Abstract
Objectives
The objective of this study was to determine disease progression, association between neurological signs and magnetic resonance imaging (MRI) findings, and long-term outcome in feline thiamine deficiency associated with defective dry food.
Methods
The clinical records of 17 cats diagnosed with thiamine deficiency related to a defective dry food were examined and data collected. The thiamine level in the food was analysed by liquid chromatography–tandem mass spectrometry.
Results
The thiamine level in the food was below the recommendation of the National Research Council. Fifteen cats were fed the food exclusively. Prior to the acute development of neurological signs, most cats displayed non-specific signs such as anorexia, lethargy or vomiting. Vestibular signs of varying severity were observed in 94% of the cats, and all but one of these presented with bilateral dysfunction. Other main neurological signs included altered mentation (76%), blindness (59%) and seizures (59%). Moreover, 80% of the cats with seizures presented with cluster seizures or status epilepticus. MRI abnormalities consistent with findings reported in the previous literature were detected in five cases. MRI was unremarkable in one cat with ongoing severe neurological signs even though thiamine had been administered. Most surviving cats recovered rapidly within 2 weeks of treatment and had either returned to normal or had minimal neurological signs at the 2 month follow-up. One cat recovered slowly over 6 months. Most cats with seizures in the initial stage of the disease remained seizure free at the 24 month follow-up.
Conclusions and relevance
This study documented the association between feline thiamine deficiency and defective dry food. MRI examination provided valuable information in the diagnosis. However, normal MRI findings do not exclude the diagnosis of feline thiamine deficiency, especially once thiamine has been supplemented. MRI findings also may not always reflect the neurological status or severity. If treated promptly, most cats will recover rapidly with a good outcome. Occasionally, recovery may be slow and take several months.
Introduction
Thiamine is required for the metabolism of glucose, lipids and amino acids, and the synthesis of neurotransmitters.1,2 Thiamine diphosphate, the biologically active form of thiamine, functions as a cofactor in important enzyme systems involving the conversion of pyruvate to acetyl-CoA, the tricarboxylic acid cycle, pentose phosphate cycle and the metabolism of branched-chain amino acids.1,3 Thiamine is therefore essential for normal cellular function in all animals. 3 The nervous system is especially sensitive to thiamine deficiency (TD) owing to its dependence on glucose metabolism. Besides the key role of thiamine in energy metabolism, its non-cofactor role is also established.4,5 For example, unphosphorylated thiamine can affect membrane ion channel activity and participate in nerve conduction and synaptic transmission.5,6
Thiamine is synthesised only in bacteria, fungi and plants. 5 Although it is synthesised by the intestinal microflora of cats, the site of synthesis is too distal for absorption. Thus, endogenously synthesised thiamine does not significantly contribute to meeting the body’s needs.7,8 Additionally, only small amounts of thiamine are stored in the body. 8 Consistent dietary thiamine is therefore necessary; it is particularly crucial for cats as their dietary requirement is relatively high compared with human beings, dogs, foxes, minks and monkeys.8–13 Clinical signs in the initial stage of feline TD are often vague and non-specific, making early diagnosis difficult. If untreated, further depletion results in progressive encephalopathy with various neurological signs, such as ventroflexion of the head and neck, blindness, mydriasis, ataxia, vestibular signs, altered mentation, seizures, coma and even death.14,15 A cardiac arrhythmic syndrome with electrocardiographic changes has also been reported in cats in the end stage of the disease. 16
Most documented issues underlying feline TD are diet related, such as food containing substances that inactivate thiamine (eg, thiaminase and sulfites), a home-prepared cooked diet and commercial canned food with insufficient thiamine.7,8,15,17–23 Food sources rich in thiamine include grains, meats, liver, poultry, fish, eggs, milk, vegetables and legumes. 24 However, thiamine is vulnerable to destruction. High temperatures, a neutral or alkaline state, and chlorinated water involved in food processing all lead to the loss of thiamine.7,8,25 For the production of commercial pet foods, the manufacturers therefore add high levels of synthetic thiamine to compensate for losses during processing.26,27 Nevertheless, TD in cats with a diet primarily consisting of commercial canned food are still documented occasionally.7,15,17,18,21 Compared with dry foods, the process of producing canned foods (eg, sterilisation with heat and/or adding alkalinising gelling agents) destroys a larger amount of thiamine. 27 In addition, a complementary diet could be mistakenly used as an exclusive diet, as previously reported. 7 Furthermore, a recent study revealed that the thiamine concentration was below the recommended allowance of the National Research Council (NRC) in 15.6% of 90 commercial canned foods that were formulated as a complete diet for cats. 28 This finding highlights the importance of analysing the final commercial food product to ensure the thiamine requirement is met.
Feline TD primarily associated with defective dry food has not been reported in the literature, although in the past 6 years two documented recalls of dry cat food by the US Food and Drug Administration were potentially due to this issue. 29 Here we report an outbreak of feline TD in Taiwan. The objectives of the study were to describe disease progression, the association between neurological signs and findings on magnetic resonance imaging (MRI), and the long-term outcome in feline TD associated with defective dry cat food.
