Abstract
The lipid A domain anchors lipopolysaccharide (LPS) to the outer membrane and is typically a disaccharide of glucosamine that is both acylated and phosphorylated. The core and O-antigen carbohydrate domains are linked to the lipid A moiety through the eight-carbon sugar 3-deoxy-d-manno-octulosonic acid known as Kdo. Helicobacter pylori LPS has been characterized as having a single Kdo residue attached to lipid A, predicting in vivo a monofunctional Kdo transferase (WaaA). However, using an in vitro assay system we demonstrate that H. pylori WaaA is a bifunctional enzyme transferring two Kdo sugars to the tetra-acylated lipid A precursor lipid IVA. In the present work we report the discovery of a Kdo hydrolase in membranes of H. pylori capable of removing the outer Kdo sugar from Kdo2-lipid A. Enzymatic removal of the Kdo group was dependent upon prior removal of the 1-phosphate group from the lipid A domain, and mass spectrometric analysis of the reaction product confirmed the enzymatic removal of a single Kdo residue by the Kdo-trimming enzyme. This is the first characterization of a Kdo hydrolase involved in the modification of gram-negative bacterial LPS.
Helicobacter pylori is a gram-negative microaerophilic rod that colonizes the human stomach and infects nearly one-half of the world's population (34). Although most infected individuals are asymptomatic, the organism has been classified as a class I carcinogen playing a major etiological role in human gastritis, peptic ulcer disease, and gastric carcinoma (4, 6, 32). Despite the hostile ecological niche of the human stomach, no other significant reservoirs of H. pylori have been identified. Therefore, a balance must be established in order to permit long-term survival of both the bacterium and its human host.
Like all gram-negative bacteria, the outer surface of H. pylori consists primarily of protein and lipopolysaccharide (LPS). During bacterial infection, the presence of LPS leads to the activation of cells of the innate immune system primarily through Toll-like receptor 4 (TLR-4), a member of a family of cell surface molecules that recognize microbial products (1, 20, 33). It has been suggested that the LPS structure of H. pylori has evolved to aid the bacterium in evading the host innate immune system, thereby helping to prolong bacterial infection and contributing to the virulence and pathogenesis of the organism. H. pylori LPS is unusual in that it contains Lewis blood antigens giving rise to a form of molecular mimicry proposed to camouflage the bacterium which may aid in persistence of the infection (2). Furthermore, H. pylori LPS contains a unique lipid A domain that shows up to 1,000 times lower immunological activity than the lipid A of the Enterobacteriaceae family (30, 31, 38).
The lipid A domain serves as the hydrophobic anchor of LPS and is the bioactive component of the molecule that is associated with gram-negative septic shock (35). The reduced endotoxicity seen with H. pylori lipid A is thought to arise from its unique chemical structure. Compared to the lipid A of Escherichia coli, the major lipid A species of H. pylori (Fig. 1) lacks the usual 4′-phosphate, as well as the 3′-ester-linked fatty acyl chains, and is derivatized with a phosphoethanolamine (pEtN) residue at the C-1 position of the proximal glucosamine (27, 38, 39). Recently, our laboratory described the biochemical mechanism for the periplasmic modification of the 1 position of H. pylori lipid A (41). The origin of the pEtN residue arises from a two-step enzymatic process involving the removal of the 1-phosphate group from H. pylori lipid A by a phosphatase, Hp0021, followed by the addition of a pEtN residue catalyzed by Hp0022 (41). Since variation in the lipid A structure affects the degree of TLR-4 signaling and resistance to antimicrobial peptides, it is important to determine the biochemical mechanisms required for modification of lipid A.
FIG. 1.
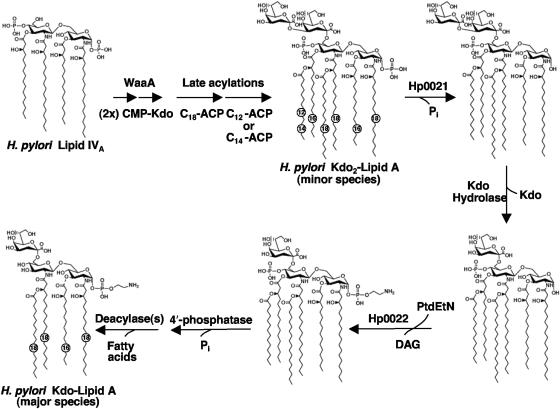
Proposed biosynthesis and modification of H. pylori Kdo2-lipid A. Following synthesis of lipid IVA, H. pylori WaaA transfers two Kdo sugars to the distal glucosamine of lipid A. Based upon the lipid A biosynthetic pathway of E. coli, this would be followed by the addition of the acyloxyacyl-linked fatty acyl chains, resulting in a lipid A species that can be detected as a minor component of H. pylori LPS (27, 38, 39). Following the constitutive biosynthetic pathway (the Raetz pathway), H. pylori Kdo2-lipid A is then modified by several enzymes. First, Hp0021 catalyzes the removal of the 1-phosphate group from H. pylori lipid A on the periplasmic side of the inner membrane (41). Other modifications include the removal of the outer Kdo sugar reported herein or the addition of a pEtN residue to the 1 position catalyzed by Hp0022 (41), both of which require prior removal of the 1-phosphate group. Additional enzymatic activities that are thought to be necessary for modification of H. pylori lipid A include a 4′-phosphatase (A. X. Tran and M. S. Trent, unpublished data) and at least one deacylase (C. M. Stead and M. S. Trent, unpublished data).
Lipid A is attached to the polysaccharide portion of LPS via the Kdo (3-deoxy-d-manno-octulosonic acid) moiety (35). Transfer of the Kdo sugars to the disaccharide backbone is catalyzed by the bifunctional glycosyltransferase WaaA (formerly KdtA) (5, 13) and is the first step of core biosynthesis in gram-negative bacteria with the remainder of the polysaccharide attached to the inner Kdo at the 5-OH group (35). In organisms having an LPS with only a single Kdo sugar, such as Bordetella pertussis and Haemophilus influenzae, WaaA has been shown to act as a monofunctional Kdo transferase (7, 21, 47). The inner core domain of H. pylori LPS also contains a single Kdo sugar, thus suggesting that H. pylori WaaA is a monofunctional transferase (3, 25, 26).
Upon investigation of the biochemical activity of H. pylori WaaA, we found that the enzyme behaved like the E. coli transferase catalyzing the addition of two Kdo moieties to lipid-IVA, a key lipid A precursor. Furthermore, we discovered an enzymatic activity in membranes of H. pylori that removes the outer Kdo from lipid A substrates containing two Kdo sugars. Therefore, the single Kdo sugar found in H. pylori LPS arises from the action of a specific Kdo hydrolase rather than from a monofunctional Kdo transferase.
MATERIALS AND METHODS
Chemicals and other materials.
[γ-32P]ATP and 32Pi were obtained from Amersham International. Silica gel 60 (0.25-mm) thin-layer plates were purchased from EM Separation Technology (Merck). Yeast extract, tryptone, and brucella broth were from Difco. Triton X-100 and bicinchoninic acid were from Pierce. All other chemicals were reagent grade and were purchased from either Sigma or Mallinckrodt.
