Abstract
Objectives
Our objective was to perform the first multicentric study in Spain to evaluate the prevalence of feline herpesvirus-1 (FHV-1), feline calicivirus (FCV), Chlamydophila felis and Mycoplasma felis in cats with upper respiratory tract disease (URTD), conjunctivitis and/or gingivostomatitis (GS) compared with control cats; and to evaluate risk factors for these clinical conditions.
Methods
Conjunctival and oropharyngeal swabs were collected and a questionnaire regarding signalment, lifestyle, vaccination history and clinical signs was obtained for each cat. Swabs were tested for each pathogen by real-time PCR.
Results
The study population consisted of 358 cats, including 98 control cats. Among the 260 diseased cats, 127 cats presented with URTD, 149 cats had conjunctivitis, 154 cats were suffering GS; many cats presented more than one clinical condition. The prevalence observed of FHV-1, FCV, C felis and M felis was, respectively, 28.3%, 48.0%, 20.5% and 46.5% in cats with URTD; 24.2%, 43.6%, 19.5% and 38.3% in cats with conjunctivitis; and 15.6%, 58.4%, 9.1% and 37.7% in cats with GS. Prevalences in the control group were 6.1%, 15.3%, 2.0% and 20.4%, respectively. Coinfections were common among all groups of cats. Risk factors were identified for all groups. FHV-1, FCV and C felis were associated with URTD and conjunctivitis. FCV was strongly associated with GS. M felis was present in a high percentage of the population in all groups, but its role in these clinical conditions remains uncertain. Vaccination was protective for URTD and GS but not for conjunctivitis.
Conclusions and relevance
This epidemiological study describes, for the first time, prevalence for FHV-1, FCV, C felis and M felis in Spain. In general, the prevalences found are similar to those reported in other countries. Factors associated with disease expression were also identified, which are relevant for practitioners.
Introduction
Upper respiratory tract disease (URTD) is a common condition, especially in cats living in communities or in close contact with other cats. 1 The disease is multifactorial and several pathogens are involved, the main ones being feline herpesvirus-1 (FHV-1) and feline calicivirus (FCV), but other pathogens such as Bordetella bronchiseptica, Chlamydophila felis or Mycoplasma species are known or suspected to play a role in the disease. 1 Clinical signs in URTD can be similar, regardless of the pathogen involved. 1
FHV-1 typically causes upper respiratory and ocular disease. Corneal dendritic ulcers are considered pathognomonic for this infection. 2 FCV produces acute oral and respiratory disease. The appearance of oral ulcers in a cat is suggestive of an infection with FCV. A limping syndrome associated with FCV is also described, with lameness and fever occurring shortly after the oral or respiratory infection. 3 C felis is a conjunctival pathogen and produces primarily severe conjunctivitis, but some cats can also manifest sneezing and nasal discharge. 4 Mycoplasma felis is part of the normal flora in the upper respiratory tract of the cat. Although its true role as a pathogen is not completely established, there is growing evidence of its association with conjunctivitis and URTD.5,6
Feline chronic gingivostomatitis (GS) is a severe chronic inflammation of the oral mucosa. Cats affected with this disease present with extreme pain, pseudoanorexia, syalorrhoea, loss of grooming and pawing at the mouth. 7 Despite being a frequent disease, the aetiopathogenesis is still unknown. It is believed to be a multifactorial disease, where some infections could play a role. Recently, several studies have correlated an increased prevalence of infection with FCV in cats with feline chronic GS.7–11
This study had two main objectives. The first was to evaluate, for the first time in Spain, the prevalence of FHV-1, FCV, C felis and M felis in a population of cats with signs of URTD, conjunctivitis or GS compared with a control population. The second objective was to study risk factors for the presence of clinical conditions and severity of disease. The main hypotheses were that the associations among pathogens and clinical syndromes reported in other countries would be similar in Spain; that M felis has a role in URTD and conjunctivitis; and, finally, that lack of a proper vaccination would be a risk factor for the presence and severity of disease.
Materials and methods
Study population
A cross-sectional study was conducted that included 30 veterinary practices in which practitioners were members of a national feline medicine study group (Grupo de Estudio de Medicina Felina de España [GEMFE AVEPA]) in Spain between September 2010 and January 2014. Each centre was asked to enrol between 10 and 20 cats. Inclusion criteria were the presence of clinical signs suggestive of URTD, conjunctivitis or GS. A control group was also recruited from some of the clinics recruiting cases in the different regions, which consisted of cats with none of the above-mentioned clinical conditions. A questionnaire for each cat included in the study was completed with information regarding signalment, lifestyle, vaccination history, description and severity of clinical signs.
Sample collection and testing
From each cat, two conjunctival and two oropharyngeal swabs were obtained and sent refrigerated in sterile dry containers to the laboratory for analysis. Real-time PCR was performed on pooled conjunctival and oropharyngeal samples from each cat for the detection of FHV-1, FCV, C felis and M felis.