Materials and methods
The medical records of the National Taiwan University Veterinary Hospital (NTUVH) were retrospectively reviewed to search for cats diagnosed with TD between October 2012 and September 2013. The diagnosis was based on the development of neurological dysfunction while being fed with a specific batch of commercial dry food. The batch was manufactured in the USA on 10 July 2012 via the process of extrusion and launched onto the market in Taiwan as a local brand at the end of October 2012 (Holistic Recipe Solution, Indoor Cat Hairball Control, Chicken & Salmon with Veg & Fruits; Isco Company). The food was later confirmed to contain insufficient thiamine by liquid chromatography–tandem mass spectrometry. Cat owners were officially warned against its use and the product was recalled from the market at the end of February 2013. Data concerning breed, age at onset, sex, diet history, duration and progression of non-specific and neurological signs, findings on neurological examination, diagnostic test results, treatment and outcome were collected. Diagnostic tests included haematology and serum biochemical analysis in all cats, and cerebrospinal fluid (CSF) analysis and MRI of the brain (0.2 Tesla Vet-MR; Esaote) in six cats. The following MRI pulse sequences were used: T1-weighted (T1W), T2-weighted (T2W) and fluid-attenuated inversion recovery (FLAIR). Additional T1W and FLAIR images were acquired following the intravenous administration of gadopentetate dimeglumine (Magnevist, Bayer Schering Pharma AG) at a dose of 0.15 mmol/kg of body weight. Follow-up information was collected via physical and neurological examination or telephone conversation with the owner at 1 week, 2 weeks, 2–4 months, 6 months and 24 months after the initial presentation.
Results
The thiamine level in this batch of food was 0.35 mg/kg dry matter (DM), below the recommendation of the NRC (⩾5.6 mg/kg DM) 11 and the Association of American Feed Control Officers (⩾5 mg/kg DM). 27 The timeline of the manufacture, launch and recall of the defective batch and the distribution of cases in the study during the outbreak is illustrated in Figure 1. Records of 17 cats (five males, 12 females) aged from 7 months to 10 years (mean ± SD 5.6 ± 2.9 years) were included in the study. Thirteen cats (76%) were domestic shorthairs and the remainder were Persians. Fifteen cats were fed this diet exclusively. In addition to the daily dry food, one cat was also fed small amount of canned food (maximum 25% of the meal volume) every few days and the other cat every 2–3 weeks. Most owners could not precisely recall the exact duration of the defective diet before their cats developed clinical signs associated with TD. However, most cases (94%) in this outbreak were diagnosed 2–4 months after the defective food launched on the local market at the end of October 2012 (Figure 1).
Figure 1.
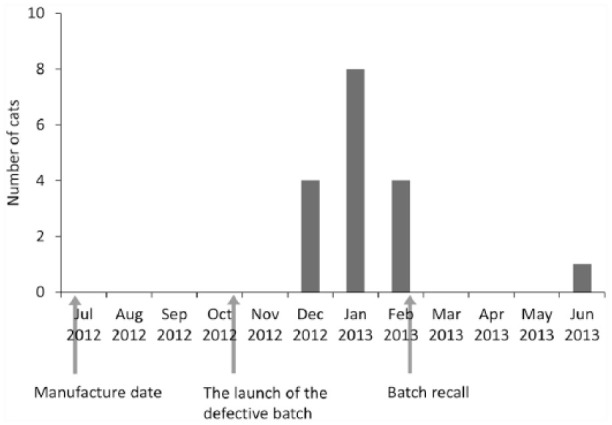
Timeline of the manufacture, launch and recall of the defective batch and the distribution of 17 cases in this study during the outbreak. The first case was seen on 25 December 2012
Non-specific signs were reported in 94% of cats, including anorexia (71%), vomiting (53%), lethargy (41%) and tachypnoea (18%). Among the 12 cats for which there was detailed information, these clinical signs preceded the onset of neurological dysfunction by 0–60 days (median 3 days).
Before presenting to the NTUVH, the duration of neurological signs ranged from 1 to 7 days (median 2 days). Vestibular signs of varying severity were observed most commonly (16/17; 94%), including ataxia, truncal ataxia, absent physiological nystagmus, spontaneous or positional nystagmus, head tilt and/or inability to walk. All but one of these 16 cats presented with bilateral vestibular dysfunction. Neurological signs in the cat without vestibular signs included seizures, blindness and altered mentation (cat 5 in Table 1 and Figure 2). Furthermore, active cervical ventroflexion and rolling forward when being lifted up was observed in some cats. Other main neurological signs included altered mentation (76%), blindness (59%) and seizures (59%). Generalised tonic–clonic seizure was the most commonly reported seizure type, and 80% of cats with seizures presented with cluster seizures or status epilepticus. The neuroanatomical localisation was determined as multifocal, consisting of forebrain and brainstem in 13 cats (76%), brainstem in three cats (18%) and forebrain in one cat (6%).
Table 1.
