Abstract
Objectives
Universal anticoagulant could be an alternative to the multiple blood sampling required for clinical pathology investigations in cats. An association of citrate, theophylline, adenosine and dipyridamole (CTAD) has been reported to be a good substitute for EDTA for haematology analysis in cats, limiting platelet clumping, and has also been shown to be valid for haematology, secondary haemostasis and some biochemical variables in humans. The aim of the study was therefore to investigate the effects of CTAD on in vitro platelet aggregation and compare results of secondary haemostasis and biochemistry tests, excluding a priori those variables not reliably measured in CTAD, such as sodium, chloride and divalent cations, in feline blood specimens collected in CTAD and paired citrate and heparin tubes.
Methods
Thirty blood specimens sampled in citrate and CTAD were analysed for in vitro platelet aggregation, and 60 blood specimens sampled in citrate or heparin and CTAD were analysed for plasma coagulation and a biochemistry panel.
Results
In vitro platelet aggregation was inhibited in CTAD compared with citrate specimens. Prothrombin time, activated partial thromboplastin time, antithrombin and fibrinogen results were similar, despite some significant differences. Measurements of triglycerides, cholesterol, glucose, urea, creatinine, phosphate, total proteins and alanine aminotransferase activity were similar and well correlated in CTAD and heparin plasmas, despite some significant differences and moderate biases. Albumin showed a marked positive proportional bias, and creatine kinase and alkaline phosphatase activities a moderate and marked negative mixed bias, respectively, but could be measured in CTAD if new reference intervals were calculated. Aspartate aminotransferase activity showed a marked negative proportional bias, along with a poor correlation and some clinical misclassifications just like the potassium concentration, and thus cannot be recommended to be measured in CTAD specimens.
Conclusions and relevance
In cats, CTAD cannot be used for primary haemostasis investigation but could be a suitable (almost) universal anticoagulant for routine haematology, as well as for plasma coagulation and many biochemistry variables.
Introduction
Blood sampling for a complete clinical pathology investigation presents a challenge in cats as multiple tubes have to be collected. This is frequently difficult because of prolonged handling and also the large volume of blood taken, especially in diseased small cats. One solution could be to use a universal anticoagulant, but no such anticoagulant has yet been found for veterinary medicine. In people, an association of citrate, theophylline, adenosine and dipyridamole (CTAD) was proposed and validated for many haematological, secondary haemostasis and some biochemical variables in a preliminary study. 1 However, the use of CTAD has not gained much acceptance in people, maybe because multiple samplings are easier to perform.
In cats, CTAD has been used as an anticoagulant for routine haematology analysis and can replace the classic EDTA as it provides a more reliable automated platelet count by limiting platelet clumping and gives similar results for the other cell counts.2–4 CTAD also reduces storage-induced changes. 5 However, the in vitro effects of CTAD on feline haemostasis, particularly its effects on platelet aggregation during primary haemostasis, and in routine plasma coagulation tests investigating secondary haemostasis, have not been studied. Possible effects of CTAD on routine plasma biochemistry in cats are also unknown; however, its use is precluded a priori for measurements of divalent cations (which make stable salts with citric acid) and sodium (present in CTAD tubes), and CTAD may also interfere with Ca2+- and Mg2+- dependent enzyme activity measurements, even although these cations are usually supplied in the reagents.
The aim of this study was thus to investigate the effects of CTAD on platelet aggregation; and to compare the results of secondary haemostasis and biochemistry measurements in cats, using CTAD specimens and paired classical sodium citrate and lithium heparin tubes.
Materials and methods
This prospective study was performed between March 2014 and July 2014 at the Central Laboratory of Medical Biology, Veterinary School of Toulouse. The two-stage experimental design consisted of an initial study to compare in vitro platelet aggregation in CTAD and citrate feline blood specimens, and a second study to compare results of secondary haemostasis tests and of a biochemistry panel in paired CTAD and citrate or heparin feline plasma specimens, respectively.