Total nucleic acid was extracted from swab samples using the QIAamp DNA Blood BioRobot MDx Kit on an automated platform (BioRobot Universal; Qiagen), according to the manufacturer’s instructions with slight modifications. Real-time PCR was performed at Vet Med Labor GmbH, a division of IDEXX Laboratories in Ludwigsburg (Germany), using the LightCycler 480 system (Roche) with proprietary forward and reverse primers and hydrolysis probes.
Target genes for pathogen detection using real-time PCR were as follows: FHV-1 B gene (S49775), FCV 5’ UTR gene (DQ424892), C felis ompA gene (AP006861) and M felis 16S rRNA gene (AY741674). The molecular diagnostics were performed with six quality controls, including a PCR-positive control of known quantity, a PCR-negative control, a negative extraction control, an internal positive control spiked into the lysis solution to monitor the nucleic acid extraction efficiency and presence or absence of inhibitory substances, an RNA quality control and an environmental contamination monitoring control.
Predictor and outcome variables
Outcome variables were clinical condition (all disease, URTD, conjunctivitis, GS) and severity of disease. The all disease group includes all those cats presenting any of the clinical conditions.
Predictor variables assessed included age (kitten: 0–0.5 years, junior 0.5–2 years, adults 2–10 years and senior >10 years); sex and neuter status; breed (purebred vs non-purebred); type of confinement (strictly indoor vs outdoor access); number of cats in the household (1, 2–6, >6); known proper vaccination; and pathogen status. Proper vaccination was considered if the adult cat had received core vaccinations (including FCV, FHV-1 and feline panleukopenia virus) in the previous 2 years and in the case of kittens and junior cats, if they had received two or three doses of primovaccination and first booster as indicated by age.
The diseased cats were further characterised according to severity of clinical signs. Rectal temperature, hyporexia and lethargy were recorded. Sneeze was graded 0–2 as none, occasional or frequent, respectively. Nasal and eye discharge were graded 0–4 as none, slight, medium, severe or very severe, respectively. Lymph nodes size was classified 0–2 as all normal, only submandibular lymph nodes enlarged or other lymph nodes enlarged, respectively. Stomatitis was graded 0–4 as none, slight reddening, moderate-to-severe reddening, severe reddening with erosions or severe reddening with ulcers, respectively.
Statistical analysis
All statistical analyses were performed using SAS 9.2. An individual cat was considered as the experimental unit, and the alpha level for determination of significance was 0.05 in all cases. Two sets of analyses were carried out using clinical condition or severity of clinical signs as outcome variables. The data set for each clinical condition only included animals with no clinical signs as the control group, and the data set for severity of clinical signs only included those animals presenting clinical signs and no control group.
Predictor variables were age, sex and neuter status, breed, lifestyle, number of cats in the household, proper vaccination and positive pathogen status. The relation between each outcome and predictor variable was calculated by univariate analysis using χ2/Fisher’s exact test in the case of categorical variables and by ANOVA for continuous variables.
Multivariable analysis of the clinical condition was carried out using logistic regression. Multivariable analysis of severity of the clinical signs was carried out by multiple linear regression for rectal temperature, and by generalised linear models for all the scores. Variables were selected for inclusion in the regression analysis if the P value calculated for their association with the outcome variable in the univariable analysis was ⩽0.25 and the variable was considered to be biologically significant. In case of co-linearity among predictors, the one to be included was selected based on its association with the outcome in the univariate analysis. The model was fitted by manual stepwise procedure. Veterinary centre of origin was initially considered in the models as a random factor but was removed as it had no significant effect (P >0.25).
Results
Characteristics of the study population
The study population consisted of 358 cats. The control group included 98 cats. The disease group included 260 cats, with 127 (35.5%) presenting with URTD, 149 (41.6%) with conjunctivitis and 154 (43.0%) with GS.
The whole population was composed of 74 (21.1%) entire males, 105 (29.9%) neutered males, 91 (25.9%) entire females and 81 (23.1%) neutered females. Most cats were not purebred (76.9%). Mean age was 3.73 years, with 96 (28.1%) kittens, 99 (28.9%) juniors, 121 (35.4%) adults and 26 (7.6%) seniors. Fifty-six percent of the cats were strictly indoor cats, whereas 44% had some outdoor access. Considering the number of cats within the household, 107 (31.9%) of them lived alone, 143 (42.7%) were in a household with 2–6 cats and 85 (25.4%) lived with more than six cats. From the whole population, only 46.1% of the cats were considered properly vaccinated.