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are summarized in Table 1. H. pylori strains J99 and 26695 were obtained from the American Type Culture Collection. Clinical isolates of H. pylori were obtained from human gastric biopsy specimens, as previously described (23). Primary plate cultures of H. pylori were grown from glycerol stock on blood agar medium at 37°C for 36 to 60 h in a microaerobic atmosphere (5% O2, 10% CO2, and 85% N2). The resultant colonies were inoculated into brucella broth supplemented with 10% fetal bovine serum (HyClone) and vancomycin (10 μg/ml). Cells were grown to an A600 of ∼1.0 at 37°C under microaerobic conditions for 24 to 36 h. Prior to every experiment, confirmation of H. pylori was performed by both Gram's stain and urease test (22). E. coli was typically grown at 37°C in Luria-Bertani broth (24). When required for selection of plasmids, cells were grown in the presence of ampicillin (100 μg/ml) or chloramphenicol (30 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| H. pylori strains | ||
| J99 | Wild type | ATCC 700824 |
| 26695 | Wild type | ATCC 700392 |
| Cp90-84 | Clinical isolate | ETSUa Clinical Microbiology Laboratory |
| Cp20-84 | Clinical isolate | ETSU Clinical Microbiology Laboratory |
| Hp7-91 | Clinical isolate | ETSU Clinical Microbiology Laboratory |
| Hp64-93 | Clinical isolate | ETSU Clinical Microbiology Laboratory |
| Hsp110-93 | Clinical isolate | ETSU Clinical Microbiology Laboratory |
| 26695/hp0021::cm | 26695 with chloramphenicol resistance cassette in hp0021 | This work |
| E. coli strains | ||
| W3110 | Wild type; F− λ− | E. coli Genetic Stock Center (Yale University) |
| XL-1 Blue | recA1 endA1 gyrA96thi-1 hsdR17 supE44 relA1 lac [F′ proAB lac1qZΔM15::Tn10 (Tetr)] | Stratagene |
| NovaBlue(DE3) | endA1 hsdR17(rK12− mK12+) supE44 thi-1 recA1 gyrA96 relA1 lac (DE3) [F′ proAB lac1qZΔM15:Tn10 (Tetr)] | Novagen |
| H. influenzae Rd | Wild type | ATCC 51907 |
| Plasmids | ||
| pET21a | Vector containing a T7 promoter; Ampr | Novagen |
| pWaaAEc | pET21a containing E. coli W3110 waaA | This work |
| pWaaAHi | pET21a containing H. influenzae waaA | This work |
| pWaaAHp1 | pET21a containing H. pylori J99 waaA | This work |
| pWaaAHp2 | pET21a containing H. pylori 26695 waaA gene | This work |
| pBluescript II SK (+) | Lac expression vector; Ampr | Stratagene |
| pBSHp0021 | pBluescript II SK (+) containing hp0021 | This work |
| pBSHp0021NX | pBSHp0021 with engineered NdeI and XhoI sites | This work |
| pBSHp0021::cm | pBSHp0021NX containing chloramphenicol resistance cassette in hp0021 | This work |
| pHel2 | E. coli-H. pylori shuttle vector; Camr | 36 |
ETSU, East Tennessee State University.
Recombinant DNA techniques.
Plasmids were isolated using the QIAGEN Spin Prep Kit. Custom primers were obtained from Integrated DNA Technologies. PCR reagents were purchased from Stratagene. PCR cleanup was performed using the QIAquick PCR Purification Kit (QIAGEN). DNA fragments were isolated from agarose gels using the QIAquick Gel Extraction Kit (QIAGEN). Restriction endonucleases, T4 DNA ligase, and shrimp alkaline phosphatase were purchased from New England Biolabs. All modifying enzymes were used according to the manufacturers' instructions.
Construction of an H. pylori 26695 1-phosphatase (hp0021)-defective mutant.
The hp0021 gene and its flanking sequences, including 654 bp upstream and 807 bp downstream, were amplified by PCR (primers 1 and 2) from H. pylori 26695 genomic DNA using Pfu Turbo (Stratagene) according to the manufacturer's instructions. The flanking DNA was digested with BamHI and KpnI, gel purified, and subsequently cloned into the high-copy phagemid vector pBluescript II SK (+) (Stratagene), resulting in the plasmid pBSHp0021. The vector pBSHp0021 was then subjected to two separate rounds of site-directed mutagenesis, using the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene), to create both NdeI (primers 3 and 4) and XhoI (primers 5 and 6) restriction sites (pBSHp0021NX). In order to disrupt the hp0021 gene, a chloramphenicol resistance cassette (cm) obtained by PCR (primers 7 and 8) from an E. coli-H. pylori shuttle vector (pHel2) (19) was inserted into the NdeI and XhoI sites of pBSHp0021NX. The resulting plasmid, pBShp0021::cm, containing an interrupted hp0021 gene was transformed into H. pylori 26695 by natural transformation (18) and resistant colonies selected on blood agar plates containing 8 μg/ml of chloramphenicol. Resistant colonies were repurified on chloramphenicol-containing plates, and the successful insertion of the resistance cassette was verified by PCR of genomic DNA.
Preparation of radiolabeled substrates.
The substrate [4′-32P]lipid IVA was generated from 125 μCi of [γ-32P]ATP and the tetraacyl-disaccharide 1-phosphate lipid acceptor (a generous gift from C. R. H. Raetz), using the overexpressed 4′-kinase present in membranes of E. coli BLR(DE3)/pLysS/pJK2 as previously described (42). Kdo2-[4′-32P]lipid IVA was prepared by adding purified E. coli Kdo transferase (KdtA) immediately after the 4′-kinase, as previously described (5). To prepare Kdo2-[4′-32P]lipid A (dilauroyl-Kdo2-[4′-32P]lipid IVA), lauroyl-acyl carrier protein (lauroyl-ACP) and membranes from E. coli overexpressing LpxL and LpxM (41, 45) were used in tandem with the Kdo transferase reaction mixture under the following conditions: 50 mM HEPES (pH 7.5), 0.1% Triton X-100, 50 mM NaCl, 50 mM MgCl2, 0.1 mg/ml bovine serum albumin, 13.3 μM lauroyl-ACP, and 0.05 mg/ml of BLR(DE3)/pLysS/pMsbB and BLR(DE3)/LysS/pHtrB membranes. Lauroyl-ACP was prepared as described by Trent and coworkers (43). Once the reaction was complete, the substrate was isolated as previously described (41). 1-Dephosphorylated-Kdo2-[4′-32P]lipid A was prepared by adding membranes of NovaBlue(DE3) overexpressing the H. pylori 1-phosphatase (Hp0021) (41) immediately following the acylation reaction using the following conditions: 0.25% Triton X-100, 50 mM morpholineethanesulfonic acid (pH 6.0), and 0.3 mg/ml NovaBlue(DE3)/pHp0021 membranes. The reaction mixture was incubated for 3 h at room temperature and the lipid isolated as previously described (41).
Overexpression of the Kdo transferase (waaA) behind a T7lac promoter.
The waaA genes of E. coli, H. influenzae, H. pylori J99, and H. pylori 26695 were separately subcloned into pET21a (Novagen) behind the T7lac promoter. The waaA genes were PCR amplified using genomic DNAs as templates. Sequences of primers are in Table 2. The forward primer contained a clamp region and NdeI site preceding the waaA coding region with its start codon. The reverse primer contained a clamp region, a BamHI site, a stop codon, a sequence for a His6 tag, and the coding region for the waaA gene. The PCR mixture contained 100 ng of genomic DNA template, 0.15 μg of each primer, 200 μM each deoxynucleoside triphosphate, 100 mM Tris-HCl (pH 8.8), 35 mM MgCl2, 250 mM KCl, and 2.5 U of Pfu DNA polymerase (Stratagene) in a reaction volume of 0.05 ml. The reaction mixture was subjected to denaturation at 95°C for 60 s, annealing at 57°C for 60 s, extension at 72°C for 80 s, and a final extension for 10 min at 72°C, using the Stratagene RoboCyler Gradient 40 PCR system. The PCR product and the vector were digested with NdeI and BamHI, ligated using a New England BioLabs Quick Ligation Kit, and transformed into XL-1 Blue (Stratagene) for propagation of the plasmid. The plasmid containing the PCR product was then transformed into NovaBlue(DE3) (Table 1) for overexpression of the protein.
