kittenhood. Cats originating from catteries (ie, pedigreed) or shelters appear to be at increased risk for becoming infected,30,41,46 presumably because of dense housing conditions and increased likelihood of fecal–oral transmission. For example, in the USA the prevalence of trichomonosis among cats in large-scale hoarding operations is 39%, 74 among pedigreed cats is 31%, 17 and among mixed populations of pet cats ranges from 4–10%.33,75 There does not appear to be any sex predilection nor consistent reports in support of any specific breed predilections for trichomonosis in cats.
A common feature of Tritrichomonas species diarrhea is that during administration of antimicrobial drugs fecal consistency improves and trichomonads are difficult to detect, but diarrhea containing trichomonads reappears shortly after treatment is discontinued. 12 This is presumably due to a decrease in the population of bacteria in the colon on which the trichomonads thrive and not a direct effect of the antimicrobial drugs on trichomonad viability.
Misdiagnosis of Giardia species infection is common in cats with trichomonosis. Cats diagnosed with Giardia species based on observation of trophozoites on a direct fecal wet mount examination, and that fail to respond to appropriate antimicrobial therapy for Giardia species, should be re-evaluated for the possibility that the observed trophozoites were Tritrichomonas species.
Pathogenesis
Great strides have been made in the past 15 years in determining the molecular identity and genetics of feline Tritrichomonas species; and also in developing diagnostic tests and, to some extent, effective treatments for this infection. However, very little is known about how these organisms actually cause diarrhea. Based on what is understood about the pathogenic mechanisms of bovine T foetus in the reproductive tract, and what can be observed in cats infected with feline Tritrichomonas species, multiple factors are likely. 76
Pathogenic factors associated with trichomonosis include interaction with endogenous bacterial flora, adherence to host mucus and epithelium, and elaboration of cytotoxins and enzymes. 77 Infection of specific-pathogen-free cats with cultures of feline Tritrichomonas species results in chronic colonization of the terminal ileum, cecum and colon, and large bowel diarrhea similar to that observed in naturally infected cats. 10 In naturally infected cats, Tritrichomonas species reside within the superficial mucus and in contact with the surface epithelium of the cecum and colon. 11 Moreover, uptake of Tritrichomonas species specific antigen by the colonic surface epithelial cells can be demonstrated. 10 This is associated histologically with infiltration of lymphocytes, plasma cells and neutrophils into the colonic lamina propria. 11 In rare instances, trichomonads can be observed invading the subepithelium. 11 Studies have demonstrated that feline Tritrichomonas species isolates adhere directly to monolayers of intestinal epithelial cells in vitro by specific ligand–receptor interactions. 25 Cysteine proteases appear to mediate both adhesion and cytotoxic effects on the intestinal epithelium, and are being explored as possible treatment targets to ameliorate the clinical signs of infection.68,78,79
Large scale studies to define the transcriptome and proteome of Tritrichomonas species to identify virulence factors and to screen large libraries of drugs for effectiveness in killing Tritrichomonas species are actively ongoing by several teams of investigators.68,80
Diagnosis of feline trichomonosis
Feline Tritrichomonas species infection is diagnosed on the basis of identifying the organism on a fecal wet mount examination, after culture of feces in media that foster the growth of Tritrichomonas species, or by PCR performed on DNA extracted from a fecal sample (Table 2).
Table 2.
Approaches used for diagnosis of feline trichomonosis, with key attributes and limitations of each method
| Diagnostic approach | Techniques and considerations |
|---|---|
| Direct fecal wet mount | • Feces must be fresh and diarrheic • Use a scant amount of feces in saline and mount under a coverslip • Lower the microscope condenser • Look for motile flagellates – distinguish trichomonads from Giardia species trophozoites • Sensitivity is ≤14% for diagnosis of infection • Difficult to differentiate Tritrichomonas species from Pentatrichomonas hominis |
| Fecal culture | • Needs to be performed ‘in-house’ (shipping not recommended) • Only use a very small amount of feces (rice grain size) • Numbers of organisms and time needed for growth depends on temperature used for incubation • Sensitivity is approximately 55% for diagnosis of infection • Does not differentiate Tritrichomonas species from P hominis |
| PCR | • Can detect both live and dead organisms • Most sensitive means for diagnosis of infection • False-negative test results are likely common – avoid by collecting the ‘best’ fecal sample (see box on page 265) |
| Histopathology | • Trichomonads can be very difficult to identify without special detection methods • Suspected to be a very insensitive means for diagnosis of infection • Microscopic examination of multiple tissue sections is required • Pathologist should be made aware that trichomonosis is a differential diagnosis |
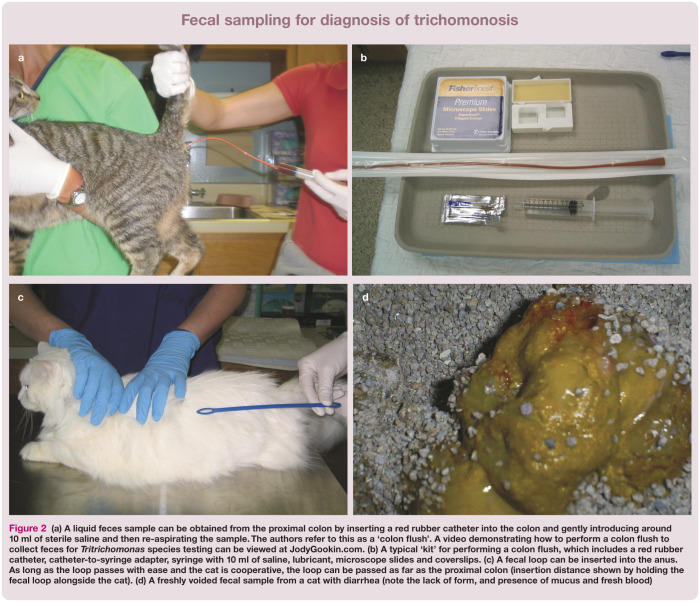
Tritrichomonas species cannot be detected by routine fecal analyses such as centrifugation flotation, and the organisms do not survive refrigeration. Suitable fecal samples may be obtained by (in order of preference): (1) passing a red rubber catheter into the proximal colon for the instillation and recovery of several milliliters of sterile saline; (2) inserting a fecal loop per rectum into the proximal colon; or (3) collecting a freshly voided specimen free of contaminating litter (Figure 2).
Figure 2.