Blood specimens were collected from 30 healthy cats obtained from the unit of a life-science services company (Amatsigroup) breeding cats for experimental purposes. This company complies with the European Directive 2010/63/EU on the protection of animals used for scientific purposes, is accredited by Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) and conforms to good laboratory practices. This site is licensed as a unit for experimental animal housing by the French authorities (Agreement number D31 188 01). All procedures were approved by the ethics committee for the protection of laboratory animals (Comité Ethique Animale no. 62) registered by the French Ministry of Higher Education and Research. Blood specimens were collected from the jugular vein through a needle and a syringe and immediately placed in a citrate tube (Venosafe 1.8 ml; Terumo) and a CTAD tube (Vacuette; Greiner Bio-One) in a randomised order. The tubes were immediately mixed by 10 inversions after collection. All tubes that were incorrectly filled or showed macroscopic clots were excluded. Specimens were analysed <9 h after blood collection. First, the platelet count was obtained with a haematology analyser, the Sysmex XT-2000iV, using a flow cytometry method. Then, platelet aggregation in whole blood specimens was assessed by a ROTEM platelet analyser with ROTEM delta (Tem International GmBH). An adenosine diphosphate (ADP) reagent (ADP-tem; Tem International GmBH) at 10 µmol/l, and a collagen solution at 5 mg/l (Collagen Horm solution; Nycomed) were used as agonists, and added to 200 µl whole blood aliquots. The analyser measured three different variables: amplitude at 6 mins in Ohm (Ω; A6), maximum slope of the aggregation graph in Ω/min (MS) and area under the curve in Ω*min (AUC).
Plasma coagulation and biochemistry variables were measured in 60 blood specimens collected from 49 healthy cats from the same unit housing experimental cats and according to the same ethical procedures as previously described, and from 11 sick cats admitted to different services of the hospital at the Veterinary School of Toulouse as part of routine diagnoses or disease monitoring. Before sampling, all owners of the sick cats signed an informed consent form to allow the use of the blood specimens for this study. Blood samples were collected from the jugular vein into a citrate vacuum tube (Venosafe 1.8 ml; Terumo), a CTAD vacuum tube (Vacuette 2ml; Greiner Bio-One) and a heparin tube (Microtubes Minicollect Plasma, 1 ml; Greiner Bio-One) with an 0.8 × 40 mm needle (Venosafe Mutisample 22 G, 0.7 × 25 mm; Terumo). The tubes were gently mixed by 10 inversions immediately after collection. All tubes that were incorrectly filled or showed macroscopic clots were excluded. All tubes were centrifuged for 10 mins at 2700 g (SIGMA 3K10 Laborzentrifugen; Bioblock Scientific). The concentrations and activities of the following biochemical analytes were measured in heparinised and CTAD plasmas with a dry-slide technology analyser and corresponding multi-layer reagents (Vitros 350; Ortho-Clinical Diagnostics): glucose, urea, creatinine, potassium, phosphate, cholesterol, triglycerides, total proteins, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine kinase (CK), alkaline phosphatases (ALP), gamma (γ)-glutamyltransferase (GGT) and total bilirubin. Quality controls were performed with Performance Verifier 1 and 2 human control solutions (Ortho Clinical Diagnostics) for each batch of analyses. Prothrombin time (PT), activated partial thromboplastin time (APTT), antithrombin (AT) and fibrinogen were measured with an automated analyser (STA-Compact; Diagnostica Stago) in citrated and CTAD plasmas by coagulometric (PT, APTT and fibrinogen) and colorimetric (AT) tests. Two human control solutions (STA coag Control N+P; Diagnostica Stago) were used as quality controls. Measurement imprecision was evaluated according to Clinical Laboratory Standards Institute (CLSI) recommendations, 6 and is reported in Tables 1 and 2.
Table 1.
Comparison of results obtained for coagulation variables in feline plasma specimens sampled in citrate and CTAD tubes
| Variables | CTAD |
Citrate |
P value CTAD vs citrate |
Passing–Bablock |
Repeatability (percentage coefficient of variation) 7 | N cases differently classified according to RI 7 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | Min–max | Median | Min–max | r | a (95% CI) | b (95% CI) | ||||
| PT (s) | 60 | 11.8 | 10.7–14.3 | 11.4 | 10.4–13.7 | <0.0001 | 0.90 | 1.00 (0.95–1.17) | 0.40 (–1.58 to 0.96) | 1.20 | 2 |
| APTT (s) | 59 | 15.1 | 11.1–116.3 | 15.1 | 12.4–99.3 | 0.7692 | 0.88 | 1.04 (0.95–1.18) | −0.60 (–2.68 to 0.78) | 0.85 | 0 |
| Fibrinogen (g/l) | 60 | 1.91 | 1.10–12.00 | 1.83 | 1.05–12.00 | 0.0012 | 0.99 | 1.05 (1.03–1.10) | −0.04 (–0.11 to 0.02) | 3.40 | 0 |
| AT (%) | 60 | 149 | 70–200 | 145 | 76–200 | <0.0001 | 0.96 | 1.02 (0.95–1.11) | 1.49 (–10.52 to 12.14) | 1.65 | 3 |
Comparison of results by Student’s paired t-test or Wilcoxon’s test according to homoscedasticity
r = Spearman’s correlation coefficient; a and b = slope and intercept of Passing–Bablock agreement, respectively; CI = confidence interval; N = number of cases which would have been classified differently according to the limits of the RI; RI = reference interval; PT = prothrombin time; APTT = activated partial thromboplastin time; AT = antithrombin; CTAD = citrate, theophylline, adenosine and dipyridamole
Table 2.