On physical examination of the diseased cats, mean rectal temperature was 38.3ºC, only 67/260 (25.8%) had lethargy and 65/260 (25.0%) presented with hyporexia. Stomatitis grading was 0 in 38.1%, 1 in 14.3%, 2 in 19.8%, 3 in 14.7% and 4 in 13.1% of the diseased cats, with a mean of 1.50. Grade of sneezing was 0 in 42.3%, 1 in 30.0% and 2 in 27.7% of the diseased cats, with a mean of 0.85. Nasal discharge grading was 0 in 51.8%, 1 in 16.5%, 2 in 19.4%, 3 in 8.3% and 4 in 4.0% of the diseased cats, with a mean of 0.96. Eye discharge grading was 0 in 37.9%, 1 in 24.9%, 2 in 18.2%, 3 in 14.7% and 4 in 4.3% of the diseased cats, with a mean of 1.23. Conjunctivitis grading was 0 in 44.7%, 1 in 16.2%, 2 in 20.6%, 3 in 13.8% and 4 in 4.7% of the diseased cats, with a mean of 1.18. Grading of lymph node abnormalities was 0 in 57.3%, 1 in 40.3% and 2 in 2.4% of the diseased cats, with a mean of 0.45.
Prevalence and correlation of infection and clinical conditions
Overall, FHV-1 was detected in 54 (15.1%) samples, FCV in 144 (40.2%), C felis in 37 (10.3%) and M felis in 117 (32.7%). The prevalence of infection for each clinical condition is shown in Table 1. The prevalence of all pathogens was significantly higher in each of the disease groups than in the control group in the univariate analysis.
Table 1.
Prevalence and univariate analysis of each pathogen for each one of the clinical conditions
| Control (n = 98) |
All disease (n = 260) |
URTD (n = 127) |
Conjunctivitis (n = 149) |
GS (n = 154) |
|
|---|---|---|---|---|---|
| FHV-1 | |||||
| Positive | 6 (6.1) | 48 (18.5) | 36 (28.3) | 36 (24.2) | 24 (15.6) |
| P value | 0.004 | <0.001 | <0.001 | 0.024 | |
| FCV | |||||
| Positive | 15 (15.3) | 129 (49.6) | 61 (48.0) | 65 (43.6) | 90 (58.4) |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Chlamydophila felis | |||||
| Positive | 2 (2.0) | 35 (13.5) | 26 (20.5) | 29 (19.5) | 14 (9.1) |
| P value | 0.002 | <0.001 | <0.001 | 0.025 | |
| Mycoplasma felis | |||||
| Positive | 20 (20.4) | 97 (37.3) | 59 (46.5) | 57 (38.3) | 58 (37.7) |
| P value | 0.002 | <0.001 | 0.003 | 0.003 |
Data are n (%). Bold indicates significant P value
URTD = upper respiratory tract disease; GS = gingivostomatitis; FHV-1 = feline herpesvirus-1; FCV = feline calicivirus
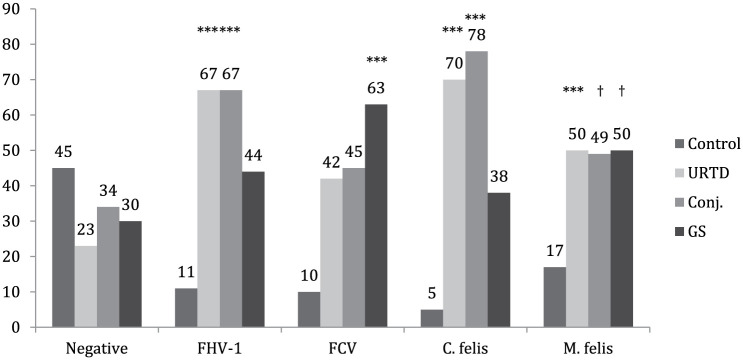
Negative results for all pathogens were obtained in 143 (39.9%) samples. From these cats, 45% were controls, 23% had URTD, 34% had conjunctivitis and 30% had GS (Figure 1). Positive FHV-1 status was significantly related to URTD (67%; P <0.001) and conjunctivitis (67%; P <0.001) but not to GS (44%; P = 0.882). Positive FCV status was significantly related to GS (63%; P <0.001) but was not related to URTD (45%; P = 0.062) or conjunctivitis (42%; P = 0.325). Positive C felis status was significantly related to URTD (70%; P <0.001) and conjunctivitis (78%; P <0.001) but not to GS (38%; P = 0.600). And, finally, positive M felis status was significantly related to URTD (50%; P <0.001) but did not attain significance for conjunctivitis (49%; P = 0.068) or GS (50%; P = 0.087).
Figure 1.
Percentages of cats presenting each clinical condition according to the positivity status for each one of the studied pathogens. Bars marked with symbols for each one of the pathogens are significantly different from the corresponding bar in the negative group (***P <0.001; †P <0.1). URTD = upper respiratory tract disease; Conj = conjunctivitis; GS = gingivostomatitis
Coinfections were very common, occurring in 28.2% of all samples tested and 47.0% of all positive samples. In the control group, coinfections were present in 9% of the cats and 23.5% of the positive samples. In the diseased cats, coinfections were present in 35.4% of the total and 50.8% of the positive samples. Coinfection was more frequent in animals showing all three clinical conditions (71% of co-infected) than in those showing two (35%) or one (28%) of the clinical conditions.