(a) A liquid feces sample can be obtained from the proximal colon by inserting a red rubber catheter into the colon and gently introducing around 10 ml of sterile saline and then re-aspirating the sample. The authors refer to this as a ‘colon flush’. A video demonstrating how to perform a colon flush to collect feces for Tritrichomonas species testing can be viewed at JodyGookin.com. (b) A typical ‘kit’ for performing a colon flush, which includes a red rubber catheter, catheter-to-syringe adapter, syringe with 10 ml of saline, lubricant, microscope slides and coverslips. (c) A fecal loop can be inserted into the anus. As long as the loop passes with ease and the cat is cooperative, the loop can be passed as far as the proximal colon (insertion distance shown by holding the fecal loop alongside the cat). (d) A freshly voided fecal sample from a cat with diarrhea (note the lack of form, and presence of mucus and fresh blood)
For the colon flush technique, approximately 10 ml of sterile saline is flushed through the catheter into the colon and then gently aspirated. A drop of the recovered solution can then be examined directly under the microscope for trichomonads or placed in a fecal culture pouch. Alternatively, the solution can be sedimented in a centrifuge at approximately 2000 x g for 5 mins and the resulting fecal pellet submitted for PCR analysis.
Fecal samples should always be fresh, devoid of contaminating litter, and kept unrefrigerated before testing. If a fecal sample is being transported to the veterinary clinic or a diagnostic laboratory, survival of trichomonads can be extended by diluting the sample with saline to prevent desiccation (3 ml of 0.9% saline per 2 g of feces). 13 After 6 h of delay, analysis results for the sample will begin to lose diagnostic sensitivity for observation or culture of live organisms. 48 Samples obtained from non-diarrheic or dry stools are not suitable for use in testing for Tritrichomonas species and rarely yield positive test results, even if infection is present. Further, concurrent administration of antibacterial drugs at the time the sample is collected appears to decrease the success of finding Tritrichomonas species. Therefore, antimicrobial therapy of any type should be discontinued for a minimum of several days before collecting samples for testing.
Direct fecal wet mount
For the direct examination of feces for the presence of motile trophozoites, commonly referred to as a ‘wet mount’, a scant amount of feces is diluted with saline solution and examined under a coverslip using a light microscope equipped with a 20 x or 40 x objective. It is imperative that the fecal sample is diarrheic and fresh (preferably examined immediately after collecting from the cat), because visualization of trichomonads is highly dependent on their motility and therefore viability. If the feces are formed or aged then motile organisms are unlikely to be present and dead organisms are very difficult to identify. The most important thing to look for is motile flagellates. Lowering the microscope condenser will increase contrast and enhance visualization of trichomonads (Figure 3).
Figure 3.
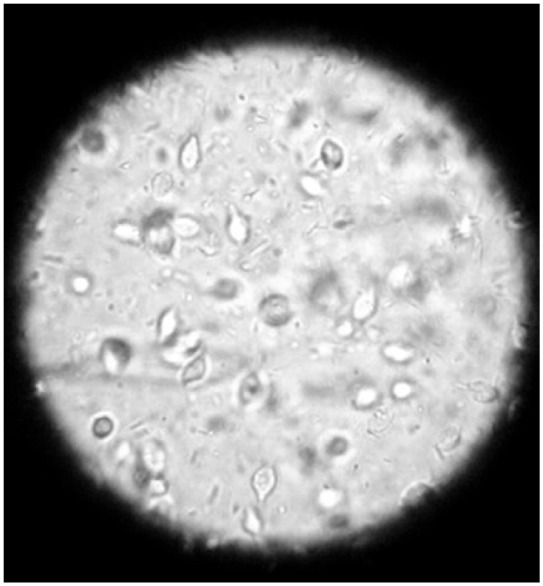
Fecal wet mount photographed through the ocular lens of a light microscope. Copious numbers of tear-drop shaped trichomonads can be observed in various depths within the saline solution. Courtesy of Dr Bronya Redden
While a direct fecal wet mount examination is the easiest way to make a diagnosis of trichomonosis in cats, it is also the least sensitive.10,17 In cats with asymptomatic, experimentally induced infection, only 2% of cases could be diagnosed on the basis of a fecal wet mount examination. 10 In cases of naturally occurring infection, diagnosis rates on the basis of a wet mount examination range from 2.6–14%.17,31,39 In other words, it is always worth performing a fecal wet mount examination because it might yield an easy diagnosis. However, due to low sensitivity, a negative wet mount examination cannot be relied upon to rule out trichomonosis. Moreover, Tritrichomonas species can be difficult to distinguish from Giardia species (see below) and also (what are assumed to be non-pathogenic) intestinal trichomonads such as P hominis based on light microscopic examination of live organisms. P hominis is diagnosed uncommonly in cats,81–83 and so feline trichomonads are generally presumed to be Tritrichomonas species. If desired, P hominis can be distinguished from Tritrichomonas species on the basis of species-specific P hominis PCR testing.16,81
Fecal culture
If repeated fecal wet mount examination results are negative for trichomonads, feces may be cultured using commercially available pouches (InPouch TF Feline; Biomed Diagnostics) 15 (Figure 4). Fecal culture using the InPouch TF is more sensitive than fecal wet mount examination for diagnosis of Tritrichomonas species infection; 17 approximately 55% of cats with naturally occurring Tritrichomonas species infection can be diagnosed using this culture system.15,17 The pouches are made of clear plastic and contain a proprietary culture medium and antibiotics that suppress unwanted growth of fecal bacteria.
Figure 4.
