Abstract
Objectives
The purpose of this study was to evaluate histopathologic aspects of, and the expression of Ki-67 and cleaved caspase-3 in, feline mammary carcinoma (FMC).
Methods
Feline mammary tumors were surgically obtained by mastectomy from 30 female cats and were fixed with formalin and embedded in paraffin wax. Four-micron sections were stained with hematoxylin and eosin for histopathologic diagnosis. Ki-67 and cleaved caspase-3 were analyzed by immunohistochemistry.
Results
Samples were histologically confirmed as FMC. Positive immunostaining was observed in all cancer samples for both nuclear Ki-67 and cleaved caspase-3, with a mean positive staining percentage of 27.5% and 21.2%, respectively. No statistically significant correlations between Ki-67 and cleaved caspase-3 were observed within FMC.
Conclusions and relevance
A high proliferation index was found in feline mammary tumors. This is the first study evaluating cleaved caspase-3 expression in FMC.
Introduction
Feline mammary tumors are the third most common neoplasm described in the female cat.1–3 Most mammary tumors occur in middle-aged to older female cats, with a mean age of 10–12 years, and are usually malignant. Siamese cats may be at a higher risk of developing mammary tumors than other breeds.3–5
Feline mammary carcinoma (FMC) is a locally infiltrative and metastasizing tumor and has been proposed as a natural model of aggressive human breast cancer.5,6 Histopathologic evaluation is not always sufficient to predict the behavior of feline mammary tumors. In recent years, new immunohistochemical methods have been developed for measuring biologic parameters (eg, cell proliferation, growth factor responsiveness and presence of proto-oncogene products), which help to obtain more accurate diagnostic and prognostic information.1,4,7 In feline mammary tumors, immunohistochemistry (IHC) has been used to assess the cell proliferation index by the detection of nuclear proteins associated with cell division (ie, Ki-67),1,5,8,9 and to detect the expression of other significant biologic markers, 10 such as the tumor suppressor protein p53. 5 These studies, however, did not include cleaved caspase-3.
The process of apoptosis is complex and regulated, in part, by molecular markers often associated with mammary carcinogenesis. An imbalance between cell proliferation and cell death by apoptosis can contribute to carcinogenesis and tumor progression.11–13
According to O’Donavan et al, 14 the marking of caspase-3 can be used to control the response to chemotherapeutic treatments as a possible drug resistance, where the decrease of expression may indicate an important mechanism of cell survival in patients with breast cancer.
The aim of this study was to evaluate the histopathologic aspects of, and expression of Ki-67 and cleaved caspase-3 in, feline mammary carcinomas.
Materials and methods
This study was approved by the ethics committee for the use of animals (CEUA, Comissão de Ética do Uso Animal) at Fluminense Federal University.
Samples and histologic examination
Thirty mammary tumors from 30 female cats, which had been submitted to surgery for primary treatment, were analyzed. The age and breed of the cats were recorded. Tumor size was measured with a caliper and the macroscopic aspects were described.
Tissue samples were fixed in 10% neutral buffered formalin and were embedded in paraffin. Data regarding tumor size were recorded. Four-micron sections were stained with hematoxylin and eosin for histologic examination and were classified according to the World Health Organization criteria for feline mammary lesions. 15 Histologic grading of carcinomas was performed according to Misdorp et al, 15 based on three morphologic features: tubule formation, nuclear pleomorphism and mitotic index.
All samples were evaluated with respect to tubule formation, pleomorphy, prominent nucleoli, mitotic figures, necrosis, inflammatory infiltration, adjacent tissue invasion and lymph node metastasis.
IHC
Ki-67 and cleaved caspase-3 were analyzed by IHC. Immunostaining was performed on 2–3 μm sections, cut from formalin-fixed, paraffin-embedded tissue, using the streptavidin–biotin peroxidase complex technique with primary monoclonal anti-Ki-67 antibody (MIB-1, 1:125 dilution; Dako) or polyclonal anti-cleaved caspase-3 antibody (Asp 175, 1:300 dilution; Cell Signaling), and DAB as chromogen.