Clinical signs, lesion localisation and imaging findings in six cats that underwent magnetic resonance imaging (MRI)
| Cat | Neurological signs and deficits | Areas with hyperintense changes on MRI T2W and FLAIR images |
|---|---|---|
| Lesion localisation based on neurological examination | Main location of MRI changes | |
| 1 | Obtundation, disorientation, head tilt, non-ambulatory tetraparesis, head swing, truncal ataxia, absent vestibular eye movement, nystagmus, mydriasis, postural reaction deficits | LGN, CC*, FN, PGM |
| Brainstem | Forebrain, brainstem | |
| 2 | Obtundation, ataxia, tetraparesis, absent menace response, absent vestibular eye movement, blindness, decreased PLR, postural reaction deficits, seizures | Cerebral cortex*, LGN |
| Forebrain, brainstem | Forebrain | |
| 3 | Disorientation, truncal ataxia, rolling, falling, absent PLR/menace response/vestibular eye movement, mydriasis, seizures, head pressing, nystagmus, blindness, tetraparesis, postural reaction deficits | Cerebral cortex*, LGN*, CC*, FN, VN* |
| Forebrain, brainstem | Forebrain, brainstem | |
| 4 | Rolling, opisthotonus, nystagmus, ataxia, decreased vestibular eye movement, head tilt (due to previous otitis media/interna), postural reaction deficits | CC |
| Brainstem | Forebrain, brainstem | |
| 5 | Seizure, obtundation, absent menace response, blindness | LGN, CC*, FN |
| Forebrain | Forebrain, brainstem | |
| 6 | Seizure, obtundation, opisthotonus, truncal ataxia, non-ambulatory tetraparesis, mydriasis, blindness, absent PLR, nystagmus, head swing, falling, circling, postural reaction deficits | NAD |
| Forebrain, brainstem | NAD |
Contrast enhancement on T1-weighted images
T2W = T2-weighted; FLAIR = fluid-attenuated inversion recovery; LGN = lateral geniculate nuclei; CC = caudal colliculi; FN = facial nuclei; PGM = periaqueductal grey matter; PLR = pupillary light reflex; VN = vestibular nuclei; NAD = no abnormality detected
Figure 2.
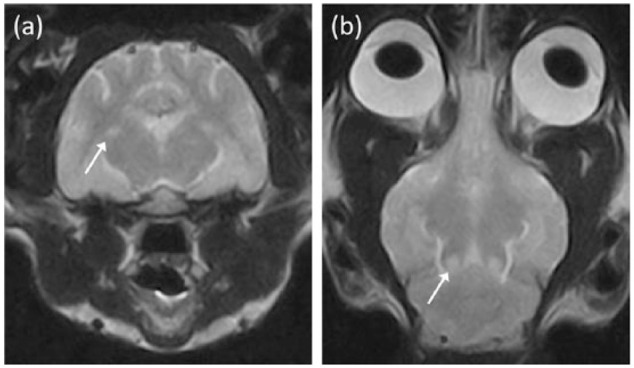
(a) Transverse T2-weighted (T2W) magnetic resonance image at the level of the thalamus and (b) dorsal T2W image at the level of the caudal colliculi of cat 5. Bilateral symmetrical hyperintense lesions are noted affecting the lateral geniculate nuceli (arrow) in (a) and the caudal colliculi (arrow) in (b). At the time of magnetic resonance imaging examination, the cat presented with seizures and blindness, without other typical signs suggestive of brainstem problems, as seen in the other thiamine-deficient cats
Laboratory abnormalities in haematology and serum biochemical analysis were noted in 12 cats at the initial presentation, including hyperglycaemia (n = 7), elevated aspartate aminotransferase (n = 7), leukocytosis (n = 5), anaemia (n = 3), elevated alanine aminotransferase (n = 1), elevated blood urea nitrogen (n = 1) and hyperalbuminaemia (n = 1).
Blood tests were repeated at various time points in 10/12 cats with initial laboratory abnormalities. In six surviving cats, all changes returned to normal values within 3 months. In the other two surviving cats, the abnormalities improved but had not resolved 1 week after the initial examination, including mildly elevated aspartate aminotransferase (n = 2), leukocytosis (n = 1) and anaemia (n = 1). In two cats that did not survive, one developed severe anaemia and leukocytosis during hospitalisation (cat 1 in Table 1) and the other continued to show moderately increased aspartate aminotransferase as the only laboratory abnormality.
CSF collected from the cisterna magna (n = 5) or caudal lumbar region (n = 1) was analysed. Albuminocytological dissociation was detected in two cats in the cisterna magna sample (protein level 38 and 41 mg/dl, respectively; reference interval <25 mg/dl). The results of the remaining CSF samples were unremarkable.
MRI of the brain was performed on six cats with ongoing neurological signs (Table 1). Abnormal findings were noted in five cats, consisting of bilateral hyperintensity affecting the lateral geniculate nuclei (n = 4), caudal colliculi (n = 4), facial nuclei (n = 3), cerebral cortex (n = 2), medial vestibular nuclei (n = 1) and periaqueductal grey matter (n = 1) on T2W and FLAIR images (Table 1, Figures 2 and 3). Mild contrast enhancement was evident on T1W images within some of the aforementioned lesions in four cats (Table 1). MRI was unremarkable in one cat, which had been treated with 5 days of thiamine supplementation prior to the MRI examination (cat 6 in Table 1 and Figure 4). Despite the treatment, it showed progressive neurological dysfunction and presented with obtundation, severe bilateral vestibular signs and seizures uncontrolled by antiepileptic drugs (AEDs) upon the MRI examination. Results of subsequent tests in this cat were all unremarkable, including CSF analysis, serum cryptococcal antigen latex agglutination serology, polymerase chain reaction tests for coronavirus and toxoplasma, and CSF bacterial culture. Based on the history of defective food and negative results from other investigations, this cat was diagnosed with TD.