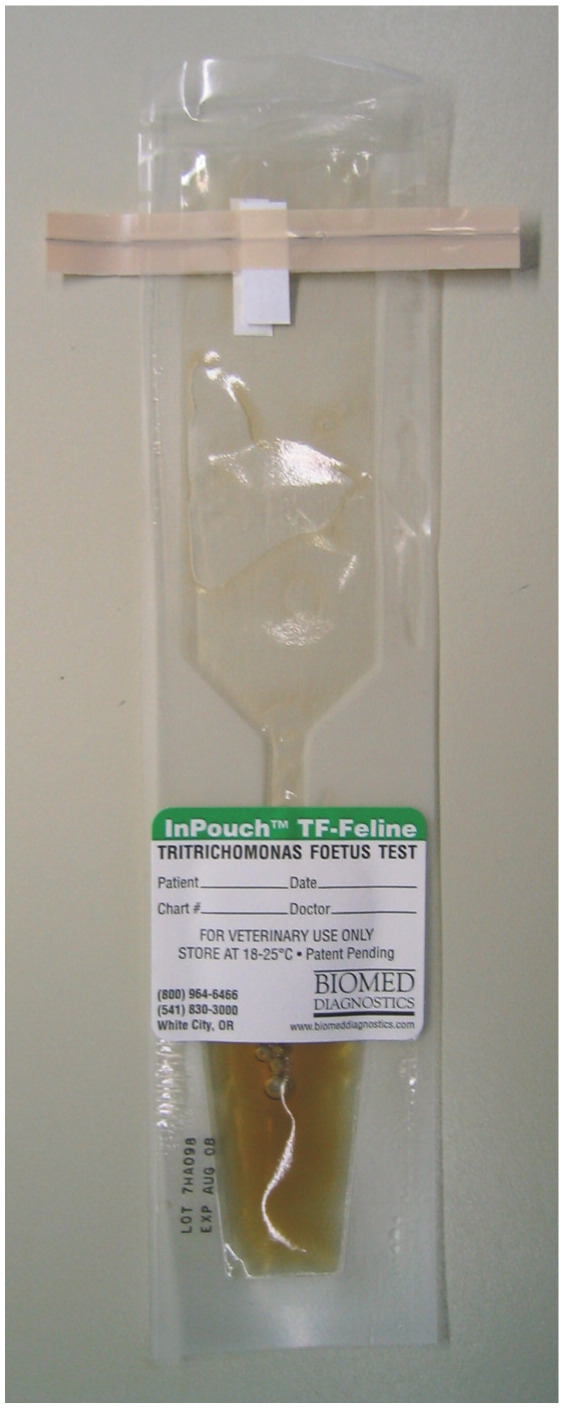
Pouch system for culture of feline Tritrichomonas species. A video demonstrating how to inoculate the pouch with a fecal sample for culture of Tritrichomonas species can be viewed at JodyGookin.com
For diagnosis of feline Tritrichomonas species, the pouches should be inoculated with 0.05 g of feces (approximately the size of a grain of rice) and incubated in an upright position in the dark at either 37°C (98.6°F) or room temperature (25°C [77°F]). At 37°C, the trichomonads will multiply quickly and many organisms can be observed by light microscopy within 72 h. Incubation at room temperature will produce fewer trichomonads, and it may take up to 12 days after inoculation to obtain positive results. The fecal sample must contain live organisms to obtain positive results from fecal culture, and optimum growth conditions for the organisms must be maintained during the test period. Therefore, care is required in handling the specimen and the pouch to avoid a false-negative result. It is strongly recommended that these cultures are performed in the clinic rather than by an external diagnostic laboratory to avoid the risk of the trichomonads dying during shipment of the fecal sample or pouch.
Trichomonads can be observed within the pouch by placing it directly on the stage of a light microscope under a 20 x or 40 x objective. Giardia species cannot survive in the culture medium for longer than 24 h 15 and therefore any trophozoites proliferating within the pouch can be presumed to be trichomonads. Both feline Tritrichomonas species and feline P hominis are able to survive in the InPouch TF medium. 82 P hominis, however, is considerably less common than Tritrichomonas species in cats.12,17,81 As mentioned, P hominis can be distinguished from Tritrichomonas species on the basis of species-specific P hominis PCR testing.16,81
Polymerase chain reaction
A sensitive and specific single-tube nested PCR based on amplification of a conserved portion of the Tritrichomonas species internal transcribed spacer region (ITS1 and ITS2) and 5.8S rRNA gene from feline feces has been described. 16 PCR-based testing is superior to fecal culture for diagnosis of naturally infected cats,17,73 can detect both live and dead organisms, and has an analytical sensitivity of 10 Tritrichomonas species organisms per 100 mg of fecal sample. 84
For PCR analysis, the authors prefer to submit feces in 70% isopropyl alcohol (ie, rubbing alcohol) in order to preserve the DNA and kill the fecal bacteria. This also allows the sample to be both held for several days and shipped at room temperature, which saves on the overall cost of PCR testing. Caution should be taken when submitting samples to a commercial laboratory that uses DNA extraction methods based on PCR diagnosis of trichomonosis in cattle. These methods may not be suited to extracting quality DNA from feline fecal samples. 84
A sensitive and specific PCR for amplification of P hominis 18S rRNA genes from DNA extracted from fecal samples is also commercially available. 81
Histopathology
Trichomonads can be observed by routine light microscopic methods in the lumen of crypts or within mucus lining the surface epithelium of the colon. However, because trichomonads are lumen-dwelling and extremely fragile, their preservation in intestinal biopsy specimens is highly unreliable. Trichomonads can also be difficult to differentiate from individually shed epithelial cells. It is advisable to indicate to the pathology service that Tritrichomonas species infection is a differential diagnosis, as a minimum of six tissue sections will need to be examined to achieve at least 95% confidence that any trichomonads would be identified. 11 Immunohistochemistry,10,11 fluorescence in situ hybridization 85 and chromogenic in situ hybridization 83 techniques have been described as means to enhance detection of Tritrichomonas species in histologic specimens, but are not commercially available. For tissue in which trichomonads are observed, DNA can be extracted from the formalin-fixed, paraffin-embedded specimen and used in PCR to identify the trichomonads as Tritrichomonas species. 85
Treatment of feline trichomonosis using ronidazole
Ronidazole (RDZ), a nitroimidazole similar to metronidazole (see box), is the only antimicrobial for which convincing efficacy for treatment of feline Tritrichomonas species infection has been demonstrated.18,31,72,87 Most cats with Tritrichomonas species infection show significant improvement in fecal consistency, or resolution of diarrhea, during the course of treatment with RDZ.
Pharmacology and pharmacokinetics
RDZ is not approved by the US Food and Drug Administration for human or veterinary use in the USA and is banned from use in food animals because of potential human hazards. Accordingly, due diligence is required to protect humans from exposure to RDZ, and veterinarians are advised to prescribe the drug only in cases of confirmed Tritrichomonas species infection after obtaining the owner’s informed consent. Several pharmacies compound chemical grade RDZ for veterinary use. Because of its foul taste and undetermined stability, compounding into gelatin capsules rather than flavored liquids is recommended. Several formulations of RDZ for treatment of trichomonosis in birds can be obtained without prescription from pigeon supply warehouses. However, these products are not recommended due to their undetermined quality, composition and low active drug concentration.
Studies investigating the pharmacokinetics of RDZ in cats suggest that 30 mg/kg PO q24h for 14 days is likely to be most effective in resolving diarrhea and eradicating Tritrichomonas species infection 88 (see box below). There is no evidence that higher or more frequent doses of RDZ, or administration over a longer period of time, is more effective. Following oral administration, RDZ is rapidly and completely absorbed by the gastrointestinal tract into the systemic circulation, where the drug has a long elimination half-life. This suggests that RDZ gains access to trichomonads in the colon by first entering the bloodstream. These properties likely predispose some cats to neurotoxicity, particularly with higher than recommended doses.
‘Colon-targeted’ formulations of RDZ have been investigated in an effort to increase the effectiveness and decrease the toxicity of RDZ.89–91 These formulations consist of encapsulating RDZ inside an indigestible coating of guar gum or chitosan. The coating prevents drug release until the capsule or tablet reaches the colon. Once in the colon, bacteria digest the coating and release RDZ directly at the site of infection.