The immunostaining of both Ki-67 and cleaved caspase-3 was scored quantitatively in neoplastic epithelial and mesenchymal cells. The number of brown-stained positive cells was subjectively evaluated in the most highly stained area of each tissue by counting 1000 cells in at least 10 high-powered microscopic fields. Immunoreactivity was assessed as (–) negative, (+) when <10% of the neoplastic cells were positive, (++) when 10–25% of the neoplastic cells were positive, (+++) when 25–50% of the neoplastic cells were positive and (++++) when >50% of the neoplastic cells were positive. 16
Statistical analysis
For statistical analysis, SPSS 12.0 for Windows (IBM) was employed and a P value <0.05 was considered significant. Kruskal–Wallis and Mann–Whitney tests were used for comparison between imunoexpressions and histologic tumor type.
Results
Of the cats evaluated, 60% were Brazilian Shorthair, 23.4% were Siamese, 13.3% were Persian and 3.3% were Ragdolls. Mean age was 10.43 years.
All histologic analyses revealed moderate pleomorphic cellular, prominent nucleoli, mitotic figures and inflammatory infiltration (Figures 1 and 2). Necrosis was present in 18 mammary tumors (60.0%).
Figure 1.
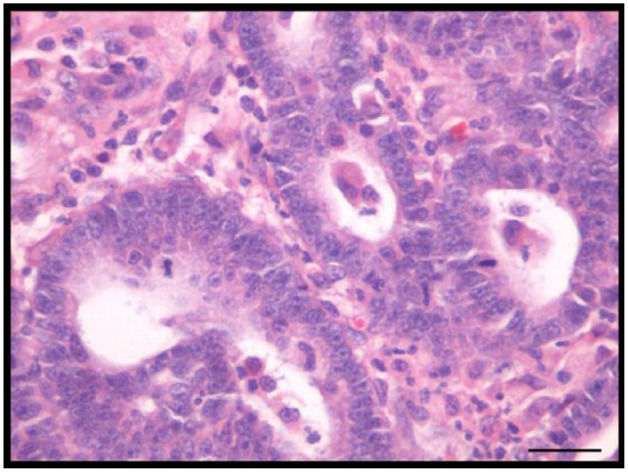
Feline tubulopapillary mammary carcinoma. Note moderate pleomorphic cells, prominent nucleoli and mitotic figures. Hematoxylin and eosin. Bar = 20 µm
Figure 2.
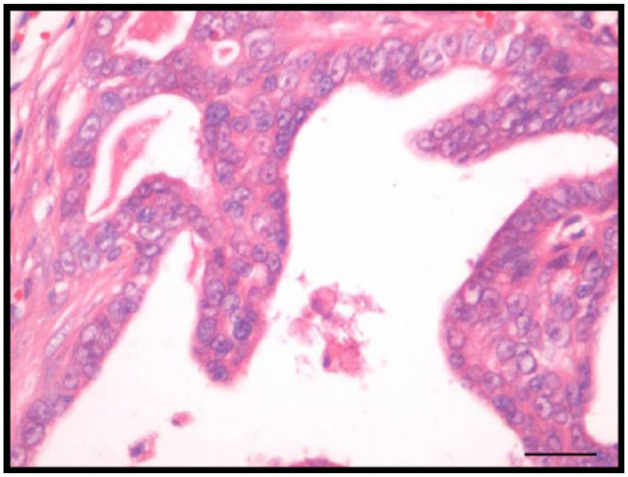
Feline tubulopapillary mammary carcinoma. Note papillary proliferation. Haematoxylin and eosin. Bar = 20 µm
Twenty-five mammary tumors (83.4%) were classified as tubulopapillary carcinoma and five (16.6%) were solid carcinoma. The relationship between the histologic type and the age of cats was not significant (P = 0.416).