Univariate and multivariable assessment of risk factors for the clinical conditions
Table 2 shows the univariate analysis of the risk factors for each of the disease groups. Age, sex, neutering status, being purebred, being kept indoors, number of cats in the household and vaccination history were variably associated with all groups. In the multivariable analysis (Table 3), only the following factors remained significant: URTD was significantly associated with positive results for FHV-1, FCV and C felis (in addition to being male, purebred and living in a multi-cat household); conjunctivitis was significantly associated with positive results for FHV-1, FCV and C felis (in addition to being purebred, having access to the outdoors and living in a multi-cat household); and GS was significantly associated with positive results for FCV and C felis (in addition to being older, male, purebred and living in a multi-cat household). Not being properly vaccinated was a significant risk factor for URTD and GS.
Table 2.
Univariate analysis of the studied risk factors for each one of the clinical conditions
| Control (n = 98) | All disease (n = 260) | URTD (n = 127) | Conjunctivitis (n = 149) | GS (n = 154) | |
|---|---|---|---|---|---|
| Age | |||||
| Kitten | 25/95 (26.3) | 71/255 (27.8) | 55/126 (43.7) | 60/148 (40.5) | 18/150 (12.0) |
| Junior | 38/95 (40.0) | 61/255 (23.9) | 28/126 (22.2) | 38/148 (25.7) | 39/150 (26.0) |
| Adult | 24/95 (25.3) | 97/255 (38.0) | 37/126 (29.4) | 45/148 (30.4) | 70/150 (46.7) |
| Senior | 8/95 (8.4) | 26/255 (10.2) | 6/126 (4.8) | 5/148 (3.4) | 23/150 (15.3) |
| P value | 0.024 | 0.008 | 0.015 | <0.001 | |
| Sex | |||||
| Male | 42/98 (42.9) | 144/260 (55.4) | 76/127 (59.8) | 76/149 (51.0) | 90/154 (58.4) |
| Female | 56/98 (57.1) | 116/260 (44.6) | 51/127 (40.2) | 73/149 (49.0) | 64/154 (41.6) |
| P value | 0.033 | 0.011 | 0.210 | 0.016 | |
| Neuter status | |||||
| Neutered | 56/98 (57.1) | 130/253 (51.4) | 46/124 (37.1) | 52/146 (35.6) | 95/148 (64.2) |
| Entire | 42/98 (42.9) | 123/253 (48.6) | 78/124 (62.9) | 94/146 (64.4) | 53/148 (35.8) |
| P value | 0.288 | 0.002 | <0.001 | 0.266 | |
| Purebred | |||||
| Yes | 12/98 (12.2) | 71/258 (27.5) | 30/125 (24.0) | 41/147 (27.9) | 50/154 (32.5) |
| No | 86/98 (87.8) | 187/258 (72.5) | 95/125 (76.0) | 106/147 (72.1) | 104/154 (67.5) |
| P value | 0.005 | <0.001 | <0.001 | 0.005 | |
| Indoor (strict) | |||||
| Yes | 68/98 (69.4) | 129/256 (50.4) | 57/125 (45.6) | 69/148 (46.6) | 79/150 (52.7) |
| No | 28/98 (28.6) | 127/256 (49.6) | 68/125 (54.4) | 79/148 (53.4) | 71/150 (47.3) |
| P value | <0.001 | <0.001 | <0.001 | 0.005 | |
| Number of cats | |||||
| 1 | 52/89 (58.4) | 55/246 (22.4) | 25/120 (20.8) | 34/138 (24.6) | 36/149 (24.1) |
| 2–6 | 21/89 (23.6) | 122/246 (49.6) | 57/120 (47.5) | 65/138 (47.1) | 72/149 (48.3) |
| >6 | 16/89 (18.0) | 69/246 (28.0) | 38/120 (31.7) | 39/138 (28.3) | 41/149 (27.5) |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Proper vaccination | |||||
| Yes | 57/98 (58.2) | 108/260 (41.5) | 46/127 (36.2) | 72/149 (48.3) | 59/154 (38.3) |
| No | 41/98 (41.8) | 152/260 (58.5) | 81/127 (63.8) | 77/149 (51.7) | 95/154 (61.7) |
| P value | 0.009 | 0.001 | 0.130 | 0.002 |
Data are n (%). Bold indicates significant P value
URTD = upper respiratory tract disease; GS = gingivostomatitis
Table 3.