Toxicity
Signs of RDZ neurotoxicity include lethargy, inappetence, ataxia and seizures. 92 Until recently, the prevalence of these side effects among cats undergoing treatment with RDZ was unknown. However, a newer retrospective study by Xenoulis et al reports discontinuation of RDZ in only 4/79 (5%) of cats due to development of anorexia or neurological signs. 93 Side effects of RDZ in cats generally resolve if the drug is withdrawn immediately, though may continue to worsen for the next few days before slowly subsiding, and may require costly and intensive emergency veterinary care.
Cats must be monitored closely while receiving RDZ. If signs of toxicity are observed, owners should be advised to discontinue treatment. Continuing treatment after onset of toxicity could result in life-threatening complications. RDZ should be avoided in cats with systemic illnesses that could confound recognition of adverse drug effects and should not be given to pregnant or nursing queens or their unweaned kittens. If treatment with RDZ must be discontinued due to clinical signs of toxicity, the cat should be retested for Tritrichomonas species infection. Many of these cats will have received sufficient RDZ to have cleared the infection.
Efficacy
Until recently, the efficacy of RDZ treatment of naturally infected cats under field conditions was unknown. In a retrospective study by Xenoulis et al, outcome data was reported for 45/79 cats diagnosed with Tritrichomonas species infection and undergoing treatment with RDZ at a dose of 30 mg/kg PO q24h. 93 A complete, or close to complete, resolution of diarrhea was reported for 64% (29/45) of cats and partial or no improvement or relapse of diarrhea in 36% (16/45) of cats. Among the cats not responding, 31% (5/16) were treated with less than the recommended dose or duration of RDZ. Follow-up testing for persistence of underlying Tritrichomonas species infection in these cats was not reported.
A study by Holliday et al reported 100% resolution or dramatic reduction in clinical signs of diarrhea in 24 cats with Tritrichomonas species treated with RDZ at 30 mg/kg PO q12h. 31 Upon completion of treatment, all cats were negative for Tritrichomonas species by culture of a rectal swab; however, positive results were observed in the only cat tested by means of PCR. Bell et al reported 100% resolution of diarrhea in 12 cats with Tritrichomonas species treated with RDZ at 30 mg/kg PO q12h. 36 After treatment, nine cats tested negative for Tritrichomonas species by means of PCR (seven cats) or fecal wet mount (two cats). Finally, Grellet et al reported treating 25 Tritrichomonas species infected cats with guar gum-coated RDZ at 30 mg/kg q24h for 14 days. After treatment, 21 cats (84%) tested negative for Tritrichomonas species by means of PCR performed on a fecal swab. The effect of RDZ on the clinical signs of diarrhea was not reported. 90
Based on these studies, a conservative estimate is that 60% of cats treated with RDZ will have close to complete resolution of clinical signs. What remains unclear is to what extent RDZ has eradicated vs merely suppressed the infection below the limit of detection. Additionally, small fecal sample sizes (eg, swab) and use of tests with lower sensitivity than PCR (wet mount or culture) are apt to increase false-negative test results in the aforementioned studies.
Post-treatment testing
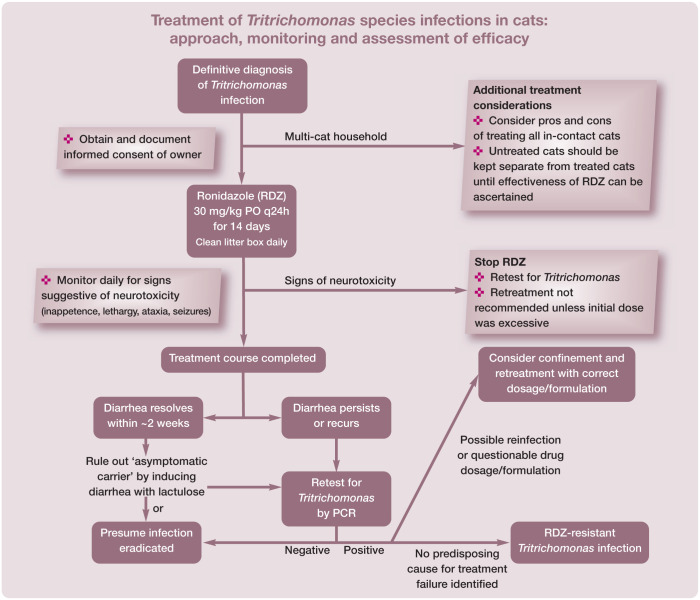
Determining whether RDZ has eliminated vs concealed Tritrichomonas species infection in any given cat remains an area of considerable frustration. If diarrhea persists or recurs ≥2 weeks after completion of RDZ treatment then cats should be retested for Tritrichomonas species by means of PCR performed on a fecal sample collected by the colon flush technique (see page 265). If this test result is negative in a cat with diarrhea then persistent infection is considered unlikely. Repeating this testing with confirmatory negative results would further support the absence of persistent infection.
The greatest difficulty arises in confidently ruling out persistent infection in a cat that no longer has diarrhea. In the authors’ experience, periods of asymptomatic infection are common in Tritrichomonas species infected cats and can be very difficult to diagnose. In cases where confirmation of Tritrichomonas species eradication is of particular relevance (eg, reintroduction of a treated cat into a cattery), the authors will treat the cat with lactulose ‘to effect’ to induce a soft diarrhea and then test a colon flush sample for Tritrichomonas species by means of PCR. Negative test results in this instance would come as close as possible to supporting the absence of infection.
Treatment failure
Treatment failure with RDZ should be established by confirmation of persistent Tritrichomonas species infection and not presumed on the basis of failure of the diarrhea to resolve. Some cats with persistent or recurrent diarrhea after treatment with RDZ may no longer have Tritrichomonas species infection. The cause of diarrhea and its clinical course in these cats remains unknown. In such cases a search for other infectious and non-infectious causes of diarrhea may be warranted, as well as empiric treatment with fenbendazole for occult parasitism. Common coinfections observed in pedigreed cats with Tritrichomonas species diarrhea include coronaviruses, Clostridium perfringens and Giardia species. 46
If Tritrichomonas species infection is confirmed following treatment with RDZ, three possibilities need to be considered (see box below). First, underdosing: the cat may have been administered an insufficient dose or duration of RDZ, or an impotent formulation of RDZ (eg, 10% powder formulation for pigeons); or it may have failed to consume sufficient amounts of the RDZ. These issues can be largely prevented by treating cats with gelatin capsule-compounded pure RDZ at the recommended dosage.
Second, treatment failure could be attributed to reinfection by another cat in the household that may or may not be showing clinical signs of diarrhea. It is a common misconception in multi-cat settings that only cats with diarrhea are infected with Tritrichomonas species. When presumed uninfected cats are allowed contact with cats that later fail treatment with RDZ, reinfection (whether likely or unlikely) can never be ruled out. This possibility can be prevented by confining Tritrichomonas species infected cats during treatment and until their treatment outcome can be assessed.