TABLE 2.
Oligonucleotides used in this study
| Name | Sequence |
|---|---|
| HP26695 kdtA Forward | 5′-GCGCGCCATATGTTTAAGTTTTTCTACCTTTTATTTTTGACTTTGGGGCAT-3′ |
| HP26695 kdtA Reverse | 5′-GCGCGCGGATCCTTAGTGGTGGTGGTGGTGGTGATGTTTAATGAAAGCGAGCAA-3′ |
| HPJ99 kdtA Forward | 5′-GCGCGCCATATGTTTAAGTTTTTCTACCTTTTATTTTTGACTTTGGGGCAT-3′ |
| HPJ99 kdtA Reverse | 5′-GCGCGCGGATCCTTAGTGGTGGTGGTGGTGGTGATGTTTGATTAATGCGAGCAA-3′ |
| EC kdtA Forward | 5′-GCGCGCCATATGCTCGAATTGCTTTACACCGCCCTTCTCTACCTTATTCAG-3′ |
| EC kdtA Reverse | 5′-GCGCGCGGATCCTCAGTGGTGGTGGTGGTGGTGATGCGTTTTCGGTGGCAG-3′ |
| HI kdtA Forward | 5′-GCGCGCCATATGTGGCGTTTTTTTTATACCAGCTTG-3′ |
| HI kdtA Reverse | 5′-GCGCGCGGATCCTCAGTGGTGGTGGTGGTGGTGTACATTGCGCTCCAAATAAGG-3′ |
| Primer 1 | 5′-GCGCGCGGATCCAACGACGGCGAAAAGAAT-3′ |
| Primer 2 | 5′-GCGCGCGGTACCAGGATAAACCCTCTCTAT-3′ |
| Primer 3 | 5′-GTTTGGGCGAGCGCCCATATGGAGGTAATTTCAAC-3′ |
| Primer 4 | 5′-GTTGAAATTACCTCCATATGGGCGCTCGCCCAAAC-3′ |
| Primer 5 | 5′-AACATGCCAAGCGGGCACTCGAGTATGGTGGGTTTGGCGGTG-3′ |
| Primer 6 | 5′-CACCGCCAAACCCACCATACTCGAGTGCCCGCTTGGCATGTT-3′ |
| Primer 7 | 5′-GCG CGC CAT ATG CCG AGA TTT TCA GGA GCT-3′ |
| Primer 8 | 5′-GCG CGC CTC GAG TTA CGC CCC GCC CTG CCA-3′ |
Preparation of cell extracts, double-spun cytosol, and washed membrane.
Typically, 500 ml of H. pylori or 200 ml of E. coli cultures was grown to an A600 of ∼1.0 at 37°C and harvested by centrifugation at 6,000 × g for 30 min. All samples were prepared at 4°C. Cell extract, membrane-free cytosol, and washed membranes were prepared as previously described (42) and stored in aliquots at −20°C. Protein concentration was determined by the bicinchoninic acid method (37), using bovine serum albumin as the standard.
Assay of Kdo hydrolase activity (formation of reaction product A).
The Kdo hydrolase activity was assayed under optimized conditions in a 10-μl reaction mixture containing 50 mM HEPES (pH 8.0), 0.1% Triton X-100, and either 5 μM [4′-32P]lipid IVA, Kdo2-[4′-32P]lipid IVA, Kdo2-[4′-32P]lipid A, or 1-dephosphorylated Kdo2-[4′-32P]lipid A (each at ∼3,000 to 5,000 cpm/nmol) as the substrate. Washed membranes typically at 1 mg/ml were employed as the enzyme source, as indicated. Enzymatic reaction mixtures were incubated at 30°C for the indicated times and terminated by spotting 4.5-μl portions of the mixtures onto silica gel 60 thin-layer chromatography (TLC) plates. The plates were dried under a cool air stream for 20 min.
When [4′-32P]lipid IVA was employed as the substrate, reaction products were separated using the solvent chloroform-pyridine-88% formic acid-water (50:50:16:5, vol/vol). The reaction products generated from substrates having the Kdo moiety were separated using the solvent chloroform-pyridine-88% formic acid-water (30:70:16:10, vol/vol). TLC plates were exposed overnight to a PhosphorImager screen and product formation detected and analyzed using a Bio-Rad Molecular Imager PhosphorImager equipped with Quantity One software. The enzyme activity was calculated by determining the percentage of the substrate converted to product.
Assay of WaaA Kdo transferase activity.
Kdo transferase activity was assayed under optimized conditions based upon the method of Brozek et al. (8, 9). Reaction mixtures (10 μl) contained 50 mM HEPES (pH 7.5), 4 mM Kdo, 10 mM CTP, 10 mM MgCl2, 1 mU of partially purified CMP-Kdo synthase, 0.1% Triton X-100, and 10 μM [4′-32P]lipid IVA (∼3,000 to 5,000 cpm/nmol). Purified CMP-Kdo synthase was prepared as previously described (8). Washed membranes at 0.05 mg/ml were employed as the enzyme source, as indicated. Enzymatic reaction mixtures were incubated at 30°C for the indicated times and terminated by spotting 4.5-μl portions of the mixtures onto silica gel 60 TLC plates. Reaction products were separated and visualized as described above.
Characterization of the in vitro Kdo hydrolase reaction product by hydrolysis at pH 4.5.
Using the assay conditions described above for the H. pylori Kdo hydrolase, a 10-μl reaction mixture (20,000 cpm) was prepared to convert the 1-dephosphorylated Kdo2-[4′-32P]lipid A substrate to the lipid labeled as product A (1-dephosphorylated Kdo-[4′-32P]lipid A). An identical reaction mixture was prepared that did not contain an enzyme source. For each reaction mixture, 4 μl of 10% sodium dodecyl sulfate (SDS) and 26 μl of 19.25 mM sodium acetate (pH 4.5) were added to achieve a final solution containing 1% SDS and 12.5 mM sodium acetate for hydrolysis of the Kdo linkage. The reaction mixtures were incubated at 100°C, and at various time points 4 μl of sample was removed and spotted onto a silica TLC plate for analysis. Lipids were separated in the solvent chloroform-pyridine-88% formic acid-water (30:70:16:10, vol/vol) and visualized as described above.
Large-scale isolation of the H. pylori Kdo hydrolase reaction product, 1-dephospho-Kdo-lipid A.
A 20-ml reaction mixture containing 50 μM 1-dephosphorylated Kdo2-lipid A (mono-lauroyl, mono-myristoyl-Kdo2-lipid IVA) substrate (41), 0.5 mg/ml H. pylori J99 membranes, 50 mM HEPES (pH 8.0), and 0.1% Triton TX-100 was incubated at 30°C for 3 h. The reaction mixture was then converted into a two-phase Bligh-Dyer mixture consisting of chloroform-methanol-0.01 M HCl (2:2:1.8 vol/vol). Phases were separated in a clinical centrifuge and the lower phase removed to a separate tube. A second extraction of the resulting upper phase was achieved by addition of fresh preequilibrated lower phase. The lower phases containing the 1-dephosphorylated Kdo-lipid A and various membrane lipids were pooled and dried under a stream of N2. Lipids were separated by DEAE anion-exchange chromatography as previously described. Fractions containing the 1-dephosphorylated Kdo-lipid A reaction product were converted to a two-phase Bligh-Dyer mixture as described above and the lipid recovered from the lower phase.