Comparison of results obtained for biochemical variables in feline plasma specimens sampled in heparin and CTAD tubes
| Variables | CTAD |
Heparin |
P
CTAD vs heparin |
Passing–Bablock |
Repeatability (percentage coefficient of variation) 7 | N cases differently classified according to RI 7 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Median | Min–max | Median | Min–max | r | a (95% CI) | b (95% CI) | ||||
| Glucose (mmol/l) | 59 | 5.29 | 1.74–10.20 | 4.70 | 1.67–10.40 | <0.0001 | 0.89 | 0.93 (0.83–1.05) | 0.79 (0.21–1.35) | 0.25 | 5 |
| Urea (mmol/l) | 59 | 6.7 | 3.2–34.0 | 6.9 | 3.2–35.8 | <0.0001 | 0.99 | 0.95 (0.93–0.98) | 0.15 (–0.03 to 0.31) | 1.00 | 0 |
| Creatinine (µmol/l) | 59 | 103.8 | 29.1–741.3 | 109.0 | 30.4–740.7 | <0.0001 | 0.99 | 0.98 (0.96–1.00) | −1.80 (–3.93 to 0.07) | 1.00 | 3 |
| Total proteins (g/l) | 59 | 74.6 | 43.0–90.1 | 73.4 | 42.2–88.5 | <0.0001 | 0.98 | 1.06 (1.02–1.12) | −3.82 (–7.56 to −0.52) | 0.30 | 1 |
| Albumin (g/l) | 59 | 36.7 | 17.7–42.8 | 30.9 | 14.3–36.3 | <0.0001 | 0.96 | 1.20 (1.11–1.30) | −0.66 (–3.74 to 2.11) | 0.95 | 4 |
| Cholesterol (mmol/l) | 59 | 3.24 | 1.44–6.40 | 3.27 | 1.41–6.44 | 0.5288 | 1.00 | 0.97 (0.95–1.01) | 0.08 (–0.02 to 0.15) | 0.75 | 0 |
| Triglycerides (mmol/l) | 59 | 0.37 | 0.20–3.03 | 0.37 | 0.19–3.15 | 0.0545 | 0.99 | 0.97 (0.95–1. 01) | 0.01 (0.00–0.01) | 0.60 | 1 |
| Potassium (mmol/l) | 58 | 3.8 | 2.7–4.8 | 3.9 | 2.8–4.8 | <0.0001 | 0.80 | 0.94 (0.74–1.11) | 0.03 (–0.67 to 0.78) | 0.75 | 6 |
| Phosphates (mmol/l) | 59 | 1.51 | 0.76–3.02 | 1.62 | 0.80–3.05 | <0.0001 | 0.98 | 0.92 (0.88–0.96) | 0.04 (–0.03 to 0.10) | 0.30 | 1 |
| AST (U/l) | 59 | 24 | 14–138 | 32 | 17–245 | <0.0001 | 0.67 | 0.74 (0.51–0.89) | 0.74 (–3.84 to 7.49) | 0.70 | 9 |
| ALT (U/l) | 59 | 71 | 42–488 | 71 | 38–484 | 0.0115 | 0.98 | 0.99 (0.94–1.01) | 1.58 (–0.06 to 5.60) | 1.25 | 0 |
| CK (U/l) | 59 | 99 | 44–3424 | 150 | 58–3302 | <0.0001 | 0.87 | 0.78 (0.71–0.89) | −11.55 (–27.55 to −1.58) | 1.25 | 1 |
| ALP (U/l) | 59 | 22 | 12–71 | 43 | 16–213 | <0.0001 | 0.95 | 0.28 (0.26–0.30) | 10.28 (9.07–11.22) | 1.55 | 45 |
Comparison of results by Student’s paired t-test or Wilcoxon’s test according to homoscedasticity
r = Spearman’s correlation coefficient; a and b = slope and intercept of Passing–Bablock agreement, respectively; N = number of cases that would have been classified differently according to the limits of the RI; RI = reference interval; AST = aspartate aminotransferase; ALT = alanine aminotransferase; CK = creatine kinase; ALP = alkaline phosphatase; CI = confidence interval; CTAD = citrate, theophylline, adenosine and dipyridamole
All concentrations and activities obtained in CTAD specimens were corrected to compensate for the dilution in CTAD tubes (1:9 [vol:vol] ratio). The results obtained with CTAD and the other anticoagulants were compared by Student’s paired t-test or by Wilcoxon’s test according to homoscedasticity, by Spearman’s correlation and by Passing–Bablock agreement analysis. The numbers of cases when the differences could account for a different ‘clinical’ classification of results, according to feline reference intervals, 7 were also counted. Calculations were carried out with an Excel spreadsheet and Analyse-It.