Finally, if other causes of treatment failure can be ruled out, persistent infection can likely be attributed to infection by a ‘strain’ of Tritrichomonas species that is resistant to RDZ. 94 The prevalence of RDZ-resistant Tritrichomonas species infection in cats is unknown, but suspected to be significant. While resistance can be documented in the laboratory, it can be largely assumed if treatment failure is observed in a cat that receives the appropriate dosage of RDZ and has not been exposed to other cats during or after treatment. In the authors’ experience, higher doses, more frequent administration or longer durations of treatment with RDZ have been ineffective in eradicating Tritrichomonas species from such cats and are apt to directly increase the risk of neurotoxicity.
Other avenues for treatment of feline trichomonosis
Other therapies for the treatment of Tritrichomonas species infection in cats are limited in their efficacy and anecdotal. Many approaches to control diarrhea have been tried without apparent success, including changes in diet, use of different antimicrobials, and supplementation with nutraceuticals and probiotics.12,13,69,95,96 However, there have been no well controlled studies of any of these therapies.
It has been suggested that frequent changes in diet and indiscriminate use of antimicrobials prolongs the time it takes for cats to resolve the diarrhea on their own. 13 Veterinarians should be cautious in embracing the success of any particular antimicrobial drug for treatment of Tritrichomonas species infection because many drugs merely suppress detection of the organisms rather than eradicate them.
To treat or not to treat
If left untreated, it is estimated that most cats (88%) with Tritrichomonas species infection will undergo spontaneous resolution of their diarrhea within 2 years (median 9 months; range 5 months to 2 years). 13 However, most of these cats will remain infected based on positive PCR test results for Tritrichomonas species. This suggests that cats do not develop an effective immune response to Tritrichomonas species infection and are incapable of eliminating the parasite. At present, no studies have been conducted to examine cats for the presence of any long-term adverse health effects of asymptomatic Tritrichomonas species infection.
Recently, the European Advisory Board on Cat Diseases recommended that treatment of Tritrichomonas species infection is indicated only in cats with diarrhea that are positive for the organism on direct fecal wet mount or culture. 97 It is the authors’ opinion that non-PCR approaches are too insensitive for use in routine diagnosis of the infection, even in cats with diarrhea. Moreover, the role of ‘asymptomatic carriers’ in disease transmission remains unclear. Asymptomatic cats can experience full relapses of diarrhea that is teeming with trichomonads as long as 6+ years after onset of their clinical ‘remission’. Accordingly, any cat harboring Tritrichomonas species, whether asymptomatic or not, should be considered a liability for transmission of infection and detection of such cats for the sake of preventing disease transmission appears warranted.
Key Points
Tritrichomonas foetus is a prevalent parasite of the feline large intestine that is recognized in cats worldwide.
The infection is chronic, can be challenging to diagnose, and is difficult to treat.
While research has come a long way in advancing our understanding of this infection, key unanswered questions remain (see box above).
We still know very little about how this pathogen causes diarrhea and are in urgent need of new, safe and effective therapeutics for the infection.
Acknowledgments
The authors acknowledge the indispensable contributions of the following individuals and funding agencies in support of research on feline trichomonosis: Edward Breitschwerdt, Adam Birkenheuer, Mark Papich, Katie Tolbert, Derek Foster, Dana LeVine, Marty Stebbins, Michael Yaeger, Mac Law, Stephen Stauffer, Maria Stone, Gigi Davidson, Mathew Poore, Henry Marr, Robin Gager, Judy Benrud, and the many veterinary students who coauthored much of the work presented here.
Footnotes
Funding: Funding for the authors’ research on feline trichomonosis has been received from the Morris Animal Foundation, Winn Feline Foundation, State of North Carolina Appropriated Research Funds, Fort Dodge Animal Health, Presutti Laboratories and North Carolina State University Veterinary Medical Foundation Support for Tritrichomonas species Research Innovation and Veterinary Education (STRIVE) Fund.
The corresponding author (JLG) offers a for-profit molecular diagnostic service for detection of feline Tritrichomonas species infection by means of PCR testing.
References
- 1. Levy MG, Gookin JL, Poore M, et al. Tritrichomonas foetus and not Pentatrichomonas hominis is the etiologic agent of feline trichomonal diarrhea. J Parasitol 2003; 89: 99–104. [DOI] [PubMed] [Google Scholar]
- 2. Da Cunha AM, Muniz J. Trabalhos do instituto Oswaldo Cruz: sobre um flagellado parasito do gato. Braz Med 1922; 36: 285–286. [Google Scholar]
- 3. Brumpt E. Recherches morphologiques et experimentales sur le Trichomonas felis da cunha et muniz, 1922, parasite du chat et du chien. Ann Parasitol 1925; 3: 239–251. [Google Scholar]
- 4. Simic T. Etude biologique et experimentale du Trichomonas intestinalis, infectant spontanement l’homme, le chat et le chien. Ann Parasitol 1932; 10: 209–224. [Google Scholar]
- 5. Kessel JF. Trichomoniasis in kittens. Trans Royal Soc Tropic Med Hyg 1928; 22: 61–80. [Google Scholar]
- 6. Hegner R, Eskridge L. Absence of pathogenicity in cats infected with Trichomonas felis from cats and Trichomonas hominis from man. Am J Hyg 1935; 22: 322–325. [Google Scholar]
- 7. Jordan HE. Trichomonas spp in feline: a case report. Vet Med 1956; 51: 23–24. [Google Scholar]
- 8. Romatowski J. An uncommon protozoan parasite (Pentatrichomonas hominis) associated with colitis in three cats. Feline Pract 1996; 24: 10–14. [Google Scholar]
- 9. Romatowski J. Pentatrichomonas hominis infection in four kittens. J Am Vet Med Assoc 2000; 216: 1270–1272. [DOI] [PubMed] [Google Scholar]