Mass spectrometry of lipid A species.
Mass spectra of purified lipids were acquired in the negative-ion linear mode using a matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometer (AXIMA-CFR; Kratos Analytical, Manchester, United Kingdom) equipped with a nitrogen laser (337 nm). The instrument was operated using a 20-kV extraction voltage and time-delayed extraction, providing a mass resolution of about ±1 atomic mass unit for compounds with an Mr of ∼2,000. Each spectrum represented the average of 100 laser shots, and saturated 6-aza-2-thiothymine in 50% acetonitrile and 10% tribasic ammonium citrate (9:1, vol/vol) served as the matrix. The samples were dissolved in chloroform-methanol (4:1, vol/vol) and deposited on the sample plate, followed by an equal portion of matrix solution (0.3 μl). The sample was dried at 25°C prior to mass analysis.
RESULTS
Modification of Kdo2-lipid A precursors by H. pylori membranes.
Like E. coli, H. pylori synthesizes a hexa-acylated bis-phosphorylated lipid A structure which can be isolated as a minor lipid A species (Fig. 1) (27). However, the vast majority of H. pylori lipid A is underacylated and lacks phosphate moieties (27, 38, 39) (Fig. 1), suggesting the bacterium expresses enzymes that modify its lipid A structure after the constitutive biosynthetic pathway. In some organisms, such as Salmonella enterica serovar Typhimurium and Pseudomonas aeruginosa, enzymes responsible for modification of the lipid A moiety have been shown to be regulated by two component systems in response to specific environmental stimuli (16, 17, 28). Modifications of H. pylori lipid A, however, appear to be constitutively present, consistent with the reduced number of two-component regulatory systems in this organism (40).
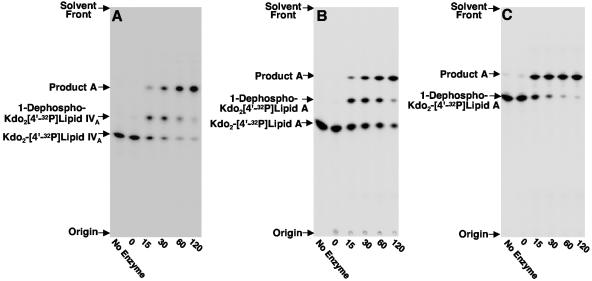
To understand how the major lipid A species of H. pylori is formed, we searched for enzymes capable of modifying key lipid A precursors. Assay of J99 membranes with a lipid A precursor containing the Kdo disaccharide Kdo2-[4′-32P]lipid IVA (Fig. 2A) resulted in the detection of two reaction products by TLC. The slower-migrating product resulted from the removal of the phosphate group from the 1 position catalyzed by the previously characterized inner membrane lipid A phosphatase Hp0021 (26695 protein designation) (41). The faster-migrating reaction product, designated product A, had not been previously detected when using [4′-32P]lipid IVA as the substrate (55), suggesting dependence upon the Kdo moiety for activity. Furthermore, it appeared that the 1-dephosphorylated Kdo2-[4′-32P]lipid IVA reaction product might be an intermediate step in the formation of product A (Fig. 2A).
FIG. 2.
Enzymatic modification of Kdo2-[4′-32P]lipid IVA (panel A), Kdo2-[4′-32P]lipid A (panel B), and 1-dephospho-Kdo2-[4′-32P]lipid A (panel C) using H. pylori membranes. Membranes from H. pylori strain J99 were assayed for enzymatic activities that modify the Kdo2-lipid A domain of LPS as described in Materials and Methods. The protein concentration was 1.0 mg/ml, and assays were carried out for the indicated times at 30°C. Reaction mixtures contained either 5 μM Kdo2-[4′-32P]lipid IVA (panel A), 5 μM Kdo2-[4′-32P]lipid A (panel B), or 5 μM 1-dephosphorylated Kdo2-[4′-32P]lipid A (panel C). Reaction products were separated by TLC and detected with PhosphorImager analysis. The previously characterized 1-dephosphorylated reaction products catalyzed by Hp0021 are indicated, and the unknown reaction product was designated product A. Similar results were obtained when membranes from H. pylori strain 26695 were used as the enzyme source (data not shown).
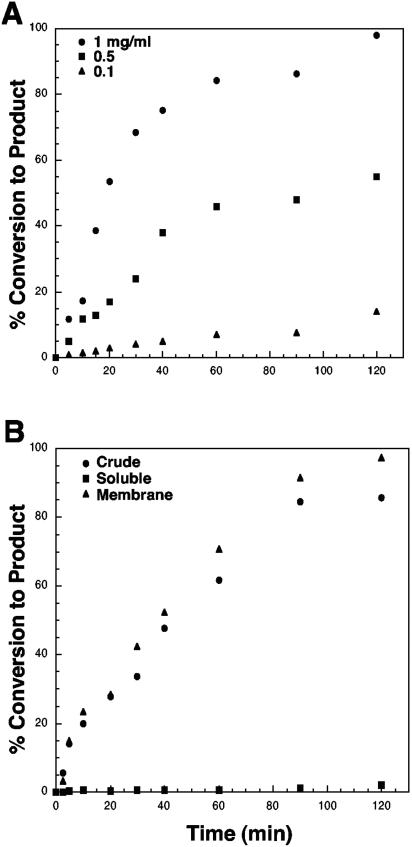
We assayed next the action of H. pylori membranes on hexa-acylated lipid A substrates. Similar amounts of enzymatic activity were seen with either Kdo2-[4′-32P]lipid IVA (Fig. 2A) or Kdo2-[4′-32P]lipid A (Fig. 2B), suggesting that the presence of the secondary acyl chains does not affect the formation of product A. Prior removal of the 1-phosphate group from Kdo2-[4′-32P]lipid A resulted in the direct conversion of 1-dephosphorylated Kdo2-[4′-32P]lipid A to product A (Fig. 2C). The conversion of 5 μM 1-dephosphorylated Kdo2-[4′-32P]lipid A to product A was linear with both protein concentration and time (Fig. 3A). When using 0.1 mg/ml H. pylori membranes as the enzyme source, conversion of the 1-dephosphorylated substrate to product A was linear with time for approximately 60 min at 30°C, having a specific activity of 0.06 nmol/min/mg. Nearly 100% conversion to product A was achieved when 1 mg/ml of membrane protein was used as the enzyme source (Fig. 3A). The enzymatic activity was localized solely to the membrane fraction (Fig. 3B).
FIG. 3.
Formation of reaction product A is dependent upon both time and protein concentration (panel A) and membrane associated (panel B). Panel A shows the formation of reaction product A assayed under standard conditions described in Materials and Methods using 5 μM 1-dephosphorylated Kdo2-[4′-32P]lipid A. Assays were carried out over a 2-h time course using H. pylori J99 membranes at the indicated concentrations. Reaction products were separated by TLC and subjected to PhosphorImager analysis. In panel B, crude extract, double-spun cytosol, or washed membranes at a protein concentration of 1 mg/ml were assayed as described for panel A for formation of reaction product A.
