Abstract
The mixed results from recent vaccine clinical trials targeting HIV-1 justify the need to enhance the potency of HIV-1 vaccine platforms in general. Use of first-generation recombinant adenovirus serotype 5 (rAd5) platforms failed to protect vaccinees from HIV-1 infection. One hypothesis is that the rAd5-based vaccine failed due to the presence of pre-existing Ad5 immunity in many vaccines. We recently confirmed that EAT-2–expressing rAd5 vectors uniquely activate the innate immune system and improve cellular immune responses against rAd5-expressed Ags, inclusive of HIV/Gag. In this study, we report that use of the rAd5-EAT-2 vaccine can also induce potent cellular immune responses to HIV-1 Ags despite the presence of Ad5-specific immunity. Compared to controls expressing a mutant SH2 domain form of EAT-2, Ad5 immune mice vaccinated with an rAd5-wild-type EAT-2 HIV/Gag-specific vaccine formulation significantly facilitated the induction of several arms of the innate immune system. These responses positively correlated with an improved ability of the vaccine to induce stronger effector memory T cell-biased, cellular immune responses to a coexpressed Ag despite pre-existing anti-Ad5 immunity. Moreover, inclusion of EAT-2 in the vaccine mixture improves the generation of polyfunctional cytolytic CD8+ T cell responses as characterized by enhanced production of IFN-γ, TNF-α, cytotoxic degranulation, and increased in vivo cytolytic activity. These data suggest a new approach whereby inclusion of EAT-2 expression in stringent human vaccination applications can provide a more effective vaccine against HIV-1 specifically in Ad5 immune subjects.
Human immunodeficiency virus/AIDS continues to be a significant health threat both in the United States and worldwide. The number of people globally living with HIV-1 was estimated to be 33.3 million in 2009 (1). HIV-1 infection is associated with high morbidity and mortality, contributing to 2 million deaths and 2.7 million people being newly infected worldwide (1). These facts emphasize the continued need to develop safe, effective, and easily administered vaccines against HIV-1. Most recently, a human clinical trial demonstrated that a prophylactic vaccine to HIV-1 may indeed be possible (2, 3). However, the results of that trial combined with some of the results derived from the Merck-sponsored STEP trial suggest that a more potent vaccine, capable of inducing greater levels of Ag-specific adaptive immune responses to HIV-1 Ags, will be needed to effectively prevent HIV-1 infections (4). Development of vaccines capable of inducing both HIV-1–specific neutralizing Ab as well as T cell responses (the latter in the hopes of reducing HIV-1 viral replication postinfection) has been a focus of HIV-1–targeted vaccine research in recent years (5-7). In particular, induction of potent effector memory T (TEM) lymphocyte responses by vaccines is hypothesized to be much more effective to protect vaccinees from infection with low levels of infectious virus typically present in the first hours and days following initial HIV-1/SIV exposure (8-10).
Recombinant adenovirus serotype 5 (rAd5)-based vaccines expressing several HIV-1 Ags have been shown to induce HIV-1–specific humoral, as well as potent cellular, immune responses in human trials (11, 12). However, pre-existing immunity against Ad5 is present in 50–90% of normal human adults (13). In vaccines given HIV-1–targeted Ad vaccines, the presence of pre-existing anti-Ad5 humoral and cellular immunity may have limited the overall efficacy of Ad5 HIV-1 vaccines in trial participants (11, 12, 14). Several approaches have therefore been developed to circumvent pre-existing anti-Ad5 immunity and/or to facilitate the induction of potent adaptive immune responses by Ad-based vaccines in general. These approaches include the use of novel Ad5-based platforms (15, 16), modification of Ad5 structure (17), and the development of alternative serotype (human- or chimpanzee-derived) adenovirus-based vectors (18).
We have previously reported that an rAd5-based vaccine platform that expresses the signaling lymphocyte activation molecule (SLAM) family of receptors adaptor molecule, EAT-2, have superior qualities relative to current generation rAd5-based vaccines (19, 20). In recent years, there has been accumulating evidence implicating a critical role for SLAM family of receptors and signaling lymphocyte activation molecule-associated protein (SAP) family of adaptors in regulating both innate and adaptive immunity (21). The members of the SLAM family of receptors were first identified as adhesion molecules on the surface of several hematopoietic cells. These receptors function as costimulatory molecules that initiate distinct signal-transduction networks in T cells, NK cells, and APCs (22). However, further functional and structural studies have demonstrated that SLAM receptors also function as critical regulators of immune cells, including NK cells, dendritic cells (DCs), neutrophils, macrophages, and platelets (22). Because EAT-2 is the only known SLAM-associated adaptor protein expressed in DCs and macrophages, it has been proposed that EAT-2 facilitates SLAM-dependent immunoregulatory function (such as proinflammatory cytokine expression) in these cell types (22). Based on these facts, we demonstrated that expression of EAT-2 from a vaccine platform triggers early activation of innate immune cells in vivo and consequently improved the induction of Ag-specific adaptive immune responses (19, 20). Specifically, Ad vaccine-mediated EAT-2 expression improved elaboration of beneficial cytokine and chemokine responses, enhanced the early activation of NK and NKT cells, and improved APC function by upregulating the expression of maturation markers, including CD40, CD80, CD86, MHC class II, and CCR7 (19). Improvements in innate immune system recruitment correlated with induction of superior cytolytic CD8+ T cell-mediated immune responses to several Ags, including HIV-1/Gag (19) and the malaria circumsporozoite protein (20).
Because EAT-2 functions as a potent T cell stimulator, we decided to examine the ability of rAd5-EAT-2 vaccines to induce potent HIV-1/Gag–specific cellular immune responses in the presence of pre-existing anti-Ad5–specific immunity, a situation that limits the induction of Ag-specific T cell responses by Ad vaccines in general (23). We also wanted to examine the role of the EAT-2 Src homology (SH)2 domain in modulating the innate and adaptive immune responses to a coadministered target Ag delivered by an Ad-based vaccine.
Materials and Methods
Vector construction
The Ad5-GFP, Ad5-null, Ad5-HIV/Gag, and Ad5-EAT-2 viruses were purified as previously described (19, 24). The rAd5-EAT-2(R31Q) vector is identical to the rAd5-EAT-2 virus, except it additionally contains an arginine 31 to glutamine (EAT-2-R31Q) mutation in the EAT-2 SH2 domain constructed as follows. Mutation at the indicated site was designed by using Vector NTI (Invitrogen, Carlsbad, CA). The open reading frame of the Eat-2 gene (GenBank accession no. NM_012009; http://www.ncbi.nlm.nih.gov/nuccore/148747581) was excised using primers flanked by XhoI and XbaI restriction endonucleases (New England BioLabs, Ipswich, MA) from a plasmid (Biomatik, Wilmington, DE) and subcloned into the pShuttle vector, which contains a CMV expression cassette. Direct sequencing and restriction enzyme mapping were carried out to confirm the integrity of the EAT-2(R31Q) sequence. The resulting pAdTrack-EAT-2 (R31Q) shuttle plasmid was linearized with PmeI restriction enzyme and homologously recombined with the pAdEasyI Ad5 vector genome, yielding pAd5-EAT-2(R31Q). HEK293 cells were transfected with PacI-linearized plasmid, and viable virus was obtained and amplified after several rounds of expanding infection. rAd5-EAT-2(R31Q) virus was purified using a CsCl2 gradient as previously described (25). To confirm that rAd5-EAT-2(R31Q) vector expresses stable protein levels of EAT-2, we performed flow cytometry analysis to validate the expression of EAT-2 protein following rAd5-EAT-2 or rAd5-EAT-2(R31Q) infection. At 72 h after infection, similar levels of EAT-2 protein were detected in both rAd5-EAT-2– and rAd5-EAT-2 (R31Q)–infected RAW264.7 cells (Supplemental Fig. 1).
All viruses were found to be replication-competent adenovirus-free by both replication-competent adenovirus PCR (E1 region amplification) and direct sequencing methods as previously described (26).