Results
One feline blood specimen was excluded and not analysed for platelet count and platelet aggregation because of the presence of some macroscopic platelet clumps, and one result for ADP platelet aggregation was not given by the analyser owing to an analytic error, and could not be included. Platelet counts in citrate and CTAD feline blood specimens were not statistically different (P = 0.3853) (Table 3). The overall degree of platelet aggregation described by AUC, MS and A6 was systematically lower in CTAD than in citrate with ADP or collagen agonists (P ⩽0.0001). Figure 1 shows a typical platelet aggregation response induced by ADP and collagen in citrate and CTAD paired blood specimens. It should be noted that one sample showed a contrary mildly lower collagen-induced platelet aggregation in citrate than in the CTAD specimen (AUC = 75 and 86; MS = 5 and 6; and A6 = 22 and 27, respectively).
Table 3.
Comparison of platelet counts obtained with Sysmex XT-2000iV and platelet aggregation assessed by a ROTEM delta platelet analyser in feline whole blood sampled in citrate and CTAD tubes
| Variables | Citrate |
CTAD |
P value citrate vs CTAD |
|||||
|---|---|---|---|---|---|---|---|---|
| n | Median | Min–max | Median | Min–max | ||||
| Platelets (109/l) | 29 | 385 | 158–662 | 381 | 172–593 | 0.3853 | ||
| ADP | A6 (Ω/min) | 28 | 16.1 | 0.0–30.2 | 2.7 | 0.0–11.4 | <0.0001 | |
| Platelet aggregation | MS (Ω) | 8 | 2–16 | 1 | 0–4 | <0.0001 | ||
| AUC (Ω*min) | 76 | 11–130 | 9 | 0–48 | <0.0001 | |||
| Collagen | A6 (Ω/min) | 29 | 28.2 | 4.9–38.0 | 7.2 | 0.0–27.0 | <0.0001 | |
| MS (Ω) | 10 | 3–16 | 2 | 1–6 | <0.0001 | |||
| AUC (Ω*min) | 86 | 5–150 | 17 | 0–86 | <0.0001 | |||
Comparison of results by Student’s paired t-test or Wilcoxon’s test according to homoscedasticity ADP = adenosine diphosphate; A6 = amplitude at 6 mins, MS = maximum slope of the aggregation graph; AUC = area under the curve; CTAD = citrate, theophylline, adenosine and dipyridamole; Ω = ohm
Figure 1.
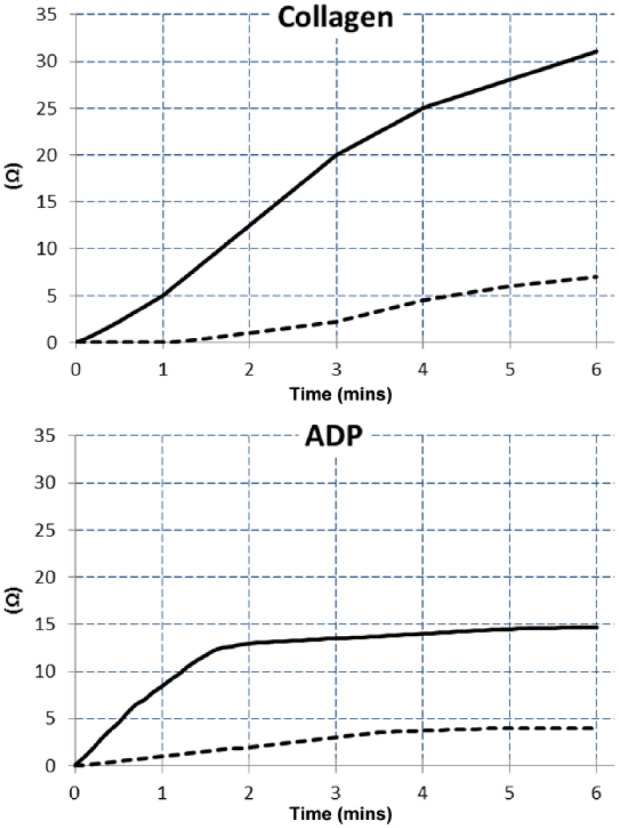
Typical example of collagen and adenosine diphosphate (ADP)-induced platelet aggregation assessed in a citrate (unbroken black line) and CTAD (dotted black line) feline whole blood sample (redrawn from ROTEM delta platelet analyser print-outs). CTAD = citrate, theophylline, adenosine and dipyridamole
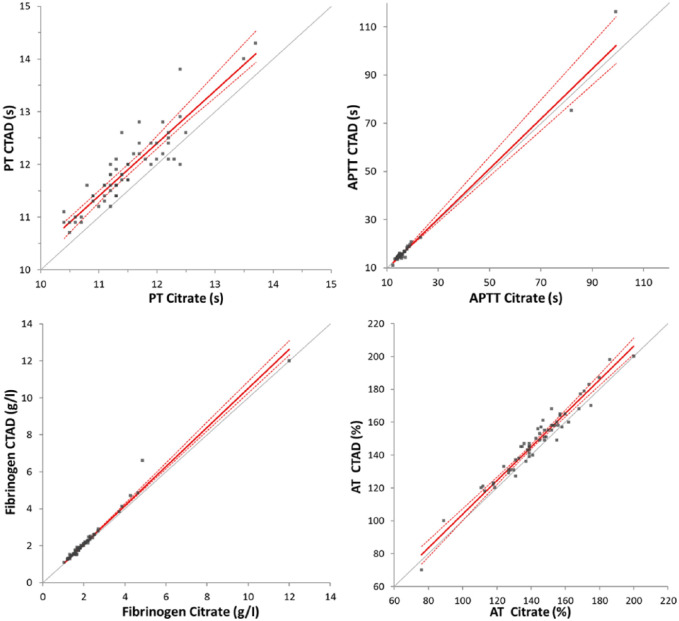