Figure 3.
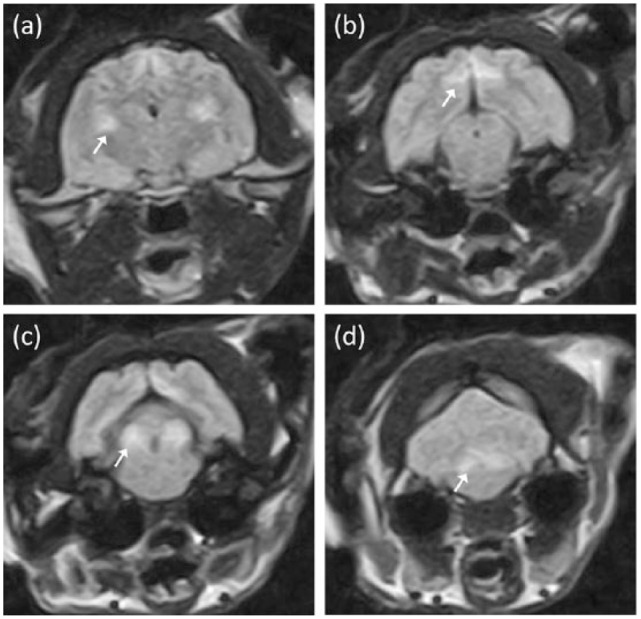
Transverse fluid-attenuated inversion recovery magnetic resonance images of cat 3 at the level of the (a) thalamus, (b) mesencephalic aqueduct, (c) caudal colliculi and (d) fourth ventricle. Bilateral symmetrical hyperintense changes are evident within the lateral geniculate nuclei (arrow) in (a), the splenial gyri (arrow) in (b), the caudal colliculi (arrow) in (c) and vestibular nuclei (arrow) in (d)
Figure 4.
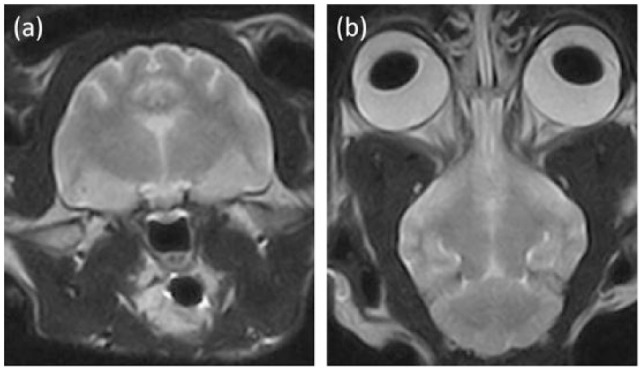
(a) Transverse T2-weighted (T2W) magnetic resonance image at the level of the thalamus and (b) dorsal T2W image at the level of the caudal colliculi of cat 6. No obvious hyperintense changes are detected. Despite 5 days of thiamine supplementation, the cat showed progressive and severe neurological signs at the time of magnetic resonance imaging examination
Treatments included thiamine supplementation, changing to a balanced diet, symptomatic treatment and supportive care. Mannitol (Taiwan Biotech), AEDs, antiemetic drugs, antibiotics, corticosteroids (methylprednisolone or prednisolone) or oxygen supplementation were administered based on clinical signs and at the discretion of clinicians. Thiamine supplementation of 100–300 mg/day (n = 14, IV or IM administration for the first 1–14 days, followed by PO at least until most clinical signs resolved) or 30 mg/day (n = 1, IV for 4 days) was prescribed and diet change (n = 16) was initiated immediately after the diagnosis was made. In the very early stage of the outbreak, when the association with the thiamine-deficient diet was still unclear, one cat without thiamine supplementation died during the clinical investigations. Half the cats with seizures were treated with monotherapy phenobarbital (n = 4; Johnson Chemical Pharmaceutical Works) or phenobarbital combined with levetiracetam (n = 1; Keppra, UCB SA). The duration of AED administration ranged from 5 weeks to 6 months (median 5 months). No recurrence of seizures was reported in the follow-up period after weaning off the AEDs. For all cats with seizure activity, seizures were only noted in the early stage of neurological manifestation (ie, no further seizure was reported once neurological signs started to improve). However, in cats treated with AEDs, it is not clear whether this observation was owing to the AED treatment or the disease course of TD.