Animal procedures
All animal procedures were approved by the Michigan State University Institutional Animal Care and Use Committee. Adult male wild-type (WT) BALB/cJ and C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). For innate immune cell responses, i.m. injection of animals (8–10 wk old) consisted of injection (into the tibialis anterior of the right hind limb) of 30 μl PBS solution (pH 7. 4) containing 2 × 1010 total virus particles (vps) of either rAd5-GFP, Ad5-EAT-2, or rAd5-EAT-2 (R31Q) as previously described (27). Plasma and tissue samples were obtained and processed at the indicated times after injection as previously described (27). rAd5-null (an [E1-,E3-]Ad5 vector not expressing any Ag) (two doses, in a 2-wk interval, of 1× 1010 vps) was injected i.m. in the right hind limb in a total of 30 μl PBS to generate animals with pre-existing Ad5 immunity (Ad5 preimmune animals) (Supplemental Fig. 2). Two immunizations with Ad5-null induced Ad5-neutralizing Ab titers that were >1/200, a level that closely parallels levels of pre-existing Ad5 immunity noted in human populations as previously described (26, 28, 29). To confirm that experimental and control groups developed equivalent amounts of anti-Ad5–specific immunity, blood samples were collected 1 d before vaccination (27 d after rAd5-null injections), and ELISA analysis for total Ad5-specific IgG was completed. Both experimental and control groups developed equal amounts of total Ad5-specific IgG Abs (Supplemental Fig. 3A). Fourteen days following the last Ad5-null injection, mice were randomly separated into three groups and i.m. vaccinated with rAd5-HIV/Gag+ rAd5-GFP, rAd5-HIV/Gag+ rAd5-EAT-2, or rAd5-HIV/Gag+ rAd5-EAT-2 (R31Q) viruses (1 × 109 total vps). Mice were boosted with the similar doses at the indicated time points (Supplemental Fig. 2).
Isolation of splenocytes
Splenocytes from individual mice were harvested and processed as follows: spleen tissues were physically disrupted by passage through a 40-μm sieve, followed by RBC lysis using 2 ml ACK lysis buffer (Invitrogen) per homogenized spleen. Splenocytes were subsequently washed twice with RPMI 1640 (Invitrogen) supplemented with 10% FBS, 2 mM l-glutamine, and 1% penicillin/streptomycin/fungizone and then resuspended and counted.
In vitro cell culture
Murine RAW264.7 macrophages (ATCC TIB71) were maintained in DMEM supplemented with 10% FBS and penicillin/streptomycin following standard procedures. Suspensions of 3 × 105 cells were seeded into each well of 12-well plates. The cells were then incubated with 600 μl culture medium alone or with medium containing rAd5-EAT-2 or rAd5-EAT-2 (R31Q) vectors at 20,000 multiplicities of infection for 48 h. EAT-2 expression was evaluated by Alexa Fluor 488 (Invitrogen)-conjugated EAT-2 Ab (sc-21572; Santa Cruz Biotechnology).
ELISPOT analysis
Splenocytes were harvested from individual mice and RBCs were lysed using ACK lysis buffer (Invitrogen). Ninety-six–well Multiscreen high protein binding Immobilon-P membrane plates (Millipore, Billerica, MA) were pretreated with ethanol, coated with mouse anti–IFN-γ or IL-2 capture Abs, incubated overnight, and blocked prior to the addition of 5 × 105 splenocytes per well. Ex vivo stimulation included the incubation of splenocytes in 100 μl media alone (unstimulated) or media containing 4 μg/ml Gag-specific peptides (Gag-AMQMLKETI constructed by Gen-Script, Piscataway, NJ) for 24 h in a 37°C, 5% CO2 incubator. Staining of plates was completed per the manufacturer’s protocol. Spots were counted and photographed by an automated ELISPOT reader system (Cellular Technology, Cleveland, OH). Ready-SET-Go! IFN-γ and IL-2 mouse ELISPOT kits were purchased from eBioscience (San Diego, CA).
Cell staining and flow cytometry
To evaluate the intracellular cytokine responses following rAd5-Gag and rAd5-EAT-2 covaccination, intracellular staining was performed as previously described (19). Briefly, cells were surface stained with allophycocyanin-Cy7-CD3, Alexa Fluor 700-CD8a, and CD16/32 Fc-block Abs, fixed with 2% formaldehyde (Polysciences, Warrington, PA), permeabilized with 0.2% saponin (Sigma-Aldrich, St. Louis, MO), and stained for intracellular cytokines with PE-Cy7-TNF-α, allophycocyanin-granzyme B, PE-perforin, FITC-IFN-γ, Pacific Blue-CD62L, PerCP-Cy5.5-CD127 (4 μg/ml) (all obtained from BD Biosciences, San Diego, CA), and PerCp-Cy5.5-IL2 (Bio-Legend, San Diego, CA). We included a violet fluorescent reactive dye (ViViD; Invitrogen) as a viability marker to exclude dead cells from the analysis (30). For innate immune cell activation studies, splenocytes were stained with various combinations of the following Abs: PE-CD69 (3 μg/ml), allophycocyanin-CD3, allophycocyanin-Cy7-CD3, Alexa Fluor 700-CD8a, PE-Cy7-NK1.1, CD11c-PE-Cy7, and CD11b-allophycocyanin-Cy7 (4 μg/ml) (all obtained from BD Biosciences). Cells were incubated on ice with the appropriate Abs for 30 min, washed, and data were collected using an LSR II instrument and analyzed using FlowJo software. For tetramer staining, blood was isolated by retro-orbital bleeds, and PBMCs were isolated using Lympholyte-Mammal (Cedarlane Laboratories, Burlington NC). Tetramer staining of PBMCs was completed using a PE-conjugated MHC class I tetramer folded with the AMQMLKETI peptide generated at the National Institutes of Health Tetramer Core Facility.
In vivo CTL assay
An in vivo CTL assay was performed as previously described (19, 20). Briefly, Ad5 preimmune BALB/cJ mice (n = 5) were covaccinated with equivalent doses of rAd5-HIV/Gag with either rAd5-GFP, rAd5-EAT-2, or rAd5-EAT-2 (R31Q) (totaling 1 × 109 vps). Fourteen days following the boost vaccine, syngeneic splenocytes were isolated and either pulsed with an irrelevant peptide specific to the Plasmodium falciparum circumsporozoite Ag (NYDNAGTNL) or with the HIV/Gag immunodominant AMQMLKETI peptide for 1 h at 37°C. Irrelevant peptide-pulsed cells were subsequently stained with 1 μM CFSE (CFSElow), whereas Gag peptide-pulsed cells were stained with 10 μM CFSE (CFSEhigh). Naive and coimmunized mice were injected with equivalent amounts of both CFSElow- and CFSEhigh-stained cells (8 × 106 total cells/mouse) via the retro-orbital sinus. After 20 h, mice were terminally sacrificed and splenocytes were recovered and analyzed on an LSRII flow cytometer. FlowJo software was used to determine percentages of CFSE-stained cells as follows: % specific killing = 1 – [(% CFSEhigh/% CFSElow)immunized/(% CFSEhigh/% CFSElow)nonimmunized].
Ab titering assay
ELISA-based titering experiments were essentially completed as previously described (26, 28). In brief, 5 × 108 vps Ad5 vector per well or 0.2 mg recombinant Gag protein per well (each diluted in PBS) was used to coat wells of a 96-well plate overnight at 4°C. Plates were washed with PBS-Tween 20 (0.05%) solution, and blocking buffer (3% BSA in PBS) was added to each well and incubated for 1–3 h at room temperature . For tittering of total IgG Abs, plasma from coinjected mice was diluted 1:10 to 1:2300 (Ad5) or 1:20 (Gag) in blocking buffer. Following dilution, plasma was added to the wells and incubated at room temperature for 1 h. Wells were washed using PBS-Tween 20 (0.05%), and HRP-conjugated rabbit anti-mouse Ab (Bio-Rad, Hercules, CA) was added at a 1:5000 dilution in PBS-Tween 20. Tetramethylbenzidine (Sigma-Aldrich) substrate was added to each well, and the reaction was stopped with a 2 N sulfuric acid. Plates were read at 450 nm in a microplate spectrophotometer.
Statistical analysis
Statistically significant differences in toxicities associated with innate immune responses were determined using a one-way ANOVA with a Student–Newman–Keuls post hoc test (p value of <0. 05 was deemed statistically significant). For ELISPOT analysis, a two-way ANOVA was used followed by Bonferroni post hoc test. For flow cytometry, a one-way ANOVA with a Student–Newman–Keuls post hoc test was used. For in vivo CTL assays, a one-way ANOVA with a Student–Newman–Keuls post hoc test was used. Statistical analyses were performed using Graph-Pad Prism (GraphPad Software).