Results for the secondary haemostasis exploration (Table 1, Figure 2), were obtained for all 60 paired specimens except one value under the limit of quantification for APTT, which was not included in the calculations. Similar APTT results were obtained in citrate and CTAD plasmas, whereas PT, fibrinogen and AT were significantly higher in CTAD than in citrate plasmas. Coefficients of correlation between citrate and CTAD samples were >0.95 for fibrinogen and AT, and 0.90 and 0.88 for PT and APTT, respectively. No bias was shown for PT, APPT and AT, and a slight proportional bias was observed for fibrinogen. Only a few clinical misclassifications of the results, according to the reference intervals, were noted for PT and AT; that is, 2/60 and 3/60 cases, respectively.
Figure 2.
Passing–Bablock agreement graphs for results of secondary haemostasis analytes in paired feline CTAD and citrate specimens. PT = prothrombin time; APTT = activated partial thromboplastin time; AT = antithrombin; grey line = identity; red line = Passing–Bablock fit; dotted red lines = 95% confidence intervals; CTAD = citrate, theophylline, adenosine and dipyridamole
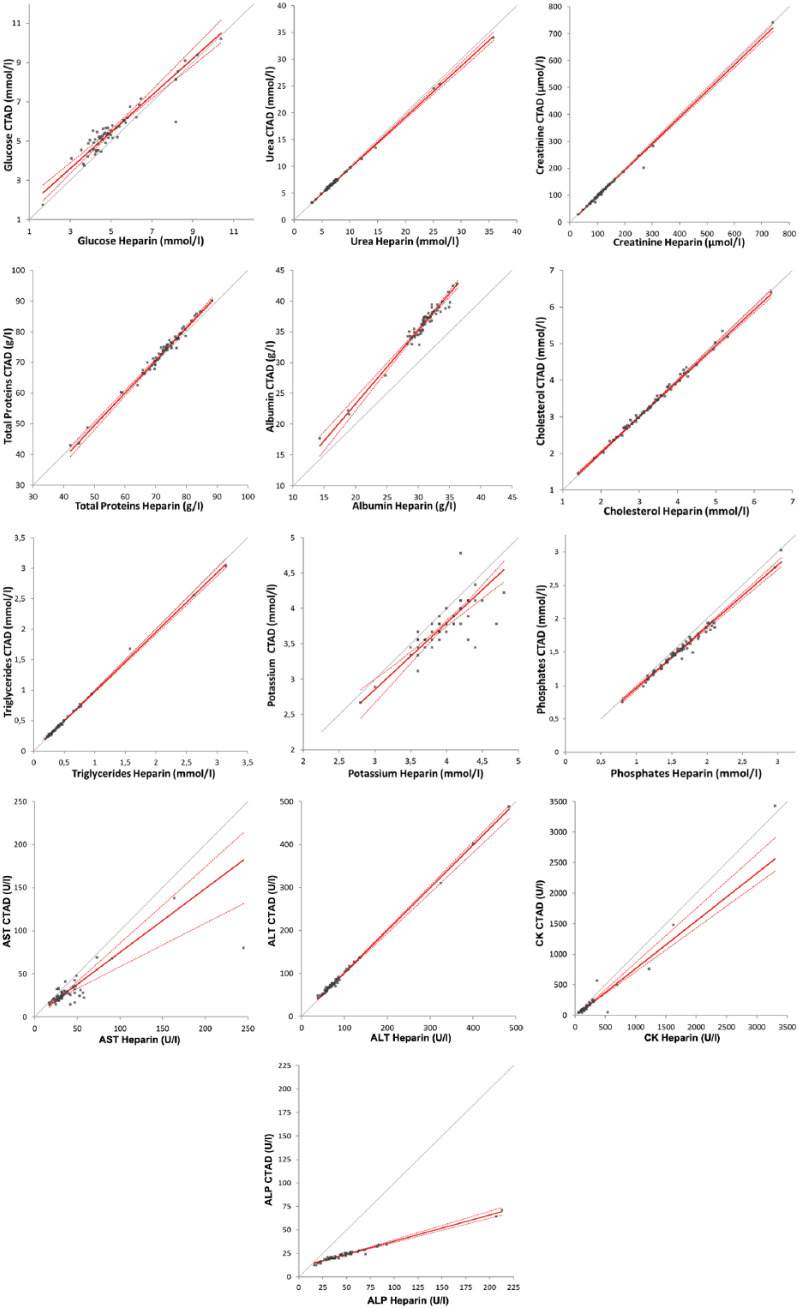
Biochemistry results were obtained for all 60 paired specimens except for one panel from a sick cat, owing to a missing heparinised sample, and for another cat, owing to the absence of measurement of a potassium concentration and GGT activity (Table 2, Figure 3). No calculation for total bilirubin and GGT activity could be performed because many values were below the limit of quantification (8/59 specimens in heparin and 22/59 specimens in CTAD plasmas for total bilirubin; 49/58 specimens in heparin and all specimens in CTAD plasmas for GGT), and only the number of cases of ‘clinical misclassification’ was estimated; that is, 21/59 specimens and 4/58 specimens, respectively. Cholesterol and triglycerides measurements in heparin and CTAD plasmas were not significantly different, whereas all the other tested analytes were significantly different (ie, glucose, urea, creatinine, potassium, phosphate, total proteins, albumin, AST, ALT, CK and ALP). Spearman’s correlation coefficients between heparin and CTAD plasmas were ⩾0.95 for 9/13 measured analytes (ie, urea, creatinine, phosphate, cholesterol, triglycerides, total proteins, albumin, ALT, and ALP), between 0.80 and 0.90 for glucose, potassium and CK, and equal to 0.67 for AST. No constant or proportional