- 10. Gookin JL, Levy MG, Law JM, et al. Experimental infection of cats with Tritrichomonas foetus. Am J Vet Res 2001; 62: 1690–1697. [DOI] [PubMed] [Google Scholar]
- 11. Yaeger MJ, Gookin JL. Histologic features associated with Tritrichomonas foetus-induced colitis in domestic cats. Vet Pathol 2005; 42: 797–804. [DOI] [PubMed] [Google Scholar]
- 12. Gookin JL, Breitschwerdt EB, Levy MG, et al. Diarrhea associated with trichomonosis in cats. J Am Vet Med Assoc 1999; 215: 1450–1454. [PubMed] [Google Scholar]
- 13. Foster DM, Gookin JL, Poore MF, et al. Outcome of cats with diarrhea and Tritrichomonas foetus infection. J Am Vet Med Assoc 2004; 225: 888–892. [DOI] [PubMed] [Google Scholar]
- 14. Levy MG, Gookin JL, Poore MF, et al. Information on parasitic gastrointestinal tract infections in cats. J Am Vet Med Assoc 2001; 218: 194–195. [PubMed] [Google Scholar]
- 15. Gookin JL, Foster DM, Poore MF, et al. Use of a commercially available culture system for diagnosis of Tritrichomonas foetus infection in cats. J Am Vet Med Assoc 2003; 222: 1376–1379. [DOI] [PubMed] [Google Scholar]
- 16. Gookin JL, Birkenheuer AJ, Breitschwerdt EB, et al. Single-tube nested PCR for detection of Tritrichomonas foetus in feline feces. J Clin Microbiol 2002; 40: 4126–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gookin JL, Stebbins ME, Hunt E, et al. Prevalence of and risk factors for feline Tritrichomonas foetus and giardia infection. J Clin Microbiol 2004; 42: 2707–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gookin JL, Copple CN, Papich MG, et al. Efficacy of ronidazole for treatment of feline Tritrichomonas foetus infection. J Vet Intern Med 2006; 20: 536–543. [DOI] [PubMed] [Google Scholar]
- 19. Stockdale H, Rodning S, Givens M, et al. Experimental infection of cattle with a feline isolate of Tritrichomonas foetus. J Parasitol 2007; 93: 1429–1434. [DOI] [PubMed] [Google Scholar]
- 20. Stockdale HD, Dillon AR, Newton JC, et al. Experimental infection of cats (Felis catus) with Tritrichomonas foetus isolated from cattle. Vet Parasitol 2008; 154: 156–161. [DOI] [PubMed] [Google Scholar]
- 21. Reinmann K, Muller N, Kuhnert P, et al. Tritrichomonas foetus isolates from cats and cattle show minor genetic differences in unrelated loci ITS-2 and EF-1alpha. Vet Parasitol 2012; 185: 138–144. [DOI] [PubMed] [Google Scholar]
- 22. Slapeta J, Muller N, Stack CM, et al. Comparative analysis of Tritrichomonas foetus (Riedmuller, 1928) cat genotype, T. foetus (Riedmuller, 1928) cattle genotype and Tritrichomonas suis (Davaine, 1875) at 10 DNA loci. Int J Parasitol 2012; 42: 1143–1149. [DOI] [PubMed] [Google Scholar]
- 23. Sun Z, Stack C, Slapeta J. Sequence differences in the diagnostic region of the cysteine protease 8 gene of Tritrichomonas foetus parasites of cats and cattle. Vet Parasitol 2012; 186: 445–449. [DOI] [PubMed] [Google Scholar]
- 24. Slapeta J, Craig S, McDonell D, et al. Tritrichomonas foetus from domestic cats and cattle are genetically distinct. Exp Parasitol 2010; 126: 209–213. [DOI] [PubMed] [Google Scholar]
- 25. Tolbert MK, Stauffer SH, Gookin JL. Feline Tritrichomonas foetus adhere to intestinal epithelium by receptor-ligand-dependent mechanisms. Vet Parasitol 2013; 192: 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walden HS, Dykstra C, Dillon A, et al. A new species of Tritrichomonas (Sarcomastigophora: Trichomonida) from the domestic cat (Felis catus). Parasitol Res 2013; 112: 2227–2235. [DOI] [PubMed] [Google Scholar]
- 27. Burgener I, Frey C, Kook P, et al. Tritrichomonas fetus: a new intestinal parasite in Swiss cats [article in German]. Schweizer Arch Tierheilkd 2009; 151: 383–389. [DOI] [PubMed] [Google Scholar]
- 28. Bissett SA, Gowan RA, O’Brien CR, et al. Feline diarrhoea associated with Tritrichomonas cf. foetus and Giardia co-infection in an Australian cattery. Aust Vet J 2008; 86: 440–443. [DOI] [PubMed] [Google Scholar]
- 29. Bissett SA, Stone ML, Malik R, et al. Observed occurrence of Tritrichomonas foetus and other enteric parasites in Australian cattery and shelter cats. J Feline Med Surg 2009; 11: 803–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gunn-Moore DA, McCann TM, Reed N, et al. Prevalence of Tritrichomonas foetus infection in cats with diarrhoea in the UK. J Feline Med Surg 2007; 9: 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holliday M, Deni D, Gunn-Moore DA. Tritrichomonas foetus infection in cats with diarrhoea in a rescue colony in Italy. J Feline Med Surg 2009; 11: 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kingsbury DD, Marks SL, Cave NJ, et al. Identification of Tritrichomonas foetus and Giardia spp. infection in pedigree show cats in New Zealand. N Z Vet J 2010; 58: 6–10. [DOI] [PubMed] [Google Scholar]
- 33. Stockdale HD, Givens MD, Dykstra CC, et al. Tritrichomonas foetus infections in surveyed pet cats. Vet Parasitol 2009; 160: 13–17. [DOI] [PubMed] [Google Scholar]
- 34. van Doorn DC, de Bruin MJ, Jorritsma RA, et al. Prevalence of Tritrichomonas foetus among Dutch cats [article in Dutch]. Tijdschr Diergeneeskd 2009; 134: 698–700. [PubMed] [Google Scholar]
- 35. Kuehner KA, Marks SL, Kass PH, et al. Tritrichomonas foetus infection in purebred cats in Germany: prevalence of clinical signs and the role of co-infection with other enteroparasites. J Feline Med Surg 2011; 13: 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bell ET, Gowan RA, Lingard AE, et al. Naturally occurring Tritrichomonas foetus infections in Australian cats: 38 cases. J Feline Med Surg 2010; 12: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lim S, Park SI, Ahn KS, et al. First report of feline intestinal trichomoniasis caused by Tritrichomonas foetus in Korea. Korean J Parasitol 2010; 48: 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miro G, Hernandez L, Montoya A, et al. First description of naturally acquired Tritrichomonas foetus infection in a Persian cattery in Spain. Parasitol Res 2011; 109: 1151–1154. [DOI] [PubMed] [Google Scholar]