From 30 primary FMCs, 17 were classified as grade I (56.7%), 12 as grade II (40.0%) and one as grade III (3.3%). Inadequate surgical margins were revealed in 9/24 cases. Two of these had microscopic invasion into muscular tissue and one had microscopic invasion into adipose tissue. Regional lymph node metastasis occurred in 3/13 (23%) cases.
Expression of Ki-67 in FMC
IHC staining for Ki-67 showed nuclear staining in all neoplastic samples, with a mean index of 27.5% (Figure 3). In some cases, cells expressing Ki-67 only showed nucleoli labeling. The mean immunostaining of Ki-67-positive FMC was scored as (+) in six cases (20.0%), (++) in 11 cases (36.6%), (+++) in 12 cases (40.0%) and (++++) in one case (3.3%).
Figure 3.
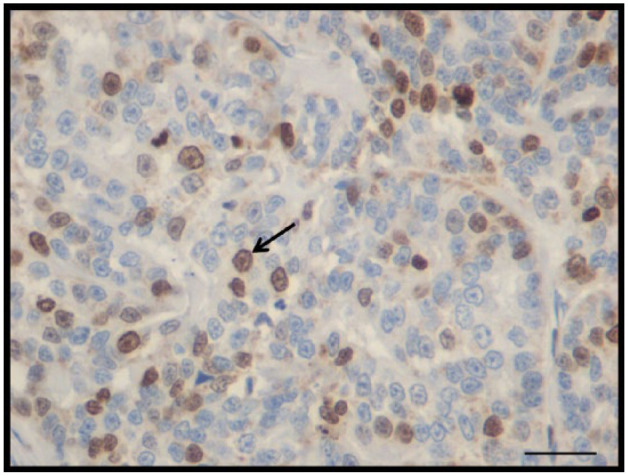
Feline mammary carcinoma immunohistochemistry. Expression of Ki-67. Bar = 20 µm
No relationship was found between Ki-67 count and histologic type (P = 0.884), or between Ki-67 count and tumor grade (P = 0.352). However, Ki-67 was significantly increased (P = 0.001) in FMC with signs of necrosis.
Expression of cleaved caspase-3 in FMC
Cleaved caspase-3 was positive in all neoplastic tissues, with an immunostaining average of 21.2%. In some cases, a granular staining pattern was seen (Figure 4), predominantly found in the cytoplasm. The mean immunostaining for cleaved caspase-3-positive FMC was scored as (+) in five cases (16.6%), (++) in 15 cases (50.0%), (+++) in nine cases (30.0%) and (++++) in one case (3.3%).
Figure 4.
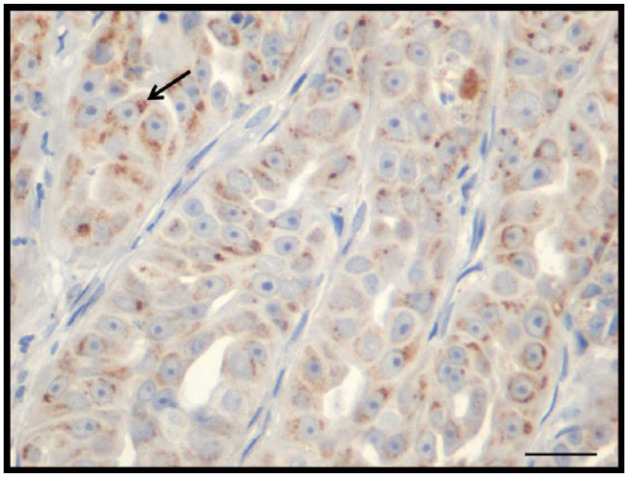
Feline mammary carcinoma immunohistochemistry. Expression of cleaved caspase-3. Bar = 20 µm
No relationship was found between cleaved caspase-3 count and histologic tumor type (P = 0.407) or between cleaved caspase-3 count and tumor grade (P = 0.521). Conversely, a positive correlation between cleaved caspase-3 and necrosis was observed (P = 0.01).