Results
Mutating the EAT-2 SH2 domain abrogates EAT-2–mediated early activation of innate and adaptive immune cells in vivo
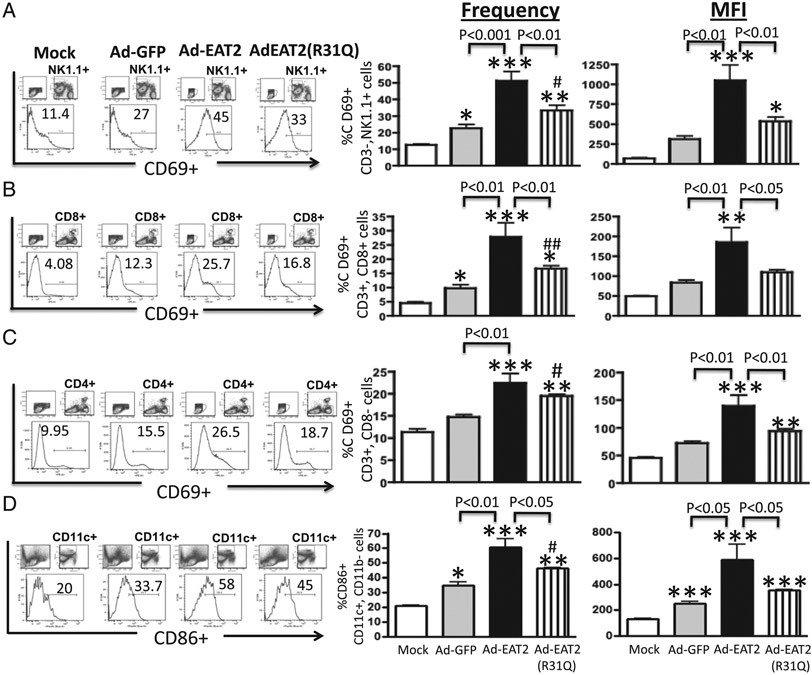
We have previously demonstrated that rAd5-based vaccines expressing the SLAM family of receptors adaptor, EAT-2, trigger early activation of both innate and adaptive immune cells, activations that were positively correlated with improved induction of cellular immune responses to coadministered Ags in Ad5-naive mice (19, 20). Several biochemical and mechanistic studies have demonstrated that SAP adaptors such as EAT-2 regulate SLAM-initiated signaling in immune cells via association of their SH2 domain to the phosphorylated immunoreceptor tyrosine-based switch motifs of SLAM receptors (31). We therefore constructed an rAd5 vector that expresses a SH2 domain mutant form of the EAT-2 adaptor (rAd5-EAT-2(R31Q)) and evaluated innate and adaptive immune cell activation following its administration into C57BL/6 mice. The location of this mutation exactly mimics a mutation in the SAP protein present in X-linked lymphoproliferative patients, a mutation that disrupts the phosphotyrosine-binding pocket of SAP adaptor, and is thus confirmed to abrogate SAP-mediated regulation of human and mouse innate and adaptive immune cells (32, 33). We first analyzed the expression of the lymphocyte activation marker CD69 in various immune cells shortly after administration of rAd5-EAT-2, rAd5-EAT-2(R31Q), or rAd5-GFP vectors into C57BL/6 mice. Consistent with our previous results (19), vaccination with rAd5-expressing WT EAT-2 induced significantly higher numbers of CD69-expressing NK (p < 0.001), CD4+ (p < 0.01), and CD8+ T (p < 0.01) cells and amounts of CD69 per cell (mean fluorescence intensity [MFI], p < 0.01) as compared with the rAd5-GFP–treated controls (Fig. 1A-C). Importantly, the activation function of EAT-2 in NK and CD8+ T cells was significantly reduced when the identical experiment was performed utilizing the rAd5 expressing the EAT-2(R31Q) mutant (Fig. 1A, 1B). Statistically significant reduction in the amount per cell (MFI, p < 0.01), but not in the number (percentages), of CD69-expressing CD4+ T cells were also observed in rAd5-EAT-2(R31Q)–injected mice as compared with WT EAT-2–expressing Ads (Fig. 1C). We also evaluated DC maturational status after rAd5 vaccinations. We observed statistically significant increases in the number and amount of CD86 (B7.1) being expressed on DCs (CD11c+CD11b−) in splenocytes derived from rAd5-EAT-2–injected mice as compared with rAd5-GFP–treated controls (Fig. 1D). In contrast, DCs derived from rAd5-EAT-2 (R31Q)–treated mice failed to significantly increase the number (p < 0.05) and amount (p < 0.05) of CD86-expressing DCs as compared with DCs derived from mice vaccinated with the rAd5-expressing WT EAT-2 (Fig. 1D).
FIGURE 1.
Intramuscular administration of WT EAT-2–expressing rAd5 vector induces innate and adaptive immune cells activation in vivo. C57BL/6 mice (n = 5) were either mock injected or i.m. injected with 2 × 1010 vps of either rAd5-EAT-2, rAd5Eat-2(R31Q), or rAd5-GFP control. CD69 expression by splenic NK cells (A), CD3+CD8+ (B), or CD3+CD8− (C) T cells was evaluated 12 h after virus injection. (D) CD86-expressing (CD11c+, CD11b−) DCs. Splenocytes were harvested, stained, and analyzed on a LSRII flow cytometer. The bars represent means ± SD. Statistical analysis was completed using one-way ANOVA with a Student–Newman–Keuls post hoc test. A p value < 0.05 was deemed statistically significant. *p < 0. 05. **p < 0. 01. ***p < 0.001 versus mock-injected mice. #p < 0. 05. ##p < 0.01 versus rAd5-GFP–injected mice.
Expressing EAT-2 protein enhances the Gag-specific cellular immune responses in Ad5 immune mice
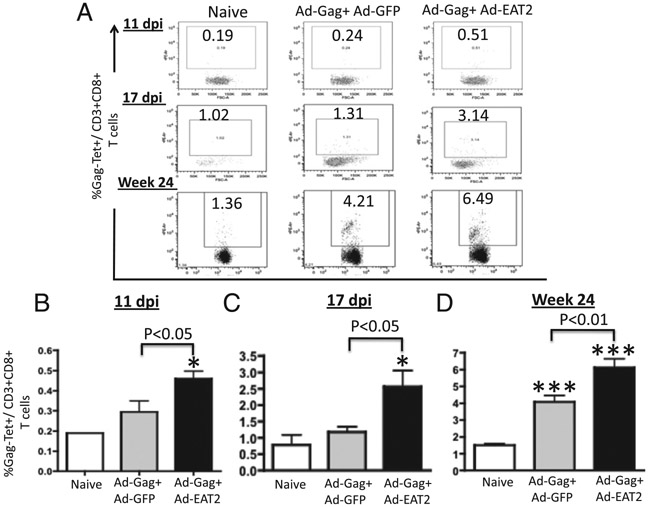
Pre-existing anti-Ad5 immunity in human populations may significantly limit the efficacy of rAd5-based vaccine approaches (34, 35). To investigate whether the enhanced innate immune profile promoted by rAd5-mediated expression of EAT-2 could still influence the adaptive immune responses to a coadministered Ag in the presence of high levels of anti-Ad5–specific immunity (Ad5 immune mice), we performed initial dose-curve studies to identify the lowest dose of rAd5-HIV/Gag that generated detectable Gag-specific cellular immune responses in Ad5 immune mice. As a result, we identified an rAd5-HIV/Gag dose of 5 × 108 vps per mouse as the most relevant experimental doses for these initial studies (data not shown). We first evaluated the ability of a single immunization with rAd5-HIV/Gag and rAd5-EAT-2 to elicit cellular immune responses at 2 wk after vaccination of Ad5 immune mice. Initial CD8+ T lymphocyte responses specific for the immunogenic HIV/Gag epitope (AMQMLKETI) were determined by multiparameter tetramer-binding assays. At 11 and 17 d postinjection (dpi), significantly increased numbers of Gag-specific CD8+ T cells were observed in PBMCs derived from rAd5-HIV/Gag– and rAd5-EAT-2–coinjected Ad5 immune mice as compared with the respective cell populations isolated from rAd5-HIV/Gag–treated controls (Fig. 2A-C).
FIGURE 2.
rAd5-HIV/Gag and rAd5-EAT-2 covaccination enhances the Gag-specific CD8+ T lymphocyte responses despite Ad5 pre-existing immunity. Ad5 preimmune BALB/c mice (n = 12) were coimmunized i.m. in the tibialis anterior with equivalent viral particles of rAd5-HIV/Gag mixed with either rAd5-EAT-2 or rAd5-GFP (total of 1 × 108 vps mixed prior to injection). PBMCs were collected from the immunized mice and stained with a PE-conjugated H2-Kd-AMQMLKETI tetramer complex together with an allophycocyanin-conjugated anti-CD3 and Pacific Blue-conjugated anti-CD8 Abs. The percentage of Gag-specific CD8+ T cells (%Tet+) is depicted. (A) Representative figures of Gag-specific tetramer+CD8+ T cells at the indicated time points are shown. (B) Gag-specific CD8+ T cell responses after prime vaccination at 11 dpi in PBMCs (pool of three mice in each group). (C) Gag-specific CD8+ T cell responses after prime vaccination at 17 dpi in PBMCs. (D) Gag-specific CD8+ T cell responses after prime-boost vaccination at week 24 in PBMCs. The bars represent means ± SD for 12 mice per group. Data were collected in an LSRII and analyzed by FlowJo software. Statistical analysis was completed using one-way ANOVA with a Student–Newman–Keuls post hoc test. A p value < 0.05 was deemed statistically significant. *p < 0.05, ***p < 0.001 versus naive animals.