bias was observed for creatinine, cholesterol, triglycerides or ALT. A negative proportional bias was observed for urea and phosphate (slopes of 0.95 and 0.92, respectively), a more marked negative proportional bias was shown for AST (slope of 0.74) and a marked positive proportional bias was shown for albumin (slope of 1.20). A positive constant bias was demonstrated for glucose (intercept of 0.79). The bias was mixed for total proteins, CK and ALP (slopes of 1.06, 0.78 and 0.28, and intercepts of about −4, −12 and 10, respectively). No clinical misclassification of the results according to the reference intervals was noted for cholesterol and ALT, whereas <10% clinical misclassifications were noted for glucose, urea, creatinine, phosphate, cholesterol, triglycerides, total proteins, albumin, ALT, CK and GGT, 10–20% clinical misclassifications were noted for potassium and AST and >35% clinical misclassifications were noted for ALP and total bilirubin.
Figure 3.
Passing–Bablock agreement graphs for results for biochemical analytes in paired feline CTAD and heparin specimens. Grey line = identity; red line = Passing–Bablock fit; dotted red lines = 95% confidence intervals; AST = aspartate aminotransferase; ALT = alanine aminotransferase; CK = creatine kinase; ALP = alkaline phosphatase; CTAD = citrate, theophylline, adenosine and dipyridamole
Discussion
The decision to test CTAD as a universal anticoagulant for feline blood samples was based on previous studies that had demonstrated that CTAD can be used as an anticoagulant for routine haematology analysis in cats, and on a study performed in human medicine that demonstrated that CTAD could be a good alternative for routine plasma coagulation and biochemistry profiles. In our study, the choice of analytes tested in the biochemistry panel was based on the decision to perform a complete biochemistry panel, as is commonly required in veterinary practice, and also on the a priori exclusion of variables such as Na, Cl and divalent cations which make stable salts with citric acid and cannot be reliably measured in CTAD plasma. To our knowledge, the effect of CTAD as a universal anticoagulant in cats has not been tested before. This is therefore the first study to investigate in vitro platelet aggregation in CTAD feline specimens, and to compare plasma coagulation and biochemistry profiles in CTAD and paired classical feline blood specimens.