Among feline mammary carcinomas, no statistically significant correlation between Ki-67 and cleaved caspase-3 expression was observed (P = 0.084).
Discussion
In our study, the Brazilian Shorthair was the most affected breed. This differs from prior studies, which showed that the Siamese is the breed most affected by mammary tumors.3–5,17,18 This may be a reflection of breed distribution in Brazil, where the Brazilian Shorthair is the most common breed.
All histologic analyses revealed moderate pleomorphic cellular, prominent nucleoli, mitotic figures and inflammatory infiltration. These features classify histologic type and grading.15,19
The histologic classification of mammary samples in this study showed that tubulopapillary carcinoma was the most common (46.5%), similar to a study by Dias Pereira et al, 20 who diagnosed tubulopapillary carcinoma in 50% of feline tumor samples. However, the histologic analyses of mammary tumors in cats are quite varied among reports. Some described a higher frequency of solid carcinomas (in 62.5%) and 31.8% of cases, but another reported a higher frequency of tubular carcinoma (31.8%).4,5,21
Although most FMCs were grade I, there were cases with inadequate surgical margins, infiltration into muscular and adipose tissue. FMC is a locally infiltrative and metastasizing tumor that has behavior similar to human breast carcinoma. It has been proposed as a useful model for developing therapeutic strategies.1,6,22,23
IHC staining for Ki-67 showed nuclear staining in all neoplastic samples, with diversified labeling intensity, as previously reported by Vörös et al. 24
As observed by Hughes and Dobson, 1 Martins 25 and Zuccari et al, 26 the pattern of Ki-67 labeling differed among canine mammary tumors, results also seen in our study. According to Yu and Filipe, 27 this variation can be explained by the antigen being placed in the nucleolus in G1, with intensity during the S and G2 phases, reaching a maximum at mitosis. Throughout the progression from S to G2, the Ki-67 antigen is closely associated with chromatin.
The mean Ki-67 index was 27.5%. Other researchers have shown a Ki-67 index in FMC similar to those seen in this study (27.4%), and they infer that this index may correlate with a poor prognosis.1,5,8,28
No relationship was found between Ki-67 count and histologic type, as in other reports,8,20 or between Ki-67 count and tumor grade, which differs from a study by Rasotto et al, 5 which found a positive correlation between these factors in feline mammary tumors. This may be related to a difference on histologic types studied and/or a variation in the degree of malignancy found. Further studies are therefore necessary.
Apoptosis can be triggered by external stimuli via specific cell surface receptors called death receptors, or by intracellular stress, such as DNA damage or disturbances in the cell cycle or metabolic pathways. These different pathways culminate with the activation of proteases known as caspases, which play a key role in the cell death process. The caspases are present in the cytosol in the form of inactive pro-enzymes, becoming active after proteolytic cleavage. 29
Current evidence suggests that there are several different routes to caspase activation, depending on the stimulus that triggers machinery death and, in general, two different apoptosis pathways may be active, via death receptors and the mitochondrial pathway.30–32 The death receptors are present on cell surfaces and are activated in response to the coupling of specific ligands, which signal aggregation and formation of a complex inductor of death. The binding of this receptor complex to pro-caspase-8 results in the activation of this enzyme by proteolytic cleavage. The caspase-8 can then, directly or through the mitochondrial pathway, activate caspase-3 (effector caspase). The mitochondrial pathway is frequently activated in response to DNA damage, involving mitochondrial membrane permeability alterations and the release of cytochrome c into the cytosol, which binds to two proteins present in the cytosol, Apaf-1 and pro-caspase-9, which, in the presence of ATP, become active. The active caspase-9 (initiator) can then cleave the subsequent effector caspases (2, 3, 6, 7, 8, 9 and 10), performing the process of apoptosis. Therefore, activation of caspase-9 mediated by cytochrome c serves as a signal amplification mechanism during apoptosis.30,31,33–38
In all cleaved caspase-3-positive neoplastic tissues evaluated, the staining resulted in diversified labeling and, in some cases, a granular staining pattern was seen predominantly in the cytoplasm, which appeared to be lower than in a previous report, where 65.7% of canine mammary tumor samples were strongly positive. 39 This difference in labeling may be related to differences among species and to individual variability of the samples analyzed.