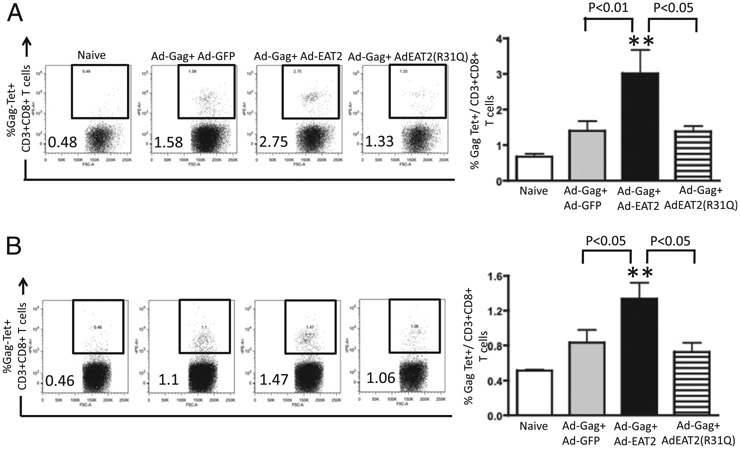
In longer term studies, we evaluated the ability of EAT-2–augmented rAd5 vaccines to induce potent Gag-specific CD8+ T lymphocyte responses following homologous rAd5-HIV/Gag prime-boost regimens in Ad5 immune mice. Two weeks following the boosting immunization, Gag-specific CD8+ T lymphocyte responses were evaluated by tetramer-binding assays. We were able to detect heightened Gag-specific tetramer-positive CD8+ T cells in PBMCs (p < 0.01) and splenocytes (p < 0.05) derived from rAd5-HIV/Gag+ rAd5-EAT-2–coinjected mice as compared with the respective cell populations isolated from control mice (Fig. 3). In contrast, CD8+ T cells derived from PBMCs and splenocytes of the rAd5-HIV/Gag+ Ad-EAT-2(R31Q)–covaccinated control mice revealed significantly reduced (p < 0.05) Gag-specific tetramer-positive CD8+ T cells as compared with the respective cell populations derived from WT EAT-2–augmented rAd5-HIV/Gag vectors, suggesting a critical role for SH2 domain-mediated signaling in EAT-2 regulating the induction of CD8+ T cell responses during vaccination (Fig. 3). Similar statistically significant results were also observed utilizing lower doses (1 × 108 total vps/mouse) of the vaccine vectors 24 wk following the homologous prime-boost immunization (Fig. 2A, 2D).
FIGURE 3.
Gag-specific CD8+ T lymphocyte responses elicited by rAd5-HIV/Gag and rAd5-EAT-2 vectors in rAd5 preimmune BALB/c mice. Ad5 preimmune BALB/c mice (n = 7) were coimmunized i.m. in the tibialis anterior with equivalent viral particles of rAd5-HIV/Gag mixed with rAd5-EAT-2, rAd5-GFP, or rAd5-EAT-2(R31Q) (total of 1 × 109 vps mixed prior to injection). HIV/Gag-specific CD8+ T lymphocyte responses from peripheral blood and spleen specific for the H2-Kd-restricted Gag epitope (AMQMLKETI) were determined at week 34 by multiparameter tetramer-binding assays. The percentage of Gag-specific CD8+ T cells (%Tet+) is depicted. At week 34, mice were sacrificed and PBMCs (A) or splenocytes (B) were harvested and stained with a PE-conjugated AMQMLKETI tetramer complex together with an allophycocyanin-conjugated anti-CD3 and Pacific Blue-conjugated anti-CD8 Abs. The bars represent means ± SD for seven mice per group for virus injected and four mice for naive animals. Data were collected in an LSRII and analyzed by FlowJo software. Statistical analysis was completed using one-way ANOVA with a Student–Newman–Keuls post hoc test. A p value < 0.05 was statistically significant. **p < 0.01 versus naive animals.
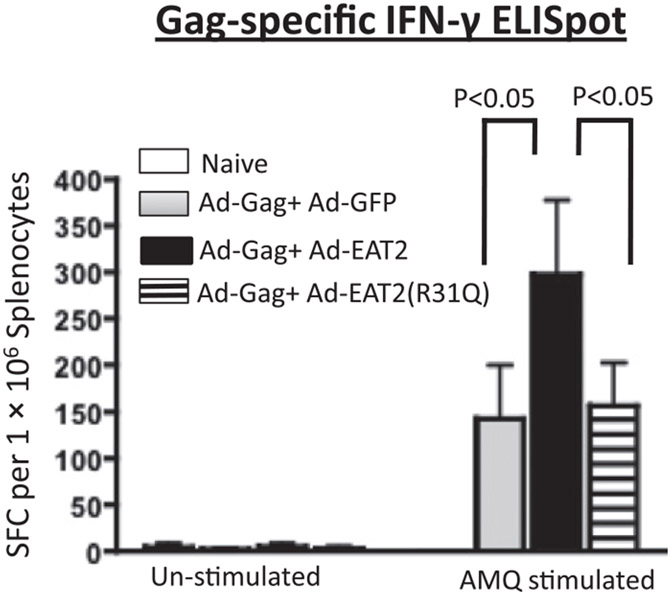
We also evaluated the functional T cell memory responses following the boosting immunization by IFN-γ ELISPOT assay. Following ex vivo stimulation with the immunodominant Gag peptide (AMQMLKETI), splenocytes derived from Ad immune mice coimmunized with rAd5-HIV/Gag and rAd5-EAT-2 contained significantly (p < 0.05) increased numbers of Gag-specific IFN-γ–secreting cells as compared with similarly treated splenocytes derived from the rAd5-HIV/Gag+ rAd5-GFP control Ad immune mice (Fig. 4). In contrast, splenocytes derived from rAd5-HIV/Gag+ rAd5-EAT-2(R31Q)–coinjected mice revealed significantly (p < 0.05) reduced numbers of Gag-specific IFN-γ–secreting cells as compared with cells derived from rAd5-HIV/Gag– and WT EAT-2–treated Ad immune mice (Fig. 4). Again, no statistically significant differences in the number of Gag-specific IFN-γ–secreting cells were observed between rAd5-EAT-2(R31Q)– and rAd5-GFP–coinjected animals (Fig. 4). We also evaluated anti-Ad responses by measuring Ad5-specific IFN-γ–secreting cells using an IFN-γ ELISPOT assay. Importantly, experimental and control groups revealed similar levels of Ad5-specific IFN-γ–secreting T cells at these and lower overall doses (data not shown). Humoral immune responses to HIV/Gag were also evaluated following single and homologous prime-boost immunizations. Consistent with our previous results (19, 20), inclusion of rAd5-EAT-2 in the vaccine mixture did not diminish the humoral immune responses (total Gag-specific IgG) to the HIV/Gag Ag (Supplemental Fig. 3B). We also evaluated the humoral immune responses to Ad5 following the homologous prime-boost immunization. Similarly, plasma levels of total Ad5-specific IgG were observed in the experimental and control groups (Supplemental Fig. 3C).
FIGURE 4.
Magnitude of HIV/Gag-specific IFN-γ–secreting T lymphocytes following rAd5-EAT-2 covaccination of Ad5 preimmune animals. Ad5 preimmune BALB/c mice (n = 7) were subjected to a homologous prime-boost rAd5 covaccination regimen. At week 34, animals were terminally sacrificed, and splenocytes were harvested and stimulated ex vivo with the 15-mer HIV/Gag-derived immunogenic peptides AMQMLKETI, and IFN-γ ELISPOT assays were completed. Bars represent means ± SD. Statistical analysis was completed using two-way ANOVA with a Bonferroni post hoc test. A p value < 0.05 was deemed statistically significant.