Odds ratio and 95% confidence intervals for the risk factors found to be significant in the multivariable analysis for each clinical condition
| Predictor variables | All disease | URTD | Conjunctivitis | GS |
|---|---|---|---|---|
| Age, adult vs kitten | 8.2 (2.6–26.1)** | |||
| Male | 2.2 (1.2–4.1)* | 3.0 (1.4–6.4)** | 3.3 (1.5–7.2)** | |
| Purebred | 4.0 (1.8–8.9)*** | 4.9 (1.8–13.4)** | 3.8 (1.7–8.6)** | 5.1 (2.0–13.0)*** |
| Outdoor | 2.6 (1.1–6.1)* | |||
| Number of cats, 2–6 vs 1 | 5.7 (2.8–11.5) *** | 7.2 (2.8–18.1)*** | 3.8 (1.8–8.0)** | 6.5 (2.6–16.2)*** |
| Lack of proper vaccination | 2.7 (1.4–5.1)** | 4.0 (1.7–9.6)*** | 2.7 (1.1–6.4)* | |
| FHV-1-positive | 3.2 (1.1–9.6)* | 3.3 (1.2–9.3)* | ||
| FCV-positive | 5.3 (2.6–11.0)*** | 4.9 (2.0–11.8)*** | 3.1 (1.4–7.2)** | 13.9 (5.3–36.1)*** |
| C felis-positive | 20.8 (2.4–182)** | 59.3 (5.5–635)*** | 27.2 (3.3–222)** | 12.3 (1.1–134)* |
Data are odds ratio (95% confidence interval)
P <0.05; **P <0.01; ***P <0.001
URTD = upper respiratory tract disease; GS = gingivostomatitis; FHV-1 = feline herpesvirus-1; FCV = feline calicivirus
Multivariable assessment of risk factors for the severity of disease
Significant predictors for severity of each clinical sign are summarised in Table 4. FHV-1 was significantly related to lethargy, and more severe sneezing, nasal and ocular discharge, and conjunctivitis. FCV was significantly related to stomatitis, hyporexia, lethargy and more severe enlarged lymph nodes. Chlamydophila felis was only significantly related to more severe ocular discharge and conjunctivitis. Finally, M felis was significantly related to more severe sneezing and nasal discharge. Age was inversely related to severity of most clinical signs, while it was related to more severe GS. Finally, lack of proper vaccination was related to more hyporexia and more severe nasal discharge.
Table 4.
Odds ratio and 95% confidence intervals for the risk factors found to be significant in the multivariable analysis for severity of disease
| Clinical sign | Significant predictors | Values | ||
|---|---|---|---|---|
| Lethargy | FHV-1** | 2.7 (1.3–5.5) | ||
| FCV* | 2.0 (1.0–3.8) | |||
| Hyporexia | Purebred* | 0.45 (0.20–1.02) | ||
| Lack of proper vaccination* | 2.22 (1.02–5.00) | |||
| FCV** | 2.4 (1.2–4.7) | |||
| Stomatitis (0–4 score) |
Age*** (vs kitten) | Junior 8.0 (3.6–17.9) |
Adult 12.1 (5.7–25.7) |
Senior 25.4 (9.3–69.5) |
| FCV*** | 4.1 (2.4–7.1) | |||
| Sneeze (0–2 score) |
Age*** (vs kitten) | Junior 0.38 (0.19–0.78) |
Adult 0.20 (0.10– 0.38) |
Senior 0.08 (0.03–0.23) |
| Indoor* | 1.96 (1.11–3.44) | |||
| FHV-1** | 2.18 (1.24–3.85) | |||
| M felis * | 2.87 (1.45–5.65) | |||
| Nasal discharge (0–4 score) |
Age*** (vs kitten) | Junior 0.35 (0.17–0.71) |
Adult 0.19 (0.10–0.37) |
Senior 0.07 (0.02–0.23) |
| Proper vaccination** | 1.85 (1.06–3.23) | |||
| FHV-1** | 2.73 (1.38–5.41) | |||
| M felis *** | 2.53 (1.46–4.59) | |||
| Ocular discharge (0–4 score) |
Age*** (vs kitten) | Junior 0.24 (0.12–0.50) |
Adult 0.23 (0.12–0.43) |
Senior 0.07 (0.02–0.22) |
| FHV-1*** | 3.88 (1.98–7.52) | |||
| FCV*** | 0.38 (0.22–0.63) | |||
| C felis * | 2.07 (1.01–4.26) | |||
| Conjunctivitis (0–4 score) |
Age*** (vs kitten) | Junior 0.29 (0.14–0.58) |
Adult 0.19 (0.10–0.38) |
Senior 0.07 (0.02–0.25) |
| FHV-1*** | 3.80 (1.96–7.25) | |||
| C felis *** | 3.38 (1.96–7.35) | |||
| Lymph nodes (0–2 score) |
FCV** | 2.22 (1.28–3.85) | ||
Data are odds ratio (95% confidence interval)
P <0.05; **P <0.01; ***P <0.001
FHV-1 = feline herpesvirus-1; FCV = feline calicivirus
Discussion
This is the first epidemiological study of feline upper respiratory pathogens in Spain. The reported prevalence in other countries has been variable and results depend, among other factors, on the specific technique used and the population of cats studied. The present study aimed at reporting prevalence in a wide segment of the population and therefore it was designed as a multicentric study that included cats from single and multi-cat households, privately owned and free-roaming cats included in a trap–neuter–return (TNR) programme, from several regions in Spain.