- 39. Tysnes K, Gjerde B, Nodtvedt A, et al. A cross-sectional study of Tritrichomonas foetus infection among healthy cats at shows in Norway. Acta Vet Scand 2011; 53: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Doi J, Hirota J, Morita A, et al. Intestinal Tritrichomonas suis (=T. foetus) infection in Japanese cats. J Vet Med Sci 2012; 74: 413–417. [DOI] [PubMed] [Google Scholar]
- 41. Hosein A, Kruth SA, Pearl DL, et al. Isolation of Tritrichomonas foetus from cats sampled at a cat clinic, cat shows and a humane society in southern Ontario. J Feline Med Surg 2013; 15: 706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Profizi C, Cian A, Meloni D, et al. Prevalence of Tritrichomonas foetus infections in French catteries. Vet Parasitol 2013; 196: 50–55. [DOI] [PubMed] [Google Scholar]
- 43. Raab O, Greenwood S, Vanderstichel R, et al. A cross- sectional study of Tritrichomonas foetus infection in feral and shelter cats in Prince Edward Island, Canada. Can Vet J 2016; 57: 265–270. [PMC free article] [PubMed] [Google Scholar]
- 44. Hinney B, Ederer C, Stengl C, et al. Enteric protozoa of cats and their zoonotic potential – a field study from Austria. Parasit Res 2015; 114: 2003–2006. [DOI] [PubMed] [Google Scholar]
- 45. Dabrowska J, Karamon J, Kochanowski M, et al. Tritrichomonas foetus infection in cat – first detection in Poland. Acta Parasitol 2015; 60: 605–608. [DOI] [PubMed] [Google Scholar]
- 46. Paris JK, Wills S, Balzer HJ, et al. Enteropathogen co-infection in UK cats with diarrhoea. BMC Vet Res 2014; 10: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mancianti F, Nardoni S, Mugnaini L, et al. A retrospective molecular study of select intestinal protozoa in healthy pet cats from Italy. J Feline Med Surg 2015; 17: 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hale S, Norris JM, Slapeta J. Prolonged resilience of Tritrichomonas foetus in cat faeces at ambient temperature. Vet Parasitol 2009; 166: 60–65. [DOI] [PubMed] [Google Scholar]
- 49. Rosypal AC, Ripley A, Stockdale Walden HD, et al. Survival of a feline isolate of Tritrichomonas foetus in water, cat urine, cat food and cat litter. Vet Parasitol 2012; 185: 279–281. [DOI] [PubMed] [Google Scholar]
- 50. Van der Saag M, McDonell D, Slapeta J. Cat genotype Tritrichomonas foetus survives passage through the alimentary tract of two common slug species. Vet Parasitol 2011; 177: 262–266. [DOI] [PubMed] [Google Scholar]
- 51. Gray SG, Hunter SA, Stone MR, et al. Assessment of reproductive tract disease in cats at risk for Tritrichomonas foetus infection. Am J Vet Res 2010; 71: 76–81. [DOI] [PubMed] [Google Scholar]
- 52. Dahlgren SS, Gjerde B, Pettersen HY. First record of natural Tritrichomonas foetus infection of the feline uterus. J Small Anim Pract 2007; 48: 654–657. [DOI] [PubMed] [Google Scholar]
- 53. Riedmuller L. Ueber die morphologie, uebertragungsversuche und klinische bedeutung der beim sporadishchen abortus des rindes vorkommenden trichomonaden. Zentralbl Bakteriol I Abt Orig 1928; 108: 103–118. [Google Scholar]
- 54. BonDurant RH, Honigberg BM. Parasitic protozoa. New York: Academic Press, 1994. [Google Scholar]
- 55. Gruby D, Delafond O. Recherches sur les animalcules se développanyt en grand nombre dan l’estomac dans les intestins, pendant la digestion des animaux herbivores et carnivores. Extrant d’une note CR Acad Sci Paris 1843; 17: 1304–1308. [Google Scholar]
- 56. Brion A, Cottereau P. Presence of Trichomonas in the nasal cavities of swine with atrophic rhinitis [article in French]. C R Seances Soc Biol Fil 1954; 148: 1415–1416. [PubMed] [Google Scholar]
- 57. Spindler LA, Shorb DA, Hill CH. The role of trichomonads in atrophic rhinitis of swine. J Am Vet Med Assoc 1953; 122: 151–157. [PubMed] [Google Scholar]
- 58. Backstrŏm L. Atrophic rhinitis in swine. Agri-Practice 1992; 13: 21–24. [Google Scholar]
- 59. Runnels LJ. Infectious atrophic rhinitis of swine. Vet Clin North Am Large Anim Pract 1982; 4: 301–319. [DOI] [PubMed] [Google Scholar]
- 60. Tachezy J, Tachezy R, Hampl V, et al. Cattle pathogen Tritrichomonas foetus (Riedmuller, 1928) and pig commensal Tritrichomonas suis (Gruby & Delafond, 1843) belong to the same species. J Eukaryot Microbiol 2002; 49: 154–163. [DOI] [PubMed] [Google Scholar]
- 61. Morin-Adeline V, Mueller K, Conesa A, et al. Comparative RNA-seq analysis of the Tritrichomonas foetus PIG30/1 isolate from pigs reveals close association with Tritrichomonas foetus BP-4 isolate ‘bovine genotype’. Vet Parasitol 2015; 212: 111–117. [DOI] [PubMed] [Google Scholar]
- 62. Cobo ER, Cano D, Campero CM. Experimental infection with Tritrichomonas suis in heifers. Vet Parasitol 2001; 99: 73–78. [DOI] [PubMed] [Google Scholar]
- 63. Kerr WR. Experiments in cattle with Tritrichomonas suis. Vet Rec 1958; 70: 613–615. [Google Scholar]
- 64. Fitzgerald PR, Johnson AE, Hammond DM, et al. Experimental infection of young pigs following intranasal inoculation with nasal, gastric, or cecal trichomonads from swine or with Trichomonas foetus. J Parasitol 1958; 44: 597–602. [PubMed] [Google Scholar]
- 65. Lun ZR, Chen XG, Zhu XQ, et al. Are Tritrichomonas foetus and Tritrichomonas suis synonyms? Trends Parasitol 2005; 21: 122–125. [DOI] [PubMed] [Google Scholar]
- 66. Frey CF, Muller N. Tritrichomonas – systematics of an enigmatic genus. Mol Cell Probes 2012; 26: 132–136. [DOI] [PubMed] [Google Scholar]
- 67. Mueller K, Morin-Adeline V, Gilchrist K, et al. High prevalence of Tritrichomonas foetus ‘bovine genotype’ in faecal samples from domestic pigs at a farm where bovine trichomonosis has not been reported for over 30 years. Vet Parasitol 2015; 212: 105–110. [DOI] [PubMed] [Google Scholar]
- 68. Morin-Adeline V, Lomas R, O’Meally D, et al. Comparative transcriptomics reveals striking similarities between the bovine and feline isolates of Tritrichomonas foetus: consequences for in silico drug-target identification. BMC Genomics 2014; 15: 955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gookin JL, Stauffer SH, Coccaro MR, et al. Efficacy of tinidazole for treatment of cats experimentally infected with Tritrichomonas foetus. Am J Vet Res 2007; 68: 1085–1088. [DOI] [PubMed] [Google Scholar]