Expressing EAT-2 induces multiple Gag-specific T lymphocyte responses in Ad5 immune mice
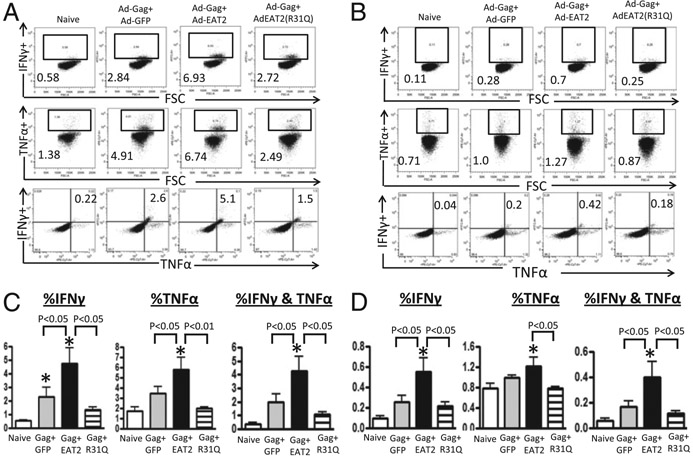
The induction of increased numbers of Ag-specific T lymphocytes that express several cytokines in response to Ags correlates with improved vaccine-induced protective immunity and positively correlates with the induction of long-lived memory responses (36, 37). Six-color flow cytometry was used to enumerate the frequency of CD8+ and CD4+ T cells producing IFN-γ, TNF-α, and/or IL-2 after ex vivo stimulation with HIV/Gag-specific peptides elicited by the use of EAT-2–augmented Ad-HIV/Gag vaccines in Ad5 immune mice. Our analysis revealed statistically higher numbers of HIV/Gag-specific IFN-γ+ (p < 0. 05), TNF-α+ (p < 0.05), and IFN-γ/TNF-α double-positive (p < 0. 05) CD8+ T cells derived from EAT-2–augmented rAd5-HIV/Gag vaccine-immunized mice as compared with mice vaccinated with control vaccines (Fig. 5A, 5C). In contrast to these robust responses, CD8+ T cells derived from mutant EAT-2(R31Q)–augmented rAd5-HIV/Gag–vaccinated, Ad immune mice elicited statistically lower magnitudes of IFN-γ (p < 0.05), TNF-α (p < 0.01), and/or IFN-γ/TNF-α double-positive (p < 0.05) cells as compared with cells derived from mice vaccinated with the EAT-2 rAd5-HIV/Gag–expressing rAd5 vectors (Fig. 5A, 5C). Although CD4+ T lymphocyte responses were several fold lower in magnitude than CD8+ T lymphocyte responses in all vaccinated groups, statistically higher number of Gag-specific IFN-γ+ (p < 0.05) and IFN-γ/TNF-α double-positive (p < 0.05) CD4+ T cells were also detected in rAd5-HIV/Gag– and rAd5-EAT-2–coimmunized mice as compared with cells derived from mice vaccinated with the control rAd5-HIV/Gag vaccines (Fig. 5B, 5D). CD4+ T lymphocytes derived from mutant EAT-2(R31Q)–augmented rAd5-HIV/Gag–injected mice express significantly (p < 0.05) lower numbers of IFN-γ, TNF-α, and IFN-γ/TNF-α double-positive cells as compared with cells derived from WT EAT-2–augmented rAd5-HIV/Gag–expressing Ad5 vectors (Fig. 5B, 5D). We also attempted to detect IL-2–expressing T lymphocytes, but none was detectable to appreciable levels in both experimental and control groups (data not shown).
FIGURE 5.
Cytokines secretion profiles of rAd5-HIV/Gag and rAd5-EAT-2 vaccine-elicited T lymphocytes. The cytokine secretion profiles of HIV/Gag-specific CD8+ and CD4+ T cells elicited by the homologous rAd5-HIV/Gag and rAd5-EAT-2 prime-boost covaccination regimen were determined by multiparameter intracellular cytokine staining assays. (A) Representative example of IFN-γ–, TNF-α–, or IFN-γ/TNF-α–producing splenic CD8+ T cells. (B) Representative example of IFN-γ–, TNF-α–, or IFN-γ/TNF-α–producing splenic CD4+ T cells. Gate were set based on negative control (naive) and placed consistently across samples. (C) The total frequency of splenic CD8+ T cells derived from Ad5 preimmune BALB/c mice expressing IFN-γ, TNF-α, or IFN-γ and TNF-α. (D) The total frequency of splenic CD4+ T cells derived from Ad5 preimmune BALB/c mice expressing IFN-γ, TNF-α, or IFN-γ and TNF-α. The bars represent means ± SD. Statistical analysis was completed using one-way ANOVA with a Student–Newman–Keuls post hoc test. A p value < 0.05 was deemed statistically significant. *p < 0.05 versus naive animals.
HIV/Gag-specific T lymphocytes elicited by rAd5-EAT-2 covaccination exhibit TEM-biased phenotype
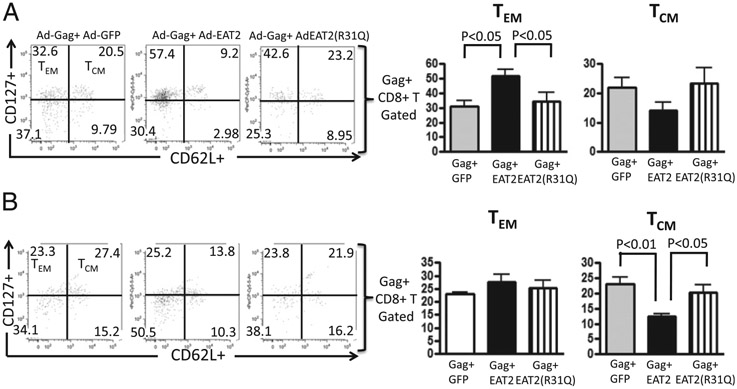
We next evaluated the phenotype and the functional characteristic of Gag-specific memory T lymphocytes induced by the EAT-2–augmented rAd5-HIV/Gag vaccinations in PBMCs and splenocytes derived from Ad5 immune animals following single or homologous prime-boost immunizations. Peripheral blood CD8+ T lymphocytes derived from Ad5 immune mice that received a single immunization with the rAd5-HIV/Gag and rAd5-EAT-2 regimen had statistically significant increases in Gag-specific tetramer-positive central memory T (TCM) (p < 0.05) (Fig. 6A) and TEM (p < 0.01) (Fig. 6) cell responses as compared with the respective cell populations isolated from control mice at this same time point after vaccination. No significant induction of Gag-specific TCM and TEM responses were observed in the splenocytes of the vaccinated mice following this priming immunization (data not shown). In contrast, 2 wk following the boosting immunization, we observed increased frequencies of Gag-specific TEM CD8+ lymphocytes in both PBMCs (Fig. 6C) and splenocytes (Fig. 6D) derived from the rAd-HIV/Gag– and rAd5-EAT-2–coinjected mice as compared with cells derived from mice vaccinated with the control rAd5-HIV/Gag vaccines. Mutating the EAT-2 SH2 domain completely abolished the ability of EAT-2–augmented vaccination to enhance the induction of these TEM CD8+ cell responses after the boost immunization in PBMCs and splenocytes of both the experimental and control groups (Fig. 6C, 6D). No statistically significant increases in Gag-specific TCM CD8+ cell responses were observed after the boost immunization in PBMCs and splenocytes of both the experimental and control groups (data not shown). Peripheral blood-derived, Gag-specific CD8+ T cells from rAd5-HIV/Gag– and rAd5-EAT-2–covaccinated mice showed a predominantly TEM phenotype as compared with cells derived from the rAd5-GFP– or r-Ad5-EAT-2 (R31Q)–coinjected mice (Fig. 7A). Interestingly, the Gag-specific CD8+ T cells derived from peripheral blood of rAd5-EAT-2–coinjected mice exhibit reduced numbers of TCM cells as compared with the control-injected mice (Fig. 7A). No significant differences in the numbers of Gag-specific TEM cells were observed in the splenocytes derived from any of the groups of treated mice at this time point (Fig. 7B). Significant reductions in the numbers of Gag-specific TCM CD8+ T lymphocytes were also observed in mice derived from rAd5-EAT-2–injected mice as compared with rAd5-GFP– and rAd5-EAT-2(R31Q)–vaccinated mice (Fig. 7B), suggesting that Ad-EAT-2–augmented vaccinations shifted the quality of the Ag-specific cellular immune responses to an effector memory, rather than a central memory, phenotype.
FIGURE 6.
EAT-2 expression increases the magnitude of Gag-specific TCM and TEM CD8+ cell responses in Ad5 immune animals. Magnitude and phenotype analysis of HIV/Gag-specific CD8+ T cells elicited by rAd5-HIV/Gag and rAd5-EAT-2 vaccination regimen were determined in peripheral blood and splenocytes of Ad5 immune mice by multiparameter tetramer-binding assays. (A) Frequency of Gag-specific tetramer-positive TCM (CD62L+CD127+) CD8+ cells 2 wk following the prime vaccination with 1 × 108 vps is shown. (B) Frequency of Gag-specific tetramer-positive TEM (CD62L−CD127+) CD8+ cells 2 wk following the prime vaccination with 1 × 108 vps is shown. (C) Frequency of Gag-specific tetramer-positive TEM (CD62L−CD127+) CD8+ cells in PBMCs 34 wk following the homologous prime-boost vaccination with 1 × 109 vps is shown. (D) Frequency of Gag-specific tetramer-positive TEM CD8+ cells in splenocytes 34 wk following the homologous prime-boost vaccination with 1 × 109 vps is shown. The bars represent means ± SD. Statistical analysis was completed using one-way ANOVA with a Student–Newman–Keuls post hoc test. A p value < 0.05 was deemed statistically significant. *p < 0.05 versus naive animals.