Prevalence data obtained in the control group is in agreement with those previously reported. FHV-1 was detected in 6% of control cats. Previous studies based on PCR have reported a wide range of results, from 2.6% in privately owned cats in the USA to 63% in a shelter in Korea.6,12 Consideration must be given to the fact that FHV-1 is shed intermittently and remains latent in the trigeminal ganglia most of the time, undetectable by PCR. 13 FCV was detected in 15% of control cats. Prevalences of 0% to 29% have been reported in healthy asymptomatic cats.11,12,14–16 Chlamydophila felis was only detected in 2% of control cats. Most studies have previously reported null prevalence in healthy cats,6,12,17,18 while two multicentric studies performed in shelters reported higher prevalences of 3.0% and 15.4%, respectively.15,17 Finally, M felis is the least studied of the four pathogens. As it is a known commensal of the feline upper respiratory tract, prevalence in healthy cats is higher than the other pathogens. 1 Our study showed a prevalence of 20%, while another study performed in shelters in the USA obtained prevalences of 0–19.4%, depending on the shelter examined. 17
In total, 127 cats with URTD were included in the study. Prevalence of the four pathogens was higher in the URTD group than in the control cats, and these differences were significant in the multivariable analysis, with the exception of M felis. Whether these pathogens were primarily responsible for the clinical signs, concomitant infections or appeared secondarily to the disease could not be established. As expected, FHV-1 infection was associated with increased severity of respiratory and ocular signs. Interestingly, M felis was significantly associated with increased severity in nasal discharge and sneezing, suggesting some role in these respiratory signs, either as a primary pathogen or, more likely, as an opportunistic secondary infection. Males had a three times higher risk of URTD than females, as reported previously in URTD and FHV-1 infection.13,19,20 The reason for this sex predisposition is unknown. In other studies, intact males were predisposed and it was assumed that they would be more likely to go outdoors and more likely to be exposed to contagious agents. However, based on our results, strictly indoor cats do not seem to be protected from infection and, therefore, the results do not support this hypothesis. However, living in a multi-cat household was, in fact, an important risk factor, as previously described, with cats from a multi-cat household having 7.2 times more of a risk of developing URTD. 15 Purebred predisposition was also demonstrated in cats entering a UK shelter, although a reason for this finding is not clear. 21
In total, 149 cats with conjunctivitis were included in the study. Prevalence of the four pathogens was also higher than in control cats, and these differences were again significant in the multivariable analysis for conjunctivitis, except for M felis. Many of the cats with conjunctivitis also had signs of URTD and therefore there is great overlap between the two groups, which could explain the apparent relationship between FCV and conjunctivitis. As expected, C felis was associated with severity of ocular signs. The role of M felis as a primary pathogen in conjunctivitis is still debated.6,17,22–26 Results from our study do not support this association. Although cats in the conjunctivitis group had M felis more commonly detected than cats in the control group (38.3% vs 20.4%), this difference did not remain significant in the multivariable analysis and M felis was not associated with increased severity of ocular signs. Being purebred was a significant risk factor for conjunctivitis. However, no breed predilection has been found in previous studies, and, again, the reason for this finding is not clear.4,27 Surprisingly, young age was not significant in the multivariable analysis. Conjunctivitis is commonly found in young cats, and most of the time it is associated with C felis or FHV-1.4,27,28 Contact with other cats seems of special importance in conjunctivitis, as being allowed outdoors and living with other cats were significant risk factors.