In cats, previous studies demonstrated that CTAD can replace EDTA as an anticoagulant for haematology routine.2–4 CTAD reduces platelet clumping assessed by blood film examination.2,4 Moreover, results of a complete blood cell count in EDTA and CTAD are not clinically different, except for a narrower platelet count’s reference interval with a higher low limit, a slightly higher haemoglobin concentration and a lower red cell distribution width – coefficient variation (RDW-CV) in CTAD than in EDTA. 4 There are also moderately different results of reticulocytes indexes. 4
In this study, CTAD markedly inhibited in vitro platelet aggregation in feline whole blood specimens, whichever agonist – ADP or collagen – was used. As expected, the overall degree of platelet aggregation was systematically lower in CTAD than in citrate specimens, except for one unexplained case. This study confirmed previously published results, based on a semi-quantitative assessment of platelet aggregation on blood smears, that platelet aggregation is limited and/or inhibited by CTAD.1,2 In humans, CTAD inhibits platelet aggregation by increasing cytoplasmic adenosine monophosphate cyclic (AMPc) concentration via the action of theophylline, dipyridamole and adenosine. This causes a sequestration of Ca2+ thereby preventing platelet adhesion, activation and aggregation. 1 In vitro studies have demonstrated platelet inactivation by CTAD via decreased expression of CD62P (P Selectine) and CD63 molecules on the platelet surface.8–10 In cats, the mechanisms of CTAD inactivation of platelets are not well known but are likely similar to those described in humans.
In human medicine, PT, APTT and fibrinogen measurements in citrate and CTAD plasmas were very similar and highly correlated (r >0.92). 1 In our study, plasma coagulation test results for PT, APTT, fibrinogen and AT were usually close, even though they were sometimes statistically different. Very few clinical misclassifications were observed for PT and AT. The corresponding reference intervals for these variables need to be recalculated according to CLSI–International Federation of Clinical Chemistry (IFCC) and American Society for Veterinary Clinical Pathology (ASVCP) recommendations,11–14 in which case CTAD could be used for routine plasma coagulation tests.
Human biochemistry profiles showed that similar values were obtained in sera and CTAD plasmas for total proteins, albumin, total bilirubin, urea, creatinine, AST, ALT, CK, glucose and potassium, whereas the values for Na and Cl differed notably, 1 which is consistent with the input of Na ions derived from the sodium citrate in the CTAD solution. In this study, biochemistry results obtained in CTAD were compared with heparin plasma and not to serum, because it is the specimen used in our institution. To our knowledge, there has been no comparison of biochemistry results between serum and plasma in feline specimens, but results were almost identical in dogs and horses.15–17 Most of the biochemistry results obtained for CTAD and heparin plasmas were similar and well correlated, that is, triglycerides, cholesterol, glucose, urea, creatinine, phosphate, total proteins and ALAT, even though they were often statistically different. Biases were observed for some of them; for example, a moderate negative proportional bias for urea and phosphate, a moderate constant positive bias for glucose and a very slight mixed bias for total proteins. Recalculation of the reference intervals for these variables according to CLSI-IFCC and ASVCP recommendations could allow the routine use of CTAD for their measurement.12–14 More intense differences were shown for the albumin and potassium measurements and enzyme activities of CK, AST and ALP. Albumin showed a marked positive proportional bias, despite a good correlation and few clinical misclassifications. The reason for this difference remains unknown, but a transfer of reference intervals could allow use of CTAD tubes to measure albumin. CK activity showed a moderate negative mixed bias, a good correlation and very few clinical misclassifications. This may result from the formation of salts between citric acid and Mg2+, which is a cofactor of CK by the citrate in CTAD. A transfer of reference intervals could, perhaps, allow the use of CTAD tubes to measure CK, but a further study, including more abnormally high values of CK in heparin plasma, should be performed to confirm this possibility. ALP activity showed a marked negative mixed bias, a very good correlation and many clinical misclassifications. The reason for this difference is likely the same as for CK. Given the high correlation, a transfer of reference intervals could allow use of CTAD tubes to measure ALP. The measurement of AST and potassium in CTAD specimens cannot be recommended. In fact, AST activity showed a marked negative proportional bias, poor correlation and some clinical misclassifications. These poor results were not only due to one very high value, which looked like an outlier (245 U/l in heparin vs 80 U/l in CTAD), as the correlation was still poor when this pair of data was removed (r = 0.65). A new study including more normal and abnormal (ie, high) values of AST in heparin plasma should be performed to see if CTAD needs to be definitively excluded from measurement of AST activity in feline specimens. Potassium measurement in heparin and CTAD showed relatively good agreement, except that the low correlation (r = 0.80) and clinical misclassifications would make the use of CTAD unacceptable. For clinical practice, the measurement of potassium must be precise as slight changes in the concentrations of electrolytes such as potassium, sodium and chloride may be critical (total allowable error about 5% 14 ). No calculations of GGT activity and total bilirubin were performed because of the very large number of values below the limit of quantification and only the number of different ‘clinical misclassifications’ was noted. Another investigation including high values of total bilirubin and GGT activity should be performed to determine the possibility of using CTAD specimens.