FIGURE 7.
Phenotype analysis of HIV/Gag-specific CD8+ T cells following the homologous prime-boost vaccination of rAd5-HIV/Gag and rAd5-EAT-2. Phenotype analysis of HIV/Gag-specific CD8+ T cells elicited by the homologous rAd5-HIV/Gag and rAd5-EAT-2 prime-boost covaccination regimen were determined at week 34 in peripheral blood and splenocytes by multiparameter tetramer-binding assays. (A) Percentages of Gag-specific TEM and TCM CD8+ cells in PBMCs are shown. (B) Percentages of Gag-specific TEM and TCM CD8+ T cells in splenocytes are shown. The bars represent means ± SD. Statistical analysis was completed using one-way ANOVA with a Student–Newman–Keuls post hoc test. A p value < 0.05 was deemed statistically significant. *p < 0.05 versus naive animals.
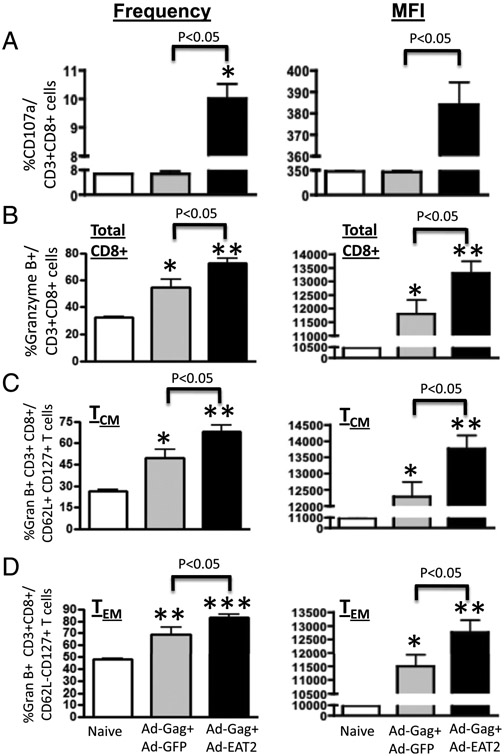
Memory CD8+ T cells of rAd5-EAT-2–vaccinated mice exhibit improved in vivo CTL activity and cytolytic degranulation
The predominant Gag-specific TEM CD8+ cell responses elicited by augmentation of vaccines with EAT-2 would predict an improved potential for induction of functional, Ag-specific cytotoxic T cell activity (38). We initially evaluated the surface mobilization (expression) of CD107a (LAMP-1) after in vitro culture of splenocytes (derived from Ad5 preimmune mice coimmunized with rAd5-HIV/Gag and rAd5-EAT-2) in the presence of the immunodominant AMQMLKETI-Gag peptide. Our results revealed that CD8+ T lymphocytes derived from rAd5-HIV/Gag– and rAd-EAT-2–coimmunized mice express significantly higher levels of CD107a (p < 0.05) as compared with cells derived from the control vaccines (Fig. 8A). Six-color flow cytometry was then used to enumerate the frequency of granzyme B and perforin-producing TCM and TEM CD8+ lymphocytes. We observed statistically significant increases in the number (p < 0.05) and amount per cell (MFI, p < 05) of Gag-specific granzyme B-producing total T (Fig. 8B), TCM (Fig. 8C), and TEM (Fig. 8D) CD8+ lymphocytes derived from EAT-2–augmented rAd5-HIV/Gag vaccine-immunized mice as compared with mice vaccinated with control vaccines. Analysis of perforin-expressing CD8+ T cells of rAd5-EAT-2–coinjected mice trended to also increase, but no statistically significant differences were observed between the experimental and control groups (data not shown).
FIGURE 8.
Gag-specific CD8+ Τ cells from rAd5-HIV/Gag– and rAd5-EAT-2–vaccinated mice exhibit improved cytolytic degranulation. Ad5 preimmune BALB/c mice (n = 6) were subjected to a homologous prime-boost rAd5 covaccination regimen. (A) At week 24, splenocytes from Ad5 preimmune BALB/c mice of rAd5-HIV/Gag– and rAd5-EAT-2–covaccinated or control groups were in vitro cultured in the presence or absence of the immunogenic Gag-specific peptide AMQMLKETI. At 72 h after infection, cells were stained with allophycocyanin-conjugated anti-CD3, Pacific Blue-conjugated anti-CD8, or FITC-conjugated anti-CD107. (B) Granzyme B-producing HIV/Gag-specific splenic CD8+ T cells elicited by the homologous prime-boost vaccination were determined at week 24 by multiparameter intracellular cytokine staining assays. Frequency and MFI of Gag-specific granzyme B-producing total CD8+ T cells is shown. (C) Frequency and MFI of Gag-specific granzyme B-producing TCM CD8+ T cells is shown. (D) Frequency and MFI of Gag-specific granzyme B-producing TEM CD8+ T cells is shown. The bars represent means ± SD. Statistical analysis was completed using one-way ANOVA with a Student–Newman–Keuls post hoc test. A p value < 0.05 was deemed statistically significant. *p < 0.05 versus naive animals.
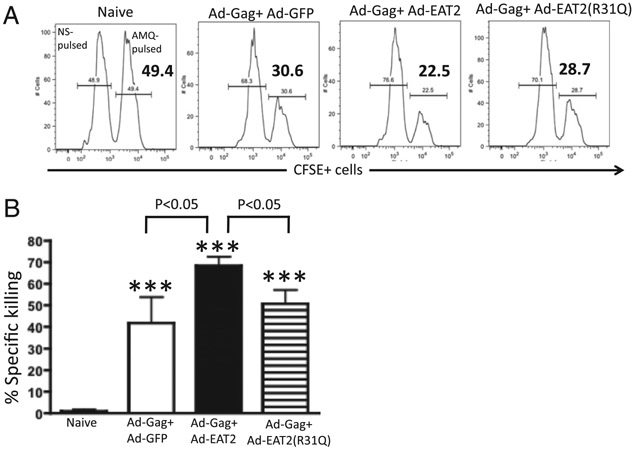
To investigate whether these Gag-specific TEM-skewed T cells also possess enhanced in vivo cytolytic activity toward Ag-presenting target cells, Ad5 preimmune BALB/c mice were primed i.m. with 1 × 109 total vps of the rAd-HIV/Gag+ Ad-EAT-2, rAd-HIV/Gag+ Ad-EAT-2(R31Q), or rAd5-HIV/Gag+ rAd5-GFP control vaccine formulations. Mice were then boosted with the same rAd5 vectors at week 32. Two weeks following the boost immunization, the three groups of mice were then injected with CFSE-labeled syngeneic splenocytes previously pulsed with the Gag-derived peptides AMQMLKETI or an irrelevant peptide (see Materials and Methods). Consistent with the results obtained in Ad5-naive animals (19, 20), HIV/Gag-specific CTL activities induced in Ad immune mice coimmunized with rAd5-HIV/Gag and rAd5-EAT-2 were significantly higher as compared with control mice (Fig. 9). Importantly, rAd5-EAT-2(R31Q)–coinjected mice exhibited significantly reduced cytolytic activity, as compared with mice coinjected with the WT EAT-2–expressing Ad5 vaccine vectors (Fig. 9).
FIGURE 9.
Increased in vivo cytolytic activity of the vaccine-elicited Gag-specific T lymphocytes in rAd5-HIV/Gag– and rAd5-EAT-2–coimmunized mice. Ad5 preimmune BALB/c (n = 5 for naives; n = 5 for rAd5-HIV/Gag+ rAd5-EAT-2; n = 3 for rAd5-HIV/Gag+ rAd5-EAT-2(R31Q); and n = 2 for rAd5-HIV/Gag+ rAd5-GFP) were coimmunized with the homologous prime-boost vaccine regimens (1 × 109 total vps) and in vivo CTL activity was determined. At week 34, syngeneic splenocytes were pulsed with either an irrelevant peptide (NYDNAGTNL peptide) and stained with 1 μM (CFSElow) or with the HIV/Gag-specific peptides (AMQMLKETI peptides) and labeled with 10 μM (CFSEhigh). Twenty hours after adoptive transfer into naive or immunized mice, splenocytes were harvested and analyzed using an LSRII flow cytometer. (A) Representative figures of percentages of CFSElow and CFSEhigh populations 20 h after adoptive transfer into naive or coimmunized mice in splenocytes are shown. (B) Statistical analysis of percentage specific killing is shown. Percentage CFSE+ cells were quantified using FlowJo software. Percentage specific killing = 1 – [(% CFSEhigh/% CFSElow)immunized/(% CFSEhigh/% CFSELow)nonimmunized]. ***p < 0.001 versus naive animals.