Two cats (12%) died within 7 days of the onset of neurological signs. One cat, as mentioned above, died during the investigation in the early stage of the outbreak. In the other cat (cat 1 in Table 1), thiamine supplementation (300 mg/day IM for 1 day, followed by 200 mg/day IM) was initiated immediately after the MRI and CSF examination that was performed 2 days after the onset of neurological dysfunction; however, the cat continued to deteriorate and died 4 days later. In 82% (14/17) of patients, significant improvement occurred within the first (n = 13) or the second week (n = 1) after thiamine supplementation. At the 2 month follow-up, these 14 cats had either returned to normal function or exhibited only mild ataxia. In contrast, the recovery was much slower in the cat that initially deteriorated progressively despite thiamine supplementation (cat 6), and it still exhibited severe ataxia 2 months after the diagnosis. At the 6 month follow-up, full neurological recovery was reported in six cats. In the remainder, the following neurological signs were reported: reluctance to jump (n = 4), mild ataxia or weakness (n = 2), mild head tilt on waking (n = 1) and occasional circling (n = 1). Interestingly, open-mouthed breathing after intense activity was reported in two cats; however, its association with TD was not confirmed. At the 24 month follow-up, eight cats had completely returned to normal, and the following neurological signs were reported in the remainder: reluctance to jump (n = 3), very mild ataxia or weakness (n = 1), mild head tilt on waking (n = 1) and occasional circling (n = 1).
Discussion
In this study, based on a cluster of cases, from different living environments, diagnosed with TD in a short period of time, a problem with the commercial diet was strongly suspected. This was further confirmed by the low thiamine content found in the dry food. Although the exact problem was not identified, it could have arisen from the manufacturing process, inadequate thiamine supplementation during manufacturing or storage. Extrusion, a process driving materials to flow through a shaped hole, is widely used in the manufacture of dry pet foods. 26 In addition to aforementioned factors (high process temperature, an alkaline buffer and chloride in water), a faster screw extruder speed is also associated with increased thiamine loss in extrusion cooking, potentially due to additional heat generated within the barrel by shear or other mechanisms. 30 Approximately 12% of thiamine loss during processing has been reported for dry cat food. 27 The duration and condition of pet food storage can also result in thiamine loss. For dry cat food, a 34% loss in thiamine after 18 months of storage has been documented. 27 Although the peak of this outbreak occurred only 5–7 months after manufacture, as illustrated in Figure 1, storage conditions such as humidity and heat can potentially hasten the destruction of thiamine. The manufacturers usually supply extra thiamine to compensate for the losses from processing and storage. However, any mismatch between supplementation and losses during processing and storage would lead to thiamine-deficient dry food.
Domestic shorthair cats were over-represented in the study. This likely reflected the breed populations in Taiwan rather than a predisposition to TD. According to previous experiments, neurological signs associated with TD appear 25–40 days from the beginning of the thiamine-deficient diet.14,15 Although it was not possible to determine exactly how long owners had been supplying the defective food before cats developed neurological signs, the lag between the launch of the diet and the peak of the outbreak fits with the literature.
One limitation of the present study is that diagnosis was based on a low thiamine level in the diet. Ideally, the diagnosis of TD should be confirmed by measuring the activity of thiamine diphosphate via erythrocyte transketolase activity assay or direct measurement of thiamine and its phosphate esters in whole blood via high-performance liquid chromatography.8,31,32 However, a presumptive diagnosis can be achieved based on case history, clinical and neurological presentation, typical MRI findings, and rapid clinical response to thiamine supplementation.19,20,33,34 Among paraclinical tests, MRI is currently considered the most valuable method for diagnosing Wernicke’s encephalopathy, the acute neuropsychiatric syndrome resulting from TD in human beings. 35 In the present study, 5/6 cats exhibited MRI changes consistent with previous reports in cats.18–21 However, no MRI abnormalities on T1W, T2W or FLAIR images were present in one cat that had received 5 days of thiamine supplementation before MRI examination (cat 6 in Table 1 and Figure 4). It is uncertain whether MRI abnormalities in this cat resolved following treatment or if there were no MRI abnormalities present even before thiamine supplementation. In people with acute Wernicke’s encephalopathy, several case reports have demonstrated that the progression of clinical signs correlates with the progression and extension of lesions on serial MRI examinations.36,37 Both the human and veterinary literature also report complete or partial resolution of MRI abnormalities following thiamine supplementation and the improvement of clinical signs.19,20,38–41 In two feline case reports, a second MRI examination performed 4 days and 3 weeks after thiamine supplementation revealed complete and nearly complete resolution of previous MRI findings, respectively.19,20 One canine case report also documented a similar phenomenon after 8 weeks of treatment. 38 Upon MRI re-examination, the clinical signs mentioned in these three reports had either dramatically improved or completely resolved. In contrast, in the present study, the cat with normal MRI findings still presented marked neurological deficits when MRI examination was performed. This unexpected finding shows that clinical neurological status may not always correlate with MRI findings in cats with TD, especially once thiamine supplementation has been administered. Moreover, in cats with MRI abnormalities in the present study, the location of hyperintense foci on T2W and FLAIR images also did not consistently correlate with clinical signs (Table 1 and Figure 2). Another speculation is that no MRI abnormalities were present even before thiamine supplementation in cat 6. In the literature regarding Wernicke’s encephalopathy in people, normal MRI findings were reported in 13–42% of patients.42,43 Furthermore, a sensitivity of 53% with a specificity of 93% was documented when using MRI to identify patients with Wernicke’s encephalopathy. 44 The value of typical MRI findings in the diagnosis of TD in dogs and cats is well recognised.18–21,38,45 However, clinicians should be aware of the potential mismatch of neurological signs and MRI findings and the possibility of unremarkable MRI findings in thiamine-deficient cats, with and potentially even without previous thiamine supplementation.