No relationship was found between cleaved caspase-3 count and histologic tumor type, or between cleaved caspase-3 count and tumor grade. This is similar to a human breast cancer report, which showed that the level of caspase-3 positivity was not related to histologic grade, lymph node status, recurrence or metastasis. 40 Furthermore, Zapata et al compared the intensity of cleaved caspase-3 immunoreactivity between normal human breast epithelium and invasive human breast carcinoma and found a stronger staining in the carcinoma samples. 41
Previous studies have already documented that alterations in caspase-3 might promote human tumorigenesis. 42 In the present study, the immunohistochemical analysis of cleaved caspase-3 revealed cytoplasmic labeling in all FMC samples, and the majority of them showed a high apoptosis index (>30%), 24 suggesting a correlation between cleaved caspase-3 expression and grade of malignancy. However, no statistical difference was found.
Among feline mammary carcinomas, no statistically significant correlation between Ki-67 and cleaved caspase-3 expression was observed. A positive statistical correlation between the apoptotic index and cell proliferation has been found in human and canine mammary tumors.39,42,43 Terzian et al found that a high number of apoptotic cells with a high rate of cell proliferation correlated with an unfavorable prognosis for canine mammary tumors. 39 However, in this study, there was no correlation between Ki-67 and cleaved caspase-3. This can be explained partly because, in poorly differentiated invasive carcinoma, apoptosis is impaired and proliferation is maintained, suggesting that proliferation-related mechanisms are of the highest importance. 43 Also, poorly differentiated, invasive human breast carcinomas are often accompanied by an increase in the number of proliferative cells and a decrease in the apoptotic fraction, which also seems to be important in carcinogenesis and progression. 43 To our knowledge, this is the first study to observe cleaved caspase-3 expression in FMC. Further studies should be conducted to confirm the expression profile of caspase-3 in FMC.
Histologic and biologic behaviors of human breast cancer are more similar to feline mammary tumors than they are to other animal species. Like human breast cancers, FMC is spontaneous, locally infiltrative and metastatic. Moreover, the Ki-67 index in feline tumor samples suggests a poor prognosis,1,5,8,24,28 as is also seen in human breast carcinoma. 44 A high apoptotic index resulting from caspase-3 activation also correlates with a poor prognosis in human breast cancer. Finally, cleaved caspase-3 expression could be related to radio- and chemo-resistance in these cancers. Thus, FMCs may be a desirable animal model in which to test innovative approaches to diagnosis and therapy of these aggressive tumors.
Conclusions
A high proliferation index and a low rate of apoptosis were found in feline mammary tumors, representative of what is seen in human breast cancer. This is the first study to evaluate cleaved caspase-3 expression in feline mammary carcinoma. Future studies should include more research on the expression of this apoptotic protein, as well as others also involved in cellular apoptosis, and their role in the pathology of feline cancer. This would certainly lead to a more accurate diagnosis.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 25 January 2016
References
- 1. Hughes K, Dobson JM. Prognostic histopathological and molecular markers in feline mammary neoplasia. Vet J 2012; 194: 19–26. [DOI] [PubMed] [Google Scholar]