Discussion
The development of an effective vaccine against HIV-1 remains elusive. Various reports in vaccinated nonhuman primates challenged with SIV suggest that putative HIV-1 vaccines should elicit potent, and durable, HIV-1–specific TEM CD8+ cell responses so as to provide protection in the earliest stages following HIV-1 infection (8-10). The promising results from recent HIV-1 vaccine trials support the need to continue attempts to enhance the potency of HIV-1–targeted vaccine platforms (39). Recombinant Ad5-based vaccines have been the focus of considerable interest for their potential application in HIV-1 vaccine development; however, early generation rAd5 platforms failed to protect vaccinees from HIV-1 infection (4, 40). One hypothesis suggests that the rAd5-based vaccine failed due to the presence of pre-existing anti-Ad5–specific immunity (23, 39). We have previously demonstrated that rAd5-based vaccine vectors expressing the SLAM family of receptors adaptor, EAT-2, induced potent cellular immune responses to a coadministered HIV-1-Gag Ag in Ad5-naive animals (19). In this study, we now demonstrate that rAd5-EAT-2 vectors also induce potent Gag-specific T cell responses in Ad5 immune mice, responses that persisted for >200 d.
Some reports have shown that nonreplicating vaccine vectors induce primarily TCM responses (10). In this study, we evaluated the phenotype and functionality of vaccine-elicited cellular immune responses 34 wk following rAd-HIV/Gag– and rAd5-EAT-2–augmented prime-boost immunizations. Our data revealed that Gag-specific T lymphocytes derived from rAd5-EAT-2–vaccinated mice are capable of producing several important analytes upon restimulation. Our data also demonstrated that following priming vaccination, the rAd5-EAT-2–augmented vaccine elicited Gag-specific CD8+ T cells exhibiting a balanced ratio of TCM and TEM CD8+ cell phenotypes. In contrast, at later time points the HIV/Gag-specific responses elicited by rAd5-EAT-2 exhibited a predominant CD8+ TEM phenotype. The importance of this transition of the Gag-specific T cells into a predominant TEM phenotype following rAd5-EAT-2 covaccination is not clear; however, published reports have demonstrated that induction of TEM cells by vaccines is a desired phenotype of Ag-specific T cells, as they may respond more rapidly upon Ag restimulation than do TCM cells (41, 42). This extends and confirms our previously published results (19) demonstrating that vaccine cocktails expressing EAT-2 along with a target Ag increased the cytolytic degranulation of Ag-specific CD8+ T cells in vivo.
The molecular mechanism as to how viral vaccine expression of the EAT-2 adaptor triggers these potent memory T cell responses is not clear; however, several reports have shown that TCM and TEM differentiation is controlled primarily by the innate immune system. Specifically, it has been shown that proinflammatory cytokines and the abundance of costimulatory molecules on the surface of APCs can play a critical role for TCM and TEM cell differentiation (38). Memory T cell differentiation has also been shown to depend on the signal strength and duration of DC/T cell interaction, a mechanism that depends primarily on proinflammatory cytokines and the presence of costimulatory molecules on APCs (43, 44). Our present and previously published data have demonstrated that expression of EAT-2 enhanced the production of proinflammatory cytokines and chemokines as well as upregulated the expression of costimulatory molecules on the surface of APCs (19). Additionally, several published reports have demonstrated that triggering SLAM receptor signaling in mouse APCs (DCs and macrophages) plays an important role in IL-12, TNF-α, IL-6, IL-8, and NO production (45, 46). Furthermore, previously published reports have shown that the extent of cell death in the contraction phase following vaccination is influenced by costimulatory molecules, cytokines, and effector molecules (7, 47). We speculate that EAT-2–mediated activation of the innate immune system may be responsible for enhancing the cellular immune responses following vaccination by any or all of these mechanisms. It is conceivable that EAT-2–mediated activation of the innate immune system also limits the contraction phase of the newly produced effector memory Gag-specific T lymphocytes.
Results from IFN-γ ELISPOT assay revealed that Gag-specific IFN-γ–secreting T cell responses are lower in magnitude than those induced by a single rAd5-HIV/Gag and rAd5-EAT-2 immunization of rAd5-naive animals (19), presumably as a result of the development of Ad5-specific T cells and neutralizing Abs generated by the priming immunization (19). Despite this, vaccination with rAd5-HIV/Gag along with EAT-2 overexpression still generates superior T cell responses as compared with the control-vaccinated Ad5 immune animals. Additionally, FACS intracellular staining analysis of Gag-specific CD8+ T cells revealed equal frequencies of IFN-γ– and TNF-α–producing CD8+ T cells in splenocytes derived from Ad5-naive (19) and Ad5 immune BALB/c mice coimmunized with EAT-2–augmented Ad5-HIV/Gag vaccines.
Although we did note improved inductions of Gag Ag-specific immune responses, we did not observe significant increases in anti-Ad–specific cellular immune responses in Ad5-EAT-2–immunized mice. This could be due to the fact that E1-deleted Ad vectors minimally express potentially immunogenic Ad5 genes, especially when compared with the high and sustained expression levels of the Gag Ag, which was under the control of the strong CMV enhancer/promoter element. However, we cannot rule out the possibility that EAT-2 did alter immune responses to Ad5 in a manner that could not be detected by the assays that we used in our studies.
Finally, although the mechanism as to how the rAd5-EAT-2 platform evaded anti-Ad5 immunity is not identified in this study, one possibility may be that despite the presence of high amounts of neutralizing Abs in the serum, these Abs are not present at high enough concentrations to fully prevent Ad transduction of APCs at the vaccination site. This, combined with an enhanced induction of innate immune responses by expression of EAT-2 from those Ad vectors successfully transducing APCs in the Ad5 immune animal, may allow for induction of beneficial innate immune responses that result in higher magnitude cellular immune responses to a coexpressed Ag, responses that remain functional and detectable for extended periods of time in Ad5 preimmune mice.
In conclusion, our results clearly establish that EAT-2 expression by vaccine platforms can significantly improve vaccine-elicited TEM CD8+ cell responses in general, and can provide a new approach for enhancing the efficacy of Ad5-based vaccines against HIV-1 specifically. Our findings also demonstrate that vaccine vectors that express EAT-2 during Ag vaccination can serve to improve the ability of a vaccine to stimulate the innate immune system, and subsequently induce potent multifunctional Ag-specific cellular immune responses even in the face of pre-existing immunity to the vaccine vector.
Supplementary Material
Acknowledgments
We thank Michigan State University Laboratory Animal Support Facilities for assistance in the humane care and maintenance of the animals used in this work.