- 70. Doi J, Abe N, Oku Y. Molecular survey of Tritrichomonas suis (=T. foetus) ‘cat’ and ‘cattle’ genotypes in pigs in Japan. J Vet Med Sci 2013; 75: 475–479. [DOI] [PubMed] [Google Scholar]
- 71. Rhyan JC, Blanchard PC, Kvasnicka WG, et al. Tissue-invasive Tritrichomonas foetus in four aborted bovine fetuses. J Vet Diagn Invest 1995; 7: 409–412. [DOI] [PubMed] [Google Scholar]
- 72. Xenoulis PG, Lopinski DJ, Read SA, et al. Intestinal Tritrichomonas foetus infection in cats: a retrospective study of 104 cases. J Feline Med Surg 2013: 15; 1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Arranz-Solis D, Pedraza-Diaz S, Miro G, et al. Tritrichomonas foetus infection in cats with diarrhea from densely housed origins. Vet Parasitol 2016; 221: 118–122. [DOI] [PubMed] [Google Scholar]
- 74. Polak KC, Levy JK, Crawford PC, et al. Infectious diseases in large-scale cat hoarding investigations. Vet J 2014; 201: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Queen EV, Marks SL, Farver TB. Prevalence of selected bacterial and parasitic agents in feces from diarrheic and healthy control cats from Northern California. J Vet Intern Med 2012; 26: 54–60. [DOI] [PubMed] [Google Scholar]
- 76. Tolbert MK, Gookin JL. Mechanisms of Tritrichomonas foetus pathogenicity in cats with insights from venereal trichomonosis. J Vet Intern Med 2016; 30: 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Felleisen RS. Host-parasite interaction in bovine infection with Tritrichomonas foetus. Microbes Infect 1999; 1: 807–816. [DOI] [PubMed] [Google Scholar]
- 78. Tolbert MK, Stauffer SH, Brand MD, et al. Cysteine protease activity of feline Tritrichomonas foetus promotes adhesion-dependent cytotoxicity to intestinal epithelial cells. Infect Immun 2014; 82: 2851–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tolbert MK, Brand MD, Gould EN. In vitro effects of cysteine protease inhibitors on Trichomonas foetus-induced cytopathic changes in porcine intestinal epithelial cells. Am J Vet Res 2016; 77: 890–897. [DOI] [PubMed] [Google Scholar]
- 80. Huang KY, Shin JW, Huang PJ, et al. Functional profiling of the Tritrichomonas foetus transcriptome and proteome. Mol Biochem Parasitol 2013; 187: 60–71. [DOI] [PubMed] [Google Scholar]
- 81. Gookin JL, Stauffer SH, Levy MG. Identification of Pentatrichomonas hominis in feline fecal samples by polymerase chain reaction assay. Vet Parasitol 2007; 145: 11–15. [DOI] [PubMed] [Google Scholar]
- 82. Ceplecha V, Svoboda M, Cepicka I, et al. InPouch TF-Feline medium is not specific for Tritrichomonas foetus. Vet Parasitol 2013; 196: 503–505. [DOI] [PubMed] [Google Scholar]
- 83. Mostegl MM, Wetscher A, Richter B, et al. Detection of Tritrichomonas foetus and Pentatrichomonas hominis in intestinal tissue specimens of cats by chromogenic in situ hybridization. Vet Parasitol 2012; 183: 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Stauffer SH, Birkenheuer AJ, Levy MG, et al. Evaluation of four DNA extraction methods for the detection of Tritrichomonas foetus in feline stool specimens by polymerase chain reaction. J Vet Diagn Invest 2008; 20: 639–641. [DOI] [PubMed] [Google Scholar]
- 85. Gookin JL, Stone MR, Yaeger MJ, et al. Fluorescence in situ hybridization for identification of Tritrichomonas foetus in formalin-fixed and paraffin-embedded histological specimens of intestinal trichomoniasis. Vet Parasitol 2010; 172: 139–143. [DOI] [PubMed] [Google Scholar]
- 86. Kather EJ, Marks SL, Kass PH. Determination of the in vitro susceptibility of feline Tritrichomonas foetus to 5 antimicrobial agents. J Vet Intern Med 2007; 21: 966–970. [DOI] [PubMed] [Google Scholar]
- 87. Lim S, Park SI, Ahn KS, et al. Efficacy of ronidazole for treatment of cats experimentally infected with a Korean isolate of Tritrichomonas foetus. Korean J Parasitol 2012; 50: 161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. LeVine DN, Papich MG, Gookin JL, et al. Ronidazole pharmacokinetics after intravenous and oral immediate-release capsule administration in healthy cats. J Feline Med Surg 2011; 13: 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Papich MG, Levine DN, Gookin JL, et al. Ronidazole pharmacokinetics in cats following delivery of a delayed-release guar gum formulation. J Vet Pharmacol Ther 2013; 36: 399–407. [DOI] [PubMed] [Google Scholar]
- 90. Grellet A, Makhlouf SE, Desquilbet L, et al. Efficacy of guar gum-based ronidazole capsules as a treatment for Tritrichomonas foetus infection in cats. J Feline Med Surg. Epub ahead of print 10 December 2015. DOI: 1098612X15621353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kavianinia I, Plieger PG, Cave NJ, et al. Design and evaluation of a novel chitosan-based system for colon-specific drug delivery. Int J Biol Macromol 2016; 85: 539–546. [DOI] [PubMed] [Google Scholar]
- 92. Rosado TW, Specht A, Marks SL. Neurotoxicosis in 4 cats receiving ronidazole. J Vet Intern Med 2007; 21: 328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Xenoulis PG, Lopinski DJ, Read SA, et al. Intestinal Tritrichomonas foetus infection in cats: a retrospective study of 104 cases. J Feline Med Surg 2013; 15: 1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gookin JL, Stauffer SH, Dybas D, et al. Documentation of in vivo and in vitro aerobic resistance of feline Tritrichomonas foetus isolates to ronidazole. J Vet Intern Med 2010; 24: 1003–1007. [DOI] [PubMed] [Google Scholar]
- 95. Gookin JL, Riviere JE, Gilger BC, et al. Acute renal failure in four cats treated with paromomycin. J Am Vet Med Assoc 1999; 215: 1821–1823. [PubMed] [Google Scholar]
- 96. Levy MG, Gookin J, Poore MF, et al. Intestinal trichomonosis in cats: pathology, diagnosis and susceptibility to antiprotozoal drugs. Proceedings of the Joint Meeting of the American Society of Parasitologists and the Society of Protozoologists; San Juan, Puerto Rico, 2000, p 108. [Google Scholar]
- 97. Gruffydd-Jones T, Addie D, Belak S, et al. Tritrichomoniasis in cats: ABCD guidelines on prevention and management. J Feline Med Surg 2013; 15: 647–649. [DOI] [PMC free article] [PubMed] [Google Scholar]