Some clues to the structure of product A can be inferred from its migration in the employed TLC systems. A faster-migrating reaction product in the TLC system results from an increase in the hydrophobic nature of the lipid A structure. Such modifications would include the removal of a phosphate group, the addition of a fatty acyl chain, or the removal of a sugar residue. In addition to the 1-phosphatase, H. pylori expresses a second phosphatase that removes the 4′-phosphate group (A. X. Tran, C. M. Stead, and M. S. Trent, unpublished data). Since the lipid substrates employed are 32P labeled at the 4′ position of the disaccharide, a 4′-phosphatase activity would cause the release of 32Pi. However, product A retained the 4′-32P-labeled phosphate group. Addition of a fatty acyl chain seemed unlikely because the chemical structure of H. pylori lipid A (27, 38, 39) does not contain acyl groups other than those resulting from the constitutive lipid A biosynthetic pathway. Since the enzymatic formation of product A required the presence of the Kdo disaccharide, we hypothesized that product A arose from the removal of the outer Kdo sugar, resulting in the formation of a more hydrophobic, faster-migrating lipid A species.
Characterization of reaction product A by pH 4.5 hydrolysis at 100°C.
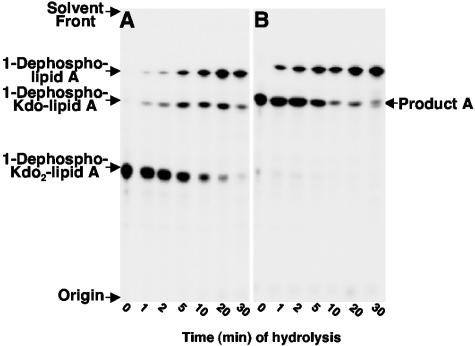
The Kdo sugars can be removed from the disaccharide backbone of lipid A by mild acid hydrolysis (pH 4.5) at 100°C without loss of the 1- or 4′-phosphate group. Under these conditions, the glycosidically linked anomeric carbons of Kdo are cleaved, removing the inner and outer Kdo sugars at comparable rates, leaving the remaining portion of the lipid A molecule intact (10, 11). As shown in Fig. 4A, incubation of 1-dephosphorylated Kdo2-[4′-32P]lipid A at 100°C results in formation of 1-dephosphorylated Kdo-lipid A that proceeds to 1-dephosphorylated lipid A lacking both Kdo residues. Unlike the initial substrate, hydrolysis of reaction product A (Fig. 4B) resulted in the formation of a single lipid species that migrated identical to that of 1-dephosphorylated lipid A. These data suggested that product A arises from removal of the outer Kdo sugar. If both Kdo sugars were present prior to chemical hydrolysis at 100°C, product A would have yielded an intermediate lipid A species containing a single Kdo sugar.
FIG. 4.
Time course of hydrolysis at pH 4.5 of 1-dephosphorylated Kdo2-[4′-32P]lipid A (panel A) and the in vitro reaction product A (panel B). Reaction product A or the initial substrate, 1-dephosphorylated Kdo2-[4′-32P]lipid A, was subjected to chemical hydrolysis at pH 4.5 (100°C) as described in Materials and Methods for the indicated times. Hydrolysis products were separated by TLC and subjected to PhosphorImager analysis.
Structural confirmation of the Kdo hydrolase reaction product by MALDI-TOF mass spectrometry.
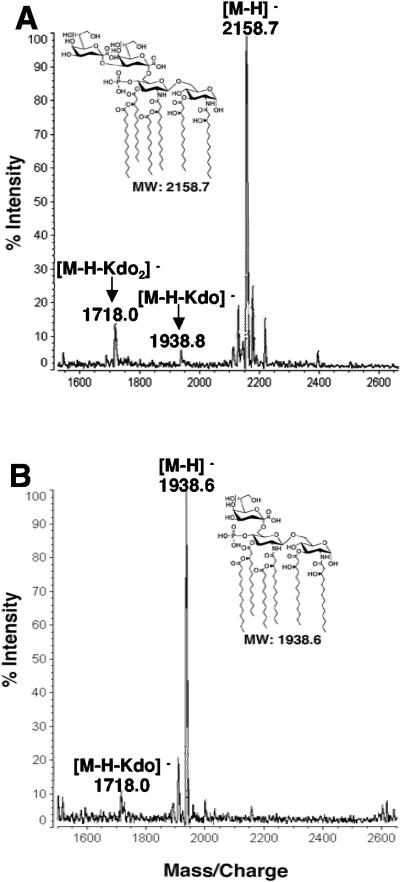
To confirm that product A arises from loss of the outer Kdo sugar, H. pylori membranes were used to convert 1-dephosphorylated Kdo2-lipid A (mono-lauroyl, mono-myristoyl-Kdo2-lipid IVA) to the proposed 1-desphosphorylated Kdo-lipid A reaction product and the lipid analyzed by MALDI-TOF mass spectrometry in the negative mode. MALDI-TOF mass spectrometry of the initial substrate, 1-dephosphorylated E. coli Kdo2-lipid A, showed major ions at m/z 2,158.7, 1,938.8, and 1,718.0 atomic mass units (Fig. 5A). The signal at m/z 2,158.7 atomic mass units is interpreted as [M-H]− of the predominant hexa-acylated Kdo2-lipid A containing a phosphate group solely at the 4′ position (Fig. 5A) (41). The minor peaks at m/z 1,938.8 and 1,718.0 atomic mass units are interpreted as [M-H-Kdo]− and [M-H-Kdo2]−, respectively, and correspond to a minor loss of the Kdo sugars during mass spectrometry (Fig. 5A). However, MALDI-TOF mass spectrometry of reaction product A revealed a predominant peak at m/z 1,938.6 atomic mass units, confirming the enzymatic removal of a single Kdo moiety (Fig. 5B). These data confirmed that H. pylori expresses a Kdo-trimming enzyme that removes the outer Kdo sugar from H. pylori LPS.
FIG. 5.
MALDI-TOF mass spectrometry of the Kdo hydrolase reaction product (product A) generated from 1-dephosphorylated Kdo2-lipid A. The reaction product generated from incubation of 1-dephosphorylated Kdo2-lipid A with H. pylori J99 membranes was purified by DEAE cellulose chromatography as described in Materials and Methods. Panel A shows the spectra of the starting 1-dephosphorylated Kdo2-lipid A substrate, and panel B shows the resulting reaction product containing a single Kdo sugar, 1-dephosphorylated Kdo-lipid A. Both spectra were acquired in the negative-ion mode. MW, molecular weight.
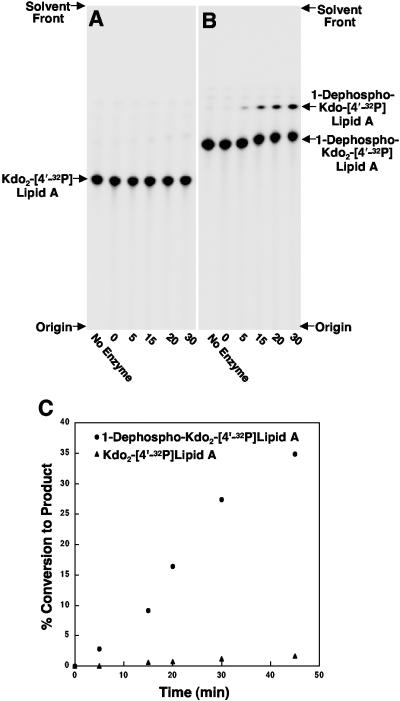
Kdo hydrolase activity in vitro requires prior removal of the 1-phosphate group.