Abbreviations used in this article:
- Ad
adenovirus
- Ad5
adenovirus serotype 5
- DC
dendritic cell
- dpi
days postinjection
- MFI
mean fluorescence intensity
- SAP
signaling lymphocyte activation molecule-associated protein
- SH
Src homology
- SLAM
signaling lymphocyte activation molecule
- TCM
central memory T
- TEM
effector memory T
- vp
virus particle
- WT
wild-type
Footnotes
A.A. was supported by the Michigan State University Foundation and by the Osteopathic Heritage Foundation. Y.A.A. was supported by a King Abdullah bin Abdulaziz scholarship, Ministry of Higher Education, Kingdom of Saudi Arabia.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Rerks-Ngarm S, Pitisuttithum P, Ganguly N, Zhang L, Tamashiro H, Cooper DA, Vun MC, Bela B, Ditangco R, Van Kinh N, et al. 2010. Defining the objectives of the AIDS vaccine for Asia network: report of the WHO-UNAIDS/Global HIV vaccine enterprise regional consultation on expanding AIDS vaccine research and development capacity in Asia. Curr. Opin. HIV AIDS 5: 435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, et al. ; MOPH-TAVEG Investigators. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med 361: 2209–2220. [DOI] [PubMed] [Google Scholar]
- 3.Pitisuttithum P, Rerks-Ngarm S, Bussaratid V, Dhitavat J, Maekanantawat W, Pungpak S, Suntharasamai P, Vanijanonta S, Nitayapan S, Kaewkungwal J, et al. 2011. Safety and reactogenicity of canarypox ALVAC-HIV (vCP1521) and HIV-1 gp120 AIDSVAX B/E vaccination in an efficacy trial in Thailand. PLoS ONE 6: e27837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, et al. ; Step Study Protocol Team. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372: 1881–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker BD, and Burton DR. 2008. Toward an AIDS vaccine. Science 320: 760–764. [DOI] [PubMed] [Google Scholar]
- 6.Korber BT, Letvin NL, and Haynes BF. 2009. T-cell vaccine strategies for human immunodeficiency virus, the virus with a thousand faces. J. Virol 83: 8300–8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson HL, and Amara RR. 2005. T cell vaccines for microbial infections. Nat. Med 11(4, Suppl.)S25–S32. [DOI] [PubMed] [Google Scholar]
- 8.Haase AT 2010. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464: 217–223. [DOI] [PubMed] [Google Scholar]
- 9.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. 2011. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, et al. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med 15: 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, et al. ; Step Study Protocol Team. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372: 1894–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priddy FH, Brown D, Kublin J, Monahan K, Wright DP, Lalezari J, Santiago S, Marmor M, Lally M, Novak RM, et al. 2008. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin. Infect. Dis 46: 1769–1781. [DOI] [PubMed] [Google Scholar]
- 13.Sumida SM, Truitt DM, Lemckert AA, Vogels R, Custers JH, Addo MM, Lockman S, Peter T, Peyerl FW, Kishko MG, et al. 2005. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol 174: 7179–7185. [DOI] [PubMed] [Google Scholar]
- 14.Haut LH, Ratcliffe S, Pinto AR, and Ertl H. 2011. Effect of preexisting immunity to adenovirus on transgene product-specific genital T cell responses on vaccination of mice with a homologous vector. J. Infect. Dis 203: 1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabitzsch ES, Xu Y, Balint JP Jr., Balcaitis S, Sanders-Beer B, and Jones FR. 2011. Induction and comparison of SIV immunity in Ad5 naïve and Ad5 immune non-human primates using an Ad5 [E1-, E2b-] based vaccine. Vaccine 29: 8101–8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemckert AA, Grimbergen J, Smits S, Hartkoorn E, Holterman L,Berkhout B, Barouch DH, Vogels R, Quax R, Goudsmit J, and Havenga MJ. 2006. Generation of a novel replication-incompetent adenoviral vector derived from human adenovirus type 49: manufacture on PER.C6 cells, tropism and immunogenicity. J. Gen. Virol 87: 2891–2899. [DOI] [PubMed] [Google Scholar]
- 17.Abe S, Okuda K, Ura T, Kondo A, Yoshida A, Yoshizaki S, Mizuguchi H, Klinman D, and Shimada M. 2009. Adenovirus type 5 with modified hexons induces robust transgene-specific immune responses in mice with pre-existing immunity against adenovirus type 5. J. Gene Med 11: 570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Xiang ZQ, Li Y, Kurupati RK, Jia B, Bian A, Zhou DM, Hutnick N, Yuan S, Gray C, et al. 2010. Adenovirus-based vaccines: comparison of vectors from three species of adenoviridae. J. Virol 84: 10522–10532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aldhamen YA, Appledorn DM, Seregin SS, Liu CJ, Schuldt NJ, Godbehere S, and Amalfitano A. 2011. Expression of the SLAM family of receptors adapter EAT-2 as a novel strategy for enhancing beneficial immune responses to vaccine antigens. J. Immunol 186: 722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuldt NJ, Aldhamen YA, Appledorn DM, Seregin SS, Kousa Y, Godbehere S, and Amalfitano A. 2011. Vaccine platforms combining circumsporozoite protein and potent immune modulators, rEA or EAT-2, paradoxically result in opposing immune responses. PLoS ONE 6: e24147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartzberg PL, Mueller KL, Qi H, and Cannons JL. 2009. SLAM receptors and SAP influence lymphocyte interactions, development and function. Nat. Rev. Immunol 9: 39–46. [DOI] [PubMed] [Google Scholar]
- 22.Calpe S, Wang N, Romero X, Berger SB, Lanyi A, Engel P, and Terhorst C. 2008. The SLAM and SAP gene families control innate and adaptive immune responses. Adv. Immunol 97: 177–250. [DOI] [PubMed] [Google Scholar]
- 23.Picker LJ, Hansen SG, and Lifson JD. 2012. New paradigms for HIV/AIDS vaccine development. Annu. Rev. Med 63: 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appledorn DM, McBride A, Seregin S, Scott JM, Schuldt N, Kiang A, Godbehere S, and Amalfitano A. 2008. Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors. Gene Ther. 15: 1606–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng P, and Graham FL. 2002. Construction of first-generation adenoviral vectors. Methods Mol. Med 69: 389–414. [DOI] [PubMed] [Google Scholar]
- 26.Seregin SS, Aldhamen YA, Appledorn DM, Zehnder J, Voss T, Godbehere S, and Amalfitano A. 2011. Use of DAF-displaying adenovirus vectors reduces induction of transgene- and vector-specific adaptive immune responses in mice. Hum. Gene Ther 22: 1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N, and Amalfitano A. 2008. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J. Immunol 181: 2134–2144. [DOI] [PubMed] [Google Scholar]
- 28.Appledorn DM, Aldhamen YA, Godbehere S, Seregin SS, and Amalfitano A. 2011. Sublingual administration of an adenovirus serotype 5 (Ad5)-based vaccine confirms Toll-like receptor agonist activity in the oral cavity and elicits improved mucosal and systemic cell-mediated responses against HIV antigens despite preexisting Ad5 immunity. Clin. Vaccine Immunol 18: 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabitzsch ES, Xu Y, Yoshida LH, Balint J, Amalfitano A, and Jones FR. 2009. Novel Adenovirus type 5 vaccine platform induces cellular immunity against HIV-1 Gag, Pol, Nef despite the presence of Ad5 immunity. Vaccine 27: 6394–6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perfetto SP, Chattopadhyay PK, Lamoreaux L, Nguyen R, Ambrozak D, Koup RA, and Roederer M. 2006. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J. Immunol. Methods 313: 199–208. [DOI] [PubMed] [Google Scholar]
- 31.Latour S, Roncagalli R, Chen R, Bakinowski M, Shi X, Schwartzberg PL, Davidson, and Veillette A. 2003. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat. Cell Biol 5: 149–154. [DOI] [PubMed] [Google Scholar]
- 32.Nichols KE, Harkin DP, Levitz S, Krainer M, Kolquist KA, Genovese C, Bernard A, Ferguson M, Zuo L, Snyder E, et al. 1998. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc. Natl. Acad. Sci. USA 95: 13765–13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roncagalli R, Taylor JE, Zhang S, Shi X, Chen R, Cruz-Munoz ME, Yin L, Latour S, and Veillette A. 2005. Negative regulation of natural killer cell function by EAT-2, a SAP-related adaptor. Nat. Immunol 6: 1002–1010. [DOI] [PubMed] [Google Scholar]
- 34.Sumida SM, Truitt DM, Kishko MG, Arthur JC, Jackson SS, Gorgone DA, Lifton MA, Koudstaal W, Pau MG, Kostense S, et al. 2004. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J. Virol 78: 2666–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seregin SS, and Amalfitano A. 2009. Overcoming pre-existing adenovirus immunity by genetic engineering of adenovirus-based vectors. Expert Opin. Biol. Ther 9: 1521–1531. [DOI] [PubMed] [Google Scholar]
- 36.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med 13: 843–850. [DOI] [PubMed] [Google Scholar]
- 37.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107: 4781–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sallusto F, Geginat J, and Lanzavecchia A. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol 22: 745–763. [DOI] [PubMed] [Google Scholar]
- 39.Barouch DH, and Korber B. 2010. HIV-1 vaccine development after STEP. Annu. Rev. Med 61: 153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lasaro MO, and Ertl HC. 2009. New insights on adenovirus as vaccine vectors. Mol. Ther 17: 1333–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sallusto F, Lenig D, Förster R, Lipp M, and Lanzavecchia A. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401: 708–712. [DOI] [PubMed] [Google Scholar]
- 42.Sallusto F, Lanzavecchia A, Araki K, and Ahmed R. 2010. From vaccines to memory and back. Immunity 33: 451–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iezzi G, Karjalainen K, and Lanzavecchia A. 1998. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 8: 89–95. [DOI] [PubMed] [Google Scholar]
- 44.Gett AV, Sallusto F, Lanzavecchia A, and Geginat J. 2003. T cell fitness determined by signal strength. Nat. Immunol 4: 355–360. [DOI] [PubMed] [Google Scholar]
- 45.Bleharski JR, Niazi KR, Sieling PA, Cheng G, and Modlin RL. 2001. Signaling lymphocytic activation molecule is expressed on CD40 ligand-activated dendritic cells and directly augments production of inflammatory cytokines. J. Immunol 167: 3174–3181. [DOI] [PubMed] [Google Scholar]
- 46.Wang N, Satoskar A, Faubion W, Howie D, Okamoto S, Feske S, Gullo C, Clarke K, Sosa MR, Sharpe AH, and Terhorst C. 2004. The cell surface receptor SLAM controls T cell and macrophage functions. J. Exp. Med 199: 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badovinac VP, Tvinnereim AR, and Harty JT. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-γ. Science 290: 1354–1358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.