Unlike the majority of surviving cats in this outbreak, the cat with normal MRI findings had a much slower recovery. The reason for this observation is unclear. As it is uncertain whether MRI abnormalities in this cat resolved following thiamine supplementation or if no MRI lesions was present even before treatment, the literature regarding Wernicke’s encephalopathy concerning both scenarios was searched for comparison or explanation for the observation in this cat. Although the correlation between absent findings on MRI and clinical outcome was not studied in Wernicke’s encephalopathy, case reports demonstrated that patients with normal MRI findings had various clinical outcomes, ranging from mild neurological deficits to death.42–44 In addition, although the literature documents resolution of MRI abnormalities following therapy and the improvement of clinical signs, reversible MRI findings do not necessarily imply a full recovery without residual neurological deficits in humans. 40 If treated promptly, acute clinical signs in Wernicke’s encephalopathy usually improve within weeks, but slow recovery has also been reported occasionally.35,41 The abovementioned information indicates that variations do exist regarding imaging findings and response to treatment in Wernicke’s encephalopathy, although most patients fit the general profiles. Many factors are discussed, including genetic alterations in the transport system of thiamine, which may contribute to how well an individual copes with TD or responds to therapy. 35 During the treatment of Wernicke’s encephalopathy, deficiency in other vitamins, especially magnesium, should also be corrected. 35 Magnesium plays a crucial role in the catalytic action of many enzymes, including transketolase in the pentose phosphate pathway and thiamine diphosphokinase in the conversion of thiamine into thiamine diphosphate. Severe magnesium depletion may lead to a refractory response to thiamine. Magnesium in blood was not measured in this cat, but the magnesium level tested in the food was above the recommendation of the Association of American Feed Control Officers. 27 In the future, thorough evaluation of general nutritional status should be considered in cats with TD.
Another limitation of this study is the use of low-field MRI. It is believed that high-field MRI can identify more subtle changes. Certainly, it would be interesting to perform such examination on cat 6, which showed no MRI abnormalities on low-field MRI (Figure 4) after thiamine supplementation while presenting marked neurological deficits. However, the imaging findings in other cats were similar to those in previously published articles.19,20 Furthermore, subtle hyperintense changes in the caudal colliculi were still detected on T2W images in a cat presenting with relatively mild clinical signs for the shortest duration in this study (cat 4 in Table 1), supporting the utility of low-field MRI as an alternative imaging modality in identifying major changes in cats with TD.
T2W hyperintense changes within the lateral geniculate nuclei and brainstem nuclei were the most common MRI findings in this study. A similar pattern was also seen after reviewing all previous feline reports; MRI changes involving the cerebral cortex and cerebellar nodules were less frequently documented.18–21 Interestingly, in Wernicke’s encephalopathy, cortical changes are also rarely reported. Moreover, such changes potentially indicate irreversible damage and a poor prognosis in humans. In contrast, cats with cerebral cortical involvement in the present study and previous case reports all recovered uneventfully. 18 Cortical involvement on MRI does not seem to be a prognostic indicator in feline TD. However, a larger sample size is needed to further confirm this observation.
Personal communication with the pet food company further revealed that in total 203 cases in Taiwan claimed to be involved in this outbreak. Compared with the total sales of food packs, it is estimated that 3–5% of cats fed the defective dry food developed clinical signs suggestive of TD. The earliest case was in mid-November 2012 and the peak of outbreak was in December 2012–January 2013. Approximately 15% of these cases developed neurological signs. For surviving cats, the majority recovered rapidly once treatment was initiated and had either completely returned to normal or showed mild clinical signs associated with balance problems, although one cat continues to exhibit intermittent seizure activities, which requires long-term AEDs. Overall, although represented as severe cases, the case distribution over time and the recovery of the 17 cats included in this study largely reflected the general presentation of this outbreak.
Conclusions
TD should be considered a differential diagnosis in cats presenting with vestibular signs, altered mentation, blindness and seizures, even with a primary diet of dry food. MRI examination provides valuable information in the diagnosis. However, normal MRI findings do not exclude the diagnosis of feline TD, especially once thiamine has been administered. MRI findings on conventional sequences also may not accurately reflect current neurological status. If treated promptly, most cats recover rapidly with no or only mild residual neurological deficits. Nonetheless, slow recovery over several months may sometimes occur. Although seizures are a common neurological sign, recurrence of seizures after withdrawing the temporary use of AEDs is rare, even when status epilepticus or cluster seizures present initially.
Acknowledgments
We would like to thank Dr Chih-Ching Wu, Dr Hsuan-Ping Hong and Dr Chi-Te Yu for patient management and data collection.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The preliminary results were presented as an abstract at the 26th Symposium of European College of Veterinary Neurology and European Society of Veterinary Neurology, Paris, 26–28 September 2013.