- 2. Jacobs TM, Hoppe BR, Poehlmann CE, et al. Mammary adenocarcinomas in three male cats exposed to medroxyprogesterone acetate (1990–2006). J Feline Med Surg 2010; 12: 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. North S, Banks T. Tumours of the urogenital tract. In: North SBT. (ed). Introduction to small animal oncology. London: Elsevier, 2009, pp 151–171. [Google Scholar]
- 4. Seixas F, Pires MA, Lopes CA. Complex carcinomas of the mammary gland in cats: pathological and immunohistochemical features. Vet J 2008; 176: 210–215. [DOI] [PubMed] [Google Scholar]
- 5. Rasotto R, Caliari D, Castagnaro M, et al. An immunohistochemical study of HER-2 expression in feline mammary tumours. J Comp Pathol 2011; 144: 170–179. [DOI] [PubMed] [Google Scholar]
- 6. Ordas J, Millan Y, Dios R, et al. Proto-oncogene HER-2 in normal, dysplastic and tumorous feline mammary glands: an immunohistochemical and chromogenic in situ hybridization study. BMC Cancer 2007; 7: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sayasith K, Sirois J, Dore M. Molecular characterization of feline COX-2 and expression in feline mammary carcinomas. Vet Pathol 2009; 46: 423–429. [DOI] [PubMed] [Google Scholar]
- 8. Castagnaro M, De Maria R, Bozzetta E, et al. Ki-67 index as indicator of the post-surgical prognosis in feline mammary carcinomas. Res Vet Sci 1998; 65: 223–226. [DOI] [PubMed] [Google Scholar]
- 9. Morris JS, Nixon C, Bruck A, et al. Immunohistochemical expression of TopBP1 in feline mammary neoplasia in relation to histological grade, Ki67, ERalpha and p53. Vet J 2008; 175: 218–226. [DOI] [PubMed] [Google Scholar]
- 10. Zappulli V, Caliari D, Rasotto R, et al. Proposed classification of the feline “complex” mammary tumors as ductal and intraductal papillary mammary tumors. Vet Pathol 2013; 50: 1070–1077. [DOI] [PubMed] [Google Scholar]
- 11. Liu S, Edgerton SM, Moore DH, 2nd, et al. Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clin Cancer Res 2001; 7: 1716–1723. [PubMed] [Google Scholar]
- 12. Bertram JS. The molecular biology of cancer. Mol Aspects Med 2000; 21: 167–223. [DOI] [PubMed] [Google Scholar]
- 13. Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature 2001; 411: 342–348. [DOI] [PubMed] [Google Scholar]
- 14. O’Donovan N, Crown J, Stunell H, et al. Caspase 3 in breast cancer. Clin Cancer Res 2003; 9: 738–742. [PubMed] [Google Scholar]
- 15. Misdorp W, Else RW, Helmen E, et al. Histological classification of mammary tumors of the dog and the cat (WHO International Classification of Tumors of Domestic Animals). Washington, DC: American Registry of Pathology, 1999. [Google Scholar]
- 16. Allred DC, Harvey JM, Berardo M, et al. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Modern Pathol 1998; 11: 155–168. [PubMed] [Google Scholar]
- 17. Ressel L, Millanta F, Caleri E, et al. Reduced PTEN protein expression and its prognostic implications in canine and feline mammary tumors. Vet Pathol 2009; 46: 860–868. [DOI] [PubMed] [Google Scholar]
- 18. Amorim FV, Souza HJ, Ferreira AM, et al. Clinical, cytological and histopathological evaluation of mammary masses in cats from Rio de Janeiro, Brazil. J Feline Med Surg 2006; 8: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Misdorp W. Tumors of the mammary gland. In: Meuten DJ. (ed). Tumors in domestic animals. Ames: Wiley Blackwell, 2002, pp 575–606. [Google Scholar]
- 20. Dias Pereira P, Carvalheira J, Gartner F. Cell proliferation in feline normal, hyperplastic and neoplastic mammary tissue – an immunohistochemical study. Vet J 2004; 168: 180–185. [DOI] [PubMed] [Google Scholar]
- 21. Seixas F, Palmeira C, Pires MA, et al. Grade is an independent prognostic factor for feline mammary carcinomas: a clinicopathological and survival analysis. Vet J 2011; 187: 65–71. [DOI] [PubMed] [Google Scholar]