To determine if the Kdo hydrolase activity required prior removal of the 1-phosphate group, we constructed an H. pylori 26695 derivative lacking a functional copy of the 1-phosphatase gene, hp0021. H. pylori mutants containing a chloramphenicol cassette in hp0021 are unable to remove the phosphate group from the 1 position of the lipid A domain (A. X. Tran and M. S. Trent, unpublished data). As shown in panel A of Fig. 6, membranes from the hp0021 mutant strain were unable to catalyze the removal of the 1-phosphate group from Kdo2-[4′-32P]lipid A. Furthermore, membranes from the 1-phosphatase mutant were unable to catalyze the removal of the outer Kdo sugar from Kdo2-[4′-32P]lipid A. This is in direct contrast to the enzymatic activities seen when using wild-type membranes (Fig. 3B). However if the lipid substrate was dephosphorylated at the 1 position prior to assay, Kdo hydrolase activity was restored (Fig. 6B). Analysis of the Kdo hydrolase activity revealed a 30-fold increase when 1-dephosphorylated Kdo2-[4′-32P]lipid A was used as the substrate (Fig. 6C). Minor amounts of hydrolase activity of up to 10 to 15% could be seen over extended assay times (over 3 h) with substrates retaining the 1-phosphate moiety (data not shown).
FIG. 6.
Membranes from an H. pylori 1-phosphatase (hp0021) mutant require prior removal of the 1-phosphate group from Kdo2-lipid A for Kdo hydrolase activity. The Kdo hydrolase activity of H. pylori membranes lacking a functional copy of hp0021 (1-phosphatase) was assayed using either 5 μM Kdo2-[4′-32P]lipid A (panel A) or 5 μM 1-dephosphorylated Kdo2-[4′-32P]lipid A (panel B). Assays were performed as described in Materials and Methods using 0.5 mg/ml of membranes for the indicated times. The reaction products were separated by TLC and subjected to PhosphorImager analysis. Panel C is a plot of Kdo hydrolase activity using the two substrates described above during a linear period of product formation.
H. pylori waaA encodes a bifunctional Kdo transferase.
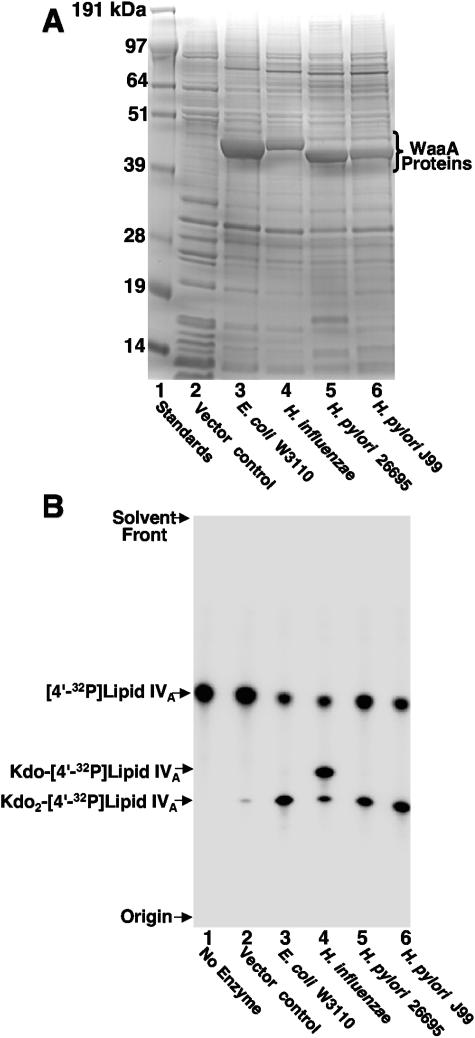
Structural data of the inner core region of H. pylori LPS reveals the presence of a single Kdo sugar, thus predicting a monofunctional Kdo transferase (WaaA) (3, 25, 26). However, if H. pylori expresses a Kdo hydrolase, one might expect that H. pylori WaaA acts as a bifunctional transferase. WaaA of H. pylori strains J99 and 26695 was overexpressed in the E. coli K-12 expression host NovaBlue(DE3) (Fig. 7A). The proteins were assayed for Kdo transferase activity along with the WaaA of both E. coli K-12 strain W3110 and H. influenzae Rd (Fig. 7B) using 32P-labeled lipid IVA as the substrate. As previously reported, the WaaA of E. coli transferred two sugars to [4′-32P]lipid IVA, forming Kdo2-[4′-32P]lipid IVA (Fig. 7B, lane 3) (5, 13), whereas the monofunctional transferase of Haemophilus (7, 47) transferred primarily one Kdo sugar (Fig. 7B, lane 4). Interestingly, the H. pylori enzymes transferred two Kdo sugars (Fig. 7B, lanes 5 and 6) as efficiently as E. coli WaaA, thus identifying the H. pylori enzyme as a bifunctional glycosyltransferase.
FIG. 7.
H. pylori WaaA is a bifunctional Kdo transferase. (A) Membranes from the NovaBlue(DE3) expression strain, containing either pET21a (vector control) or WaaA plasmids (Table 1), were isolated, and 15-μg samples of protein were analyzed by SDS-polyacrylamide gel electrophoresis using 12% polyacrylamide. The gel was stained with Coomassie blue. The positions of the molecular weight standards and WaaA proteins are indicated. (B) Membranes from NovaBlue(DE3) expressing WaaA from the indicated gram-negative organisms were assayed for Kdo transferase activity as previously described (8, 47). The protein concentration was 0.05 mg/ml, and assays were carried out for 1 h at 30°C with 5 μM [4′-32P]lipid IVA substrate. Under these conditions, the endogenous Kdo transferase activity (lane 2) from expression of chromosomal waaA was minimal (<3%). Products were separated by TLC and detected with PhosphorImager analysis.
Presence of Kdo hydrolase activity in clinical isolates of H. pylori.
To determine if the Kdo-trimming activity was widely found in various human isolates, H. pylori isolates from gastric biopsy samples of five different patients were cultured in liquid medium (23). Assay of the membrane fraction from each clinical isolate showed a Kdo hydrolase activity when 1-dephosphorylated Kdo2-[4′-32P]lipid A was used as the substrate (C. M. Stead and M. S. Trent, unpublished results). Secondly, the level of Kdo-trimming activity was impressive for all clinical isolates and in some cases stronger than that previously seen with strains J99 and 26695. Therefore, the enzymatic removal of Kdo from H. pylori LPS appears to be a conserved feature of H. pylori LPS biosynthesis. Prior to this report, a putative Kdo-trimming activity was reported in membranes of Francisella novicida (46). The identification of the H. pylori activity along with the biophysical analysis of its reaction product opens the possibility of a Kdo hydrolase in other gram-negative bacterial pathogens.
DISCUSSION
The core and lipid A domains of LPS are synthesized on the cytoplasmic side of the inner membrane of the gram-negative bacterial cell envelope (35, 36). MsbA is an essential ABC transporter that is required for flipping LPS to the periplasmic side of the inner membrane (12, 14, 15). In the human pathogen H. pylori, transport of LPS across the inner membrane is followed by the removal of the 1-phosphate group by a specific lipid A 1-phosphatase, Hp0021. Removal of the 1-phosphate group allows the periplasmic addition of a pEtN residue to the C-1 hydroxyl catalyzed by Hp0022 (41).