Accepted: 11 December 2015
References
- 1. Abdou E, Hazell AS. Thiamine deficiency: an update of pathophysiologic mechanisms and future therapeutic considerations. Neurochem Res 2015; 40: 353–361. [DOI] [PubMed] [Google Scholar]
- 2. Plaitakis A, Hwang EC, Woert MH, et al. Effect of thiamin deficiency on brain neurotransmitter systems. Ann N Y Acad Sci 1982; 378: 367–381. [DOI] [PubMed] [Google Scholar]
- 3. Jhala SS, Hazell AS. Modeling neurodegenerative disease pathophysiology in thiamine deficiency: consequences of impaired oxidative metabolism. Neurochem Int 2011; 58: 248–260. [DOI] [PubMed] [Google Scholar]
- 4. Bettendorff L. Thiamine in excitable tissues: reflections on a non-cofactor role. Metab Brain Dis 1994; 9: 183–209. [DOI] [PubMed] [Google Scholar]
- 5. Manzetti S, Zhang J, van der Spoel D. Thiamin function, metabolism, uptake, and transport. Biochemistry 2014; 53: 821–835. [DOI] [PubMed] [Google Scholar]
- 6. Tallaksen CM, Tauboll E. Excitatory effect of thiamin on CA1 pyramidal neurones in rat hippocampal slices in vitro. Eur J Neurol 2000; 7: 693–698. [DOI] [PubMed] [Google Scholar]
- 7. Davidson MG. Thiamin deficiency in a colony of cats. Vet Rec 1992; 130: 94–97. [DOI] [PubMed] [Google Scholar]
- 8. Markovich JE, Heinze CR, Freeman LM. Thiamine deficiency in dogs and cats. J Am Vet Med Assoc 2013; 243: 649–656. [DOI] [PubMed] [Google Scholar]
- 9. National Research Council. Foxes: recommended dietary allowances. In: Nutrient requirements of mink and foxes. 2nd revised ed. Washington, DC: National Academies Press, 1982, pp 27–28. [Google Scholar]
- 10. National Research Council. Vitamins. In: Nutrient requirements of nonhuman primates. 2nd ed. Washington, DC: National Academies Press, 2003, pp 129–130. [Google Scholar]
- 11. National Research Council. Nutrient requirements and dietary nutrient concentrations. In: Nutrient requirements of dogs and cats. Washington, DC: National Academies Press, 2006, pp 354–370. [Google Scholar]
- 12. Leoschke WL, Elvehjem CA. The thiamine requirement of the mink for growth and fur development. J Nutr 1959; 69: 211–213. [DOI] [PubMed] [Google Scholar]
- 13. Ziporin ZZ, Nunes WT, Powell RC, et al. Thiamine requirement in the adult human as measured by urinary excretion of thiamine metabolites. J Nutr 1965; 85: 297–304. [DOI] [PubMed] [Google Scholar]
- 14. Everett GM. Observations on the behavior and neurophysiology of acute thiamin deficient cats. Am J Physiol 1944; 141: 439–448. [Google Scholar]
- 15. Loew FM, Martin CL, Dunlop RH, et al. Naturally-occurring and experimental thiamin deficiency in cats receiving commercial cat food. Can Vet J 1970; 11: 109–113. [PMC free article] [PubMed] [Google Scholar]
- 16. Toman JEP, Everett GM, Oster RH, et al. Origin of cardiac disorders in thiamine-deficient cats. Proc Soc Exp Biol Med 1945; 58: 65–67. [Google Scholar]
- 17. Baggs RB, deLahunta A, Averill DR. Thiamine deficiency encephalopathy in a specific-pathogen-free cat colony. Lab Anim Sci 1978; 28: 323–326. [PubMed] [Google Scholar]
- 18. Marks SL, Lipsitz D, Vernau KM, et al. Reversible encephalopathy secondary to thiamine deficiency in 3 cats ingesting commercial diets. J Vet Intern Med 2011; 25: 949–953. [DOI] [PubMed] [Google Scholar]
- 19. Moon SJ, Kang MH, Park HM. Clinical signs, MRI features, and outcomes of two cats with thiamine deficiency secondary to diet change. J Vet Sci 2013; 14: 499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palus V, Penderis J, Jakovljevic S, et al. Thiamine deficiency in a cat: resolution of MRI abnormalities following thiamine supplementation. J Feline Med Surg 2010; 12: 807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Penderis J, McConnell JF, Calvin J. Magnetic resonance imaging features of thiamine deficiency in a cat. Vet Rec 2007; 160: 270–272. [DOI] [PubMed] [Google Scholar]
- 22. Steel RJ. Thiamine deficiency in a cat associated with the preservation of 'pet meat' with sulphur dioxide. Aust Vet J 1997; 75: 719–721. [DOI] [PubMed] [Google Scholar]
- 23. Studdert VP, Labuc RH. Thiamin deficiency in cats and dogs associated with feeding meat preserved with sulphur dioxide. Aust Vet J 1991; 68: 54–57. [DOI] [PubMed] [Google Scholar]
- 24. Wooley JA. Characteristics of thiamin and its relevance to the management of heart failure. Nutr Clin Pract 2008; 23: 487–493. [DOI] [PubMed] [Google Scholar]
- 25. Kimura M, Itokawa Y, Fujiwara M. Cooking losses of thiamin in food and its nutritional significance. J Nutr Sci Vitaminol (Tokyo) 1990; 36 Suppl 1: 17–24. [DOI] [PubMed] [Google Scholar]
- 26. Tran QD, Hendriks WH, van der Poell AFB. Effects of extrusion processing on nutrients in dry pet food. J Sci Food Agric 2008; 88: 1487–1493. [Google Scholar]
- 27. Crane SW, Cowell CS, Stout NP, et al. Commercial pet foods. In: Hand MS, Thatcher CD, Remillard RL, et al. (eds). Small animal clinical nutrition. 5th ed. Topeka, KS: Mark Morris Institute, 2010, pp 178–188. [Google Scholar]
- 28. Markovich JE, Freeman LM, Heinze CR. Analysis of thiamine concentrations in commercial canned foods formulated for cats. J Am Vet Med Assoc 2014; 244: 175–179. [DOI] [PubMed] [Google Scholar]
- 29. US Food and Drug Administration. Animal and veterinary recalls archive. http://www.fda.gov/AnimalVeterinary/SafetyHealth/RecallsWithdrawals/ucm393160.htm (accessed June 18, 2015).