Finally, 154 cats with GS were included in the study. In this population, the prevalence of FCV was much higher than in control cats and the highest of all groups. FCV was significantly associated with GS and was related to general signs of malaise such as hyporexia, increased lymph nodes or lethargy, as well as being associated with severity of stomatitis. The increased severity of hyporexia is probably related to the presence of lesions in the oral cavity, frequently associated with this viral infection. 3 Although some previous studies did not find a correlation between FCV and chronic feline GS, there is growing evidence of a link between the two.7–11 However, only 58% of the cats with GS were positive for FCV and, therefore, there is still a large proportion of cats with GS that cannot be explained by FCV infection alone. Other infectious diseases, as well as the cat’s own immune response, have been implicated.11,29 C felis was also, surprisingly, significantly associated with GS, although the confidence interval for the odds ratio has a lower limit close to 1, which could indicate a weaker association. Male, purebred and older cats were associated with GS. Feline chronic GS is known to be a disease of mature cats, but no sex and breed predispositions have been reported. 29 Cats not living alone were 6.5 times more likely to develop GS. The reason for this finding is not obvious but could relate to the increase risk of FCV infection and the development of escaping quasi-species in multi-cat environments.30,31
The lack of a proper vaccination protocol was a risk factor for cats with URTD and GS. However, it was not associated with conjunctivitis. This could be explained because of the important relationship between conjunctivitis and C felis, with cats with C felis being much more likely to present conjunctivitis. Vaccination against C felis is considered non-core and, therefore, it was not included in the definition of proper vaccination. 32 Twenty-three percent of the cats properly vaccinated were not vaccinated against C felis, and this might explain the apparent lack of efficacy of vaccination in preventing conjunctivitis. Looking at severity of disease, vaccination protected against hyporexia and reduced the severity of nasal discharge. However, it did not protect against ocular discharge and conjunctivitis, probably for the same reasons mentioned above. It is worth mentioning that the definition of properly vaccinated adult cats included only those that had received a booster in the past 2 years. Current guidelines for vaccination of cats recommend tri-annual revaccination for the general feline population, but some guidelines recommend more frequent boosters (ie, annual) in cats at high risk. Therefore, 2 years were chosen arbitrarily to improve the ability to detect an effect of vaccination in the whole population.2,3,32,33
The study has some limitations. First, PCR was performed on pooled samples from conjunctival and oropharyngeal mucosa. It would have been interesting to obtain separate results but it would have increased costs of the study. Second, although questionnaires were obtained with all the samples, some of the information was missing, especially in animals from TNR programmes where no vaccination history or age was known. Finally, the questionnaire was not designed to differentiate cats with acute stomatitis usually associated with FCV infection from cats with chronic feline GS syndrome, and they were all grouped together in the GS group. However, most of the cats in this group were suffering from the chronic form and therefore conclusions from this group may be extrapolated to cats suffering chronic feline GS syndrome.
Conclusions
This epidemiological study describes, for the first time, prevalence for FHV-1, FCV, C felis and M felis infections in Spain. These prevalences are, in general, similar to prevalences reported in other countries. Some risk factors are described for these clinical conditions. FHV-1, FCV and C felis were associated with URTD and conjunctivitis. FCV was strongly associated with GS. M felis was present in a high percentage of the population in all groups, but its role in these clinical conditions remains uncertain. Finally, proper core vaccination (as defined above) was protective for URTD and GS but not for conjunctivitis.
Acknowledgments
We thank all participating veterinarians for collecting the samples and for providing the background information on the cats.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The study was partially funded by Merial.
Accepted: 26 January 2016
References
- 1. Cohn LA. Feline respiratory disease complex. Vet Clin Small Anim 2011; 41: 1273–1289. [DOI] [PubMed] [Google Scholar]
- 2. Thiry E, Addie D, Bélak S, et al. Feline herpesvirus infection. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Radford AD, Addie D, Bélak S, et al. Feline calicivirus infection. ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sykes JE. Feline chlamydiosis. Clin Tech Small Anim Pract 2005; 20: 129–134. [DOI] [PubMed] [Google Scholar]
- 5. Chandler JC, Lappin MR. Mycoplasma respiratory infections in small animals: 17 cases (1988–1999). J Am Anim Hosp Assoc 2002; 38: 111–19. [DOI] [PubMed] [Google Scholar]
- 6. Low HC, Powell CC, Veir JK, et al. Prevalence of feline herpesvirus 1, Chlamydophila felis and Mycoplasma spp DNA in conjunctival cells collected from cats with and without conjunctivitis. Am J Vet Res 2007; 68: 643–648. [DOI] [PubMed] [Google Scholar]
- 7. Dolieslager SMJ, Lappin DF, Bennett D, et al. The influence of oral bacteria on tissue levels of Toll-like receptor and cytokine mRNAs in feline chronic gingivostomatitis and oral health. Vet Immunol Immunopathol 2013; 151: 263–274. [DOI] [PubMed] [Google Scholar]