By assaying H. pylori membranes for enzymes capable of further modifying LPS precursors, we detected the presence of a membrane-bound enzyme that removes the outer Kdo sugar from H. pylori LPS. Analysis of the reaction product by chemical hydrolysis and mass spectrometry confirmed the removal of a single Kdo sugar. Under long periods of incubation (up to 6 h), we did not detect any significant removal of the inner Kdo sugar (data not show). Based upon its requirement for 1-dephosphorylated substrates (Fig. 6) for enzymatic activity, we propose that the Kdo hydrolase functions after Hp0021 in the biosynthesis of H. pylori LPS (Fig. 1), suggesting the enzyme's active site also lies in the extracytoplasmic region of the bacterium. At this time we are unable to determine if the H. pylori lipid A pEtN transferase, Hp0022, functions before or after removal of the outer Kdo sugar. Since H. pylori synthesizes a minor lipid A species resembling that found in E. coli (Fig. 1), further processing of H. pylori LPS/lipid A includes the removal of the 4′-phosphate and removal of specific fatty acyl chains (Fig. 1).
The inner core region of H. pylori LPS has been reported to contain a single Kdo sugar predicting the presence of a monofunctional Kdo transferase. Monofunctional Kdo transferases have been identified in other gram-negative bacteria such as B. pertussis (21) and H. influenzae (47). However, if a Kdo-trimming enzyme exists in H. pylori one would expect a bifunctional Kdo transferase to be present in the organism. Comparison of H. pylori WaaA with previously characterized mono- and bifunctional Kdo transferases showed that H. pylori WaaA transfers two Kdo sugars to the lipid A precursor lipid IVA (Fig. 7). Clearly, the number of Kdo sugars in the core oligosaccharide of LPS can no longer conclusively predict the functionality of WaaA of gram-negative bacteria.
The chemical composition of the lipid A moiety of gram-negative bacterial LPS is critical for the endotoxic properties of the molecule. However, it is not clear if variation of the core oligosaccharide plays an important role in host cell activation (29, 44). Recently, it was reported that the Kdo sugars of Neisseria meningitidis lipooligosaccharide were important for activation of human and murine macrophages via the TLR-4/MD-2 pathway (48). Perhaps in conjunction with other modifications of the H. pylori Kdo2-lipid A structure, removal of the outer Kdo sugar leads to a reduction in the agonistic activity of H. pylori LPS, contributing to persistence of the organism. Secondly, it is possible that reduction of the number of Kdo sugars could aid in resistance to cationic antimicrobial peptides since the Kdo sugars are negatively charged. Identification of the structural gene encoding the H. pylori Kdo hydrolase will be required to determine the role this unique LPS-modifying enzyme plays in the pathogenesis of gram-negative bacterial infections.
Acknowledgments
C. M. Stead and M. S. Trent thank M. E. Lester for critical reading of the manuscript.
This work was supported by National Institutes of Health grants K22-AI53645 (to M.S.T.) and RO1-GM6440 (to R.J.C.).
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Appelmelk, B. J., M. A. Monteiro, S. L. Martin, A. P. Moran, and C. M. Vandenbroucke-Grauls. 2000. Why Helicobacter pylori has Lewis antigens. Trends Microbiol. 8:565-570. [DOI] [PubMed] [Google Scholar]
- 3.Aspinall, G. O., M. A. Monteiro, R. T. Shaver, L. A. Kurjanczyk, and J. L. Penner. 1997. Lipopolysaccharides of Helicobacter pylori serogroups O:3 and O:6—structures of a class of lipopolysaccharides with reference to the location of oligomeric units of d-glycero-alpha-d-manno-heptose residues. Eur. J. Biochem. 248:592-601. [DOI] [PubMed] [Google Scholar]
- 4.Bayerdorffer, E., A. Neubauer, B. Rudolph, C. Thiede, N. Lehn, S. Eidt, and M. Stolte. 1995. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. Lancet 345:1591-1594. [DOI] [PubMed] [Google Scholar]
- 5.Belunis, C. J., and C. R. Raetz. 1992. Biosynthesis of endotoxins. Purification and catalytic properties of 3-deoxy-d-manno-octulosonic acid transferase from Escherichia coli. J. Biol. Chem. 267:9988-9997. [PubMed] [Google Scholar]
- 6.Blaser, M. J. 1996. The bacteria behind ulcers. Sci. Am. 274:104-107. [DOI] [PubMed] [Google Scholar]
- 7.Brabetz, W., S. Muller-Loennies, and H. Brade. 2000. 3-Deoxy-d-manno-oct-2-ulosonic acid (Kdo) transferase (WaaA) and Kdo kinase (KdkA) of Haemophilus influenzae are both required to complement a waaA knockout mutation of Escherichia coli. J. Biol. Chem. 275:34954-34962. [DOI] [PubMed] [Google Scholar]
- 8.Brozek, K. A., K. Hosaka, A. D. Robertson, and C. R. Raetz. 1989. Biosynthesis of lipopolysaccharide in Escherichia coli. Cytoplasmic enzymes that attach 3-deoxy-d-manno-octulosonic acid to lipid A. J. Biol. Chem. 264:6956-6966. [PubMed] [Google Scholar]
- 9.Brozek, K. A., and C. R. Raetz. 1992. 3-Deoxy-d-manno-octulosonate transferase and late acyltransferases of lipopolysaccharide biosynthesis. Methods Enzymol. 209:476-485. [DOI] [PubMed] [Google Scholar]
- 10.Caroff, M., C. Deprun, D. Karibian, and L. Szabo. 1991. Analysis of unmodified endotoxin preparations by 252Cf plasma desorption mass spectrometry. Determination of molecular masses of the constituent native lipopolysaccharides. J. Biol. Chem. 266:18543-18549. [PubMed] [Google Scholar]
- 11.Caroff, M., A. Tacken, and L. Szabo. 1988. Detergent-accelerated hydrolysis of bacterial endotoxins and determination of the anomeric configuration of the glycosyl phosphate present in the “isolated lipid A” fragment of the Bordetella pertussis endotoxin. Carbohydr. Res. 175:273-282. [DOI] [PubMed] [Google Scholar]
- 12.Chang, G., and C. B. Roth. 2001. Structure of MsbA from E. coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science 293:1793-1800. [DOI] [PubMed] [Google Scholar]
- 13.Clementz, T., and C. R. Raetz. 1991. A gene coding for 3-deoxy-d-manno-octulosonic-acid transferase in Escherichia coli. Identification, mapping, cloning, and sequencing. J. Biol. Chem. 266:9687-9696. [PubMed] [Google Scholar]
- 14.Doerrler, W. T., H. S. Gibbons, and C. R. Raetz. 2004. MsbA-dependent translocation of lipids across the inner membrane of Escherichia coli. J. Biol. Chem. 45102-45109. [DOI] [PubMed]
- 15.Doerrler, W. T., M. C. Reedy, and C. R. Raetz. 2001. An Escherichia coli mutant defective in lipid export. J. Biol. Chem. 276:11461-11464. [DOI] [PubMed] [Google Scholar]
- 16.Gunn, J. S., K. B. Lim, J. Krueger, K. Kim, L. Guo, M. Hackett, and S. I. Miller. 1998. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 27:1171-1182. [DOI] [PubMed] [Google Scholar]
- 17.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276:250-253. [DOI] [PubMed] [Google Scholar]
- 18.Haas, R., T. F. Meyer, and J. P. van Putten. 1993. Aflagellated mutants of Helicobacter pylori generated by genetic transformation of naturally competent strains using transposon shuttle mutagenesis. Mol. Microbiol. 8:753-760. [DOI] [PubMed] [Google Scholar]
- 19.Heuermann, D., and R. Haas. 1998. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 257:519-528. [DOI] [PubMed] [Google Scholar]
- 20.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 21.Isobe, T., K. A. White, A. G. Allen, M. Peacock, C. R. Raetz, and D. J. Maskell. 1999. Bordetella pertussis waaA encodes a monofunctional 2-keto-3-deoxy-d-manno-octulosonic acid transferase that can complement an Escherichia coli waaA mutation. J. Bacteriol. 181:2648-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jerris, R. C. 1995. Helicobacter, p. 492-498. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. American Society of Microbiology, Washington, D.C.