- 30. Alam MS, Kaur J, Khaira H, et al. Extrusion and extruded products: changes in quality attributes as affected by extrusion process parameters: a review. Crit Rev Food Sci Nutr 2016; 56: 445–475. [DOI] [PubMed] [Google Scholar]
- 31. Talwar D, Davidson H, Cooney J, et al. Vitamin B(1) status assessed by direct measurement of thiamin pyrophosphate in erythrocytes or whole blood by HPLC: comparison with erythrocyte transketolase activation assay. Clin Chem 2000; 46: 704–710. [PubMed] [Google Scholar]
- 32. Galvin R, Brathen G, Ivashynka A, et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol 2010; 17: 1408–1418. [DOI] [PubMed] [Google Scholar]
- 33. Chung SP, Kim SW, Yoo IS, et al. Magnetic resonance imaging as a diagnostic adjunct to Wernicke encephalopathy in the ED. Am J Emerg Med 2003; 21: 497–502. [DOI] [PubMed] [Google Scholar]
- 34. Elefante A, Puoti G, Senese R, et al. Non-alcoholic acute Wernicke's encephalopathy: role of MRI in non typical cases. Eur J Radiol 2012; 81: 4099–4104. [DOI] [PubMed] [Google Scholar]
- 35. Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol 2007; 6: 442–455. [DOI] [PubMed] [Google Scholar]
- 36. Kim K, Shin DH, Lee YB, et al. Evolution of abnormal eye movements in Wernicke's encephalopathy: correlation with serial MRI findings. J Neurol Sci 2012; 323: 77–79. [DOI] [PubMed] [Google Scholar]
- 37. Liu YT, Fuh JL, Lirng JF, et al. Correlation of magnetic resonance images with neuropathology in acute Wernicke's encephalopathy. Clin Neurol Neurosurg 2006; 108: 682–687. [DOI] [PubMed] [Google Scholar]
- 38. Garosi LS, Dennis R, Platt SR, et al. Thiamine deficiency in a dog: clinical, clinicopathologic, and magnetic resonance imaging findings. J Vet Intern Med 2003; 17: 719–723. [PubMed] [Google Scholar]
- 39. Murata T, Fujito T, Kimura H, et al. Serial MRI and (1)H-MRS of Wernicke's encephalopathy: report of a case with remarkable cerebellar lesions on MRI. Psychiatry Res 2001; 108: 49–55. [DOI] [PubMed] [Google Scholar]
- 40. Sakurai K, Sasaki S, Hara M, et al. Wernicke's encephalopathy with cortical abnormalities: clinicoradiological features: report of 3 new cases and review of the literature. Eur Neurol 2009; 62: 274–280. [DOI] [PubMed] [Google Scholar]
- 41. Zhong C, Jin L, Fei G. MR imaging of nonalcoholic Wernicke encephalopathy: a follow-up study. AJNR Am J Neuroradiol 2005; 26: 2301–2305. [PMC free article] [PubMed] [Google Scholar]
- 42. Weidauer S, Nichtweiss M, Lanfermann H, et al. Wernicke encephalopathy: MR findings and clinical presentation. Eur Radiol 2003; 13: 1001–1009. [DOI] [PubMed] [Google Scholar]
- 43. Lough ME. Wernicke’s encephalopathy: expanding the diagnostic toolbox. Neuropsychol Rev 2012; 22: 181–194. [DOI] [PubMed] [Google Scholar]
- 44. Antunez E, Estruch R, Cardenal C, et al. Usefulness of CT and MR imaging in the diagnosis of acute Wernicke's encephalopathy. AJR Am J Roentgenol 1998; 171: 1131–1137. [DOI] [PubMed] [Google Scholar]
- 45. Singh M, Thompson M, Sullivan N, et al. Thiamine deficiency in dogs due to the feeding of sulphite preserved meat. Aust Vet J 2005; 83: 412–417. [DOI] [PubMed] [Google Scholar]