- 8. Addie DD, Radford A, Yam PS, et al. Cessation of feline calicivirus shedding coincident with resolution of chronic gingivostomatitis in a cat. J Small Anim Pract 2003; 44: 172–176. [DOI] [PubMed] [Google Scholar]
- 9. Lommer MJ, Verstraete FJM. Concurrent oral shedding of feline calicivirus and feline herpesvirus 1 in cats with chronic gingivostomatitis. Oral Microbiol Immunol 2003; 18: 131–134. [DOI] [PubMed] [Google Scholar]
- 10. Dowers KL, Hawley JR, Brewer MM, et al. Association of Bartonella species, feline calicivirus and feline herpesvirus 1 infection with gingivostomatitis in cats. J Feline Med Surg 2010; 12: 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Belgard S, Truyen U, Thibault JC, et al. Relevance of feline calicivirus, feline immunodeficiency virus, feline leukemia virus, feline herpesvirus and Bartonella henselae in cats with chronic gingivostomatitis. Berl Münch Tierärztl Wochenschr 2010; 123: 369–376. [PubMed] [Google Scholar]
- 12. Kang BT, Park HM. Prevalence of feline herpesvirus-1, feline calicivirus and Chlamydophila felis in clinically normal cats at a Korean animal shelter. J Vet Sci 2008; 9: 207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Binns SH, Dawson S, Speakman AJ, et al. A study of feline upper respiratory tract disease with reference to prevalence and risk factors for infection with feline calicivirus and feline herpesvirus. J Feline Med Surg 2000; 2: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henzel A, Sperotto Brum MC, Lautert C, et al. Isolation and identification of feline calicivirus and feline herpesvirus in southern Brazil. Braz J Microbiol 2012; 43: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Helps CR, Lait P, Damhuis A, et al. Factors associated with upper respiratory tract disease caused by feline herpesvirus, feline calicivirus, Chlamydophila felis and Bordetella bronchiseptica in cats: experience from 218 European catteries. Vet Rec 2005; 156: 669–673. [DOI] [PubMed] [Google Scholar]
- 16. Quimby JM, Elston T, Hawley J, et al. Evaluation of the association of Bartonella species, feline herpesvirus 1, feline calicivirus, feline leukemia virus and feline immunodeficiency virus with chronic feline gingivostomatitis. J Feline Med Surg 2008; 10: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bannasch MJ, Foley JE. Epidemiologic evaluation of multiple respiratory pathogens in cats in animal shelters. J Feline Med Surg 2005; 7: 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mochizuki M, Kawakami K, Hashimoto M, et al. Recent epidemiological status of feline upper respiratory infections in Japan. J Vet Med Sci 2000; 62: 801–807. [DOI] [PubMed] [Google Scholar]
- 19. Dinnage JD, Scarlett JM, Richards JR. Descriptive epidemiology of feline upper respiratory tract disease in an animal shelter. J Feline Med Surg 2009; 11: 816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong WT, Kelman M, Ward MP. Surveillance of upper respiratory tract disease in owned cats in Australia, 2009–2012. Prev Vet Med 2013; 112: 150–155. [DOI] [PubMed] [Google Scholar]
- 21. Edwards DS, Coyne K, Dawson S, et al. Risk factors for time to diagnosis of feline upper respiratory tract disease in UK animal adoption shelters. Prev Vet Med 2008; 87: 327–339. [DOI] [PubMed] [Google Scholar]
- 22. Veir JK, Ruch-Gallie R, Spindel ME, et al. Prevalence of selected infectious organisms and comparison of two anatomic sampling sites in shelter cats with upper respiratory tract disease. J Feline Med Surg 2008; 10: 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holst BS, Hanas S, Berndtsson LT, et al. Infectious causes for feline upper respiratory tract disease – a case-control study. J Feline Med Surg 2010; 12: 783–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hartmann AD, Hawley J, Werckenthin C, et al. Detection of bacterial and viral organisms from the conjunctiva of cats with conjunctivitis and upper respiratory tract disease. J Feline Med Surg 2010; 12: 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gourkow N, Lawson JH, Hamon SC, et al. Descriptive epidemiology of upper respiratory disease and associated risk factors in cats in an animal shelter in coastal western Canada. Can Vet J 2013; 54: 132–138. [PMC free article] [PubMed] [Google Scholar]
- 26. Lee-Fowler T. Feline respiratory disease: what is the role of Mycoplasma species? J Feline Med Surg 2014; 16: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sykes JE, Anderson GA, Studdert VP, et al. Prevalence of feline Chlamydia psittaci and feline herpesvirus 1 in cats with upper respiratory tract disease. J Vet Intern Med 1999; 13: 153–162. [DOI] [PubMed] [Google Scholar]
- 28. Wills JM, Howard PE, Gruffydd-Jones TJ, et al. Prevalence of Chlamydia psittaci in different cat populations in Britain. J Small Anim Pract 1988; 29: 327–339. [Google Scholar]
- 29. Healey KAE, Dawson S, Burrow R, et al. Prevalence of feline chronic gingivostomatitis in first opinion veterinary practice. J Feline Med Surg 2007; 9: 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Radford AD, Turner PC, Bennet M, et al. Quasispecies evolution of a hypervariable region of the feline calicivirus capsid gene in cell culture and in persistently infected cats. J Gen Virol 1998; 79: 1–10. [DOI] [PubMed] [Google Scholar]
- 31. Coyne KP, Gaskell RM, Dawson S, et al. Evolutionary mechanisms of persistence and diversification of a calicivirus within endemically infected natural host populations. J Virol 2007; 81: 1961–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vaccination Guidelines Group, Day MJ, Horzinek MC, et al. WSAVA guidelines for the vaccination of dogs and cats. J Small Anim Pract 2010; 51: 1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scherk MA, Ford RB, Gaskell RM, et al. 2013 AAFP Feline Vaccination Advisory Panel Report. J Feline Med Surg 2013; 15: 785–808. [DOI] [PMC free article] [PubMed] [Google Scholar]