- 22. De Maria R, Olivero M, Iussich S, et al. Spontaneous feline mammary carcinoma is a model of HER2 overexpressing poor prognosis human breast cancer. Cancer Res 2005; 65: 907–912. [PubMed] [Google Scholar]
- 23. Misdorp W, Weijer K. Animal model of human disease: breast cancer. Am J Pathol 1980; 98: 573–576. [PMC free article] [PubMed] [Google Scholar]
- 24. Vörös A, Csorgo E, Kovari B, et al. The use of digital images improves reproducibility of the Ki-67 labeling index as a proliferation marker in breast cancer. Pathol Oncol Res 2014; 20: 391–397. [DOI] [PubMed] [Google Scholar]
- 25. Martins DC. Avaliação imuno-histoquímica da proliferação e morte celular em neoplasias mamárias malignas caninas. Universidade Federal Fluminense, 2008. [Google Scholar]
- 26. Zuccari DAPC, Pavam MV, Terzian ACB, et al. Immunohistochemical evaluation of Ki-67 and PCNA in canine mammary neoplasias: correlation with prognostic factors and clinical outcome. Pesquisa Vet Brasil 2008; 28: 207–215. [Google Scholar]
- 27. Yu CC, Filipe MI. Update on proliferation-associated antibodies applicable to formalin-fixed paraffin-embedded tissue and their clinical applications. Histochem J 1993; 25: 843–853. [PubMed] [Google Scholar]
- 28. Preziosi R, Sarli G, Benazzi C, et al. Multiparametric survival analysis of histological stage and proliferative activity in feline mammary carcinomas. Res Vet Sci 2002; 73: 53–60. [DOI] [PubMed] [Google Scholar]
- 29. Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 1972; 26: 239–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slee EA, Adrain C, Martin SJ. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ 1999; 6: 1067–1074. [DOI] [PubMed] [Google Scholar]
- 31. Slee EA, Harte MT, Kluck RM, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol 1999; 144: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hengartner MO. The biochemistry of apoptosis. Nature 2000; 407: 770–776. [DOI] [PubMed] [Google Scholar]
- 33. Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997; 91: 479–489. [DOI] [PubMed] [Google Scholar]
- 34. Green DR, Martin SJ. The killer and the executioner: how apoptosis controls malignancy. Curr Opin Immunol 1995; 7: 694–703. [DOI] [PubMed] [Google Scholar]
- 35. Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell 2003; 3: 17–22. [DOI] [PubMed] [Google Scholar]
- 36. Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol 2000; 10: 369–377. [DOI] [PubMed] [Google Scholar]
- 37. Kuida K. Caspase-9. Int J Biochem Cell Biol 2000; 32: 121–124. [DOI] [PubMed] [Google Scholar]
- 38. Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood 2001; 98: 2603–2614. [DOI] [PubMed] [Google Scholar]
- 39. Terzian ACB, Zuccari DAPC, Pereira RS, et al. Avaliação da caspase 3 e Ki-67 como marcadores prognósticos nas neoplasias mamárias em cadelas. Braz J Vet Res Anim Sci 2007; 44: 96–102. [Google Scholar]
- 40. Yang XF, Xin Y, Mao LL. Clinicopathological significance of PTEN and caspase-3 expressions in breast cancer. Chinese Med Sci J 2008; 23: 95–102. [DOI] [PubMed] [Google Scholar]
- 41. Zapata JM, Krajewska M, Krajewski S, et al. Expression of multiple apoptosis-regulatory genes in human breast cancer cell lines and primary tumors. Breast Cancer Res Treat 1998; 47: 129–140. [DOI] [PubMed] [Google Scholar]
- 42. McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harbor Perspect Biol 2013; 5: a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mommers EC, van Diest PJ, Leonhart AM, et al. Balance of cell proliferation and apoptosis in breast carcinogenesis. Breast Cancer Res Treat 1999; 58: 163–169. [DOI] [PubMed] [Google Scholar]
- 44. Offersen BV, Sorensen FB, Knoop A, et al. The prognostic relevance of estimates of proliferative activity in early breast cancer. Histopathology 2003; 43: 573–582. [DOI] [PubMed] [Google Scholar]