One limit of this study was the limited range of values obtained for the different analytes. A new study including more abnormal values should be carried out to confirm these preliminary results.
Conclusions
In cats, CTAD cannot be used for primary haemostasis investigation but could be a suitable (almost) universal anticoagulant for routine haematology, as previously reported, as well as for plasma coagulation and many biochemistry variables as demonstrated in this study. This is an improvement on the current situation as use of CTAD tubes could reduce the number of cases for which multiple sampling is required, and thus improve animal welfare.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 2 January
References
- 1. Yokota M, Tatsumi N, Tsuda I, et al. CTAD as a universal anticoagulant. J Autom Methods Manag Chem 2003; 25: 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Norman EJ, Barron RC, Nash AS, et al. Evaluation of a citrate-based anticoagulant with platelet inhibitory activity for feline blood cell counts. Vet Clin Pathol 2001; 30: 124–132. [DOI] [PubMed] [Google Scholar]
- 3. Granat F, Geffré A, Braun JP, et al. Comparison of platelet clumping and complete blood count results with Sysmex XT-2000iV in feline blood sampled on EDTA or EDTA plus CTAD (Citrate, Theophylline, Adenosine, and Dipyridamole). J Feline Med Surg 2011, 13: 953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Granat F, Geffré A, Bourges-Abella N, et al. Feline reference intervals for the Sysmex XT-2000iV and the ProCyte DX haematology analysers in EDTA and CTAD blood specimens. J Feline Med Surg 2014; 16: 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Granat F, Geffré A, Bourges-Abella N, et al. Changes in haematology measurements with the Sysmex XT-2000iV during storage of feline blood sampled in EDTA or EDTA plus CTAD. J Feline Med Surg 2013, 15: 433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. NCCLS. Document EP5-A2 evaluation of precision performance of quantitative measurement methods; approved guideline. 2nd ed. Vol 24, No 25. Wayne, PA: NCCLS, 2004. [Google Scholar]
- 7. Reynolds BS, Geffré A, Bourgès-Abella NH, et al. Effects of intravenous, low-dose ketamine-diazepam sedation on the results of hematologic, plasma biochemical, and coagulation analyses in cats. J Am Vet Med Assoc 2012; 240: 287–293. [DOI] [PubMed] [Google Scholar]
- 8. Macey M, Azam U, McCarthy D, et al. Evaluation of the anticoagulants EDTA and citrate, theophylline, adenosine, and dipyridamole (CTAD) for assessing platelet activation on the ADVIA 120 hematology system. Clin Chem 2002; 48: 891–899 [PubMed] [Google Scholar]
- 9. Mody M, Lazarus AH, Semple JW, et al. Preanalytical requirements for flow cytometric evaluation of platelet activation: choice of anticoagulant. Transfus Med 1999; 9: 147–154. [DOI] [PubMed] [Google Scholar]
- 10. Kuhne T, Hornstein A, Semple J, et al. Flow cytometric evaluation of platelet activation in blood collected into EDTA vs Diatube-H, a sodium citrate solution supplemented with theophylline adenosine, and dipyridamole. Am J Hematol 1995; 50: 40–45. [DOI] [PubMed] [Google Scholar]
- 11. CLSI. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. 3rd ed. Vol C28-A3. Wayne, PA: CLSI, 2008. [Google Scholar]
- 12. Geffré A, Friedrichs K, Harr K, et al. Reference values: a review. Vet Clin Pathol 2009; 38: 288–298. [DOI] [PubMed] [Google Scholar]
- 13. Friedrichs KR, Harr KE, Freeman KP, et al. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 2012; 41: 441–453. [DOI] [PubMed] [Google Scholar]
- 14. Harr KE, Flatland B, Nabity M, et al. ASVCP guidelines: allowable total error guidelines for biochemistry. Vet Clin Pathol 2013; 42: 424–436. [DOI] [PubMed] [Google Scholar]
- 15. Ceron JJ, Martinez-Subiela S, Hennemann C, et al. The effects of different anticoagulants on routine canine plasma biochemistry. Vet J 2004; 167: 294–301. [DOI] [PubMed] [Google Scholar]
- 16. Thoresen SI, Havre G, Morber GH, et al. Effects of storage time on chemistry results from canine whole blood, serum and heparinized plasma. Vet Clin Pathol 1992; 21: 88–94. [DOI] [PubMed] [Google Scholar]
- 17. Lindner A. Comparison of clinical chemical variables in blood plasma and serum of horses. Eur J Clin Chem Clin Biochem 1992; 29: 837–840. [PubMed] [Google Scholar]