- 23.Li, C., D. A. Ferguson, Jr., T. Ha, D. S. Chi, and E. Thomas. 1993. A highly specific and sensitive DNA probe derived from chromosomal DNA of Helicobacter pylori is useful for typing H. pylori isolates. J. Clin. Microbiol. 31:2157-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, J. R. 1972. Experiments in molecular genetics. Cold Springs Harbor Laboratory, Cold Springs Harbor, N.Y.
- 25.Monteiro, M. A., B. J. Appelmelk, D. A. Rasko, A. P. Moran, S. O. Hynes, L. L. MacLean, K. H. Chan, F. S. Michael, S. M. Logan, J. O'Rourke, A. Lee, D. E. Taylor, and M. B. Perry. 2000. Lipopolysaccharide structures of Helicobacter pylori genomic strains 26695 and J99, mouse model H. pylori Sydney strain, H. pylori P466 carrying sialyl Lewis X, and H. pylori UA915 expressing Lewis B classification of H. pylori lipopolysaccharides into glycotype families. Eur. J. Biochem. 267:305-320. [DOI] [PubMed] [Google Scholar]
- 26.Moran, A. P., Y. A. Knirel, S. N. Senchenkova, G. Widmalm, S. O. Hynes, and P. E. Jansson. 2002. Phenotypic variation in molecular mimicry between Helicobacter pylori lipopolysaccharides and human gastric epithelial cell surface glycoforms. Acid-induced phase variation in Lewis(x) and Lewis(y) expression by H. pylori lipopolysaccharides. J. Biol. Chem. 277:5785-5795. [DOI] [PubMed] [Google Scholar]
- 27.Moran, A. P., B. Lindner, and E. J. Walsh. 1997. Structural characterization of the lipid A component of Helicobacter pylori rough- and smooth-form lipopolysaccharides. J. Bacteriol. 179:6453-6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moskowitz, S. M., R. K. Ernst, and S. I. Miller. 2004. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J. Bacteriol. 186:575-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muroi, M., and K. Tanamoto. 2002. The polysaccharide portion plays an indispensable role in Salmonella lipopolysaccharide-induced activation of NF-κB through human Toll-like receptor 4. Infect. Immun. 70:6043-6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa, T., Y. Asai, Y. Sakai, M. Oikawa, K. Fukase, Y. Suda, S. Kusumoto, and T. Tamura. 2003. Endotoxic and immunobiological activities of a chemically synthesized lipid A of Helicobacter pylori strain 206-1. FEMS Immunol. Med. Microbiol. 36:1-7. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa, T., Y. Suda, W. Kashihara, T. Hayashi, T. Shimoyama, S. Kusumoto, and T. Tamura. 1997. Immunobiological activities of chemically defined lipid A from Helicobacter pylori LPS in comparison with Porphyromonas gingivalis lipid A and Escherichia coli-type synthetic lipid A (compound 506). Vaccine 15:1598-1605. [DOI] [PubMed] [Google Scholar]
- 32.Parsonnet, J., G. D. Friedman, D. P. Vandersteen, Y. Chang, J. H. Vogelman, N. Orentreich, and R. K. Sibley. 1991. Helicobacter pylori infection and the risk of gastric carcinoma. N. Engl. J. Med. 325:1127-1131. [DOI] [PubMed] [Google Scholar]
- 33.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 34.Pounder, R. E., and D. Ng. 1995. The prevalence of Helicobacter pylori infection in different countries. Aliment. Pharmacol. Ther. 9(Suppl. 2):33-39. [PubMed] [Google Scholar]
- 35.Raetz, C. R., and C. Whitfield. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71:635-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raetz, C. R. H. 1996. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles, p. 1035-1063. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 37.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 38.Suda, Y., Y. M. Kim, T. Ogawa, N. Yasui, Y. Hasegawa, W. Kashihara, T. Shimoyama, K. Aoyama, K. Nagata, T. Tamura, and S. Kusumoto. 2001. Chemical structure and biological activity of a lipid A component from Helicobacter pylori strain 206. J. Endotoxin Res. 7:95-104. [PubMed] [Google Scholar]
- 39.Suda, Y., T. Ogawa, W. Kashihara, M. Oikawa, T. Shimoyama, T. Hayashi, T. Tamura, and S. Kusumoto. 1997. Chemical structure of lipid A from Helicobacter pylori strain 206-1 lipopolysaccharide. J. Biochem. (Tokyo) 121:1129-1133. [DOI] [PubMed] [Google Scholar]
- 40.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, J. C. Venter, et al. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 41.Tran, A. X., M. J. Karbarz, X. Wang, C. R. Raetz, S. C. McGrath, R. J. Cotter, and M. S. Trent. 2004. Periplasmic cleavage and modification of the 1-phosphate group of Helicobacter pylori lipid A. J. Biol. Chem. 279:55780-55791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trent, M. S., W. Pabich, C. R. Raetz, and S. I. Miller. 2001. A PhoP/PhoQ-induced lipase (PagL) that catalyzes 3-O-deacylation of lipid A precursors in membranes of Salmonella typhimurium. J. Biol. Chem. 276:9083-9092. [DOI] [PubMed] [Google Scholar]
- 43.Trent, M. S., L. M. Worsham, and M. L. Ernst-Fonberg. 1998. The biochemistry of hemolysin toxin activation: characterization of HlyC, an internal protein acyltransferase. Biochemistry 37:4644-4652. [DOI] [PubMed] [Google Scholar]
- 44.Ulmer, A. J., H. Heine, W. Feist, M. H. Wang, H. Loppnow, T. Kirikae, F. Kirikae, S. Kusumoto, T. Kusama, H. Brade, et al. 1994. Biological activity of lipid A partial structures. Prog. Clin. Biol. Res. 388:71-83. [PubMed] [Google Scholar]
- 45.Vorachek-Warren, M. K., S. Ramirez, R. J. Cotter, and C. R. Raetz. 2002. A triple mutant of Escherichia coli lacking secondary acyl chains on lipid A. J. Biol. Chem. 277:14194-14205. [DOI] [PubMed] [Google Scholar]
- 46.Wang, X., M. J. Karbarz, S. C. McGrath, R. J. Cotter, and C. R. Raetz. 2004. MsbA transporter dependent lipid A 1-dephosphorylation on the periplasmic surface of the inner membrane: topography of Franciscella novicida LpxE expressed in Escherichia coli. J. Biol. Chem. 279:49470-49478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White, K. A., I. A. Kaltashov, R. J. Cotter, and C. R. Raetz. 1997. A mono-functional 3-deoxy-d-manno-octulosonic acid (Kdo) transferase and a Kdo kinase in extracts of Haemophilus influenzae. J. Biol. Chem. 272:16555-16563. [DOI] [PubMed] [Google Scholar]
- 48.Zughaier, S. M., Y. L. Tzeng, S. M. Zimmer, A. Datta, R. W. Carlson, and D. S. Stephens. 2004. Neisseria meningitidis lipooligosaccharide structure-dependent activation of the macrophage CD14/Toll-like receptor 4 pathway. Infect. Immun. 72:371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]